(C) 2012 Takafumi Nakano. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new quadrannulate species of Orobdella, Orobdella ketagalan sp. n., from Taipei, Taiwan, is described. This is the first record of Orobdella and the family Orobdellidae from Taiwan. This new species possesses small, paired sperm duct bulbs in the male reproductive system. In addition to these bulbs, the following combination of characters distinguishes this new species from other quadrannulate species: somite IV uniannulate, male gonopore at XI b6, female gonopore at XIII a1, 1/2 + 4 + 1/2 between gonopores, simple tubular gastroporal duct, lacking epididymides, and undeveloped atrial cornua. Phylogenetic analyses using nuclear 18S rDNA and histone H3 as well as mitochondrial COI, 12S rDNA, tRNAVal, and 16S rDNA markers showed that Orobdella ketagalan is related to the two Ryukyu Archipelago species Orobdella dolichopharynx Nakano, 2011 and Orobdella shimadae Nakano, 2011.
Hirudinida, Orobdellidae, Orobdella, new species, first record, gastroporous, Taiwan
Species of the genus Orobdella Oka, 1895 are large annelids that feed on earthworms. They are usually 10–20 cm in length (except for Orobdella koikei Nakano, 2012, approx. 5 cm) and they inhabit the banks of mountain streams in East Asia (
Taxonomic and inventory studies on Orobdella have progressed recently, and this genus now includes ten species (
All of the known Orobdella species have been described based on specimens collected from Japan, and eight of the ten species have been reported only from Japanese islands (
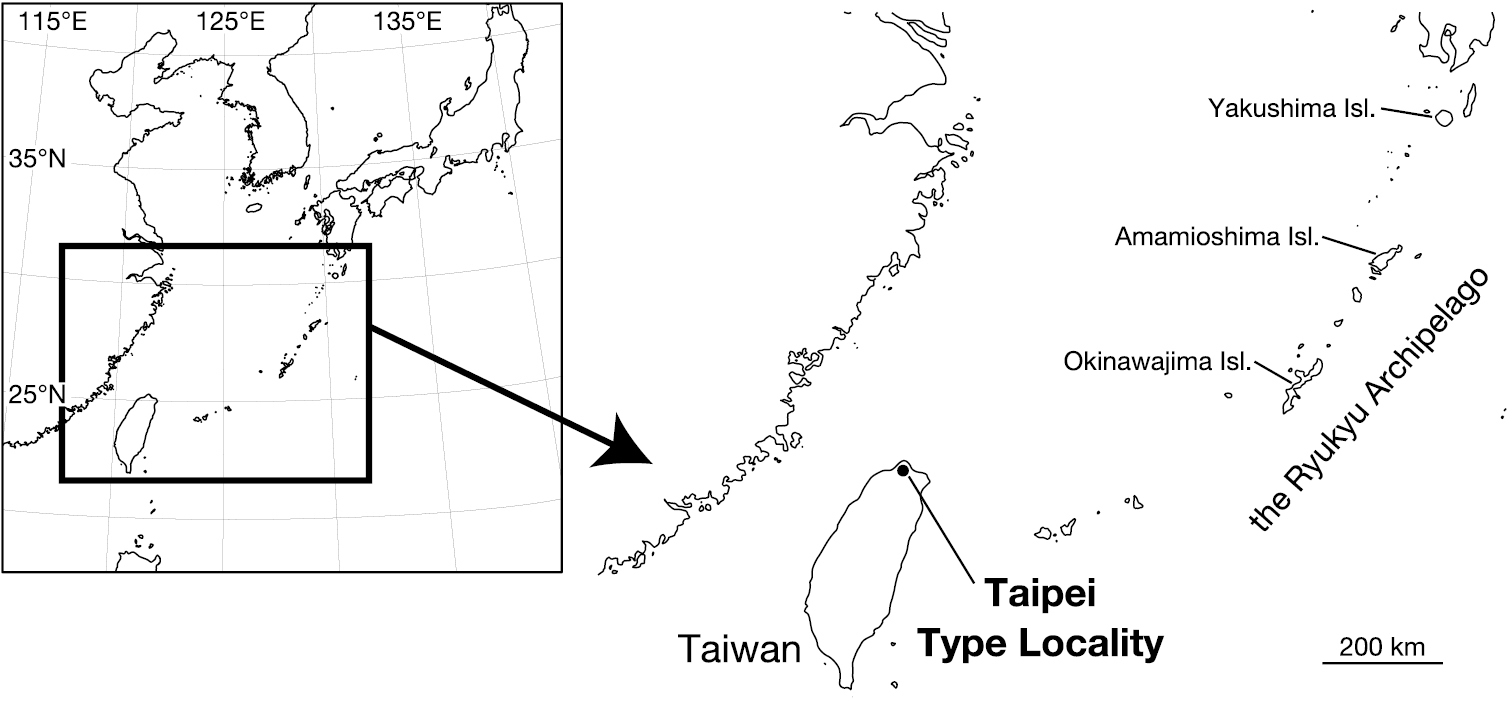
Leeches were collected from Taipei, Taiwan (Fig. 1). Botryoidal tissue was taken from specimens, which were fixed in ethanol, for DNA extraction. All of the specimens were preserved in 70% ethanol. Two measurements were taken: body length (BL) from the anterior margin of the oral sucker to the posterior margin of the caudal sucker, and maximum body width (BW). Examination, dissection, and drawings of the specimens were accomplished under a stereoscopic microscope with a drawing tube (Leica M125). The specimens have been deposited in the Zoological Collection of Kyoto University (KUZ).
