(C) 2011 Jeffrey V. Freeman. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A canopy trap and aerial nets led to finding 8 species of Tabanidae. There was an abundance of calyptrate muscoid flies. Camel’s Hump is in the Green Mountains of western New England, USA. Discovering Diptera on Camel’s Hump involved sixteen visits over 40 years. Upwards of 23 other Diptera species are listed. Habitats on the east side and above 762 m (2500 ft) elevation on Camel’s Hump differ from the west slope but the boreal forest on both sides is influenced by cloud and fog precipitation on trees. The cliffs just above the 900 m level along the east side are often overlooked, are not seen from the summit and provide access to morning sun for insects. Recent visits explored the role of polarized skylight in relation to the canopy trap, the boreal forest environment and flies found there.
Tabanidae, Muscidae, Tachinidae, Camel’s Hump, polarized light, Vermont
Ross T. Bell introduced a group of students to the ecology of invertebrates on Camel’s Hump (Chittenden County, 44°19'N, 72°53'W) in 1972 and I was one of them. The purpose here is to bring together results of visits in 1972 (Freeman, 1973), 1998, 1999 and 2010 with use of nets and traps. I found eight species of tabanids but few individuals. Upwards of 15 species of calyptrate muscoid flies have been found in the large batches caught in a canopy trap. Officially Camel’s Hump (1244 m) from its summit down to 762 m is a natural area accessible only by four trails: Burrows Trail from the west, the Monroe Trail from the east and the Long Trail from north and south. It takes about two hours from a trail head to get on station and collecting for a day. The important question posed in our 1972 course was, what species of our chosen group are present on Camels Hump? Then, how do these invertebrates interact with their physical and biological environment on Camel’s Hump? More recently, how does the geomorphology of Camel’s Hump relate to these invertebrates? Also, what is the role of polarized skylight in the lives of the flies found here? Some aspects of human ecology enter this study as well.
After initial successful use of the canopy trap in 1970 and 1971 in studying Tabanidae it was a natural response to apply this method to Camel’s Hump in 1972.
Collecting with an aerial net was the usual method of collecting flies, and most often with sweeps but for whole morning or whole afternoon sessions the canopy trap was in operation. The Hut Clearing offered an open space in the boreal forest and was the junction of four trails. Net sweeps in the Hut Clearing especially but along trails as well and on the summit provided additional samples. Most labels show elevation. Another net made of nylon marquisette netting caught black flies and other very small Diptera. Wind can preclude use of the trap on the summit. With so many people there it is not advisable to leave the trap untended. The Hut Clearing is open to flies entering the clearing from four directions and above. It was possible to purchase dry ice in Burlington, wrap it in insulating material and have it on the trap in the Hut Clearing within three hours. Octenol (1-octen-3-ol, http://sigmaaldrich.com/united-states.html ), is a compound attractive to biting flies. In more recent years it was more convenient than dry ice.
