(C) 2011 Drew A. Hildebrandt. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of Bembidion (Trichoplataphus Netolitzky) from the Ozark Plateau of Missouri and Arkansas is described (Bembidion ozarkense Maddison and Hildebrandt). It is distinguishable from the closely related species, Bembidion rolandi Fall, by characteristics of the male genitalia, and sequences of the genes cytochrome oxidase I and 28S ribosomal DNA. A brief review of the North American species of Trichoplataphus is presented, including a key to species.
Bembidion, Bembidiini, Trechinae, COI, 28S rDNA, taxonomy, systematics
While identifying beetles of the genus Bembidion Latreille that the senior author collected in Arkansas and Missouri some years ago, he came across a series of specimens of subgenus Trichoplataphus Netolitzky that keyed to Bembidion rolandi Fall using the latest revision of the group (
The purpose of this paper is to describe the new species that was discovered and provide a review of the North American species of the subgenus Trichoplataphus. The most recent revision of the subgenus was included in Lindroth’s treatment of the genus for the northern U.S. and Canada (
It is with great pleasure that we dedicate this paper to Dr. Ross Bell. For one of us he has been a very generous, informative and helpful correspondent, for the other a mentor and close colleague. Both of us have benefited immeasurably by our interactions with him. This paper is a small contribution to the fauna of an area that has interested Ross since his youth, when he first collected the endemic Chlaenius viduus Horn in the Ozarks.
MethodsApproximately 580 specimens of Bembidion (Trichoplataphus) were examined as part of this review; no effort was made to do a complete survey of specimens in existing collections. Specimens were examined from the following collections; each collection listing begins with the codon used in the text.
CMNH Carnegie Museum of Natural History, Pittsburgh, PA, USA (Robert L. Davidson)
DAHC Drew A. Hildebrandt collection, Clinton, MS. USA
OSAC Oregon State Arthropod Collection, Oregon State University, Corvallis, OR (David R. Maddison)
TAMU Texas A&M University, College Station, TX, USA (Edward C. Riley)
Collecting methods. Specimens were collected by hand, by splashing or pouring water on gravel bars or by raking the gravel by hand during the day to dislodge the beetles hiding under the gravel; or at night when the beetles were out, actively moving about on the surface. During the day, raking the gravel by hand works best for collecting specimens of this subgenus because of their behavior when disturbed. Unlike members of most other Bembidiini, individuals of Trichoplataphus tend to run under the water and cling to stones, and thus they are more difficult to catch (
Specimens for morphological studies were killed and preserved in sawdust or woodchips to which ethyl acetate was added. Specimens for DNA sequencing were collected into 95% or 100% ethanol, with best results obtained if the abdomen was slightly separated from the rest of the body to allow better penetration.
Morphological methods. Methods for studying adult structures, and terms used, are given in
All measurements were made on dried, pinned or pointed specimens using an Olympus SZ6045 zoom stereo microscope with a calibrated ocular micrometer. Character examinations were made at magnifications ranging from 10x to 63x; all measurements were made at 30x. Standardized body length (SBL) was measured following the protocol of
Taxon sampling for DNA studies. We sequenced DNA from 18 specimens of Bembidion (Trichoplataphus), including all known North American species, as well as Bembidion mimekara Toledano and Schmidt from China (Table 1). Preliminary analyses of multiple genes across Bembidion (Maddison, unpublished) indicate that the sampled Trichoplataphus form a clade, and that, among the species sampled, B. mimekara is the sister group to the North American species. DNA vouchers are housed in the David Maddison voucher collection at Oregon State University.
DNA sequencing. Methods for obtaining DNA sequences are described in
Assembly of multiple chromatograms for each gene fragment and initial base calls were made with Phred (
Sequences have been deposited in GenBank with accession numbers JF800039 through JF800074.
Alignment. Alignment for both genes could be unambiguously determined, as there were no insertion or deletion events evident in the COI sequences, and only one in 28S, in which Bembidion mimekara has 2 bases not present in the other species.
