(C) 2011 Mariana Baptista Lacerda. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Pseudaeginella montoucheti (Quitete, 1971) is redescribed based on newly collected specimens from red and brown algae and tubiculous polychaete colony that were obtained from shallow waters at Tamboretes Archipelago, Balneário Barra do Sul and Sepultura Beach, Bombinhas, Santa Catarina State, Brazil. Of 10 species of Pseudaeginella so far reported, Pseudaeginella montoucheti is closest to Pseudaeginella sanctipauli Laubitz, 1995, but differs from the latter by having more numerous body spines including ventro-lateral ones over gills on pereonites 3 and 4, and the antenna 1 length measuring half body length. An identification key for Pseudaeginella species and a checklist of Caprellidea occurring along the Brazilian coasts are also presented.
Amphipoda, Pseudaeginella montoucheti (Quitete 1971) , Redescription, Santa Catarina, Brazil
The knowledge on ecology and biology of Brazilian caprellids is restricted to some areas and substrata, mainly those living on macroalgae from southeastern and southern coast (
Of 19 species of caprellids so far recorded from Brazilian coasts (Table 1), Pseudaeginella montoucheti was firstly described as Fallotritella montoucheti Quitete, 1971 (
Checklist of the Brazilian Caprellidae with their distribution in Brazil
| Species | Distribution in Brazil (references) |
|---|---|
|
Caprellidae Caprella Lamarck, 1801 |
|
| Caprella aculeata (Dana, 1853) | Rio de Janeiro ( |
| Caprella andreae Mayer, 1890 | Torres and Tramandaí, RS ( |
| Caprella danilevskii Czerniavski, 1868 | Ubatuba, SP ( |
| Caprella dilatata Krøyer, 1843 | Arraial do Cabo, RJ ( |
| Caprella equilibra Say, 1818 | Ubatuba, SP ( |
| Caprella globiceps Dana, 1853 | Rio de Janeiro ( |
| Caprella penantis Leach, 1814 | Paranaguá, PR ( |
| Caprella scaura Templeton, 1836 | Arraial do Cabo, RJ ( |
| Hemiaegina Mayer, 1890 | |
| Hemiaegina minuta Mayer, 1890 (= Hemiaegina costai Quitete, 1972) | Bahia and Pernambuco States ( |
| Liropus Mayer, 1890 | |
| Liropus nelsonae Guerra-García, 2003 |
7°58'S, 34°17'W – 7°50'S, 34°17'W ( |
| Monoliropus Mayer, 1903 | |
| Monoliropus enodis Rayol & Serejo, 2003 | Guanabara Bay, RJ ( |
| Orthoprotella Mayer, 1903 | |
| Orthoprotella melloi Quitete, 1975 | Pernambuco State ( |
| Paracaprella Mayer, 1890 | |
| Paracaprella digitimanus Quitete, 1971 |
1°21'S, 43°50'W ( |
| Paracaprella pusilla Mayer, 1890 | Arraial do Cabo, RJ ( |
| Paracaprella tenuis Mayer, 1903 | Ubatuba SP ( |
| Parvipalpus Mayer, 1890 | |
| Parvipalpus colemani Guerra-García, 2003 |
7°58'S, 34°17'W – 7°50'S, 34°17'W ( |
|
Pseudaeginella Mayer, 1890 Pseudaeginella montoucheti (Quitete, 1971) |
Itamaracá, PE, Mar Grande and Olivença, BA, Guarapari and Vitória, ES ( |
|
Phtisicidae Phtisica Slabber, 1769 |
|
| Phtisica marina Slabber, 1769 | Arraial do Cabo, RJ ( |
| Phtisica verae Quitete, 1979 | Rio de Janeiro State ( |
BA, Bahia State; ES, Espírito Santo State; PE, Pernambuco State; RJ, Rio de Janeiro State; SC, Santa Catarina State; SP, São Paulo State
Collections were conducted at two Islands - Pássaros Island (26°22'S, 48°31'W) and Araras Island (26°27'S, 48°34'W) – in Tamboretes Archipelago, municipality of Balneário Barra do Sul (16th May 2009) and at Sepultura Beach, Bombinhas (30th June 2011) Santa Catarina State, southern Brazil. Caprellideans were found in the phytal of the red algae Amphiroa beauvoisii Lamouroux and Spyridia aculeata (Schimper) Kützing, of the brown alga Sargassum cymosum C. Agardh, 1820 and as the associate fauna of a tubiculous polychaete colony; these communities were living over rocky surface in infralittoral depths, from 0.5 to 7.0 m. The biological substrates were carefully wrapped up in a plastic bag and scraped from the rocky surface with a spatula by scuba divers. In laboratory, the plastic bag content was very kindly washed in dilute formalin. The deposited material was sieved, sorted and caprellids were fixed and preserved in ethyl alcohol 70%.
From a total of 54 examined specimens (31 males and 23 females), several specimens of male and female were selected to be dissected under stereomicroscope. The dissected material was mounted in polyvinyl lactophenol. All figures were drawn with the aid of a camera lucida. Specimens are deposited in Museum of Natural History of Capão da Imbuia (MHNCI) and in Center for Zoological Studies (CEZ), from Institute of Biology, Federal University of Rio de Janeiro.
