(C) 2012 Teresa Bonacci. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Cucujus tulliae sp. n. is described as a new member of genus Cucujus Fabricius, 1775 (Coleoptera, Cucujidae), which enumerates at present eleven species distributed in Eurasia and northern America. This saproxylic beetle is the first Cucujus species known only from Mediterranean and it is probably endemic to Calabria (Italy). The species was found especially in old–growth mountain forests of high conservation value (i.e. national parks) dominated by Calabrian pine (Pinus laricio calabrica). We hypothesize that Cucujus tulliae sp. n. probably evolved from isolated populations of Cucujus haematodes Erichson, 1845. The species is thus relictual and of high conservation value, corresponding at least to endangered (EN) category with respect to recent IUCN criterion. Cucujus tulliae sp. n. is here compared with two species native to Europe – Cucujus haematodes and Cucujus cinnaberinus (Scopoli, 1763) and with the Caucasian Cucujus haematodes caucasicus Motschulsky, 1845, which is confirmed as a valid subspecies. The male genitalia of this Caucasian form have been examined and illustrated for the first time. A comprehensive key to adults and larvae of European species is provided.
Calabria, Cucujus cinnaberinus, Cucujus haematodes caucasicus, Italy, Sila National Park, old–growth forests, Pinus laricio, relict species, larval taxonomy
The genus Cucujus F. enumerates at the moment 11 known species, distributed throughout the Holarctic region and highly concentrated in Asia, with many endemics especially in India, Nepal, Myanmar, China, Taiwan and Japan (
Cucujus cinnaberinus is an endemic taxon to Europe, distributed from Spain to Ukraine and Sweden, its populations are more densely diffused only in eastern Europe, from Austria and Bavaria eastwards (
Cucujus haematodes is a palearctic species ranging from Bavaria and Southern Italy to Japan, it comprises at least one well differentiated subspecies, Cucujus haematodes opacus Lewis, 1988, found in Japan and Taiwan, whereas the nominal form is known from Southern Italy (Calabria), Greece and Eastern Europe until the Primorskiy Region of far eastern Russia and China (
During a long term survey of the Cucujus populations of the Sila National Park, started 2009 as reported in
All the adult specimens have been obtained by rearing the larval material collected in the Sila National Park under the bark of an endemic pine, Pinus laricio var. calabrica and occasionally of silver fir, Abies alba fallen trees. Adult specimens are in our study area quite uneasy to collect in nature, probably because of their early appearance in spring and short reproductive season. On the contrary, the preimaginal stages are well known for their underbark life, in the literature they are sometimes defined as “scavengers”, but also predators on pupae and small larvae of other insects (
All the larval specimens were kept at a temperature of 20°C with fresh beef meat, their development lasted about 8 months and a maximum of six larval molts were observed before pupation.
The dissections of adult male specimens were normally performed after short KOH treatment of the terminalia and total abdomen removal. The male genitalia are in fact highly delicate and a less careful extraction may cause the loss of the typical long “flagellum”, well described by
The terminology of adult genitalia follows
Museo Civico di Storia Naturale, Verona, Italy (MCV); Department of Entomology the National Museum, Prague, Kunratice 1, Czech Republic (NMP); collection P. Brandmayr, conserved in the Department of Ecology of the University of Calabria (PBC). The larval material used for the taxonomic key is conserved in 70% alcohol in the “Tullia Zetto” larval collection (TZC) in the Department of Ecology of the University of Calabria.
Resultsurn:lsid:zoobank.org:act:1CC7BB12-8520-4DD0-890F-BDEB5C0FBD82
Italy, Central Calabria, Sila National Park, mountains between the Cecita Lake and the Longobucco municipality, 1300–1600 m a. s. l., forests between 39°23'/25'N and 16°32'/35'E.
Holotype male: Sila National Park, Calabria, Italy, Vallone Freddo, Spezzano Sila (CS), 1300 m a. s. l., larva collected at instar five 18.10.2010, lg. Mazzei, adult emerged 28.12.10 in laboratory, prep. N. 3, PBC. Paratypes: one female, Vallone Freddo, reared from an aged larva collected 05.04.2011, lg. Mazzei, PBC. Larval specimens: 12 larvae (III–VI instar), Calabria, Sila, Vallone Freddo, Spezzano della Sila (CS), 1300 m a. s. l., lg. Mazzei, 05.05.2011; 2 larvae (V–VI), Calabria, Sila, Golia Corvo Natural Reserve, Spezzano della Sila (CS), 1300 m a. s. l., lg. Bonacci; 15.07.2011; 1 larva (V), Calabria, Aspromonte, Gambarie (RC), 1350 m a. s. l., lg. Mazzei, 07.06.2009.
