(C) 2012 Mauro Gobbi. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
In this paper we report about 88 longhorned beetles (Cerambycidae) species found in 6929 hectares and distributed along an altitudinal gradient of 1500 m of an Italian alpine valley (Val Genova, central-eastern Italian Alps). The species richness, result merging data from sixty years (1947–2007) of entomological surveys, corresponds to the 32% of the Italian cerambycid fauna confirming the high richness/surface ratio, probably unique in the Alps. The effect of thirteen environmental variables was tested on the species richness, but only the elevation resulted able to affect it. The species richness decrease with altitude not gradually, but experience a strong step above 1700 m a.s.l.. The highest species richness (average values of 42 species) was recorded at the lowest and mid elevations (between 800 and 1600 m a.s.l.). The species turnover along the altitudinal gradient is low suggesting moderate habitat turnover along the valley.
One of the eighty-eight observed species, Tragosoma depsarium, is classified near threatened by the IUCN. Our data suggest that the wilderness of the valley close to the suitable management of grasslands and forests, help to support high level of cerambycids diversity. This biodiversity is good indicators of health of the wood saproxylic assemblages, as well an important food source for many vertebrate predators.
Cerambycids, saproxylic, species richness, protected areas, Val Genova, Alps
The longhorned beetles (Coleoptera: Cerambycidae) can be considered one of the richest families of animals with about 35, 000 described species (Hurka 2006). The European species richness amounts to a total of 677 species (
From the ecological point of view, longhorned beetles might potentially be excellent indicator species of the health of the wood saproxylic assemblages (
In the central-eastern Italian Alps there is a valley belonging to the Adamello-Brenta Natural Park (Trentino – Alto Adige Province), named Val Genova which is characterized by the total absence of urbanized areas, and that is characterized by a mosaic of different natural and human-managed habitats which have not suffered modifications for at least one century. The human activities are limited to the hay meadows, pastures and to the cut of some forested areas. This valley had attracted the attention of many entomologists since the middle of the last century. In the last sixty years many surveys was done by different entomologists to catch cerambycids, therefore we merged presence/absence historical data with those collected by us. The obtained database was used to test the following hypothesis: i) the species richness decreases gradually along the altitudinal gradient, ii) the presence of a mosaic of natural and human-managed habitats supports high values of species richness.
Methods Study areaThe study area is an alpine valley named Val Genova (46°09'N, 10°40'E). It is located in the central-eastern Italian Alps, in the Trentino - Alto Adige Region, and belongs to the Adamello-Brenta Natural Park. The valley is about 20 Km long, and the area considered for the cerambycids catchment it is distributed along an altitudinal gradient of about 1500 metres (800–2200 m a.s.l.), and inside an area of 6929 hectares. The average precipitation is over 1000 mm, and the most rainy periods are during May and October with precipitation over 100 mm. Different climatic factors determine the vegetation gradient along the valley. At the lowest elevation (800-1000 m a.s.l.) there is dominance of broadleaf woods with Fagus sylvatica, Alnus incana, Acer pseudoplatanus, Corylus avellana, Fraxinus excelsior, Carpinus betulus, Laburnum anagyroides, Betula pendula, Salix alba, Robinia pseudoacacia. At the mid-elevations (1100-1800 m a.s.l.) the broadleaf forests (mainly Betula) are mixed with conifers characterized by the presence of Abies excelsa, Abies alba and Pinus sylvestris. The timberline is around 1900 m a.s.l., while the treeline (with Larix decidua) is around 2100 m a.s.l.. Above 2100 m shrubs of Alnus viridis, Rhododendron ferrugineum and rare Pinus mugo appear. Along this altitudinal and vegetation gradient there are many grasslands (e.g. pastures, meadows) human-managed for at least one century. In particular the hay meadows are located at the low-mid elevation (< 1500 m a.s.l.), whereas the pastures are from the mid to the highest elevation (1300–2200 m a.s.l.). Studies performed in the Adamello-Brenta Natural Park evidenced just an increase of 10% of the forest coverage since the Second World War up today due to the increase of neglected areas, in particular at the highest elevations (
The database has been created by merging data collected during the field surveys performed by
Our survey of longhorned beetles was carried out from May to September 2007, two-three times a week along the bottom of the valley and at the same elevation chosen by Moscardini in 1947 (
The surveys performed during these sixty years are not comparable with the aim to describe the species richness trend along the time because the sites visited by each entomologist have been not always the same, and the sampling effort was different.
