(C) 2012 Ricardo Pérez-de la Fuente. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The Albian amber from Spain presently harbors the greatest number and diversity of amber adult fossil snakeflies (Raphidioptera). Within Baissopteridae, Baissoptera? cretaceoelectra sp. n., from the Peñacerrada I outcrop (Moraza, Burgos), is the first amber inclusion belonging to the family and described from western Eurasia, thus substantially expanding the paleogeographical range of the family formerly known from the Cretaceous of Brazil and eastern Asia. Within the family Mesoraphidiidae, Necroraphidia arcuata gen. et sp. n. and Amarantoraphidia ventolina gen. et sp. n. are described from the El Soplao outcrop (Rábago, Cantabria), whereas Styporaphidia? hispanica sp. n. and Alavaraphidia imperterrita gen. et sp. n. are describedfrom Peñacerrada I. In addition, three morphospecies are recognized from fragmentary remains. The following combinations are restored: Yanoraphidia gaoi Ren, 1995, stat. rest., Mesoraphidia durlstonensis Jepson, Coram and Jarzembowski, 2009, stat. rest., and Mesoraphidia heteroneura Ren, 1997, stat. rest. The singularity of this rich paleodiversity could be due to the paleogeographic isolation of the Iberian territory and also the prevalence of wildfires during the Cretaceous.
Holometabola, taxonomy, paleontology, paleogeography, Mesozoic, Albian
Raphidioptera (snakeflies) are regarded as one of the most primitive lineages of holometabolous insects, their fossil record dating back to the Early Jurassic (
Raphidioptera currently comprises six families, i.e., four extinct families, Mesoraphidiidae Martynov, 1925 (Late Jurassic – Late Cretaceous), Baissopteridae Martynova, 1961 (Late Jurassic – Early Cretaceous), Priscaenigmatidae Engel, 2002 (Early Jurassic; classified into its own suborder and considered the most primitive raphidiopterans), and Metaraphidiidae Bechly and Wolf-Schwenninger, 2011 (Early Jurassic); and two extant families that date back to the Eocene, Raphidiidae Latreille, 1810 and Inocelliidae Navás, 1913 (
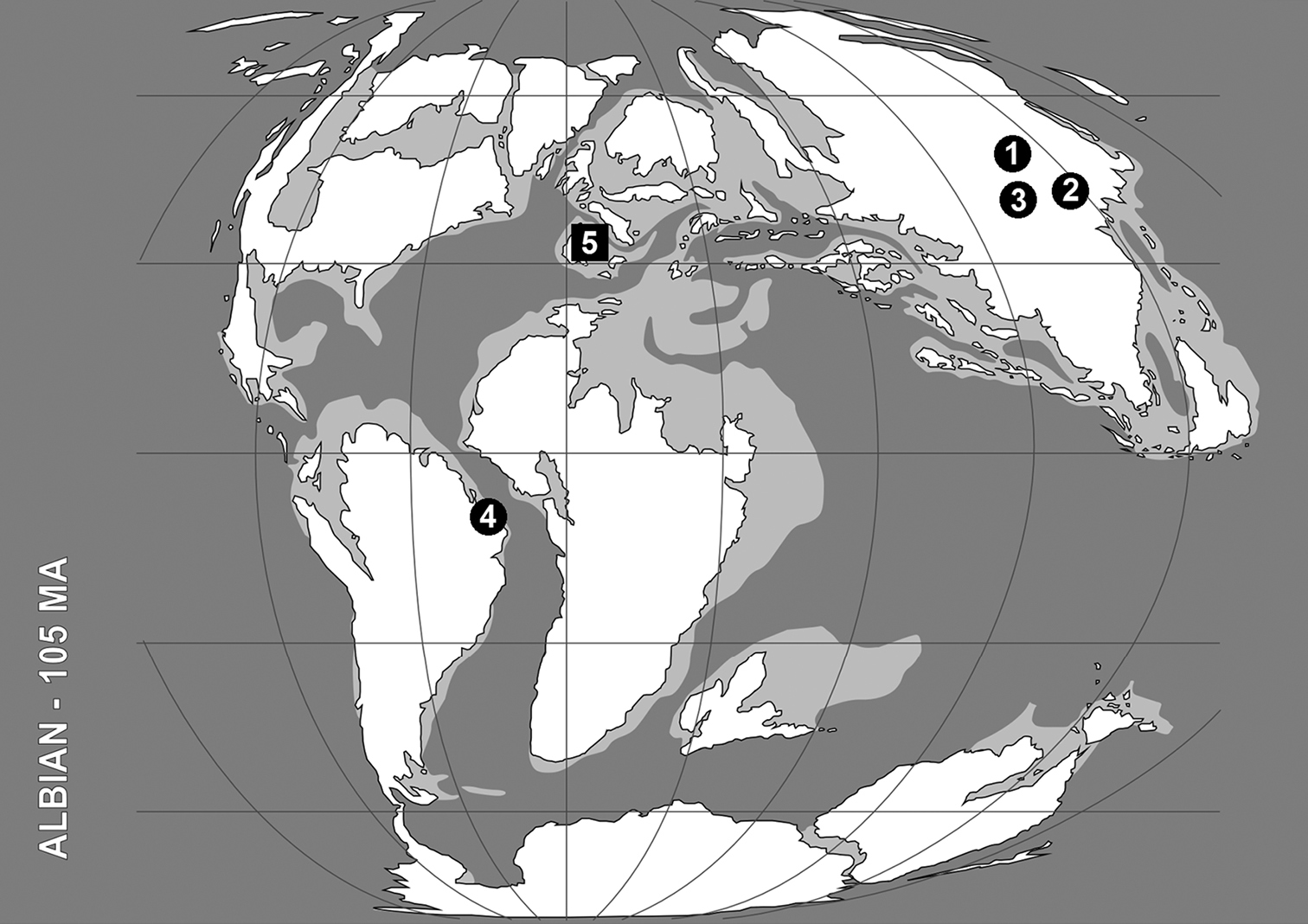
Distribution of the Early Cretaceous snakeflies currently classified within the Baissopteridae. The paleogeographic map (redrawn from Blakey 2008) corresponds to middle Albian (ca. 105 Ma). Data summarized in
Currently recognized genera classified within the extinct families Baissopteridae and Mesoraphidiidae.
| Family Baissopteridae Martynova, 1961 |
| Genus Austroraphidia Willmann, 1994 |
| Genus Baissoptera Martynova, 1961 |
| Genus Cretoraphidia Ponomarenko, 1993 |
| Genus Cretoraphidiopsis Engel, 2002 |
| Genus Lugala Willmann, 1994 |
| Family Mesoraphidiidae Martynov, 1925 |
| Genus Alavaraphidia Pérez-de la Fuente, Peñalver, Delclòs & Engel, gen. n. |
| Genus Alloraphidia Carpenter, 1967 |
| Genus Amarantoraphidia Pérez-de la Fuente, Peñalver, Delclòs & Engel, gen. n. |
| Genus Archeraphidia Ponomarenko, 1988 |
| Genus Baisoraphidia Ponomarenko, 1993 |
| Genus Cantabroraphidia Pérez-de la Fuente, Nel, Peñalver & Delclòs, 2010 |
| § Genus Cretinocellia Ponomarenko, 1988 |
| Genus Grimaldiraphidia Bechly and Wolf-Schwenninger, 2011 |
| Genus Huaxiaraphidia Hong, 1992 |
| Genus Iberoraphidia Jepson, Ansorge & Jarzembowski, 2011 |
| Genus Jilinoraphidia Hong and Chang, 1989 |
| Genus Kezuoraphidia Willmann, 1994 |
| Genus Lebanoraphidia Bechly & Wolf-Schwenninger, 2011 |
| Genus Mesoraphidia Martynov, 1925 |
| Genus Nanoraphidia Engel, 2002 |
| Genus Necroraphidia Pérez-de la Fuente, Peñalver, Delclòs & Engel, gen. n. |
| Genus Ororaphidia Engel & Ren, 2008 |
| Genus Pararaphidia Willmann, 1994 |
| Genus Proraphidia Martynova, 1947 |
| Genus Siboptera Ponomarenko, 1993 |
| Genus Sinoraphidia Hong, 1982 |
| Genus Styporaphidia Engel & Ren, 2008 |
| Genus Xuraphidia Hong, 1992 |
| Genus Yanoraphidia Ren, 1995 |
§ Recently transferred from Baissopteridae by Bechly and Wolf-Schwenninger (2011).
Cretaceous amber snakeflies (Neuropterida: Raphidioptera).<br/>
| Age, taxa | Amber locality (country) |
|---|---|
| Neocomian (Barremian–Aptian) | |
| Lebanoraphidia nana Bechly and Wolf-Schwenninger, 2011 | Jezzine (Lebanon) |
|
Mesoraphidiid larva (in |
Jezzine (Lebanon) |
| Albian | |
| Baissoptera? cretaceoelectra sp. n. | Peñacerrada I (Spain) |
| Alavaraphidia imperterrita gen. et sp. n. | Peñacerrada I (Spain) |
| Styporaphidia? hispanica sp. n. | Peñacerrada I (Spain) |
| Mesoraphidiid gen. et sp. indet. 1 (MCNA 9218) | Peñacerrada I (Spain) |
| Mesoraphidiid gen. et sp. indet. 3 (MCNA 9316) | Peñacerrada I (Spain) |
| Cantabroraphidia marcanoi Pérez-de la Fuente et al., 2010 | El Soplao (Spain) |
| Amarantoraphidia ventolina gen. et sp. n. | El Soplao (Spain) |
| Necroraphidia arcuata gen. et sp. n. | El Soplao (Spain) |
| Mesoraphidiid gen. et sp. indet. 2 (CES 376) | El Soplao (Spain) |
| Nanoraphidia electroburmica Engel, 2002 | Hukwang (Myanmar) |
| Mesoraphidiid larva 1 (in |
Hukwang (Myanmar) |
| Mesoraphidiid larva 2 (in |
Hukwang (Myanmar) |
| Mesoraphidiid larva 3 (in |
Hukwang (Myanmar) |
| Late Albian | |
| Mesoraphidiid larva (in |
Archingeay-Les Nouillers (France) |
| Turonian | |
| Grimaldiraphidia luzzii (Grimaldi, 2000) | New Jersey (USA) |
| Mesoraphidiid larva (in |
New Jersey (USA) |
| Campanian | |
| Mesoraphidiid larva (in |
Grassy Lake (Canada) |
The recent discovery of a significant paleodiversity of snakeflies in Spanish amber stimulated the present work. Herein we describe this diversity and place it in the context of other Mesozoic snakeflies, highlighting some factors that could explain the uniqueness of this record when compared to other Cretaceous ambers.
Material and methodsSamples designated by the institutional abbreviation CES are housed in the laboratory of the El Soplao Cave, Celis, Cantabria (Spain) encompassing the Institutional Collection from the El Soplao outcrop; whereas those designated as MCNA are housed in the Museo de Ciencias Naturales de Álava, Vitoria-Gasteiz, Spain. The higher classification followed herein is modified from that of
Drawings were prepared with a camera lucida attached to an Olympus BX51 microscope. Photomicrographs were prepared using a Nikon D1x digital camera attached to an Infinity K-2 long-distance microscopic lens. Images were merged using Combine ZP and Helicon Focus 4.2.1 (HeliconSoft Ltd.) softwares. All measurements are in millimeters and were taken using an ocular graticule.