ResultsFamily Caprellidae Leach, 1814
Genus Pseudaeginella Mayer, 1890
http://species-id.net/wiki/Pseudaeginella_montoucheti
Figs 1–4MHNCI 2844 One female from the phytal of red alga Spyridia aculeata, 7 m deep, Araras Island (26°27'S, 48°34'W), Tamboretes Archipelago, Santa Catarina, Brazil, 16th May, 2009.
MHNCI 2845 One male and two females from the phytal of the calcareous red alga Amphiroa beauvoisii, 4 m deep.
MHNCI 2846 Three males and two females from the phytal of Amphiroa beauvoisii, 1.5 m deep, Pássaros Island (26°22'S, 48°31'W), Tamboretes Archipelago, Santa Catarina, Brazil, 16th May, 2009.
MHNCI 2847 Four males and two females from the phytal of brown alga Sargassum cymosum, Bombinhas (27°08'28"S, 48°28'42"W), Santa Catarina, Brazil, 30th June, 2011.
CEZ 968 Holotype male from of Sargassum, Itamaracá, Pernambuco, Brazil 5th August, 1968. Collector: Dr. Pierre Montouchet.
CEZ 971 Two paratypes males and three paratypes females from of Sargassum, Mar Grande, Bahia, Brazil, 22th January, 1968. Collector: Dr. Pierre Montouchet.
CEZ 972 12 paratypes males and eight paratypes females from of Sargassum, Itamaracá, Pernambuco, Brazil 5th August, 1968. Collector: Dr. Pierre Montouchet.
CEZ 973 Seven paratypes males and four paratypes females from of Sargassum, Mar Grande, Bahia, Brazil, 22th January, 1968. Collector: Dr. Pierre Montouchet.
CEZ 974 One paratype male and one paratype female from of Sargassum, Guarapari, Espírito Santo, Brazil, 6th September, 1968. Collector: Dr. Pierre Montouchet.
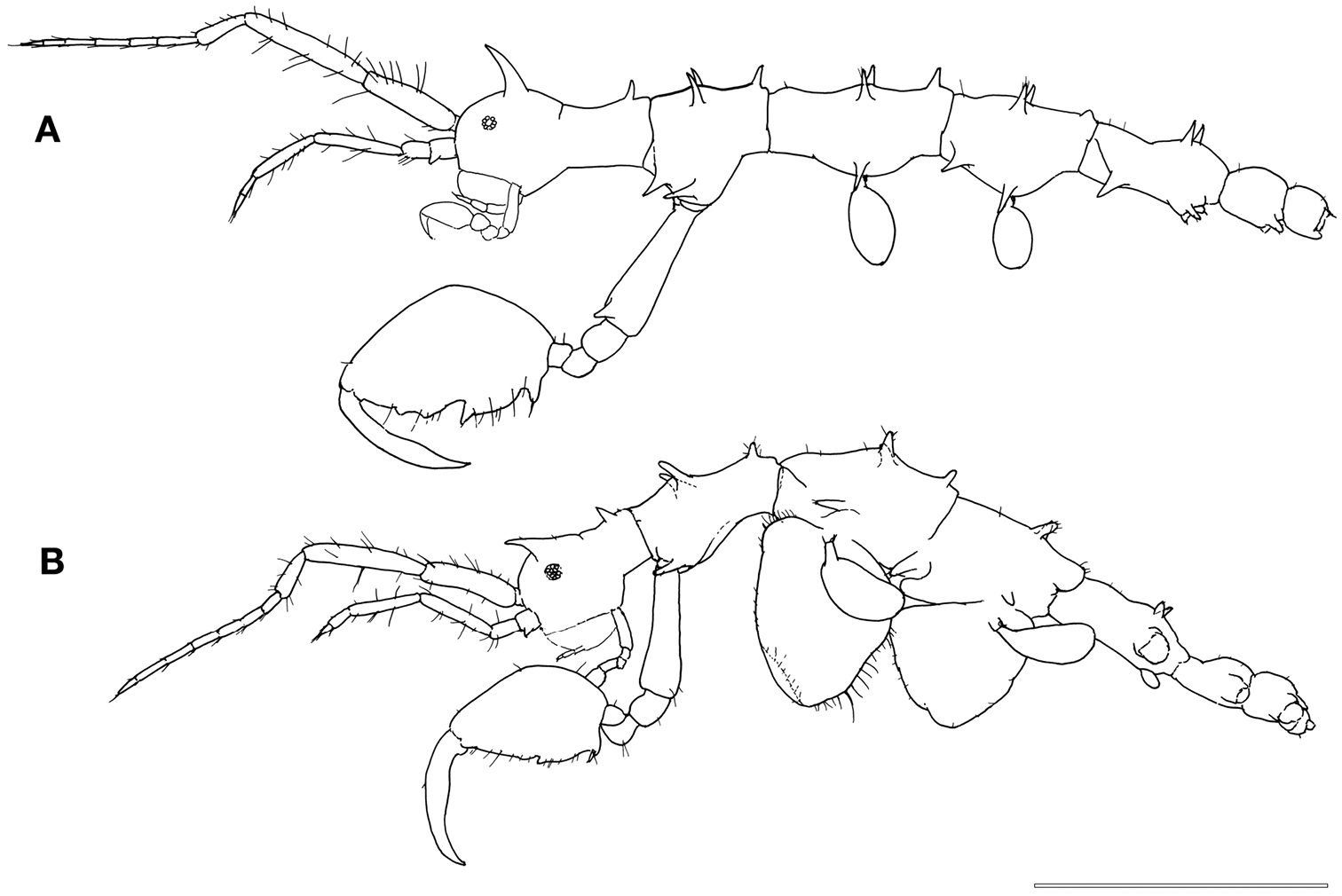
Male (Fig. 1A). Body length 3.0 mm. Pereonites 3 and 4 the longest, followed by pereonites 2 and 5. Head and pereonite 1 (suture clearly present) concave along dorsal margin, head with an anteriorly curved mid-dorsal projection, pereonite 1 with a small postero-dorsal projection. Pereonite 2with paired mid-dorsal projections, 1 postero-dorsal projection, paired antero-lateral projections and paired mid-lateral projections. Pereonite 3 with paired mid-dorsal projections, 1 postero-dorsal projection and paired mid-lateral projections. Pereonite 4 with paired mid-dorsal projections, 1 weak postero-dorsal projection, paired antero-lateral projections and paired mid-lateral projections. Pereonite 5 withpaired mid-dorsal projections, paired antero-lateral projections and paired mid-lateral projection near the swollen basal part of pereopod 5. Pereonite 6 with paired postero-lateral projections near the basal part of pereopod 6.
