(C) 2011 Saša Širca. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new needle nematode, Longidorus carniolensis sp. n., recovered from the soil around the roots of grapevine Vitis vinifera L. from Slovenia, is described and illustrated. Longidorus carniolensisis an amphimictic species, characterised by females with a moderately long (L=5.6–8.2 mm) and plump (a=51–72.4, ave. 66.3) body, assuming a spiral to C-shape when heat relaxed. Head region continuous, anteriorly almost flat, lip region 23–25 µm wide; guiding ring situated posteriorly (42–47 μm, 43–50 μm in males), odontostyle long (ave. 146.6 (136–157) μm); pharyngeal glands with normal location, their nuclei of approximately equal size; tail bluntly conoidal to almost hemispherical. Males abundant, spicules slender and long (122–145 μm), ventromedian supplements 13–17, irregularly spaced, preceded by an adanal pair. Four juvenile stages present, the first stage juvenile with bluntly conoidal tail. Codes for identifying the new species when using the key by
grapevine, morphology, taxonomy, 28S rDNA
The nematodes of the genus Longidorus Micoletzky, 1922 cause damage to many economically important crops by direct feeding on their roots. Additionally, they can cause indirect damage to the host plants by transmitting plant viruses. To date, six Longidorus species have been reported from Slovenia (
Soil samples were collected in July 2008 and October 2009 from the rhizosphere of Vitis vinifera L. in Drašiči and Krmačina localities in the southern part of Slovenia. The sampling was performed by digging holes beneath grapevine plants and carefully collecting soil around the roots at 40–50 cm depth. Approximately 500 cm3 of a collected soil sample was gently mixed and two 200 cm3 sub-samples were processed. Nematodes were extracted from the soil using a decanting method followed by the Baermann funnel technique. Longidorid nematodes for morphological study were hand-picked, fixed in TAF (7 ml 40% formalin, 2 ml tri- ethanolamine, and 91 ml distilled water), processed to glycerol (
Drawings and photographs were taken using an Olympus BX51 compound microscope powered with differential interference contrast (DIC). Images were taken with a ColorView IIIu camera and cell^P software (Olympus Soft Imaging Solutions Gmbh). Measurements were made using an Olympus BX 41 light microscope, a digitising tablet (CalComp Drawing Board III, GTCO CalCom Peripherals, Scottsdale, AZ, USA), and Digitrak 1.0f programme (Philip Smith, Scottish Crop Research Institute, Dundee, UK).
Total DNA extraction and amplificationExtracted female nematodes for molecular study were transferred into 1.5 ml tube in a 1 ml drop of sterile water. DNA was extracted from a single female nematode from type-locality Drašiči and from Krmačina locality; 10 ml 1M EDTA pH 8 and 50 ml nucleic lysis solution (Promega Wizard DNA purification kit) mixture was added to each tube and homogenised with micropestle. Isolation of DNA was continued according to manufacturer’s instructions. Isolated DNA was re-suspended in 10 µl of distilled water of which 2 µl was used in each PCR reaction. A fragment of the D2 and D3 expansion region of the 28S rDNA gene was amplified using the primers D2A (5’-ACA AGT ACC GTG AGG GAA AGT TG-3‘) and D3B (5‘-TCG GAA GGA ACC AGC TAC TA-3‘) (
Obtained PCR products were purified using the JetQuick PCR purification spin kit (Genomed) and sequenced on an ABI PRISM 310 DNA Sequencer using BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems), the sequences obtained were submitted to the GenBank. Cluster analyses were performed using sequences of several Longidorus species from the NCBI GenBank (http://www.ncbi.nlm.nih.gov/) obtained from different phylogenetic studies (
Species of fam. Longidoridae used in phylogenetic reconstructions.
| GenBank accession number | Nematode species | Origin | Reference |
|---|---|---|---|
| AY601583 | Longidorus africanus Merny, 1966 | California, USA |
|
| AY494715 | Longidorus americanum Handoo, Carta & Skantar, 2005 | Georgia, USA |
|
| AY601571 | Longidorus apulus Lamberti & Bleve-Zacheo, 1977 | Mola di Bari, Italy |
|
| AY601570 | Longidorus arthensis Brown, Grunder, Hooper, Klingler & Kunz, 1974 | Suter, Switzerland |
|
| AY601574 | Longidorus athesinus Lamberti, Coiro & Agostinelli, 1991 | Italy |
|
| AY601572 | Longidorus attenuatus Hooper, 1961 | Germany |
|
| AY601576 | Longidorus breviannulatus Norton & Hoffmann, 1975 | Nebraska, USA |
|
| HM447030 | Longidorus caespiticola | Brdo, Slovenia |
|
| AY601585 | Longidorus camelliae Zheng, Peneva & Brown, 2000 | Hangzhou, China |
|
| JN631811 | Longidorus carniolensis sp. n. | Krmačina, Slovenia | This study |
| JN631812 | Longidorus carniolensis sp. n. | Drašiči, Slovenia | This study |
| AF480072 | Longidorus carpathicus Lišková, Robbins & Brown, 1997 | Germany | Rubtsova 2001 |
| EF654539 | Longidorus distinctus Lamberti, Choleva & Agostinelli, 1983 | Kráľovský Chlmec, Slovakia |
|
| AY593057 | Longidorus dunensis Brinkman, Loof & Barbez, 1987 | Holterman et al. Unpublished |
|
| AY601575 | Longidorus edmundsi Hunt & Siddiqi | Caribbean sea beach, Cuba |
|
| HM447032 | Longidorus elongatus | Maribor, Slovenia |
|
| AY601573 | Longidorus euonymus Mali & Hooper, 1973 | Zabagr, Hungary |
|
| AY601581 | Longidorus goodeyi Hooper, 1961 | Peebles, Scotland, UK |
|
| HM447031 | Longidorus helveticus | Trška gora, Slovenia |
|
| AF480074 | Longidorus intermedius Kozlowska & Seinhorst, 1979 | Germany |
|
| DQ364599 | Longidorus juvenilis | Svetinje, Slovenia |
|
| AY601568 | Longidorus latocephalus Lamberti, Choleva & Agostinelli, 1983 | Greece |
|
| DQ364600 | Longidorus leptocephalus | Juršinci, Slovenia |
|
| AY601565 | Longidorus macrosoma Hooper, 1961 | Switzerland |
|
| HM447029 | Longidorus moesicus | Vrhpolje, Slovenia |
|
| AY601577 | Longidorus piceicola Lišková, Robbins & Brown, 1997 | Branisko, Slovakia |
|
| EF538750 | Longidorus poessneckensis Altherr, 1974 | Cerne Voderady, Czech Republic |
|
| AF480073 | Longidorus profundorum Hooper, 1965 | Germany |
|
| AF480071 | Longidorus sturhani Rubtsova, Subbotin, Brown & Moens, 2001 | Belgium |
|
| EF538754 | Longidorus uroshis Krnjaic, Lamberti, Krnjaic, Agostinelli & Radicci, 2002 | Velke Pole, Slovakia |
|
| AY601628 | Xiphinema index Thorne & Allen | Argentina |
|
urn:lsid:zoobank.org:act:546D321E-CF14-46C1-A623-73A1F76839BD
http://species-id.net/wiki/Longidorus_carniolensis
Figs 1–12See Table 2.
Measurements of females, males and juvenile stages of Longidorus carniolensis sp. n., from Slovenia (mean ± standard deviation, with range). All measurements in micrometers.