The canopy trap was similar to that used by
The study area was in the area above 762 m elevation partly as studied by
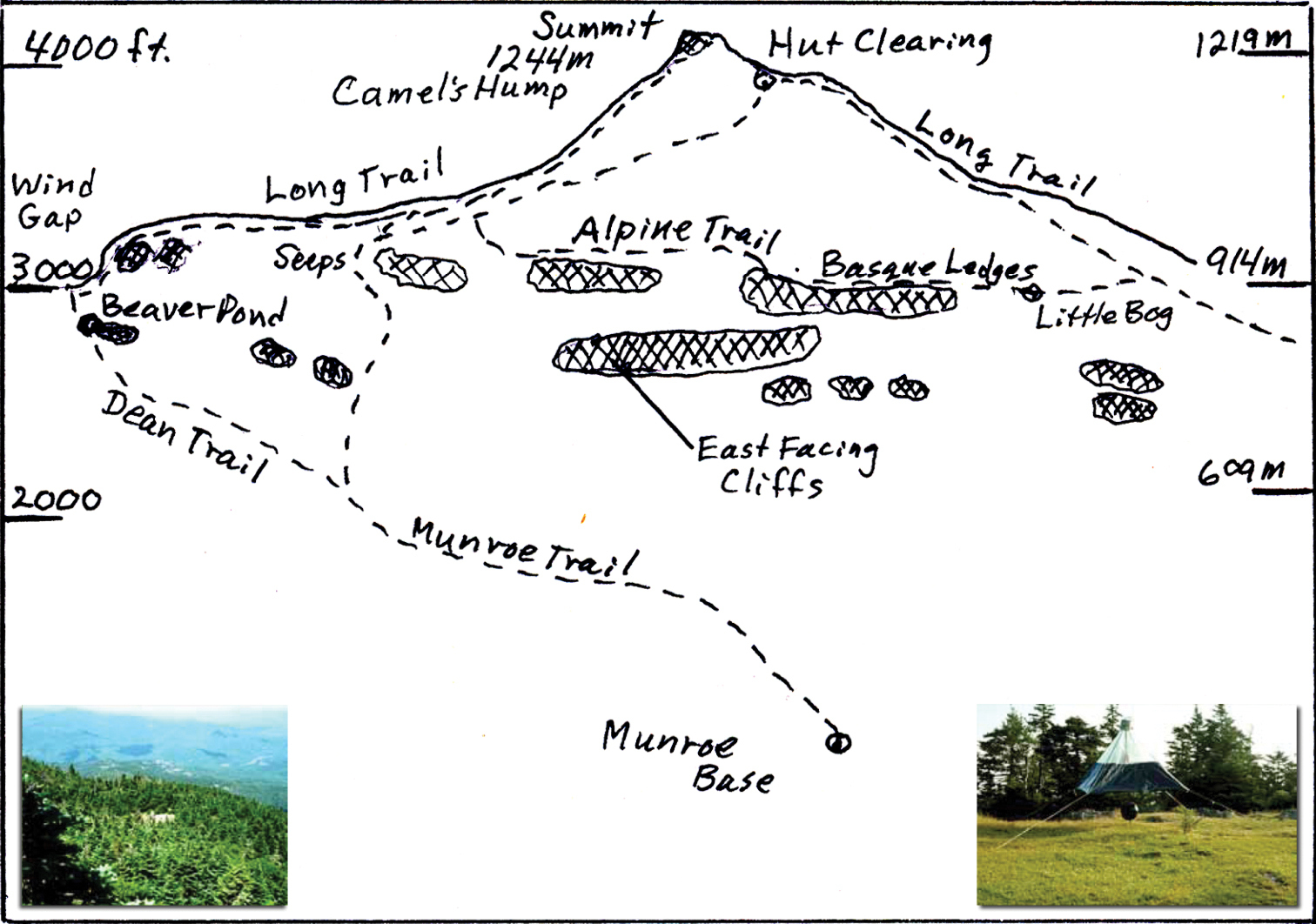
Figure 1. Camel’s Hump seen from the east. The Monroe Trail ascends to the cliffs and passes seeps, then crosses the Alpine Trail and ends at the Hut Clearing. The Long Trail follows the ridge line and goes up over the summit. The Dean Trail branches from the Monroe Trail and passes the Beaver Pond and reaches the Wind Gap nearby. The Little Bog is toward the northern end of the less-traveled Alpine Trail which provides access to some open rocky treeless areas (Basque Ledges) above the east cliffs and is a bad weather bypass around the summit. The Seeps may provide limited habitat for certain black flies (Simuliidae). Lower right inset shows the canopy trap in Hut Clearing as Ross Bell saw it in 1972. Lower left is a view of the Hut Clearing with canopy trap seen from near the summit with I-89 about 11 km away and no evidence of cliffs from here.
Figure 2. Canopy trap in Hut Clearing. Elevation 1158 m. Lower black part of trap changes bright reflection at right to a more “flat” black at left with a 90 degree turn of polarizing filter on camera lens. Photo here is from opening as Long Trail enters from the north. The Hut Clearing is a four-way junction of trails on Camel’s Hump and a convenient place for a trap, fully visible to flies.
