(C) 2012 Benjamin W. Price. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The monotypic South African alderfly genus Leptosialis Esben-Petersen, 1920 is reviewed and Leptosialis africana Esben-Petersen, 1920 is redescribed. In the process a new species of alderfly Leptosialis necopinata sp. n. from the Eastern Cape and KwaZulu-Natal provinces of South Africa is recognised and described. Within Sialidae the new species most closely resembles Leptosialis africana. A key to the two species of Leptosialis using both adult and larval characters is provided.
Sialidae, Leptosialis, taxonomy, South Africa
The Afrotropical Megaloptera fauna have historically received very little attention and only four species of alderfly (Sialidae) are currently recognised: Sialis vanderweelei Aspöck & Aspöck, 1983 (Egypt), Protosialis afra Navás, 1936 and Protosialis madegassa Navás, 1927 (Madagascar), and Leptosialis africana Esben-Petersen, 1920 (South Africa). Leptosialis Esben-Petersen was established from adult specimens collected in the Cedarberg Mountains of the Western Cape Province. Additional localities and illustrations were provided by
With very few specimens in museum collections, it is clear that these African alderflies are rarely encountered insects. The adults have historically been collected in summer amongst riparian vegetation associated with the still reaches of slow-flowing streams, and the larvae inhabit slow flowing streams with clay or silt substrates (
While examining the Iziko South African Museum and Albany Museum collections, two distinct phenotypes of putative Leptosialis africana adults were found, corresponding to the two disjunct regions from which they have been recorded. The specimens from the Eastern Cape and KwaZulu-Natal provinces are distinct enough from Western Cape Leptosialis africana to warrant recognition as a new species.
The specimens included in the present study are deposited in three collections: the Albany Museum, Grahamstown (AMGS), the Natural History Museum, London (NHM), and the Iziko South African Museum, Cape Town (SAMC). Label data for the specimens are presented in quotation marks; information from different labels is separated by a virgule (/), and information on different lines of a label is separated by commas. Information in square brackets clarifies or augments the often cryptic text on the specimen label(s) and provides their geographic coordinates.
Terminalia preparations were made by clearing the distal half of the abdomen in a cold, saturated potassium hydroxide (KOH) solution for 8–10 h. After neutralising the KOH with acetic acid and water, the distal half of the abdomen was transferred to glycerine for further dissection and examination. Following examination, the cleared terminalia was placed in a microvial containing glycerine and pinned beneath the specimen. The terminologies of venation and terminalia follow
http://species-id.net/wiki/Leptosialis
The adults of Leptosialis are characterized by the following morphological traits: the narrowly elongated forewing, which is about 3.0–4.0 times longer than wide; the distally branched Rs; the MA either unbranched, bifurcated or trifurcated; the MP distally branched or with one or both main branches bifurcated; the male 9th tergum with a pair of posterolateral, digitiform processes; the paired ectoproct; the 11th gonocoxite in caudal view ventrally with a pair of acute, hook-like processes. At present no characters can be used to distinguish the larvae of the genus Leptosialis from other alderfly genera, many of which currently lack larval description.
Forewing length ~8–11 mm in males; ~11–12 mm in females. Body: generally brown or blackish-brown. Head: antenna pilose approximately half the length of the forewing; ocelli absent, or represented by three very small tubercles on vertex; labrum ~4.0–5.0 times wider than long, lateral margins rounded, front margin slightly emarginated. Prothorax > two times wider than long. Legs: yellow or dark brown, bearing dense setae; tarsal claws reddish brown. Wings: Forewing 3.0-4.0 times longer than wide, minutely hirsute, margins pilose; costal area broadened basally; subcostal area with five to eight distinct costal crossveins proximally; sc-r absent; Rs distally branched, MA either unbranched, bifurcated or trifurcated, MP distally branched or with one or both main branches bifurcated, CuA bifurcated; three or four crossveins between R and Rs. Hindwing as broad or slightly broader than forewing, about 3.0 to 3.5 times as long as wide; two or three distinct costal crossveins proximally; venation similar to forewing, with three crossveins between R and Rs. Male genitalia: 9th tergum transversely arched, with a pair of posterolateral, digitiform processes; ectoproct paired, small, roundly inflated ventrad; 11th gonocoxite in caudal view ventrally with a pair of acute, hook-like processes. Female genitalia: 7th sternum broad, posterior margin distinctly produced; 9th gonocoxite broad, apex bearing small, stout gonostylus.