Map showing the collection localities in this study.
We used the numbering convention of
The extraction of genomic DNA followed (
PCR and cycle sequencing (CS) primers used in this study. Sources: a
| Gene | Primer name | Reaction | Primer sequence (5’ → 3’) |
|---|---|---|---|
| 18S | |||
| 1 | Aa | PCR & CS | AACCTGGTTGATCCTGCCAGT |
| La | PCR & CS | CCAACTACGAGCTTTTTAACTG | |
| 2 | Ca | PCR & CS | CGGTAATTCCAGCTCCAATAG |
| Ya | PCR & CS | CAGACAAATCGCTCCACCAAC | |
| 3 | Oa | PCR & CS | AAGGGCACCACCAGGAGTGGAG |
| Ba | PCR & CS | TGATCCTTCCGCAGGTTCACCT | |
| Histone H3 | |||
| H3aFb | PCR & CS | ATGGCTCGTACCAAGCAGACVGC | |
| H3bRb | PCR & CS | ATATCCTTRGGCATRATRGTGAC | |
| COI | |||
| 1 | LCO1490c | PCR & CS | GGTCAACAAATCATAAAGATATTGG |
| HCO2198c | CS | TAAACTTCAGGGTGACCAAAAAATCA | |
| 2 | LCO-ind | CS | TCCAGAACGTATTCCATTATTTG |
| HCO-outd | PCR & CS | TCTGGGTAGTCAGAATATCG | |
| 12S–16S | |||
| 12SA-ind | PCR & CS | AATTAAAACAAGGATTAGATACCC | |
| 12SB-outd | PCR & CS | AACCCATAATGCAAAAGGTAC | |
Samples used for the phylogenetic analyses. Information on vouchers, collection localities, and GenBank accession numbers is provided.UNIMAS, the Universiti Malaysia Sarawak. Sources: a
| Species | Voucher | 18S | Histone H3 | COI | 12S–16S |
|---|---|---|---|---|---|
| Orobdella ketagalan sp. n. | KUZ Z208 Holotype | AB704785 | AB704786 | AB704787 | AB704788 |
| Orobdella ketagalan sp. n. | KUZ Z209 Paratype | AB704789 | AB704790 | ||
| Orobdella ketagalan sp. n. | KUZ Z210 Paratype | AB704791 | AB704792 | ||
| Orobdella ketagalan sp. n. | KUZ Z211 Paratype | AB704793 | AB704794 | ||
| Orobdella esulcata | KUZ Z29 Holotype | AB663655c | AB698873b | AB679664a | AB679665a |
| Orobdella dolichopharynx | KUZ Z120 Holotype | AB663665c | AB698876b | AB679680a | AB679681a |
| Orobdella ijimai | KUZ Z110 Topotype | AB663659c | AB698877b | AB679672a | AB679673a |
| Orobdella kawakatsuorum | KUZ Z167 Topotype | AB663661c | AB698878b | AB679704a | AB679705a |
| Orobdella koikei | KUZ Z156 Holotype | AB698883b | AB698882b | AB679688a | AB679689a |
| Orobdella mononoke | KUZ Z224 Holotype | AB698868b | AB698869b | AB698866b | AB698867b |
| Orobdella octonaria | KUZ Z181 Topotype | AB698870b | AB698871b | AB679708a | AB679709a |
| Orobdella shimadae | KUZ Z128 Holotype | AB663663c | AB698875b | AB679676a | AB679677a |
| Orobdella tsushimensis | KUZ Z134 Holotype | AB663653c | AB698872b | AB679662a | AB679663a |
| Orobdella whitmani | KUZ Z45 Topotype | AB663657c | AB698874b | AB679668a | AB679669a |
| Erpobdella japonica | KUZ Z178 | AB663648c | AB698879b | AB679654a | AB679655a |
| Gastrostomobdella monticola | UNIMAS/A3/BH01/10 | AB663649c | AB698880b | AB679656a | AB679657a |
| Mimobdella japonica | KUZ Z179 | AB663650c | AB698881b | AB679658a | AB679659a |
H3 and COI sequences were aligned by eye because there were no indels. Nuclear 18S and mitochondrial 12S–16S sequences were aligned using MAFFT X-INS-I (
Phylogenetic trees were constructed using maximum likelihood (ML) and Bayesian inference (BI). ML phylogenies were calculated using TREEFINDER v October 2008 (
Nodes with bootstrap (BS) values higher than 70% were considered sufficiently resolved (
urn:lsid:zoobank.org:act:5F5BABE8-BD26-4FC7-9593-F73E62E26122
Genus Orobdella Oka, 1895
urn:lsid:zoobank.org:act:FA8333ED-8C17-41FD-AFC1-62A4F98D4AC1
urn:lsid:zoobank.org:act:AFF291DF-E13F-46A3-A965-14B92E23F520
http://species-id.net/wiki/Orobdella_ketagalan
Figs 2–4Somite IV uniannulate, somites VIII–XXV quadrannulate. Pharynx reaching to posterior of XIV to anterior of XV. Gastropore conspicuous at XIII a1. Gastroporal duct simple, tubular. Male gonopore at XI b6, female gonopore at XIII a1, gonopores separated by 1/2 + 4 + 1/2 annuli. Small paired sperm duct bulbs in XV. Epididymis absent. Atrial cornua, coniform, undeveloped.
