Research Article |
|
Corresponding author: Yuh-Wen Chiu ( chiuywlab@gmail.com ) Academic editor: Ingo S. Wehrtmann
© 2019 Hsi-Te Shih, Yixiong Cai, Yuh-Wen Chiu.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Shih H-T, Cai Y, Chiu Y-W (2019) Neocaridina fonticulata, a new land-locked freshwater shrimp from Hengchun Peninsula, Taiwan (Decapoda, Caridea, Atyidae). ZooKeys 817: 11-23. https://doi.org/10.3897/zookeys.817.29332
|
Abstract
A new species of land-locked freshwater shrimp, Neocaridina fonticulata sp. n. (Atyidae), is described from Kenting, Hengchun Peninsula, Pingtung County, southern Taiwan. This new species can be distinguished from its congeners by rostrum structure, pereiopods, and male first and second pleopods. The molecular evidence of mitochondrial cytochrome oxidase subunit I (COI) also supports the establishment of a new species. This is the third endemic species of Neocaridina known from Taiwan.
Keywords
Neocaridina fonticulata , mitochondrial cytochrome oxidase subunit I, new species, morphology
Introduction
The genus Neocaridina Kubo, 1938 is a group of small-sized shrimps with a land-locked habit, inhabiting in the middle and upper reaches of rivers in East Asia, with more than 30 species recorded (
A recent survey of the species diversity of freshwater shrimps of Taiwan showed an undescribed species from southern Taiwan with different morphological characters compared to other known species of Neocaridina, which was supported by molecular evidence. This species is herein described as a new species, endemic to Taiwan Island, which brings the total number of Taiwanese species of Neocaridina to four.
Materials and methods
Specimens of the genus Neocaridina examined in this study were collected from a spring in Sheding, Kenting, Hengchun Peninsula, Pingtung County, Taiwan and preserved in 70%–95% ethanol after collection. Some specimens were selected and illustrated with the help of a drawing tube attached to a Nikon stereo microscope (model SMZ 1000), and deposited in the Zoological Collections of the Department of Life Science, National Chung Hsing University, Taichung, Taiwan (NCHUZOOL) and the Zoological Reference Collection of the Lee Kong Chian Natural History Museum, National University of Singapore, Singapore (formerly the Raffles Museum of Biodiversity Research) (ZRC). Carapace length is abbreviated cl, and the mode refers to the most frequently number occurring. The rostral formula was counted based on all specimens available. The egg measurements were based on five eggs each from four ovigerous females (see material examined).
Sequences of mitochondrial cytochrome oxidase subunit I (COI) were obtained following the method described by
The best-fitting model for sequence evolution was determined by MrModeltest (version 2.2,
Other analyses, including the nucleotide composition, variable and parsimony informative positions, Kimura 2-parameter (K2P) distance (
Systematic account
Family Atyidae De Haan, 1849
Neocaridina Kubo, 1938
Neocaridina fonticulata sp. n.
Material examined
Holotype: male, cl 3.4 mm, NCHUZOOL 15004, a spring at Sheding, Kenting, Pingtung County, Taiwan, 21°57'26.7"N, 120°48'35.5"E, elevation of 150 m, coll. H.-T. Shih and Y. C. Gan, 1 July 2015. Paratypes: 13 males, cl 2.5–3.3 mm, NCHUZOOL 15005, 5 females, cl 2.6–3.8 mm, 2 ovigerous females, cl 3.6–3.7 mm, NCHUZOOL 15006; 1 male, cl 4.2 mm, NCHUZOOL 15007; 1 male, cl 3.9 mm, NCHUZOOL 15008; 7 males, cl 2.7–3.3 mm, 2 females, cl 3.6–3.8 mm, 2 ovigerous females, cl 3.5–3.6 mm, ZRC 2018.1013, same collection data as for holotype. 1 male, cl 3.5 mm, 1 damaged specimen, cl 4.4 mm, NCHUZOOL 15009, Sheding, Kenting, Pingtung County, Taiwan, 5 May 2015, coll. Y. C. Gan.
Neocaridina fonticulata sp. n.: A carapace and cephalic appendages, lateral view B telson, dorsal view C preanal carina, lateral view D right scaphocerite and antenna, ventral view E right mandible F right maxillula G right maxilla H right 1st maxilliped I right 2nd maxilliped J right 3rd maxilliped. Scale bars: 1.5 mm (A); 0.5 mm (B, E–J); 1 mm (C, D) (male, cl 3.0 mm, paratype, ZRC 2018.1013).
