(C) 2012 Mohammed A. Hannan. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The large carpenter bees (Xylocopinae, Xylocopa Latreille) occurring in central Saudi Arabia are reviewed. Two species are recognized in the fauna, Xylocopa (Koptortosoma) aestuans (Linnaeus) and Xylocopa (Ctenoxylocopa) sulcatipes Maa. Diagnoses for and keys to the species of these prominent components of the central Saudi Arabian bee fauna are provided to aid their identification by pollination researchers active in the region. Females and males of both species are figured and biological notes provided for Xylocopa sulcatipes. Notes on the nesting biology and ecology of Xylocopa sulcatipes are appended. As in studies for this species from elsewhere, nests were found in dried stems of Calotropis procera (Aiton) (Asclepiadaceae) and Phoenix dactylifera L. (Arecaceae).
Apoidea, Anthophila, Xylocopini, Arabian Peninsula, systematics, biology, host plants, nesting
The tribe Xylocopini comprises the large carpenter bees (Xylocopinae: Xylocopa Latreille) species of which principally nest in dead wood (including the wood of human constructions), bamboo culms, and other similar substrates (e.g.,
As part of an on-going effort to survey the bee fauna and pollinator resources of the Kingdom of Saudi Arabia and to eliminate the taxonomic impediment for working on this diverse region, we have begun with surveys of the melittofauna from central Saudi Arabia. Herein we provide a brief contribution to this larger effort by documenting the species of the large carpenter bees occurring in this area and as an aid to studies of wild bee pollination already underway (Hannan et al. in prep.). Two species are recognized from the region, Xylocopa (Koptortosoma) aestuans (Linnaeus) and Xylocopa (Ctenoxylocopa) sulcatipes Maa, although the latter may be frequently found misidentified in some collections as Xylocopa (Xylomelissa) hottentotta Smith or XC.) fenestrata (Fabricius) (e.g.,
Material examined herein is deposited in the King Saud University Museum of Arthropods, Plant Protection Department, College of Food and Agriculture Sciences, King Saud University, Riyadh, Kingdom of Saudi Arabia (KSMA) and Division of Entomology (Snow Entomological Collections), University of Kansas Natural History Museum, Lawrence, Kansas, USA (SEMC). Photomicrographs were prepared using a Nikon D1x digital camera attached to an Infinity K-2 long-distance microscope lens. Morphological terminology in the diagnoses follows that of
The nesting biology of Xylocopa sulcatipes was studied in Amariah, approximately 25 km northwest of Riyadh, from September 2010 through December 2011. Nests were found on 6 June 2011 at the base of a large hill near an agricultural farm near Wadi Amariah and the highway to Riyadh. Prior to collection the nests were observed for at least an hour to note the coming and going of bees. Most nests were located around 9:00am and collected around 12:00pm. Nests were sealed with plastic and brought to the lab for dissection and study. During four visits (6, 12, 19 June and 28 September 2011) a total of 13 nests were collected (Table 1). Nests were in the dead branches of local milkweeds [Calotropis procera (Aiton) (Asclepiadaceae), more widely known as the “Apple of Sodom”] growing in a sparsely vegetative desert area and among date palms, Phoenix dactylifera L. (Arecaceae). Nests were measured, sketched, and photographed, and the inhabitants deposited in the KSMA repository.
Measurements of sampled nests of Xylocopa (Ctenoxylocopa) sulcatipes Maa from central Saudi Arabia collected in dead wood of two plants. Means are given with standard deviations. n = number of nests sampled for each metric.