Holotype. KUZ Z208, mature specimen of 70.9 mm length, dissected, collected from Yangmingshan National Park (alt. 779 m, 25°11'07"N, 121°31'10"E), Taipei City, Taiwan, by Win-Je Chi on March 24, 2011. Paratypes (a total of five specimens collected from Taiwan in 2005–2011): KUZ Z197, from Jinsan Township, Taipei County (alt. 739 m, 25°11'01"N, 121°30'54"E), on March 18, 2005; KUZ Z207, from the type locality (alt. 776 m, 25°09'49"N, 121°33'10"E) by Chi-Lun Lee and Win-Je Chi on July 30, 2010; KUZ Z209 (alt. 779 m, 25°11'07"N, 121°31'10"E), dissected, KUZ Z210 (alt. 600 m, 25°11'11"N, 121°31'10"E), dissected, from the type locality by Win-Je Chi on March 24, 2011; and KUZ Z211 from the type locality (alt. 737 m, 25°10'55"N, 121°30'50"E) by Win-Je Chi on April 24, 2011.
The specific name is taken from the native Taiwanese tribe Ketagalan. The type locality of this new species is in an area settled by this aboriginal tribe. The specific name is a native word, not a Latin or Latinized word.
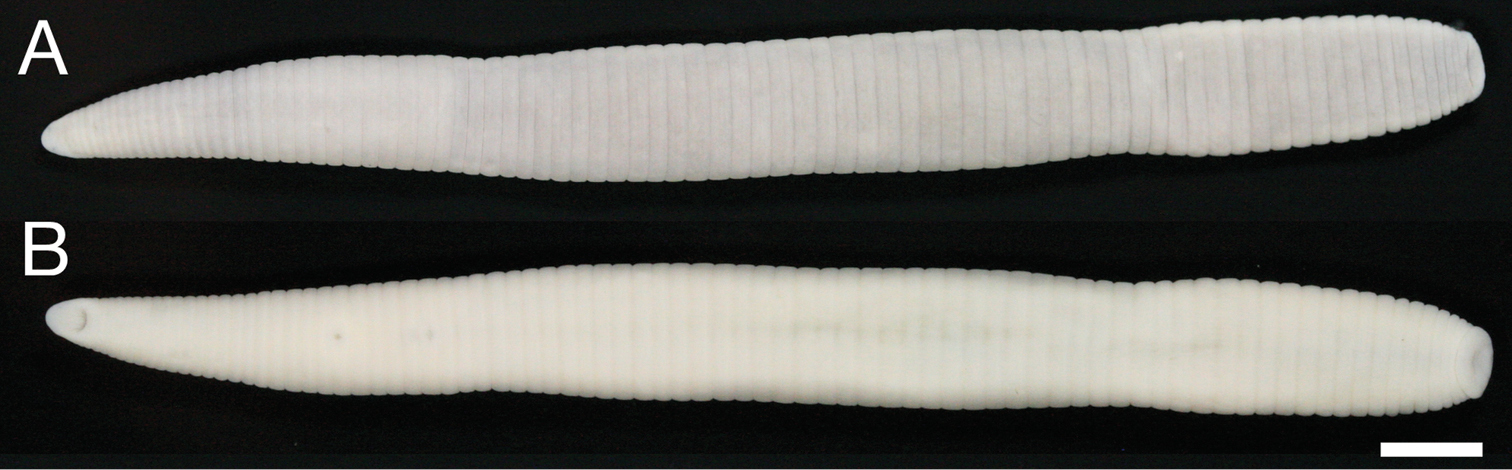
Body firm, muscular, elongated, gaining regularly in width in caudal direction, dorso-ventral depressed, sides nearly parallel from mid-length to point just anterior to caudal sucker, BL 70.9 mm, BW 6.4 mm (Fig. 2). Caudal sucker ventral, oval, diameter smaller than BW (Figs 2B, 3D). Color faded in preservative (Fig. 2).
Orobdella ketagalan sp. n., holotype, KUZ Z208. A Dorsal and B ventral views. Scale bar, 5 mm.
