(C) 2012 Sergei Zonstein. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The Chinese representatives of Raveniola Zonstein, 1987 are currently recognized to comprise seven species. Four new species – Raveniola montana sp. n. (♂♀), Raveniola shangrila sp. n. (♂), Raveniola songi sp. n. (♂) and Raveniola yunnanensis sp. n. (♂) – are described from the highlands of Yunnan Province, China. According to some characters (shape of the palpus, palpal tibia and tibia I in males) they can be placed together with Raveniola hebeinica Zhu, Zhang & Zhang, 1999 and with Raveniola guangxi (Raven & Schwendinger, 1995), comb. n., transferred here from Sinopesa Raven & Schwendinger, 1995. The current generic position of Raveniola xizangensis (Hu & Li, 1987) is confirmed. Other Chinese nemesiids referred previously to Raveniola are transferred to Sinopesa: Sinopesa chinensis (Kulczyński, 1901), comb. n., Sinopesa sinensis (Zhu & Mao, 1983), comb. n. and Sinopesa chengbuensis (Xu & Yun, 2002), comb. n. The relationships between these Asian genera and their relations to Afrotropical nemesiids are discussed.
Araneae, spiders, taxonomy, Nemesiidae, Raveniola, Sinopesa
Raveniola Zonstein, 1987 with 20 named species is the fifth largest genus of the globally distributed Nemesiidae, encompassing 356 species belonging to 43 genera (
Among the countries the highest number of Raveniola species, five out of 20, is reported from China (cf.
The study began with an examination of several nemesiid series donated to us by Russian entomologists who had visited Yunnan Province in the People’s Republic of China in 2005. One of us (YM), had additionally collected nemesiid material while visited China in 2011. One species (Raveniola hebeinica) was obtained courtesy of our Chinese colleagues (Shuqiang Li and Zhang Feng). A rich collection of comparative material, including the majority of known Raveniola species, as well as representatives of the nemesiid genera Hermacha Simon, 1889, Entypesa Simon, 1902, Lepthercus Purcell, 1902, Pionothele Purcell, 1902and Sinopesa (4, 8, 1, 1 and 3 species, respectively), was obtained from the collections listed below.
Institutional acronyms used here are: ARC – Agriculture Research Council, Pretoria, South Africa; BDSU – Biology Department of Shandong University, China; FMNH – The Field Museum of Natural History, Chicago, USA; HUB – Hebei University, Baoding, China; IZAS – Institute of Zoology, Chinese Academy of Sciences, Beijing, China; MHNG – Muséum d’histoire naturelle, Genève, Switzerland; MNHN – Muséum national d’Histoire naturelle, Paris, France; NHM – Natural History Museum, London, UK; NMW – Naturchistorisches Museum Wien, Austria; TAU – Zoological Museum, Tel Aviv University, Israel; ZMMU – Zoological Museum of Moscow University, Russia.
Other abbreviations are as follows. Eyes: ALE – anterior lateral; AME – anterior median, PLE – posterior lateral, PME – posterior median. Spinnerets: PLS – posterior lateral, PMS – posterior median. Spine shape and position: d – dorsal; M – megaspine; p – prolateral; pd – prodorsal; pv – proventral; r – retrolateral; rd – retrodorsal; rv – retroventral; v – ventral.
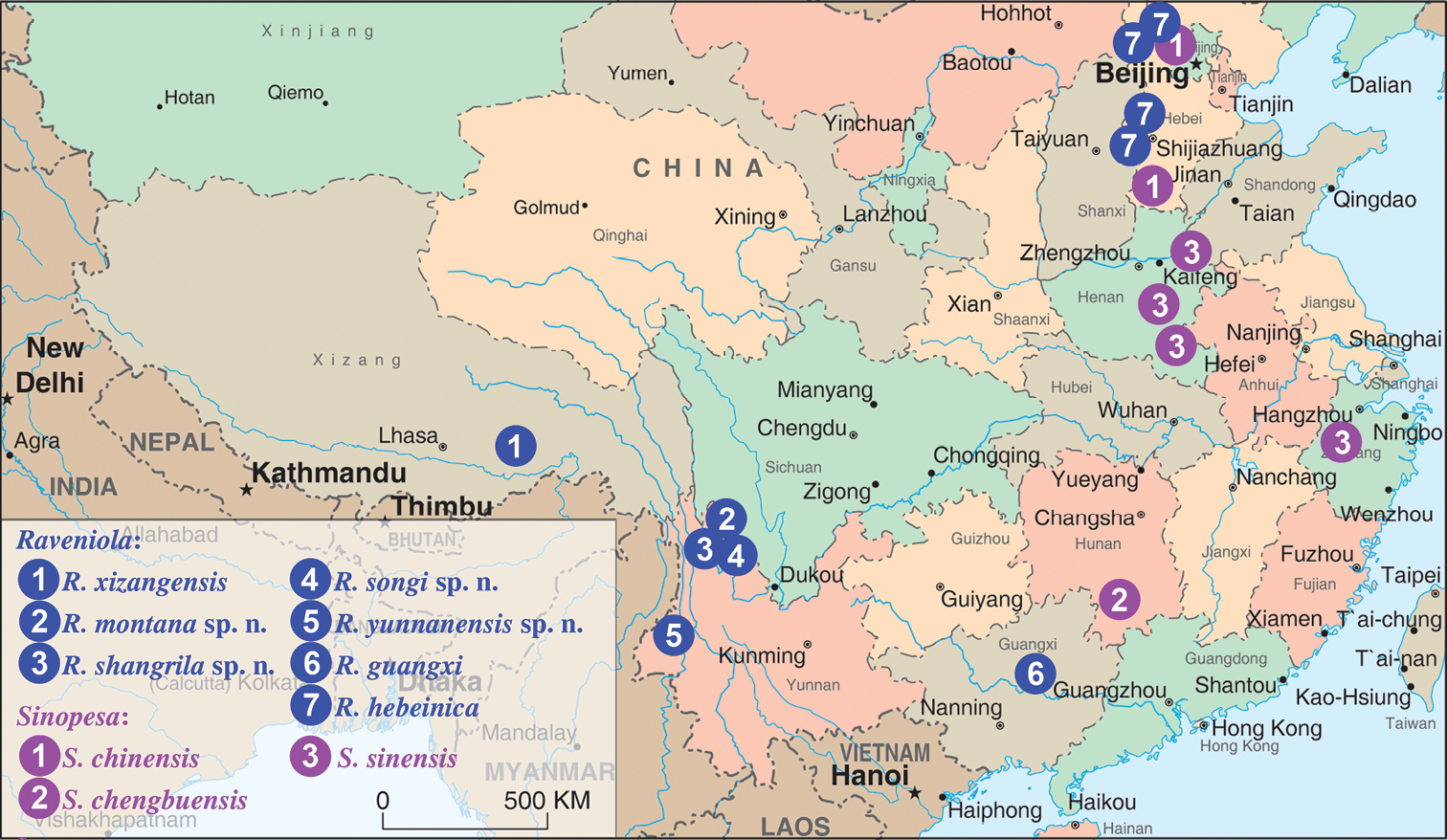
Photographs were taken using a Canon 500D digital camera with a 100 mm Canon macro lens and a Zeiss Discovery V20 stereomicroscope with a Canon PowerShot G9 digital camera attached to it. Measurements were taken to an accuracy of 0.01 mm. All measurements are given in millimetres. Total body length includes chelicerae but not spinnerets. Diameter of AME is given usually as a diameter of a sharply edged AME pupil. When the eye dome was mounted well and could be measured, the corresponding data follow in parentheses. Any measurements for this parameter are also given in parentheses. The length of sternum was measured along the straight line between the posterior tip of the sternum and the hindmost part of the labium. Lengths of leg and palp segments were measured on the dorsal side, and lengths of spinneret segments on the ventral side, from midpoint of anterior margin to midpoint of posterior margin. Lengths of palps and legs are given as: total (femur, patella, tibia, metatarsus and tarsus). Fig. 1 was created on the base of a small tourist map located online at http://www.homepages.ucl.ac.uk/~zczcc07/maps.htm and claimed to be free to reproduce and use.