Larvae (Figs 22–23).Head: yellow to reddish brown. Thorax: pro-, meso- and metathorax orange to reddish brown, with distinct reticulated patterns of yellowish marks. Legs: pale yellow, bearing dense setae; tarsal claws reddish brown; Abdomen: dark purplish or blackish brown dorsally with paired, pale, submedian, comma-shaped marks on each segment; anal prolegs and hooked claws absent; elongated caudal filamentous appendage present; 7 pairs of pale yellow lateral abdominal gills present.
The genus Leptosialis is the only representative of the Sialidae in South Africa. Because wing venation is quite different between the two Leptosialis species, it is difficult to find a stable morphological diagnosis for this genus. The male ninth tergum with a pair of digitiform processes could be the most important character to distinguish Leptosialis from its closely related genera, Stenosialis and Austrosialis.The two species may be distinguished using the following key.
Habitus images of Leptosialis species. 1 Leptosialis africana Esben-Petersen, female, holotype 2 same, male 3 Leptosialis necopinata sp. n. male, holotype 4 same, female. Scale bars = 5.0 mm
| 1 | Adults: (Figs 1, 2) Forewing narrowly rounded with MA and both branches of MP simple ( |
Leptosialis africana |
| – | Adults: (Figs 3, 4) Forewing broadly rounded with MA branches bi- and trifurcated and with one or both branches of MP bifurcated (Fig. 9). Male: terminalia with 11th gonocoxite narrowly triangular dorsoventrally, with a pair of long, hook-like processes with additional subterminal hook ventrally, visible in caudal view (Figs 18-19). Female: 8th gonocoxite large, terminally divided to form a pair of subtriangular lobes, obtusely protruding posterolaterally (Fig. 21). Larvae: (Fig. 23) Head orange, slightly darkened medially on frons; prothorax orange, dorsally with indistinct marks, meso- and metathorax castaneous with reticulated yellowish marks; abdomen dark purplish-brown with paired, pale, submedian, comma-shaped marks on each segment (Fig. 1: |
Leptosialis necopinata sp. n. |
http://species-id.net/wiki/Leptosialis_africana
Figures 1–2, 5–6, 10–15, 22Holotype, male [see remarks below] (pinned; Fig. 1), South Africa: Western Cape: “G[rea]t. Winterhoek Mts., Tulbagh [33°8'S, 19°10'E], XI.1916, K.H. Barnard / SAM-MEG-A000041 / Holotype” (SAMC).
1 male (pinned, Fig. 2), South Africa: Western Cape: “G[rea]t. Winterhoek Mts., Tulbagh, [33°8'S, 19°10'E], XI.1932, K.H. Barnard / SAM-MEG-A000042” (SAMC); 1 female (pinned), South Africa: Western Cape: “Hottentots Holland Mts., Steenbras [34°6'S, 18°58'E], XI.1932, K.H. Barnard, SAM-MEG-A000043” (SAMC); 1 male (pinned), South Africa: Western Cape: “Caledon Distr., R. Zonder End, Oudebosch [34°9'S, 19°54'E], XII.1919, K.H. Barnard / 1930-131” (NHM); 2 larvae (in alcohol), South Africa: Western Cape: “Bettys Bay, Small Vlei [34°21'S, 18°56'E], 18.I.1957, [A.D. Harrison and J.D. Agnew] / FRW 128C” (AMGS).
The adults of Leptosialis africana may be easily distinguished from adults of Leptosialis necopinata sp. n. by the shape and venation of the forewing, where the wings of Leptosialis africana are narrowly rounded compared to being broadly rounded to truncated in Leptosialis necopinata sp. n. In addition, the wings of Leptosialis africana show MA and both branches of MP all simple, while Leptosialis necopinata sp. n. has MA and one or both branches of MP all bearing additional forks. The larvae of Leptosialis africana may be distinguished by the reddish brown head and thorax dorsally with distinct yellowish marks that are lacking in Leptosialis necopinata sp. n.