| Character | Holotype | Females | Males | J1 | J2 | J3 | J4 |
|---|---|---|---|---|---|---|---|
| n | n=1 | n=13 | n=14 | n=15 | n=6 | n=11 | n=9 |
| L | 7089 | 7447.5±679.0 5653-8226 |
7917.7±753.9 6702-9525 |
1349.9±53.8 1283-1449 |
2584.2±228.0 2329-2872 |
3692.2±238.0 3305-4149 |
5441.2±700.7 4677-6647 |
| a | 65.2 | 66.3±6.1 51.0-72.4 |
72.5±6.4 59.6-81.2 |
44.0±2.3 39.3-46.2 |
46.5±4.3 39.8-50.6 |
50.5±4.7 42.8-57.4 |
60.6±3.7 53.2-65.6 |
| b | 14.2 | 12.7±0.9 11.6-14.3 |
12.8±0.8 11.8-14.9 |
4.9±0.4 4.3-5.6 |
6.3±0.3 5.8-6.6 |
7.9±0.7 7.0-9.0 |
9.7±1.7 8.4-13.9 |
| c | 165.7 | 177.9±35.5 108.1-224.5 |
173.7±27.5 127.6-241.8 |
41.2±2.7 36.8-45.9 |
71.9±4.8 66.4-79.4 |
94.2±9.2 82.5-113.6 |
132.6±17.6 98.2-155.1 |
| c’ | 0.6 | 0.6±0.1 0.5-1.0 |
0.8±0.1 0.6-1.1 |
1.4±0.1 1.2-1.5 |
0.8±0.04 0.7-0.9 |
0.7±0.1 0.6-0.8 |
0.7±0.04 0.6-0.8 |
| V (%) | 49.7 | 49.4±1.4 47.1-51.5 |
|||||
| G1 (%) | 11.9 | 13.4±2.6 10.6-17.4 |
|||||
| G2 (%) | 11.8 | 13.3±2.6 9.9-17.3 |
|||||
| d | 2.0 | 1.8±0.1 1.7-2.0 |
2.1±1.1 1.0-5.8 |
2.0±0.1 1.8-2.2 |
1.9±0.1 1.8-2.1 |
1.9±0.1 1.7-2.1 |
1.7±0.6 0.3-2.0 |
| d’ | 2.0 | 2.0±0.2 1.8-2.3 |
1.9±0.1 1.8-2.1 |
1.7±0.1 1.6-1.9 |
1.8±0.2 1.7-2.1 |
1.9±0.1 1.7-2.2 |
1.9±0.4 0.8-2.2 |
| Anterior end to guiding ring | 45.4 | 44.6±1.6 42-47 |
46.5±2.3 43-50 |
21±0.7 20–22 |
28.7±1.6 26.5-30.5 |
33.9±1.0 32-35 |
39.8±1.2 38-42 |
| Anterior end to nerve ring | 267.5 | 258.6±15.4 220-275 |
270.7±9.5 249-289 |
129.6±8.9 114-145 |
182.1±31.0 153-228 |
207.5±24.1 176-273 |
231.1±15.9 213-252 |
| Hemizonid | 237.5 | 253.7±18.9 204-270 |
10.4±0.5 10-11 |
205.9±7.4 195-216, n=7 |
234.6±19.2 210-216, n=4 |
||
| Odontostyle | 144 | 147.5±4.7 136-157 |
149.1±6.6 132-159 |
81.9±3.5 76-88 |
88.2±5.0 79-94 |
107.7±4.4 98-114 |
125.9±3.6 120-131 |
| Replacement odontostyle | 87.6±2.5 85-93 |
104.9±2.4 101-108 |
123.7±3.2 119-130 |
146.0±3.9 142-152 |
|||
| Odontophore | 92 | 91.4±4.1 85-97 |
90.2±6.4 75-99 |
49.6±3.9 43-56 |
62.4±2.3 59-66 |
72.9±4.2 64-78 |
85.1±3.4 81-91 |
| Neck length | 500 | 590.5±59.9 462-674 |
618.6±56.6 510-703 |
274.1±20.8 237-317 |
408.3±33.4 370-448 |
472.5±48.7 409-524 |
553.6±47.5 478-628 |
| Pharyngeal bulb length | 139 | 142.7±6.0 133-152 |
141.1±6.9 129-157 |
72.6±2.3 67-75 |
89.6±3.1 84-93 |
106.8±6.1 96-114 |
123.6±5.6 118-135 |
| Pharyngeal bulb width | 39 | 40.2±2.8 36-44 |
37.9±2.1 32-41 |
15.6±0.9 14-17 |
22.7±1.4 21-24 |
28.1±1.6 26-31 |
33.3±1.9 31-37 |
| DO* | 11.4 | 11.6±0.3 11.2-11.9 |
11.0±0.8 9.8-11.8 |
14.0±2.4 11.3-18.1 |
10.9±1.3 9.1-12.0 |
11.3±0.7 10.3-11.9 |
11.7±1.6 9.9-14.8 |
| DN | 37.1 | 38.1±2.6 32.8-40.2 |
37.9±3.0 33.2-42.9 |
38.6±1.9 36.7-42.7 |
37.2±1.7 35.5-40.3 |
37.0±1.0 35.6-38.4 |
36.8±3.7 30.0-42.6 |
| LS1N | 53.7 | 56.1±2.6 52.3-61.0 |
55.0±3.0 49.6-62.3 |