Molecular phylogenetic analysis. Models of nucleotide evolution were chosen with the aid of ModelTest version 3.7 (
Likelihood analyses of nucleotide data were conducted using Garli version 1.0.699 (
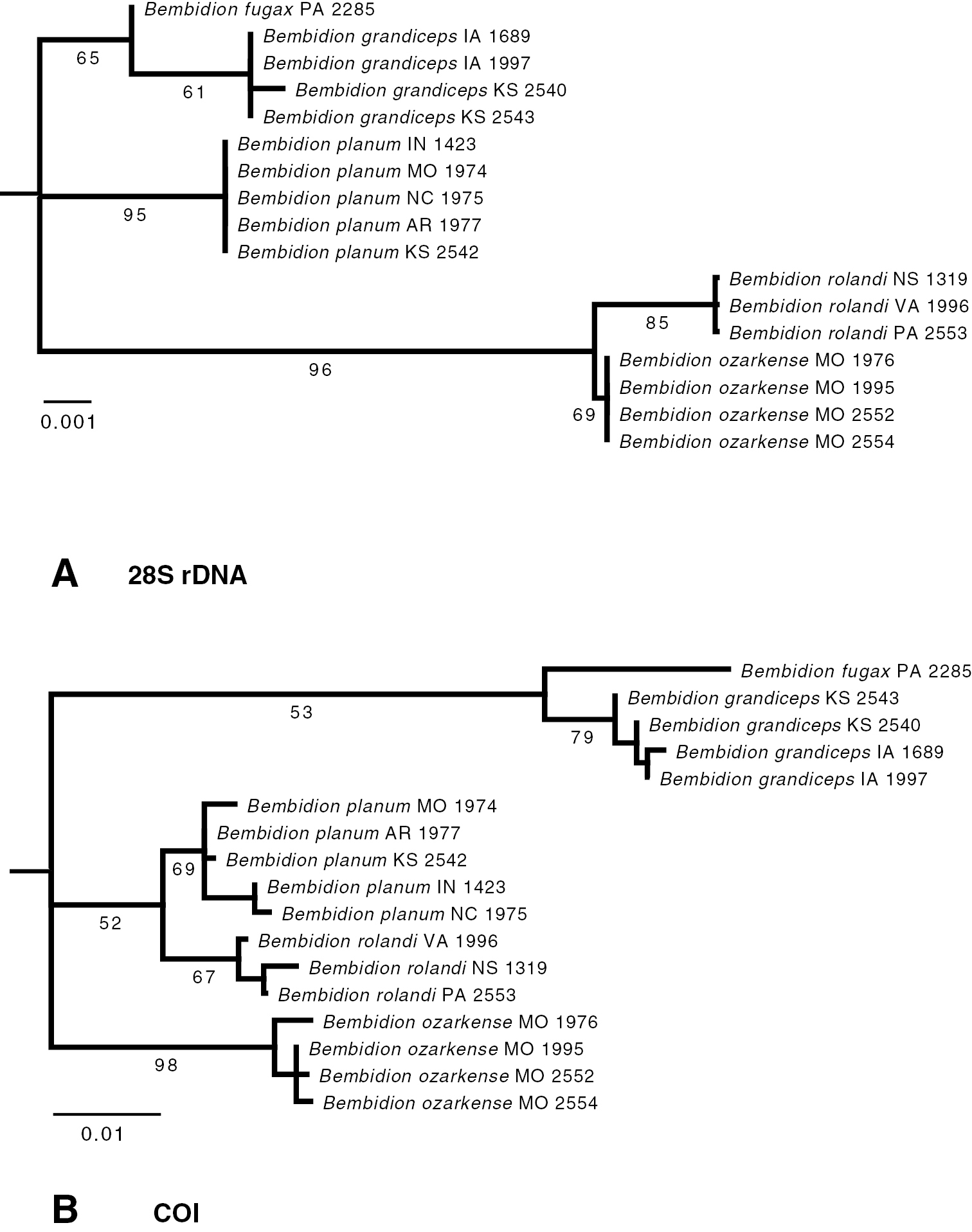
Specimens of each of the species have distinctive COI and 28S sequences (Fig. 1). Bembidion rolandi shows consistent differences from similar specimens from the Ozarks, which we are describing as Bembidion ozarkense. All specimens of Bembidion rolandi differ from all specimens of Bembidion ozarkense at 18 bases among the 766 sequenced sites in COI (2.3% divergent), as well as 2 of the 995 sites sampled of 28S rDNA (0.2%). These two species are reciprocally monophyletic in the inferred phylogenetic trees (Fig. 1).
Maximum-likelihood bootstrap trees for A 28S rDNA and B COI. Each terminal taxon is a single specimen, whose state or province of origin is shown, using standard abbreviations, after the species name; the four digits at the end of the name form the voucher number. Numbers below each branch are the percentage of bootstrap replicates showing that branch; values are not shown within each species. Branch lengths are proportional to number of substitutions per site, as reconstructed by Garli. Bembidion (Trichoplataphus) mimekara, the outgroup, is not shown because of its long branch length.
Bembidion (Trichoplataphus) specimens sampled for DNA sequences. Four-digit numbers under “#” are D.R. Maddison DNA voucher numbers. The specimen marked with a “*” is the holotype of Bembidion ozarkense, n.sp.