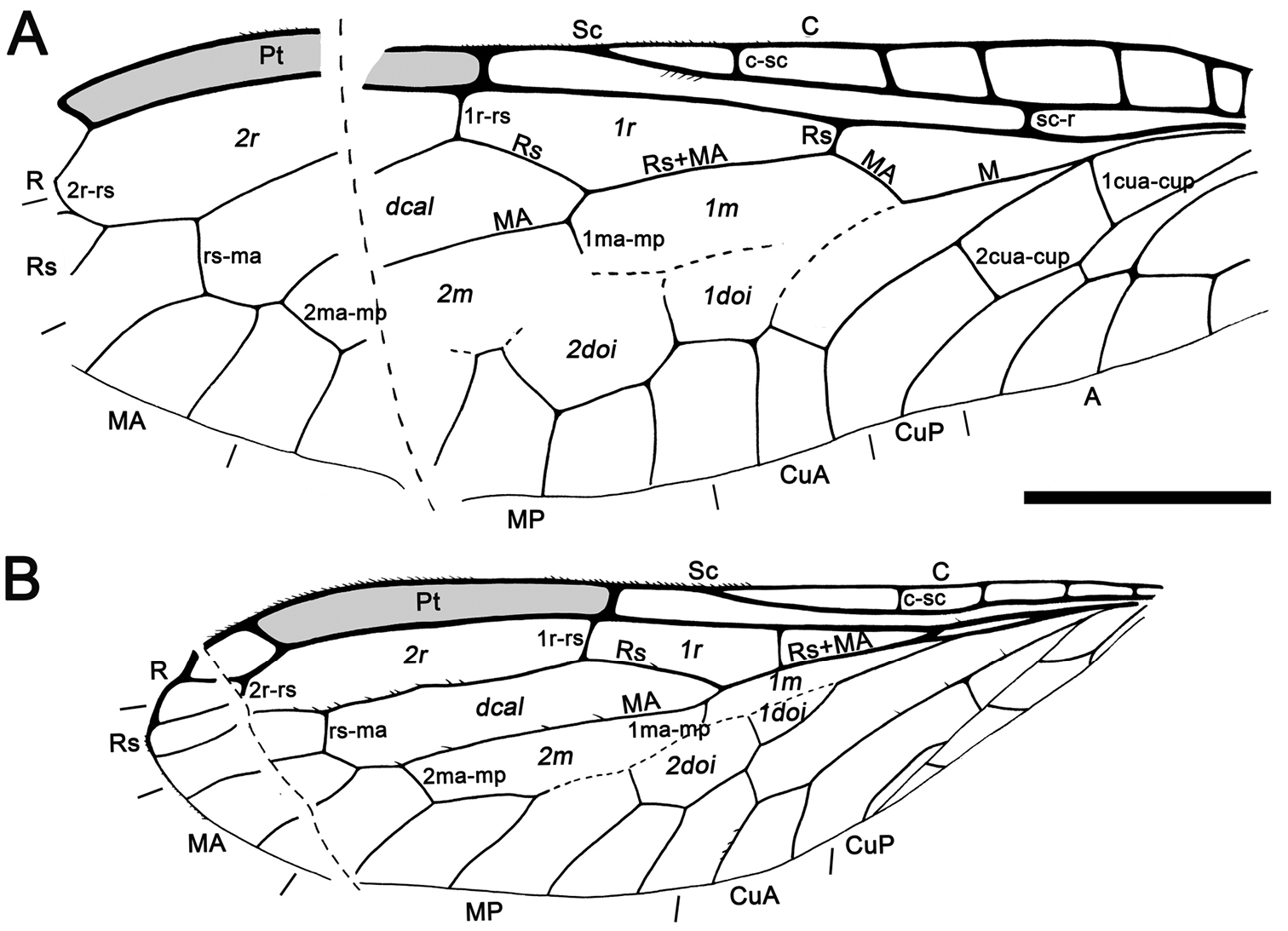
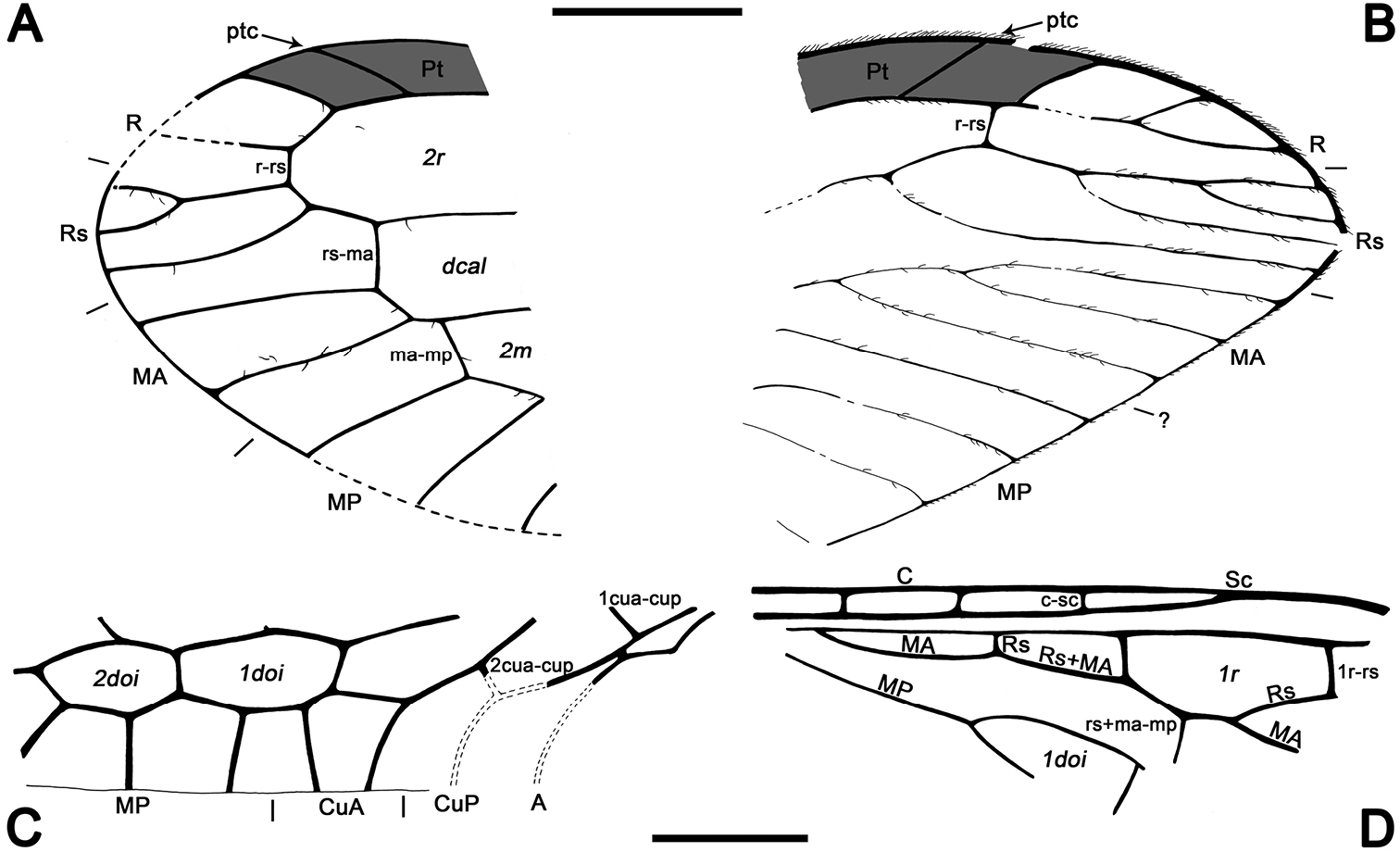
Abbreviations. Veins: A, anal; C, costa; CuA, cubital anterior; CuP, cubital posterior; MA, medial anterior; MP, medial posterior; ptc, pterostigmal crossvein; R, radial; Rs, radial sector; Sc, subcosta. Wing fields (in italics): dcal, discal cell; doi, discoidal cell; m, medial cell; pt, pterostigma; r, radial cell; sr, subradial cell.
Geological settingThe Peñacerrada I (in Moraza, Burgos. 42°40'22"N, 2°42'57"W) and El Soplao (in Rábago, Cantabria. 43°18'20"N, 4°26'50"W) amber-bearing deposits are included within the Basque-Cantabrian Basin (BCB) in the north of the Iberian Peninsula. To the south, the BCB constitutes a thrust sheet on the Cenozoic Duero and Ebro basins, while to the north it extends offshore to the Gulf of Biscay. BCB’s oriental limit is in the Pyrenees, and its occidental limit is in the Asturian Paleozoic Massif. The evolution of the BCB during the Late Jurassic-Early Cretaceous was contextualized into a stretching rift setting related with the opening of both the North Atlantic Ocean and the Biscayan Gulf. This regional extension produced a complex distribution of sub-basins and depositional areas, with different sedimentation features between them.