Tullia Zetto was an active zoologist and teacher of the Department of Ecology of the University of Calabria, who endeavoured for more than 20 years larval morphology and behaviour of carabids and other predatory beetles in Calabria and in several Mediterranean lands. She was born in Trieste 15.01.1949 and deceased in Cosenza 24.11.2010.
Cucujus tulliae is clearly related to the cinnaberinus–haematodes species group, its distribution seems to be restricted to the mountains of Calabrian peninsula. The closest taxon could be Cucujus haematodes, from which it can easily be distinguished by the less prominent postgenae, the arrow head shaped prosternal apophysis, the smaller and less spiny pronotum, the typical median lobe of the aedeagus and the larval morphology, that is apparently unique because of its slender body and occipital furrows.
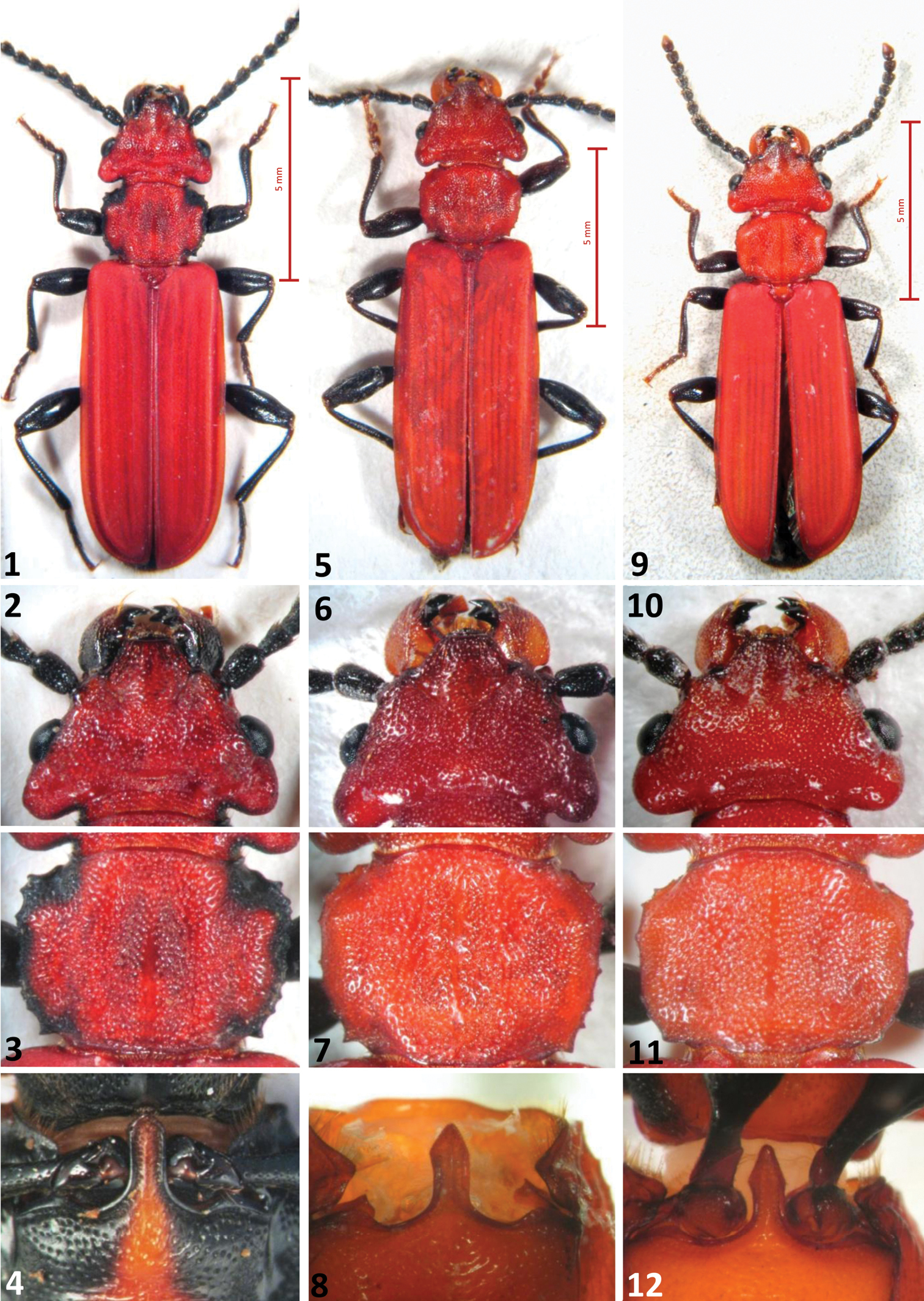
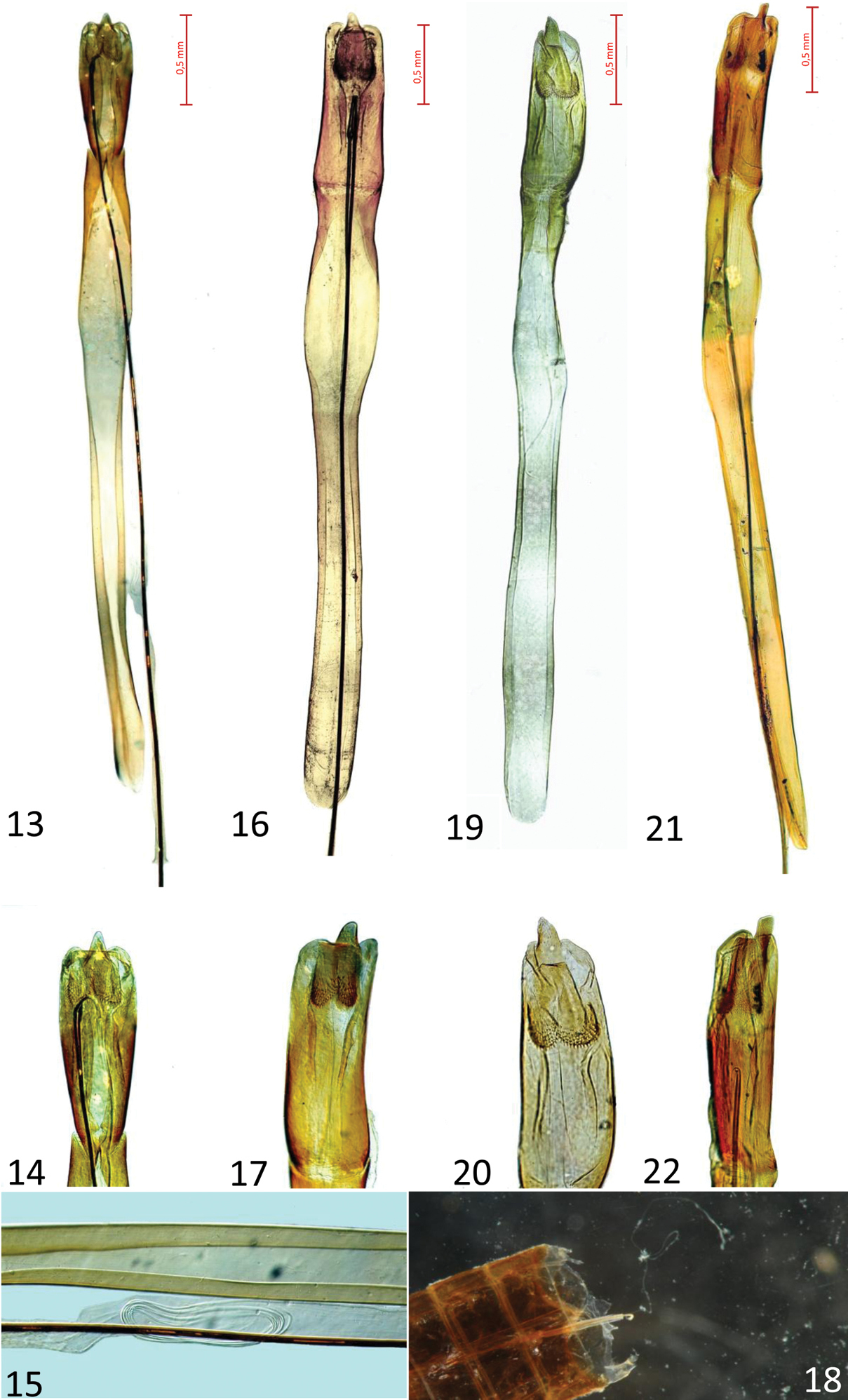
A bright red species, resembling a small–sized haematodes in colour, but with a less serrate pronotum. Length 11.2–12.5 mm. Colour light red, legs dark brown/black, tarsi brown. Prosternum of the same colour of pronotum. Antennae black, mandibles red/orange with black apex. Head distinctly wider than pronotum, with two longer setae after the posterior border of eyes. Postgenae less swollen than in Cucujus haematodes (Figs 1, 5, 9). Occipital furrow deep and long as one third of the posterior head width. Frontoclypeus with a gentle longitudinal swelling. Pronotum less spiny than in Cucujus