(C) 2012 Vladimir Pešić. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
New records of water mites (Acari: Hydrachnidia) from Baishih River drainage of north Taiwan, are presented. Twelve species are recorded, of which ten are new for Taiwan; two of them, Torrenticola projectura and Hygrobates taiwanicus are described as new for science.
Acari, water mites, new species, running waters, Baishih River drainage, Taiwan
Taiwan is a large island (35, 881 km2) located in the Asian Pacific region. As the Tropic of Cancer bisects the Island, the climate changes from subtropical to tropical from the northern to southern parts of the island. The climate of Taiwan is heavily influenced by monsoons and typhoons which bring an annual rainfall of ~ 2150 mm, with 80% of precipitation concentrated in the summer (or wet season), i.e. May–October. As there is a central mountain range extending from the north to the south of the island with surrounding plateau and hills, two third of the island is covered by mountainous area with an elevation 100–>3000 m. All rivers in Taiwan are characterized by short length and steep gradient with rapid flows and small drainage basins associated with the steep terrain. Such diverse patterns of geographical typology and hydrology result in the high levels of habitat heterogeneity and aquatic biodiversity of the Taiwan rivers (
Water mites are one of the most ubiquitous components of the lotic communities. However, detailed investigation on their taxonomy, distribution and ecology is generally lacking in the Asian Pacific region. Furthermore, the water mite fauna of Taiwan is very incompletely known. Recently, we published the results of the first collection of water mites from Taiwan, listing three species of the family Torrenticolidae, two of them were new to science (
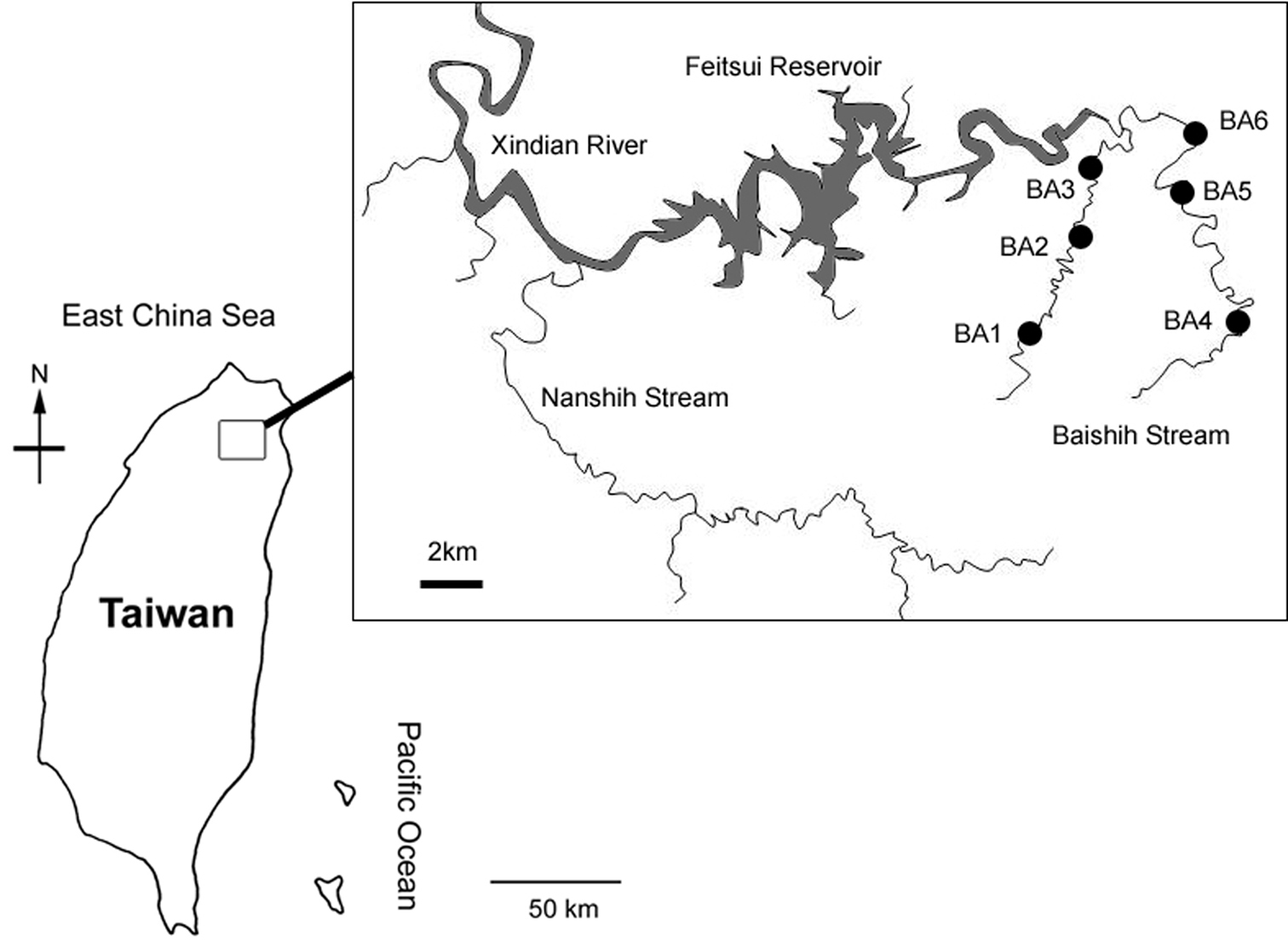
Map of study area showing location of the six sampling sites.
Baishih River system is located in the northern Taiwan. It is one of the major upstream feeder tributary of the urbanised Xindian River which runs into Danshui River in the New Taipei City. Baishih River originates from both Mount Sanfonsun and Mount Yingtzuling, and drains southwestward into Feitsui Reservoir which provides water supply for the population of the Taipei metropolitan area. Baishih River is ~50 km long and the catchment area is about 310 km2. The present study focused on two major tributaries Chinkualiao Stream and Diyu Stream of Baishih River drainage. All study sites are relatively undisturbed by human activities as both Chinkualiao Stream and Diyu Stream are located within the protected area. The riparian area is dominated by natural forest with discontinuous distribution of cultivated land owned by local villagers.
The paper aims to describe the diversity and distribution pattern of water mites from the northern Taiwan. In the present study, twelve species are identified, two of them are new to science. Descriptions of these species are given in this paper.
Material and methodsIn this study, water mites were collected at six sampling sites (Table 1; Figs 1, 2A–F) using standard Surber sampling method with WaterMark® Surber Type Stream Bottom Sampler (500 μm mesh). Water mites were sorted in the laboratory with the aid of a stereo microscope and preserved in 90% ethanol. All the material has been collected by Rita Yam and this is not repeated in the text. Holotypes and paratypes of the new species are deposited in the National Museum of Natural Science (NMNS), Taichung, Taiwan. Other materials are kept in the collections of the Ecology and Conservation Laboratory, Department of Bioenvironmental Systems Engineering, National Taiwan University (ECL).