52.0±2.6 47.9-57.3 |
50.6±1.7 48.6-52.7 |
52.9±2.1 48.1-55.3 |
54.3±2.2 52.2-57.7 |
| RS1N | 53.7 | 54.6±2.5 51.5-58.6 |
55.5±2.6 52.4-61.2 |
51.5±2.7 46.3-55.3 |
50.9±1.4 48.9-52.7 |
53.3±2.3 48.1-56.3 |
53.5±2.5 50.7-57.4 |
| S2O | 83.4 | 84.9±1.6 82.3-87.1 |
86.3±5.7 81.9-102.5 |
84.2±1.0 82.7-85.8 |
84.8±2.7 83.1-90.2 |
85.0±1.6 81.6-87.1 |
82.8±0.9 81.6-84.1 |
| Prerectum | 347.5 |
419.0±85.9 280-550 |
576.8±182.2 248-832 |
141.7±60.7 81-290 |
183.8±30.1 150-224 |
289.1±50.0 175-363 |
363.6±91.7 275-538 |
| Rectum | 51.5 |
50.3±3.0 47-57 |
- | 14.3±1.9 12-19 |
22.8±0.8 22-24 |
32.6±2.6 30-38 |
45.3±2.4 42- 49 |
| Tail | 42.8 | 43.3±9.2 34-69 |
46.4 ± 6.8 32-61 |
32.9±1.4 31-35 |
35.9±1.6 34-38 |
39.4±2.9 35.5-45 |
41.2±3.2 38-48 |
| Length of hyaline part | 20.2 | 17.8±0.7 17-19 |
16.9±1.2 14-19 |
7.9±0.9 6-9 |
10.4±0.7 9-11 |
13.1±0.9 11-15 |
14.9±0.8 13-16 |
| Body diameter at: - lip region |
22.9 | 24.2±0.8 23-25 |
24.8±1.3 22-26.5 |
10.6±0.4 10-11 |
15.0±0.5 15-16 |
18.4±1.1 17-20 |
20.9±0.5 20-21 |
| - guiding ring | 46.6 | 48.5±3.3 44-55 |
48.0±2.3 45-52 |
18.3±0.5 18-19 |
26.8±2.2 25-31 |
35.7±2.7 31-41 |
43.0± 3.9 38.7-51 |
| - base of pharynx | 95.4 | 93.4±4.5 89-101 |
93.8±4.8 84-103 |
30.2±1.2 29-33 |
50.5±3.4 46-55 |
63.9±3.8 59-73 |
76.4±5.6 69.5-84 |
| - mid-body/at vulva | 108.7 | 112.9±9.5 97-127 |
109.3±5.4 98-117 |
30.7±1.2 29-33 |
55.8±4.0 49-60 |
73.5±5.6 66-85 |
89.8±10.0 79-105 |
| - anus | 68.5 | 68.2±4.3 60-75 |
61.2±3.2 55-66 |
24.3±1.0 22-26 |
44.4±1.7 42-46 |
55.2±3.6 51.3-60.5 |
63.1±3.6 59-70 |
| - hyaline part | 49.5 | 49.0±1.7 47-52 |
39.5±2.2 37-43 |
16.0±0.9 15-17 |
26.8±2.7 24-32 |
37.6±2.5 32-41 |
42.9±2.7 39-47 |
| Spicules | 126.9±5.8 122-145 |
* Following
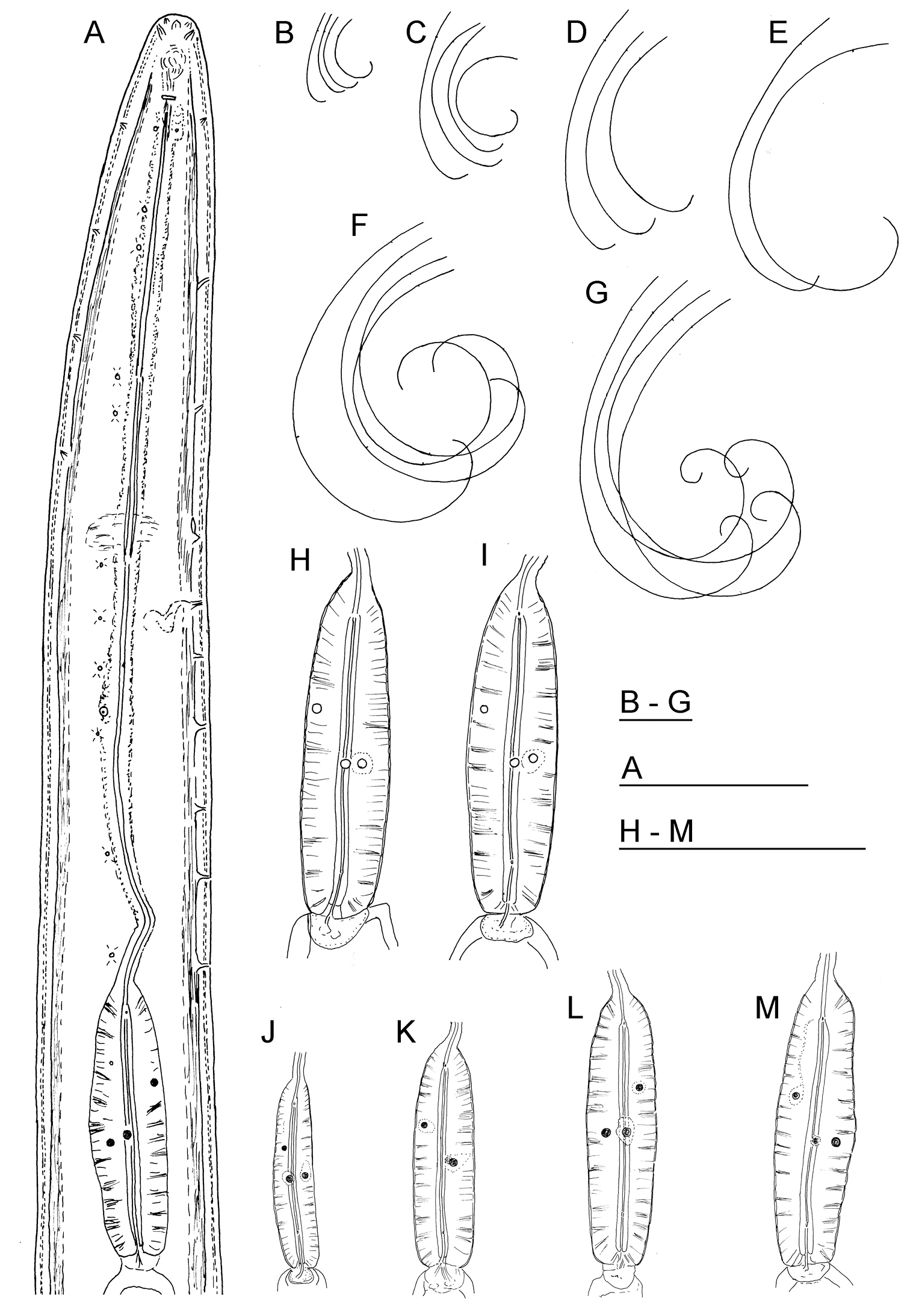
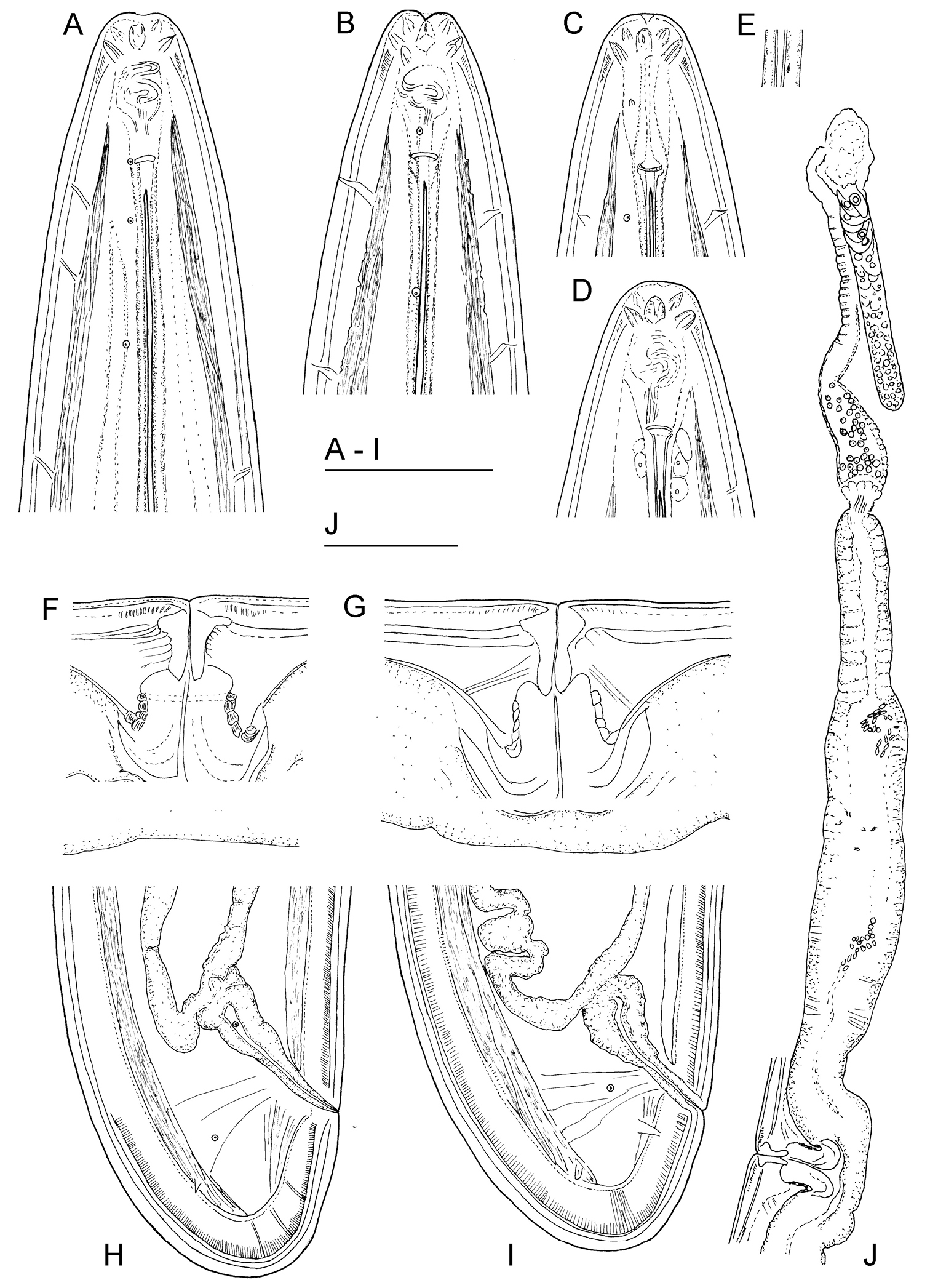
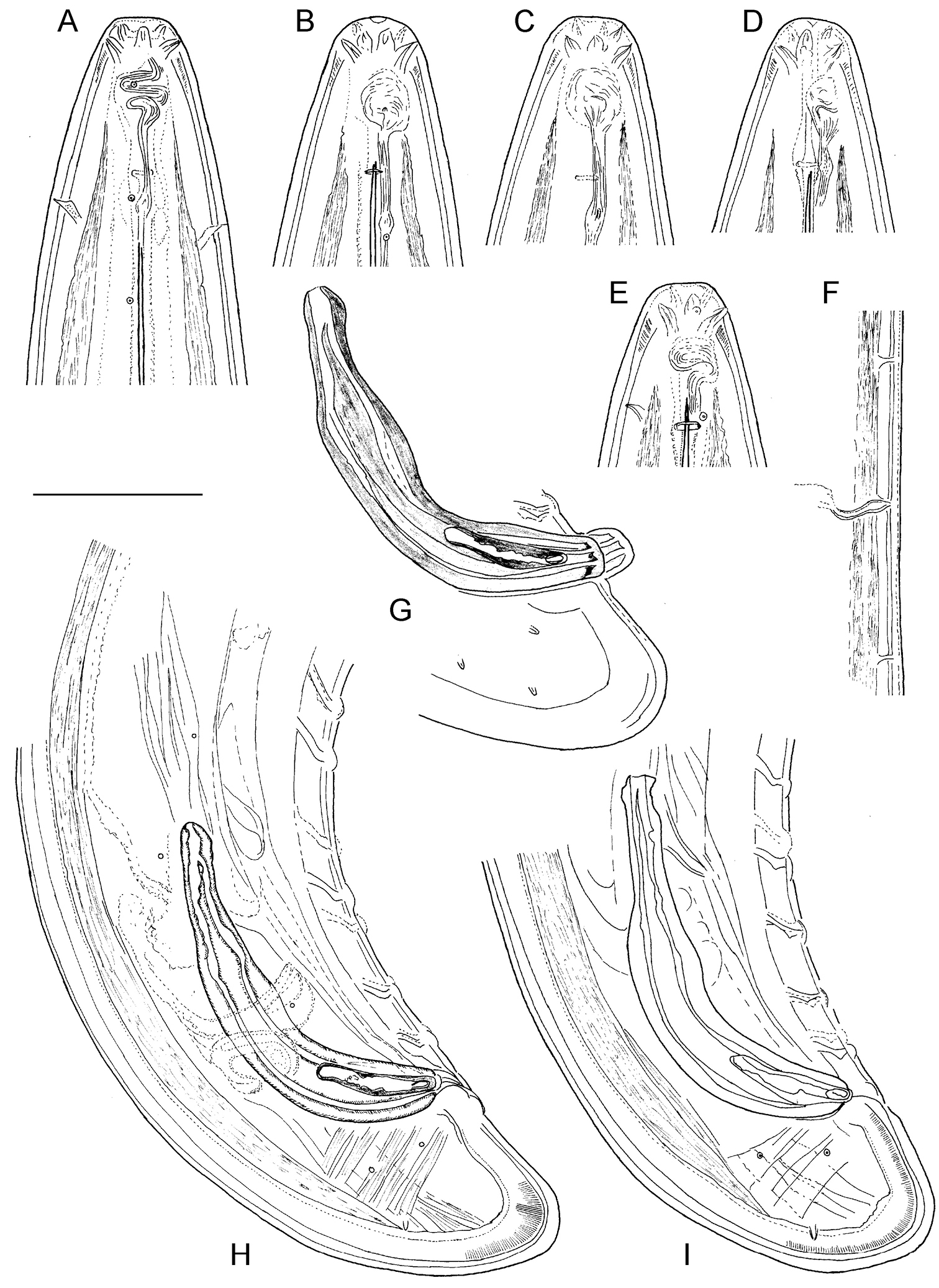
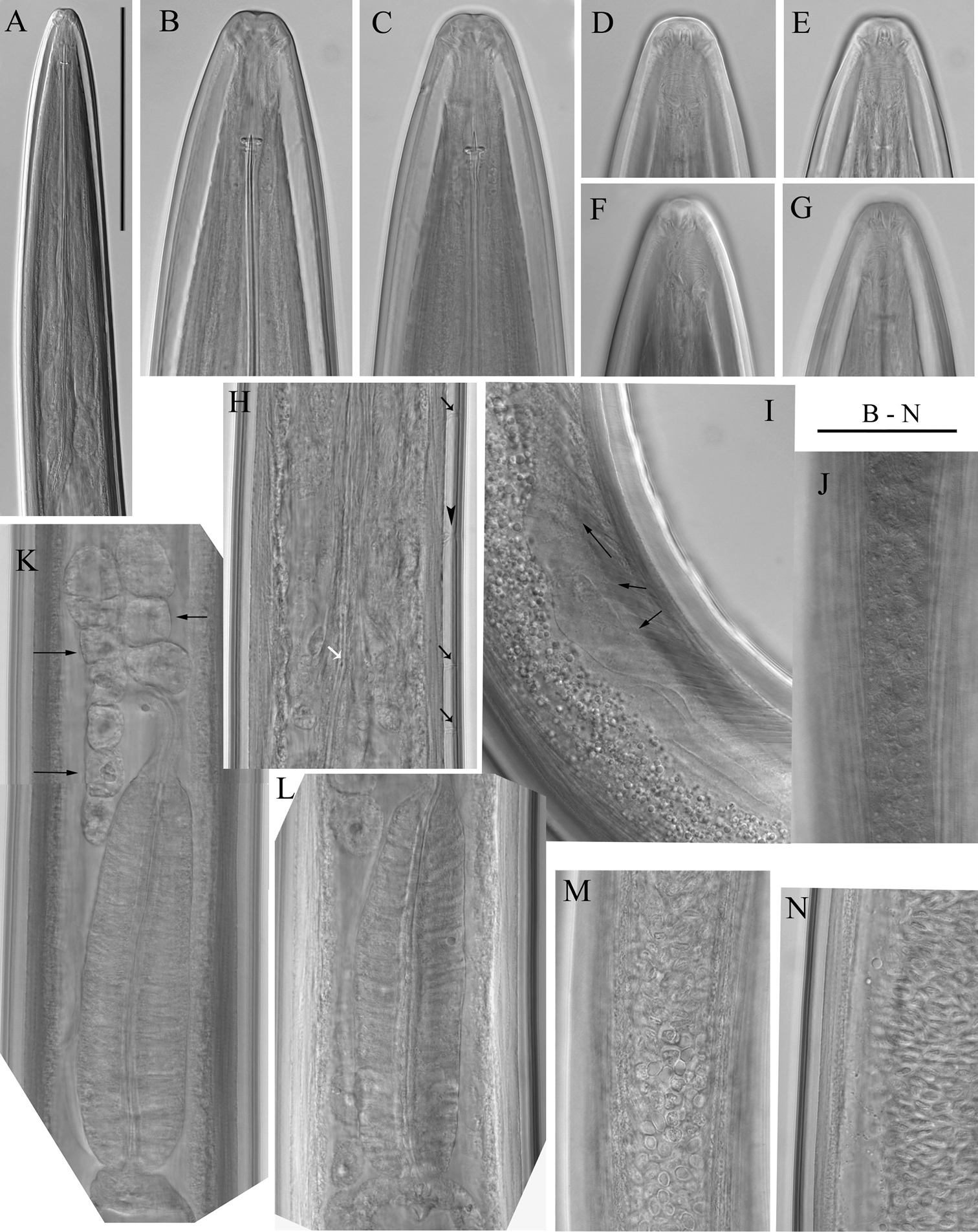
Female. Body moderately long (L=5.6–8.2 mm) and plump (a=51–72.4), assuming a spiral to C shape when heat relaxed. Cuticle consisting of several layers under light microscope: 11–14 µm thick at guiding ring level; 7–8 µm along the body; 13–15 µm on tail posterior to anus. Lateral pores number 10–14 in pharyngeal region: a single pore in front of guide ring, rarely two or none; 3–5 in odontostyle and 1–3 in odontophore regions; 3–4 dorsal pores and 7–10 ventral pores; numerous lateral body pores. Usually the fifth ventral pore (sometimes the fourth) differs in size (Figs 1A, 4F and 6H) compared to the other ventral pores. Lip region continuous, anteriorly almost flat, 7–9 μm high. Labial papillae prominent. Amphid aperture assumed to be a minute pore, difficult to be observed under light microscope. Pouch-like amphidial fovea with convoluted fine dendritic branches (receptors), extending to 1/2 - 2/3 the distance between anterior end and guiding ring, fovea slightly longer (15–18 μm, n=5) than wide (14–16 μm, n=4) with no distinct margins. Fusus (sensillium pouch) at 57±1.9 (55–60) μm from anterior end. Guiding ring 7–9 μm wide. Odontostyle long and very slender, 2 μm wide at the base. Odontophore with weakly developed flanges. In all females a small (2–3 μm long) rudimentary odontostyle tip (vestigium) present, directed forward, and observed in the slender pharynx at 300.5±40.3 (224–350) μm from anterior end; in two specimens the vestigium located in odontophore area. Slender pharynx often coiled in its posterior part. In this region 5–7 glandular bodies are observed in all females. Nerve ring surrounding odontophore base, rarely surrounding mid-odontophore, or just behind it, second nerve ring at a distance of 85.2±6.6 (78–98) μm behind the first one. Hemizonid flat, 10–11 μm long. Pharyngeal bulb about 1/4 of the neck length. Normal arrangement of pharyngeal glands, the nuclei of dorsal and ventrosublateral glands approximately the same size, their diameters 3.4±0.4 (3–4) μm, n=7 and 3.9±0.2 (3.5–4) μm, n=11, respectively. Cardia small, broadly rounded, wider than long, variable in size: 20.1±1.8 (10–23) × 10.1±1.8 (7–12) μm . Reproductive system amphidelphic, varying in dimensions due to the stage of maturity of female. Vagina extending about half body width. Pars distalis vaginae with characteristic shape (Fig. 2F, G), 26–28 µm and pars proximalis vaginae 32–38 µm long, respectively; muscular walls of the latter almost parallel. Uteri very long, anterior uterus 494.6±52 (430–563) µm long, posterior uterus 510.0±88.7 (357–643) µm long, differentiated, filled with sperm cells in all females examined; well developed sphincter between uterus and pars dilatata oviductus also containing numerous sperm cells. Anterior and posterior oviduct of similar size, measured in four specimens: 275–348 μm, and 283–330 μm. Anterior ovarium 263.4±51.8 (210–347) μm long, n=7, posterior ovarium 234.3±35.8 (183–309) μm long, n=5; in older mature specimens the length is about 3 times greater (1055–1060 μm for anterior and 1020 μm for posterior ovary). One egg in anterior pars dilatata oviductus measuring 227 × 87.5 μm and one uterine egg measuring 225 × 77.5 μm. A weakly developed ovijector present, 112.0±12 (95–125) μm long. In one female a rudimentary adanal pair of supplementary papillae was observed (Fig. 9E). Prerectum variable in length; rectum 0.7±0.1 (0.6–0.8) body width at anus. A short post-intestinal sac present. Tail bluntly conoidal, rounded to almost hemispherical; ventral side straight or slightly convex, the dorsal curvature greater. Two pairs of lateral pores.
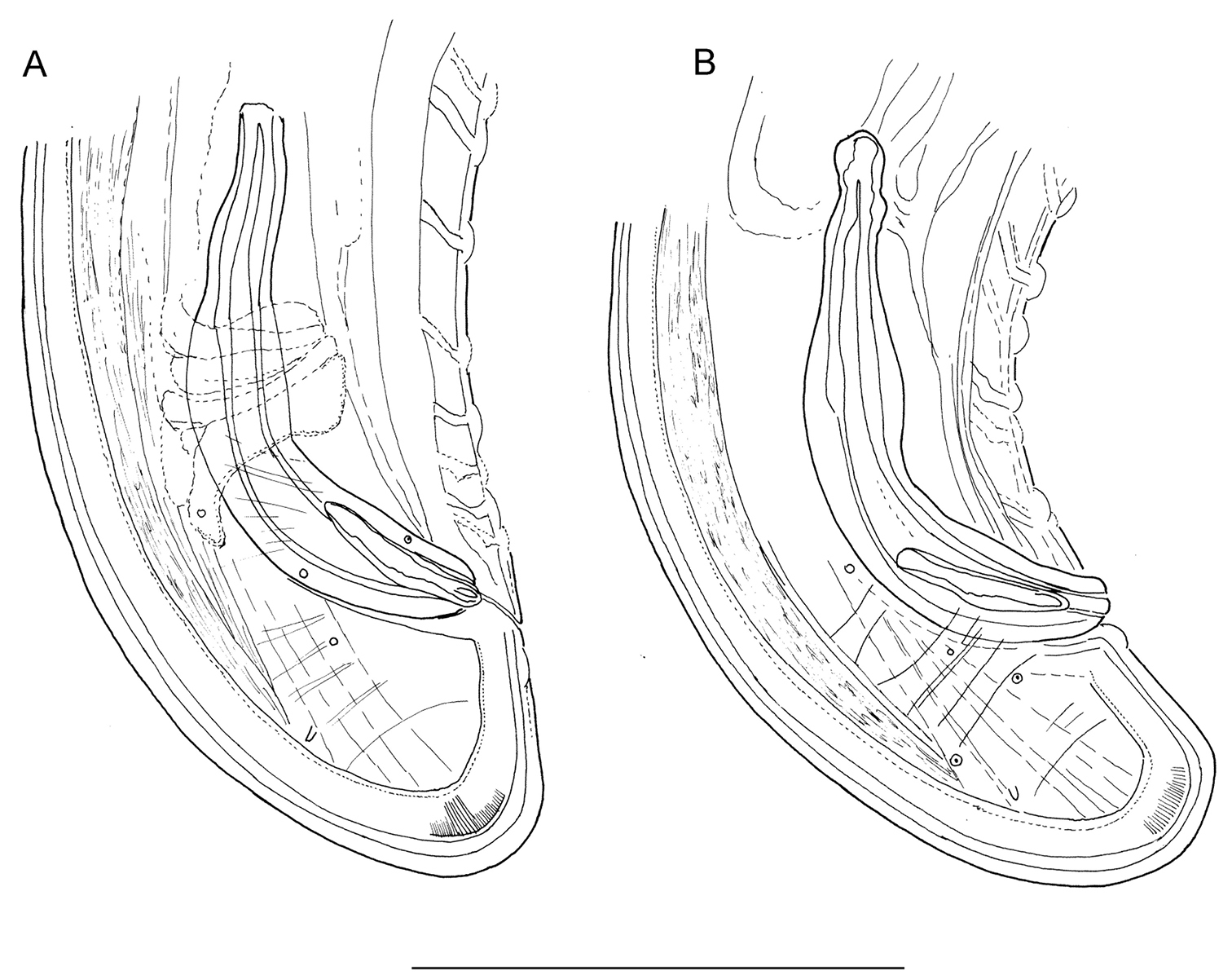