Forewing length 8.1 mm, hindwing length 7.0 mm (n = 1). Head (Fig. 5): pale to dark brown, frons and clypeus black; vertex with a pair of raised black vittae medially and several small, raised, black protrusions laterally. Compound eyes blackish brown, strongly produced. Antennae dark brown, pilose, approximately half the length of the forewing. Mouthparts yellowish brown, diminutive. Thorax (Fig. 5): entirely blackish brown. Legs yellow, bearing dense yellowish setae; coxae blackish brown; femora pale brown; 1st-3rd tarsomeres distally pale brown; 4th and 5th tarsomeres brown; tarsal claws reddish brown. Wings narrowly rounded, brown, slightly darker proximally; veins brown. Forewing nearly 4.0 times as long as wide; five to eight distinct costal crossveins proximally; sc-r absent; Rs distally branched, MA unbranched, MP distally branched, and CuA bifurcated; three or four crossveins between R and Rs. Hindwing slightly broader than forewing, about 3.5 times as long as wide; two or three distinct costal crossveins proximally; venation similar to forewing, with three crossveins between R and Rs. Abdomen: blackish brown. Terminalia (Figs 10–13) with 9th tergum transversely arched, with a pair of digitiform processes posterolaterally that curve slightly ventromedially; anterior and posterior margins slightly concave arcuately in dorsal view; 9th sternum slightly longer than 9th tergum; posterior margin moderately produced; 9th gonocoxite in lateral view nearly elliptical; ectoproct paired, small, roundly inflated ventrad; 11th gonocoxite broadly triangular dorsoventrally, dorsal margin slightly sinuous in dorsal view, with a pair of short and acutely hook-like processes ventrally in caudal view.
Adult female (Figs 1, 6).Forewing length 11.1 mm, hindwing length 10.2 mm (n = 2). Larger than male, but similar in colouration and venation. Terminalia (Figs 14-15) with 7th sternum broad, posterior margin distinctly produced; 8th gonocoxite rather small, subtriangular; 9th gonocoxite broad; apex with small, stout gonostylus, ectoproct feebly sclerotized, small, suboval.