Other material
3 males, 9 females, NCHUZOOL 15010, Sheding, Kenting, Pingtung County, Taiwan, coll. Y. C. Gan, 5 May 2015. 3 males, 11 females, 2 ovigerous females, NCHUZOOL 15011, two damaged males, ZRC 2018.1014, same collection data as for holotype.
Comparative material
Neocaridina ikiensis: 1 male, cl 4.6 mm, ZRC 2017.0960, 1 female, cl 5.1 mm, ZRC 2017.0961, 8 males, cl 3.0–5.4 mm, 8 females, cl 2.9–5.1 mm, ZRC 2017.0962, small stream at Kugiyama-hure, Gonoura Town, Iki City, Nagasaki Prefecture, Japan, coll. Yasuhiko Nakahara, 23 November 2015.
Diagnosis
Rostrum short, straight, slightly sloping downwards, reaching mostly to end of 1st segment of antennular peduncle, rostral formula 1–3+8–15/1–4. Pterygostomian margin armed with an indistinct spine. 1st pereiopod carpus 1.2–1.5 × as long as high; chela 2.0–2.1 × as long as broad; fingers slightly longer than palm. 2nd pereiopod carpus 1.1–1.2 × as long as chela, 3.9–4.3 × as long as high; chela 2.1–2.3 × as long as broad; fingers 1.3–1.4 × as long as palm. 3rd pereiopod with propodus straight in females, slightly incurved in males, 2.7–3.0 × as long as dactylus; dactylus terminating in two claws, 4–6 accessory spines on flexor margin, strongly incurved in males. 5th pereiopod propodus 2.7–2.8 × as long as dactylus, dactylus terminating in one claw, with 46–54 spinules on flexor margin. Endopod of male 1st pleopod extending to 0.8 × exopod length, inflated at distal ¾, pyriform, 1.7 × as long as wide, appendix interna at base of inflated part, short. Appendix masculina of male 2nd pleopod cylindrical, reaching to 0.7 length of endopod, appendix interna reaching to 0.6 length of appendix masculina. Uropodal diaeresis with 13–14 movable spinules. Eggs 1.10 × 0.68 to 1.20 × 0.75 mm in diameter.
Description
Rostrum short, straight, slightly sloping downwards, without distinct postrostral ridge, reaching slightly short of or slightly beyond end of 1st segment of antennular peduncle, occasionally reaching to, rarely beyond end of 2nd segment of antennular peduncle; armed dorsally with 9–18 (mode 13–15) very small teeth, including 1–3 (mode 2) on carapace, ventrally with 1–4 small teeth (mode 2–3). Antennal spine fused with inferior orbital angle. Pterygostomian margin sub-rectangular, armed with an indistinct spine.
Sixth pleomere in male 0.43cl, 1.40 × as long as 5th pleomere, slightly shorter than telson; 6th pleomere in female 0.48cl, 1.38 × as long as 5th pleomere, slightly shorter than telson. Telson 3.0 × as long as wide, with four or five pairs of dorsal spinules and one pair of dorsolateral spinules; posterior margin rounded, lined with four or five pairs of simple setae, lateral pair distinctly longer than intermediate pairs. Pre-anal carina moderately high, lacking spine.
Eyes well developed, anterior corneal margin reaching to 0.6 × length of basal segment of antennular peduncle. Antennular peduncle 0.6 × as long as carapace; basal segment of antennular peduncle longer than combined length of 2nd and 3rd segments, anterolateral angle reaching 0.3 length of 2nd segment, 2nd segment distinctly longer than 3rd segment. Stylocerite reaching 0.7–0.8 length of basal segment of antennular peduncle. Scaphocerite 3.5 × as long as wide, with extension of the distolateral spine reaching end of antennular peduncle.
Mandible with incisor process ending in irregular teeth; molar process truncated. Maxillule lower lacinia broadly rounded; upper lacinia elongate, with a row of 30 distinct spiniform setae on inner margin; palp short. Maxilla distal endite subdivided; palp short; scaphognathite tapering posteriorly with some long, curved setae at posterior end. 1st maxilliped with stout palp. 2nd maxilliped typical of genus, endopod with fused dactylus and propodal segments. 3rd maxilliped reaching to end of antennular peduncle, with ultimate segment slightly longer than penultimate segment.