Localities of Raveniola and Sinopesa species in China. Raveniola: 1 XIZANG/TIBET: Jansha County 2–4 YUNNAN: Shika Mts. 5 YUNNAN:Finchuiyanou Mts. 6 GUANGXI: Liuzhou 7 BEIJING municipality: Mt. Xialongmen; HEBEI: Tuoliang, Bai’an, Damaqun Shan Mts. Sinopesa: 1 BEIJING municipality: Chanping; HEBEI: Pingxiang 2 HUNAN: Chengbu County 3 ZHEJIANG: Lin’an; HENAN: Huaiyang, Xiping, Yueyang, Shāngchéng.
http://species-id.net/wiki/Raveniola
By retroventral position of the male mating spur on tibia I, Raveniola differs essentially from the majority of the Holarctic and Asian nemesiid genera: from Mediterranean Nemesia Audouin, 1826, Iberesia Decae & Cardoso, 2006 and Brachythele Ausserer, 1871 as well as from the Nearctic Calisoga Chamberlin, 1937 and from Asian Atmethochilus Simon, 1887 and Damarchus Thorell, 1891. Males in all these genera possess mating spurs located on the process ventrally or prolaterally. In addition, in the two latter genera males have paired tarsal claws provided with a single S-shaped tooth row instead of the biserial dentition typical for male nemesiids.
Within the rest of this group of genera, in which males are also known to possess the retrolateral or retroventral megaspines on tibia I, Raveniola can be distinguished from Central American Mexentypesa Raven, 1987 by having the unpaired tarsal claw (absent in the latter genus) and integral tarsi (pseudosegmented in Mexentypesa); from African Hermacha Simon, 1889, Entypesa Simon, 1902, Lepthercus Purcell, 1902 and Pionothele Purcell, 1902 – by much smaller PMS (from first three of them) or longer apical segment of PLS (domed in Pionothele). Moreover, males of Raveniola differ from males of all the above-mentioned genera by their elongate, cylindrical and strongly spinose palpal tibiae. The congeneric females have no unique distinctive characters.
East-Asian Sinopesa Raven & Schwendinger, 1995 is the only genus that has been found to share with Raveniola the above-listed definitive features. These partially sympatric genera differ from each other by the characters shown in the table in the Discussion below.
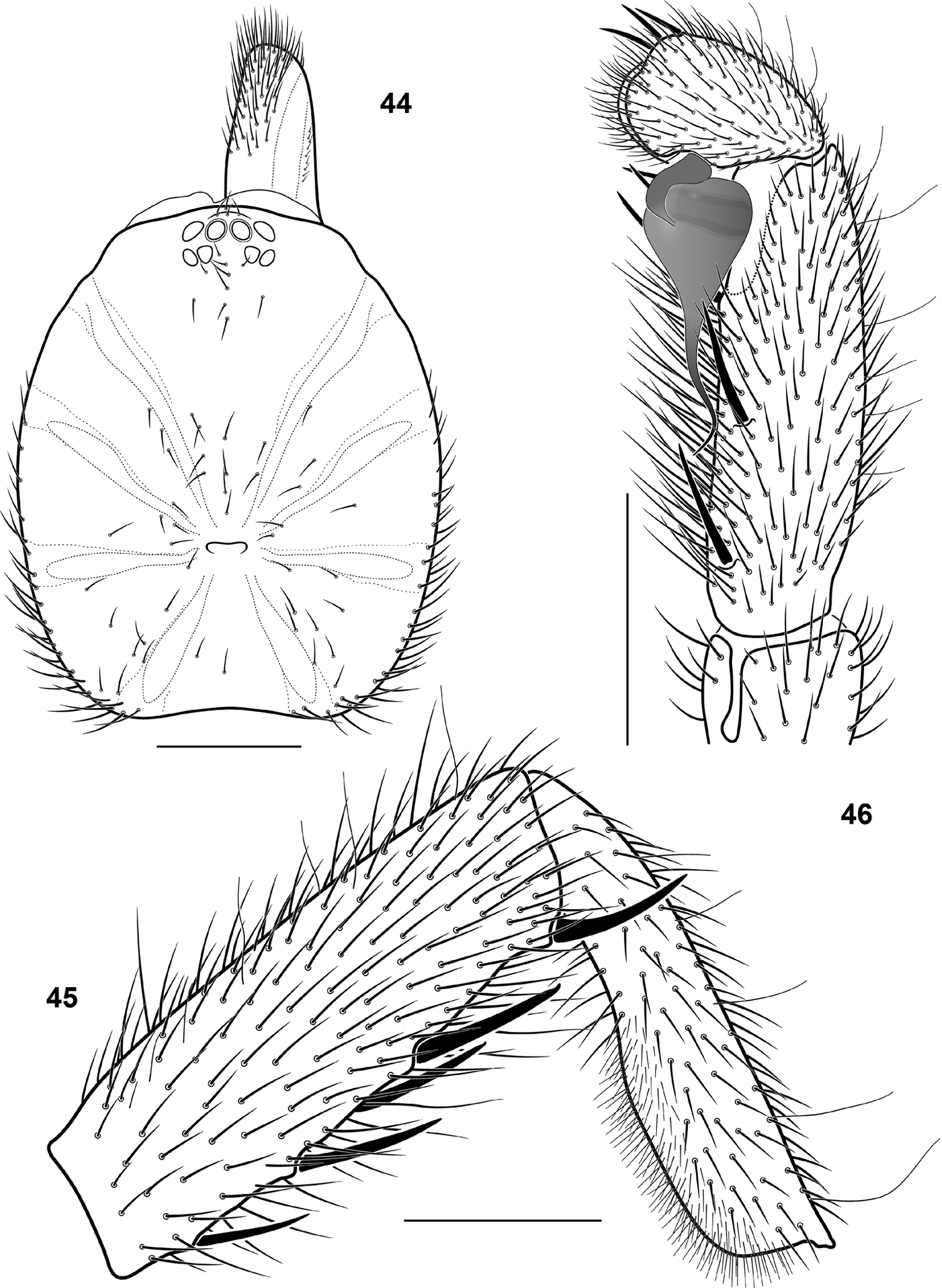
Medium-sized to large nemesiids with carapace 4–14 mm long. Carapace hirsute. Eye tubercle low to moderately developed. Chelicerae in most species without rastellum. Maxillae rectangular with few to numerous cuspules. Serrula not evident. Labium twice wider than long with no cuspules. Paired sternal sigillae small round submarginal to marginal. Leg formula 4123 or 1423. Metatarsal preening combs absent. Leg tarsi integral (not cracked or pseudosegmented), aspinose in most species. In males scopula on tarsi I always entire; tarsi II with entire or narrowly divided scopula; tarsi III and IV with widely divided scopula or without it; conspecific females with weaker scopula on posterior tarsi. Paired tarsal claws biserially toothed both in males and females; claw apex long and moderately curved. Unpaired tarsal claw small and sharply bent. Males: intercheliceral tumescence if present located ventrally; palpal tibia ±long, spinose; cymbium rather short with or without spines; tibia I with 2(3) sequential megaspines. Females: each paired spermatheca with 2–3 individual diverticulae. PMS small to absent. PLS: apical segment triangle to digitiform.
Over 20 species are currently known in the south Palearctic, from Turkey to China (see
Unfortunately, we have no direct label data shoving peculiarities in the habitats and ecology of Chinese species of Raveniola. The only male of Raveniola yunnanensis sp. n.was found under the rock in the mixed mountainous broad-leaved forest (I. Kabak, personal communication). The representatives of Raveniola montana sp. n., Raveniola shangrila sp. n. and Raveniola songi sp. n. were collected with pitfall traps together with highland ants Myrmica pleiorhytida Radchenko & Elmes, 2009 (Hymenoptera, Formicidae) by the same collectors and in the same biotope. The latter species was noted inhabiting mountainous meadows (see
Females of Raveniola guangxi, Raveniola shangrila sp. n., Raveniola songi sp. n. and Raveniola yunnanensis sp. n. are unknown.