The Tabanidae were readily identified in keys by
With many male and female Muscidae and Anthomyiidae in batches from the canopy trap the determining to species was a major task and must continue. The specialists working with Anthomyiidae are now deceased and revised keys to species by
The key to genera for Anthomyiidae in the Manual of Nearctic Diptera (MND) (Huckett, 1987 in McAlpine 1987) can be used but cautiously due to changes. I used the key in the MND for Muscidae supplemented by visits to collections and consulting Dr. Jade Savage.
The large quantities of muscids and anthomyiids actually pinned or pointed in 1972 were a sampling out of hundreds that were discarded. For Simuliidae the keys provided by
A Berlese-Tulgren funnel set up in the laboratory at University of Vermont allowed extracting tabanid larvae from habitat material from the Little Bog. Identification to species could be done with
An Olympus SZ stereozoom microscope worked well on specimens with an AO fiber optics high intensity illuminator. Markings on some adult Muscidae and Anthomyiidae can appear differently depending on direction viewed and kind of lighting. A 14× Hastings Triplet hand lens works with most Tabanidae and some previewing and checking of calyptrate muscoid flies.
Results Canopy trap and net sweepsTable 1 summarizes samples collected by canopy trap and net sweeps mostly from the Hut Clearing where hiking trails intersect on Camel’s Hump. In these collections both males and females were evident. Calliphorids were much more common on the summit or near feces. Tachinids were collected more with sweeps. Anthomyiids were present in most trap samples but muscids dominated. Identification of Muscidae to genus is difficult. Determining species is more difficult. While (
Diptera families in collections on Camel’s Hump, Vermont between 1972 and 2010. Emphasis on the Hut Clearing, separated by male and female. Elevation in meters, locations, elevation, method and batches (011, 9809, 1004), species, and discards (NP). CT = canopy trap; Sw = net sweeps; GM = Gressitt Malaise trap; Cmb = combined batches; St = Stonemyia tranquilla; Hm = Hybomitra microcephala; Ht = Hybomitra pechumani; Cm = Chrysops mitis. NP = Not pinned. Estimated number discarded only as mentioned in 1972 notes (
| Meter | |||||||||
| Date | Location | Elev | Trap/SW | TAB | MUSC | ANTH | TACH | CAL | NP |
| 7/11/1972 | BvrPnd | 853 | CT, 011 | 1Cm | 0 | 0 | 0 | 0 | 128 |
| 7/13/1998 | FrnGlade | 1036 | CT 9805 | 0 | 5♂13♀ | 3♂21♀ | 0 | 0 | 0 |
| 7/15/2010 | AlpnTr | 975 | GM, 1004 | 2St | 5♂5♀ | 0 | 6♂4♀ | 2 | 0 |
| 7/15/2010 | MnroTr | 853 | SW, 1002 | 0 | 0♂10♀ | 0 | 0 | 0 | 0 |
| 7/17/1972 | HutClg | 1158 | CT, 018 | 3Hm | 0 | 0 | 0 | 0 | many |
| 7/18/1972 | HutClg | 1158 | CT018, 030 | 4 Hp | 12♂16♀ | 3♂20♀ | 2♂8♀ | 0 | 226 |
| HutClg | O23, Cmb | ||||||||
| 7/21/1972 | HutClg | 1158 | CT, 9906 | 3Hm | 4♂16♀ | 5♀ | 0♂1♀ | 3♀ | 0 |
| 7/21/1999 | HutClg | 1158 | Sw, 9914 | 0 | 0♂ 2♀ | 2♂6♀ | 0 | 0 | 0 |
| 7/21/1999 | HutClg | 1158 | Sw, 9907 | 3Hm ♀ | 5♂15♀ | 0♂4♀ | 0 | 0 | 0 |