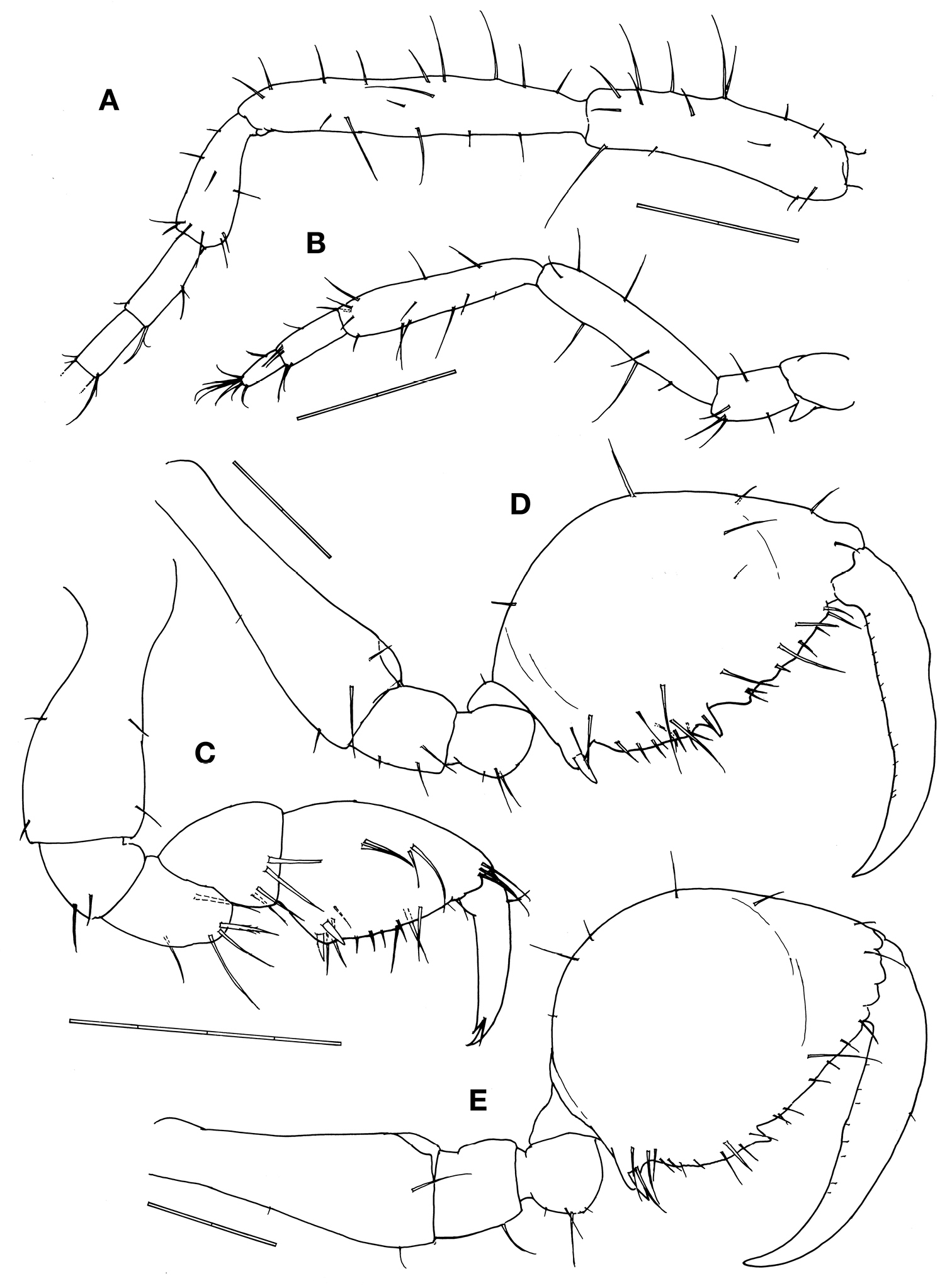
Antennae (Figs. 2A, 2B). Antenna 1 about half body length. Peduncular articles with ca. 10 to 20 simple setae of varied length; peduncular article 2 the longest followed by article 1. Flagellum 6-articulate with 4/5 of peduncular length. Antenna 2 about 4/5 of antenna 1 length, without swimming setae; peduncular setose in varied length; flagellum with 8 and 6 simple setae in the proximal and distal articles.