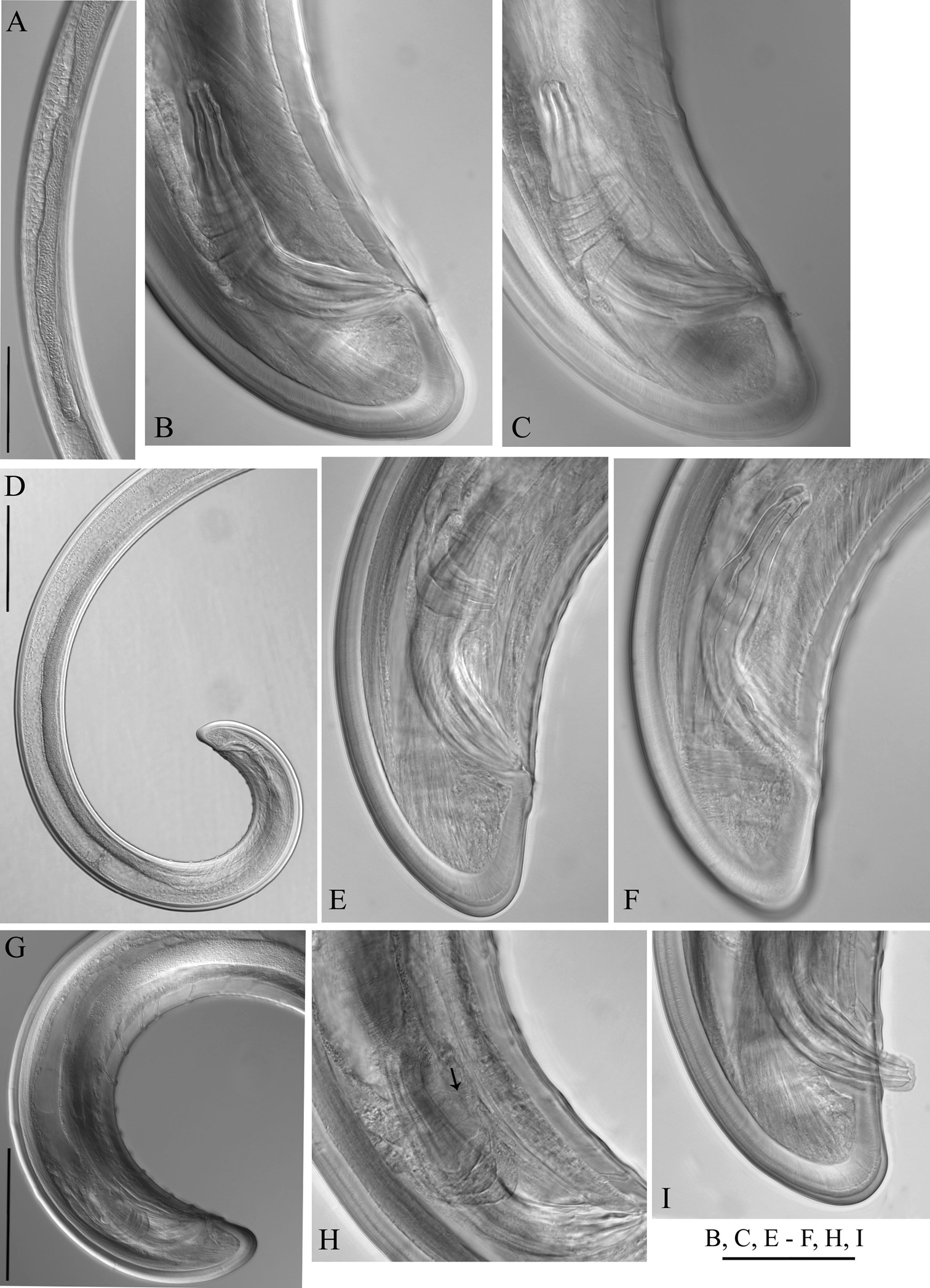
Male. Body C shaped when heat relaxed, posterior part more strongly coiled ventrally. Similar to females in general morphology except for genital system. Lateral pores number 10–15 in pharyngeal region: a single pore in front of guide ring, 3–5 in odontostyle and 1–2 in odontophore regions; 2–5 dorsal pores, mostly 3–4, and 7–10 ventral pores. Cuticle in post-labial region at the guiding ring level 10.5–13.5 µm thick, 6.5–9 µm along body, 9–10 µm in post-cloacal area. Second nerve ring at 80.7±14 (50–100) μm behind the first one (n=14). In all males a small vestigium (2–3 μm, in one specimen 6 μm long), directed forward (in two specimens directed rearward), is observed in the slender pharynx at 300.5±40.3 (224–350) μm from anterior end; in two specimens the vestigium detected in odontophore area. Two to eleven glandular bodies observed in all males in posterior part of the slender pharynx and pharyngeal bulb. In two specimens lens-like hemizonion at a distance of 242 and 271 μm from anterior end observed. Pharyngeal bulb slightly less than 1/4 of neck length (22.9±1.6 (20.9–26.7%). Ventromedian supplements composed of one adanal pair and a row of 13–17 irregularly spaced single ones, the first three appear as double in some specimens. Spicules comparatively slender, of almost equal width along the length, curved to almost at right angle. Lateral guiding piece not bifid, with uneven internal walls. Post-cloacal papilla well developed. Tail short, bluntly conoidal, ventral side almost straight, dorsal side convex. Two or three pairs of lateral caudal pores.
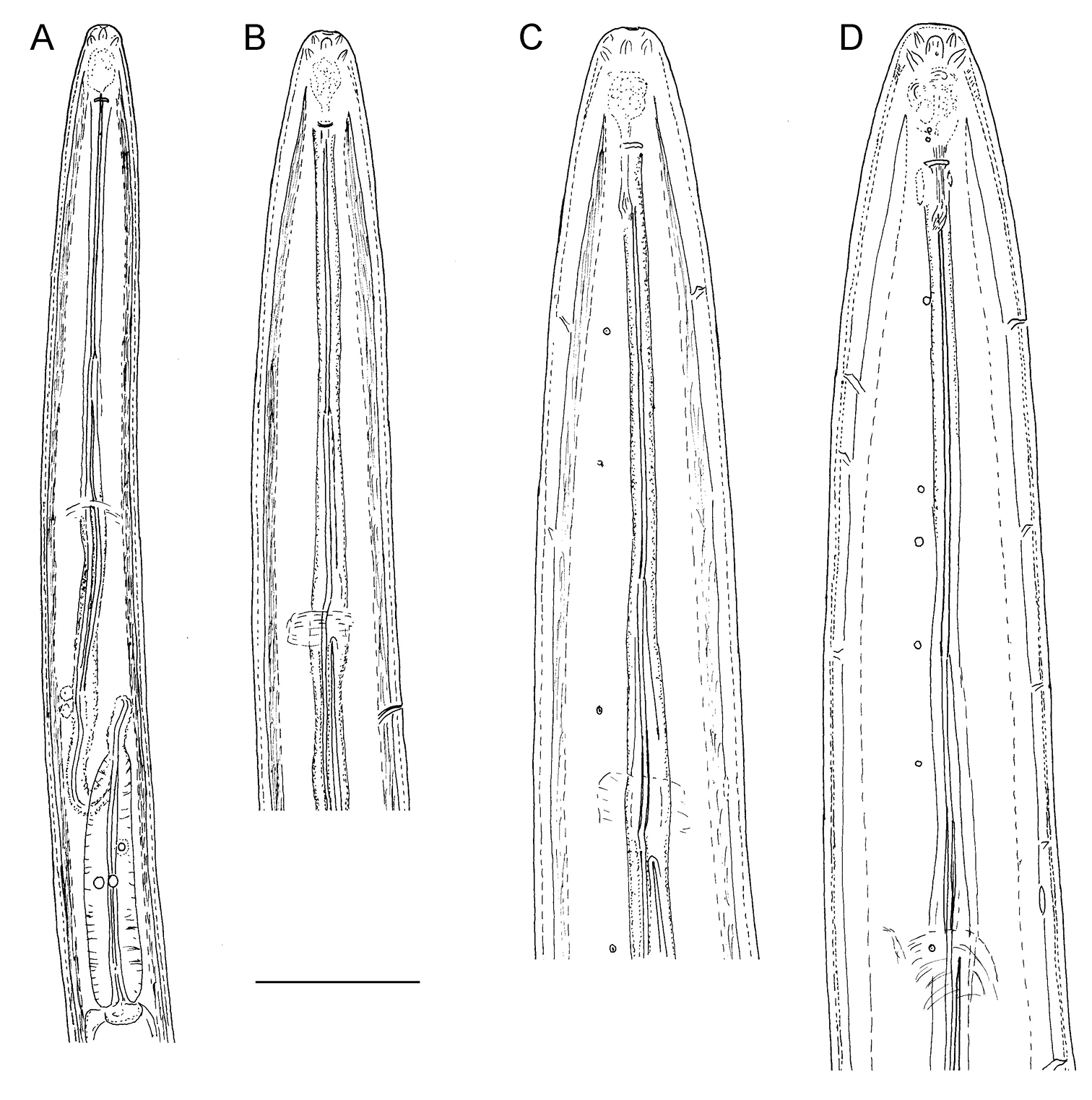
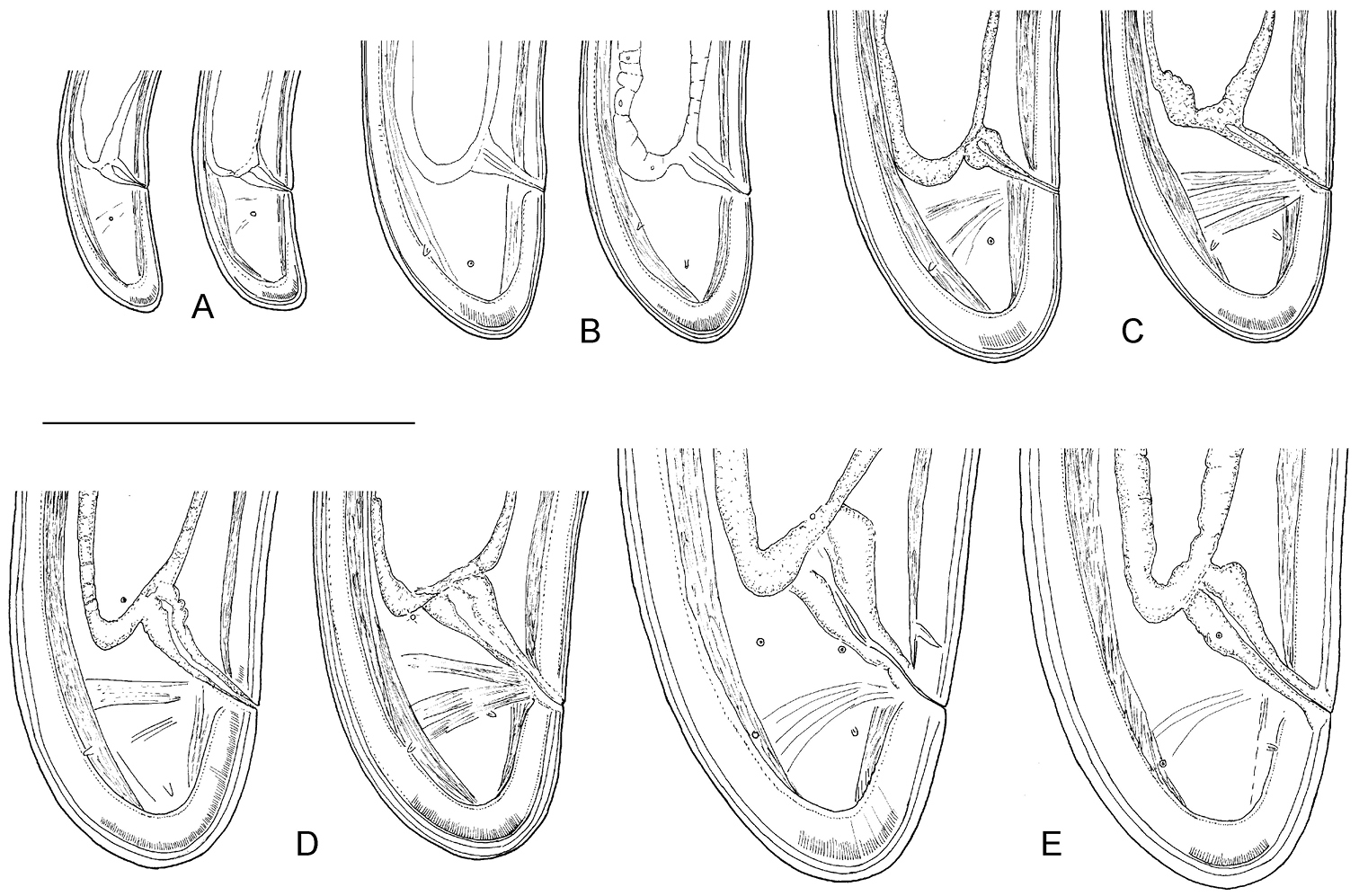
Juveniles. Four developmental stages clearly present (Fig. 11) as determined from the position of the replacement odontostyle and the principal morphometric characters of body, odontostyle and replacement odontostyle lengths, and developing gonad (genital primordium) size. The habitus of juveniles not changing considerably during successive stages, assuming J or C shape. In first stage juvenile, lip region somewhat different from the next stages, it is rounded with a very weak depression after the second circle of labial papillae, the latter slightly protruding and changing the lip region outline. Amphidial fovea in first two stages has no clearly visible receptors, only small refractive elements discernable. Both the tail and body width at anus is increasing in length and c’ ratio is decreasing. Tail shape in J1 is conoidal, ventrally almost straight or slightly concave, dorsally convex, which gives asymmetrical appearance, in successive stages it gradually becomes rounded but always with the dorsal curvature more strongly expressed.