First four pereiopods with epipod. 1st pereiopod reaching slightly beyond distal end of basal segment of antennular peduncle; merus 1.8–2.1 × as long as broad, as long as carpus; carpus excavated anteriorly, shorter than chela, 1.2–1.5 × as long as high; chela 2.0–2.1 × as long as broad; fingers slightly longer than palm. 2nd pereiopod reaching end of antennular peduncle; merus shorter than carpus, 3.6–4.1 × as long as broad; carpus 1.1–1.2 × as long as chela, 3.9–4.3 × as long as high; chela 2.1–2.3 × as long as broad; fingers 1.3–1.4 × as long as palm. 3rd pereiopod reaching beyond end of antennular peduncle by dactylus; merus stout; propodus straight in females, slightly incurved in males, 2.7–3.0 × as long as dactylus (terminal claw included), 7.2–7.5 × as long as broad, numerous spinules on posterior margin; dactylus terminating in two claws, 4–6 accessory spines on flexor margin, strongly incurved in males. 4th pereiopod similar to 3rd pereiopod in form and length. 5th pereiopod reaching to end of 2nd segment of antennular peduncle, propodus 8.0–9.5 × as long as broad, 2.7–2.8 × as long as dactylus, dactylus 2.9–3.4 × as long as wide (spinules included), terminating in one claw, with 46–54 spinules on flexor margin.
Endopod of male 1st pleopod extending to 0.8 × exopod length, inflated at distal ¾, pyriform , 1.7 × as long as wide, with tiny spinules on distal margin of dorsal surface, appendix interna at base of inflated part, short. Appendix masculina of male 2nd pleopod cylindrical, reaching to about 0.7 length of endopod, inner and distal surface densely lined with long, stout spines, appendix interna reaching to 0.6 length of appendix masculina.
Uropodal diaeresis with 13–14 movable spinules.
Eggs 1.10 × 0.68 to 1.20 × 0.75 mm in diameter.
Colour in life
Body colour varying from translucent to light blue, with darker red-brown spots on dorsal surface and lighter red-brown spots on lateral surface of carapace; pleon usually with several dark red-brown vertical stripes on lower lateral surface, and white star-shaped pigment scattered on whole body (Figure
Etymology
Neocaridina fonticulata is named after its known habitat, from the Latin root, fonticulus, for little spring.
Ecological notes
Specimens of the new species were collected from leaf litter layer of a small stream (Figure
Distribution
Presently known only from Sheding, Kenting, southern Taiwan.
Remarks
With the short rostrum, Neocaridina fonticulata sp. n. is morphologically most similar to the insular Chinese species Neocaridina zhoushanensis Cai, 1996, originally described as a subspecies of N. denticulata, from Zhoushan Islands of Zhejiang Province. It can be differentiated by the more slender chela of the 1st pereiopod (2.0–2.1 × as long as wide in the new species vs. 1.6–1.7 × in N. zhoushanensis; cf. Figures
Neocaridina fonticulata sp. n.: pereiopods in lateral view. A right 1st pereiopod B right 2nd pereiopod C right 3rd pereiopod D same, dactylus E right 5th pereiopod F same, dactylus G right male 1st pleopod, front view H right male 2nd pleopod, internal view I diaeresis of left uropodal exopod. Scale bars: 1 mm (A–C, E); 0.2 mm (D, F); 0.5 mm (G, H); 0.2 mm (I) (male, cl 3.0 mm, paratype, ZRC 2018.1013).
With the relatively short rostrum, Neocaridina fonticulata sp. n. morphologically resembles two Taiwanese species, N. saccam Shih & Cai, 2007 and N. ketagalan Shih & Cai, 2007. It differs from N. saccam (cf.
Neocaridina fonticulata sp. n. can be separated from N. ketagalan (cf.
Neocaridina fonticulata sp. n.: A carapace and cephalic appendages, lateral view B right 1st pereiopod C right 2nd pereiopod D left 3rd pereiopod E same, dactylus F left 5th pereiopod G same, dactylus. Scale bars: 1.5 mm (A); 1 mm (B–D, F); 0.2 mm (E, G) (female, cl 3.8 mm, paratype, ZRC 2018.1013).
With its relatively short rostrum, Neocaridina fonticulata sp. n. morphologically also resembles the recently described Japanese species Neocaridina ikiensis Shih, Cai, Niwa & Nakahara, 2017. It can be differentiated from the latter by its shorter rostrum (reaching from slightly short of to slightly beyond the end of the 1st segment of antennular peduncle vs. reaching slightly short of to distinctly beyond the end of the 2nd segment of antennular peduncle; cf. Figures
With the relatively slender endopod of the male 1st pleopod, the new species is similar to N. koreana Kubo, 1938. It can be separated from the latter by the relatively shorter rostrum, which mostly reaches to or slightly beyond the end of the 1st segment of antennular peduncle vs. almost reaching to or slightly beyond antennular peduncle in N. koreana (cf.