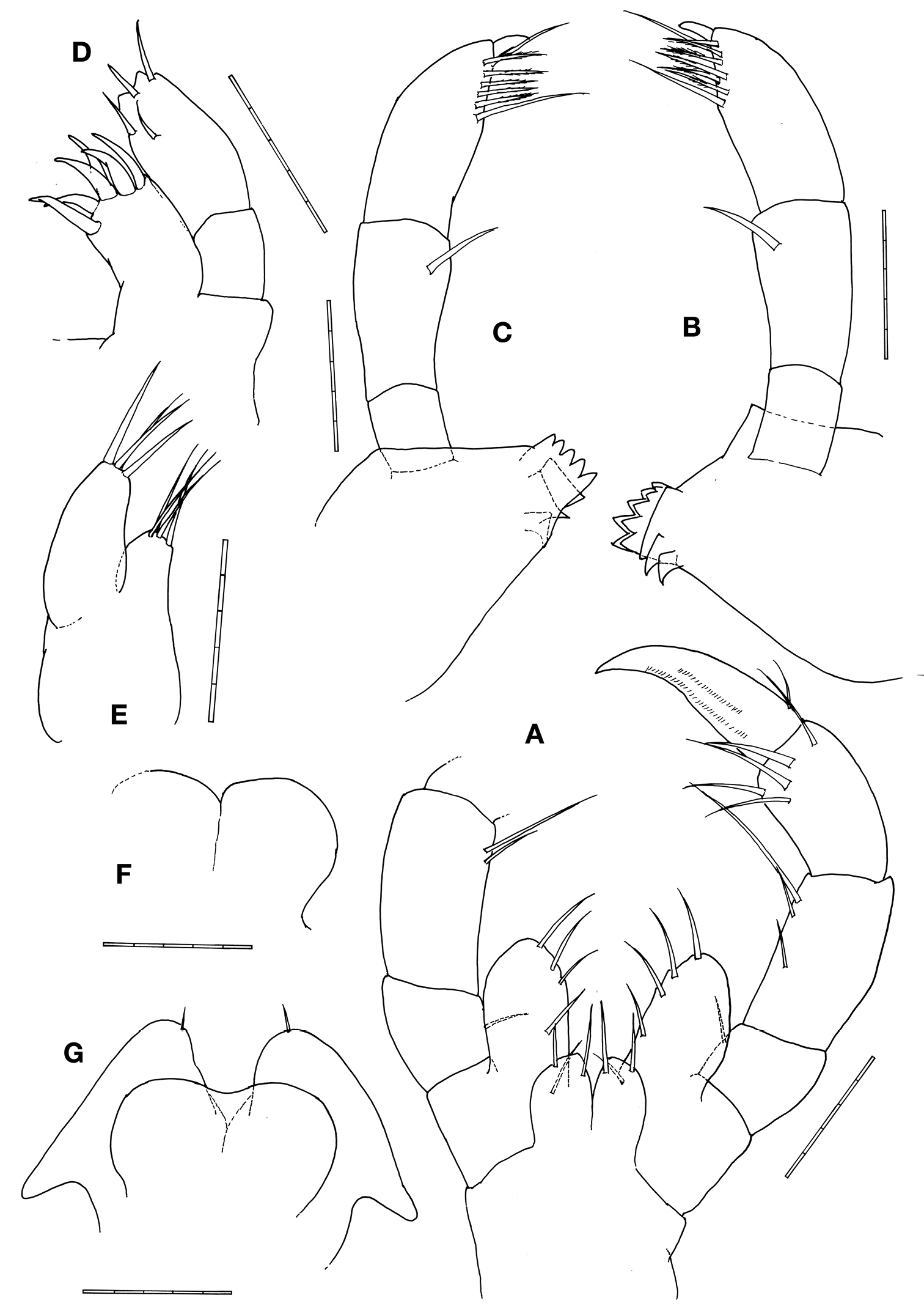
Mouthparts (Figs. 3A–G). Upper lip notched, forming rounded projections. Right mandiblewith incisor with 5 teeth and followed by lacinia mobilis with 5 teeth and 3 trapezoid plates; palp article 2 with 1 lateral seta; palp article 3 setal formula 1–6–1 with a distal knob. Left mandible incisor with 5 teeth followed by 3 trapezoid plates; palp article 2 with 1 lateral seta; palp article 3 setal formula 1–6–1 with a distal knob. Lower inner lips round and fused each other, outer lobes round with 1 apical seta. Maxilla 1outer plate with 6 stout apical setal-teeth; palp distal margin with 4 setae. Maxilla 2inner plate triangular with 4 apical setae; outer plate elongate with about 4 apical setae. Maxilliped basal endite (inner plate) with 2 setae on outer margin; ischial endite (outer plate) oval, 2 times longer than inner plate, with 4 or 5 setae on inner margin; palp article 2 with 2 or 3 setae on inner margin; palp article 3 with 5 distal setae; palp article 4 (dactylus) weakly falcate.