Longidorus carniolensis sp. n. Female: A Neck region F Habitus H Pharyngeal bulb Male: G Habitus I Pharyngeal bulb; Juveniles: B–E Habitus of first, second, third and forth juvenile stages J–M Pharyngeal bulb of first, second, third and forth juvenile stages. Scale bars: B–G 1 mm; A, H–M 100 μm.
Longidorus carniolensis sp. n. Female: A–D Anterior ends E Vestigium in the walls of the slender part of pharynx F, G Vulval region G Anterior genital branch. Scale bars: A–I 50 μm, J 100 μm.
Longidorus carniolensis sp. n. Female: A Anterior region B–D Amphidial fovea E Vestigium F–H Vulval region I Vulval region, uterus and egg J Pharyngeal bulb, dorsal and subventral glands K, L Tail – different optical sections M Sphincter N Prerectum O–Q Variation in tail shape. Scale bars: I, N 200 μm; A–G, H–M, O–Q 50 μm.
Longidorus carniolensis sp. n. Male: A–E Anterior end B, D, E in sublateral view F Excretory pore and ventral pores G Partly protracted spicules H–I Tail end. Scale bar: 50 μm.
Longidorus carniolensis sp. n. Male: A, B Variation in tail shape. Scale bar: 50 μm.
Longidorus carniolensis sp. n. Male: A Anterior region B, C Head region D–G Amphidial fovea H Vestigium (white arrow), excretory pore (thick arrow) and ventral pores (slender arrows) I Ejaculatory glands (marked by arrows) J Lateral field K, L Pharyngeal bulb with glandular bodies (marked by arrows) M, N Sperm cells at different stage of development. Scale bars: A 200 μm; B–N 50 μm.
Longidorus carniolensis sp. n. Male: A Posterior genital branch B, C, E, F Tail and copulatory apparatus – different optical sections D, G Posterior end H Rectum (marked by arrow), spicules and lateral piece I Partly protracted spicules. Scale bars: A, D, G – 200 μm; B, C, E–F, H, I – 50 μm.
Longidorus carniolensis sp. n. Juveniles: A Neck region of first stage B–D Spear region of second, third and fourth stage. Scale bar: 50 μm.
Longidorus carniolensis sp. n. Evolution of the tail. A–D Tail of first–fourth juvenile stage E Tail of female. Scale bar: 100 μm.
Longidorus carniolensis sp. n. Juvenile: A–D Anterior region of first, second, third and forth stages H–K Pharyngeal bulb of first, second, third and forth juvenile stages M, F, G, R genital primordium of first, second, third and forth stages N, S Tail shape of first stage O, T Tail shape of second stage P, U Tail shape of third stage Q, V Tail shape of forth stage Female: E Anterior region L Pharyngeal bulb W Tail shape. Scale bar: 50 μm.
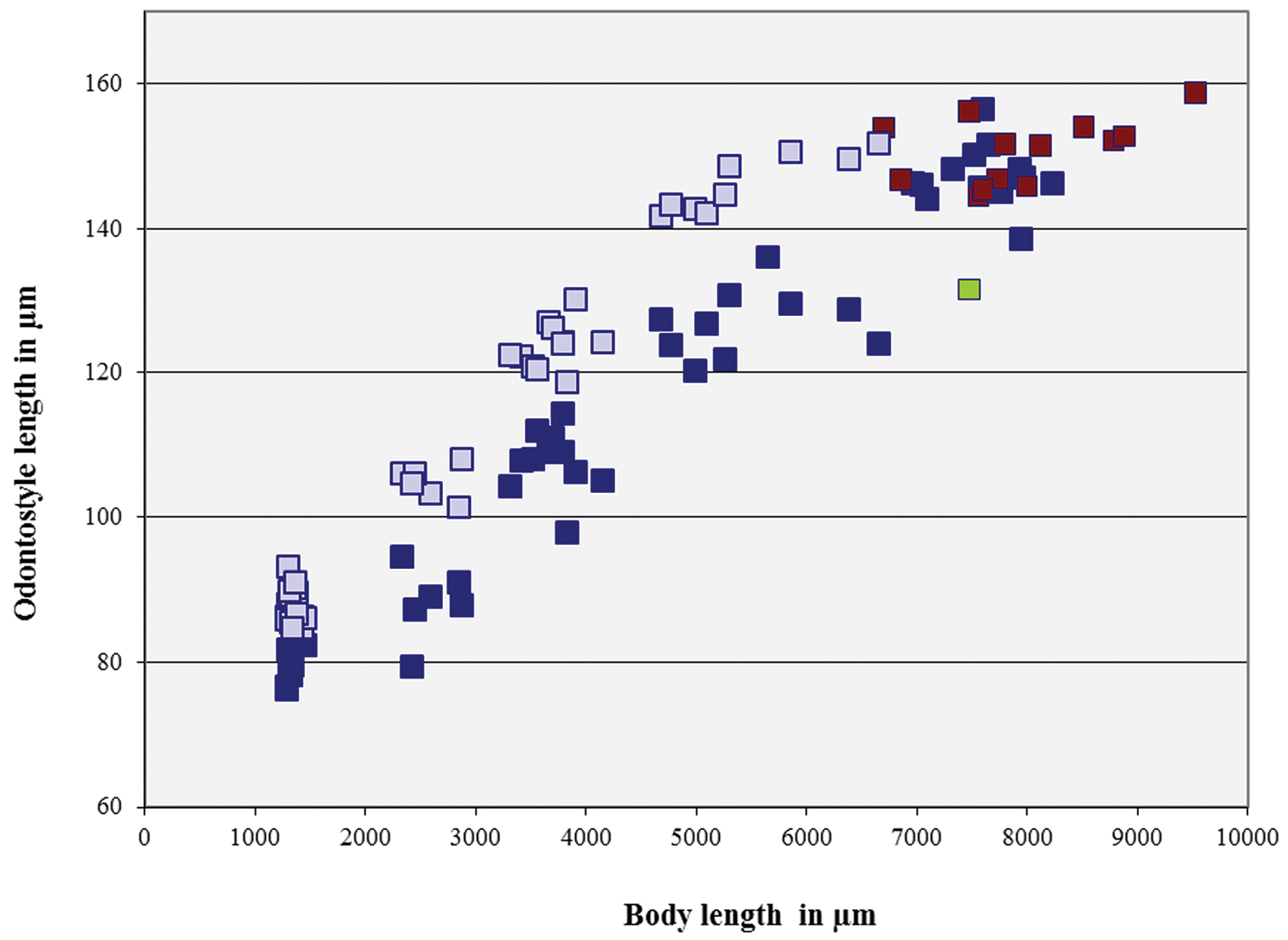
Longidorus carniolensis sp. n. Scatter plot of the functional (■, dark blue) and replacement odontostyle (■, light blue) in relation to the body length of the juvenile stages and adults: females (■, dark blue) and males (■, red), female with very short odontostyle (■, green).