List of the sampling sites in the present study.
| Site code | Latitude | Longitude | Catchment | Subcatchment | Altitude (m asl) |
|---|---|---|---|---|---|
| BA-1 | 121.656242°E, 24.882695°N | Baishih River | Chinkualiao Stream | 365 | |
| BA-2 | 121.656328°E, 24.883970°N | Baishih River | Chinkualiao Stream | 343 | |
| BA-3 | 121.674029°E, 24.924912°N | Baishih River | Chinkualiao Stream | 250 | |
| BA-4 | 121.701640°E, 24.908065°N | Baishih River | Diyu Stream | 232 | |
| BA-5 | 121.697561°E, 24.915911°N | Baishih River | Diyu Stream | 210 | |
| BA-6 | 121.696603°E, 24.933962°N | Baishih River | Diyu Stream | 190 | |
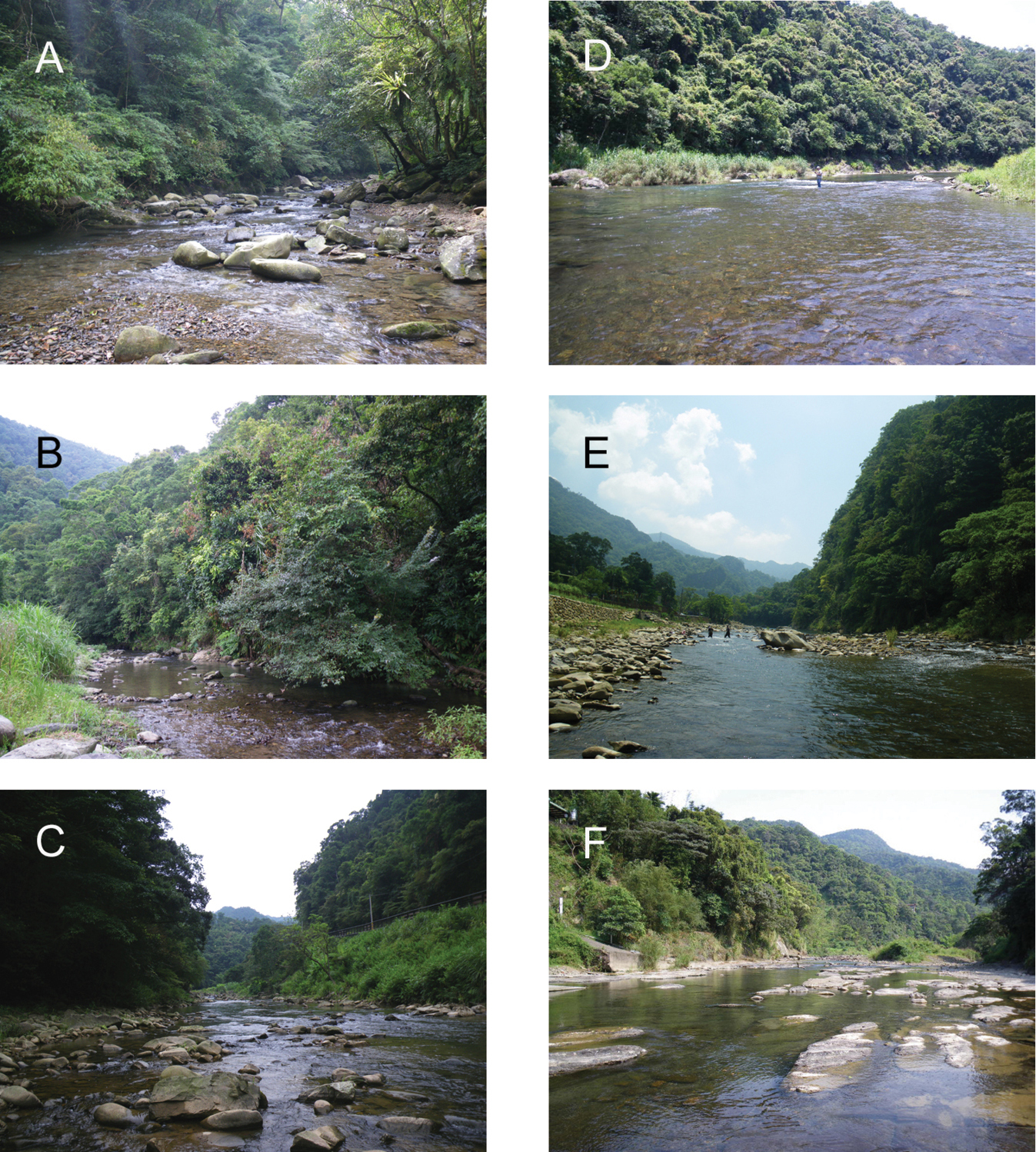
Photographs of the six study sampling sites in the present study. A = BA-1 B = BA-2 C = BA-3 D = BA-4 E = BA-5 F = BA-6.
In the section ‘Material examined’, the sampling site codes were derived from the geographical database Rita Yam (see Table 1). The composition of the material is given as: males/females/deutonymphs or adults/deutonymphs. All measurements are given in µm. The following abbreviations are used: asl = above sea level, Cx-I = first coxae, Cxgl-4 = coxoglandularia of fourth coxae, dL = dorsal length, H = height, L = length, W = width, I-L-6 = Leg 1, sixth segment (tarsus), mL = medial length, n = number of specimens examined, P-1 = palp, first segment, Vgl-2 = ventroglandulare 2.
Systematics Family Hydrodromidae K. Viets Genus Hydrodroma Koch, 1837ECL-BA-1: 01.vii.2010 0/1/0 (mounted).
Due to the presence of a single, short swimming hair on segments four and five of the third and fourth legs and the shape of palp (Fig. 3A), the single specimen from the Chinkualiao stream shows general conformity with Hydrodroma rheophila. As
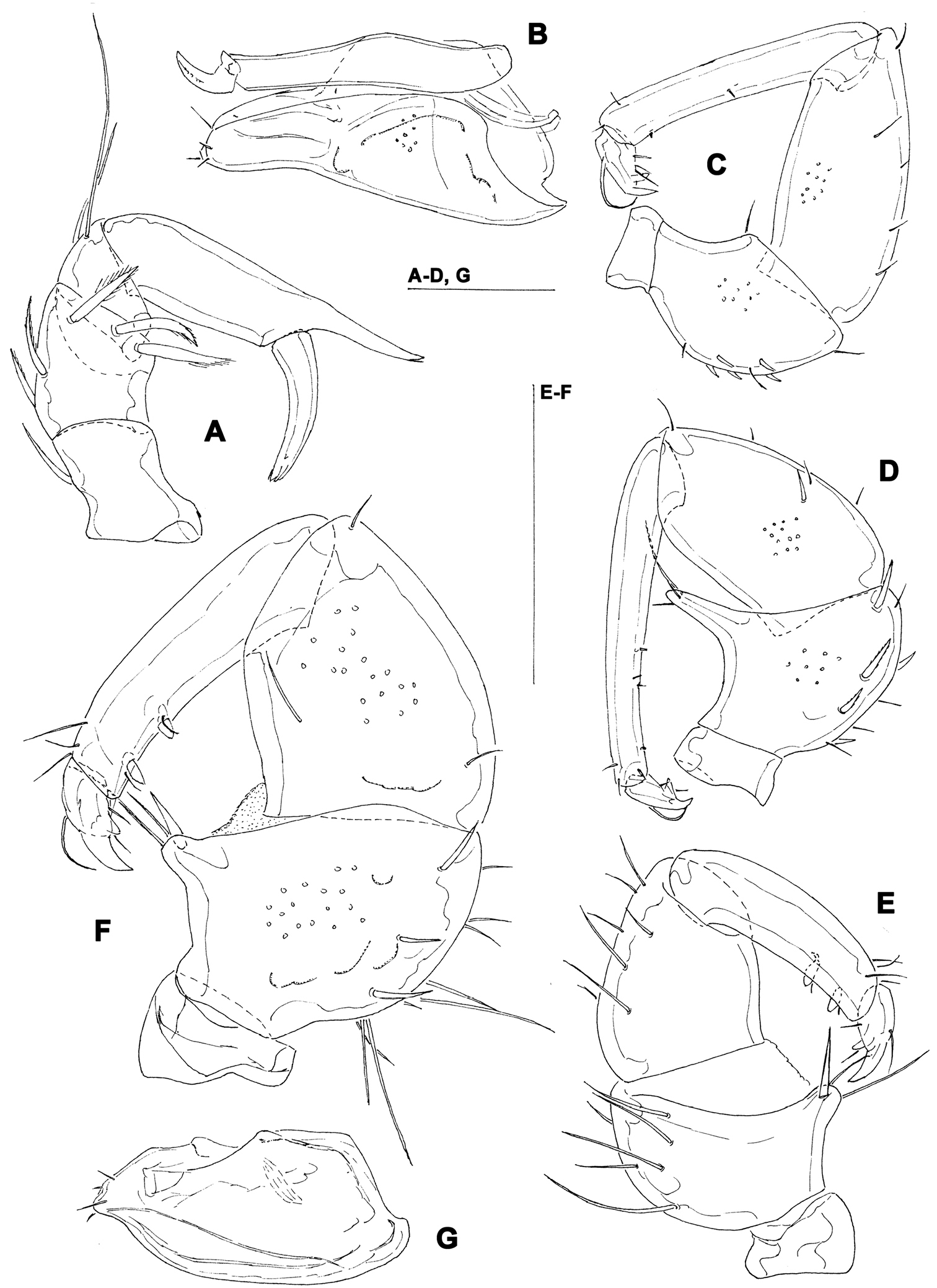
A Hydrodroma cf. rheophila Cook, 1967, female: palp. B–C Sperchon rostratus Lundblad, 1969, female: B = palp C = capitulum and chelicera D Sperchon cf. gracilipalpis Lundblad, 1941, female: palp E–G Sperchon cornutoides Lundblad, 1941 (F–G = female, E = male) E–F = palp G = capitulum. Scale bars = 100 µm.