Gnathopod 1 basis as long as ischium, merus and carpus combined, covered by sparse setae of varied length; propodus subtriangular, palm with a pair of proximal stout setae (grasping spines) and a row of 8 simple setae; dactylus with sparse and short setae, inner margin smooth with a teeth subdistally (Fig. 2C).
Gnathopod 2 inserted in the pereonite 2 at 2/5 from anterior margin (Fig. 1A); coxa vestigial; basis 1.3 times of pereonite 2 length, with a spiny projection near antero-distal corner; ischium rectangular; merus rounded; carpus triangular and provided with scarce simple setae; propodus oval, ratio between width: length = 0.57, inner margin provided with 1 stout setae proximally, 3 triangular projections medially and distally and numerous setae: few simple setae on the outer margin; dactylus shorter than palm and slightly curved with a row of setulae alongside the inner margin (Fig. 2D).
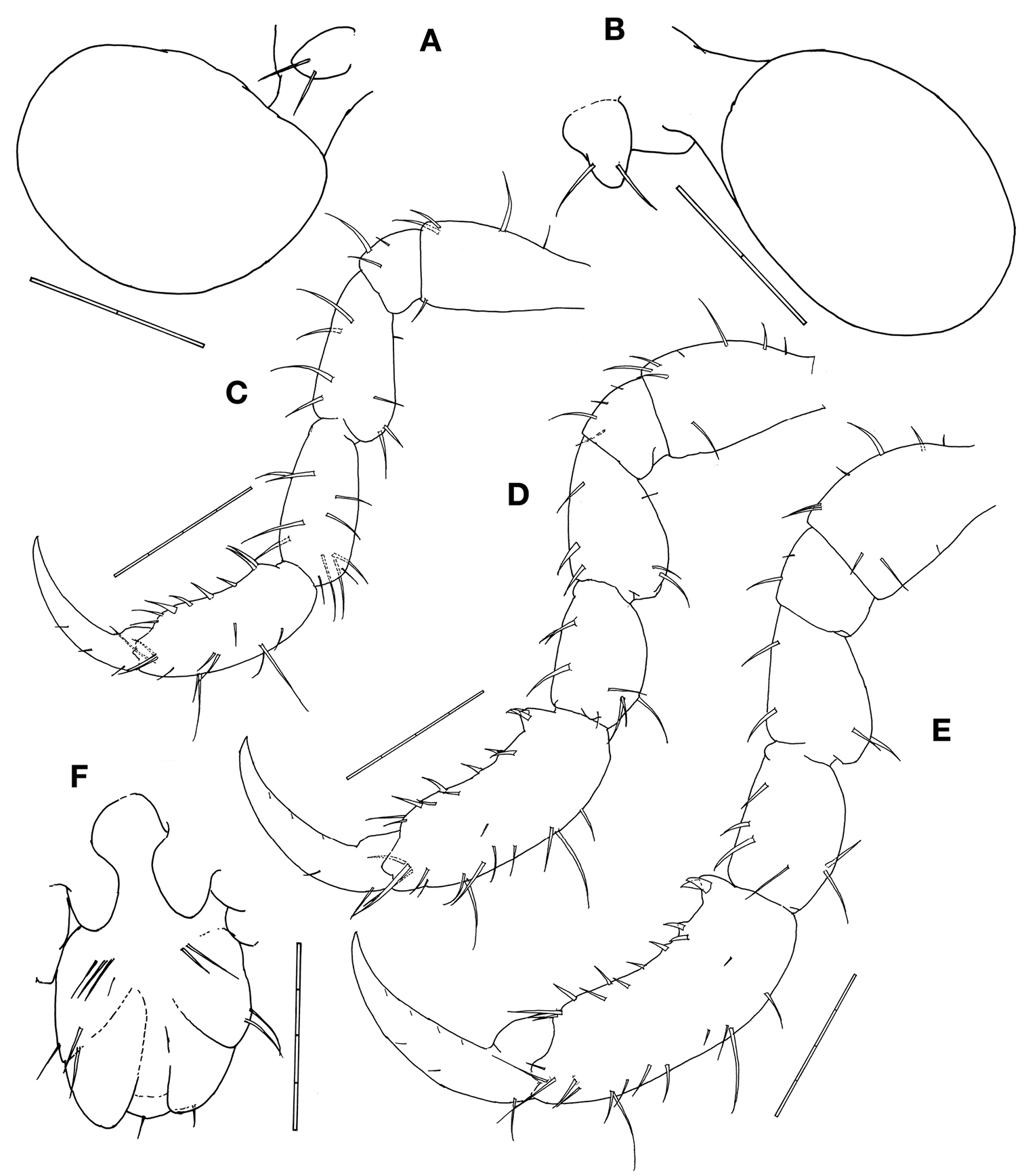
Gill 3length 2/5 of corresponding pereonite, elliptical (Fig. 1A), pereopod 3 tiny with 2 simple setae apically (Fig. 4A). Gill 4length 1/3 of corresponding pereonite, elliptical (Fig. 1A), pereopod 4 similar to pereopod 3 (Fig. 4B).
Pereopod 5 basis to carpus furnished with 3–10 setae of varied length; palm of propodus very slightly concave with 2 setae proximally and a row of 7 robust setae alongside; dactylus slightly curved (Fig. 4C). Pereopods 6 and 7 similar to pereopod 5 in feature but increasing in size (Figs. 4D, 4E).