The amber localities are Albian in age, about 110 Mya (
From Peñacerrada I two different vegetational assemblages were distinguished in palynological analyses (
Different tree groups have been interpreted as the resin producers. For the oriental area of the BCB (Peñacerrada I), araucariaceans close to the Recent genus Agathis were suggested as original producers of the resin (
urn:lsid:zoobank.org:act:94EB4ADD-CCC8-46CB-98AF-F2EC4D47BDDC
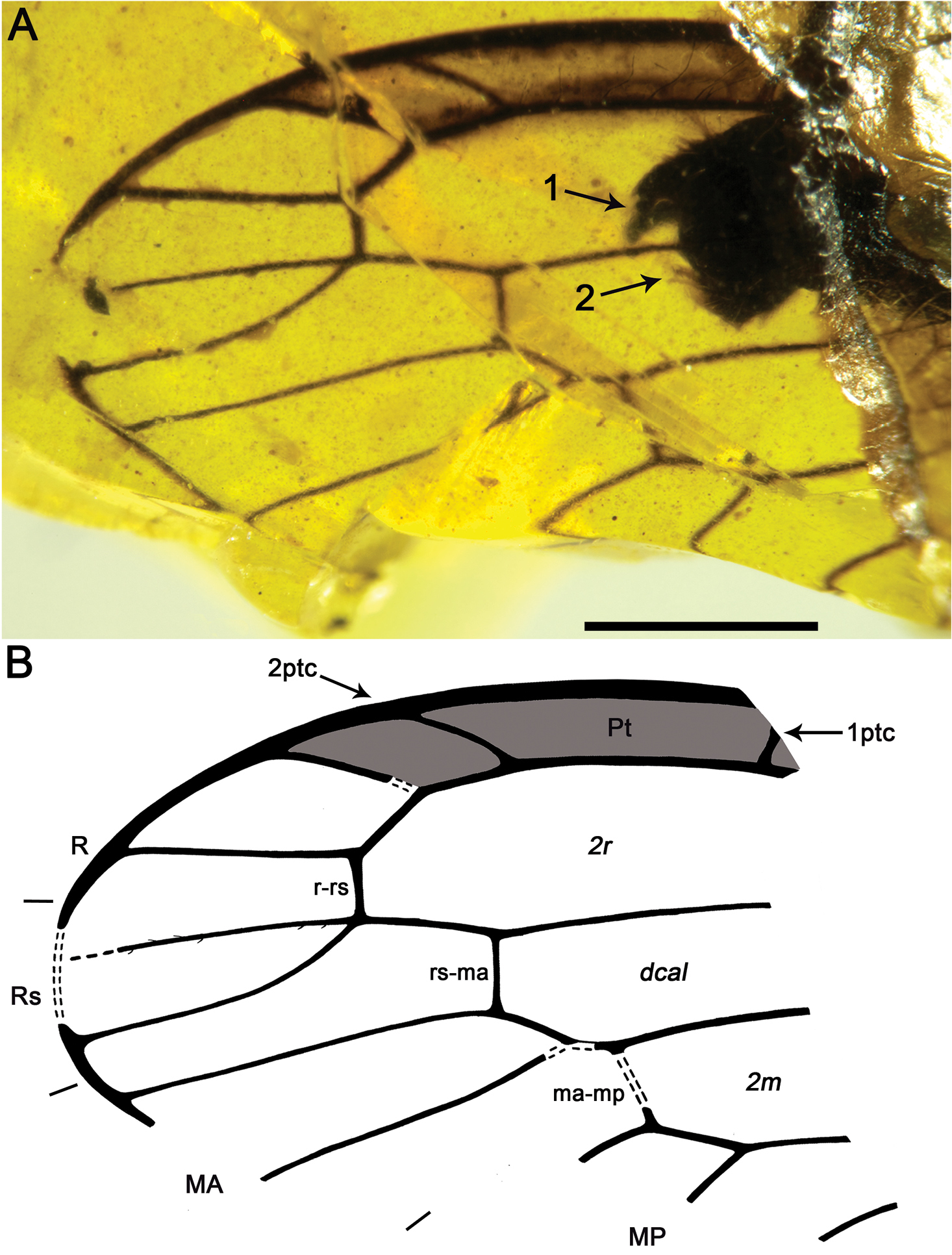
Figs 2 , 3MCNA 12068.4, from Peñacerrada I amber; fore- and hind wing distal fragments. Three associated hymenopterans are preserved as syninclusions.
Fore- and hind wing with a relatively long pterostigma with a strongly oblique and slightly sinusoid pterostigmal crossvein placed beyond pterostigmal midlength; fore- and hind wing with one closed radial cell distal to pterostigma; forewing Rs with six branches; forewing with at least eight closed subradial cells.
Sex unknown. Veins with some strong, very short setae preserved, membrane hyaline. Forewing.Length of preserved fragment 6.4, maximum width 2.8;wing apex relatively rounded; pterostigma relatively long (2.4 long, length ca. eight times basal pterostigmal width), slightly widening distally, not conspicuously infumate as preserved; pterostigma with a strongly oblique and slightly sinusoid pterostigmal crossvein placed beyond pterostigmal midlength, basally closed by a crossvein; pterostigma longer than any radial cell; R with two branches beyond pterostigma;at least three radial cells present, one closed radial cell partly distal to pterostigma; Rs with six branches and at least eight closed subradial cells;MA at least with two branches; gradate seriesveryregular, almost following a staircase-like pattern. Hind wing.Length of preserved fragment 5.9, maximum width as preserved 2.7;wing apex more pointed than in forewing;costal field distinctly narrower than in forewing; one c-sc crossvein preserved;pterostigma relatively long (2.5 long, length ca. 10 times basal pterostigmal width), slightly widening distally, not conspicuously infumate as preserved, starting 0.5 (twice pterostigmal basal width) beyond termination of Sc; pterostigma with a strongly oblique and slightly sinusoid pterostigmal crossvein placed beyond pterostigmal midlength, basally closed by a crossvein;pterostigma longer than any radial cell;R with two branches beyond pterostigma;at least five radial cells present, one small closed radial cell distal to pterostigma;Rs with five branches and at least seven closed subradial cells; MA at least with two branches; gradate seriesveryregular, almost following a staircase-like pattern.
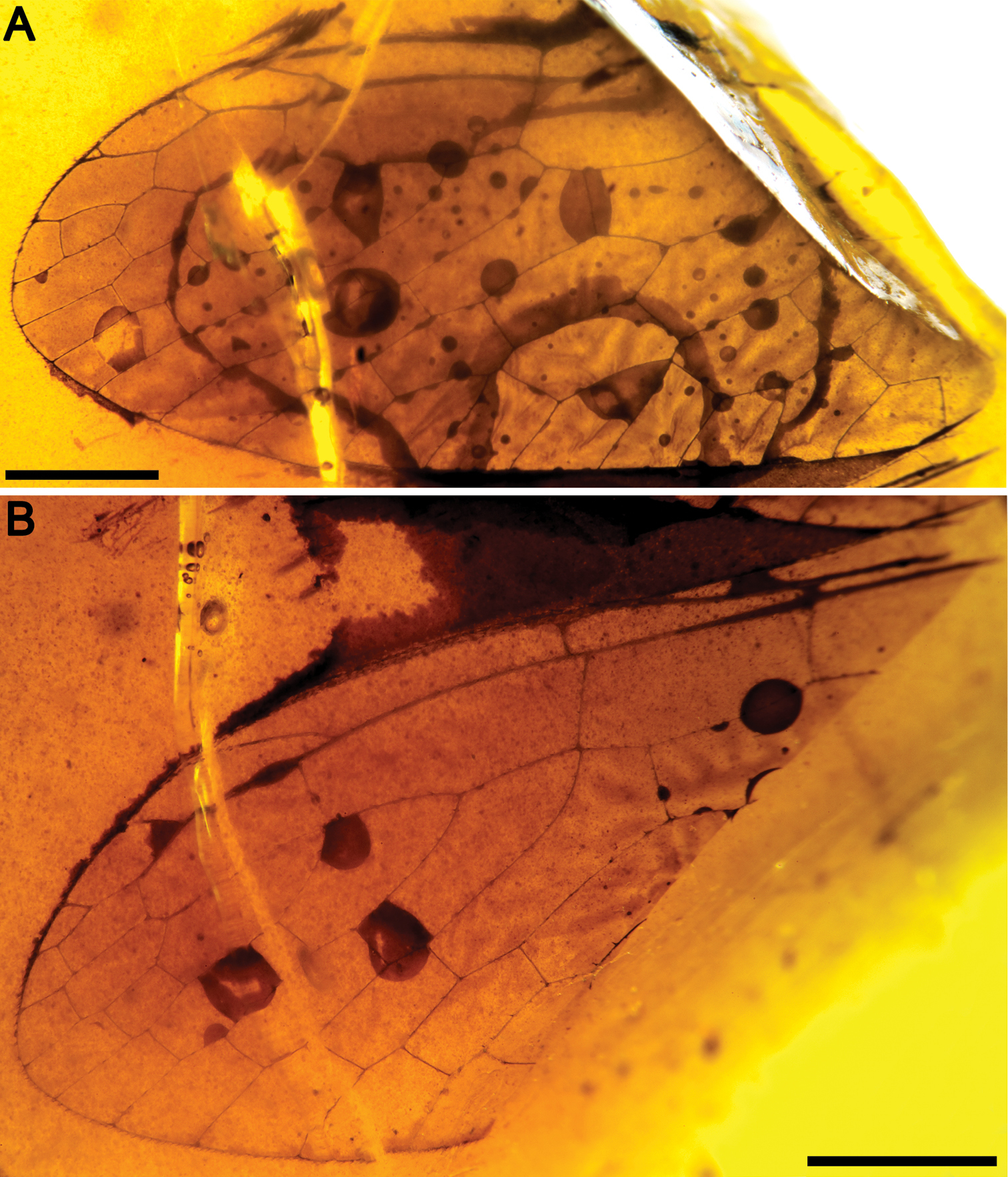
Baissoptera? cretaceoelectra sp. n., holotype MCNA 12068.4. A forewing B hind wing. Scale bars = 1 mm.
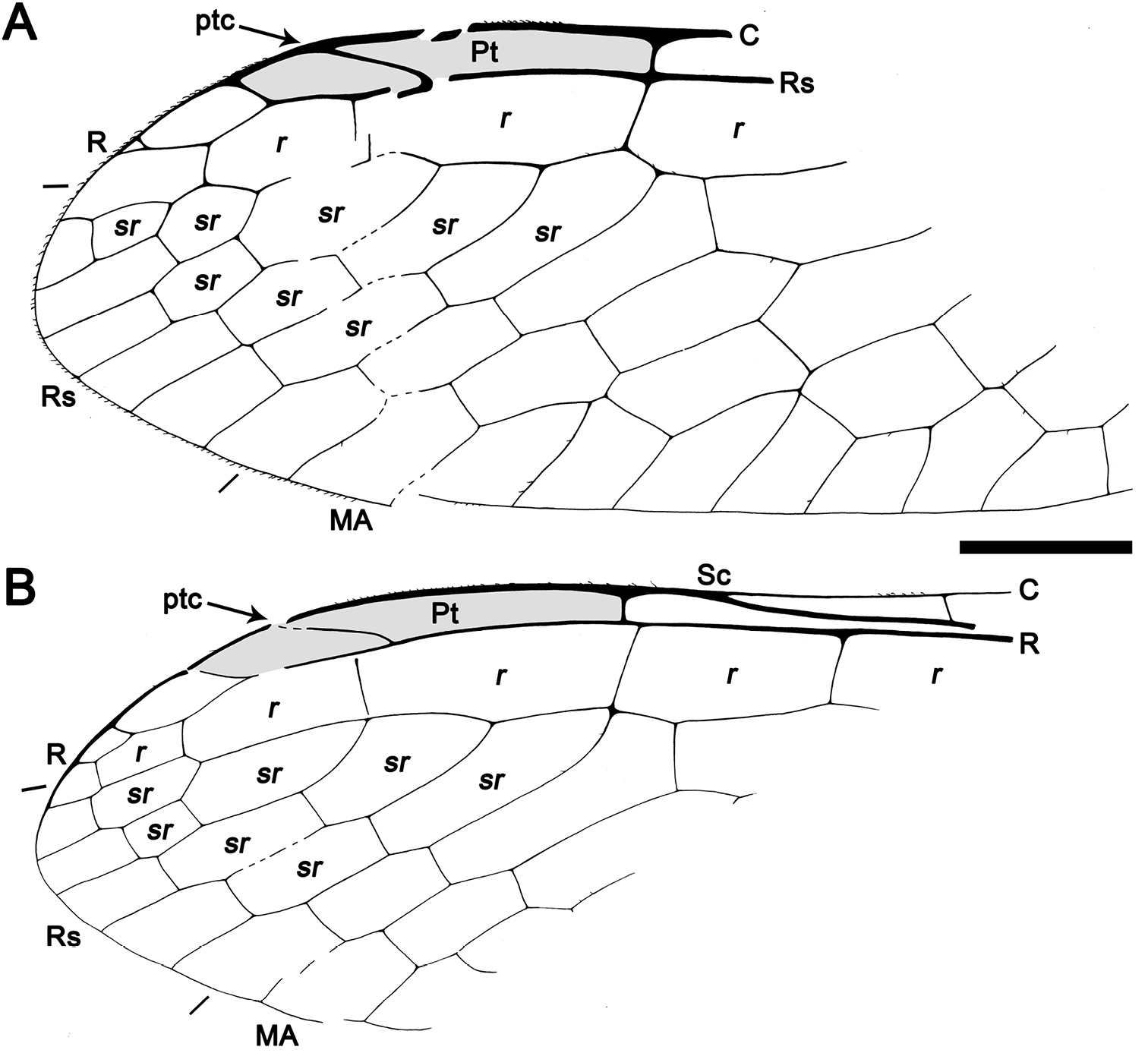
Drawings of Baissoptera? cretaceoelectra sp. n., holotype MCNA 12068.4. A, forewing B hind wing. Scale bar = 1 mm (both wings at the same scale).