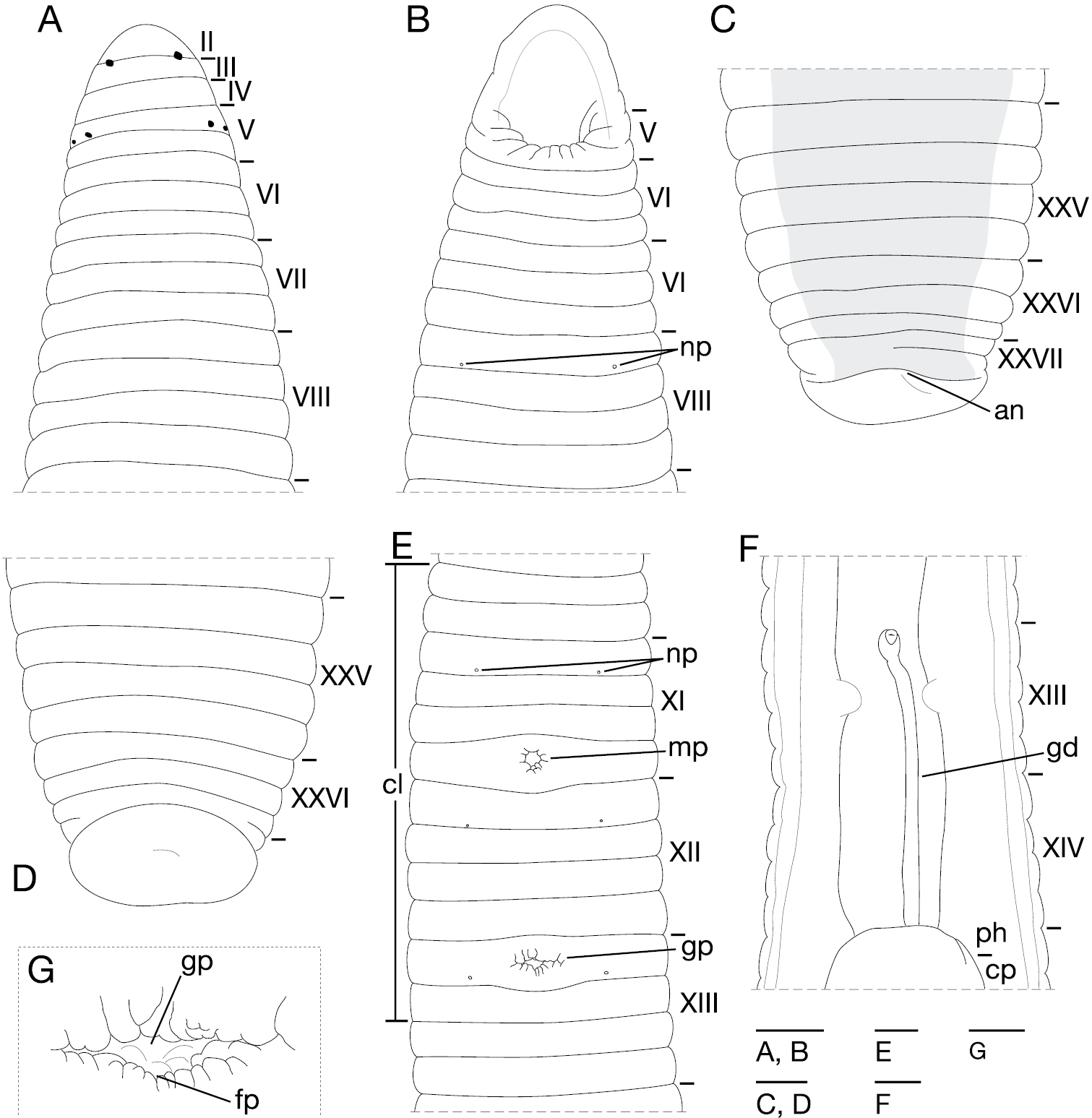
Somite I completely merged with prostomium (Fig. 3A). Somite II uniannulate, not separated from I (Fig. 3A). Somites III and IV uniannulate (Fig. 3A). Somite V biannulate, (a1 + a2) = a3, a3 forming posterior margin of oral sucker (Fig. 3A, B). Somites VI and VII triannulate, a1 = a2 = a3 (Fig. 3A, B). Somites VIII–XXV quadrannulate, a1 = a2 = b5 = b6 (Fig. 3A–E); b5 of X being first annulus on clitellum, a2 of XIII being last annulus of clitellum (Fig. 3E). Somite XXVI triannulate, a1 > a2 > a3, a3 being last complete annulus on venter (Fig. 3C, D). Somite XXVII incomplete uniannulate with slight furrow (Fig. 3C); anus behind it with no post-anal annulus (Fig. 3C).