| Species | # | Locality |
| Bembidion mimekara Toledano & Schmidt | 1366, 2448 | China: Yunnan: Gongshan County, Dulongjiang Township, Kongdan, east bank of Dulong Jiang. 1510 m, 27.87764°N, 098.33618°E |
| Bembidion planum (Haldeman) | 1977 | USA: Arkansas: Henderson Creek at route 23, Ozark Mountains |
| Bembidion planum (Haldeman) | 1423 | USA: Indiana: Crawford Co., English, Camp Fork Creek, 150m 38.3334°N, 86.4646°W |
| Bembidion planum (Haldeman) | 2542 | USA: Kansas: Jefferson Co., Little Slough Ck, W of Kiowa Rd. 39.23355°N, 95.39497°W |
| Bembidion planum (Haldeman) | 1974 | USA: Missouri: Washington Co., Big River at route 21, 245m, 37.8121°N, 90.7723°W |
| Bembidion planum (Haldeman) | 1975 | USA: North Carolina: Mitchell Co., Penland, North Toe River, 744m, 35.9293°N, 82.1149°W |
| Bembidion fugax (LeConte) | 2285 | USA: Pennsylvania: Perry Co., Susquehanna River 5 km N New Buffalo, 40.4909°N, 76.9535°W |
| Bembidion grandiceps Hayward | 1689 | USA: Iowa: Webster Co., Des Moines River near Stratford, 275m, 42.3101°N, 93.9371°W |
| Bembidion grandiceps Hayward | 1997 | USA: Iowa: Webster Co., Des Moines River near Stratford, 275m, 42.3101°N, 93.9371°W |
| Bembidion grandiceps Hayward | 2540 | USA: Kansas: Jefferson Co., Little Slough Ck, W of Kiowa Rd. 39.23355°N, 95.39497°W |
| Bembidion grandiceps Hayward | 2543 | USA: Kansas: Jefferson Co., Little Slough Ck, W of Kiowa Rd. 39.23355°N, 95.39497°W |
| Bembidion ozarkense, sp. n. | 1976 | USA: Missouri: Carter Co., Current River at Van Buren, 135m, 36.9904°N, 91.0100°W |
| Bembidion ozarkense, sp. n. | 1995 | USA: Missouri: Washington Co., Irondale, Big River, 230m, 37.8302°N, 90.6895°W |
| Bembidion ozarkense, sp. n. | 2552* | USA: Missouri: Carter Co., Current River at Van Buren, 135m, 36.9904°N, 91.0100°W |
| Bembidion ozarkense, sp. n. | 2554 | USA: Missouri: Carter Co., Current River at Van Buren, 135m, 36.9904°N, 91.0100°W |
| Bembidion rolandi Fall | 1319 | Canada: Nova Scotia: Economy River at route 2, 45.3868°N, 63.8992°W |
| Bembidion rolandi Fall | 1996 | USA: Virginia: Rockbridge Co., Maury River, Glasgow, 215m, 37.6329°N, 79.4431°W |
| Bembidion rolandi Fall | 2553 | USA: Pennsylvania: Perry Co., Susquehanna River 5 km N New Buffalo, 40.4909°N, 76.9535°W |
The subgenus Trichoplataphus of the genus Bembidion (Coleoptera: Carabidae: Trechinae: Bembidiini) contains 19 described species in the Palaearctic Region (