The specific epithet is a combination of the Greek words cretaceus (taken from the period name, although specifically meaning “chalky”) and elektron, meaning “amber”.
Within the current taxonomic framework, the numerous crossveins of MCNA 12068.4 are indicative of placement in the Baissopteridae. Unfortunately, neither base of the wing is preserved, thus important characters such as the maximum width of the costal field, the pattern of distribution of c-sc crossveins (= costal crossveins), the separation between M and CuA in the forewing, and the shape of the basal piece of MA, are unknown. Also, the infumation of the pterostigma is not evident in the holotype, but it is uncertain if this could have been caused by taphonomical processes and is, therefore, not used as a diagnostic character although if the absence of infumation is true of the species in life, then it would represent a remarkable difference from all other described baissopterids. Fortunately, the pterostigma can be delimited thanks to the relative parallelness of C and R (R tends to conspicuously change its slope beyond the pterostigma in the other baissopterids) and also the greater thickness of both veins.
The specimen is tentatively classified within the genus Baissoptera as it has the pterostigmal crossvein most similar to the diversity found within this genus. Today just two of the 12 species currently classified within the genus Baissoptera, i.e., Baissoptera brasiliensis Oswald, 1990 and Baissoptera lisae Jepson, Ansorge & Jarzembowski, 2011, lack a pterostigmal crossvein in both fore- and hind wings (
urn:lsid:zoobank.org:act:BF4F7D74-232F-4F2E-8754-E3940CD9D8B0
Necroraphidia arcuata sp. n.
Small size; costal field very broad; pterostigma with a single, subdistal, strongly oblique, slightly sinuose to arcuate crossvein; pterostigma with a diffuse base; forewing with Rs and MA each forked twice; forewing with second radial cell proximally broad.
The new genus-group name is a combination of the Greek word nekros, meaning, “dead”, and Raphidia, common generic stem for snakeflies. The name is feminine.
Necroraphidia gen. n. is most similar to Ororaphidia and Styporaphidia from the Late Jurassic of Inner Mongolia, China (
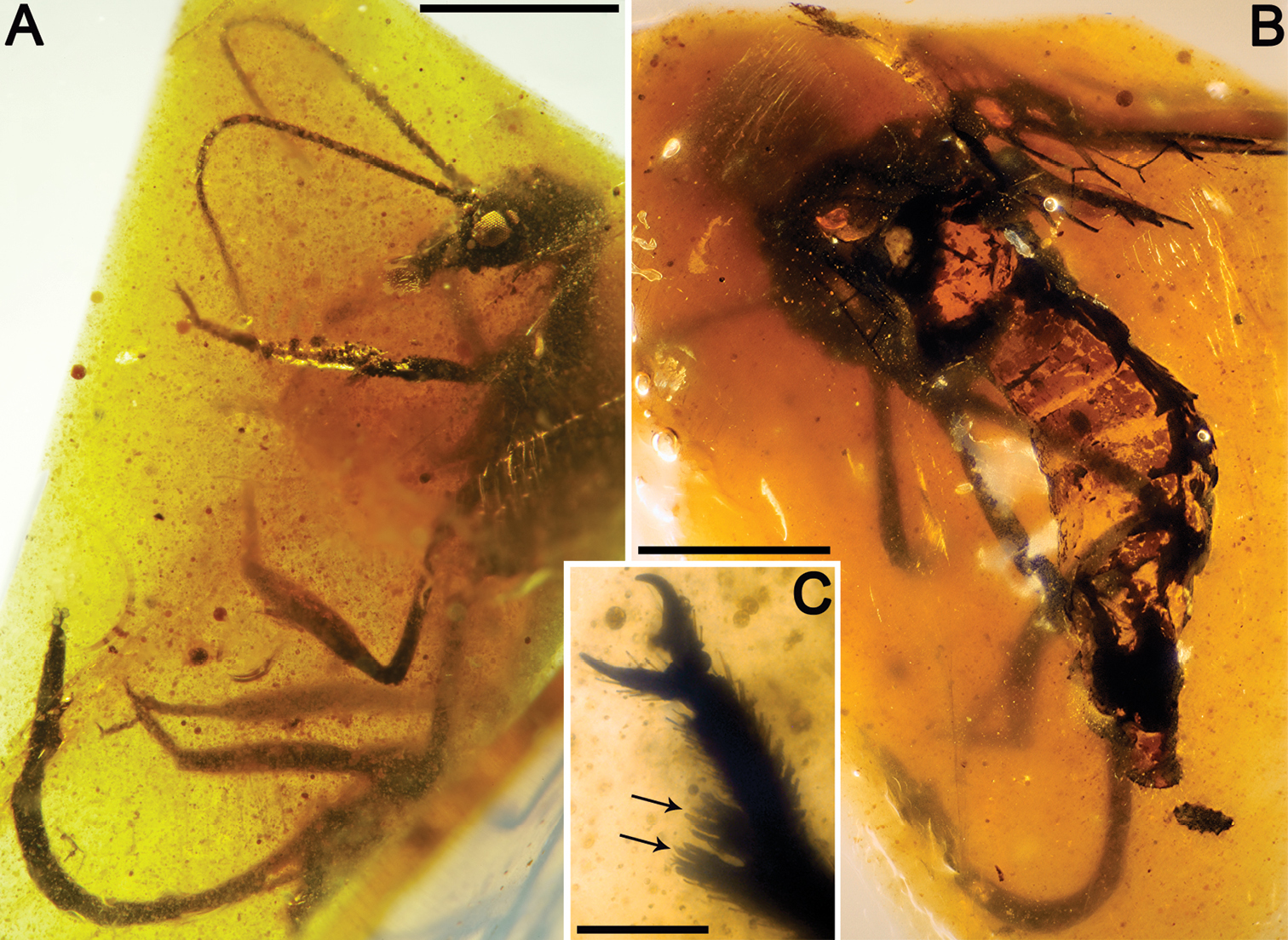
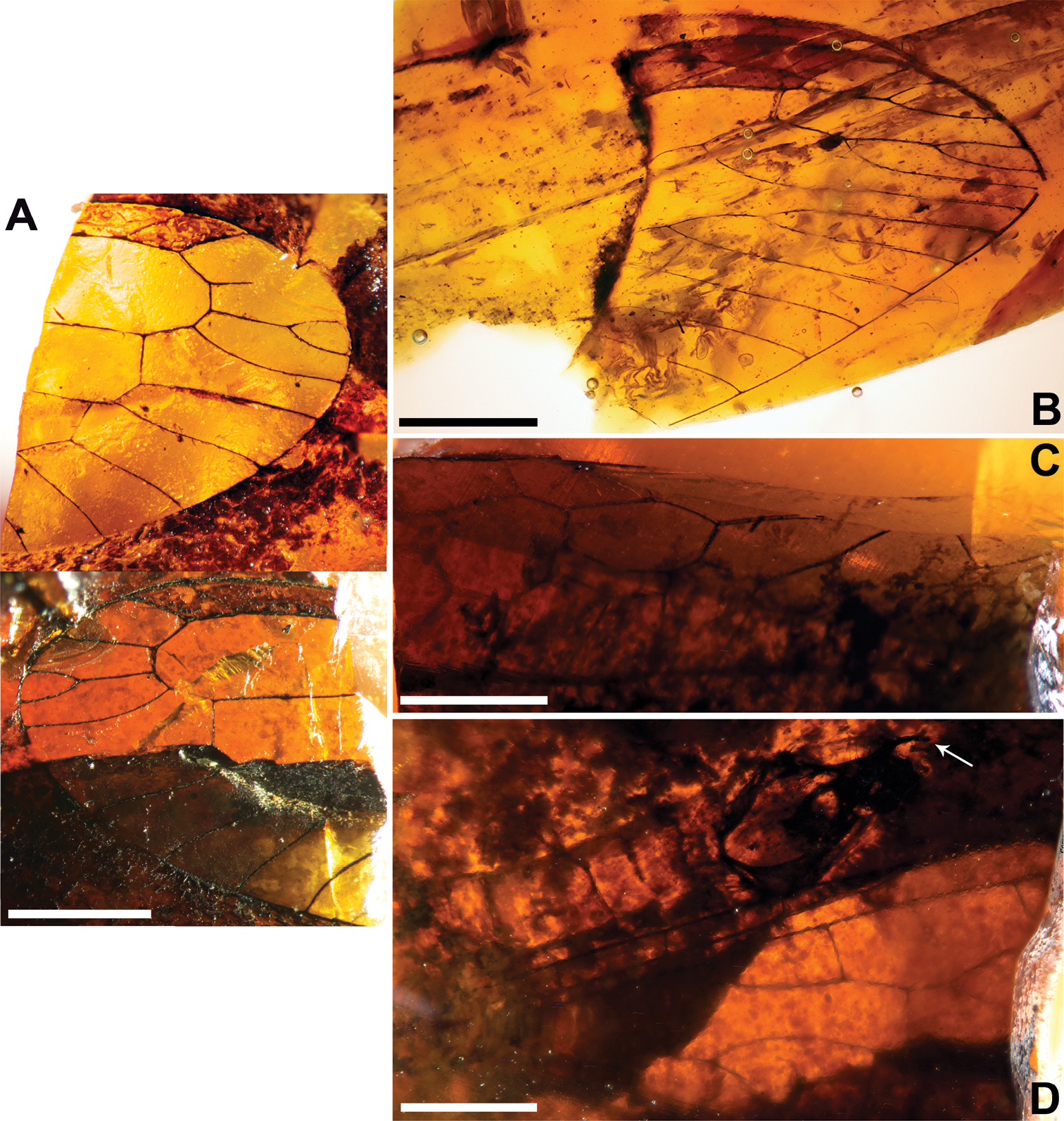
Necroraphidia arcuata gen. et sp. n., holotype CES 391.1. A ventral habitus, note some charcoalified plant fibersnearby the specimen (arrow) B right forewing pterostigmal crossvein C right hind wing pterostigmal crossvein D right forewing pterostigmal diffuse base E right hind wing pterostigmal diffuse base. Scale bars: A = 2 mm; B, C, D, E = 0.5 mm.
urn:lsid:zoobank.org:act:A0CA422D-FD60-4753-B8DE-7C5A4E75BDAE
http://species-id.net/wiki/Necroraphidia_arcuata
Figs 4 , 5CES 391.1, from El Soplao amber; incomplete specimen, almost complete left fore- and hind wings (lacking their basalmost part), distal half of right hind wing and apex of right forewing, partial abdomen, and two leg fragments. Dense fungal hyphae infestate the abdomen and wings. The specimen is preserved together with the following syninclusions: two coleopterans, two hymenopterans (one of them, CES 391.2, belonging to the Megalyridae;
As for the genus (vide supra).