| 7/21/1999 | HutClg | 1158 | Sw, 9909 | 1Hm♀ | 4♂8♀ | 5♂13♀ | 0 | 1 | 0 |
| 7/22/1998 | FrnGlade | 1036 | CT, 9809 | 0 | 14♂ 16♀ | 4♂19♀ | 0 | 0 | 0 |
| 7/25/1972 | HutClg | 1158 | CT, 038 | 0 | 12♂ 30♀ | 12♂30♀ | 0 | 0 | 365 |
| 8/2/1972 | HutClg | 1158 | Sw, 052 | 0 | 14♂38♀ | 0 | 0 | 0 | 0 |
| 8/3/1998 | HutClg | 1158 | Sw, 9813 | 0 | 1♂8♀ | 0♂2♀ | 1♂0♀ | 4 | 0 |
| 8/3/1972 | BvrPnd | 853 | CT | 0 | 12♂8♀ | 9♂8♀ | 0 | 0 | 0 |
| 8/9/1972 | HutClg | 1158 | SW 052 | 0 | 11♂ 35♀ | 0 | 0 | 0 | 0 |
| Total | 101♂220♀ | 28♂121♀ | 9♂13♀ |
Larvae of Chrysops lateralis were collected from habitat material from the Little Bog. This species is collected here by net sweeps rather than by trap. (No Cl in Table 1) Hikers experience attacks from deer flies when leaving the Monroe Base. The two kinds of black flies, “buzzers” and “biters”, are part of life on the trails of Camel’s Hump. Biters leave their red marks on the skin of legs, arms and neck. The buzzers near the head are more easily collected with a net than biters and gain our attention.
Annotated list of species found on Camel’s Hump, Duxbury, VermontSIMULIIDAE, Black flies (
Prosimulium mixtum Syme & Davies, 1958, Species complex. Common near a seep and stream at 854 m, Monroe Trail; 011 Beaver Pond July 11, 1972; 048 Hut Clearing 1158 m, Aug 2, 1972; 033, Hut Clearing 1158 m, canopy trap, July 25, 1972
Simulium parnassum Malloch, 1914. 014, East side of summit, 1189 m, July 17, 1972
CULICIDAE, Mosquitoes. Small numbers on Camel’s Hump. http://www.vermontagriculture.com/ARMES/plantindustry/entomology/mosquito/MosquitoControl.html
Aedes vexans (Meigen, 1830) July 13, 1998
Ochlerotatus provocans (Walker, 1848) July 25, 1972
BIBIONIDAE
Bibio femoratus Wiedemann, 1820. Summit 1244 m, collected by HP Wimmer, 1972.
TABANIDAE, Deer flies and horse flies. (
Stonemyia tranquilla (Osten Sacken, 1875) Caught by trap. Male on summit by net. Trap at Hut Clearing and Monroe Base. Non-biting small brown tabanid. Found as “Pangonia” in
Chrysops carbonarius Walker, 1848. Unknown other person. July 2, 1972. June species.
Chrysops geminatus Wiedemann, 1828.By net at Monroe Base 462 m; Monroe Trail 853 m, Hut Clearing 1158 m as females attacking person. Male on summit.
Chrysops lateralis Wiedemann, 1828. Monroe Base, Alpine Trail 945 m, Hut Clearing. Larva at Little Bog.
Chrysops mitis Osten Sacken, 1875. Mainly a June species, black coloration, by net at Beaver Pond, 853 m on Dean Trail in July.
Chrysops sordidus Osten Sacken, 1875. Netted by classmate “on Camels Hump”, has label with “Bolton, VT” but no elevation given. Found also on Mt. Mansfield (
Hybomitra microcephala (Osten Sacken, 1876). By trap. Monroe Base and Hut Clearing. Male photographed waiting on summit rock. By net sweeps at Hut Clearing. Larvae in well rotted log on wooded hillside (
Hybomitra pechumani Teskey & Thomas, 1979. Determined in 1972 as Hybomitra typhus (Whitney, 1904) Hybomitra pechumani was described when Hybomitra typhus became 2 species. Collected by net sweeps and trap at Hut Clearing, 1158 m.
LONCHOPTERIDAE, Lonchoptera furcata (Fallén, 1823) 038 July 25, 1972. Hut Clearing. 1158 m.