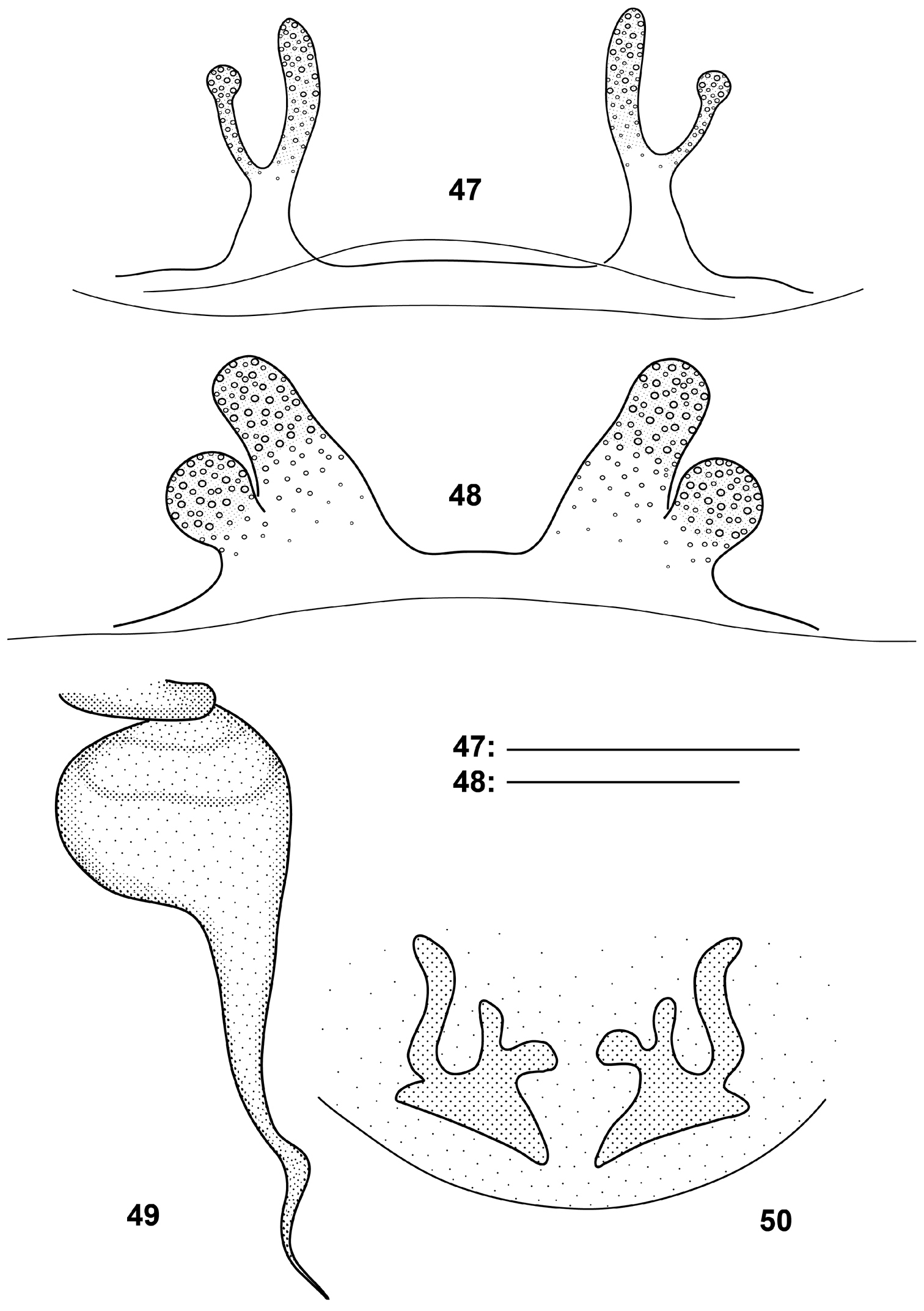
| 1 | Large species: carapace length > 10 mm. Males: embolus with subapical keel (Fig. 49). Females: basal receptacle bifurcate (Fig. 50) | Raveniola xizangensis |
| – | Smaller: carapace length 4.6–7.3 mm. Males: subapical embolic keel absent or vestigial. Females: basal receptacle entire | 2 |
| 2 | Males | 3 |
| – | Females | 7 |
| 3 | PMS absent | 4 |
| – | PLS (sometimes tiny) present | 5 |
| 4 | Maxillae with few (3–4 in the holotype) cuspules. Embolus as shown on Figs 37 and 38 | Raveniola guangxi |
| – | Maxillae with numerous (>13) cuspules. Embolus as in Fig. 41 | Raveniola shangrila sp. n. |
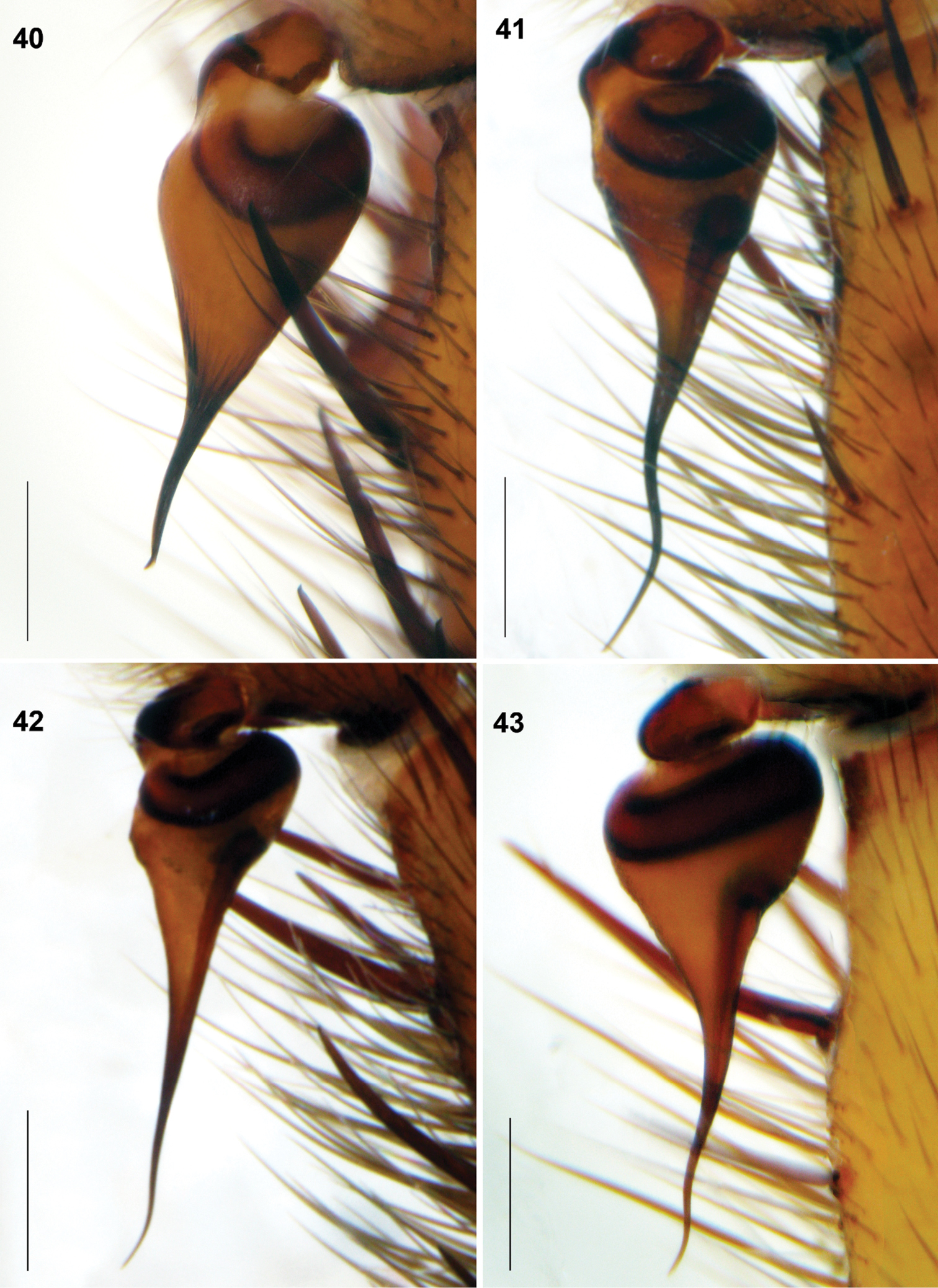
| 4 | PLS: apical segment digitiform. Embolus ±twisted (Figs 40–43) | 5 |
| – | PLS: apical segment triangle. Embolus curved apically (Fig. 39) | Raveniola hebeinica |
| 5 | Leg I: tibia incrassate, equal in length with, or shorter than metatarsus (Figs 26, 28). Few spines on cymbium (Figs 32–34) | 6 |
| – | Leg I: tibia very long and slender, considerably longer than metatarsus (Fig. 29). Cymbium with numerous dorsal spines (fig. 35) | Raveniola yunnanensis sp. n. |
| 6 | Leg I: metatarsus straight, longer than tibia (Fig. 26). Cymbium with long spines (Fig. 32). Embolus short with well developed embolic ridges (Fig. 40) | Raveniola montana sp. n. |
| – | Leg I: metatarsus ±curved, equal in length to tibia (Fig. 28). Spines on cymbium shorter (Fig. 34). Embolus longer without or with very weak embolic ridges (Fig. 42) | Raveniola songi sp. n. |
| 7 | PLS: apical segment triangle. Spermathecae as shown in Fig. 47 | Raveniola hebeinica |
| – | PLS: apical segment digitiform. Spermathecae as shown in Fig. 48 | Raveniola montana sp. n. |
http://species-id.net/wiki/Raveniola_guangxi
Figs 7, 15, 24, 30, 37, 38Holotype ♂ – CHINA: GuangxiProvince: Liuzhou, Dragon Lake (24°16'N, 109°24'E); in MCZ; examined.