Sex unknown.Legs patterned as follows (at least in the preserved fragments): femur with three dark areas, tibia with proximal area darkened and a dark area beyond midlength.Wing veins brown; veins with strong, very short setae, especially abundant on C; membrane hyaline. Forewing.Length of preserved fragment 6.9 (estimated total wing length > 9), maximum width 2.7; costal field very broad (costal field about two times wider than pterostigmal base at widest preserved point; Sc terminating into C around two-thirds of estimated wing length; three c-sc crossveins preserved; single, proximal sc-r crossvein; pterostigma elongate (3.2 long, longer than either radial cell), widening distally (maximum width almost twice basal width), and faintly infumate, starting at termination of Sc; pterostigma with a single, subdistal, strongly oblique, slightly sinuose crossvein; pterostigma with a diffuse base (i.e., lacking a crossvein as proximal boundary of this wing region); Rs with three branches, distalmost fork very short; two large radial cells present; first radial cell about 1.3 times longer than second radial cell; second radial cell proximally broad; MA arising slightly distad midpoint of first radial cell, with three branches; three discoidal cells posterior to MP; 1cua-cup crossvein not preserved; anal veins not preserved; jugal lobe not visible. Hind wing.Length of preserved fragment 6.6 (estimated total wing length 8–9), maximum width 2.7;costal field distinctly narrower than in forewing;four c-sc crossveins preserved;pterostigma elongate (2.9 long, longer than either radial cell), widening distally (maximum width almost twice basal width), and faintly infumate, starting at termination of Sc; pterostigma with a single, subdistal, strongly oblique, arcuate crossvein; pterostigma with a diffuse base; Rs with two branches; two radial cells present; MA with three branches; two discoidal cells posterior to MP, the first one trianguloid, not much smaller than second one; 1ma-mp crossvein not especially close to fork between Rs and MA; anal area not preserved. Abdomen. Length 3.8. Genitalia degraded, with dorsal part missing, and badly seen due to presence of dense fungal hyphae.
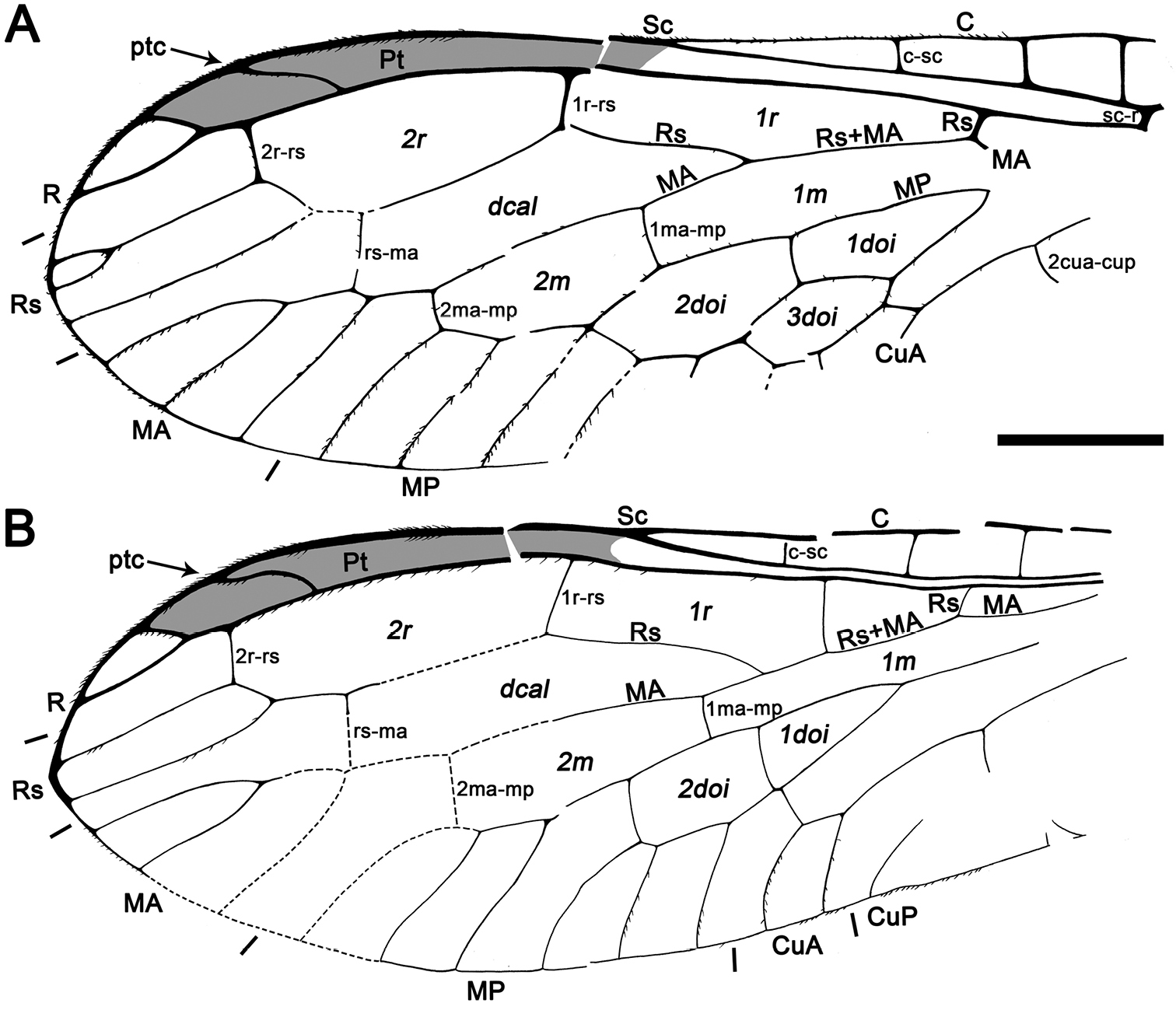
Drawings of Necroraphidia arcuata gen. et sp. n., holotype CES 391.1. A right forewing B right hind wing with reconstructed part taken from the left hind wing (dashed line). Only the distalmost c-sc crossvein has been tagged for both wings. Scale bar = 1 mm (both wings at the same scale).
The specific epithet is the Latin term arcuatus, meaning “bent”, and refers to the arcuate form of the pterostigmal crossvein.
urn:lsid:zoobank.org:act:0AAD19B5-EF08-4B10-B704-DBF6D56A0D6F
Fig. 6MCNA 9343, from Peñacerrada I amber; hind wing fragment and abdominal apex, including the genitalia.
The new species is similar to Styporaphidia magia Engel and Ren, 2008 from the Late Jurassic of Inner Mongolia, China, in the presence of two pterostigmal crossveins. Styporaphidia? hispanica sp. n.differs in that the distance between 1ptc and 2ptc is three times the distance between 2ptc and the end of the pterostigma (two times in Styporaphidia magia), the forking of Rs at the apicalmost r-rs crossvein (rather than prior to it in Styporaphidia magia), and R meeting the apicalmost r-rs crossvein beyond the pterostigma (within in Styporaphidia magia).
Male. Hind wing. Length as preserved 3.5, maximum width as preserved 2.5; wing apex relatively rounded; C especially thick when compared to other veins; pterostigma almost with constant width along its entire length, infumate; pterostigma with two crossveins, distalmost crossvein oblique and slightly arcuate, proximal crossvein apparently straight, distance between 1ptc and 2ptc three times distance between 2ptc and end of pterostigma; Rs with two branches, forking at r-rs crossvein; R meeting apicalmost r-rs crossvein beyond pterostigma; rs-ma crossvein meeting MA after its distalmost fork; MA with two branches. Abdomen. Gonocoxites 9 with a few long setae;gonostyli 9 segment rather short, rounded (not acute), slightly upcurved;tergite 10 (+11?) with distal setae;paired, contiguous, acute genital structures located dorsad to gonostyli 9, interpreted as distalmost part of parameres (Fig. 6A).
Styporaphidia? hispanica sp. n., holotype MCNA 9343. A hind wing apical fragment and genitalia; arrow 1 points to gonostyli 9, whereas arrow 2 points to the paired genital sclerites interpreted as the distalmost part of the parameres B drawing of preserved hind wing apical fragment. Scale bar = 1 mm.
The specific epithet refers to the occurrence of this species in ancient Spain (Hispania in Latin).
Although the base of the pterostigma is not preserved and it is accordingly impossible to ascertain if it was diffuse (i.e., lacking a crossvein), this species is tentatively placed in the genus Styporaphidia owing to the presence of two pterostigmal crossveins. The presence of two pterostigmal crossveins is a rare feature among the Raphidioptera and otherwise known in a few other raphidiopterans, i.e, namely the baissopterids Baissoptera kolosnitsynae and Baissoptera pulchra (
urn:lsid:zoobank.org:act:EA2F19D0-E3C8-43A7-BA92-BFC73C2FA208
Amarantoraphidia ventolina sp. n.
Minute size. Head ovoid, with the portion posterior to the compound eyes longer than the eye diameter and tapering caudad; three large ocelli present, situated between anterior half of compound eyes; antennae with a low number of flagellomeres (i.e., ≤ 26). Pronotum slightly longer than head, with a constant height along its entire length. Mesotibiae especially swollen; process at midlength of the metatibiae absent. Forewing with costal field moderately broad; pterostigma elongate, without crossveins; Sc terminating into C slightly distad wing midlength; six c-sc crossveins present; two discoidal cells posterior to MP; apicalmost branch of CuA simple; 1cua-cup crossvein located at the M-CuA separation.
The new genus-group name is a combination of the Greek term amarantos, meaning “that never fades, ageless”, and Raphidia, common generic stem for snakeflies. The name is feminine.