Orobdella ketagalan sp. n., holotype, KUZ Z208. A Dorsal and B ventral views of somites I–VIII C dorsal and D ventral views of somites XXV–XXVII and caudal sucker E ventral view of somites X b5–XIII F ventral view of gastroporal duct; and G ventral view of gastropore and female gonopore. Scale bars, 1 mm (A–F) and 0.25 mm (G). Abbreviations: an, anus; cl, clitellum; cp, crop; fp, female gonopore; gd, gastroporal duct; gp, gastropore; mp, male gonopore; np, nephridiopore; and ph, pharynx.
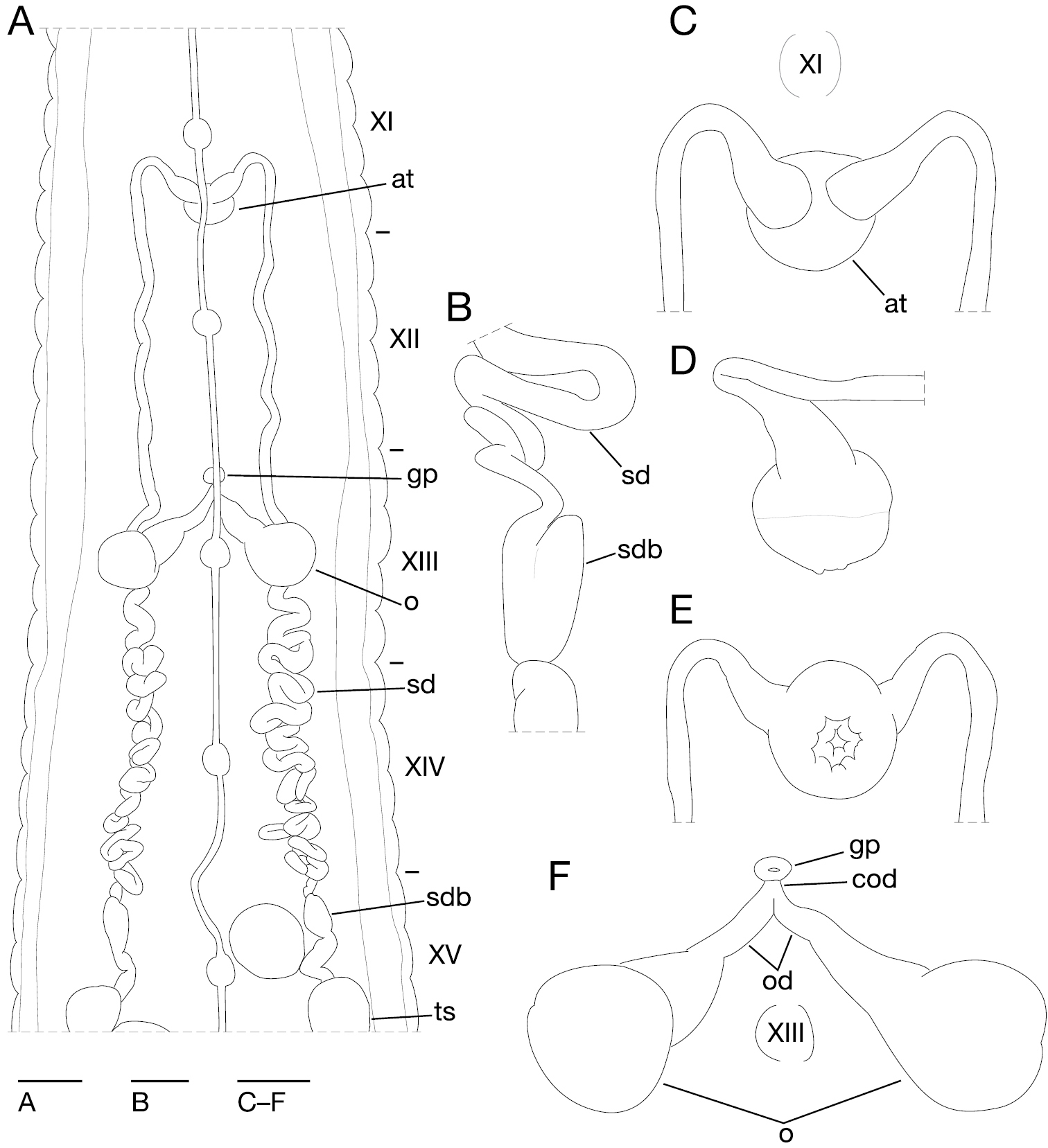
Anterior ganglionic mass in VI a2 and a3. Ganglia VIII–XXI in a2 of each somite (Fig. 4A). Ganglion XIII in a2 and b5 (Fig. 4A). Ganglia XIV–XXIII in a2 of each somite (Fig. 4A). Ganglia XXIV and XXV in a1 and a2 of each somite. Ganglion XXVI in b6 of somite XXV. Posterior ganglionic mass in XXVI a1–a3.