Longhorned beetles were identified using
The Incidence-based Coverage Estimator of species richness (ICE) has been used to estimate the gamma-biodiversity (
Analyses have been computed using SPSS 13.0 (SPSS, Inc., Chicago IL), PAST 2.0 (http://folk.uio.no/ohammer/past ) and Estimate S (http://viceroy.eeb.uconn.edu/EstimateS ).
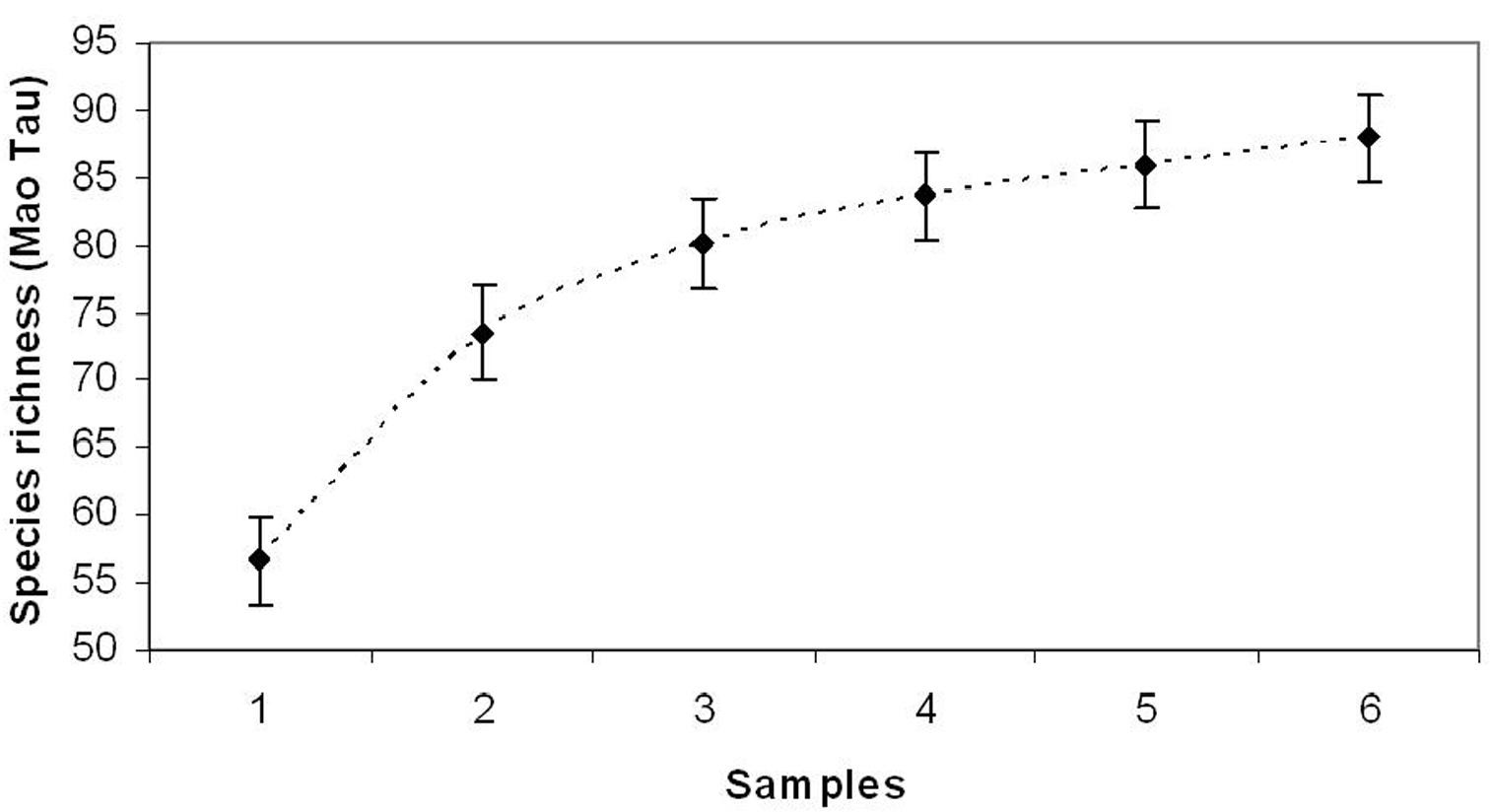
ResultsThe database realized merging data collected in sixty years (1947-2007) by different entomologists produced a checklist of 88 species (Table 1) observed along the altitudinal gradient comprise between 800 and 2200 m a.s.l.. A new species, never recorded before, has been observed during the last sampling season performed in 2007; the species is Phytoecia cilindrica (Linnaeus, 1758). The ICE index estimated for the valley a total of 93 species indicating that about the 91% of the species has been sampled; the species accumulation curve is not tending to the asymptote (Fig. 1), but it is gradually increasing confirming that more species could be cached, yet.
Cerambycids observed along the altitudinal gradient of the Val Genova (* = presence) The species are ordered on the base of their frequency at each elevational sampling point. (IUCN abbreviations: LC = least concern; NT = near threatened; EU endem = European endemism).
| Species / Elevation (m) | 800 | 900 | 1060 | 1100 | 1250 | 1430 | 1500 | 1640 | 1790 | 2000 | 2200 | IUCN Red List |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pachytodes cerambyciformis (Schrank, 1781) | * | * | * | * | * | * | * | * | * | * | ||
| Tetropium castaneum (Linné, 1758) | * | * | * | * | * | * | * | * | * | * | ||
| Monochamus sutor (Linné, 1758) | * | * | * | * | * | * | * | * | * | LC | ||
| Alosterna tabacicolor (De Geer, 1775) | * | * | * | * | * | * | * | * | ||||