Adult specimens of the subgenus are easily separated from most other groups in North America by the irregular scattering of setiferous punctures on the abdominal sterna (
Members of Trichoplataphus are found on open, bare gravel bars and banks, sometimes mixed with clay or sand (Fig. 2;
The North American species of Trichoplataphus are:
Bembidion ozarkense sp. n.
Bembidion rolandi Fall, 1922
Bembidion grandiceps Hayward, 1897
Bembidion fugax (LeConte, 1848)
Bembidion planum (Haldeman, 1843)
Identification of Species Using Morphological DataSpecies of this subgenus are very difficult to tell apart using morphological features, as the known differences are very subtle. Future morphological studies may reveal better characters to separate them. In the meantime, the key we present below (a modified version of couplets 77 through 80 in
Type locality of Bembidion ozarkense, the Current River at Van Buren (USA: Missouri: Carter County).
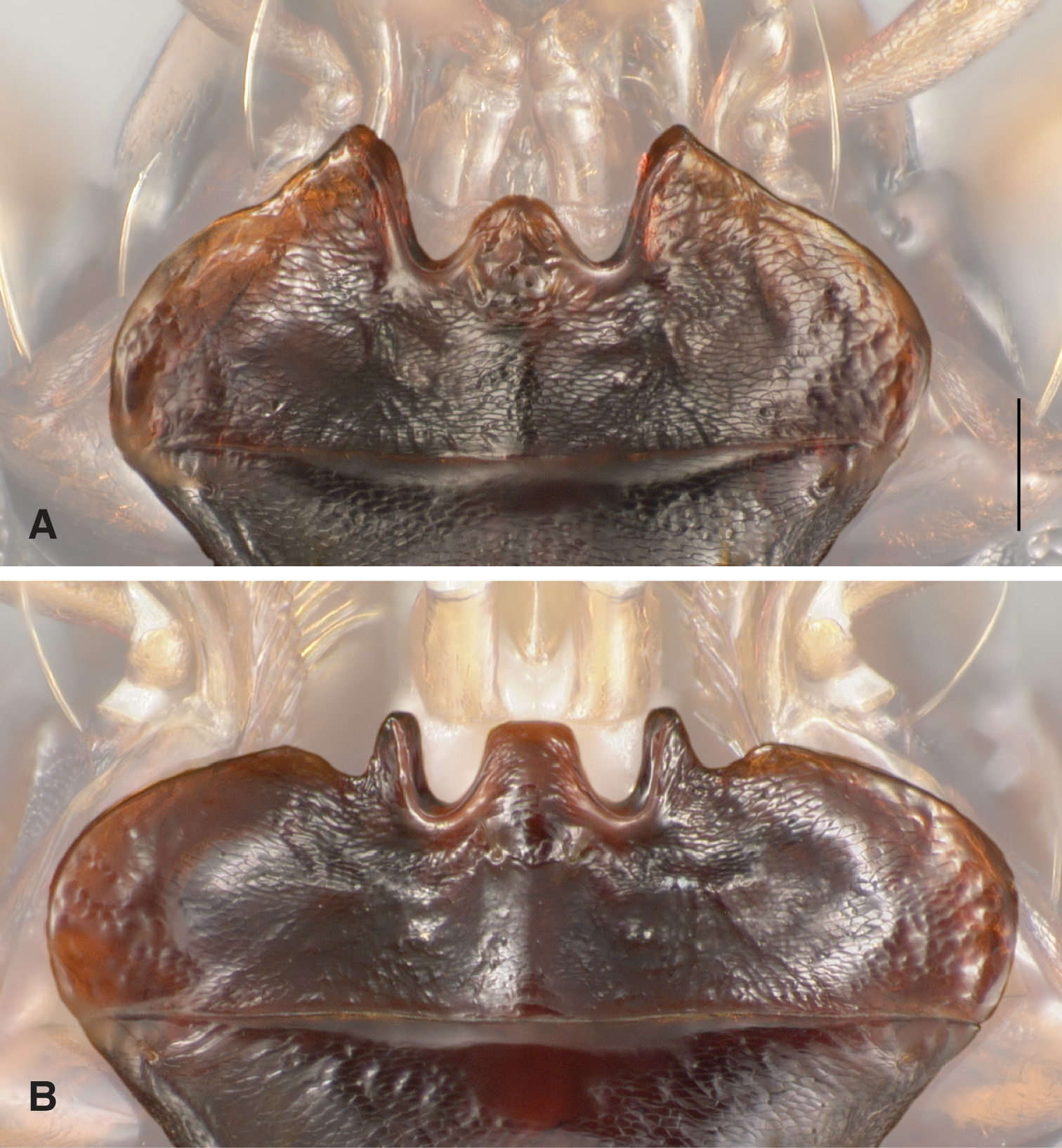
| 77 | Mentum with epilobes of normal size for a Bembidion, projected notably anteriad of the mentum tooth (Fig. 3A) | 77a |
| – | Mentum with epilobes much reduced, projected about as far anteriad as the mentum tooth, and with a prominent concavity on the lateral side of each epilobe (Fig. 3B) | 78 |
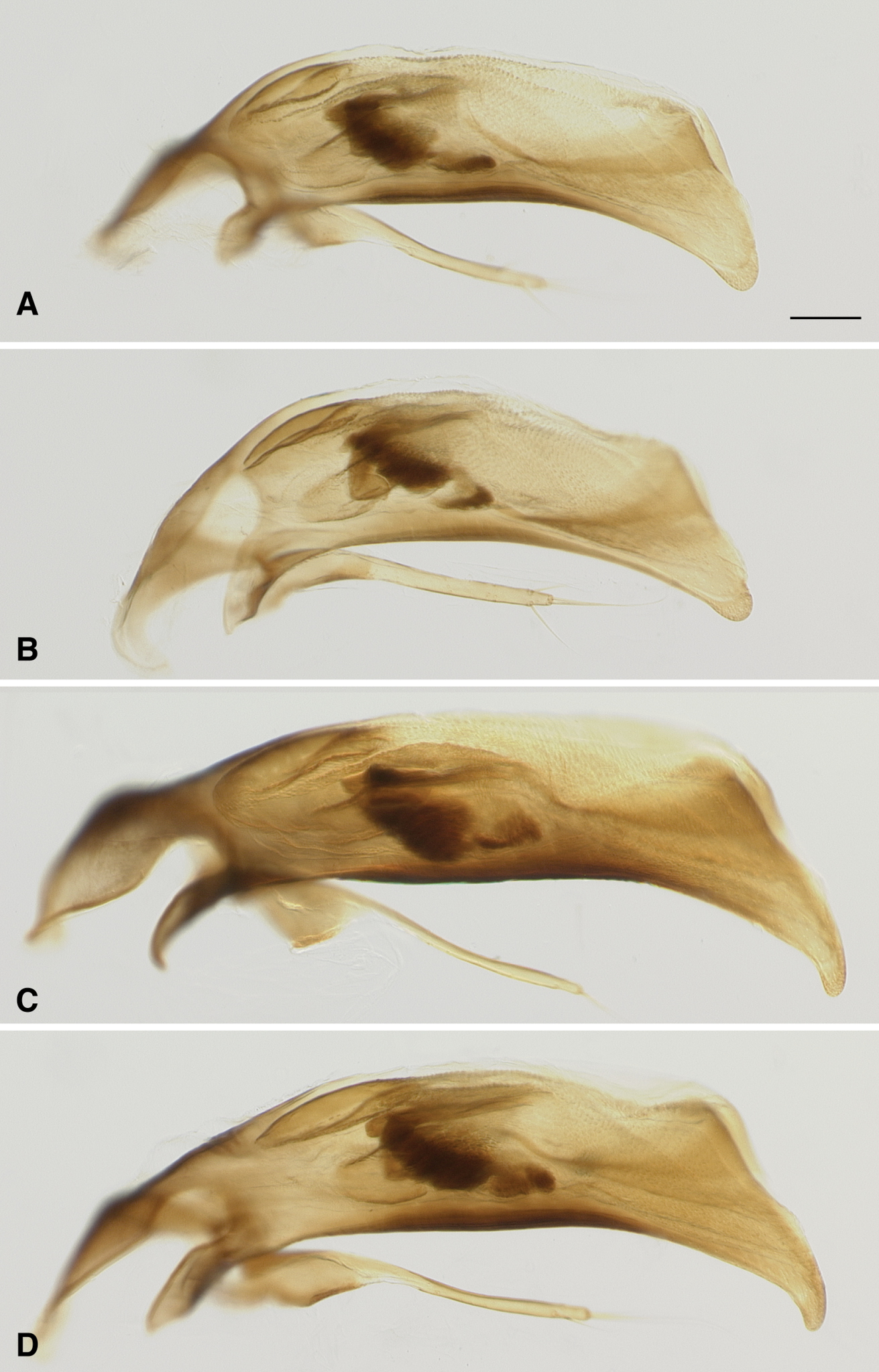
| 77a | From the Ozark Plateau of Missouri and Arkansas; aedeagus with tip relatively blunt, and not sharply bent downward (Fig. 4A and 4B) | Bembidion ozarkense |
| – | From east of the Mississippi River; aedeagus with tip more narrowly pointed, abruptly bent downward (Fig. 4C and 4D) | Bembidion rolandi |
| 78 | Prothorax strongly constricted at base ( |
Bembidion grandiceps |
| – | Prothorax less constricted. Elytra slightly sloping at extreme tip. | 79 |
| 79 | 6th elytral stria hardly weaker than 5th behind shoulder but obliterated towards apex. Frontal furrows prolonged and strongly diverged behind posterior supra-orbital punctures | Bembidion fugax |
| – | 6th elytral stria much weaker than 5th, often obliterated throughout. Frontal furrows ended just behind posterior supra-orbital puncture and little diverged | Bembidion planum |
Menta of Bembidion (Trichoplataphus). Scale bar is 0.1 mm. Features other than the menta have been digitally faded A Bembidion ozarkense, USA: Missouri: Carter Co., Current River at Van Buren, 135m, 36.9904°N, 91.0100°W B Bembidion grandiceps, USA: Iowa: Webster Co., Des Moines River near Stratford, 275m, 42.3101°N, 93.9371°W, voucher DNA1689.