Orobdella ketagalan sp. n., holotype, KUZ Z208. A Dorsal view of reproductive system including ventral nervous system B lateral view of bulb of right sperm duct C dorsal D lateral, and E ventral views of male atrium: C including position of ganglion XI; and F dorsal view of female reproductive system including position of ganglion III. Scale bars, 1 mm (A), 0.5 mm (C–F), and 0.25 mm (B). Abbreviations: at, atrium; cod, common oviduct; gp, gastropore; o, ovisac; od, oviduct; sd, sperm duct; sdb, sperm duct bulb; and ts, testisacs.
Eyes, three pairs, first pair dorsally in furrow of II/III, second and third pairs dorsolaterally on posterior margin of V (a1 + a2) (Fig. 3A). Nephridiopores, 17 pairs, ventrally at posterior margin of a1 of each somite of VIII–XXIV (Fig. 3B, E). Papillae numerous, minute, hardly visible, one row on every annulus.
Pharynx agnathous, euthylaematous, reaching to XV a1 (Fig. 3F). Crop tubular, acecate, in XV a1 to XXI a2. Gastropore conspicuous, ventral, located middle of XIII a1 (Fig. 3E, G). Gastroporal duct narrow, simple tubular, joining with crop in XIV/XV (Fig. 3F). Intestine tubular, acecate, in XXI a2 to XXIV b5/b6. Rectum tubular, thin-walled.
Male gonopore located at middle of XI b6 (Fig. 3E). Female gonopore at middle of XIII a1, inconspicuous, located behind gastropore (Fig. 3E, G). Gonopores separated by 1/2 + 4 + 1/2 annuli (Fig. 3E). Testisacs multiple, one or two testisacs on each side in each annulus, in XV a2 to XXV b5 (Fig. 4A). Sperm ducts in XI b5 to XV a2, coiled in XIII b5 to XV a1 (Fig. 4A): small paired sperm duct bulbs in XV a1 (Fig. 4A, B). Epididymides absent. Ejaculatory bulbs absent. Paired atrial cornua in XI b5 and b6, undeveloped, coniform (Fig. 4A, C). Atrium body short, muscular, globular in XI b5 and b6 (Fig. 4A, C–E). Penis sheath and penis absent. Ovisacs, one pair, thin-walled, globular, in XIII a2 and b5 (Fig. 4A, F). Oviducts thin-walled, right oviduct crossing ventrally beneath nerve cord, both oviducts converging into common oviduct in XIII a1/a2 (Fig. 4A, F). Common oviduct thin-walled, very short, directly ascending to female gonopore (Fig. 4F).
Maximum BL 111.7 mm, maximum BW 10.3 mm (KUZ Z210). In life, dorsal surface grayish, slightly darker in first third of dorsum, ventral surface whitish. Somite XXVI dorsally quadrannulate, ventrally triannulate (KUZ Z197, Z207, Z211) or quadrannulate (KUZ Z210). Somite XXVII incomplete biannulate. Pharynx reaching to XIV a1/b5–b6. Crop reaching to XXI a2/b5–XXI/XXII. Gastroporal duct joining with crop in XIV b5–XIV b5/b6. Intestine reaching to XXIV a2/b5–XXV a2. Testisacs in XV a2–XVI b6 to XXIII a1–XXV a2. Paired sperm duct bulbs in XV a1 and a2 (KUZ Z209), in XV b5 (KUZ Z210). Right or left oviducts crossing ventrally beneath nerve cord.
Known from Yangmingshan National Park and adjacent areas in northern Taipei City, Taiwan (Fig. 1).
Orobdella ketagalan differs from the five other quadrannulate Orobdella species (i.e., Orobdella esulcata Nakano, 2010, Orobdella kawakatsuorum Richardson, 1975, Orobdella koikei, Orobdella tsushimensis, and Orobdella whitmani) in the following combination of characteristics (Table 3): IV uniannulate, gonopores separated by 1/2 + 4 + 1/2 annuli, XXV quadrannulate, gastroporal duct simple and tubular, paired sperm duct bulbs in XV, epididymides absent, and atrial cornua undeveloped. Because Orobdella ketagalan possesses quadrannulate mid-body somites, this new species is easily distinguishable from the four sexannulate species (i.e., Orobdella dolichopharynx Nakano, 2011, Orobdella ijimai Oka, 1895, Orobdella mononoke Nakano, 2012, and Orobdella shimadae Nakano, 2011) and one octannulate species, Orobdella octonaria Oka, 1895.