haematodes, distinctly prolonged at the level of the neck as a short collar (Figs 3, 7, 11). Prosternal apophysis elongated as an arrow head, slender than in Cucujus haematodes (Figs 4, 8, 12). Elytral sides distinctly carinate at its upper external margin, almost until the apex. Elytral surface opaque, density of punctures similar to that of Cucujus haematodes. Apex of elytra broadly rounded and with short pubescence. Metathoracic wings well developed and robust. Sutural stria slightly convex. Median lobe distinctly wider than median strut, less restricted at its apex than in haematodes (Figs 13, 16, 19, 21).Apical process well protruding, more than in haematodes, and triangular at the tip, like in Cucujus cinnaberinus (Figs 14, 17, 20, 22). Median strut 4.5 times longer than median lobe and particularly thin. Flagellum longer than the entire body, connected at its base with the male deferents, its more chitinized trait reaches the median lobe at the level of the two chitinous plates of the endophallus and bends backwards to the median strut. After bending the flagellum becomes transparent and runs across the endophallus, where, at the middle of its length, it rolls up in a sort of “ball”, like a wire (Fig. 17). In figure 2 the “flagellum ball” is photographed in the median strut of Cucujus cinnaberinus (Fig. 18).
The larvae of this species have been collected under the bark of fallen pine trees of at least 25 cm diameter or on dead silver fir fallen trunks, but only on single locations of the National Park. The species seems to prefer cooler, northern exposed slopes and high air humidity at major elevations. In laboratory most larvae kept at 20°C died before pupation, a fact that could be explained by lower temperature preferences of the immature stages. The low number of larvae collected and the successful rearing of only two adults makes hypothesis much more difficult. Concerning geographic distribution, at the moment this taxon is known only from Calabria, in the highest part of the Sila plateau in the core of the Sila National Park, from two sites: Vallone Freddo and Serra Vurga. A single larva has been collected in the surroundings of Gambarie, in the Aspromonte National Park, at 07.06.2009, but this specimen died before pupation. There is little doubt that this new taxon may be an endemic saproxylic beetle of central and southern Calabria, with larval populations depending on the availability of high amounts of dead wood, especially of Calabrian pine, Pinus laricio var. calabrica.
The three European Cucujus species, adults. 1–4 Cucujus cinnaberinus 1 Total body, dorsal view 2 Head 3 Pronotum 4 Prosternal apophysis 5–8 Cucujus haematodes 5 Total body, dorsal view 6 Head 7 Pronotum 8 Prosternal apophysis 9–12 Cucujus tulliae 9 Total body, dorsal view 10 Head 11 Pronotum 12 Prosternal apophysis.
We examined several specimens of this taxon, both from Italy as well as from the National Museum of Prague. This species is very abundant in the pine forest of the Sila National Park in Calabria.