urn:lsid:zoobank.org:act:042B6160-C581-4028-BC44-421C1448C831
http://species-id.net/wiki/Bembidion_ozarkense
Fig. 3A, 4A, 4B, 5A, 6Male (in OSAC), here designated, labeled “USA: Missouri: Carter Co., Current River at Van Buren, 135m, 36.9904°N, 91.0100°W, 24.iv.2005. DRM 05.015. D.R. Maddison” / “David R. Maddison DNA2552 DNA Voucher” [pale green paper] / “HOLOTYPE Bembidion ozarkense Maddison & Hildebrandt” [red paper]”. Genitalia in glycerine vial with specimen; extracted DNA stored separately. GenBank accession numbers for DNA sequences of the holotype are JF800056 (28S) and JF800065 (COI).
221 specimens as follows: USA: Missouri: Carter Co., Current River at Van Buren, 135m, 36.9904°N, 91.0100°W (88 specimens); Carter Co., Current River at Van Buren, 135m, 36.9924°N, 91.0167°W (3); Carter Co., Current River at Van Buren, 135m, 36.9911°N, 91.0133°W (3); Maries Co., Maries River near Argyle, 200m, 38.2700°N, 92.0007°W (10); Reynolds Co., Clark National Forest. Sutton’s Bluff Campground (6); Washington Co., Irondale, Big River, 230m. 37.8302°N, 90.6895°W (16); Washington Co., Big River at route 21, 245m. 37.8121°N, 90.7723°W (4); Washington Co., Big River at route 21, 245m, 37.8132°N, 90.7734°W (1); Arkansas: Crawford Co., Lee Creek (1); Marion Co., Buffalo Point St.Pk. (42); Searcy Co., 5 mi. W. Big Flat. Big Creek (47). Male genitalia have been examined from at least one specimen from each paratype locality.
Paratypes of Bembidion ozarkense have been deposited in the CMNH, DAHC, OSAC and TAMU, and in the collections of The Natural History Museum, London (BMNH), the California Academy of Sciences (CAS), the Field Museum of Natural History (FMNH), the Museum of Comparative Zoology (MCZ), Muséum National d’Histoire Naturelle in Paris (MNHN), the University of Arizona (UAIC), the University of Alberta Strickland Museum (UASM), and the National Museum of Natural History, Smithsonian Institution (USNM).
Eighty-two specimens examined but not designated as paratypes are: USA: Missouri : Pulaski Co., Devil’s Elbow. Big Piney River. 10km E. Waynesville. 734 ft. 37 50 52.4 N 92 03 42.0 W (2); Arkansas: Marion Co., Buffalo Point S.P. (15); Searcy Co., 5.3 mi. W. Big Flat. Big Creek. (65). The Missouri specimens are omitted from the paratype series as there are no males whose genitalia could be examined; the Arkansas specimens are omitted as they are slightly damaged.