| Anastrangalia dubia (Scopoli, 1763) | * | * | * | * | * | * | * | * | ||||
| Anastrangalia sanguinolenta (Linné, 1758) | * | * | * | * | * | * | * | * | ||||
| Evodinus clathratus (Fabricius, 1792) | * | * | * | * | * | * | * | * | ||||
| Leptura quadrifasciata (Linné, 1758) | * | * | * | * | * | * | * | * | ||||
| Monochamus sartor (Fabricius, 1787) | * | * | * | * | * | * | * | * | * | LC - European endemism | ||
| Rhagium inquisitor (Linné, 1775) | * | * | * | * | * | * | * | * | ||||
| Ruptela maculata (Poda, 1761) | * | * | * | * | * | * | * | * | ||||
| Saperda scalaris (Linné, 1758) | * | * | * | * | * | * | * | * | LC | |||
| Stenostola dubia (Laichrting, 1784) | * | * | * | * | * | * | * | * | * | |||
| Stenurella melanura (Linné, 1758) | * | * | * | * | * | * | * | * | ||||
| Acmaeops pratensis (Laicharting, 1784) | * | * | * | * | * | * | * | |||||
| Aromia moschata moschata (Linné, 1758) | * | * | * | * | * | * | * | LC | ||||
| Callidium violaceum (Linnaeus, 1758) | * | * | * | * | * | * | * | LC | ||||
| Gaurotes virginea (Linné, 1758) | * | * | * | * | * | * | * | |||||
| Hylotrupes bajulus (Linné, 1758) | * | * | * | * | * | * | * | LC | ||||
| Lepturobosca virens (Linné, 1758) | * | * | * | * | * | * | * | * | ||||
| Oxymirus cursor (Linné, 1758) | * | * | * | * | * | * | * | * | ||||
| Agapanthia villosoviridescens (De Geer, 1775) | * | * | * | * | * | * | ||||||
| Judolia sexmaculata (Linné, 1758) | * | * | * | * | * | * | * | |||||
| Molorchus minor (Linné, 1758) | * | * | * | * | * | * | LC | |||||
| Paracorymbia hybrida (Rey, 1885) | * | * | * | * | * | * | ||||||
| Paracorymbia maculicornis (De Geer, 1775) | * | * | * | * | * | * | ||||||
| Pidonia lurida (Fabricius, 1776) | * | * | * | * | * | * | ||||||