DNA analyses and discussion
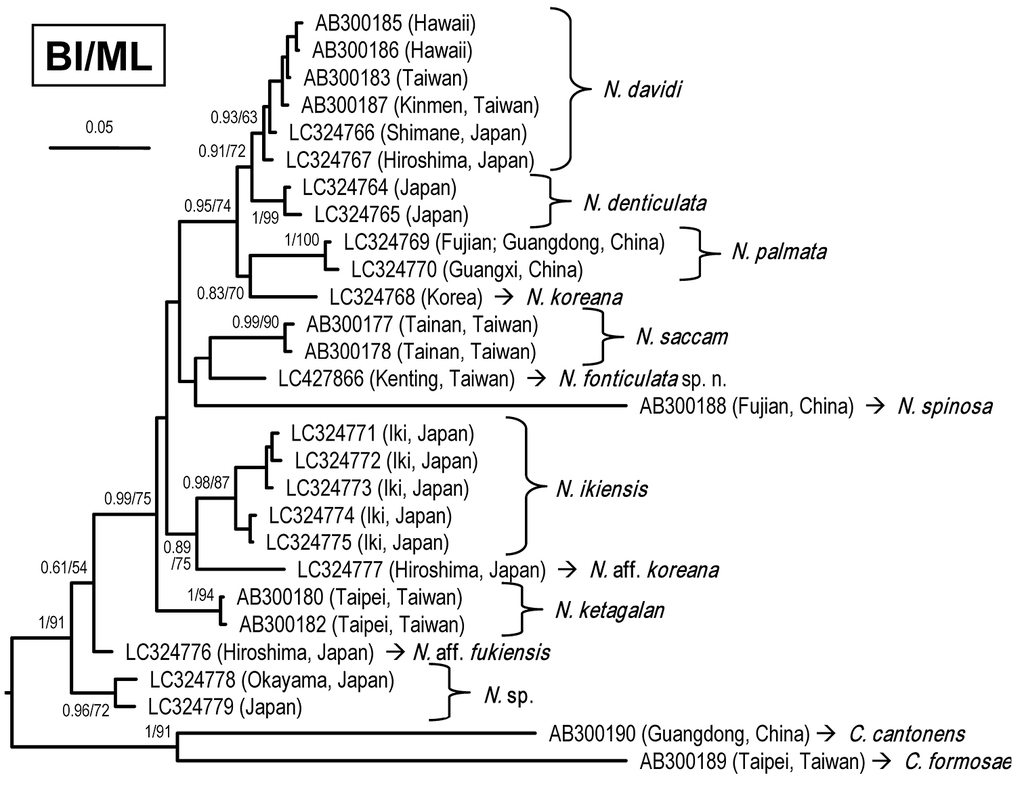
A total of four specimens from Sheding, Kenting, were used in the molecular phylogenetic analysis. A 658-bp segment of COI was amplified, resulting in one haplotype (accession number LC427866). Based on the COI haplotypes, the phylogenetic tree was reconstructed using BI analysis, with the support values from the BI and ML analyses shown in Figure
Matrix of percentage pairwise nucleotide divergences (lower left) and mean number of differences (upper right) based on COI within and between some species of Neocaridina from East Asia. Values of range are shown in parentheses.
| Intraspecific | Interspecific | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nucleotide divergence | Mean nucleotide difference | N. davidi | N. denticulata | N. koreana | N. palmata | N. fonticulata sp. n. | N. ketagalan | N. saccam | |
| N. davidi | 0.67 | 4.39 | 17.75 | 29.88 | 30.63 | 36.5 | 44.46 | 45.67 | |
| (0–1.54) | (0–10) | (14–22) | (28–32) | (28–34) | (35–39) | (41–48) | (42–50) | ||
| N. denticulata | 0.46 | 3 | 2.77 | 31.5 | 33.17 | 38.5 | 48.83 | 48.83 | |
| (0–0.77) | (0–5) | (2.17–3.46) | (30–33) | (31–36) | (36–41) | (46–52) | (47–51) | ||
| N. koreana | 0 (0) | 0 (0) | 4.73 | 5 | 35.67 | 46 | 46.33 | 52.33 | |
| (4.42–5.07) | (4.75–5.25) | (35–37) | (46–46) | (46–47) | (52–53) | ||||
| N. palmata | 0.41 | 2.67 | 4.83 | 5.26 | 5.67 | 47 (47) | 48.33 | 53 (53–53) | |
| (0–0.61) | (0–4) | (4.4–5.39) | (4.9–5.73) | (5.56–5.89) | (48–49) | ||||
| N. fonticulata sp. n. | 0 (0) | 0 (0) | 5.82 | 6.16 | 7.41 | 7.56 | 34.67 | 34.33 | |
| (5.57–6.24) | (5.74–6.59) | (7.41) | (7.56–7.56) | (34–35) | (34–35) | ||||
| N. ketagalan | 0.1 | 0.67 | 7.17 | 7.94 | 7.49 | 7.82 | 5.53 | 38 | |
| (0–0.15) | (0–1) | (6.58–7.78) | (7.44–8.5) | (7.44–7.61) | (7.76–7.93) | (5.42–5.58) | (37–39) | ||
| N. saccam | 0.31 | 2 | 7.39 | 7.94 | 8.51 | 8.62 | 5.49 | 6.08 | |
| (0–0.46) | (0–3) | (6.75–8.14) | (7.62–8.32) | (8.45–8.63) | (8.62–8.62) | (5.43–5.6) | (5.91–6.25) | ||
The discovery of the new species increases the number of Neocaridina species in Taiwan to four, i.e., N. davidi, N. saccam, N. ketagalan, and N. fonticulata sp. n. (
Acknowledgements
This study was supported by grants from the Ministry of Science and Technology (MOST 105-2621-B-005-002-MY3), Executive Yuan, Taiwan, to HTS; and the Forestry Bureau, Council of Agriculture (103 Forestry Development-07.1-Conservation-60), Executive Yuan, Taiwan to YWC. Thanks are also due to Ye Chen Gan for helping collection and Pei-Yi Hsu for measuring specimens. We acknowledge the subject editor Ingo Wehrtmann, Charles Fransen, Tomoyuki Komai, and one anonymous reviewer who greatly improved the manuscript.