USA: Missouri: Carter Co., Current River at Van Buren, 135m, 36.9904°N, 91.0100°W. At the type locality (Fig. 2), adults of Bembidion ozarkense were common on 24 April 2005; specimens occurred in company with Bembidion (Trichoplataphus) planum, Bembidion (Pseudoperyphus) antiquum Dejean, and Bembidion (Pseudoperyphus) chalceum Dejean.
Derived from the Ozark Plateau of Missouri and Arkansas, which encompasses the known range of this species.
Bembidion ozarkense males can be recognized among North American Trichoplataphus by the combination of epilobes of the mentum of normal size for a Bembidion (Fig. 3A), and aedeagus with the tip relatively thick and not abruptly bent downward (Fig. 4A and 4B).
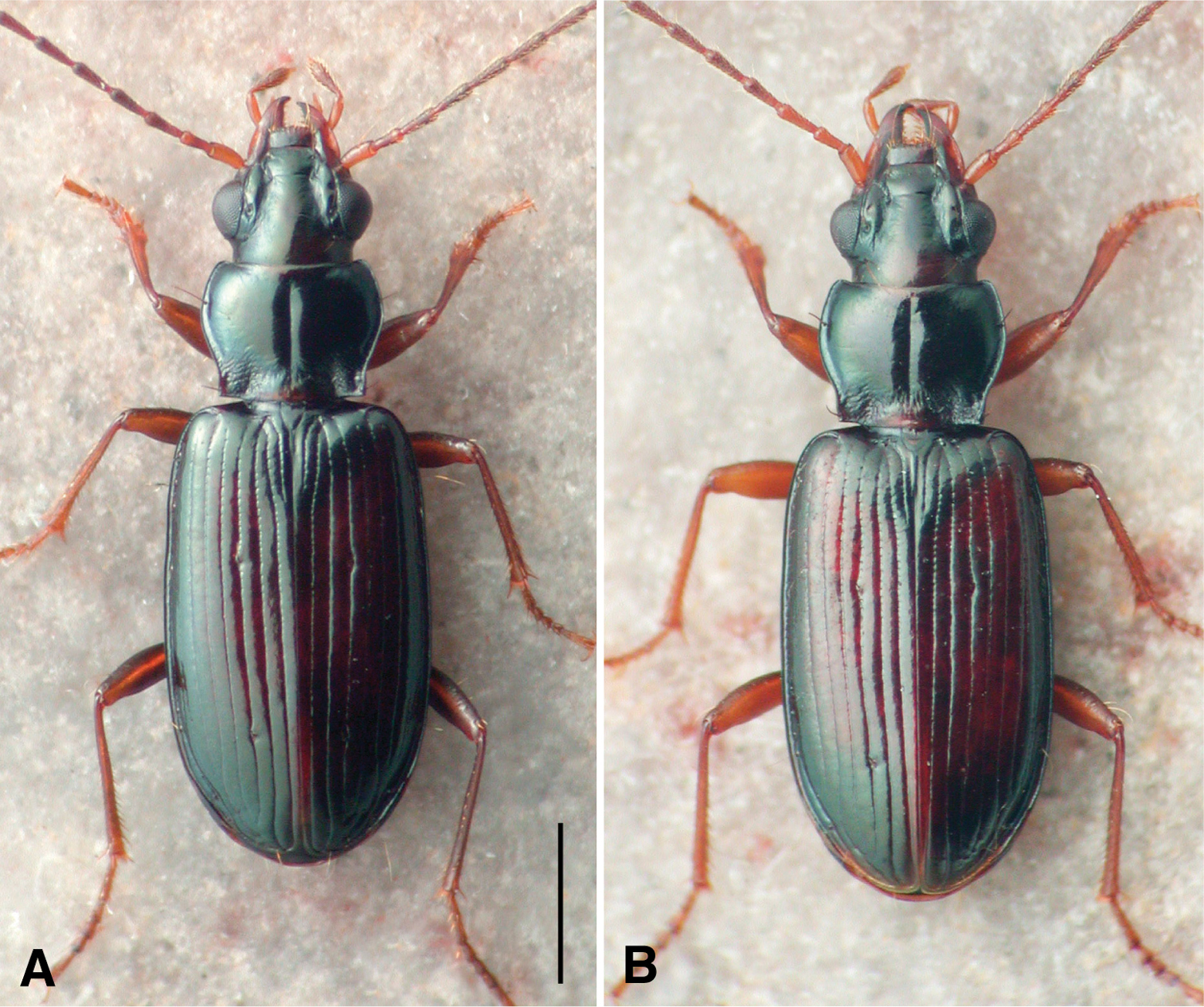
This species is markedly similar to Bembidion rolandi in external form (Fig. 5), sharing with it normal mentum epilobes; narrow, flat body, with long antennae; the pronotal base notably constricted, hind angles right, with the lateral margins in front of the hind angle parallel and straight. We have not yet found any reliable external characters to separate the two species, although there are some traits by which they tend to differ. Specimens of Bembidion ozarkense tend to be slightly smaller (average SBL of males is 4.4 mm, of females 4.7 mm) than those of Bembidion rolandi (4.6 and 4.8 mm, respectively), but there is a broad range of overlap, as adults of both species range in length from 4.2 to 5.1 mm. Bembidion ozarkense tends to be darker than Bembidion rolandi, with less-rufous elytra, and with the posterior medial portion of the dorsal surface of the head darker, rarely with the rufous region of many Bembidion rolandi; the second antennomere of Bembidion ozarkense is often infuscated centrally, whereas it is usually entirely pale rufous in Bembidion rolandi. However, while these tendencies are noticeable in larger series, there is enough overlap between species that they are of marginal use when comparing individual specimens.