| Rhagium bifasciatum (Fabricius, 1775) | * | * | * | * | * | * | ||||||
| Stenocorus meridianus (Linné, 1758) | * | * | * | * | * | * | ||||||
| Tetropium fuscum (Fabricius, 1787) | * | * | * | * | * | * | * | |||||
| Achanthoderes clavipes (Schrack, 1781) | * | * | * | * | * | |||||||
| Anastrangalia reyi (Heyden, 1889) | * | * | * | * | * | |||||||
| Clytus arietis (Linné, 1758) | * | * | * | * | * | LC | ||||||
| Pachyta quadrimaculata (Linné, 1758) | * | * | * | * | * | |||||||
| Stenurella bifasciata (Muller, 1776) | * | * | * | * | * | |||||||
| Tetropium gabrieli (Weise, 1905) | * | * | * | * | * | |||||||
| Cortodera femorata (Fabricius, 1787) | * | * | * | * | ||||||||
| Pseudalosterna livida (Fabricius, 1776) | * | * | * | * | ||||||||
| Rhagium mordax (De Geer, 1775) | * | * | * | * | ||||||||
| Stictoleptura rubra (Linné, 1758) | * | * | * | * | ||||||||
| Anaglyptus mysticus (Muller, 1766) | * | * | * | LC | ||||||||
| Asemum striatum (Linné, 1758) | * | * | * | |||||||||
| Dinoptera collaris (Linné, 1758) | * | * | * | |||||||||
| Lamia textor (Linné, 1758) | * | * | * | |||||||||
| Obrium brunneum (Fabricius, 1792) | * | * | * | LC | ||||||||
| Pachyta lamed (Linnè, 1758) | * | * | * | |||||||||
| Pogonocherus hispidulus (Piller & Mitterpacher, 1783) | * | * | * | |||||||||
| Prionus coriarius (Linné, 1758) | * | * | * | LC | ||||||||
| Saphanus piceus (Licharting, 1784) | * | * | * | |||||||||
| Spondylis buprestoides (Linné, 1758) | * | * | * | |||||||||
| Strangalia attenuata (Linné, 1758) | * | * | * | |||||||||
| Tragosoma depsarium (Linné, 1767) | * | * | * | NT | ||||||||
| Cerambyx scopolii Fuesslins, 1775 | * | * | LC | |||||||||
| Cholorophorus figuratus (Scopoli, 1763) | * | * | LC | |||||||||