PHORIDAE, Humpbacked flies. Smaller than a black fly.
Megaselia pulicaria (Fallén, 1823) 035. July 25, 1972. Little Bog 945 m.
SYRPHIDAE, Hover flies.
Sericomyia militaris Walker, 1849
SEPSIDAE, Black scavenger flies
Sepsis punctum (Fabricius, 1794) Known for wing waving. Found near larval habitat such as feces or decaying plant/animal material.
ANTHOMYIIDAE, Flower flies, Root Maggot Flies
Leucophora (Proboscimyia) brevis (Huckett, 1940)
Delia platura (Meigen, 1826) Seed corn maggot. Genus includes economically important anthomyiids.
Hylemya alcathoe (Walker, 1849)
MUSCIDAE, “House flies”
Hydrotaea ponti Vockeroth, 1995 Sweat fly, shiny black
Hydrotaea militaris (Meigen, 1826) Sweat fly, shiny black.
Thricops albibasalis (Zetterstedt, 1849)
Thricops spiniger (Stein, 1904) Very common near or above tree line (
Musca autumnalis de Geer, 1776. Very occasional but distinctively marked.
Helina sp. 1 Robineau-Desvoidy, 1830
Helina sp. 2 Robineau-Desvoidy, 1830
OESTRIDAE, Bot flies
Cephenemya phobifer (Clark, 1815). Deer nasal or pharyngeal bot fly. Hovering over summit rocks, July 7 to October 21, 1245 m.
Gasterophilus intestinalis (DeGeer, 1776) Collected on summit while hovering.
CALLIPHORIDAE, Blow flies or bottle flies
Calliphora vomitoria (Linnaeus, 1758)
Lucillia illustris Meigen, 1826. Feces, Monroe Trail, July 22, 1998
TACHINIDAE, Parasitic Flies (Larvaevoridae) www.nadsdiptera.org/Tach/home.htm Collected in the Hut Clearing, 1210 m.
Lixophaga unicolor (Smith, 1917), Most numerous. No host data. (NHD)
Panzeria platycarina (Tothill, 1921) NHD
Billaea trivittata (Curran, 1929) Hosts include Cerambycidae, long-horned beetles. (
Eulasiona comstocki Townsend, 1892 Hosts include certain leaf tier (Oecophorid) moths.
Periscepsia (Ramonda) clesides (Walker, 1849) NHD
Gymnosoma par Walker, 1849 NHD
Oswaldia sp. Undescribed species. Parasitoids of Lepidoptera (
Several aspects of human ecology on Camels Hump are that people prefer the summit, they stay on trails, they are available to biting flies but sometimes make use of off trail areas by leaving feces. When people defecate in the woods they commonly do not bury their feces and they leave toilet paper. Later feces can be attractive to blow flies (Calliphoridae). Deer flies (Tabanidae) and black flies (Simuliidae) find potential hosts along trails. Since there is no overnight camping allowed currently on Camel’s Hump above 609 m, hiker activity appears on the summit around 9 AM or about two hours after people start from trail heads. Warmed and sweaty hikers can be attractive to certain sweat-loving “person flies” or “sweat flies” (Hydrotaea militaris and Hydrotaea ponti, Muscidae).
Cliffs are sunning places for Diptera (Lindner, 1973) and rocky summits provide rendezvous sites for males and females of certain flies.
Primary production mainly is the balsam fir (Abies balsamea), red spruce (Picea rubens), mountain paper birch (Betula cordifolia), moss mats and spinulose wood ferns (Dryopteris spinulosa) of the boreal forest. Sugar maple (Acer saccharum), yellow birch (Betula alleghaniensis) and American beech (Fagus grandifolia) occur in the lower northern hardwood forest. One large fern patch (0.1 hectare) just above the junction of the Alpine Trail with the Monroe Trail yielded anthomyiid flies in the trap. Certain species can affect ferns (
There is an observed preference by people to look up at the summit, to keep on trying to get there, and to enjoy the view. Biologists, however, think about where they might not yet have collected or explored for flies on Camel’s Hump and to go there. This might be a fair weather bias and a time of day bias. Collecting in fog or rain, however, is not productive.