The only morphological characteristic that we have found to reliably distinguish the two species is in the male aedeagus: the tip of Bembidion rolandi is thin, and abruptly bent downward (Fig. 4C and 4D; n=8), traits not found in Bembidion ozarkense (Fig. 4A and 4B; n=30). DNA sequence data can also be definitively used to identify the species; either COI or 28S rDNA will suffice.
However, the known distribution ranges are distinctly separate, and can be used in the absence of male genitalia or DNA sequences to identify specimens.
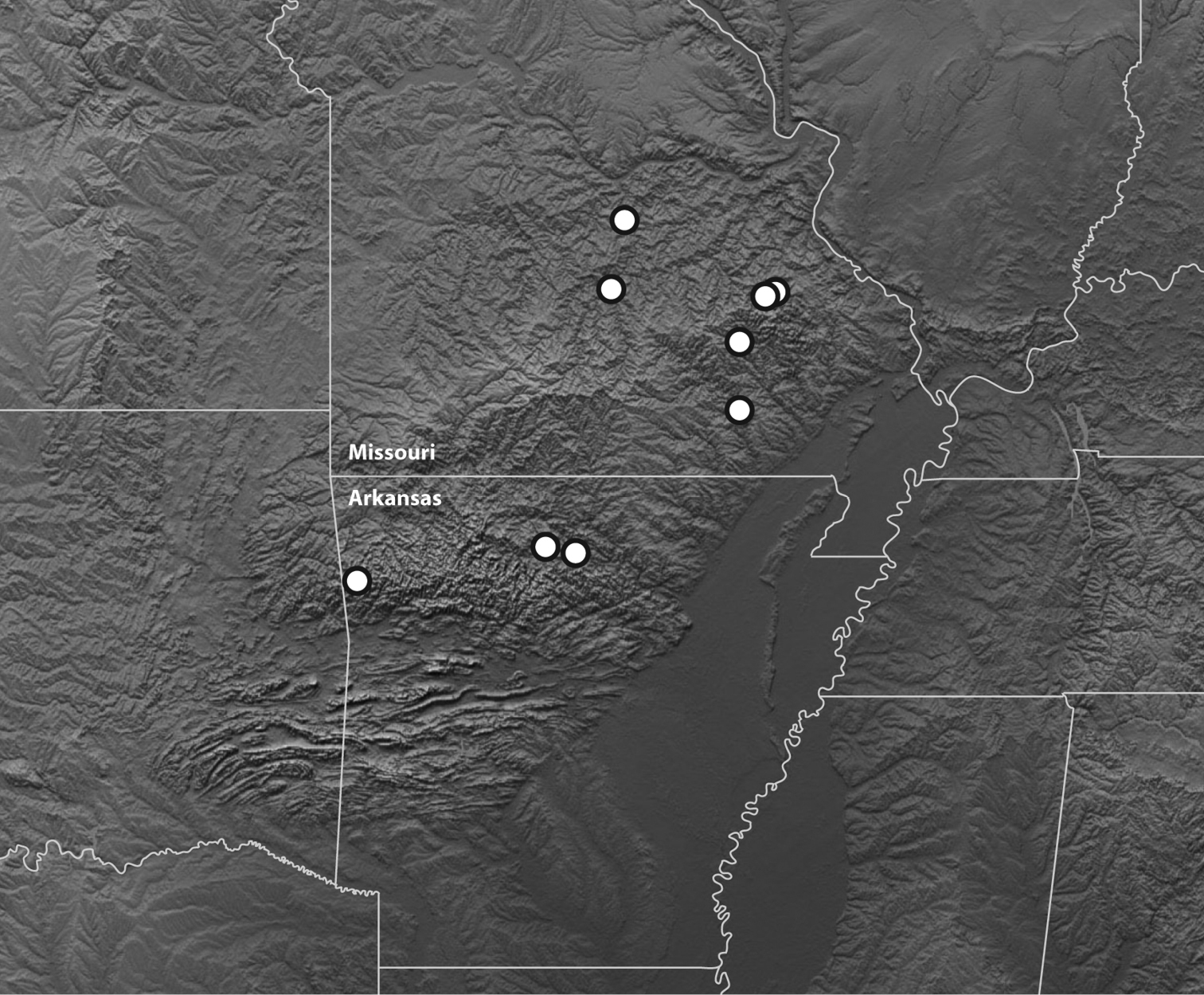
The known specimens of this species are from the Ozark Plateau of Missouri and Arkansas (Fig. 6).