PBC: Cucujus cinnaberinus: 1 ♂, Calabria, Sila, Vallone Freddo, Spezzano della Sila (CS), 1300 m a. s. l., lg. Mazzei, 05.04.2011, aedeagus slide n. 2. 1 ♀, Calabria, Sila, Vallone Freddo, Spezzano della Sila (CS), 1300 m a. s. l., lg. Mazzei, 06.05.2011, slide n. 6. 1 ♂, Calabria, Sila, Vallone Freddo, Spezzano della Sila (CS), 1300 m a. s. l., lg. Mazzei, 05.04.2011, slide n. 9. 2 ♂, Calabria, Sila, Monte Pettinascura, San Giovanni in Fiore (CS), 1650 m a. s. l., lg. Mazzei, 19.08.20092 ♀, Calabria, Sila, Monte Pettinascura, San Giovanni in Fiore (CS), 1650 m a. s. l., lg. Mazzei, 29.05.2009. 1♀, Calabria, Sila, Vallone Freddo, Spezzano della Sila (CS), 1300 m a. s. l., lg. Mazzei, 05.04.20113 ♂, Calabria, Sila, Vallone Freddo, Spezzano della Sila (CS), 1300 m a. s. l., lg. Mazzei, 05.04.2011. 1 ♂, Calabria, Sila, Arnocampo, San Giovanni in Fiore (CS), 1250 m a. s. l., lg. Mazzei, 06.07.2009. 1 ♀, Calabria, Sila, Cozzo del Principe, Spezzano della Sila (CS), 1350 m a. s. l., lg. Mazzei, 12.08.2009. 1 ♂, Calabria, Sila, Cozzo del Principe, Spezzano della Sila (CS), 1350 m a. s. l., lg. Mazzei, 12.08.2009. 4 ♂, Calabria, Sila, Golia Corvo Natural Reserve, Spezzano della Sila (CS), 1300 m a. s. l., lg. Mazzei, 07.07.2009. 4♀, Calabria, Sila, Golia Corvo Natural Reserve Spezzano della Sila (CS), 1300 m a. s. l., lg. Mazzei, 07.07.2009.
MCV: Cucujus cinnaberinus, 1 ♀ ex coll. Brasavola, Sila, Bosco Gariglione, IX. 1 ♂, Calabria, Sila, Silvana Mansio, VIII–1960, det. Ratti, 1971.
NMP: about 120 specimens from many countries of Europe.
Cucujus cinnaberinus larvae: 5 larvae (V–VI instar), Calabria, Sila, Vallone Freddo, Spezzano della Sila (CS), 1300 m a. s. l., 29.6.2009, lg. Mazzei; 18 larvae (IV–VI), Calabria, Sila, Monte Pettinascura, San Giovanni in Fiore (CS), 1650 m a. s. l., 12.09.2009, lg. Mazzei. 7 larvae (V–VI), Calabria, Sila, Golia Corvo Natural Reserve, Spezzano della Sila (CS), 1300 m a. s. l., 30.05.2009, lg. Mazzei.
Male genitalia of four Cucujus species/subspecies. 13–15 Cucujus cinnaberinus (Sila N. Park) 13 Median lobe and median strut with flagellum, dorsal view 14 Median lobe 15 flagellum “ball” inside the endophallus 16–18 Cucujus haematodes (Sila N. Park) 16 Median lobe and median strut with flagellum, dorsal view 17 Median lobe 18 Abdominal end of flagellum, with the basal part of the endophallus and genital duct 19–20 Cucujus tulliae (Sila N. Park) 19 Median lobe and median strut with flagellum removed 20 Median lobe 21–22 Cucujus caucasicus (“Caucasus”) 21 Median lobe and median strut with flagellum, dorsal view 22 Median lobe.
This species is abundant in the Sila National Park, but less than Cucujus cinnaberinus. We collected numerous larval stages also in the Aspromonte National Park.
PBC: Cucujus haematodes: 1 ♂, Calabria, Sila, Vallone Freddo, Spezzano della Sila (CS), 1300 m a. s. l., lg. Mazzei, 12.09.2009, slide n. 4. 1♂, Calabria, Sila, Vallone Freddo, Spezzano della Sila (CS), 1300 m a. s. l., lg. Mazzei, 05.04.2011, slide n. 1. 1 ♀, Calabria, Sila, Cozzo del Principe, Spezzano della Sila (CS), 1350 m a. s. l., lg. Bonacci, 12.08.2009, slide n. 7. 1 ♂, Calabria, Sila, Vallone Freddo, Spezzano della Sila (CS), 1300 m a. s. l., lg. Mazzei, 10.12.10, slide n. 8. 1 ♀, Calabria, Sila, Valle di Casu, Longobucco (CS), 1380 m a. s. l., lg. Brandmayr, 11.02.11. 1 ♂ Calabria, Sila, Valle di Casu, Longobucco (CS), 1380 m a. s. l., lg. Brandmayr, 11.02.11. 1 ♂, Calabria, Sila, Monte Pettinascura, San Giovanni in Fiore (CS), 1650 m a. s. l., lg. Bonacci, 29.05.2009. 1 ♀, Calabria, Sila, Cozzo del Principe, Spezzano della Sila (CS), 1350 m a. . l.s, lg. Bonacci, 18.08.2009. 2 ♂, Calabria, Aspromonte, Gambarie (RC), 1350 m a. s. l., lg. Mazzei, 07.06.2009. 3 ♀, Calabria, Aspromonte, Gambarie (RC), 1350 m a. s. l., lg. Mazzei, 07.06.2009. 3 ♂, Calabria, Sila, Colle Vurga, Longobucco (CS), 1550 m a. s. l., lg. Brandmayr, 04.07.2011.