Amarantoraphidia gen. n.is first compared with the other described minute mesoraphidiid genera, which have a forewing length around 6 or less. They include amber inclusions but also some compression fossils. Apart from the minute size, in all of these taxa the pterostigma is very elongate, without crossveins, and basally closed by a crossvein. Regarding those as amber inclusions, Amarantoraphidia is readily distinct from the other two currently described Spanish mesoraphidiid genera, Cantabroraphidia and Alavaraphidia gen. n., as well as Lebanoraphidia, by its ovoid head shape (subquadrate in Cantabroraphidia, and rhomboidal in the other two genera) and by its lesser number of antennal flagellomeres (26 flagellomeres in Cantabroraphidia, 38 flagellomeres or more in Lebanoraphidia, and 44 in Alavaraphidia). The two genera Nanoraphidia (type species Nanoraphidia electroburmica Engel, 2002, Burmese amber, latest Albian in age) and Grimaldiraphidia (type species Grimaldiraphidia luzzi (Grimaldi, 2000), New Jersey amber, Turonian in age)share with Amarantoraphidia the ovoid head shape. However, in both of these genera the ocelli are placed between the posterior half of the compound eyes, not between the anterior part as in Amarantoraphidia. Additionally, whereas Amarantoraphidia has two discoidal cells posterior to MP in the forewing, Grimaldiraphidia has three and Nanoraphidia just a single cell. Also, in Nanoraphidia the M-CuA separation is located near midpoint of the first and second cua-cup crossveins (
In addition to the aforementioned taxa, five mesoraphidiids with minute size have been hitherto described from compression fossils: Grimaldiraphidia parvula (Martynov, 1925), from Karatau (South Kazakhstan), Late Jurassic in age; Grimaldiraphidia mitchelli (Jepson, Coram and Jarzembowski, 2009), Grimaldiraphidia purbeckensis Jepson, Coram and Jarzembowski, 2009, and Mesoraphidia websteri Jepson, Coram and Jarzembowski, 2009, the three species from the Purbeck Limestone Group, Dorset (UK), Berriasian in age; and Nanoraphidia lithographica Jepson, Ansorge and Jarzembowski, 2011 (tentatively assigned to this genus), from El Montsec (Spain), Early Barremian in age. Grimaldiraphidia parvula (described from a complete specimen but its wings unresolved dorsoproximally) has more proximal positions of the fork of Rs and the 2r-rs and rs-ma crossveins than Amarantoraphidia (
urn:lsid:zoobank.org:act:79F0A7A3-789D-4B3F-8E34-A021073A86AD
http://species-id.net/wiki/Amarantoraphidia_ventolina
Figs 7 –9CES 364.1, from El Soplao amber; almost complete female, just lacking the distalmost portion of both forewings beyond the end of the pterostigma and the distal third of the right hind wing. The first left leg is disarticulated. The specimen is preserved together with the following syninclusions: one evaniid (a new Cretevania species; CES 364.2,
As for the genus (vide supra).
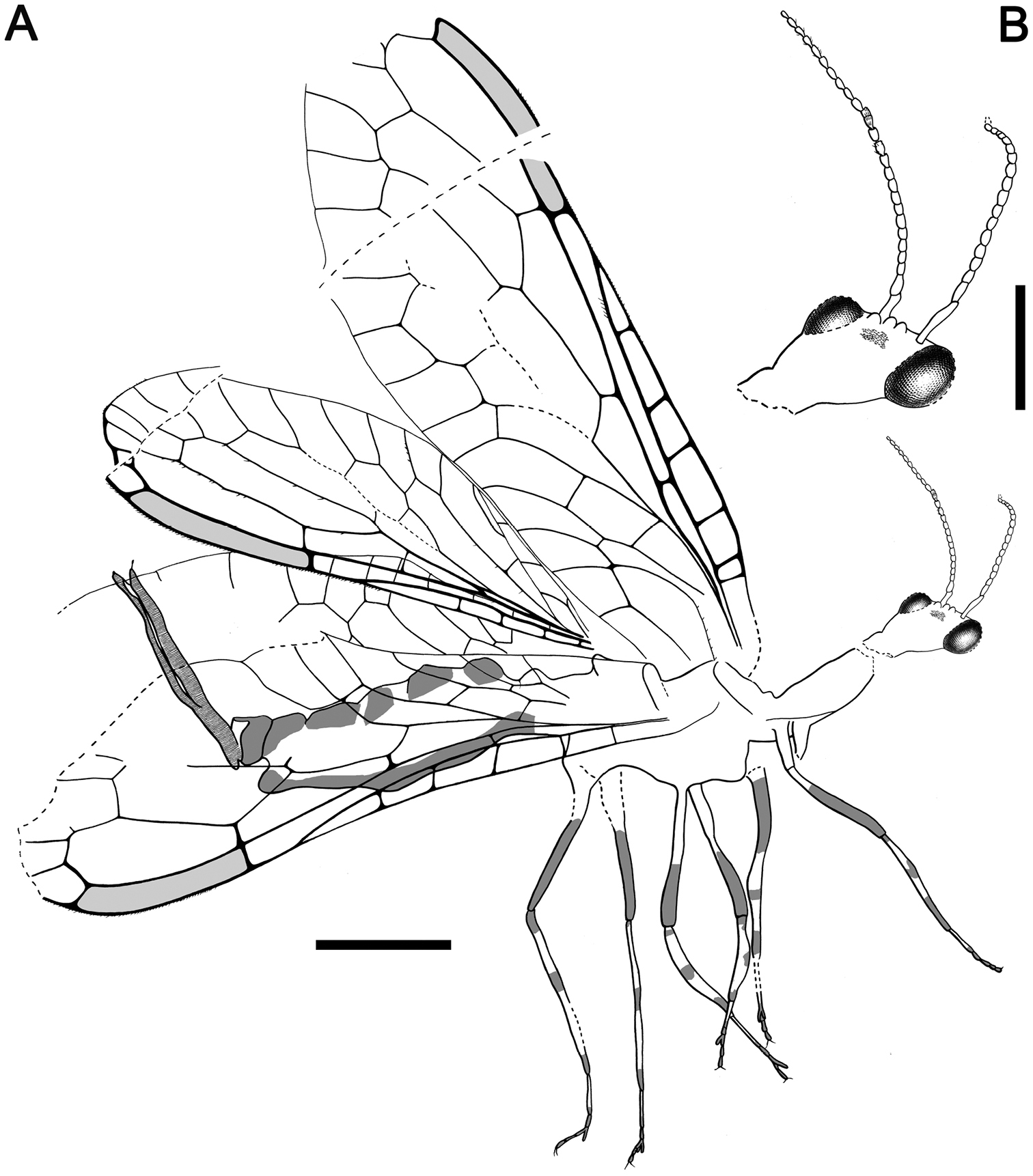
Female.Integument dark brown; legs patterned as follows: femora darkened from just before their midlength to their end; three dark areas on tibiae, proximally, medially and distally; tarsomere 1 not darkened, distal tarsomeres darkened. Head.Ovoid, about 0.7–0.8 long, with portion posterior to compound eyes longer than eye diameter and tapering caudad; three large ocelli present, situated between anterior half of compound eyes; mandibles with teeth not visible;palps short;compound eyes large and exopthalmic, separated by distance slightly greater than compound eye length; antennae inserted around anterior tangent of compound eyes (exact insertion not visible); scape and pedicel gracile, both measuring about length of four flagellomeres and being subequal in thickness to them; 24 flagellomeres present, slightly longer than wide, with sparse, minute setae; coronal ecdysial cleavage line not evident; posterior border of head with a distinct collar-like lip.Thorax.Prothorax about 1.1 long, meso- plus metathorax 1.4 long; pronotum slightly longer than head, with a constant height along its entire length (i.e., without a distinct change of slope in lateral view); a few spines visible on prothorax, dorsoanterior mesothorax apparently with a few small spines; all tibiae with apical spines; mesotibiae especially swollen; metatibiae significantly more elongate and thinner than the other tibiae; process at midlength of metatibiae absent; five tarsomeres, third with bilobed extensions lacking digitiform processes (Fig. 7B); pretarsal claws simple, with a basal enlargement; arolium large.Wing veins brown, meeting wing margins without bifurcating; veins with strong, very short setae, especially abundant on C; membrane hyaline. Forewing.Length about 5.6 (tip not preserved), maximum width 1.9; costal field moderately broad (at widest point costal field about 1.4 wider than pterostigma); six c-sc crossveins present, two basalmost c-sc crossveins particularly close to each other; Sc terminating into C slightly distad wing midlength; single, proximal sc-r crossvein; pterostigma elongate (1.5 long), slightly longer than either radial cell; pterostigma with constant width along its entire length, faintly infumate, starting 0.4 (about three times pterostigmal width) beyond termination of Sc; pterostigma without crossveins, basally closed by a crossvein; Rs with two branches; two large radial cells present; first radial cell nearly as long as second radial cell, with MA arising slightly distad its midpoint; MA with two branches; two discoidal cells posterior to MP; apicalmost branch of CuA unforked; 1cua-cup crossvein located at M-CuA separation; 2A arcuate; jugal lobe not visible. Hind wing. Length about 4.1, maximum width 1.3; costal field distinctly narrower than in forewing; four c-sc crossveins present; sc-r crossvein absent; pterostigma elongate (1.5 long), slightly longer than second radial cell; pterostigma with about a constant width along its entire length, faintly infumate, starting 0.3 (slightly more than two times pterostigmal width) beyond termination of Sc; pterostigma without crossveins, basally closed by a crossvein; Rs with two branches; two radials cells present; MA with two branches; two discoidal cells posterior to MP, first one smaller and trianguloid; 1ma-mp crossvein close to fork between Rs and MA; anal area folded. Abdomen. Length 2.4; ovipositor robust, 1.7 long as preserved, 0.1 thick (about 15 times as long as wide); ovipositor showing dense annulations (Fig. 7C); ovipositor with faint, stiff, short sensory setae along its entire length; ovipositor gonostyli most likely club-shaped.
Amarantoraphidia ventolina gen. et sp. n., holotype CES 364.1. A lateral habitus, note a charcoalified plant fibernearby the specimen (arrow) B left metatarsi showing bilobed third tarsomere C distal portion of ovipositor, note its dense annulation; arrow points to a gonostylus. Scale bars: A = 1 mm; B, C = 0.1 mm.
Drawings of Amarantoraphidia ventolina gen. et sp. n., holotype CES 364.1. A lateral habitus B head, magnified. Scale bars: A = 1 mm; B = 0.5 mm.
Figure 9. Drawings of Amarantoraphidia ventolina gen. et sp. n., holotype CES 364.1. A left forewing B left hind wing, depicted with its preservational folding, i.e., the basal part of MA is superimposed by the basal part of MP and the anal field is folded upwards. Only the distalmost c-sc crossvein has been tagged for both wings. Scale bar = 1 mm (both wings at the same scale).
In the Cantabrian mythology, the “ventolines” are tenacious and always cheerful fairy-like air beings that dwell in the depths of the sky and, when summoned, help defenseless fishermen by placidly steering their boats to the shore while embracing them with their warm green wings. The term has been singularized and feminized for combination.
In extant snakeflies, the dense annulations of the ovipositor (cf. Fig. 7C) provide the flexibility necessary for introduction into irregular cavities, similar to a flexible metallic hose (
urn:lsid:zoobank.org:act:070A3E8E-1F78-4663-A909-1872E5EEA80E
Alavaraphidia imperterrita sp. n.
Minute size. Head rhomboidal, with clypeus especially elongate and the portion posterior to the compound eyes shorter than the eye diameter and strongly tapering caudad; three large ocelli present, situated near the posterior tangent of compound eyes; antennae extremely elongate, with a high number of flagellomeres (i.e., ≥ 38). Pronotum shorter than head, with a constant height along its entire length. All tibiae especially swollen medio-apically; process at midlength of the metatibia indistinct. Bilobed extensions of third tarsomeres with distal digitiform processes.
The new genus-group name is a combination of Álava, from Álava amber (the name of the group for Peñacerrada I and Peñacerrada II amber localities), and Raphidia, common generic stem for snakeflies. The name is feminine.