Differs from all other known Chinese congeners except Raveniola shangrila sp. n. by absence of PMS. From the latter species Raveniola guangxi may be distinguished by shape of the embolus and by fewer maxillary cuspules – 3–4 vs. 15–20 (cf. Figs 15, 30, 37 and 19, 33, 41, respectively).
The holotype male was described in detail by Raven & Schwendinger, 1995. Carapace, sternum with labium and maxillae, tibia and metatarsus I, palpal tibia and cymbium, and bulbus (in retrolateral and ventral aspects) as shown in Figs 7, 15, 24, 30, 37 and 38. Female unknown.
Known only from the type locality (Fig. 1).
http://species-id.net/wiki/Raveniola_hebeinica
Figs 8, 16, 25, 31, 39, 47Holotype ♂ and paratypes ♀♀ from Mt. Tuolang (Hebei Province, Pinshang County, 38°45'N, 113°49'E, 1500–2000 m); supposed to be in HUB, but was not found (Feng Zhang, personal communication).
Beijing Municipality, Mt. Xialongmen (39°58'N, 115°27'E), 1000–1300 m, 60–65 km W Beijing, 21–23.09.2001, coll. Y. D. Yu – 1♂ (IZAS). Hebei Province: Xingtai County, Taihang Mts., surroundings of Bai’an 60 km W Xingtai City (approximately 37°04'N, 113°48'E), 600–1000 m, 16.07.2007, coll. Jiao Guobin – 1♀, 1♀ subad. (HUB); Damaqun Shan Mts. (40°31'N, 115°49'E, 1000–1200 m) 75 km NW Beijing, 12.08.2010, coll. Yu. M. Marusik – 2♀ subad., 1 juv. (TAU).
The species differs from all known Chinese Raveniola species by small lateral receptacles in females (cf.
♂♀ were well described by
The only examined male has carapace 7.02 mm long (7.29 in the holotype). The carapace of the noticeably smaller examined adult female measures only 5.17 mm (vs. 6.67 in the female paratype used at the description).
CHINA: Hebei Province and Beijing Municipality (Fig. 1).
urn:lsid:zoobank.org:act:3EC3FB22-1D28-43BC-9A80-99C452367601
http://species-id.net/wiki/Raveniola_montana
Figs 5, 9, 10, 17, 18, 26, 32, 40, 48Holotype ♂ and paratype ♀ – CHINA: Yunnan Province, Sueshan Mt. Ridge, Shika Mts. 10–15 km W Zhongdian (approximately 27°48'N, 99°35'E), 3800–4300 m, 25.05–6.06.2005, coll. I. Shokhin & S. Murzin (IZAS).
The specific epithet montana is derived from the Latin montanus (pertaining to the mountains), referring to the mountain habitat of this species.
The species differs from all other Chinese species of Raveniola by having a short embolus provided with deep ridges in males (Fig. 40); the specific configuration of female spermathecae is shown in Fig. 48.
Male (holotype). Body length 15.50. Colour in alcohol: carapace, chelicerae, palps and first pair of legs dorsally intense reddish brown; eye tubercle with darker spots surrounding AMEs and lateral eyes; sternum, labium, maxillae and legs II–IV light reddish brown; dorsal abdomen uniformly light greyish brown, ventral abdominal surface and spinnerets pale greyish brown.
General appearance as in Fig. 5. Carapace (Fig. 9) 6.35 long, 5.51 wide; covered with semi-adpressed dark hairs. Eye diameters (AME, ALE, PLE, PME): 0.19(0.26), 0.37, 0.19, 0.11. Interdistances: AME–AME 0.13(0.07), ALE–AME 0.09(0.06), ALE–PLE 0.04, PLE–PME 0.03, PME–PME 0.48. Cheliceral furrow with 9 promarginal teeth and 5 mesobasal denticles each. Labium (Fig. 17) 0.59 long, 1.08 wide. Maxillae with 15 cuspules each. Sternum 2.66 long, 2.65 wide. Palp: 8.66 (3.39, 1.59, 2.51, –, 1.17). Leg I: 20.23 (5.18, 3.06, 4.50, 4.82, 2.67). Leg II: 18.52 (4.97, 2.61, 4.13, 4.29, 2.52). Leg III: 18.15 (4.63, 2.48, 3.66, 4.65, 2.73). Leg IV: 23.41 (5.70, 2.66, 5.04, 6.88, 3.13). Leg I: tibia slightly incrassate, metatarsus slightly curved retroventrally (Fig. 26).
Spination. Palp: femur d1–1–1–1, pd1, rd1; patella p1–1–1, r1; tibia d1–1–2, p2–2–1, r0–1–1, v3–1–3; cymbium d5. Leg I: femur d1–1–1–1, pd1–1–1; rd 1–1–1; patella p1–1; tibia p2–1–0, pv1–1–0–0, rv1–1–M–M; metatarsus v0–0–2. Leg II:hg femur d1–1–1–1; pd1–1–1; patella p1–1; tibia p2(1)–1(0)–1, v2–2–3; metatarsus p1(0)–1–0; v2–2–3. Leg III: femur d1–1–1–1, pd0–1–1, rd0–1–1; patella p1–1, r1; tibia d1–1, p1–1–1, r1–1–1, v2–2–2(3); metatarsus d1–1–2, p1–1–1, r1–1–1, v2(3)–2–3. Leg IV: femur d1–1–0–0, pd0–1–1, rd0–1–1; patella p1, r1; tibia d1–1–2, p1–1–1, r1–1–1, v2–2–2(3); metatarsus pd1–1–2, p1–1–1, r1–1–1, v2–1–2–1(0)–3. Tarsi I–IV aspinose.
Scopula: distally on metatarsus I, entire on tarsus I, divided by setae on tarsus II; elsewhere absent. Paired claws: inner row with 5–6, outer row with 6–7 teeth. Trichobothria: 2 rows of 9–11 per row on tibiae, 12–17 on metatarsi, 11–14 on tarsi, 8 on cymbium.
Palpal tibia shortened, cymbium with few long spines (Fig. 32). Bulb provided with well-developed ridges; embolus short slightly twisted (Fig. 40).
Spinnerets. PMS: length 0.38; diameter 0.12. PLS: maximum diameter 0.35; length of basal, medial and apical segments 0.61, 0.63, 0.71; total length 1.95; apical segment digitiform.
Female (paratype): Body length 12.90. Colour in alcohol as in male, but slightly paler.
Carapace (Fig. 10) 4.66 long, 3.75 wide. Eye diameters (AME, ALE, PLE, PME): 0.16(0.20), 0.22/0.23, 0.14, 0.10. Interdistances: AME–AME 0.10(0.06), ALE–AME 0.07(0.05), ALE–PLE 0.05, PLE–PME 0.03, PME–PME 0.43. Cheliceral furrow with 9 promarginal teeth and 5 mesobasal denticles. Labium (Fig. 18) 0.50 long, 1.03 wide. Maxillae with 15 cuspules each. Sternum 2.16 long, 1.99 wide. Palp: 7.45 (2.64, 1.44, 1.74, –, 1.63). I: 12.00 (3.55, 1.70, 2.72, 2.33, 1.70). II: 10.86 (3.10, 1.63, 2.29, 2.17, 1.67). III: 10.62 (2.75, 1.49, 2.05, 2.60, 1.73). IV: 14.09 (3.70, 1.57, 3.01, 3.76, 2.05).
Spination. All femora with a few stiff bristles (undeveloped spines) located medially and distally; palpal patella, patella I and tarsi I–IV aspinose. Palp: femur pd0–0–1; tibia p2–2, v1–1–3; tarsus v2. Leg I: femur pd0–0–1; tibia v1(2)–1(2)–2; metatarsus v2–2–2. Leg II: femur pd0–0–1; patella p1; tibia p1–1, v1–1–2; metatarsus p0–1–0, v2–2–2. Leg III: femur pd 0–1–1, rd 0–1–1; patella p1, r1; tibia d0–1–1, p1–1–1, r1–1–1, v2–2–3; metatarsus d0–1–1, p1–1–1, r1–1–1, v2–2–3. Leg IV: femur pd0–1–1, rd0–0–1; patella r1; tibia d0–1–0, p1–1(0)–1, r0–1–1, v2–2–3; metatarsus d0–1–0, p1–1–1–1, r1–1–1, v2–1–2–3.
Scopula: distal on metatarsus I, narrowly divided by setae on palpal tarsus and tarsus I, widely divided and vestigial on tarsus II, elsewhere absent. Paired claws: promargin and retromargin on tarsi I and II with 6–7 teeth, on tarsi III and IV with 4–6 teeth each, respectively; palpal claw with 4 teeth on promargin. Trichobothria: 2 rows of 8–10 per row on tibiae, 14–16 on metatarsi, 11–12 on tarsi, 8 on palpal tarsus.