MCV: Cucujus haematodes: 1 ♀, Calabria, Sila, Bosco Gariglione, IX, coll. ex Brasavola. 1 ♂, Basilicata, Mt. Pollino, Piani del Pollino, VI ’51, lg. Ruffo. 1 ♂, Basilicata, Mt. Pollino, Piano Ruggio, VI–1953, lg. Ruffo.
NMP: About 100 specimens from the Czech Republic and to Far East. Cucujus haematodes larvae: 3 larvae (V–VI instar), Calabria, Sila, Vallone Freddo, Spezzano della Sila (CS), 1300 m a. s. l., lg. Mazzei, 12.08.2009; 3 larvae (V–VI), Calabria, Sila, Monte Pettinascura, San Giovanni in Fiore (CS), 1650 m a. s. l., lg. Mazzei, 12.08.2009; 1 larva (V instar) Calabria, Sila, Cozzo del Principe, Spezzano della Sila (CS), 1350 m a. s. l., lg. Mazzei, 10.07.2009.
Cucujus haematodes caucasicus stat. nov.: dorsal view of male specimen. Locality: Kaukasus, N. W. – Kuban – lg. C. Rost, Berlin.
At the beginning of this investigation we faced the possibility that the newly described taxon may belong to another already known form of the haematodes group. In Europe only a putative subspecies or relative of haematodes was known: Cucujus caucasicus Motschulsky, 1845.
1 ♂ and 1 ♀: Caucasus, “Reitter. Leder.”, determined as: Cucujus haematodes v. caucasicus Motsch.”. 1 ♂: Caucasus, “Reitter. Leder.”, determined as: “ v. caucasicus – Det. Dr. Obenberger”. 1 ♂: Kaukasus, N. W. – Kuban – C. Rost, Berlin. – ex coll. J. Hlisnikowski. Determined as: “Cucujus haematodes Er.”. 1 ♀: Caucasus – Krasnaja Poljana – R. Rous legit. 6.1967. All individuals are conserved in NMP.
A larger Cucujus haematodes with a broad pronotum, almost as wide as the head. Median lobe of the same general shape of Cucujus haematodes, but the apical process of the inner sac vertical, paddle like in lateral view.
Length 16.0–15.5 mm, colour light red, as in Cucujus tulliae sp. n. or in Cucujus haematodes, mandibles yellow with brownish/black apex. Head slightly wider than pronotum, postgenae less protrudent sidewards and more rounded (Fig. 23). Prothorax anteriorly not restricted, as it happens in true Cucujus haematodes, and proportionally larger, the external borders with pronounced, evident teeth. Prosternal apophysis of the same shape of Cucujus haematodes, at the end shortly triangular.
Aedeagus of the same general form of Cucujus haematodes, but a little longer, (Figs 21–22), total length mm 5, 2 – 5, 3, median lobe ending with a vertically flattened apical process, in lateral view distinctly paddle shaped.
This subspecies or allopatric species inhabits the Caucasian mountains – namely Georgia, Armenia and the three Russian regions – Republic of Adigeyia, Krasnodarskiy Kray and Stavropol’skiy Kray (
The three European Cucujus species, larvae. 24–27 Cucujus cinnaberinus 24 Head, dorsal view 25 Head, ventral view 26 Tergum IX and urogomphi, dorsal view 27 Tergum IX and urogomphi, ventral view 28–31 Cucujus haematodes 28 Head, dorsal view 29 Head, ventral view 30 Tergum IX and urogomphi, dorsal view 31 Tergum IX and urogomphi, ventral view 32–35 Cucujus tulliae 32 Head, dorsal view 33 Head, ventral view 34 Tergum IX and urogomphi, dorsal view 35 Tergum IX and urogomphi, ventral view.