| Clytus lama (Mulsant, 1847) | * | * | LC - European endemism | |||||||||
| Exocentrus punctipennis Mulsant & Guillebeau, 1856 | * | * | ||||||||||
| Leiopus nebulosus (Linné, 1758) | * | * | ||||||||||
| Mesosa nebulosa (Fabricius, 1781) | * | * | ||||||||||
| Oberea pupillata (Gyllenhal, 1817) | * | * | ||||||||||
| Paracorymbia fulva (De Geer, 1775) | * | * | ||||||||||
| Parmena unifasciata (Rossi, 1790) | * | * | ||||||||||
| Phytoecia cylindrica (Linnaeus, 1758) | * | * | ||||||||||
| Phytoecia nigricornis (Fabricius, 1781) | * | * | ||||||||||
| Pogonocherus fasciculatus (De Geer, 1775) | * | * | ||||||||||
| Saperda carcharias (Linné, 1758) | * | * | ||||||||||
| Stenopterus rufus (Linné, 1767) | * | * | LC | |||||||||
| Stenostola ferrea (Schrank, 1776) | * | * | ||||||||||
| Acmaeops septentrionis (Thompson, 1666) | * | |||||||||||
| Aegomorphus clavipes (Schrank, 1781) | * | |||||||||||
| Anoplodera rufipes (Schaller, 1783) | * | |||||||||||
| Anoplodera sexguttata (Fabricius, 1775) | * | |||||||||||
| Arhopalus ferus (Mulsant, 1839) | * | |||||||||||
| Arhopalus rusticus (Linné, 1758) | * | |||||||||||
| Brachyta interrogationis (Linné, 1758) | * | * | ||||||||||
| Callidium aeneum (De Geer, 1775) | * | LC | ||||||||||
| Cholorophorus sartor (Muller, 1766) | * | LC | ||||||||||
| Corymbia scutellata scutellata (Fabricius, 1781) | * | |||||||||||
| Exocentrus lusitanus (Linné, 1767) | * | |||||||||||
| Glaphyra umbellatarum (Schreber, 1759) | * | LC | ||||||||||
| Grammoptera ruficornis (Fabricius, 1781) | * | |||||||||||
| Mesosa curculionoides (Linné, 1758) | * | |||||||||||
| Oberea oculata (Linné, 1758) | * | |||||||||||
| Oplosia cinerea Mulsant, 1839 (=fennica Paykull, 1800) | * | |||||||||||
| Saperda octopunctata (Scopoli, 1772) | * | LC | ||||||||||