Spermathecae as in Fig. 48.
Spinnerets. PMS: length 0.32; diameter 0.13. PLS: maximum diameter 0.37; length of basal, medial and apical segments 0.59, 0.37, 0.50; total length 1.46; apical segment digitiform.
CHINA: Yunnan Province (Fig. 1).
Male nemesiids, dorsal view. 2 Sinopesa maculata, Thailand 3 Entypesa schoetedeni, South Africa 4 Raveniola virgata, Kyrgyzstan 5 Raveniola montana sp. n., South China (scale bar = 5 mm).
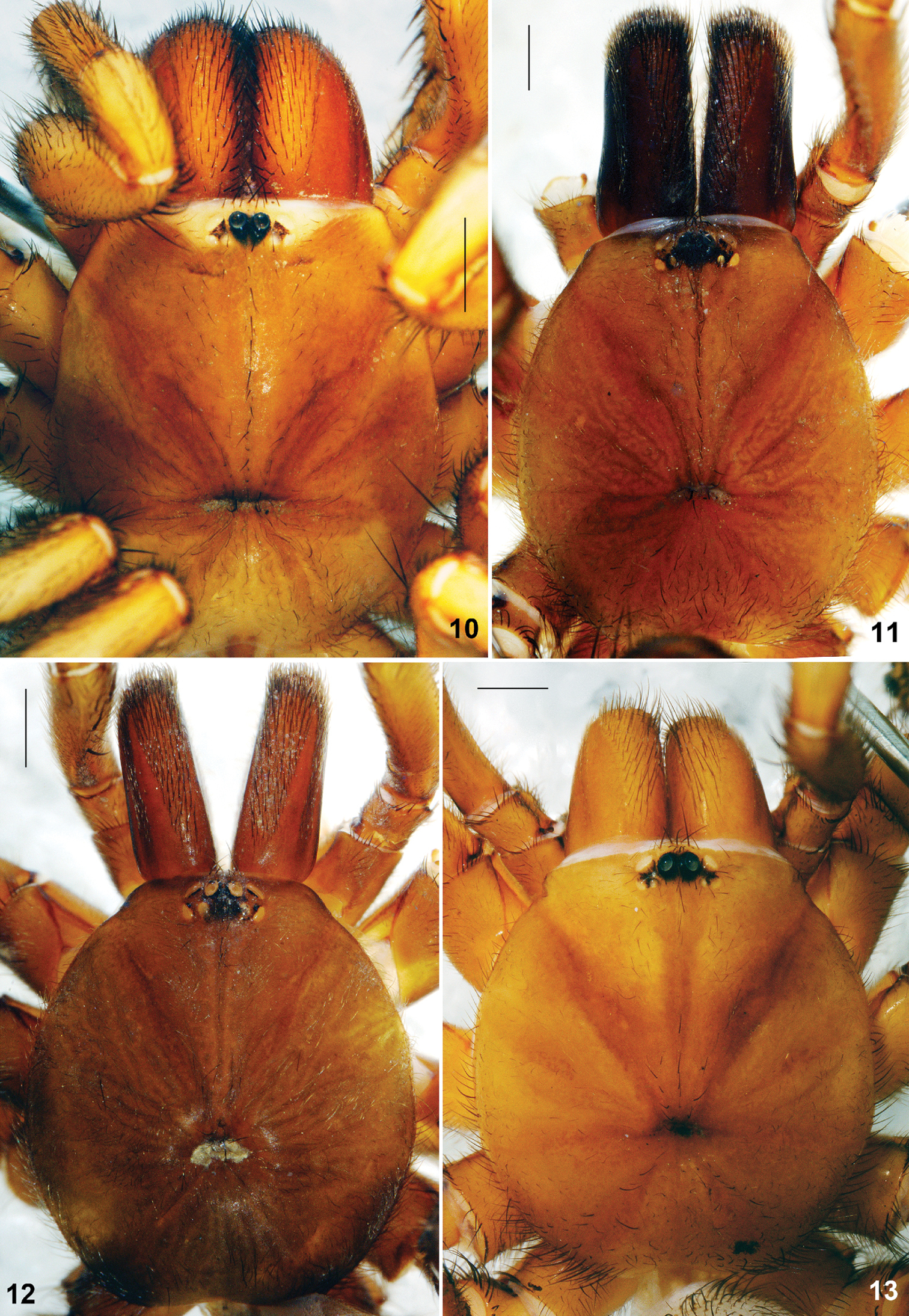
Figures 6-9. Sinopesa and Raveniola, holotype (7, 9) and conspecific (6, 8) males: carapace, dorsal view. 6 Sinopesa maculata 7 Raveniola guangxi 8 Raveniola hebeinica 9 Raveniola montana sp. n. (scale bar = 1 mm).
Raveniola, holotype (13) and paratype (10–12) males (11–13) and female (10) carapace, dorsal view 10 Raveniola montana sp. n. 11 Raveniola shangrila sp. n. 12 Raveniola songisp. n. 13 Raveniola yunnanensis sp. n. (scale bar = 1 mm).
urn:lsid:zoobank.org:act:1C0D32BA-DC72-471C-847C-A783549E2A13
http://species-id.net/wiki/Raveniola_shangrila
Figs 11, 19, 27, 33, 41Holotype ♂ – CHINA: Yunnan Province, Sueshan Mt. Ridge, Shika Mts. 10–15 km W Zhongdian (approximately 27°48'N, 99°35'E), 3800–4300 m, 25.05–6.06.2005, coll. I. Shokhin & S. Murzin (IZAS). Paratypes. 5♂ with the same collecting data are shared between MNHG, MNHN, NHML, TAU and ZMMU.
The specific epithet is given in honour of the mythical Tibetan land Shangri-La attributed to the highland region located in the far eastern part of Tibet (Xizang) and north-western part of Yunnan, i.e., including the type locality of this species.
The full reduction of PMS allows to place this species together with Raveniola guangxi; Raveniola shangrila can be distinguished from the latter species by shape of the embolus and larger number of maxillary cuspules – 15–20 vs. 3–4 (cf. Figs 15, 30, 37 and 19, 33, 41, respectively). In general, specimens of Raveniola shangrila sp. n. appear poorly distinguishable from those of Raveniola songi sp. n., but certain distinctions in the configuration of male bulb and metatarsus I are evident (cf. Figs 15, 16, 23 and 24).
Male (holotype). Body length 16.10. Colour in alcohol: carapace (with lighter spotted pattern), legs I partially, legs II–IV mostly middle foxy brown; sternum, labium and maxillae lighter coloured; chelicerae, all femora dorsally, tibiae and metatarsi I dark reddish brown; eye tubercle blackish brown; abdomen uniformly light brownish grey; genital area, booklungs and spinnerets pale yellowish grey.
Carapace (Fig. 11) 5.91 long, 5.45 wide; covered with semi-adpressed dark hairs. Eye diameters (AME, ALE, PLE, PME): 0.20(0.25), 0.31, 0.20, 0.20. Interdistances: AME–AME 0.15(0.11), ALE–AME 0.12(0.10), ALE–PLE 0.09, PLE–PME 0.03, PME–PME 0.57. Cheliceral furrow with 9 promarginal teeth and 4–5 mesobasal denticles. Labium (Fig. 19) 0.60 long, 1.03 wide. Maxillae with 17–19 small cuspules in wide triangle area. Sternum 2.85 long, 2.75 wide. Palp: 9.19 (3.56, 1.78, 2.77, –, 1.08). Leg I: 18.61 (5.02, 2.85, 4.09, 4.27, 2.38). Leg II: 16.11 (4.65, 2.56, 3.25, 3.46, 2.19). Leg III: 13.48 (3.71, 1.97, 2.47, 3.16, 2.17). Leg IV: 17.57 (4.72, 2.40, 3.81, 4.23, 2.41). Leg I: tibia incrassate, metatarsus curved retroventrally (Fig. 27).
Spination. All femora with a few stiff bristles (undeveloped spines) located medially and distally; patella IV and tarsi I–IV aspinose. Palp: femur pd1; patella p1; tibia d1–1–1, p1–1–1, r0–1–1, v1–2–0; cymbium d4. Leg I: femur pd1–0–1; patella p1; tibia p1–1–1, v2–2–M–M; metatarsus rv1. Leg II: femur pd1–0–1, rv0–0–1; tibia p1–1–1, v2–2–2; metatarsus p0–1–1; v2–2–3. Leg III: femur pd1–0–1, rd1–0–1; patella p1–1, r1–1; tibia d1–0, p1–1, r0–1, v2–2–2; metatarsus d0–1–1, p1–1–1, r0–1–1, v2–2–2(3). Leg IV: femur pd0–0–1, rd0–0–1; tibia p0(1)–1–1, r0–0(1)–1, v2–2–2; metatarsus p0–1–1, r0–1–1, v2–2–2.