The three European Cucujus species, larvae. 36–38 Cucujus cinnaberinus 36 Urogomphi, lateral view and conical appendage (black arrow) 36 Conical appendage 38 Lateral end of tergum IX, sclerotized thorn 39–41 Cucujus haematodes 39 Urogomphi, lateral view and conical appendage (white arrow) 40 Conical appendage 41 Lateral end of tergum IX, sclerotized thorn 42–44 Cucujus tulliae 42 Urogomphi, lateral view and conical appendage (black arrow) 43 Conical appendage 44 Lateral end of tergum IX, sclerotized thorn.
| 1 | Body bright red on the dorsal side, with exception of the apex of the mandibles, that are often lighter, orange. Pronotum entirely reddish, always somewhat restricted at the front border, sides with more or less protruding red teeth | 2 |
| – | Sides of pronotum, inner side of postgenae and mandibles black, mandibles before the apex somewhat lighter coloured. Maximum width of the pronotum at the front border, ventral side of the same part black, with a median yellow stripe that prolonges onto the prosternal apophysis. Head triangular, postgenae obliquely protruding backwards, occipital groove well marked and reaching postgenae, nevertheless deeper in the middle, where the punctuation is well developed. Body length 12–15.5 mm, median lobe of the aedeagus evidently restricted at the basis (Fig. 14) | Cucujus cinnaberinus Scop. |
| 2 | Pronotum robust, with distinct lateral spines, prosternal apophysis with parallel sides and short, triangular end (Fig. 8). Postgenae well developed, body length 13–16 mm | 3 |
| – | Pronotum more rounded, lateral borders with small, obtuse spines, on the head side restricted in a short neck. Pronotal apophysis ending with a prolonged, arrowhead like point (Fig. 12). Body length 11.5–12.5 mm | Cucujus tulliae sp. n. |
| 3 | Pronotum of normal size, distinctly narrower than the head, apical process of the median lobe tongue like, rounded at the tip (Fig. 17) | Cucujus haematodes Er. |
| – | Body size 15.5–16 mm, pronotum broad and robust, little smaller than the head at its posterior end. Postgenae well developed, but less protruding laterally and more backwards oriented, not surpassing the head width at the level of the eyes. Apical process of the median lobe compressed, paddle like (Fig. 22) | Cucujus haematodes caucasicus Motsch. |
Larvae extremely flat bodied, length 7–8 mm in young stages, around 25–30 mm in aged ones. Colour from pale yellow to dark reddish brown. Head prognathous, always broader than pronotum, maximum width at the occiput (parietale), postgenae inflated. Epicranial suture sinuate, coronal suture short but distinct. Stemmata normally in number of six, located more or less at half of the length of head. Antennae of variable length; first antennomere robust, distal antennomere much slender and setose only at the apex. Second antennomere with a flat sensorium bordered by a chitinous ring. Fronto clypeal suture more less concave in the middle; front margin of the labrum with 4 macrochaetae; frontoclypeal suture absent. Mandibles asymmetrical, broadly triangular and irregular bidentate, the teeth of the left one not at the same level (dorsal view). Two setae on the external side of each mandible; prostheca thin and hook like, broad based. Mola tuberculate, long, anteriorly with a penicillum formed by a dense brush of short setae.
The complex of mouth parts well protruding and occupying more or less one third of the head width. Maxillary and labial palps very short. Legs very short and robust, ending with one tarsungulus.
Notal sclerites distinctly bordered. Abdominal segment VIII longer than precedent, lateral margin with two processes bearing setae. Lateral portion of segment VIII with a stout protrusion below the spiraculum (“spiracular process”). Between segment VIII and IX there is a strong conical appendage with many apical setae. Tergum IX with one pair of strong urogomphi bearing a robust bifid spine at their base. Lateral ends of tergum IX with a strongly sclerotized thorn.