Longidorus carniolensisis an amphimictic species, characterized by females with a moderately long (L=5.6–8.2 mm) and plump (a=51–72) body, assuming a spiral to C-shape when heat relaxed; head region continuous, anteriorly almost flat, lip region 23–25 µm wide, guiding ring situated posteriorly (42–47 μm, 43–50 μm in males), long odontostyle (146.6 (136–157) μm), distribution of pharyngeal glands normal, nuclei of approximately equal size, tail bluntly conoid to hemispherical. Males abundant, spicules slender and long (122–145 μm), ventromedian supplements 13–17, irregularly spaced and preceded by an adanal pair. Postembrional development through four juvenile stages.
The codes for identifying the new species when using the polytomous key by
Longidorus poessneckensis – by its somewhat longer odontostyle (ave. 147.5 (136–157) vs ave. 133 (122–142), ave. 126 (122–130) and ave. 140.2 (132–148) μm); more posteriorly sitated guiding ring (ave. 44.6 (42–47) vs ave. 40 (36–43) and 39 ave. (37–40) μm); tail short conoidal vs elongate conoid in J1 (c’=1.2–1.5 vs 1.8–2.2 and 1.8–2.5); males abundant vs males very rare (
Longidorus macrosoma – by its shorter (5.6–8.2 vs 8.4–11.9 mm) and more plump (a=51–72.4 vs 77–113 and 105–126) body; differently shaped lip region (laterally rounded vs truncated, flattened); somewhat longer odontostyle (136–157 vs 113–148 μm); different tail shape in J1, bluntly conoidal vs mucronate; longer spicules in males 122–145 vs 105 μm and ave. 116.2 (112–121) μm (
Longidorus caespiticola – by its longer odontostyle (136–157 vs 100–120, 96–109 μm); different numbers of dorsal (2–5 vs a single pore) and ventral (7–10 vs 5–6) pores; more posteriorly sitated guiding ring (42–47 vs 30–41, 37, 42.5 μm); longer spicules in males (122–145 vs 90, 80–95 μm), smaller c’ value in J1 (1.2–1.5 vs almost 2) (
Longidorus helveticus – by different tail shape of J1 being bluntly conoid vs mucronated; longer (122–145 vs 104–118 μm ) and differently shaped spicules (
Longidorus macroteromucronatus – by having more posteriorly situated guide ring (42–47 vs 38 μm); thicker cuticle along the body (6–7 vs 4 μm) and on tail region (9–10.5 vs 13–15 μm); longer odontostyle (136–157 vs 133 μm) (
Longidorus raskii – by its wider lip region (23–25 vs 15–19 and 14–16 μm); more posteriorly situated guiding ring (42–47 vs 33–38 and 33–43 μm); longer odontostyle (136–157 vs 90–103 and 98–100 μm);longer spicules (122–145 vs 82–103 and 79–90 μm) (
Longidorus kheirii – by itslonger odontostyle (136–157 vs 111–130 μm); different tail shape in J1 (bluntly conoidal vs mucronated); males abundant vs males rare; longer spicules (122–145 vs 80 μm) (
Longidorus pius – by its more posterior position of the guiding ring (42–47 vs 35–42 and 37–42.5 μm); different tail shape in J1 (bluntly conoidal vs mucronated); males abundant vs males absent (
Longidorus nevesi – by having wider lip region (23–25 vs 16–22 μm), different amphidial fovea shape (pouch like, not bilobed vs bilobed); differently shaped and longer spicules in males (122–145 vs 87–100 μm) (
Longidorus major – by having shorter body (L=5.6–8.2 vs 8.5–12 mm); somewhat narrower lip region (23–25 vs 22–27 μm); different tail shape in J1 (bluntly conoidal vs mucronate) and amphidial fovea (pouch like, not bilobed vs bilobed), males abundant vs males absent (
Longidorus carpathicus – by its longer body (L=5.6–8.2 mm vs 6.2–6.5 mm); wider (23–25 vs 16–18 μm) and differently shaped lip region; lower c’ value (c’= ave. 0.6 (0.5–1.0) vs c’= 0.8); different shape in J1 (bluntly conoidal vs mucronated with a rather long mucro); males abundant vs males absent (
Longidorus piceicola – by having longer body (L=5.6–8.2 vs 4.2–6.5, 4.4–8.0 and 5.2–7.9 mm); wider (23–25 vs 14–17 μm) and differently shaped lip region (continious, almost flat vs broadly rounded); lower c’ value (c’=ave. 0.6 (0.5–1.0) vs c’=0.9–1.3); differently shaped tail in J1 (bluntly conoidal vs elongate conoid) (
Longidorus vinearum – by having different lip region shape (abruptly vs gradually tapering), different shape of amphidial fovea (pouch like not bilobed vs irregularly bilobed); longer odontostyle (136–157 vs 105.5–132.5 μm); different tail shape in J1 (bluntly conoidal vs conical, c’=1.2–1.5 vs 1.9–2.8) (
Longidorus pauli – by having different (continious vs slightly offset) and wider (23–25 vs 14–17 μm) lip region, amphidial fovea pouch like, not bilobed vs bilobed; longer odontostyle (136–157 vs 102–118 μm); lower a and c’ values (a=51.0–72.4 vs a=120.3–143.5; c’= ave. 0.6 (0.5–1.0) vs c’=0.9 (0.8–1.0), respectively); more posteriorly situated guiding ring (42–47 vs 27–36 μm); longer spicules (122–145 vs 61–69 μm); different tail shape in J1 (bluntly conoidal vs subdigitate) (
Longidorus arthensis – by itswider (23–25 vs 14–17 μm) lip region, amphidial fovea pouch like not bilobed vs bilobed; longer odontostyle (136–157 vs 102–111 μm); lower c’ values (c’=av 0.6 (0.5–0.1) vs c’=av 0.9 (0.8–1.1); more posteriorly sitated guiding ring (42–47 vs 30–38 μm); longer spicules (122–145 vs 60–66 μm); different tail shape in J1 (bluntly conoidal vs mucronated) (
Longidorus iuglandis – by its wider lip region (23–25 vs 14–16 μm); amphidial pouches not bilobed vs bilobed; longer odonostyle (136–157 vs 112–128 μm); more posterior position of the guiding ring (42–47 vs 31–41 μm); longer tail (34–69 vs 33–41 μm); longer spicules (122–145 vs 93–99 μm); different tail shape in J1 (bluntly conoidal vs mucronated) (
Longidorus picenus - by its wider lip region (23–25 vs 19–22 μm); amphidial fovea not bilobed vs bilobed; more posterior position of the guiding ring (42–47 vs 31–41 μm); longer spicules (122–145 vs 103–112 μm); different tail shape in J1 (bluntly conoidal vs mucronated) (
Longidorus silvae - by its more plump body (a=51.0–72.4 vs a=87.5–137.5 in Italian population and a=87.4–116 in Serbian populations), wider lip region (23–25 vs 14–17 μm); amphidial fovea not bilobed vs bilobed; longer odontostyle (136–157 vs 113.5–133 μm (Italian population) and 108–136 μm (Serbian populations)); different tail shape in J1 (bluntly conoidal vs mucronated); males abundant vs males rare; longer spicules (122–145 vs 77–78 μm) (
Longidorus uroshis – by having wider (23–25 vs 15–20.5 μm) lip region; lower a values (a=51.0–72.4 vs a=96.9–108.9); different tail shape in J1 (bluntly conoidal vs mucronated); longer spicules (122–145 vs 59–72 μm) (
Longidorus saginus – by having longer body (L=5.6–9.5 vs 4.8–6.4 mm); amphidial fovea pouch shaped not bilobed vs asymetrically bilobed; longer tail (34–69 vs 21–33 μm) (
Longidorus orongorongensis – by its more anterior position of the guiding ring (42 -47 vs 63–73 μm); shorter odontostyle (136–157 vs 152–166 μm); longer spicules (122–145 vs 84–87 μm) (
Longidorus cretensis – by having normal vs abnormal location of pharyngeal glands; wider lip region (23–25 vs 17–21 μm); longer spicules (122–145 vs 71–91 μm); different tail shape in J1 (bluntly conoidal vs conoid pointed) (
Longidorus cylindricaudatus – by having lip region abruptly vs gradually tapering; amphidial fovea not bilobed vs bilobed; shorter odontostyle (136–157 vs 164–178 μm); lower a values (a=51–72.4 vs a=94.4–113.4); males abundant vs males absent (
Longidorus fasciatus – by its wider lip region (23–25 vs 12–14 μm); different amphidial poches (not bilobed vs asymmetrically bilobed); longer odontostyle (136–157 vs 102–119 μm; male abundant vs males absent (
Longidorus litchii – by its somewhat shorter odontostyle (136–157 vs 138–171 mm); different amphidial poches (not bilobed vs bilobed); more anterior postion of the guiding ring (42–47 vs 82.5–96.5 μm); different tail shape in J1 (bluntly conoidal vs elongate conoid with long digitate tip, c’=1.2–1.5 vs c’=2.7–3.4); longer spicules (122–145 vs 68.5–71 μm) (
An old vineyard with roots of several Vitis vinifera varieties close to Drašiči village in southern part of Slovenia (45°39'N; 15°23'E), 229 m above sea-level.