Scopula: long, distal 1/2 on metatarsus I and II, entire on tarsi I and II, mixed with setae on tarsi III and IV. Paired claws with 6–8 teeth on promargin and retromargin. Trichobothria: 2 rows of 7–9 per row on tibiae, 12–15 on metatarsi, 10–12 on tarsi, 7 on cymbium.
Cymbium with few rather short spines (Fig. 33). Bulb without ridges; embolus long and twisted (Fig. 41).
Spinnerets. PMS: absent. PLS: maximum diameter 0.55; length of basal, medial and apical segments 0.84, 0.54, 0.75; total length 2.13; apical segment digitiform.
Female unknown.
Carapace length in males varies from 5.03 to 5.95 (n=5).
CHINA: Yunnan Province (Fig. 1).
urn:lsid:zoobank.org:act:6B13CF73-BC7B-4901-85E6-5ED8DF4C8A9C
http://species-id.net/wiki/Raveniola_songi
Figs 12, 20, 28, 34, 42Holotype ♂ – CHINA: Yunnan Province, Sueshan Mt. Ridge, Shika Mts. 10–15 km W Zhongdian (approximately 27°48'N, 99°35'E), 3800–4300 m, 25.05–6.06.2005, coll. I. Shokhin & S. Murzin (IZAS). Paratypes. 11♂ with the same collecting data are shared between IZAS (2), MNHG (2), MNHN (1), NHML (1), TAU (4) and ZMMU (1).
Etymology. The specific name is given in honour and memory of Prof. Daxiang Song (宋大祥; 1935–2008), for his immense contribution to Chinese arachnological research.
Males of Raveniola songi sp. n.habitually resemble those of Raveniola shangrila sp. n.but unlike them possess small PMS; other distinctive features are shown above (cf. Figs 15, 16, 23 and 24).
Male (holotype). Body length 13.50. Colour in alcohol: carapace, chelicerae and legs I medium reddish brown; eye tubercle somewhat darker; sternum, labium, maxillae and legs II–IV even lighter reddish brown; abdomen light greyish brown with darker pattern consisting of a weak longitudinal median spot and few transverse fasciae dorsally, and small irregularly shaped spots laterally and ventrally; genital area, book-lungs and spinnerets pale yellowish brown.
Carapace (Fig. 12) 5.10 long, 4.24 wide; covered with moderately dense and thin semi-adpressed dark hairs. Eye diameters (AME, ALE, PLE, PME): 0.18(0.24), 0.24, 0.20, 0.20. Interdistances: AME–AME 0.15(0.10), ALE–AME 0.06(0.04), ALE–PLE 0.08, PLE–PME 0.02, PME–PME 0.30. Cheliceral furrow with 9 promarginal teeth and 4–5 mesobasal denticles. Labium (Fig. 20) 0.33 long, 0.77 wide. Maxillae with 24–26 cuspules arranged in triangle area. Sternum 2.40 long, 2.28 wide. Palp: 7.86 (3.16, 1.59, 2.31, –, 0.80). Leg I: 15.32 (4.38, 2.12, 3.48, 3.25, 2.09). Leg II: 13.73 (3.91, 1.86, 3.19, 2.77, 2.00). Leg III: 13.11 (3.47, 1.85, 2.40, 3.31, 2.08). Leg IV: 16.63 (4.40, 1.95, 3.39, 4.60, 2.29). Leg I: tibia incrassate, metatarsus slightly curved retroventrally (Fig. 28).
Spination. Palp: femur d0(1)–1–1–1, pd0–1–1; patella p1–1; tibia d1–1–1, r0–1(0)–1, pv1–1–1, rv1–1–1; cymbium d2(4). Leg I: femur d1–1–1–1, pd0–1–1; rd 0–0–1; tibia p1–1–0, pv1–1; rv1–1–M–M; metatarsus v0–0–2. Leg II: femur d1–1–1–1; pd1–1–1; patella p1–1; tibia p1–1–1, v1–2–1(2)–3; metatarsus p0–1–0; v1–2–2. Leg III: femur d1–1–1–1, pd1–1–1, rd1–1–1; patella p1–1, r1; tibia d1–1–1, p1–1–1, r1–1–1, v2–2–3; metatarsus d1–1–1, p1–1–1, r1–1–1, v2–2–3. Leg IV: femur d1–1–1–1, pd1–1–1, rd1–1–1; patella p1, r1; tibia d1–1–1(0), p1–1–1, r1–1–1, v2–2–3; metatarsus pd1–1–1, p1–1–1, r1–1–1, v2–2–2–3. Patella I and tarsi I–IV aspinose.
Scopula: entire distal 2/3 and 1/2 on metatarsi I and II, respectively; entire on tarsi I and II, widely divided by setae on tarsus III; vestigial on tarsus IV. Paired claws on tarsi I-III and IV with 8–10 and 8–10 teeth per row, respectively. Trichobothria: 2 rows of 7–8 per row on tibiae, 8–11 on metatarsi, 8–9 on tarsi, 6 on cymbium.
Palpal tibia long, cymbium with few short spines (Fig. 34). Bulb without ridges; embolus long, acuminate and slightly twisted (Fig. 42).
Spinnerets. PMS: length 0.41; diameter 0.14. PLS: maximum diameter 0.46; length of basal, medial and apical segments 0.70, 0.44, 0.42; total length 1.56; apical segment short-digitiform.
Female unknown.
Carapace length in males varies from 4.80 to 5.43 (n=12).
CHINA: Yunnan Province (Fig. 1).
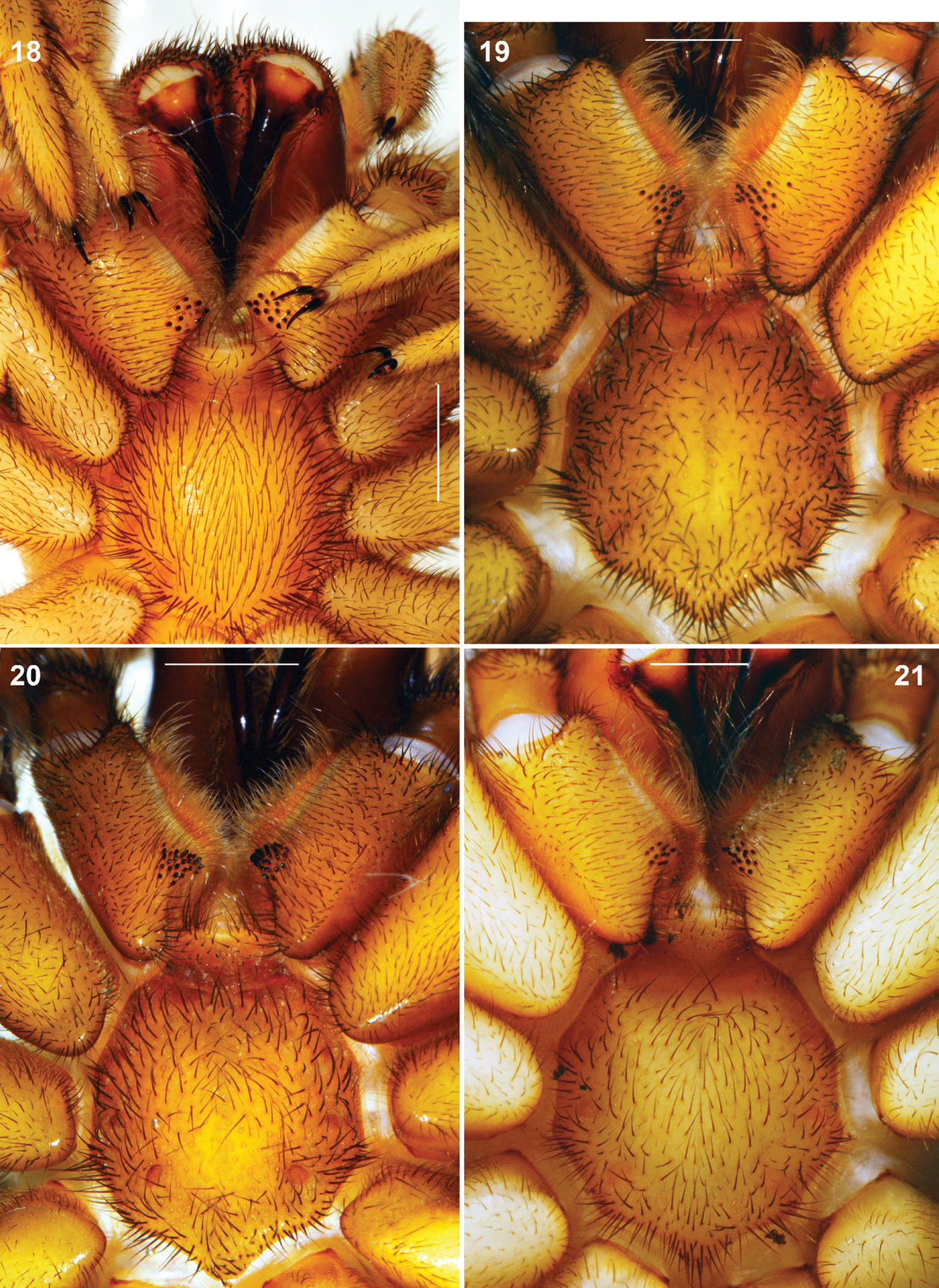
Sinopesa and Raveniola, holotype (15, 17) and conspecific (14, 16) males: sternum, labium and maxillae, ventral view 14 Sinopesa maculata 15 Raveniola guangxi 16 Raveniola hebeinica 17 Raveniola montana sp. n. (scale bar = 1 mm).