Comparison of morphological characters between Orobdella ketagalan sp. n. and five quadrannulate congeneric species.
| Character | Orobdella ketagalan sp. n. | Orobdella esulcata | Orobdella kawakatsuorum | Orobdella koikei | Orobdella tsushimensis | Orobdella whitmani |
|---|---|---|---|---|---|---|
| Annulation of IV | uniannulate | uniannulate | biannulate | uniannulate | uniannulate | uni- or biannulate |
| Number of annuli between gonopores | 1/2 + 4 + 1/2 | 2/3 + 4 + 1/3 | 6 | 1/2 + 4 + 1/2 | 1/2 + 5 | 1/2 + 4 +1/2 |
| Annulation of XXV | quadrannulate | quadrannulate | quadrannulate | triannulate | quadrannulate | quadrannulate |
| Gastroporal duct | simple tubular | tubular, but bulbous at junction with gastropore | simple tubular | tubular, but bulbous at junctions with gastropore and crop | bottle-shaped | bulbiform |
| Paired sperm duct bulbs | in XV | absent | absent | absent | absent | absent |
| Epididymides | absent | XVI to XX | XVI to XVII | XVII to XIX | XVI to XIX | XVI to XVIII |
| Atrial cornua | undeveloped | ovate | undeveloped | ovate | coniform | ovate |
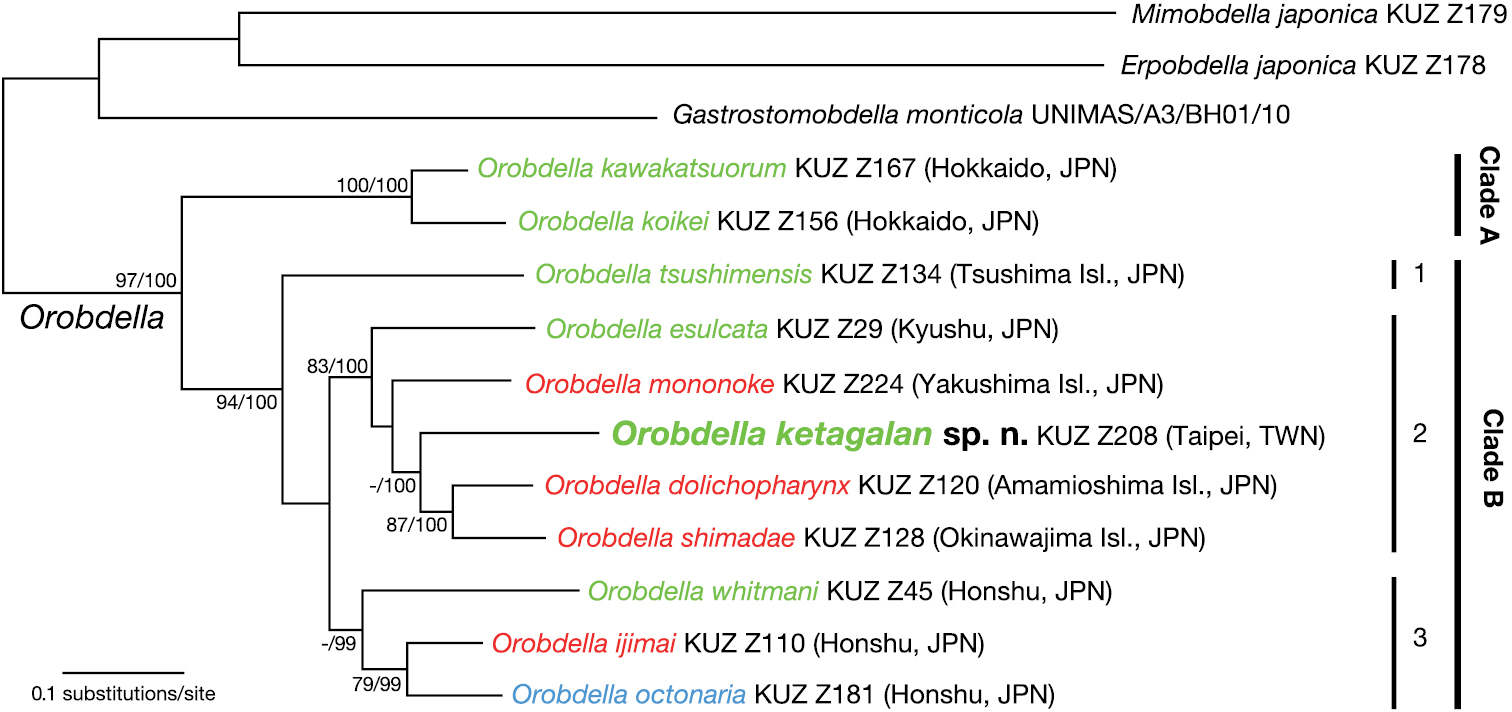
The BI tree (Fig. 5) was nearly identical to the ML tree with ln L = -12357.61 (not shown). Monophyly of the genus Orobdella was well supported (BS = 97%, BPP = 100%). Orobdella then divided into two clades: clade A (BS = 100%, BPP = 100%) consisted of two species from Hokkaido, Japan, Orobdella kawakatsuorum and Orobdella koikei; and clade B (BS = 94%, BPP = 100%) included the other nine Orobdella species. Clade B was split into three subclades: subclade B1 included only Orobdella tsushimensis (from Tsushima Island, Japan); subclade B2 (BS = 83%, BPP = 100%) included Orobdella esulcata (from Kyushu, Japan), Orobdella mononoke (from Yakushima Island, Japan), Orobdella dolichopharynx (from Amamioshima Island, Japan), Orobdella shimadae (from Okinawajima Island, Japan), and Orobdella ketagalan (from Taipei, Taiwan); and subclade B3 (BS = 69%, BPP = 99%) consisted of three species (from Honshu, Japan), Orobdella whitmani, Orobdella ijimai, and Orobdella octonaria. Subclades B2 and B3 formed a monophyletic clade in both analyses, but with low support (BS = 67%, BPP = 89%).