| Asclepiadaceae | Arecaceae | |
|---|---|---|
| Metric | Calotropis procera (Aiton) | Phoenix dactylifera L. |
| Branch length (cm) | 147.75±74.59 (n=8) | 57.33±6.53 (n=6) |
| Nest entrance (mm) | 9.06±0.78 X 8.85±0.86 (n=8) | 10.17±2.17 X 11±2 (n=7) |
| Height of nest from ground (cm) | 83.50±30.3 (n=8) | 400±0 (n=6) |
| Length of nest (cm) | 23.61±14.93 (n=7) | 11.05±4.36 (n=6) |
| Branch diameter at nest (cm) | 1.88±0.38 (n=8) | 6.83±0.41 (n=6) |
| Internal diameter of nest (cm) | 1.28±0.19 (n=8) | 1.77±0.12 (n=6) |
| Number of cells/nest | 6.60±5.6 (n=5) | 4.50±1.64 (n=6) |
| Length of cells (mm) | 18.8±1.63 (n=30) | 20.11±1.37 (n=19) |
This is the largest and most widespread subgenus of carpenter bees, with at least 196 recognized species ranging throughout Subsaharan Africa to the Mediterranean countries of that continent, Dalmatia, the Arabian Peninsula, southwestern Asia, and southern Asia east to the Philippines, Taiwan, and Japan, and south through Indonesia, New Guinea, and the Bismarck Archipelago to southernmost Australia (
http://species-id.net/wiki/Xylocopa_aestuans
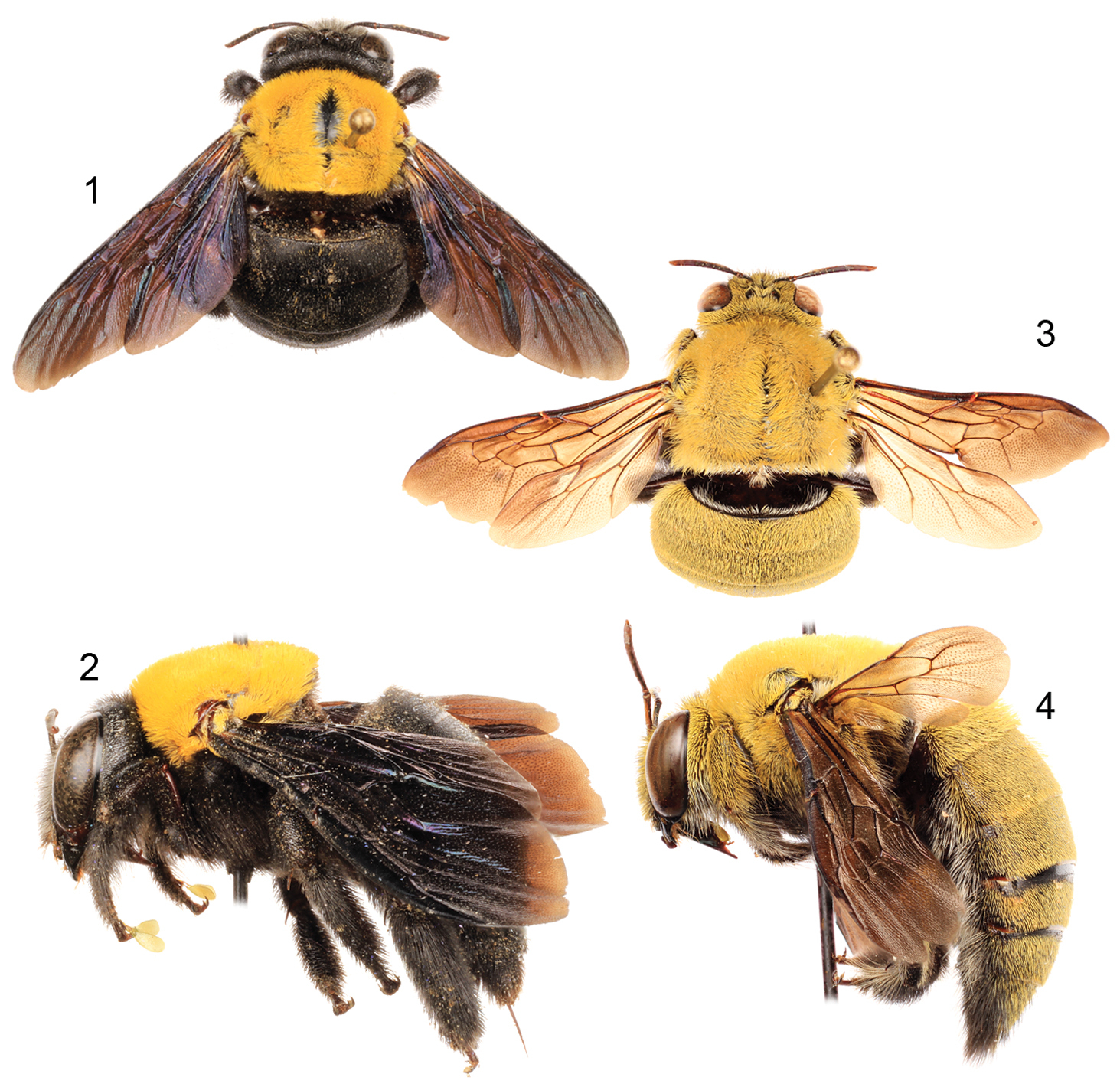
Figs 1–11Diagnosis. Xylocopa aestuans can be most readily distinguished from other Saudi Arabian large carpenter bees by the following: female face with largely white or pale pubescence (Fig. 5), mesosomal dorsum densely covered by yellow pubescence obscuring underlying integument (Figs 1, 2); mandible bidentate at apex; posterodorsal margin of mesoscutellum projecting beyond posterior margin of metanotum; pygidial plate unarmed. Male covered by dense yellow pubescence (Figs 3, 4, 6); first metasomal tergum with subhorizontal dorsal surface abruptly and angulately separated from declivitous anterior surface; gradulus of first metasomal tergum transverse, lateral extremities not directed posteriorly; male terminalia as in figures 7–11.