Raveniola, holotype (21) and paratype (18–20) males (19–21) and female (18): sternum, labium and maxillae, ventral view 18 Raveniola montana sp. n. 19 Raveniola shangrila sp. n. 20 Raveniola songisp. n. 21 R. yunnanensis sp. n. (scale bar = 1 mm).
Entypesa, Sinopesa and Raveniola, holotype (24) and conspecific (22, 23, 25) males: tibia and metatarsus I, retrolateral view. 22 Entypesa schoetedeni 23 Sinopesa maculata 24 Raveniola guangxi 25 Raveniola hebeinica (scale bar = 1 mm).
urn:lsid:zoobank.org:act:BCC3B9B7-A813-46D3-9DB1-3C9884038FCA
http://species-id.net/wiki/Raveniola_yunnanensis
Figs 13, 21, 29, 35, 43Holotype ♂ – CHINA: Yunnan Province, Finchuiyanou Mts. 40 km NNW of Baoshan, 25°28'54"N, 99°05'05"E, 3200 m, 10.05.2005, coll. I. Kabak & I. Belousov (IZAS).
The specific epithet is given after the name of the inhabited region (Yunnan).
Males differ from all other Chinese congeners by lighter body colouration and longer legs (tibia I 5.5 times as longer than wide vs. 4–5 times in other species) and more spinose embolus armed with ca. 25–30 spines (vs. 3–7).
Male (holotype). Body length 12.85. Colour in alcohol: carapace, chelicerae, palps and first pair of legs dorsally intense yellowish orange; eye tubercle with darker spots surrounding AMEs and lateral eyes; sternum, labium, maxillae and legs light yellowish orange; abdomen dorsally uniformly light grey, ventral abdominal surface and spinnerets pale greyish yellow.
Carapace (Fig. 13) 5.73 long, 4.60 wide; covered with moderately dense and thin semi-adpressed dark hairs. Eye diameters (AME, ALE, PLE, PME): 0.17 (0.24), 0.23, 0.13, 0.08/0.09. Interdistances: AME–AME 0.15 (0.10), ALE–AME 0.13 (0.10), ALE–PLE 0.12, PLE–PME 0.05, PME–PME 0.57. Cheliceral furrow with 9–10 promarginal teeth and 6–7 mesobasal denticles. Labium (Fig. 21) 0.43 long, 0.78 wide. Maxillae with 13–16 cuspules in compact area confined to basal maxillary edge. Sternum 2.32 long, 2.34 wide. Palp: 8.29 (3.52, 1.54, 2.41, –, 0.82). Leg I: 18.72 (5.17, 2.86, 4.49, 3.77, 2.43). Leg II: 17.70 (4.84, 2.52, 4.24, 3.67, 2.43). Leg III: 16.52 (4.24, 2.05, 3.52, 4.10, 2.61). Leg IV: 21.57 (5.22, 2.30, 5.02, 6.26, 2.77). Leg I: tibia 5.48 times longer than broad, slightly arcuate, metatarsus slightly curved retroventrally (Fig. 29).
Spination. Palp: femur d1–1–1–1, pd1–1–1; patella p1; tibia d2–1–2, p1–0–1–1–1, pv1–1–2–1, rv1–1–0; cymbium d ca.20. Leg I: femur d1–1–1(0)–0, pd1–1–1; rd 1(0)–1–1; patella p1; tibia p1–1–0, pv1–0–1–1, rv 1–1–0–M–M; metatarsus v0–1–2. Leg II: femur d1–1–1(0)–0; pd1–1–1; tibia p1–1–1, v2(3)–2–3; metatarsus p1–1; v2–2–3. Leg III: femur d1–1–1–1, pd1–1–1, rd1–1–1; patella p1, r1; tibia d1–1–1, p1–1–1, r1–1–1–1, v2–2–3; metatarsus d1–1–1, p1–1–2, r1–1–1–1, v2–2–3. Leg IV: femur d1–1–1–1, pd1–1–1, rd1–1–1; patella p1; tibia d1–1–0, p1–1–1, r1–1–1–1, v2–2–3; metatarsus pd1–1–1, p1–1–1, r1–1–1–1, v2–1–1–3. Tarsi I–IV aspinose.
Scopula: moderately dense and long – entire covering whole ventral metatarsus I and distal 2/3 of metatarsus II, entire on tarsi I–II, divided by setae on tarsus III; widely divided and vestigial on metatarsus III and tarsus IV. Paired claws: legs I–III with 6–8 teeth, leg IV with 9 teeth in two rows on each claw. Trichobothria: 2 rows of 6–8 per row on tibiae, 10–12 on metatarsi, 8–10 on tarsi, 6 on cymbium.
Palpal tibia long; cymbium strongly spinose (Fig. 35). Bulb without ridges; embolus gradually tapering and slightly twisted (Fig. 43).
Spinnerets. PMS: length 0.30; diameter 0.12. PLS: maximum diameter 0.48; length of basal, medial and apical segments 0.83, 0.62, 0.77; total length 2.22; apical segment digitiform.
Female unknown.
CHINA: Yunnan Province (Fig. 1).
Raveniola, holotype (26, 29) and paratype (27, 28) males: tibia and metatarsus I, retrolateral view. 26 Raveniola montana sp. n. 27 Raveniola shangrila sp. n. 28 Raveniola songisp. n. 29 Raveniola yunnanensis sp. n. (scale bar = 1 mm).
Raveniola, holotype (30, 32, 35), paratype (33, 34) and conspecific (31) males: palpal tibia, cymbium and bulbus, retrolateral view. 30 Raveniola guangxi 31 Raveniola hebeinica 32 Raveniola montana sp. n. 33 Raveniola shangrila sp. n. 34 Raveniola songisp. n. 35 Raveniola yunnanensis sp. n. (scale bar = 1 mm).