The BI tree of 3790 bp of nuclear 18S rDNA and histone H3, and mitochondrial COI, 12S rDNA, tRNAVal, 16S rDNA. A species name in green indicates a quadrannulate species; in red, sexannulate; and in blue, octannulate. The numbers associated with the nodes represent the bootstrap values for ML (BS) and Bayesian posterior probabilities (BPPs). BSs higher than 70 % and/or BPPs higher than 95 % are indicated. Abbreviations: JPN, Japan; and TWN, Taiwan.
In subclade B2, three species from the Ryukyu Archipelago, Orobdella mononoke, Orobdella dolichopharynx, and Orobdella shimadae, and the Taiwanese Orobdella ketagalan formed a monophyletic clade, but this clade was also not sufficiently supported (BS = 55%, BPP = 82%). Monophyly of Orobdella ketagalan, Orobdella dolichopharynx, and Orobdella shimadae was supported in the BI analyses (BPP = 100%), but was not recovered in the ML analyses (BS = 46%). Monophyly of Orobdella dolichopharynx and Orobdella shimadae was confirmed (BS = 87%, BPP = 100%).
DiscussionThe phylogenies obtained in this study are nearly identical to those obtained in other phylogenetic analyses of the genus Orobdella (
Orobdella ketagalan possesses small, paired sperm duct bulbs in XV (Fig. 4A, B). Such small bulbs have never before been reported in Orobdella. Hence, small sperm duct bulbs could be considered an apomorphy of the Taiwanese Orobdella ketagalan. Orobdella species generally possess eipididymides in their male reproductive systems (
This is the first record of the genus Orobdella from Taiwan. Moreover, we collected several other specimens that appear to be undescribed species of Orobdella (Nakano and Lai, unpublished observation). Further faunal and systematic studies will reveal the species diversity of Taiwanese Orobdella and further elucidate the biogeographical and evolutionary history of these macrophagous leeches.
Key to the known species of the genus Orobdella| 1 | Mid-body somites more than quadrannulate | 2 |
| – | Mid-body somites quadrannulate | 6 |
| 2 | Mid-body somites sexannulate | 3 |
| – | Mid-body somites octannulate | Orobdella octonaria Oka, 1895 |
| 3 | Pharynx reaching to XIV | 4 |
| – | Pharynx reaching to XVI | 5 |
| 4 | Gonopores separated by 1/2 + 7 + 1/2 annuli | Orobdella ijimai Oka, 1895 |
| – | Gonopores separated by 8 + 1/2 annuli | Orobdella mononoke Nakano, 2012 |
| 5 | Gonopores separated by 8 annuli | Orobdella dolichopharynx Nakano, 2011 |
| – | Gonopores separated by 9 annuli | Orobdella shimadae Nakano, 2011 |
| 6 | Color yellowish | 7 |
| – | Color grayish blue or brown | 9 |
| 7 | Gonopores separated by 1/2 + 4 + 1/2 annuli | 8 |
| – | Gonopores separated by 1/2 + 5 annuli, gastroporal duct bottle-shaped | Orobdella tsushimensis Nakano, 2011 |
| 8 | Gastroporal duct bulbiform, epididymides in XVI to XVIII | Orobdella whitmani Oka, 1895 |
| – | Gastroporal duct simple tubular, epididymides absent, small paired sperm duct bulbs in XV | Orobdella ketagalan sp. n. |
| 9 | Color grayish blue | 10 |
| – | Color brown, gonopores separated by 1/2 + 4 + 1/2 annuli | Orobdella koikei Nakano, 2012 |
| 10 | Gonopores separated by 2/3 + 4 + 1/3, gastroporal duct simple tubular but bulbous at junction with gastropore | Orobdella esulcata Nakano, 2010 |
| – | Gonopores separated by 6 annuli, gastroporal duct simple tubular | Orobdella kawakatsuorum Richardson, 1975 |
The authors are grateful to Professor Tsutomu Hikida (Kyoto University; KU) for his helpful comments and suggestions to improve this manuscript. We are also grateful to Win-Je Chi (National Taiwan University; NTU) and Chi-Lun Lee (NTU) for providing type specimens of the new species, to two anonymous reviewers and Dr Fredric R. Govedich (Southern Utah University) for their constructive comments on this manuscript, and to Hiroshi Noda (KU) and Eri Kawaguchi (KU) for their technical support. This study was financially supported in part by a Grant-in-Aid for Biodiversity and Evolutionary Research of Global COE (A06) from the Ministry of Education, Culture, Sports Science and Technology (MEXT), Japan, to Kyoto University.