Comments. Xylocopa aestuans is one of the widespread and ubiquitous of large carpenter bee species. There has been considerable debate regarding the identity of the species of Koptortosoma similar to Xylocopa aestuans (i.e., considering them synonyms, subspecies, or separate species), with different authors of varying opinions how to segregate the minor variation into natural taxonomic entities (e.g.,
Habitus photomicrographs of Xylocopa (Koptortosoma) aestuans (Linnaeus) from central Saudi Arabia. 1 Female, dorsal 2 Female, lateral 3 Male, dorsal 4 Male, lateral.
Faces of Xylocopa (Koptortosoma) aestuans (Linnaeus) from central Saudi Arabia. 5 Female 6 Male.
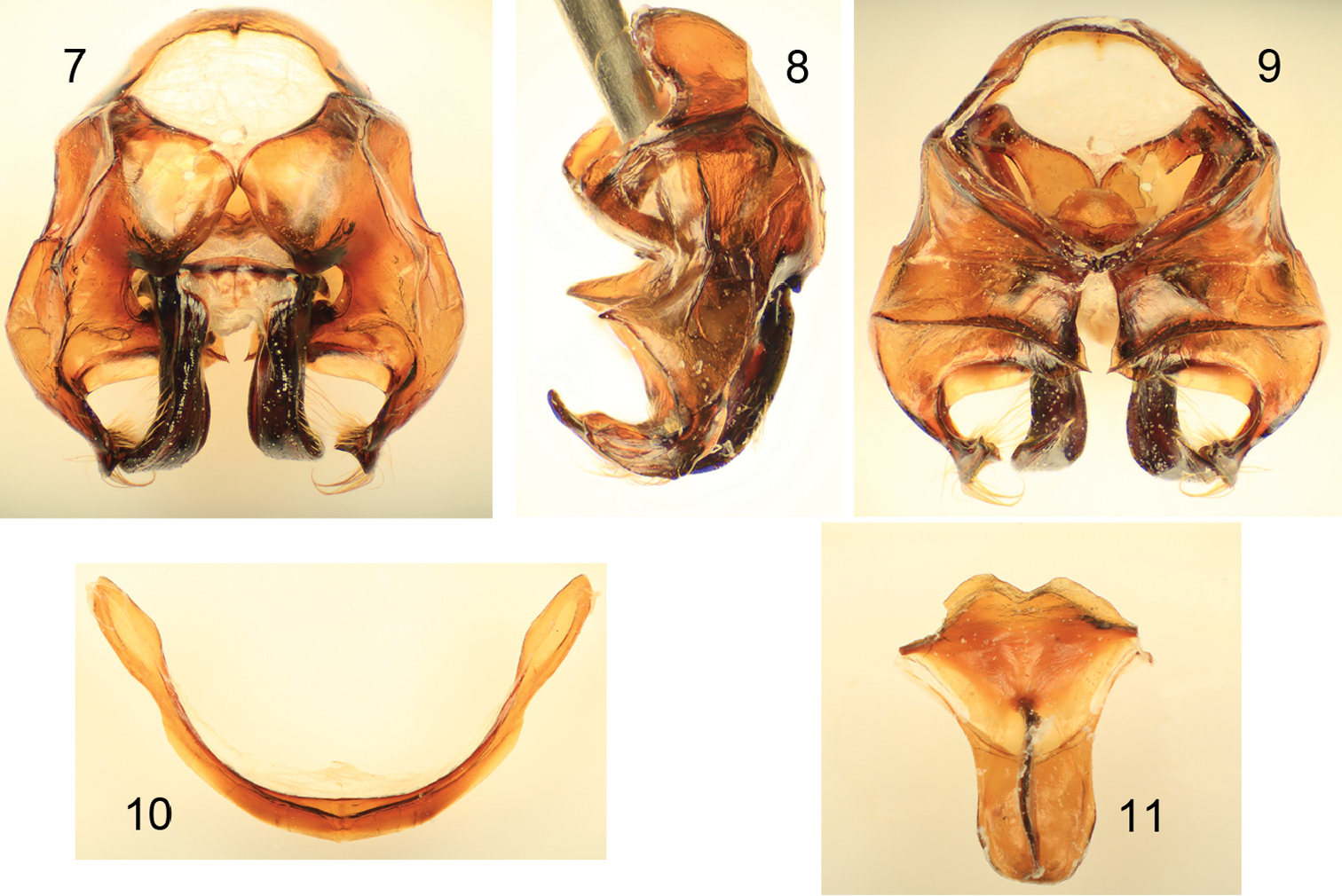
Male terminalia of Xylocopa (Koptortosoma) aestuans (Linnaeus) from central Saudi Arabia. 7 Genital capsule, dorsal aspect 8 Genital capsule, lateral aspect 9 Genital capsule, ventral aspect 10 Seventh metasomal sternum 11 Eighth metasomal sternum.
This is a widespread, albeit not very diverse, subgenus of Old World carpenter bees (
http://species-id.net/wiki/Xylocopa_sulcatipes
Figs 12–22Xylocopa sulcatipes can be most readily distinguished from other Arabian large carpenter bees by the following: Female with face with largely black pubescence (Fig. 16), mesosomal dorsum largely covered by black pubescence not obscuring underlying integument (Figs 12, 13); mandible tridentate at apex; mesoscutellum not projecting over metanotum, apical margin rounded in profile; pygidial plate armed on each side with subapical spine. Male covered by largely fuscous to black pubescence except