India, Indonesia, Iran, Oman, Balkan. New for Taiwan.
http://species-id.net/wiki/Sperchon_rostratus
Figs 3B–CECL-BA-3: iii.2010 0/1/0 (mounted). ECL-BA-5: iii.2010 0/1/0.
The two females examined in the present study fit well the description of Sperchon rostratus Lundblad, 1969. Figures 3B–C show some morphological details of the specimen from Chinkualiao stream.
Burma, China (Guizhou Province), Turkey, Iran. New forTaiwan.
http://species-id.net/wiki/Sperchon_gracilipalpis
Fig. 3DECL-BA-2: iii.2010 1/1/0. ECL-BA-3: vi.2010 0/1/0 (mounted). ECL-BA-4: iii.2010 5/0 1/0/0 (mounted); iv.2010 0/1/0; 27.vii.2010 1/1/0. ECL-BA-5: iii.2010 1/1/0; iv.2010 0/1/0. ECL-BA-6: iv.2010 0/2/0.
The specimens from Baishih River drainage are provisionally assigned to the Oriental species Sperchon gracilipalpis Lundblad, 1941. However, they resemble both Sperchon gracilipalpis and the Palaearctic Sperchon hispidus Koenike, 1895 (common character state: dorsum of both sexes with seven paired and one unpaired (occasionally paired) muscle attachment plates; III/IV-L-3-5 with numerous pinnate dorsal setae; similar shape of palp (Fig. 3D) and male ejaculatory complex). The diagnostic differences separating these two species have never been discussed. More material of Sperchon gracilipalpis from the type area should be investigated in order to get an insight on further diagnostic differences.
SE Asia, China (Guizhou Province). New forTaiwan.
ECL-BA-1: 10.viii.2009 0/1/0; iii.2010 0/1/0; 01.vii.2010 0/1/0 (mounted); 02.vii.2010 0/2/0; 30.x.2010 1/0/0. ECL-BA-2: 27.vii.2010 2/0/0 (1/0/0 mounted). ECL-BA-3: 23.ix.2009 0/1/0; iii.2010 0/1/0. ECL-BA-4: iii.2010 0/1/0; 27.vii.2010 1/2/0 (1/1/0 mounted). ECL-BA-5: iv.2010 0/1/0. ECL-BA-6: iii.2010 0/1/0.
The specimens examined from Baishih River drainage agrees with the description of Sperchon cornutoides Lundblad, 1941, a species known only from Java (
Indonesia. New for Taiwan.
ECL-BA-1: 07.vii.2009 1/1/0; 23.ix.2009 2/0; iv.2010 13/0; 01.vi.2010 1/0; vii.2010 29/0; 27.vii.2010 2/1/0. ECL-BA-2: 02.ix.2009 11/2(1juvenile)/0; ii.2010 57/0; iii.2010 3/1/1; iv.2010 0/2/0; vi.2010 2/2/0; 27.vii.2010 17/0; 29.ix.2010 5/0; 30.x.2010 1/0. ECL-BA-3: 01.vii.2009 1/0/0; 23.vii.2009 1/0/0; ii.2010 4/11/0; ii.2010 10/0; iv.2010 0/1/0; 27.vii.2010 1/0/0. ECL-BA-4: 02.vii.2009 1/0/0; ii.2010 2/1/0; iii.2010 6/4/0; iv.2010 0/1/0; vi.2010 1/0/0; vii.2010 19/0; 27.vii.2010 5/0/0; 30.x.2010 1/0. ECL-BA-5: iii.2010 1/1/0; 01.vi.2010 4/0/0; vii.2010 11/0; 07.vii.2010 1/0/0; 30.x.2010 1/0/0. ECL-BA-6: 17.vi.2009 3/0; iii.2010 2/9/0; vi.2010 0/1/0; 01.vii.2010 1/0/0; 27.vii.2010 1/0/0; 29.ix.2010 1/0.
It is the most abundant species in our material. For an analysis of diagnostic characters of populations from Taiwan tentatively assigned to Monatractides circuloides see:
Malaysia, Thailand, Taiwan.
ECL-BA-1: 07.vii.2009 1/0/0. ECL-BA-3: 27.vii.2010 1/4/0. ECL-BA-4: 02.vii.2009 0/2/0; ii.2010 0/1/0; iii. 2010 0/4/0; iv.2010 0/1/0 (mounted); vi.2010 1/1/0; 27.vii.2010 0/1/0; ECL-BA-5: 21.vii.2009 1/0/0; iii.2010 0/2/0; 01.vi.2010 1/1/0; 07.vii.2010 0/1/0. ECL-BA-6: ii.2010 1/0/0; iii. 2010 0/6/0; 27.vii.2010 0/1/0; 29.ix.2010 3/0.