Although most of the wings are not preserved, the other features of Alavaraphidia gen. n. are distinctive enough to justify the creation of a distinct taxon. The minute size of Alavaraphidia mainly limits its comparison to other minute taxa mainly described from amber inclusions (refer to comments for Amarantoraphidia gen. n.). Although a few other taxa based on wings from compressions are minute in size it is not possible to compare them with the new genus and species due to the absence of most of its wings. The genus Lebanoraphidia shares with Alavaraphidia the rhomboidal shape of the head with the compound eye length greater than that of the head posterior to the eyes (
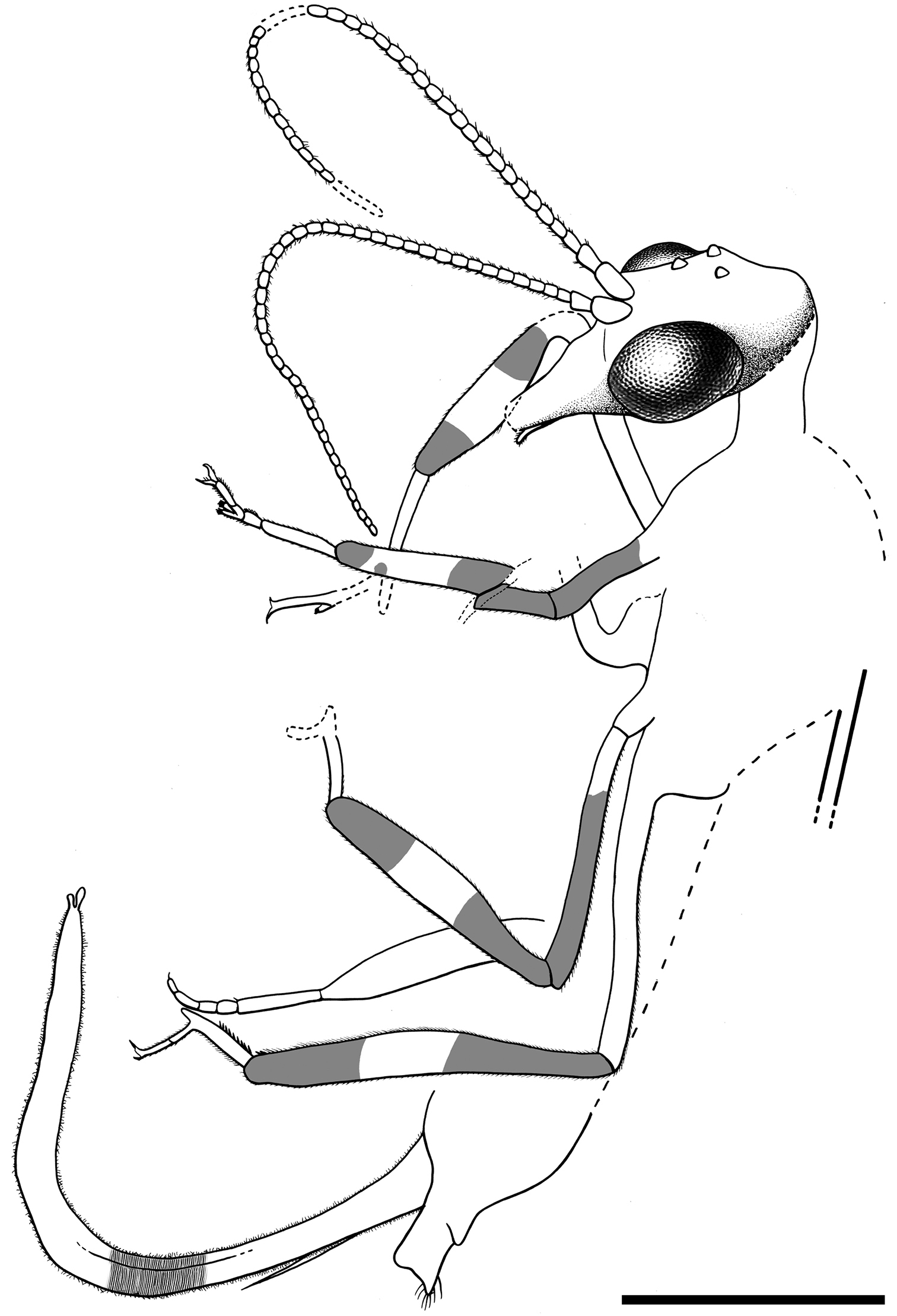
Alavaraphidia imperterrita gen. et sp. n., holotype MCNA 13608. A lateral habitus B dorsal habitus C left distal foretarsus, showing bilobed third tarsomere with digitiform processes (arrows). Scale bars: A, B = 1 mm; C = 0.1 mm.
urn:lsid:zoobank.org:act:E1406721-46BE-4F26-869E-EC6C5AE6CD01
http://species-id.net/wiki/Alavaraphidia_imperterrita
Figs 10 , 11MCNA 13608, from Peñacerrada I amber; partial specimen showing head and ventral parts of thorax and abdomen, including the ovipositor. Only the basalmost part of the wings is preserved.
As for the genus (vide supra).
Female.Body length excluding ovipositor 5.7. Integument dark brown; legs patterned as follows: femora darkened from just before their midlength to their end; two dark regions on tibiae, proximally and distally (medial dark region absent); tarsomere 1 not darkened, distal tarsomeres darkened. Head. Rhomboidal, about 1.2 long, with portion posterior to compound eyes shorter than eye diameter (about 0.7 times) and strongly tapering caudad; three large ocelli present, situated near posterior tangent of compound eyes; mandible with teeth not visible; palps short; clypeus especially elongate; compound eyes separated by about compound eye length; three large ocelli present, situated near posterior tangent of compound eyes; antennae inserted posterior to clypealfrons sulcus, basad anterior tangent of compound eyes; antennae extremely elongate, with 44 flagellomeres; flagellomeres elongate, about 1.5 times longer than wide; scape and pedicel thicker than flagellomeres, scape measuring about two flagellomeres, pedicel measuring slightly more than a flagellomere; coronal ecdysial cleavage line not evident; posterior border of head not visible. Thorax. Prothorax about 0.8 long; meso- and metathorax about 1.1 long; pronotum shorter than head, with a constant height along its length (i.e., without a distinct change of slope in lateral view); thoracic dorsal spines not visible, if present; all tibiae especially swollen medio-apically, with apical spines, spines also visible on metatarsomeres; process at midlength of metatibia indistinct; five tarsomeres, third with bilobed extensions having six to eight distal digitiform processes (Fig. 10C), different in shape than regular leg setae (not tapering apically); pretarsal claws simple, with a basal enlargement; arolium large.Preserved wing veins brown, with strong, very short setae; preserved membrane hyaline.Forewing. Costal field not especially broad. Three c-sc crossveins preserved.Hind wing. Costal field distinctly narrower than in forewing. Three c-sc crossveins preserved.Abdomen.Length 2.7; ovipositor robust but rather elongate, about 2.7 long as preserved, 0.2 thick (about 15 times as long as wide);ovipositor with dense annulations; ovipositor with conspicuous faint, stiff, small sensory setae along its entire length; ovipositor gonostyli club-shaped; tergite 10 (+11?) with a distalmost stripe of stiff trichobothria (some probably from tergite 9).
Drawing of Alavaraphidia imperterrita gen. et sp. n., holotype MCNA 13608. Lateral habitus. The annulations of the ovipositor have been depicted partially. Scale bar = 1 mm.
The specific epithet is the Latin term imperterritus, meaning “fearless”, and symbolizes the unalterable condition of an organism entrapped in amber.
http://species-id.net/wiki/Cantabroraphidia
Fig. 12According to the original diagnosis, this monospecific genus was characterized by the following combination of characters: minute size (forewing length 5.5). Head more or less quadrangular; with portion posterior to the compound eyes slightly shorter than the eye diameter and not tapering caudad; three large ocelli present, situated between anterior half of the compound eyes; posterior border of head with a distinct collar-like lip. Pronotum subequal in length to head length, with anterior half narrowed dorsoventrally relative to posterior half (i.e., with slight downward curve in lateral view). Process present at midlength of the metatibiae in a posterior position. Forewing with costal field relatively broad (at widest point costal field as broad as pterostigma); pterostigma elongate, without crossveins; Sc terminating into C in distal two-thirds of wing length; four c-sc crossveins; two discoidal cells posterior to MP; 1cua-cup crossvein strongly basad M-CuA separation; posterior branch of MP forked. Refer to
Cantabroraphidia marcanoi Pérez-de la Fuente et al., 2010. Laterodorsal habitus. Sex unknown. Note the abundant presence of timber debris in the amber piece, surrounding the specimen. Scale bar = 1 mm.
Figs 13A, 14A
MCNA 9218, from Peñacerrada I amber; wing apex plus two minute wing fragments lacking formal descriptive significance. The sample consists of part and counterpart after the amber piece broke following the plane of the inclusion.
Sex unknown. Hind wing(?). Length of preserved fragment 2.4, maximum width of preserved fragment 2.4;wing apex rounded; pterostigma slightly increasing in width distally, infumate; pterostigma with an almost straight subdistal crossvein; all apical branches relatively short; Rs with three branches, distalmost fork very short; apicalmost r-rs crossvein (2r-rs?) meeting R distal to the pterostigma; rs-ma crossvein situated at distalmost fork of MA; MA with two branches.
The present material is distinct from other Spanish amber snakeflies but does not preserve enough detail to permit formal designation as a taxonomic entity. The presence of a pterostigmal crossvein immediately discounts Cantabroraphidia and Amarantoraphidia gen. n. MCNA 9218 highly resembles the hind wing of Styporaphidia in the shape and location of the distalmost pterostigmal crossvein. In fact, its venation is very similar to Styporaphidia? hispanica sp. n., though in the present material Rs is forked very close to the wing margin and the shape of its distalmost radial cell (most likely the second one) is somewhat different. Furthermore, the less pointed wing apex, shorter apical branches, and apical shapes of the radial, discal, and presumed second medial cells immediately distinguish MCNA 9218 from Necroraphidia gen. n. and genus and species indet. 2 (vide infra), and the almost straight pterostigmal crossvein and positions of the apicalmost r-rs (2r-rs?), rs-ma, and apicalmost ma-mp (2ma-mp?) crossveins further differentiate MCNA 9218 from Necroraphidia.
Fragmentary wing remains, genus and species indeterminate: CES 9218 part and counterpart (A), CES 376 (B), and MCNA 9316 (C–D). A distal part of a hind wing(?), part (above) and counterpart (below) B preserved distal wing fragments C preserved part of forewing D preserved part of hind wing and distal part of abdomen. Arrow points to the tip of right gonostylus 9. Scale bars = 1 mm.
Drawings of fragmentary wing remains, genus and species indeterminate: CES 9218 counterpart (A), CES 376 (B), and MCNA 9316 (C–D). A distal part of hind wing(?) B best preserved distal wing fragment C preserved part of forewing D preserved part of hind wing; only the distalmost c-sc crossvein has been tagged; basalmost preserved c-sc crossvein not depicted. Scale bars = 1 mm (A and B; C and D at the same scale).