face, dorsum of mesosoma, and apicolateral patches of first metasomal tergum with predominantly white or pale setae (Figs 14, 15, 17); first metasomal tergum with subhorizontal dorsal surface rounding into declivitous anterior surface; gradulus of first metasomal tergum laterally curved posteriorly; male terminalia as in figures 18–22.
Habitus photomicrographs of Xylocopa (Ctenoxylocopa) sulcatipes Maa from central Saudi Arabia. 12 Female, dorsal 13 Female, lateral 14 Male, dorsal 15 Male, lateral.
Faces of Xylocopa (Ctenoxylocopa) sulcatipes Maa from central Saudi Arabia. 16 Female 17 Male.
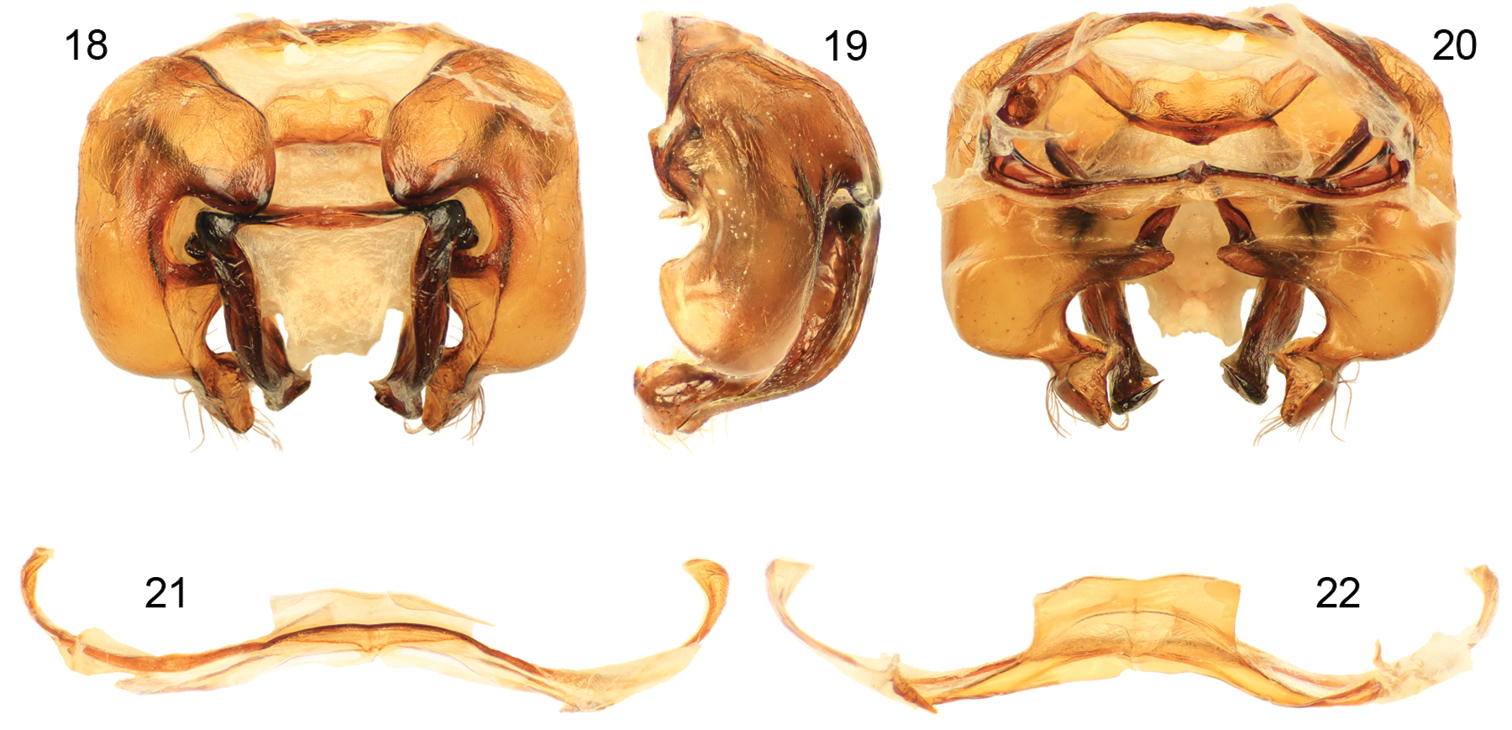
Male terminalia of Xylocopa (Ctenoxylocopa) sulcatipes Maa from central Saudi Arabia. 18 Genital capsule, dorsal aspect 19 Genital capsule, lateral aspect 20 Genital capsule, ventral aspect 21 Seventh metasomal sternum 22 Eighth metasomal sternum.
| 1 | Males | 2 |
| – | Females | 3 |
| 2 | Bee covered by dense yellow pubescence; first metasomal tergum with subhorizontal dorsal surface abruptly and angulately separated from declivitous anterior surface; gradulus of first metasomal tergum transverse, lateral extremities of gradulus not directed posteriorly; terminalia as in figures 7–11 | Xylocopa aestuans (Linnaeus) |
| – | Bee covered by largely fuscous to black pubescence except face, dorsum of mesosoma, and apicolateral patches of first metasomal tergum with predominantly white or pale setae; first metasomal tergum with subhorizontal dorsal surface rounding into declivitous anterior surface; gradulus of first metasomal tergum laterally curved posteriorly; terminalia as in figures 18–22 | Xylocopa sulcatipes Maa |