The studied material was collected from February to September. For an analysis of diagnostic characters of Torrenticola taiwanicus see:
Taiwan.
urn:lsid:zoobank.org:act:41E5931A-EFE2-42B6-B0D5-A86EB897B9FF
http://species-id.net/wiki/Torrenticola_projectura
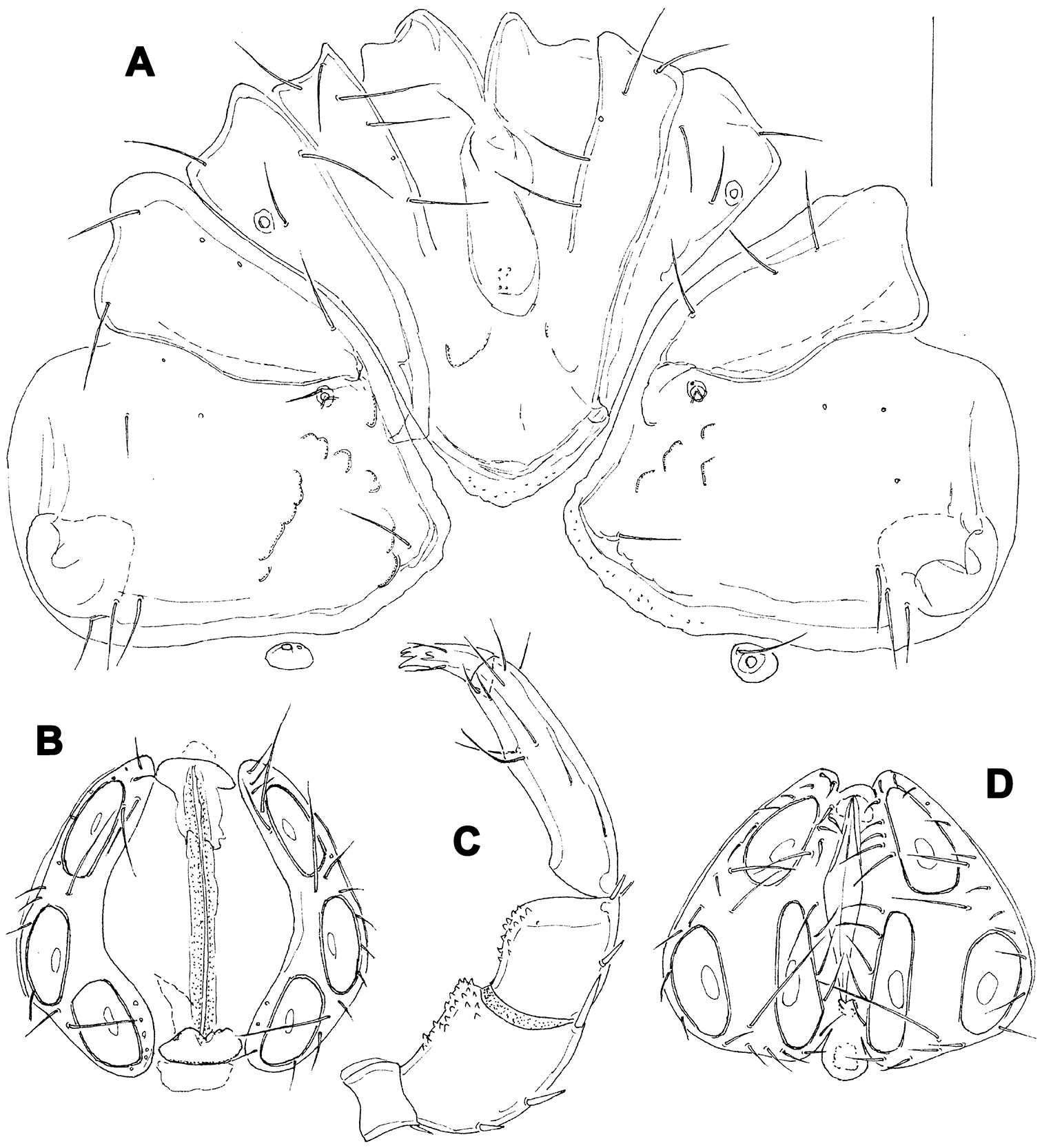
Figs 4A–EHolotype male (NMNS-6894-002), dissected and slide mounted, Taiwan, BA-1, Baishih River drainage, Chinkualiao Stream, 27.vii.2010. Paratype: one male, same data as holotype; one male, dissected and slide mounted, BA-2, Chinkualiao Stream, 02.ix.2009.
Male (female unknown). Cx-IV extending posterior to genital field; excretory pore posterior to line of primary sclerotization, Vgl-2 slightly posterior to excretory pore; capitulum with a short rostrum; medial suture line of Cx-II+III short; P-2 ventrodistal projection cone-shaped, pointed towards distal, P-3 ventrodistal projection larger than projection of P-2, P-3 with a long tapering ventral protrusion which curves distally.
Male (holotype, in parentheses measurements of paratype, n = 1) — Idiosoma (ventral view: Fig. 4B) L 788 (738), W 675 (625); dorsal shield (Fig. 4A) L 709 (658), W 544 (525), L/W ratio 1.3 (1.25); dorsal plate 663 (614); shoulder plate L 197-200 (189), W 78-82 (71-75), L/W ratio 2.4-2.6 (2.5-2.7); frontal plate L 163 (154-158), W 71-76 (70-72), L/W ratio 2.1-2.3 (2.1-2.3); shoulder/frontal plate L ratio 1.2 (1.2). Capitular bay L 150 (153), Cx-I total L 270 (263), Cx-I mL 119 (109), Cx-II+III mL 80 (70); ratio Cx-I L/Cx-II+III mL 3.4 (3.8); Cx-I mL/Cx-II+III mL 1.5 (1.6). Genital field L/W 156 (159)/124 (128), L/W ratio 1.26 (1.24); ejaculatory complex normal in shape, L 239 (211); distance genital field-excretory pore 180 (150), genital field-caudal idiosoma margin 272 (234). Capitulum (Fig. 4E) ventral L 231 (225); chelicera total L 275 (269), claw L 65 (65), basal segment L 212 (214), L basal segment/claw ratio 3.3 (3.3); palp (Figs 4C-D) total L 257 (256), dL: P-1, 35 (34); P-2, 72 (70); P-3, 52 (53); P-4, 72 (72); P-5, 26 (27); dL P-2/P-4 ratio 1.0 (0.97); P-2 ventrally slightly convex, dorsally convex in the proximal, straight in the distal part, ventrodistal projection cone-shaped, pointed towards distal, P-3 with a long tapering ventral protrusion which curves distally, P-4 distally slightly tapering, slightly curved, ventral setae (one long and three short) on flat hump.
Torrenticola projectura sp. n., male: A = dorsal shield B = idiosoma, ventral view C = palp, medial view D = palp (P-1 missing), medial view E = capitulum. Scale bars = 100 µm.
The species is named after the distinctive shape of ventrodistal projection of P-3; ‘projectura’ - Latinised form of ‘projection’.
The shape of ventrodistal projection of P-3 of the new species is very distinctive and can separate it from all other Torrenticola species.
Taiwan.