Larva (Fig. 22).Head reddish brown; thorax reddish brown, distinctly marked with complicate patterns of yellowish marks; legs pale yellow, bearing dense setae, tarsal claws reddish brown. Abdomen blackish brown dorsally with pale yellow lateral abdominal gills.
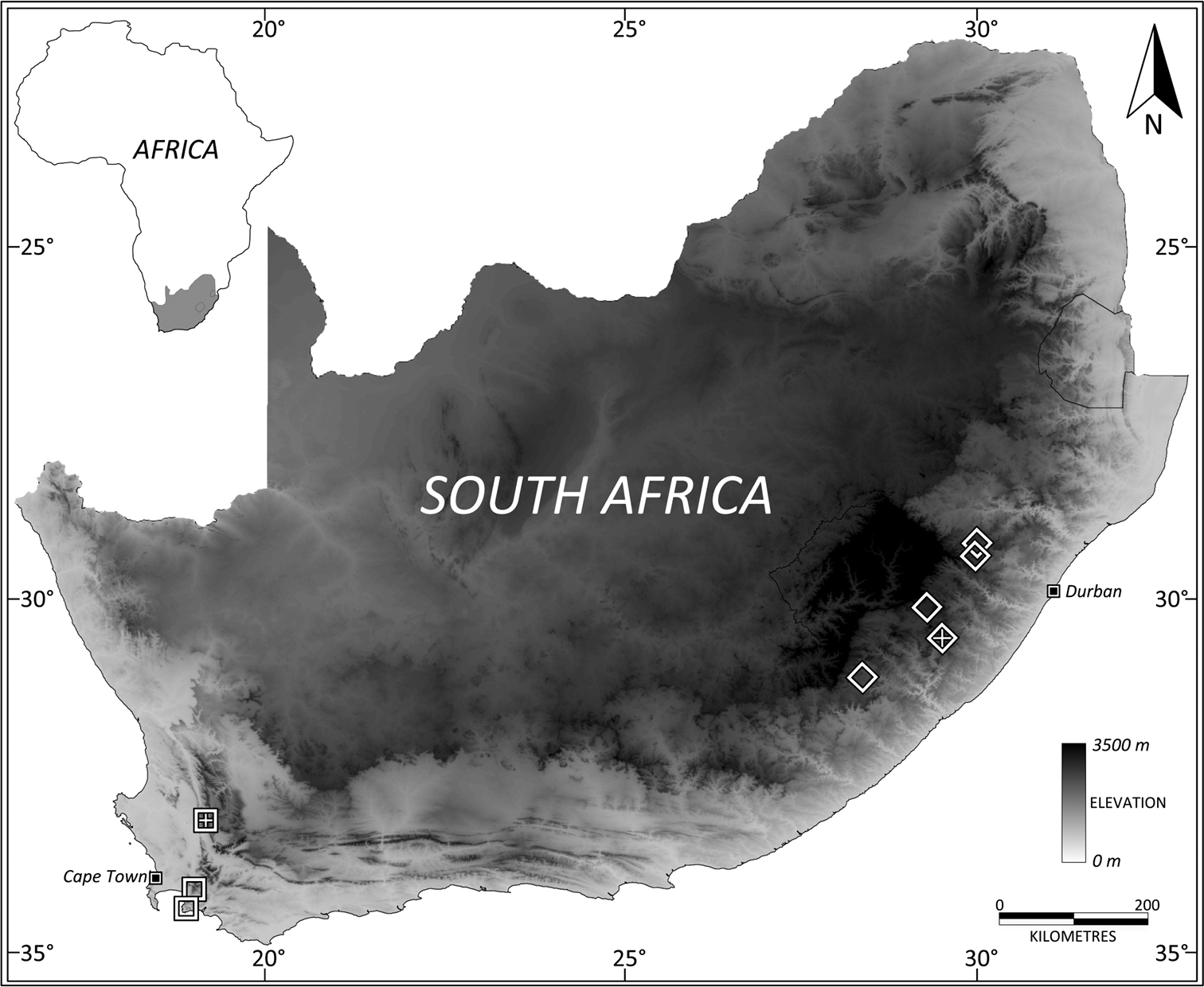
South Africa: Western Cape (Fig. 24).
The type specimen was noted by Esben-Petersen in his description as male, but its abdomen and genitalia are now lost. This species of alderfly shows sexual dimorphism, with females being larger than males. Although it lacks an abdomen, the holotype is probably a female, based on comparison with confirmed male and female specimens, and contrary to the original description.
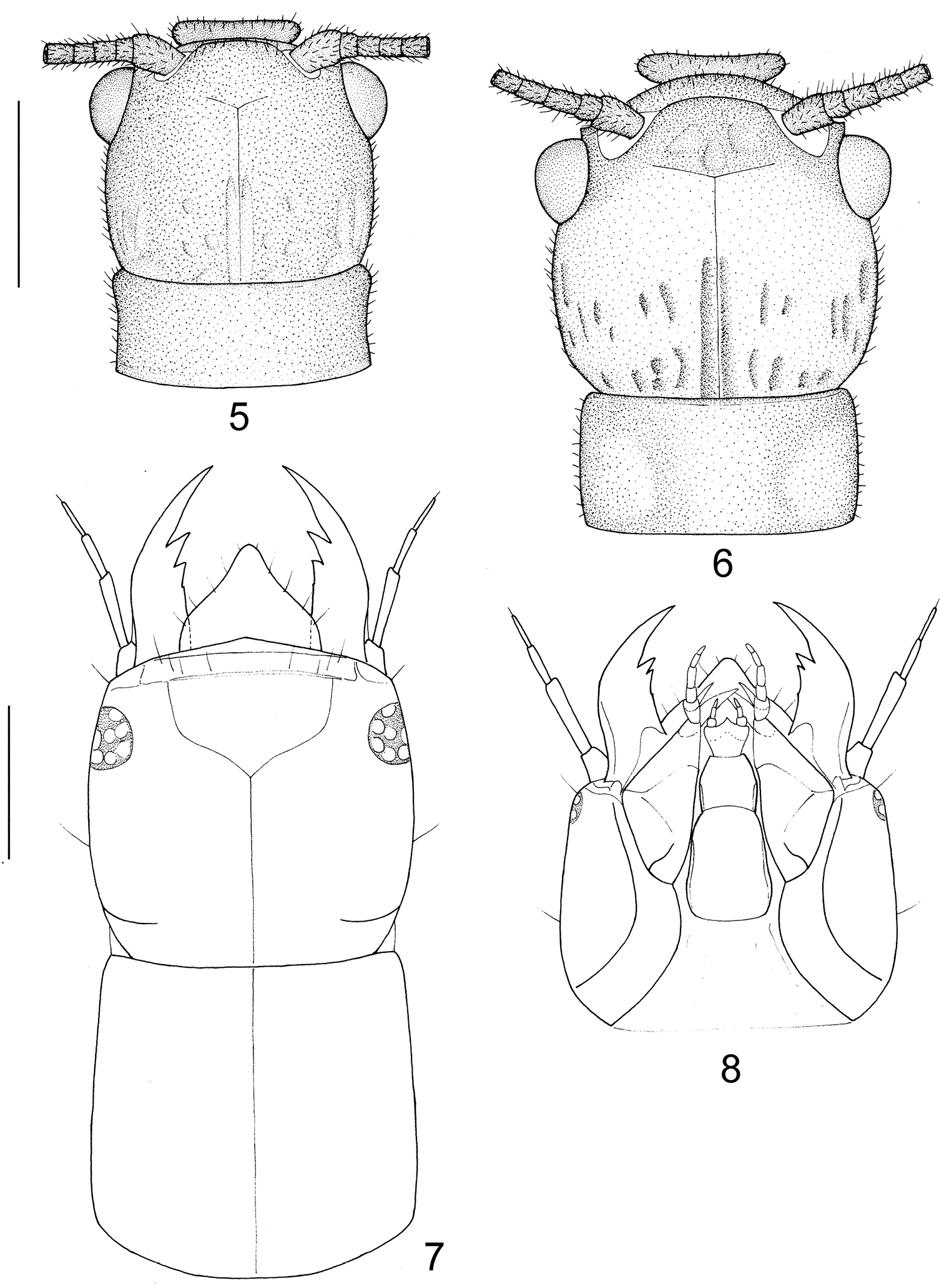
Head and prothorax of Leptosialis. 5 Leptosialis africana, head and pronotum of male adult, dorsal view 6 same of female adult, dorsal view 7 Leptosialis necopinata sp. n., head and pronotum of larva, dorsal view 8 same, head of larva, ventral view. Scale bar = 1.0 mm.