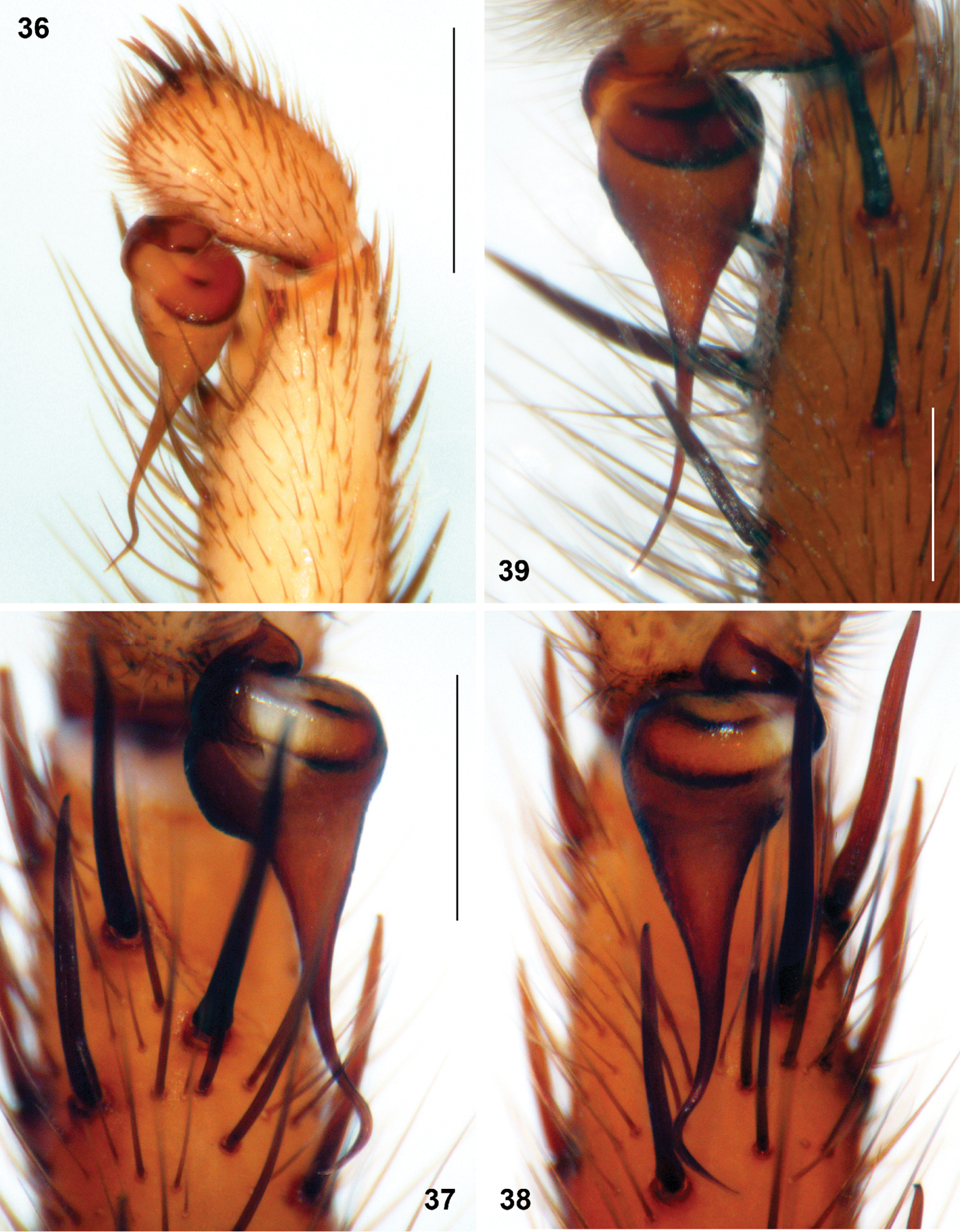
Sinopesa and Raveniola, holotype (37, 38) and conspecific (36, 39) males: bulbus, ventral (38) retroventral (36, 37) and retrolateral (39) view. 36 Sinopesa maculata 37, 38 Raveniola guangxi 39 Raveniola hebeinica (scale bar = 0.5 mm). Note: Figs 37 and 38 show right and left palpi of the same specimen, respectively.
Raveniola, holotype (40, 43) and paratype (41, 42) males: palpal bulbus, retrolateral view. 40 Raveniola montana sp. n. 41 Raveniola shangrila sp. n. 42 Raveniola songisp. n. 43 Raveniola yunnanensis sp. n. (scale bar = 0.5 mm).
http://species-id.net/wiki/Raveniola_xizangensis
Figs 49, 50♀ holotype and ♂ paratype from Jancha County, Tibet; dep. BDSU, not examined.
Raveniola xizangensis differs from all other Chinese congeners as well as from north-west-Himalayan Raveniola concolor Zonstein, 2000 by the developed subapical embolic keel in males and the bifurcate basal receptacle in females (cf. Figs 37–43, 47-50, and
This largest Chinese nemesiid with carapace 11-12 mm long was well described by
Known only from the type locality – Jancha County, Tibet (see Fig. 1, Raveniola loc. 1).
Sinopesa chinensis (Kulczyński, 1901) comb.n., conspecific male (sensu
Raveniola, bulbus (49, holotype male, ventral view) and spermathecae (47, 48, 50, conspecific/paratype females, ventral view): 47 Raveniola hebeinica 48 montana sp. n. 49, 50 Raveniola xizangensis (from
Three nemesiid genera have been reported from China to date. The first is Nemesia Audouin, 1826, represented here by the enigmatic Nemesia sinensis, known only from the holotype female (
While establishing Sinopesa, Raven and Schwendinger separated it from the related genus Entypesa on the basis of two characters: the absence of PMS and of serrula. The apical segment of PLS in Sinopesa spp. was found to be digitiform. Later,
It should be noted that in the mentioned African genera the spinneret morphology retains the more plesiomorphic condition: PMS are relatively large and fully functional, and the apical segment of PLS in all these genera except Pionothele is long and slender (digitiform). The spinneret morphology in Sinopesa is noted above. In Raveniola species PMS are small to tiny; within the species described to date they are absent in Raveniola fedotovi (Charitonov, 1946) and Raveniola kopetdaghensis (Fet, 1984); the apical segment of PLS varies, with few exceptions (see Zonstein 2009), from short-digitiform to triangular.
Although Sinopesa and Raveniola have never hitherto been compared to one another, they share a number of apomorphies that might bring them closer to each other than to the mentioned genera. Some of these characters, such as the absence of the maxillary serrula and the metatarsal preening combs, are also shared with Lepthercus and Pionothele; whereas other features appear to be unique. Males of both Asian nemesiid genera share the presence of 2–3 (vs. one in the mentioned African nemesiids, as shown in Fig. 22) retroventral megaspines on tibia I. The congeneric females possess the divided spermathecae (that are entire in Entypesa and Hermacha - see
Differences between Chinese members of Sinopesa and Raveniola are summarised below:
In the course of this study the characters of three Chinese nemesiids previously placed in Raveniola were found to correspond to the diagnostic features of Sinopesa. Hence, they are transferred here to the latter genus: Sinopesa chinensis (Kulczyński, 1901) comb. n., Sinopesa sinensis (Zhu & Mao, 1983) comb. n. and Sinopesa chengbuensis (Xu & Yun, 2002) comb. n. Moreover, using the same definitive criteria, one of the existing members of Sinopesa should be transferred to Raveniola: Raveniola guangxi (Raven & Schwendinger, 1995) comb. n. The current generic position of Raveniola xizangensis (Hu & Li, 1987) and Raveniola hebeinica Zhu et al., 1999, whose features do not contradict the generic characters, is presently confirmed.
Being compared by shape of the male embolus and female spermathecae with other Raveniola species, the Chinese representatives showed a closer similarity to the Central Asian congeners and especially to North-West Himalayan Raveniola concolor (cf.
Seven true Chinese species of Raveniola were revealed in course of this study, which engages here, however, only with the material and information previously available. Currently, we cannot estimate the true diversity of Raveniola species within the country, but expect it to be much higher. This expectation is based, first and foremost, on the fact that many parts of the region were not specially investigated; while a rather small amount of material from two geographically close localities has already revealed four new species. An additional possible factor relates to the limited and often sympatric character of their distribution, specific to this group of spiders. Numerous mountain ridges in central and southern parts of China (which provide both the mosaic character and richness of habitats) and lack of special collection efforts suitable for these mygalomorphs, reinforces the prediction regarding their probably higher species diversity.
The prospective areas in which new findings might be expected are the provinces lying between the two main groups of the known localities (see Fig. 1), especially Sichuan and southern part of Gansu. It should be noted that the members of this genus may also occur in the furthest north-western part of China. According to our data (Zonstein, in prep.), some Raveniola species were observed in Kyrgyzstan, inhabiting Alai and Trans-Alai Mt. Ridges, both adjoining Xingjian.
We are grateful to the various curators and collection managers who provided us with the necessary type and comparative material, and to Naomi Paz for her linguistic help. Laura Leibensperger generously lent for study the holotype of Sinopesa guangxi kept in MCZ, for study. Jürgen Gruber helped to examine the assumed male and female of Brachythele chinensis sensu Kritscher, 1957, preserved in the NMW. Peter Schwendinger kindly lent the conspecific specimens of Sinopesa maculata, collected from the type locality and deposited in the MHNG. Types of Entypesa nebulosa and Sinopesa kumensis, preserved in MNHN, were examined thanks to the generous help of Christine Rollard and Elise-Ann LeGuin. Janet Beccaloni kindly helped us to study several representatives of Hermacha and Entypesa kept in NHM. Specimens of Hermacha bicolor, Entypesa schoutedeni and Lepthercus sp. were borrowed from ARC courtesy of Ansie Dippenaar-Schoeman and Petro Marais. Shuqiang Li and Feng Zhang kindly provided us with male and females of Raveniola hebeinica. Rudy Jocqué and Domir De Bakker helped with the lending of specimens of Entypesa and Pionothele from the spider collection of MRAC. Petra Sierwald and James Boone generously lent for study several dozen of Entypesa specimens kept at the spider collection of FMNH. We are also thankful to the anonymous reviewers for their helpful comments. This study received financial support from the Ministry of Absorption, Israel and from the Russian Foundation for Fundamental Research (grants # 11–04–01716 and 12–04–01548).