DiscussionSpecies presented here came from the east side of Camel’s Hump and the summit. The work of
(
Working with flies in Anthomyiidae involved several approaches. Visiting two nearby collections (New York State Museum, Albany; University of Massachusetts at Amherst) allowed seeing specimens of species found on Mt. Katahdin and the Presidential Range. The key to genus in the MND (Huckett, 1987) for Anthomyiidae is said to be not reliable. Delia platura, the seed corn maggot, is abundantly present on both Mt. Katahdin ( 44♂, 18♀) and the Presidential Range (266♂, 282♀) as adults (
Locating and identifying larvae of Chrysops lateralis in the Little Bog suggests that future habitat sampling of some sort might help to learn where and how the abundance of flies in Muscidae and Anthomyiidae comes from.
The keys by
The canopy trap provided many calyptrate muscoid flies for sorting and pinning a representative sample. Most lowland trap collections had mostly tabanids and few muscoid flies. This canopy trap lasted 30 years until disposal in 2001. The bias established by the phrase “summer is July” resulted mostly from scheduling of Ross Bell’s course but takes advantage of abundance of many insects at the height of summer at higher elevations. Conditions on the mountain change in August. Collecting in June will help to confirm seasonal succession already known for tabanids. Future lists of Muscidae, Anthomyiidae and Tachinidae may lead to establishing their seasonal succession. Both Chrysops carbonarius and the black species Chrysops mitis are common spring deer fly species but can hold over into July. Significant adaptive advantages of calyptrate muscoid flies with fewer larval instars (four) than tabanids (seven) are small size, overwintering as larvae, the power of flight, ability to lay hundreds of eggs or larvae (Tachinidae) in very specific locations or on hosts may help to account for the large numbers on Camel’s Hump in the short summer.
The nose bots or deer nasal bot flies collected over the years mostly have the hair on the thorax worn down. The very first couplet in the key to species uses the nearly solid yellow hair of the thorax with a black patch over each wing base to separate Chrysops phobifer from the other Nearctic species (
Visits to the summit of Camel’s Hump often involve interacting with people and this can detract from field time. The summit presents other problems, some related to wind, that make use of the canopy trap less attractive than, for example, the Hut Clearing.
Setting up and leaving the labeled canopy trap in the Hut Clearing was far more practical and was a more sheltered place. Careful observation of Hybomitra microcephala waiting on summit rocks or hovering of the bot flies, however, was more productive than use of a trap. The observer must be there to see and photograph them.
Hydrotaea militaris and the less common Hydrotaea ponti are of personal interest because they are obvious bothersome 5 mm shiny black muscid sweat flies that do not bite.
Hovering and waiting are behaviors common to male tabanids. But Hydrotaea microcephala and Chrysops geminatus show waiting behavior on this summit as they do at Mt. Rigaud west of Montreal (
Changes in the plant communities on Camel’s Hump have been found by
An older map of Camel’s Hump (
Polarized skylight refers to the band of maximum polarization of light overhead at 90 degrees to the sun. Reflections from water, honeydew, or the canopy trap may also affect flies. Polarized light is part of the sensory ecology of invertebrates. With land sloping away on this mountain there is a maximum view of the eastern sky. How it influences flies here remains to be determined.
In mid morning the band of polarized light would sink behind the summit in the west and northwest for sites on the east slope of Camel’s Hump above 762 m. Observing fly behavior with polarized light in mind is one more aspect of their ecology.
More specifically
Change of appearance of the canopy trap in the Hut Clearing can be seen in Fig. 2. How flies might process this visual difference remains to be explored experimentally.
Whatever light reaches the insect compound eye might otherwise be reflected except for structural features preventing reflection.
The designation of “taxonomic impediment” refers to the lack of taxonomic expertise in research on biodiversity and ecology. Declining numbers of taxonomists and increasing demand for identifications have had their effect on this study. Workers with tabanids have had access to help from several taxonomists. This directly affects our ability to list species as found in new places and results in unevenness in representation on checklists.