Key to the larvae of the European species of genus Cucujus Fabricius, 1775| 1 | Head very wide and flat, posterior margin of the head not excavate, without posterior furrows. Epistomal lateral edge not or less oblique from the antennal basis to the dorsal articulation of mandible. Mouthparts occupant more than a half of the front margin of the head. Antennae not longer than the head. Lateral chitinous thorns of tergum IX gently curved backwards | 2 |
| – | Head wide and flat, with distinctly furrowed postgenae at the posterior margin, forming a separate swelling on the occipital part of the head. Mouthparts more protruding, lateral border of epistomal margin distinctly oblique from the antennal basis to the insertion of mandible. Mouthparts complex slender, antennae as long as the whole head. Lateral thorn of the tergum IX strongly bent backwards | 3 |
| 2 | Antennae slender, second joint distinctly longer than the first one, apical joint thin five times longer than wide at the basis. Lateral border of parietale a little swollen in correspondence of the stemmata. Epistomal front margin moderately oblique towards the mandibular basis (Figs 24–25). Urogomphi well curved and apically a little converging to the median plane (Figs 26–27). Basal tooth with minor spine directed outwards and far from the apex. Spiracular process small and little pronounced (Figs 36–38). Conical appendage very large at the basis, distinctly setose around the apical part, chitinous apex short (Fig. 37) | Cucujus cinnaberinus (Scop.) |
| – | Antennae very short, second joint longer like the first one. Apical antennomere four times as wide as at the basis. Head robust and not swollen in the stemmata area (Figs 28–29). Front epistomal margin straight from antennal basis to mandibular insertion. Maximum head width behind the stemmata. Urogomphi well curved but apically not converging to the median plane (Figs 30–31). Basal tooth with less distant apical spines. Conical appendage slender and of different shape (Figs 39–41). Spiracular process inconspicuous | Cucujus haematodes Er. |
| 3 | Head posteriorly sinuate and marked by very long antennae. Second antennomere a little longer than the first one. Apical antennomere six times longer than wide at the basis. Epistomal front margin strongly oblique towards the mandibular insertion. Frontal suture less sinuate than in previous species (Figs 32–33). Urogomphi strong and a little converging at the end, apex with three evident macrochaetae (Figs 34–35). Basal tooth of normal shape and slender. Spiracular process distinctly protrudent backwards (Figs 42–43). Lateral thorn of tergum IX strongly bent to the rear, forming an angle of ninety degrees (Fig. 44) | Cucujus tulliae sp. n. |
The new described taxon of saproxylic beetle, Cucujus tulliae, seems to be of limited distribution, at the moment the only two adult specimens, obtained by rearing, are known from Sila National Park, and a single larva has been captured in the Aspromonte National Park, always on dead Calabrian Pine trunks. It is not astounding that this very cryptic species escaped the capture by previous collectors, most of them were occasional visitors of the Sila plateau and this area has never been the object of a long term faunal survey. Moreover, in the older Italian reports (
The new species seems to be also habitat restricted, in fact the larvae have been found only at three sites in Sila National Park, on northern exposures or at high elevations. Thus, further research is needed to assess the ecological requirements of the species.
The systematic position of Cucujus tulliae sp. n. indicates an affinity with Cucujus haematodes – body colour and male genital parts, especially the connection between median lobe and median strut (phallobase of other authors) are similar in both species. The new species may have evolved during a cold climate phase (glacial period?) from isolated Cucujus haematodes populations. The smaller body size (if confirmed by new findings) speaks for less favorable conditions, as prey density or trunk sizes.
Concerning a first hypothesis on the conservation status of this southern Italian endemic, the evaluation as endangered (EN) in the IUCN categories seems appropriate – e.g. with respect to endemic Osmoderma spp. (
The second outstanding result of this study is that for the first time Cucujus haematodes caucasicus Motsch. has been recognized as a valid subspecies, or perhaps as an allopatric species of the haematodes “species aggregate”, the heterogeneity of which has been emphasized especially by
The Cucujus haematodes group reveals to be not only extremely widespread in his Palaearctic distribution, but also highly influenced by isolation and tending to local speciation. A revision of this species aggregate could be of importance for conservation biology and for the relationships between saproxylic predators and history of palearctic forests.
The authors are particularly indebted with the Direction of the Sila National Park and its personnel. President of the Park, prof. Sonia Ferrari and director Dr. Michele Laudati kindly gave permissions for this research. Dr. Jiří Hájek, responsible of the Department of Entomology of the National Museum in Prague made it possible to see all the Cucujus collection material of this Museum. Dr. Leonardo Latella of the Museo Civico di Storia Naturale in Verona helped us in the same way. Our friend Prof. Ettore Contarini, Bagnacavallo, Ravenna, participated vigorously to the first field trips of this study and provided useful literature. Two anonymous reviewers highly contributed to the quality of this paper.