| 3 | Mesosomal dorsum densely covered by yellow pubescence obscuring underlying integument; face with largely white or pale pubescence; pygidial plate unarmed; posterodorsal margin of mesoscutellum projecting beyond posterior margin of metanotum; mandible bidentate at apex | Xylocopa aestuans (Linnaeus) |
| – | Mesosomal dorsum largely covered by black pubescence not obscuring underlying integument; face with largely black pubescence; pygidial plate armed on each side with subapical spine; mesoscutellum not projecting over metanotum, apical margin rounded in profile; mandible tridentate at apex | Xylocopa sulcatipes Maa |
The biology of Xylocopa sulcatipes has been the focus of several extensive ecological and behavioral studies, principally in Israel (e.g.,
Although Xylocopa sulcatipes, like other Xylocopa, is polylectic, females were observed foraging mostly from Calotropis procera and it was there that males were seen to approach and grab females for mating. In addition to foraging at Calotropis procera, females were observed visiting Reseda alba L. (Resedaceae) and radish [Raphanus sativus L. (Brassicaceae)]. Given that species of Xylocopa may be useful for agricultural pollination (
Photographs of nests of Xylocopa (Ctenoxylocopa) sulcatipes Maa in stems of Calotropis procera (Aiton) in central Saudi Arabia. 23 Nest entrance in stem of Calotropis procera in the wild 24 Opened nest with series of larvae in individual cells 25 Opened nest with pupae 26 Individual pollen mass with egg situated on top.