urn:lsid:zoobank.org:act:8A67823A-EC83-418D-80B8-2934848B1D9D
http://species-id.net/wiki/Hygrobates_taiwanicus
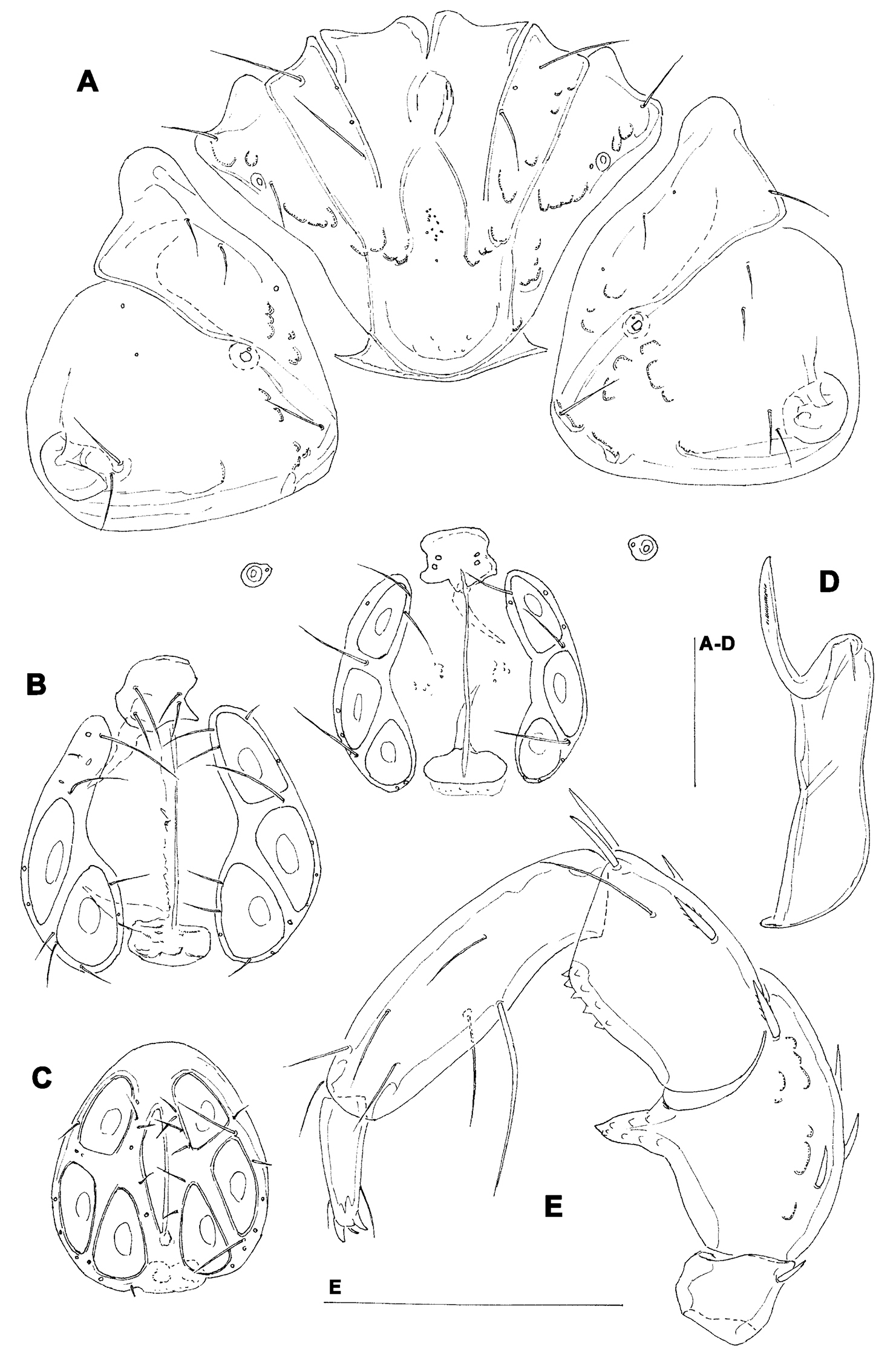
Figs 5 –6Holotype female (NMNS-6894-001), dissected and slide mounted, Taiwan, BA-3, Chinkualiao Stream, vi.2010. Paratypes: 0/1/0, same data as holotype; 1/1/0 (mounted), BA-1, Chinkualiao Stream, iii. 2010; 0/3/0, BA-4, Diyu Stream, iii.2010; 0/1/0, BA-5, Diyu Stream, iv.2010.
Idiosoma L > 750; acetabula arranged in an obtuse angle; female pregenital sclerite with four setae, its anterior margin extending beyond anterior margin of genital plates; female genital plates longer than gonopore; posterior end of female genital plates posterior to postgenital sclerite; P-2 ventrodostal projection elongate, cone-shaped; proximoventral seta on P-4 longer and thicker than distoventral seta.
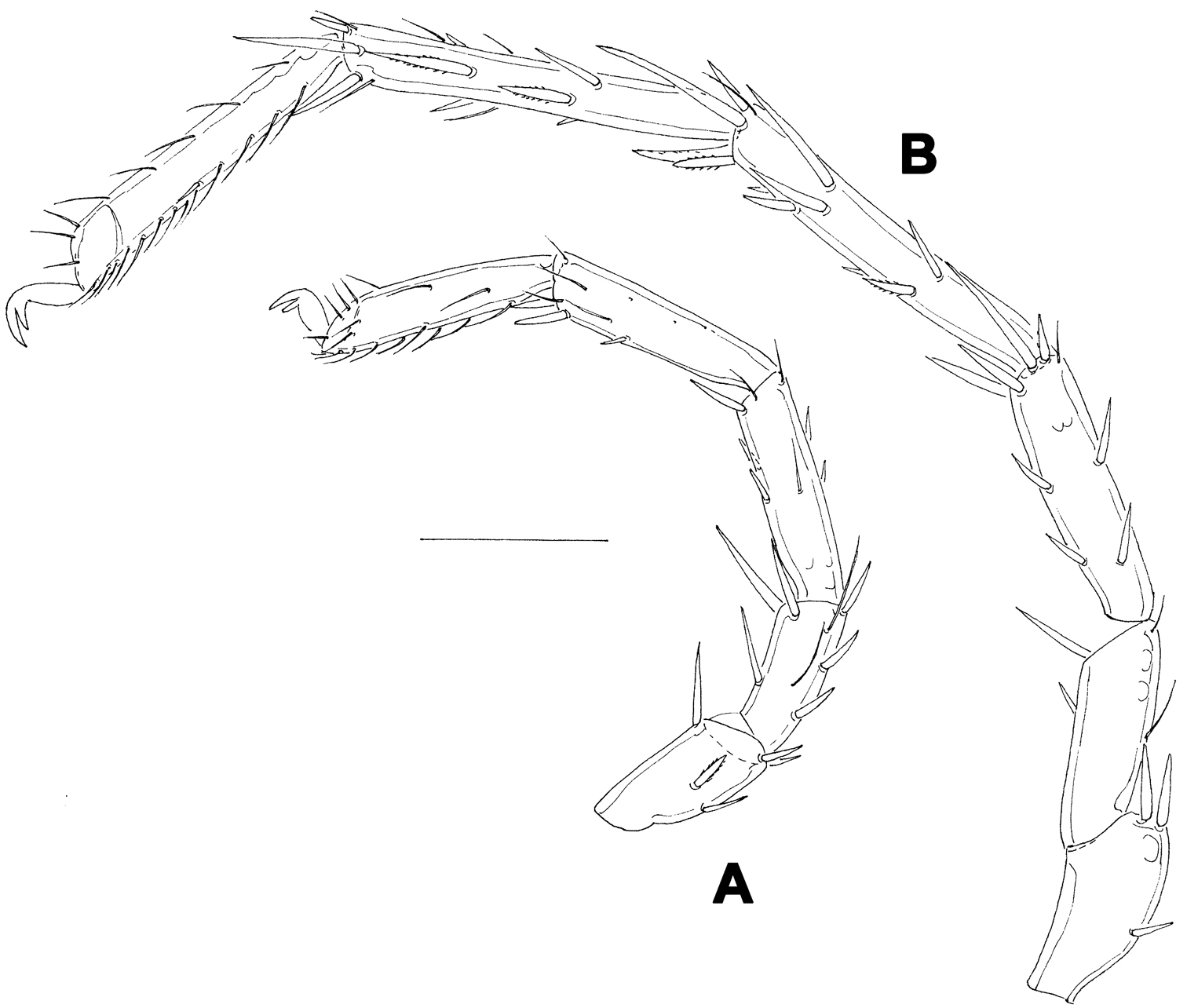
Female (holotype, in parentheses paratype). Idiosoma L 775 (778), W 613 (706); integument soft, with very fine striation. Capitulum broadly fused with Cx-I. Coxae (Fig. 5A): coxal field L 322 (359), Cx-III W 472 (500), posterior end of anterior coxal group rounded-truncate, Cx-I+II L/W 238 (259)/319 (344), posterolateral apodemes extending slightly beyond sclerotization. Suture line of Cx-III and Cx-IV nearly straight, incomplete, extending to near Cxgl-IV; medial margin of Cx-IV rounded-triangular. Genital field (Fig. 5A-B): L/W 181 (213)/173 (203), acetabular plates with smooth border, L 143-147 (175); acetabula arranged in an obtuse triangle, L Ac-1-3: 55-56 (66), 55-58 (72), 44 (66-68); pregenital sclerite with four setae, its anterior margin extending beyond anterior margin of genital plates. Excretory pore smooth. Palp (Fig. 5E): total L 358 (423), dL: P-1, 26 (34); P-2, 98 (111); P-3, 70 (85); P-4, 121 (136); P-5, 43 (57); dL P-2/P-4 ratio, 0.81 (0.82); chelicera (Fig. FD) total L 250 (319), basal segment L 157 (200), claw L 100 (125), L ratio basal segment/claw 1.6 (1.6). Legs: dL of I-L-2-6 (Fig. 6A): 69 (89), 90 (109), 131 (156), 134 (159), 138 (161); dL of IV-L (Fig. 6B): 108 (128), 103 (128), 151 (184), 213 (263), 225 (272), 193 (234).