| Saperda populnea (Linnè; 1758) | * | |||||||||||
| Stenopterus ater (Linné, 1767) | * | LC | ||||||||||
| Stenurella nigra (Linnè, 1758) | * | |||||||||||
| alpha diversity | 43 | 41 | 46 | 38 | 48 | 49 | 32 | 37 | 12 | 11 | 2 |
Accumulation curve on the number of species observed during the surveys.
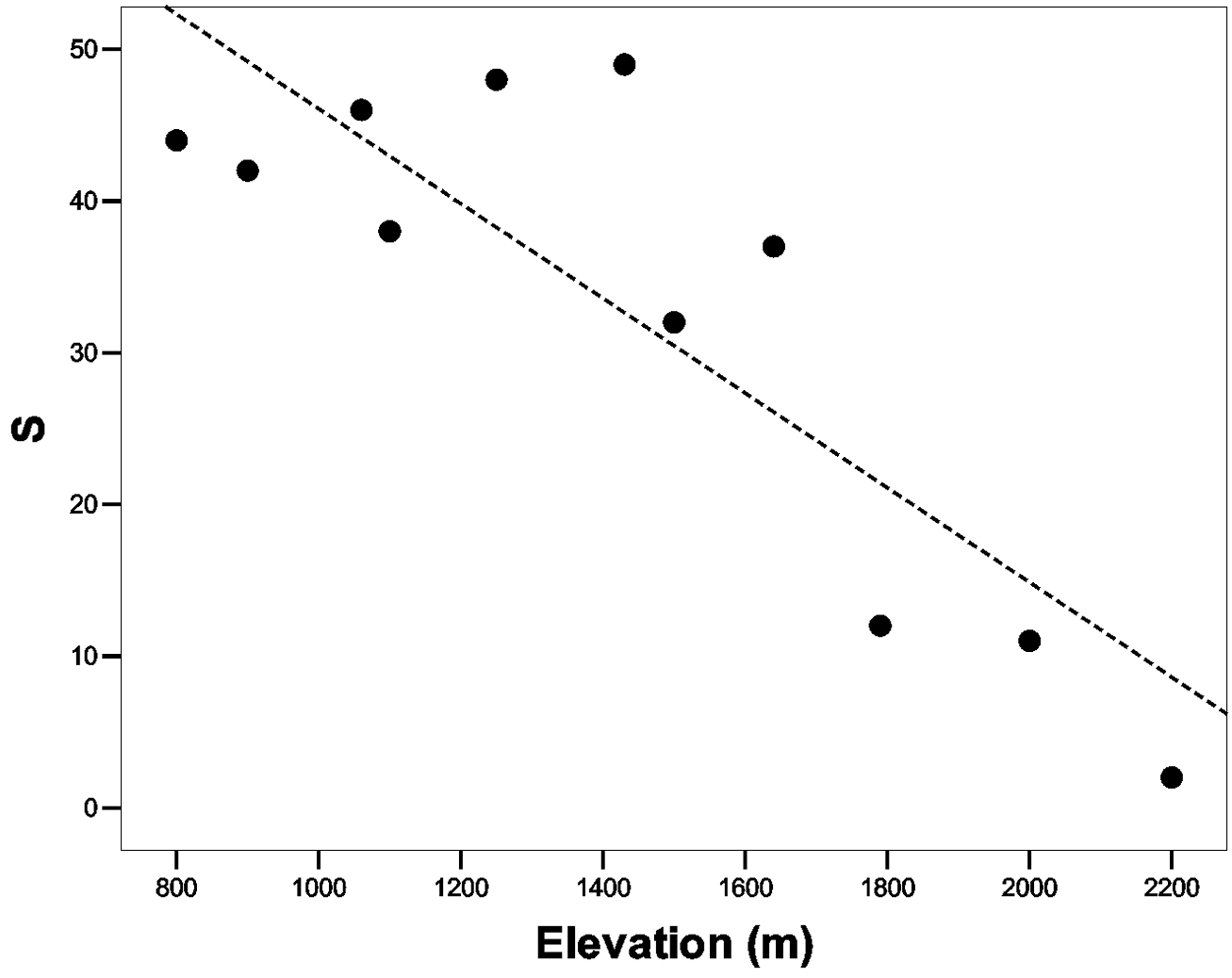
The Spearman’s correlation analysis highlighted that species richness is correlated to two variables: the elevation (rho = -0, 74; P = 0, 01), and of the distance from the secondary roads (rho = -0, 65; P = 0, 03), but elevation and the distance from the secondary roads are positively auto-correlated (rho = 0, 73; P = 0, 01). The Linear Regression Analyses performed to test the effect of elevation and distance from the secondary roads on the species richness show that elevation is the only variable able to affect the negatively the specie richness (ANOVA test: F2, 10 = 10, 27; P = 0, 006; elevation: t = -2, 58; P = 0, 033; distance from the secondary roads: P = 0, 95). In particular, species richness decreased with increasing elevation, but this trend is not gradual; the highest values of S are between 800 and 1600 m a.s.l. (Smean = 41, 14). Within this elevational gradient, any significant trend in the species richness resulted (P = 0, 45) (Fig. 2).The species richness decrease strongly above 1600 metres, and between 1700 and 2200 metres; the average species richness is 8.
Relationship between the species richness (S) and the altitudinal gradient.
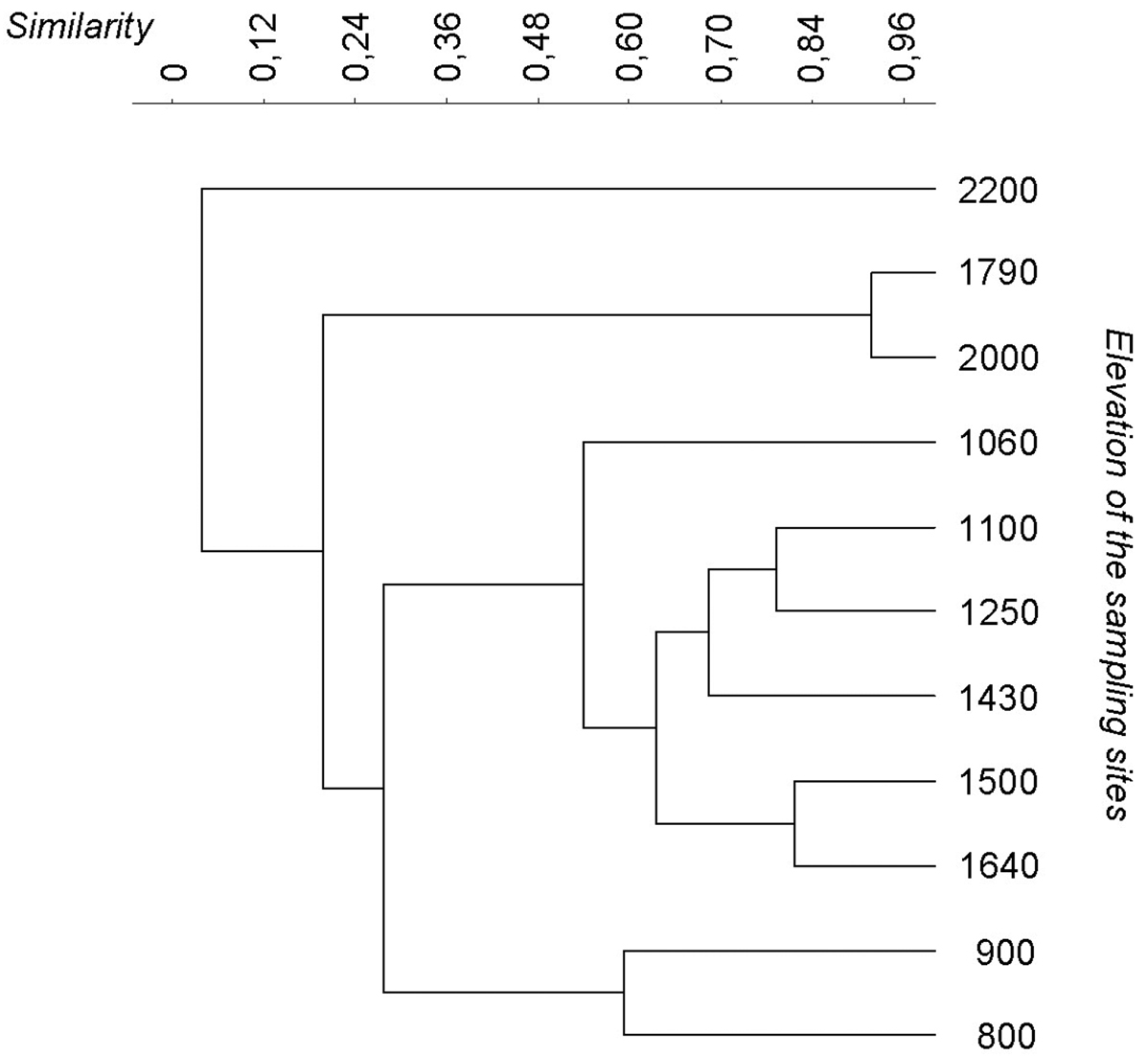
ANOSIM test demonstrated that species composition along the valley varied with a significantly low turnover (ANOSIM, r = 0.21; P < 0.01). The dendrogram built on the base of the Jaccard similarity showed the cluster of three main groups (Fig. 3): the first one is between 800 and 900 m asl, the second one is between 1060 and 1640 m, and the third is above 1790 m a.s.l..
Dendrogram drawn on the base of the Jaccard similarities between the sites.
The high species richness (S = 88) found in Val Genova valley reflect the high wilderness of this area due to the cerambycids being excellent indicators of the health of the wood decomposer community because of their habitat specificities (
The effects of the environmental variables on the species richness showed that only the elevational gradient determines a decrease in the number of species, and a low spatial assemblage’s turnover. This result is in agreement with
Twenty-five percent of the species observed in the Val Genova have been evaluated in the IUCN redlist of saproxylic beetles (
The forest management is known to negatively affect saproxylic beetles (
In conclusion, the biodiversity of longhorned beetles observed in the Val Genova valley can be considered surprising due to the high number of species living into an area with the following features: small size and distributed along a wide elevational gradient.
The possibility to observe so many cerambycids in a limited space is to our knowledge, unique in the Alps. It suggest that other taxonomic groups should be considered with the purpose to increase the entomological knowledge of this valley that probably is the only one not urbanized, at least in the Italian Alps.
The authors thank Adamello-Brenta Natural Park for permission to carry out this research. We are indebted to Carlo Pesarini (Museo Civico di Storia Naturale di Milano, Italy) to help us in the species identifications. We thank the anonymous referee for his review. Part of the results was included in the masters’ thesis of the second author.