Penes length about 2 times width (Fig. 4F).
Abdomen with a pair of lateral lobes and dorsal lobe with a pair of dorsal setae (Fig. 4F).
Female. Body length 3.1 mm (Fig. 1B). Pereonites 3 and 4 subequal and the longest, followed by pereonite 2. Clear suture between head and pereonite 1, head with 1 anteriorly curved mid-dorsal projection. Antenna 1 flagellum 7-articulate. Pereonite 1with 1 postero-dorsal projection. Pereonite 2with paired mid-dorsal projections, 1 postero-dorsal projection and paired antero-lateral projections. Pereonite 3with paired mid-dorsal projections, 1 postero-dorsal projection, paired mid-lateral projections and paired postero-lateral projections. Pereonite 4with paired mid-dorsal projections and paired mid-lateral projections. Pereonite 5with paired mid-dorsal projections. Gnathopod 2 propodus length 1.5 times width (Fig. 2E), with grasping spine proximally followed by a serrated margin; two smooth triangular projections medially.
In adult males and females including those collected by Quitete (Quitete, 1971a) in Pernambuco State, the number of articles in the flagellum of antenna 1 varies from 5 to 7 during growth. The size reduction of the mid-dorsal projections on pereonites 3 and 5 mentioned by this author was only found among specimens studied by her. Setal formula for terminal article of mandibular palp can be 1–5-1 or 1–6-1. The body spination is rather constant among individuals summing up 30 spines in males.
Itamaracá, Pernambuco State, Brazil.
Western South Atlantic. Brazil. Itamaracá, Pernambuco State; Olivença, Ilhéus, Bahia State; Vitória and Guarapari, Espírito Santo State (
Amongst thallii of the brown seaweed Sargassum sp. (
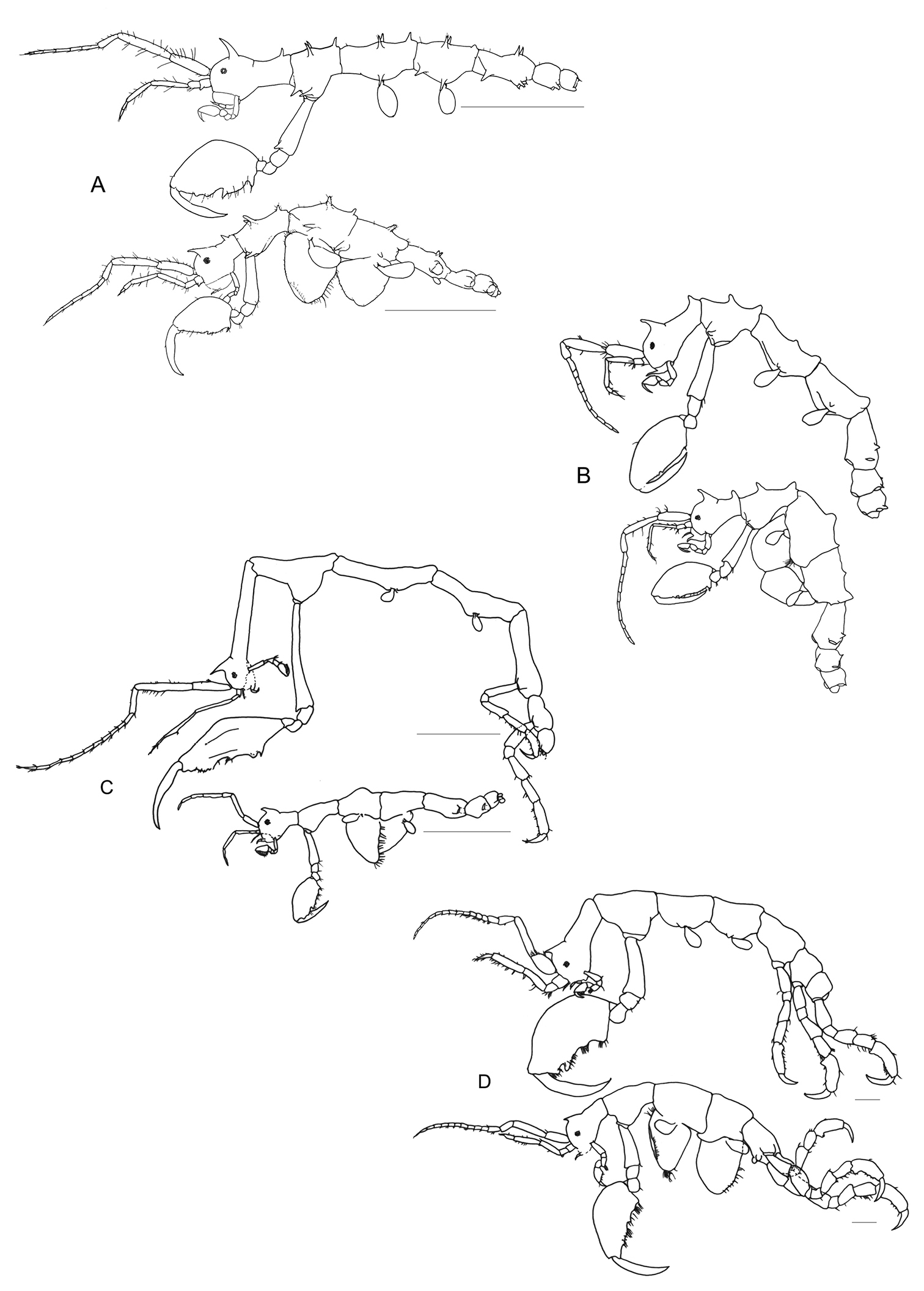
Pseudaeginella montoucheti (Quitete, 1971). A male, lateral view B female, lateral view. Scale bar: 1.0 mm.