Males (n = 2): similar to female, except for genital field. Idiosoma L 775-794, W 625-656; coxal field L 338, Cx-III W 463, Cx-I+II L/W 231/334; genital field L/W 160-170/148-151, anterior margin convex, L Ac-1-3: 51-58, 63-66, 63-66; ejaculatory complex L 169. Palp (Fig. 5C): total L 365-373, dL: P-1, 28; P-2, 99-100; P-3, 71-75; P-4, 119-123; P-5, 48-47; dL P-2/P-4 ratio, 0.81-0.83; chelicera: basal segment L 194, claw L 103, L ratio basal segment/claw 1.9. Legs: dL of I-L-2-6: 74, 91-97, 138-141, 139-141, 142-144; dL of IV-L: 114, 106, 156, 228, 232, 203.
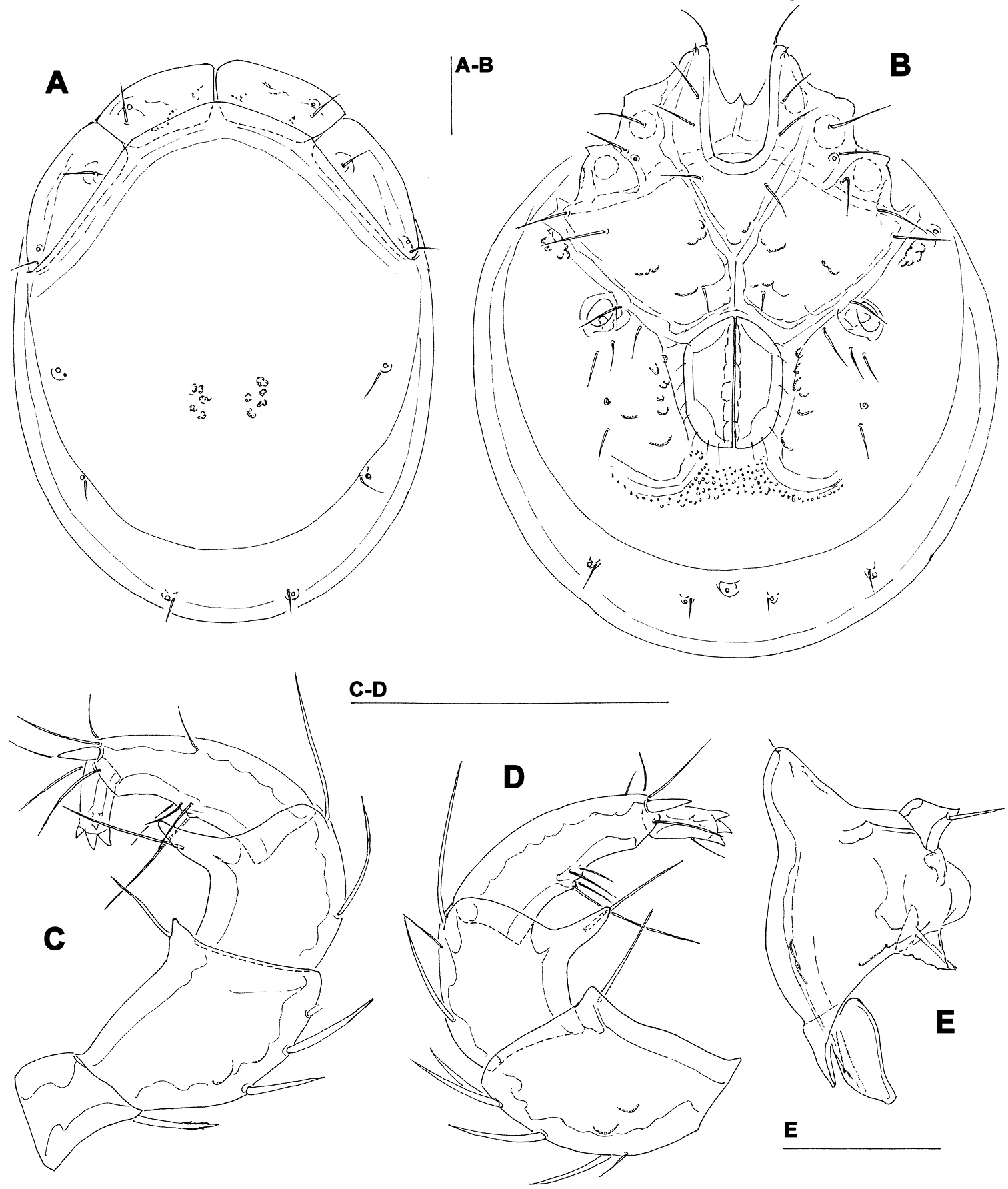
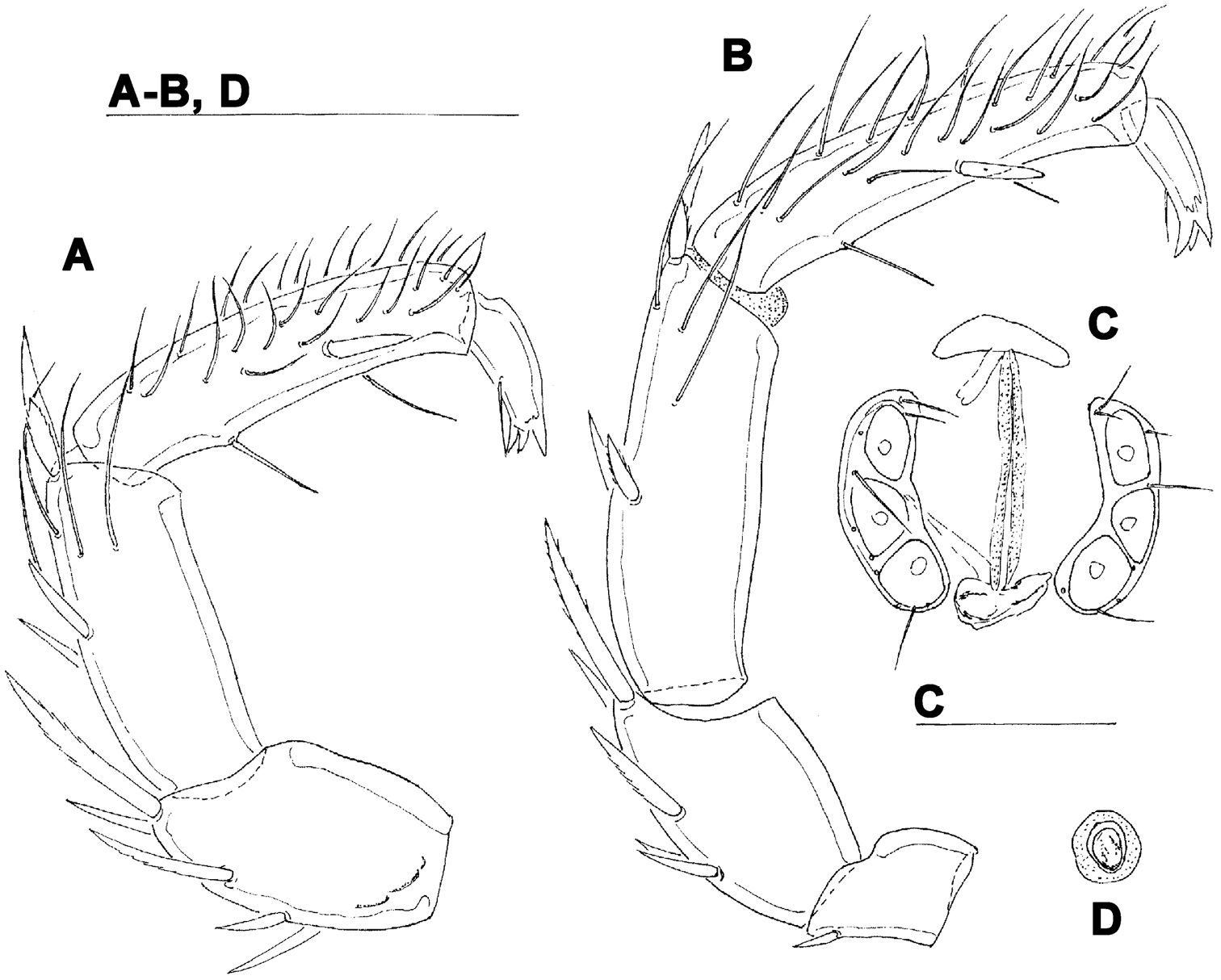
Hygrobates taiwanicus sp. n. (A–B, D–E = female; C = male): A = coxal and genital field B–C = genital field (paratypes) D = chelicera E = palp. Scale bars = 100 µm.