The difficulty in determining species in Muscidae and Anthomyiidae from adult specimens leaves this study with many undetermined specimens. That
The much closer look at larvae and puparia of Muscidae by
First, what Ross Bell started must continue as we build on the basis begun here. Second, the huge quantities of Muscidae and Anthomyiidae emphasize that the nature of larval habitats is yet to be determined. Third, further more targeted collecting is needed, especially of spring species of Tabanidae, in June on Camel’s Hump. Fourth, the relation to flies of east-facing cliffs of the summit and cliffs at elevation 850 m below the Alpine Trail needs further study. Fifth, as it becomes available after completion of building renovations, the collection of Muscidae at Bishop’s University deserves a visit and further study. Sixth, revised keys to Anthomyiidae should continue to be improved to continue the work of GCG Griffiths.
Huckett’s (1965) keys to species of northern Muscidae were done when Anthomyiidae was included as a subfamily of Muscidae. Access to “known’s” in various collections did, however, include species determined by HC Huckett. Revised keys to genus for Muscidae and Anthomyiidae are a major need.
I thank Jade Savage, Bishop’s University, Sherbrooke, QC, for determinations of Muscidae. James O’Hara and Monty Wood, Canadian National Collection, Ottawa, determined the Tachinidae. Visits to collections at the New York State Museum, Albany, hosted by Tim McCabe and University of Massachusetts hosted by Benjamin Normark are appreciated. These visits provided access to some Anthomyiidae and Muscidae. Alan Graham of the Vermont Agency of Agriculture determined the species of Mosquitoes. Douglas Currie of the Royal Ontario Museum determined the Simuliidae. I thank Bryan Gee for help with graphics. I thank Jack Blount, Gary Salmon and Kristin Freeman who helped with initial reviews. Fran Ryan and Sandy Duling at the Castleton State College Library helped with access to journals and interlibrary loans.
A canopy trap is composed of 182 cm equilateral triangular faces with upper half as clear “6 mil” (0.15 mm) plastic and the lower half of “6 mil” black plastic. A pipe clamp holds all four sides onto the base of an outer 15 cm diameter galvanized sheet metal sleeve. Another slightly smaller cylindrical sleeve must slide into the lower sleeve and have above it a tilted cone of aluminum mosquito screen that leads into a 15 cm powder funnel firmly supported by a bracket with side braces. The screen funnel seam can be sewn with pulled out aluminum wire and sealed to funnel with silicone adhesive. The bracket has the lid of the collecting jar bolted below. Other attachments involve drilling holes and peening over rivets made from aluminum tacks or using pop rivets.
A Mason jar can then be fitted with the killing agent. Radiating hem lines are stitched with durable fabric and a brass grommet is placed at each corner. The lower black portion can be lined with fabric to maintain strength under the tension of tie-downs. Midway down in the top sleeve four sets of drilled holes allow installing wires attached to a central circle of metal (“spider”) to allow the spike in top of support pole to hold trap at desired height. Plastic tent pegs allow staking the trap out so as to keep the canopy approximately level. A plastic beach ball spray painted with glossy black enamel and inflated each time can hang below the trap. When flies pass under the trap they tend to buzz upward to find a way out. Eventually they reach the leaning screen cone and the powder funnel where light comes through and the fumes from either calcium cyanide or DDVP (Dichlorvos 18.6%, No-Pest Strip) causes them to die. Sometimes clear plastic wrap will work as a cover. Either a bag of dry ice or a small vial with a pipe cleaner wick charged with Octenol will enhance the catch. A canopy trap apparently provides a producer of heat from black plastic in sunshine, a sharp and non-plant outline, and reflection of polarized light like hair on a large mammal. With carbon dioxide it imitates exhaled breath of a large mammal. This trap generally catches more horse flies than deer flies and has served as a good survey tool. This trap allows the collector to go elsewhere catching deer flies or other flies with an aerial net.