Other-locality: a vineyard close to Krmačina village in southern part of Slovenia.
Longidorus carniolensis n. sp were detected in 6 out of 10 soil samples from locations of Drašiči and Krmačina. Population density was 4–15 specimens of all developmental stages per 200 cm3 of soil sample.
Holotype female and 2 female, 5 male and 8 juvenile (3 J1, 1 J2, 2 J3, 2 J4) paratypes deposited in the Nematode Collection of Agricultural Institute of Slovenia, Ljubljana, Slovenia; two female, two male and 10 juvenile (3 J1, 2 J2, 2 J3, 2 J4) paratypes - in the Wageningen Nematode Collection (WaNeCo), Wageningen, the Netherlands; one female, one male and 6 juvenile (3 J1, 1 J2, 1 J3, 1 J4) paratypes - in the Nematode Collection of The Food and Environment Research Agency, Sand Hutton, UK (former Rothamsted Nematode Collection); one female, three male and 6 juvenile (3 J1, 2 J3, 1 J4) paratypes - in the Nematode Collection of the Zoology Museum, Ghent University, Belgium; one female, three male and two juvenile (1 J3, 1 J4) paratypes - in the Nematode Collection of the Institute of Plant Protection, Bari, Italy; two female, one male and 6 juvenile (3 J1, 1 J2, 1 J3, 1 J4) paratypes - in the Nematode Collection of the University of California at Riverside, USA; one female, one male and two juvenile (1 J3, 1 J4) paratypes - in the USDA Nematode Collection, Beltsville, Maryland, USA; 4 female, 5 male and 8 juvenile (3 J1, 1 J2, 2 J3, 2 J4) paratypes - in the Nematode Collection of the Institute of Biodiversity and Ecosystem Research, BAS, Sofia.
The species epithet carniolensis was derived from Carniola which is the Latin name of the Kranjska province, a historical region that comprised parts of what is now Slovenia.
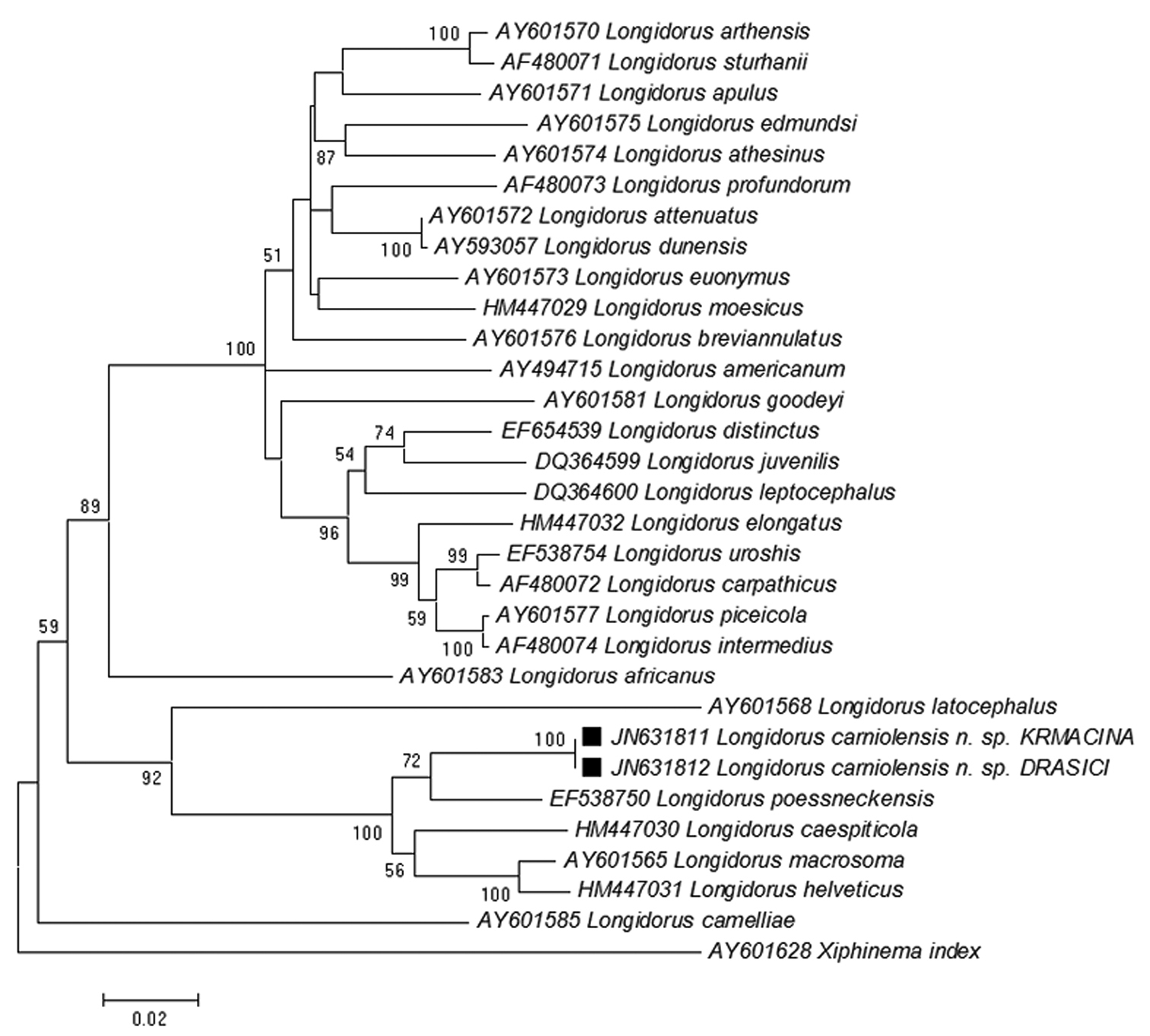
Cluster analyses of the D2-D3 expansion regions of the 28S rDNA nuclear gene sequences of Longidorus carniolensis sp. n. and closely related species (Table 1) were performed and a phylogenetic tree was constructed (Fig. 12). The sequences of both populations of Longidorus carniolensis sp. n.from Drašiči and Krmačina were identical. They formed a distinct clade within a cluster of the closely related sequences of Longidorus poessneckensis, Longidorus helveticus, Longidorus macrosoma, Longidorus caespiticola and Longidorus latocephalus. The closest sequence to Longidorus carniolensis sp. n.was the sequence of Longidorus poessneckensis (Acc. No EF538750) with 91.9% of similarity.
Phylogenetic tree of rDNA D2/D3 expansion region sequences of Longidorus carniolensis sp. n. from Slovenia (square mark) and sequences of closely related Longidorus species (NCBI GenBank). Sequences were analysed using Neighbour Joining Method. Bootstrap support values higher than 50% are presented.
There are some characteristic morphological features observed in Longidorus carniolensis sp. n. such as the presence of vestigium, hemizonid and hemizonion, and the abberant ventral pore. The vestigium was present in all specimens (males and females), it was located in the slender pharynx, behind odontophore, in few specimens in the odontophore area. Such a vestigium has been reported also for Longidorus fursti Heyns, Coomans, Hutsebaut et Swart, 1987 from South Africa; two Chinese Longidorus species (
Hemizonid and hemizonion are not commonly observed structures in dorylaimids (
The only available data on the excretory system in Longidorus refers to Longidorus macrosoma in which a ventral excretory pore at the level of the nerve ring, leading to a non-canalicular tissue in its anterior part has been observed together with two nucleated glands embedded in the tissues of a ventrally located ampulla-like structure (
The data on D2D3 rDNA regions of majority of longidorid species, particularly of those belonging to the genus Longidorus is far to be complete; this does not facilitate the reconstruction of the phylogenetic relationships among the members of this widely distributed group of ectoparasitic nematodes. Despite of this, based on the rDNA results as well as a combination of morphological features the new species is included in a clearly defined group of closely related species (Longidorus poessneckensis (92% similarity), Longidorus macrosoma and Longidorus caespiticola (90%), Longidorus helveticus (89%), sharing some common characters – amphids with pouch-like fovea, not bilobed, amphidial duct well discernable, tapering lip region, which is continuous with the rest of body, normal arrangement of pharyngeal glands, bluntly conoidal to hemispherical tail, much shorter or equal to the anal body width; and the development through 4 juvenile stages. All these species occur in Europe, more frequently in West and Central Europe. The correlation between the amphid structure and clustering of longidorid species has been underlined by
The study was supported by the National Science Fund, project N DO 02-101/2008 and Slovenian Research Agency, project BI-BG/09-10-009. We are grateful to Dr Nathalie Yonow, Swansea University, Wales, UK for linguistic editing as well as to Dr Plamen Pankov, IBER, BAS, Sofia for his technical assistance.