Hygrobates taiwanicus sp. n., female: A = I-L-2-6 B = IV-L. Scale bar = 100 µm.
The species is named for its occurrence in Taiwan.
The new Hygrobates species belong to the group of the species characterized byt the presence of two or three setae upon, and inside the margin of, the female pregenital sclerite and proximoventral seta on P-4 longer and thicker than distoventral seta. This group includes three species (
Distribution. Taiwan.
http://species-id.net/wiki/Hygrobates_longiporus
Figs 7A–DECL-BA-3: 23.ix.2009 4/0; ii.2010 0/3/0; vi.2010 2/0. ECL-BA-4: 17.vi.2009 1/0/0. ECL-BA-5: iv.2010 1/0/0; 07.vii.2010 5/0; 27.vii.2010 19/0; 30.x.2010 0/2/0. ECL-BA-6: 17.vi.2009 3/2/0; 17.vi.2009 7/0; vi.2010 0/3/0; 01.vii.2010 2/0/0; 27.vii.2010 12/0 (0/1/0 mounted); 29.ix.2010 0/1/0.
The most abundant Hygrobates species from the Baishih River drainage resemble to the Palaeractic Hygrobates longiporus Thor. In shape of coxal (Fig. 7A) and genital field (Figs 7B, D) and palp (Fig. 7C), no differences can be found to differentiate the specimens from Taiwan. Two additional species closely related to Hygrobates longiporus are described from Oriental part of China (
Hygrobates cf. longiporus Thor, 1898 (A-C = female, D = male): A = coxal field C = palp B, D = genital field. Scale bar = 100 µm.
Palaearctic. New for Taiwan.
http://species-id.net/wiki/Hygrobates_hamatus
Figs 8A–CECL-BA-3, vi.2010 0/1/0 (mounted). ECL-BA-5, 20.viii.2009 0/1/0.
The specimens from Taiwan are in a good agreement with description of the Oriental Hygrobates hamatus. This species is very similar to Hygrobates soari K. Viets, 1911, a species widspread in the Afrotropical region, reaching in its distribution to northern Oman (
In addition, we gave some measurements of the specimen of Hygrobates hamatus from Baishih River drainage which represented the easternmost record finding of this species.
Female. Idiosoma L/W 881/625. Coxae (Fig. 8A): coxal field L 353, Cx-III W 463, Cx-I+II L/W 260/337. Genital field (Fig. 8B): L/W 145/182, acetabular plates L 101-105; L Ac-1-3: 35-37, 38-39, 29-32. Palp (Fig. 8C): total L 442, dL: P-1, 26; P-2, 118; P-3, 101; P-4, 163; P-5, 34; dL P-2/P-4 ratio, 0.72.
Hygrobates hamatus K. Viets, 1935, female: A = coxal field B = genital field C = palp. Scale bars = 100 µm.
SE Asia, India, New Guinea, Australia, Iran. New for Taiwan.
http://species-id.net/wiki/Atractides_spatiosus
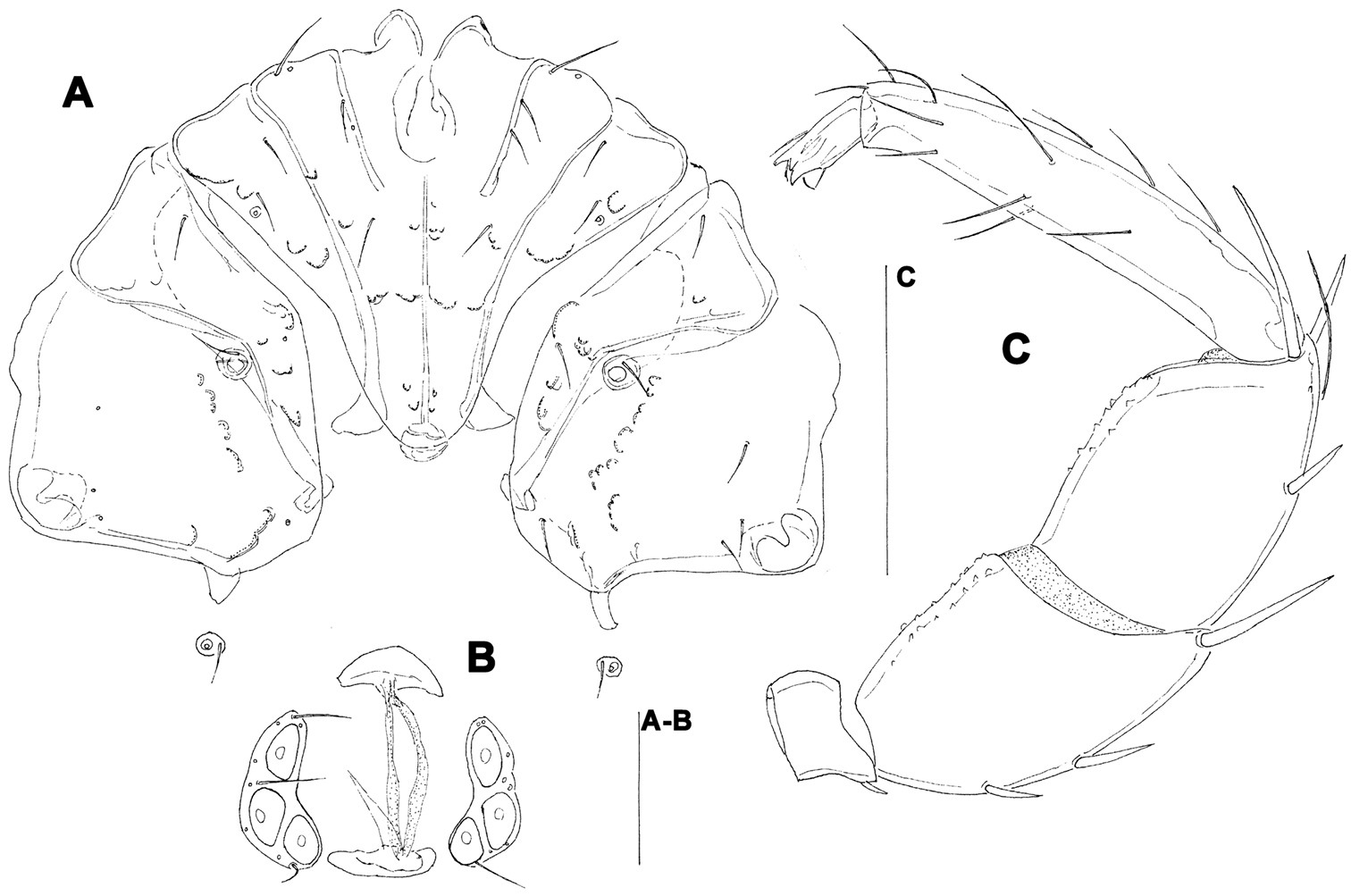
Figs 9A , 10BECL-BA-1: 01.vi.2009 0/1/0; 02.vii.2010 0/1/0. ECL-BA-2: iv.2010 0/2/0; vi.2010 0/1/0. ECL-BA-3: 03.ix.2009 0/3/0. ECL-BA-4: iii.2010 0/4/0 (0/1/0 mounted); iii.2010 0/1/0. ECL-BA-5: iv.2010 0/1/0; 07.vii.2010 0/4/0. ECL-BA-6: iii.2010 0/1/0; iv.2010 0/2/0; vi.2010 0/1/0; ix.2010 0/1/0.