Figs 13B, 14B
CES 376, from El Soplao amber; forewing apex and the area surrounding pterostigma from an additional wing. Some additional dorsoproximal parts of the wing are also present but with a very poor preservation, so just a few more characters can be elucidated. An indeterminate hymenopteran is present as a syninclusion.
Length as preserved ca. 5.0 (wing well preserved only in 3.4 of that length), maximum width as preserved 2.7; wing apex pointed, positioned within Rs series; wing veins brown, meeting wing margins without bifurcating; veins with strong, short setae, especially abundant on C; Sc ending and proximal r-rs crossvein (1r-rs?; very faintly preserved) situated at about same wing length, pterostigma slightly widening distally, infumate; pterostigma with a very faint subdistal, rather straight (not conspicuously arcuate), strongly oblique crossvein; uncertain if pterostigmal division present; apical branches of Rs, MA and MP subparallel; two apical branches of R distal to pterostigma; Rs with four branches, posteriormost originating before distalmost r-rs crossvein (2r-rs?), separated from it by much more than its length; rs-ma and distalmost ma-mp crossveins lacking in preserved wing fragment, so most likely with a proximal position; MA most likely with three branches.
Despite the fact that CES 376 is distinct from the other taxa in Spanish amber, it is not named as its preserved parts are not enough to resolve its affinities. The wing fragments show a high resemblance with some mesoraphidiids such as Mesoraphidia obliquivenatica (Ren, 1994) and Caloraphidia glossophylla, both from the Cretaceous compression deposit of Liaoning (China), as long as all of them share the presence of a strongly oblique, rather straight pterostigmal crossvein in a rather distal position and a distal portion of the wing with long apical branches and without crossveins other than the 2r-rs crossvein (
Figs 13C–D, 14C–D
MCNA 9316, from Peñacerrada I amber; fore- and hind wing fragments from the same side of the body and a poorly preserved part of the abdomen, although the genitalia is somewhat visible. The amber piece contains abundant organic remains. The specimen is preserved together with legs of a spider as syninclusions.
Male.Small size (inferred from preserved wing fragments). Forewing. Length as preserved about 4.5, maximum width not measurable. Two discoidal cells posterior to MP; posterior branch of MP unforked; M-CuA separation not preserved; 2A arcuate. Hind wing.Length as preservedabout 4.7, maximum width not measurable. Costal field relatively narrow; four c-sc crossveins preserved; Sc ending into C at length of first radial cell’s midlength; pterostigma not evidently infumate, not closed basally by a crossvein, at least proximally; first discoidal cell not especially small (or compact) (small in Cantabroraphidia marcanoi); rs+ma-mp crossvein present. Abdomen. Length as preserved (including genitalia) 3.8. Gonocoxites 9 with paired basal inner tubercles with small dark teeth; gonocoxites 9 elongate, with very long, stiff setae distally; gonostyli 9 very elongate, slightly upcurved; parameres not conspicuous; tergite 10 (+11?) with distalmost two or three stripes of trichobothria.
Comments. The preserved characters of MCNA 9316 are not enough to create a new taxon. The combined presence of two discoidal cells posterior to MP as in Cantabroraphidia and Amarantoraphidia gen. n., and the lack of a crossvein closing the pterostigma basally in the preserved wing length as in Necroraphidia gen. n., Ororaphidia, and Styporaphidia (showing three discoidal cells), precludes assignment to any of these genera. The lack of a crossvein closing the pterostigma basally can mean that the base of the pterostigma may have been diffuse or that the pterostigma may have been placed in a more distal position, as occurs in some mesoraphidiids. However, the inferred size of MCNA 9316 would have not been as reduced as in the minute mesoraphidiids but would have better fit with that of those mesoraphidiids with a diffuse pterostigmal base (refer to comments for Necroraphidia gen. n.). Furthermore, the presence of a rs+ma-mp crossvein in the hind wing as occurs in MCNA 9316 is a very rare character among described Mesoraphidiidae but more common in modern snakeflies, although it is also present in Styporaphidia magia. The morphology of the genitalia appear distinctive enough as to be recognizable in future findings. It is interesting to note that
The following keys include only those species sufficiently diagnostic to name. As such, the additional morphospecies are not considered here and so all new material should be cross-referenced with the above accounts on those more fragmentarily known taxa. Note that Alavaraphidia imperterrita sp. n. is absent from the first key given that the diagnostic parts of the forewings are not preserved.
Key to genera and species of Spanish amber Raphidioptera based on wings| 1 | Fore-/hind wing with pterostigmal crossvein | 2 |
| – | Fore-/hind wing without pterostigmal crossvein (Mesoraphidiidae) | 4 |
| 2 | Fore-/hind wing with sparse or moderate crossvenation; at most with two radial cells, three medial cells, and three discoidal cells posterior to MP; often with no closed subradial cells (i.e., cells between Rs branches) (Mesoraphidiidae) | 3 |
| – | Fore-/hind wing with relatively rich crossvenation; with at least three radial cells, and numerous subradial, medial, and discoidal cells posterior to MP (Baissopteridae) | Baissoptera? cretaceoelectra sp. n. |
| 3 | Fore-/hind wing with a single pterostigmal crossvein; distalmost r-rs crossvein meeting R within pterostigma, Rs (proximally) forking before distalmost r-rs; rs-ma crossvein meeting MA before its distalmost fork | Necroraphidia arcuata gen. et sp. n. |
| – | Hind wing with two pterostigmal crossveins; distalmost r-rs crossvein meeting R beyond pterostigma, Rs forking at r-rs crossvein; rs-ma crossvein meeting MA after its distalmost fork | Styporaphidia? hispanica sp. n. |
| 4 | Forewing with four c-sc crossveins; apicalmost branch of CuA forked near wing margin; M-CuA separation between 1cua-cup and 2cua-cup crossveins (closer to latter) | Cantabroraphidia marcanoi Pérez-de la Fuente et al., 2010 |
| – | Forewing with six c-sc crossveins; apicalmost branch of CuA unforked; M-CuA separation at the 1cua-cup crossvein | Amarantoraphidia ventolina gen. et sp. n. |
| 1 | Head not rhomboidal; compound eyes shorter, equal, or not much longer than head posterior to eyes; antennae with a low number of flagellomeres (≤ 26) | 2 |
| – | Head rhomboidal; compound eyes longer than head posterior to eyes; antennae very elongate, with a high number of flagellomeres (≥ 38) | 5 |
| 2 | Head ovoid; compound eyes shorter or equal than head posterior to eyes; antennae with less than 26 flagellomeres | 3 |
| – | Head quadrangular; compound eyes slightly longer than head posterior to eyes;antennae with 26 flagellomeres | Cantabroraphidia Pérez-de la Fuente et al., 2010 |
| 3 | Compound eyes not distinctly shorter than head posterior to eyes; ocelli positioned between posterior half of compound eyes | 4 |
| – | Compound eyes distinctly shorter than head posterior to eyes; ocelli positioned between anterior half of compound eyes | Amarantoraphidia gen. n. |
| 4 | Compound eyes nearly equal than head posterior to eyes; antennae longer than head length; posterior border of head without a collar-like lip | Nanoraphidia Engel, 2002 |
| – | Compound eyes apparently slightly longer or equal than head posterior to eyes; antennae shorter than head length; posterior border of head with a collar-like lip | Grimaldiraphidia Bechly & Wolf-Schwenninger, 2011 |
| 5 | Compound eyes two times head posterior to eyes; clypeus short; antennae with around 38 flagellomeres; pronotum longer than head; bilobed extensions of third tarsomere lacking digitiform processes | Lebanoraphidia Bechly & Wolf-Schwenninger, 2011 |
| – | Compound eyes ca. 1.4 times head posterior to eyes; clypeus especially elongate; antennae with more than 38 flagellomeres; pronotum shorter than head; bilobed extensions of third tarsomere with distal digitiform processes | Alavaraphidia gen. n. |
Recently,
Seven larval mesoraphidiids have been described up to now from five Cretaceous amber localities around the world (Table 2). This circumstance contrasts with the lack of larval records as compressions, mostly due to the decreased fidelity of preservation of the soft body tissues in such deposits. Taking into account that the larval record of the remainder of holometabolous orders is similarly not that abundant in Cretaceous ambers, the relatively high number of larval mesoraphidiids most likely indicates that at least some, if not all, were corticolous, i.e., they lived under bark, as is well known in extant snakeflies (
Like many moderate- to large-sized insects, snakeflies are difficult to find as amber inclusions, particularly complete specimens in Cretaceous ambers. It is not surprising that those individuals recovered as complete, or relatively complete, inclusions are among the smallest members of the order. Nonetheless, immatures and fragments of much larger snakefly species are also found as amber inclusions as evidenced by the rich Spanish fauna. What is more curious is that among the more abundant Cretaceous amber sources such as Lebanon, Myanmar, New Jersey, and Canada, the reported snakeflies remain relative rarities (Table 2; Engel pers. obs.), and the fragmentary remains of numerous, sometimes larger, species that are observed in the Spanish deposits are not seen in these other outcrops. Why should the Iberian fauna be so particularly rich? It is similarly enigmatic that as of yet the younger French ambers (late Albian to Cenomanian), with the exception of two larval head capsules from the late Albian Archingeay - Les Nouillers outcrop in southwestern France (
However, another hypothesis could explain the high abundance/diversity of snakeflies in Spanish amber, apart from its insularity. Perhaps this abundance is partly owing to the common occurrence of wildfires in the Spanish amber environment, inferred from evidence at several Spanish amber localities (see
The discovery of the first amber baissopterid is especially remarkable. Furthermore, the presence of the family in the Iberian territory during the Early Cretaceous completes the remarkable preexisting paleogeographic baissopterid hiatus between the Cretaceous localities from eastern Asia and Brazil.
With the fossils herein described, the Albian amber of Spain currently harbors the greatest abundance and diversity of snakeflies in Cretaceous resins. As is also suggested by other paleoentolomological records, this significant snakefly paleodiversity may reflect a faunistic singularity of the Iberian territory during the Albian perhaps as a consequence of its geographic isolation during the Jurassic and the Cretaceous, or a combination of this with an environment resulting from episodic wildfires. Wildfires would have increased the availability of dead wood as a substrate for the xylophagous or xylophilous insects to develop, which, in turn, could have aided the increase in the xylophilous, predatory snakefly populations.
We thank the Museo de Ciencias Naturales de Álava for providing samples from the Peñacerrada I outcrop, and the staff from El Soplao Cave and the Instituto Geológico y Minero de España (IGME) for providing samples from the El Soplao outcrop. The authors are indebted to R. López-del Valle for the preparation of the samples, and to two anonymous reviewers for their helpful comments. This paper is part of the first author’s doctoral dissertation, supported by an APIF grant from the University of Barcelona. This work is a contribution to the projects CGL2008-00550/BTE and CGL2011-23948/BTE, together as “The Cretaceous amber of Spain: A multidisciplinary study”, of the Spanish Ministry of Economy and Competitivity, and the IGME project 491-CANOA 35015 “Investigación científica y técnica de la Cueva de El Soplao y su entorno geológico” (to EP). This is also a contribution of the Division of Entomology, University of Kansas Natural History Museum, partial support for which was provided by US National Science Foundation grant DEB-0542909 (to MSE).