Aedeagus of male Bembidion ozarkense and Bembidion rolandi. Scale bar is 0.1 mm A Bembidion ozarkense, USA: Missouri: Carter Co., Current River at Van Buren, 135m, 36.9904°N, 91.0100°W Voucher DNA1976 B Bembidion ozarkense, USA: Missouri: Washington Co., Irondale, Big River, 230m, 37.8302°N, 90.6895°W, Voucher DNA1995 C Bembidion rolandi, USA: Virginia: Rockbridge Co., Maury River, Glasgow, 215m, 37.6329°N, 79.4431°W, Voucher DNA1996 D Bembidion rolandi, Canada: Nova Scotia: Economy River at route 2, 45.3868°N, 63.8992°W, Voucher DNA1319.
Habitus photographs. Specimens at same scale; scale bar is 1.0 mm A Bembidion ozarkense, USA: Missouri: Carter Co., Current River at Van Buren, voucher DRMV100311 B Bembidion rolandi, USA: Virginia: Rockbridge Co., Maury River at Glasgow, voucher DRMV100193.
Map of the Ozark Plateau and surrounding regions showing the known distribution of Bembidion ozarkense. Topographic image modified from NASA/JPL, http://photojournal.jpl.nasa.gov/catalog/pia03377
As discussed above, specimens of this species cannot be separated from Bembidion ozarkense using external characteristics, although they can be distinguished by male genitalia; see the diagnosis under Bembidion ozarkense for details.
Geographic distribution. Currently recorded from Canada: NB, NS, ON, QC; USA: DC, MA, MD, ME, NJ, NY, OH, PA, VA, VT, WV (
Most specimens can be separated from Bembidion fugax and Bembidion planum by a combination of: broad head and pronotum; prothorax markedly constricted at base with the lateral margins just in front of the hind angle parallel, although this parallel region is much shorter than in Bembidion rolandi or Bembidion ozarkense; elytra quite flat at apex; and broad, markedly delimited frontal furrows that are clearly diverging between eyes and extended beyond the posterior supraorbital puncture.
We have seen specimens from USA: TX, KS, IA. Reports from the literature (
Octhedromus planipennis LeConte, 1850; Bembidion champlaini Casey, 1918.
Most specimens can be separated from Bembidion grandiceps using the characters listed for that species. Also, specimens of Bembidion fugax have a more narrow head and pronotum and the elytral apex slopes slightly.
This species can be separated from Bembidion planum by the 6th elytral stria being as, or nearly as, impressed behind the shoulder as the 5th, and the frontal furrows are prolonged and markedly diverging behind the posterior supra-orbital puncture. In addition, Bembidion fugax has deeper furrows with steeper sides, more polished especially at the bottom, and more sharply etched at the bottom, with the raised area between the eye and the frontal furrow being more bulbous, and shinier; Bembidion planum has broad, shallow furrows, sides gently sloped, more microsculpture on the slopes and especially bottoms, not sharply etched at the bottom, and with the raised area between the eye and the frontal furrow being flatter and not as shiny. Specimens tend to be darker and slightly larger than those of Bembidion planum and in most specimens the pronotal microsculpture is weakly impressed and obsolete on the disk.
Recorded from USA: DC, IL, IN, MA, MD, NJ, NY, OH, PA, TN, VA, VT (
Bembidium guexii Chaudoir, 1868: 242; Bembidion vulsum Casey, 1918: 55; Bembidion filicorne Casey, 1918: 56.
Most specimens of this species can be separated from Bembidion grandiceps using the characters listed for that species. Also, specimens of Bembidion planum have a narrower head and pronotum and the elytral apex slopes slightly.
Most specimens of this species can be separated from Bembidion fugax by the more weakly impressed 6th compared with 5th elytral stria, and the frontal furrows end just behind the posterior supra-orbital puncture and are little divergent. Specimens tend to be paler and slightly smaller than those of Bembidion fugax, and in most specimens have strongly-impressed microsculpture over the entire pronotum.
Recorded from Canada: NB, NS, ON, QC; USA: AR, CT, DC, IA, IL, IN, KY, MA, MD, ME, MI, MN, MO, MS, NC, NH, NJ, NY, OH, PA, RI, SC, TN, VA, VT, WI, WV (
We thank the curators for the collections from which we borrowed specimens.
We owe special thanks to Robert Davidson (CMNH) for sharing his particular knowledge of the habitats and habits of this group, for informing of us of some characters that help separate B. fugax and B. planum, and for providing a thoughtful review of the manuscript.
We also thank the following for collecting specimens of Trichoplataphus for our DNA studies: Kojun Kanda, Taro Eldridge, and Zach Falin (B. grandiceps from Kansas); Monica Hughes (B. planum from Arkansas); David Kavanaugh (B. mimekara fromChina). Thanks as well to A.E. Arnold for help in collecting at numerous other localities.
This project was enabled by funds generously provided by the University of Arizona, and the Harold E. and Leona M. Rice Endowment Fund at Oregon State University.