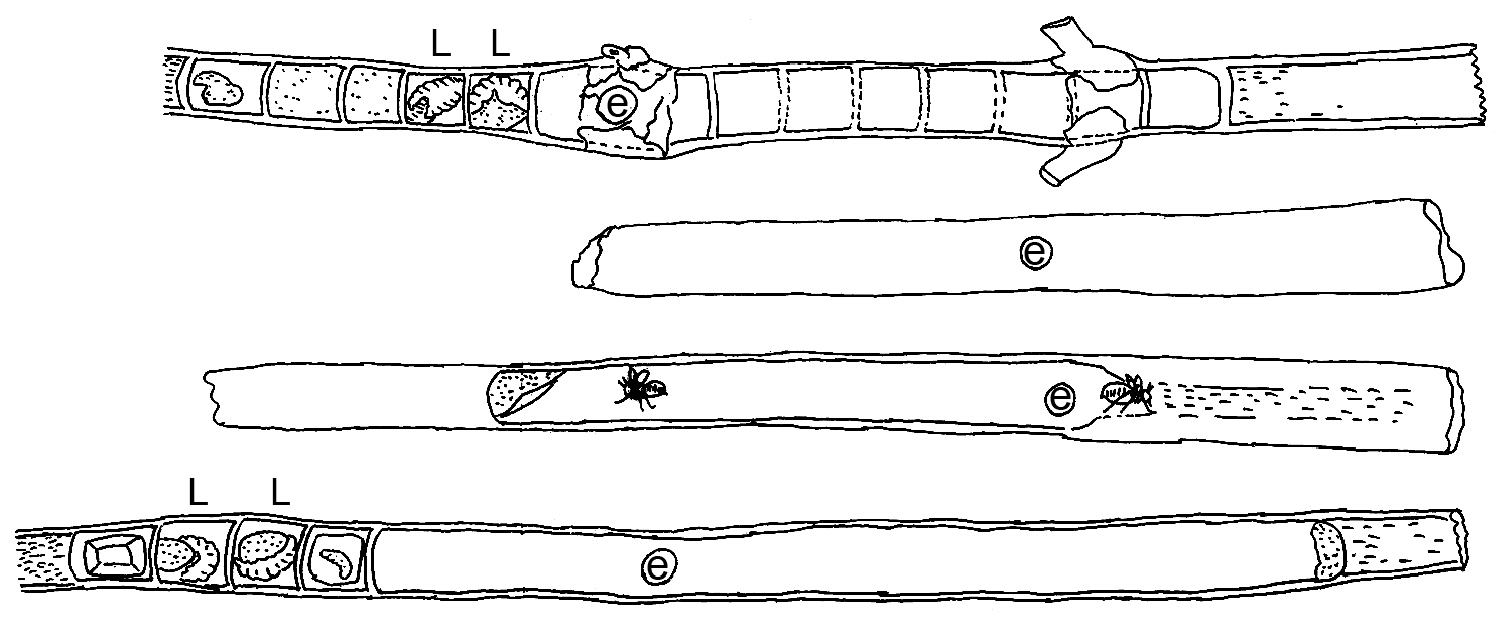
Diagrams of representative nests of Xylocopa (Ctenoxylocopa) sulcatipes Maa in stems of Calotropis procera (Aiton) in central Saudi Arabia. L = larva; e = nest entrance. Line illustrations by M.A. Hannan.
This work was supported by King Saud University, Deanship of Scientific Research, College of Food and Agriculture Sciences Research Center. We are thankful to Naser Al-Ghoson and Hassan Balhareth who helped us during the course of this study, to Prof. Charles D. Michener for encouragement, to Dr. Ismael A. Hinojosa-Díaz for assistance with photomicrography, support for which was provided by the Engel Illustration Fund of the University of Kansas College of Liberal Arts and Sciences, and to two anonymous reviewers for their comments on the manuscript. Lastly, we are grateful to the owner and employees of the agricultural farm Mazra’ah Al-Gasim who generously permitted us to work on their property. This is a contribution of the Division of Entomology, University of Kansas Natural History Museum.