Pseudaeginella montoucheti (Quitete, 1971). A–D male. A antenna 1 B antenna 2 C gnathopod 1 D gnathopod 2. E female gnathopod 2. Scale bars: A–E: 0.2 mm.
Pseudaeginella montoucheti (Quitete, 1971). Male. A maxilliped B left mandible C right mandible D maxilla 1 E maxilla 2 F upper lip G lower lip. Scale bars: A–G: 0.05 mm.
Pseudaeginella montoucheti (Quitete, 1971). Male. A pereopod 3 and gill 3 B pereopod 4 and gill 4 C pereopod 5 D pereopod 6 E pereopod 7 F abdomen (ventral view). Scale bars: A, B: 0.1 mm. C–F: 0.2 mm.
The above treatment of Fallotritella and Pseudaeginella performed by
The genus Fallotritella was established based on Fallotritella biscaynensis McCain, 1968 collected from Florida, U.S.A, Antigua & Barbuda and St. Lucia (
Pseudaeginella montoucheti (Quitete, 1971) is a tiny caprellidean that measures less than 3.5 mm in body length (see Fig. 1). Within this genus, Pseudaeginella montoucheti is the second spiniest species (total of 30 spines on the head and pereonites 1–7 of males) and only surpassed by Pseudaeginella sanctipauli that has a total of 33 spines on body surface. In the drawing of Pseudaeginella montoucheti from Pernambuco State performed by
Although restricted to the Atlantic coast of Brazil, the present study showed that Pseudaeginella montoucheti is distributed along more than 2, 600 km, from tropical (Itamaracá Island, Pernambuco State, 7°44'S, 34°49'W) to subtropical (municipalities of Barra do Sul, 26°27'S, 48°34'W and Bombinhas, 27°08'S, 48°28'W, Santa Catarina State) latitudes. Recently, Caprella dilatata Krøyer, 1843 was also reported showing wide distribution from Sao Paulo State, Brazil (
Pseudaeginella Mayer, 1890 is currently composed of 10 species: Pseudaeginella antiguae Barnard, 1932 from Antigua and Barbuda, Pseudaeginella biscaynensis (McCain, 1968) from Florida, U.S.A., Pseudaeginella campbellensis Guerra-García, 2003b from subantarctic islands of New Zealand, Pseudaeginella colombiensis Guerra-García, Krapp-Schickel & Müller, 2006 from Colombia, Pseudaeginella inae Krapp-Schickel & Guerra-García, 2005 from Indonesia, Pseudaeginella montoucheti (Quitete, 1971) from Brazil, Pseudaeginella polynesica (Müller, 1990) from Bora Bora and Moorea, French Polynesia, Pseudaeginella sanctipauli Laubitz, 1995 from St. Paul and Amsterdam Islands, France, Pseudaeginella tristanensis (Stebbing, 1888) from Tristan da Cunha, and Pseudaeginella vaderi Guerra-García, 2004 from Australia.
Of 10 species of Pseudaeginella, the closest species to Pseudaeginella montoucheti can be considered Pseudaeginella sanctipauli that wasdescribed from St. Paul and Amsterdam Islands, South Indian Ocean (
A key to the species of Pseudaeginella is presented below; it was mainly based on the characteristics of body somites because these can be observed without dissections of mouthparts.