The examined female specimens from Baishih River system resemble Oriental Atractides spatiosus (K. Viets, 1935). A problem exists regarding the similarity between Atractides spatiosus and Atractides cognatus (K. Viets, 1935), a further taxon originally introduced as a subspecies and later elevated to species rank by Pešić and Smit (2009). However, the populations attributed by Pešić and Smit (2009) to Atractides cognatus differ from the original description of the later (see: K.
A Atractides cf. spatiosus (K. Viets, 1935), female: A = palp, medial view (P-1 missing) B–D Atractides cf. cognatus (K. Viets, 1935), female: B = palp, medial view C = genital field D = excretory pore. Scale bars = 100 µm.
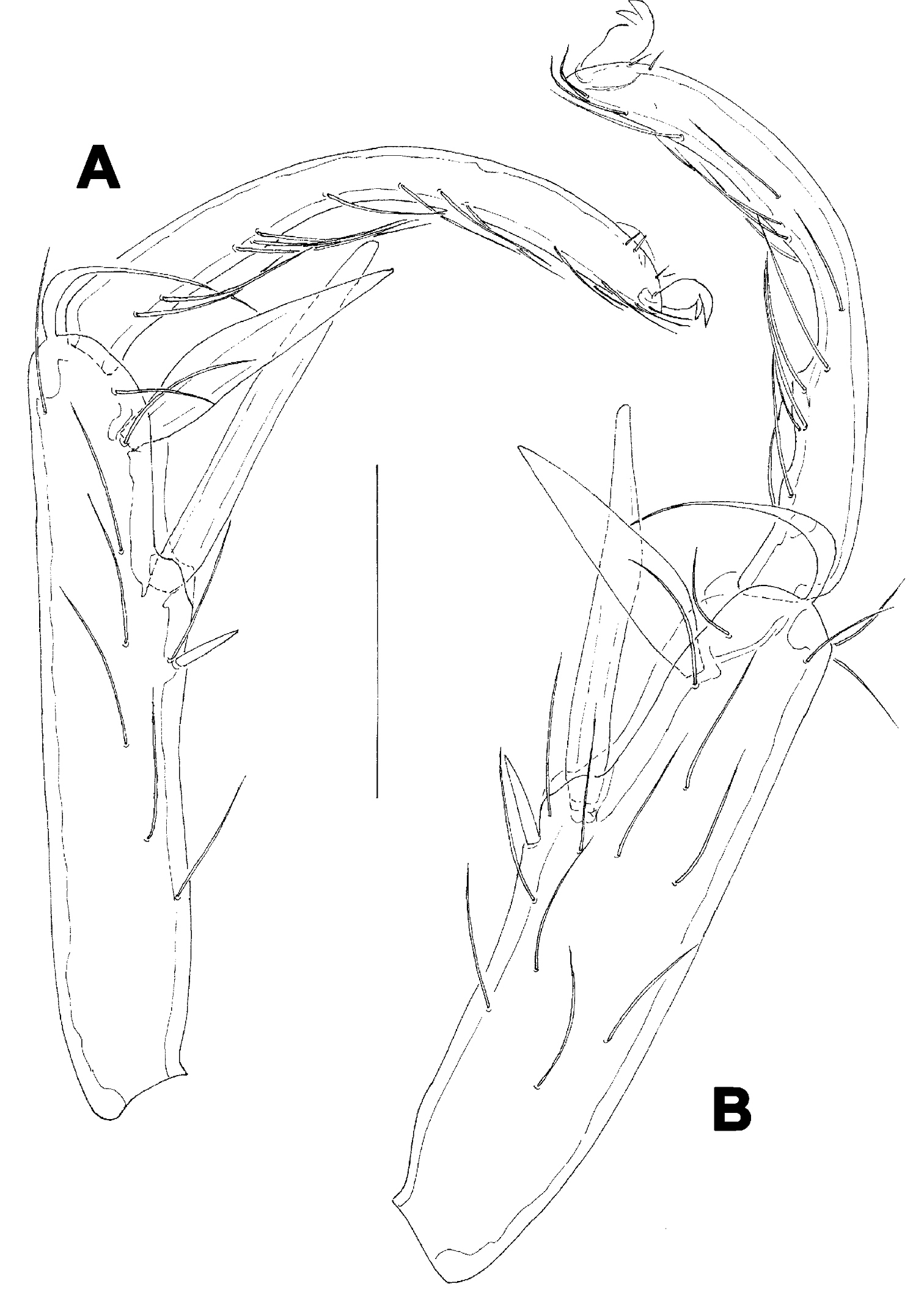
A Atractides cf. spatiosus (K. Viets, 1935), female: A = I-L-5 and -6 B Atractides cf. cognatus (K. Viets, 1935), female: B = I-L-5 and -6. Scale bar = 100 µm.
SE Asia. New for Taiwan.
http://species-id.net/wiki/Atractides_cognatus
Figs 9B–D , 10AECL-BA-1: 23.ix.2009 0/1/0. ECL-BA-4: 20.viii.2009 0/1/0; 27.vii.2010 0/2/0. ECL-BA-5: 27.vii.2010 0/3/0; x.2010 0/1/0. ECL-BA-6: 01.vii.2010 0/1/0; 27.vii.2010 0/1/0; 30.x.2010 0/1/0 (mounted).
See discussion under Atractides cf. spatiosus.
SE Asia. New for Taiwan.
This study was supported by the research grants NSC100-627-B-002-009- and NSC101-2923-B-002-001-MY3 from the National Science Council, ROC. The authors also acknowledged the Agriculture Department from the New Taipei City Government for issuing the field sampling permit at the Baishih River during the study period. We are thankful to the anonymous referee for the careful work and valuable comments.