References
- Cai Y (1996) A revision of the genus Neocaridina (Crustacea: Decapoda: Atyidae). Acta Zootaxonimica Sinica 21: 129–160. [In Chinese]
- De Grave S, Fransen CHJM (2011) Carideorum catalogus: the recent species of the dendrobranchiate, stenopodidean, procarididean and caridean shrimps (Crustacea: Decapoda). Zoologische Mededelingen, Leiden 85: 195–588.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299.
- Jang-Liaw NH, Lee TH, Chou WH (2008) Phylogeography of Sylvirana latouchii (Anura, Ranidae) in Taiwan. Zoological Science, Tokyo 25: 68–79. https://doi.org/10.2108/zsj.25.68
- Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16: 111–120. https://doi.org/10.1007/BF01731581
- Kubo I (1938) On the Japanese atyid shrimps. Journal of the Imperial Fisheries Institute, Tokyo 33: 67–100.
- Liang XQ (2004) Fauna Sinica. Invertebrata: Crustacea: Decapoda: Atyidae. Science Press, Beijing, 375 pp. [In Chinese]
- Naruse T, Shokita S, Cai Y (2006) Neocaridina iriomotensis, a new species of land-locked freshwater shrimp (Crustacea: Decapoda: Atyidae) from Iriomote island, southern Ryukyus, Japan. Proceedings of the Biological Society of Washington 119: 25–31. https://doi.org/10.2988/0006-324X(2006)119[25:NIANSO]2.0.CO;2
- Nylander JAA (2005) MrModeltest version 2.2. Evolutionary Biology Centre, Uppsala Univ., Uppsala.
- Ronquist F, Huelsenbeck JP, van der Mark P (2005) MrBayes 3.1 manual. http://mrbayes.csit.fsu.edu/manual.php
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MRBAYES 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. https://doi.org/10.1093/sysbio/sys029
- Shih HT, Cai Y (2007) Two new species of land-locked freshwater shrimp genus Neocaridina Kubo, 1938 (Decapoda: Caridea: Atyidae) from Taiwan, with notes on the speciation within Taiwan Island. Zoological Studies 46: 680–694.
- Shih HT, Cai Y, Niwa N, Nakahara Y (2017) A new species of land-locked freshwater shrimp of the genus Neocaridina (Decapoda: Caridea: Atyidae) from Iki Island, Kyushu, Japan. Zoological Studies 56: 30.
- Shih HT, Hung HC, Schubart CD, Chen CA, Chang HW (2006) Intraspecific genetic diversity of the endemic freshwater crab Candidiopotamon rathbunae (Decapoda, Brachyura, Potamidae) reflects five million years of geological history of Taiwan. Journal of Biogeography 33: 980–989. https://doi.org/10.1111/j.1365-2699.2006.01472.x
- Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. https://doi.org/10.1093/bioinformatics/btl446
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28: 2731–2739. https://doi.org/10.1093/molbev/msr121