Key to species of the genus Pseudaeginella
| 1a | Antenna 1 longer than half of body length | 2 |
| 1b | Antenna 1 equal or shorter than half of body length | 5 |
| 2a | Basis of gnathopod 2 longer than propodus length | Pseudaeginella sanctipauli (Fig. 5A) |
| 2b | Basis of gnathopod 2 shorter than propodus length | 3 |
| 3a | Basis of gnathopod 2 approximately the length of pereonite 2 | Pseudaeginella biscaynensis (Fig. 5B) |
| 3b | Basis of gnathopod 2 longer than pereonite 2 length | 4 |
| 4a | Pereonites 2, 3, 4 and 5 with lateral projections near the insertion of gnathopod 2, gills and pereopods 5 | Pseudaeginella colombiensis (Fig. 5C) |
| 4b | Pereonites 2, 3, 4 and 5 without lateral projections | Pseudaeginella polynesica(Fig. 5D) |
| 5a | Pereonites with dorsal projections | 6 |
| 5b | Pereonites without any dorsal projections | 8 |
| 6a | Basis of gnathopod 2 provided with a rounded projection proximally | Pseudaeginella campbellensis (Fig. 5E) |
| 6b | Basis of gnathopod 2 without any projection | 7 |
| 7a | Pereonites 4 and 5 with a paired antero-lateral projections, body somites with a total of more than 30 projections | Pseudaeginella montoucheti (Fig. 6A) |
| 7b | Pereonites 4 and 5 without any antero-lateral projections, body somites with a total of less than 30 projections | Pseudaeginella tristanensis (Fig. 6B) |
| 8a | Basal article of antenna 2 peduncle with a distal projection, well marked suture between head and pereonite 1 | Pseudaeginella vaderi (Fig. 6C) |
| 8b | Basal article of antenna 2 peduncle without any projection, discrete suture between head and pereonite 1 | Pseudaeginella inae (Fig. 6D) |
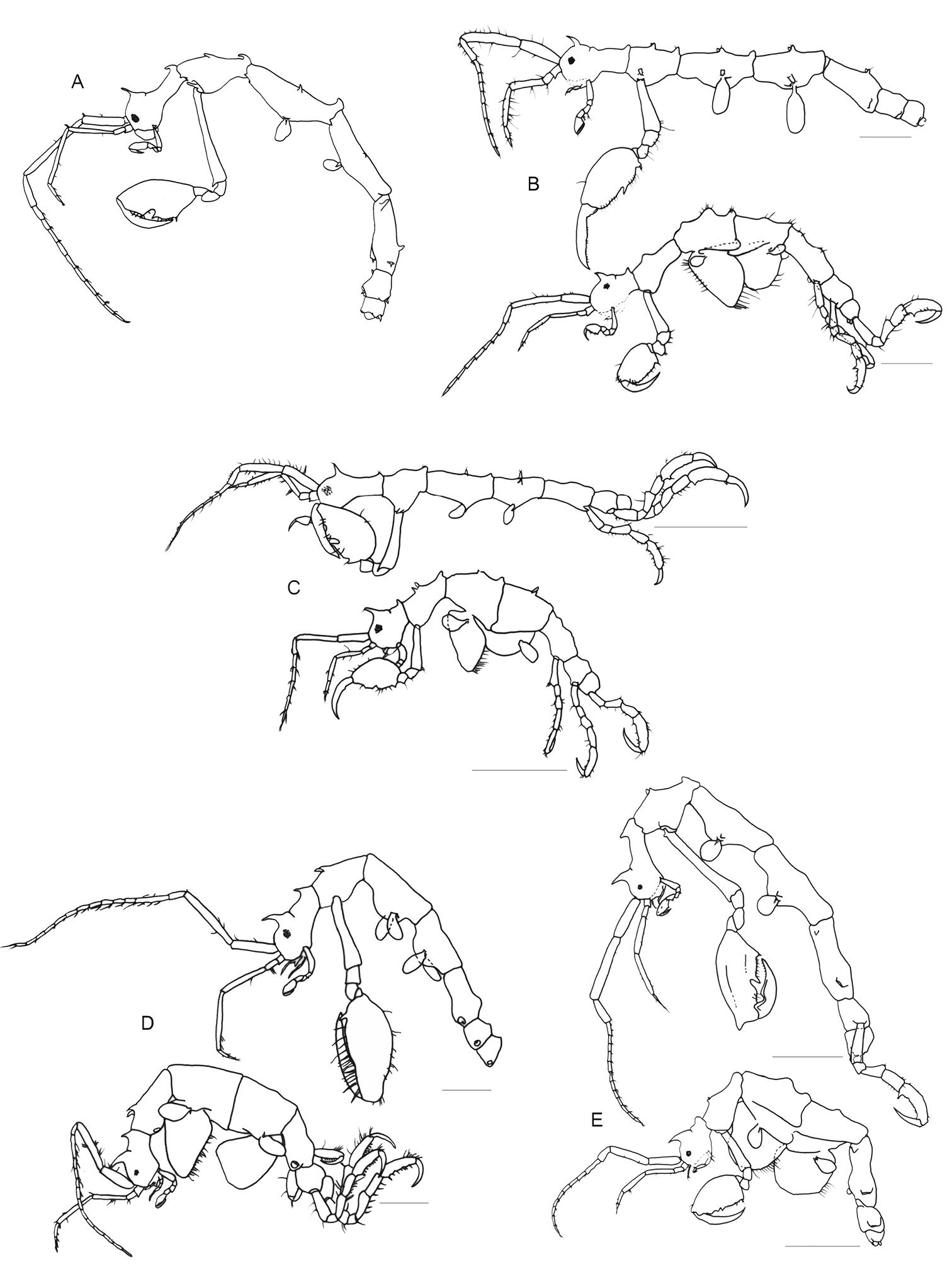
Pseudaeginella spp. A Pseudaeginella sanctipauli Laubitz, 1995 (Redraw from
Pseudaeginella spp. A Pseudaeginella montoucheti (Quitete, 1971). Scale bar 1 mm B Pseudaeginella tristanensis (Stebbing, 1888) (Redraw from
We are grateful to Prof. Dr. Danúncia Urban for the critical reading and to Prof. Dr. Luís Amilton Foerster for language revision, both from Federal University of Paraná. All biological sample collecting of the present study complies with the current laws of Paraná State and Brazilian Federal Government, and was conducted with the permission of the Brazilian Institute of Environment and Renewable Natural Resources–IBAMA–of Paraná and Santa Catarina State (Authorization 16247–1 DIFAP/IBAMA, Data 15/08/2008 and Authorization 23180–1 DIFAP/IBAMA, Data 29/04/2010). This is Contribution N˚1820 of Department of Zoology, Federal University of Paraná.