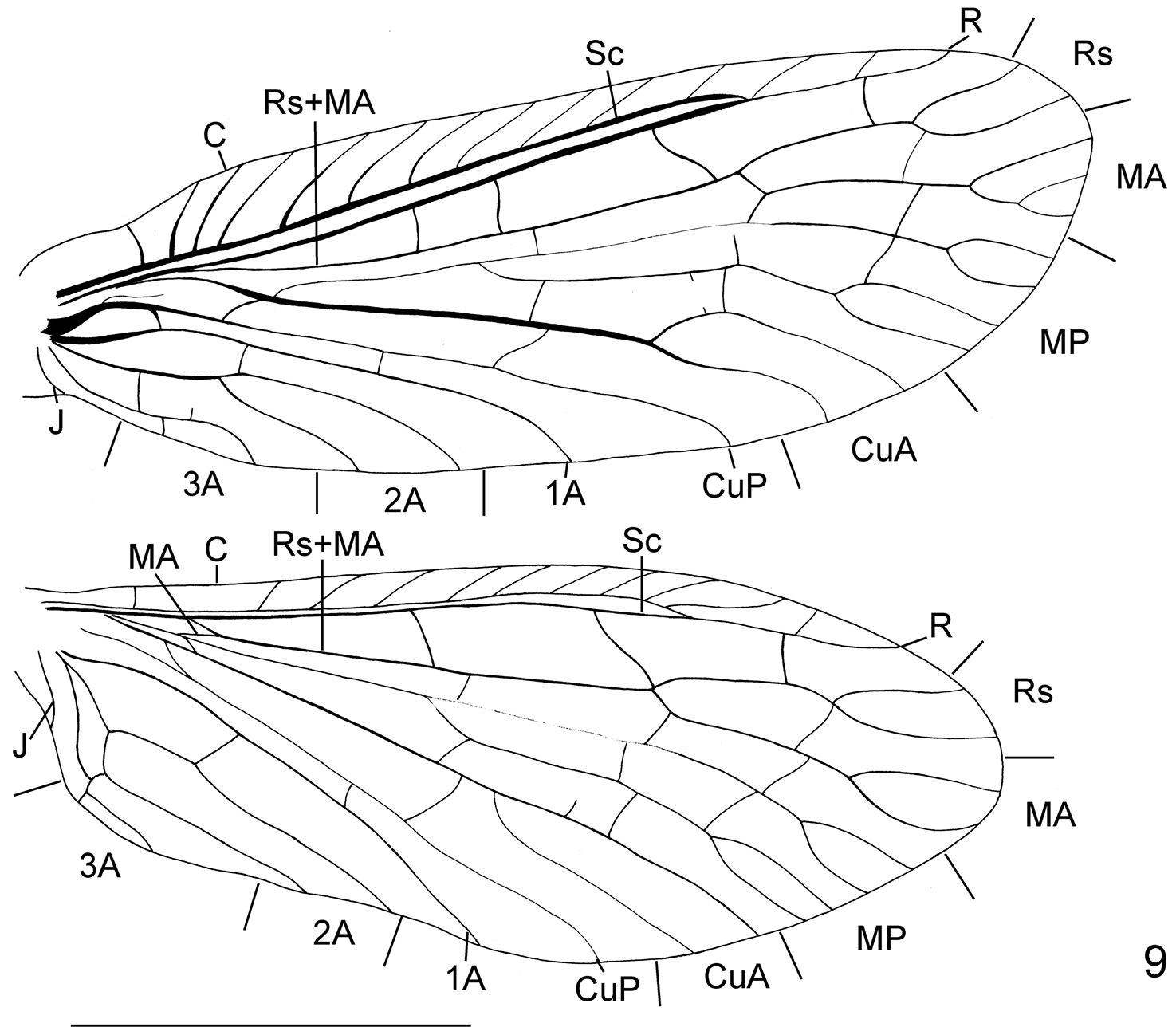
Wing venation of Leptosialis necopinata sp. n. Scale bar = 5.0 mm.
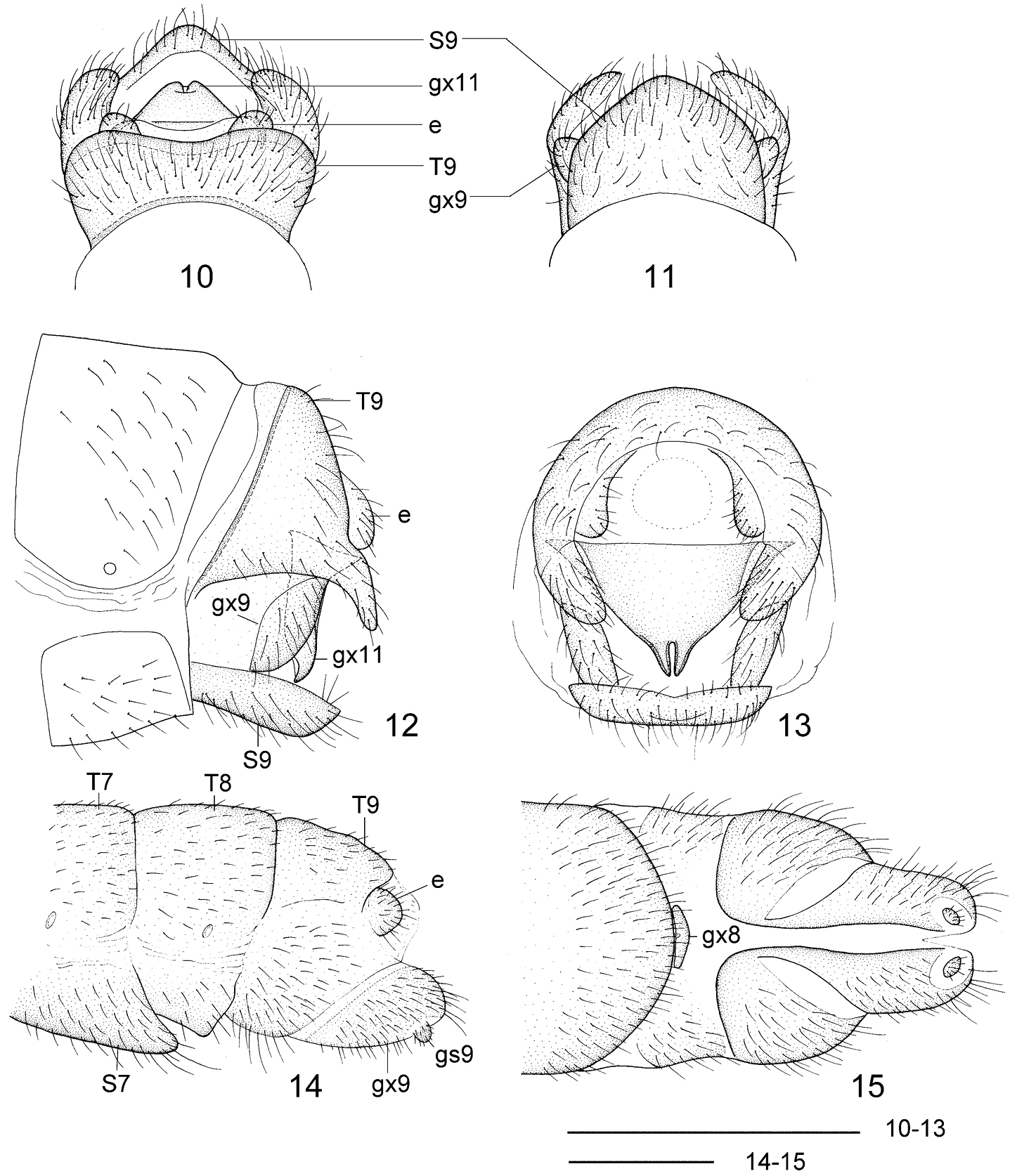
Leptosialis africana Esben-Petersen. 10 male terminalia, dorsal view 11 male terminalia, ventral view 12 male terminalia, lateral view 13 male terminalia, caudal view 14 female terminalia, lateral view 15 female terminalia, ventral view. T7-9: seventh to ninth tergum; S7-9: seventh to ninth sternum; gx8, 9 11 eighth, ninth and eleventh gonocoxite; gs9: ninth gonostylus; e: ectoproct. Scale bars = 0.5 mm.
urn:lsid:zoobank.org:act:0DC77737-04AC-49B5-A2CF-3B2A09201DEA
http://species-id.net/wiki/Leptosialis_necopinata
Figures 3–4, 7–9, 16–21, 23South Africa: KwaZulu-Natal: Kokstad [30°33'S, 29°25'E].
Holotype, male (pinned; Fig. 3), SOUTH AFRICA: KwaZulu-Natal: “Kokstad [30°33'S, 29°25'E], I.1941, [possibly R.S. Crass] / GEN 2081A / HOLOTYPE” (AMGS). Paratypes, 1 male, 5 female (all pinned), same data as holotype, male “GEN 2081B / PARATYPE” females “GEN 2081C / PARATYPE” (AMGS).
SOUTH AFRICA: 1 female (pinned), Eastern Cape: “Maclear Municipal Dam [31°4'0"S, 28°18'36"E], 27.III.1993 [F.C. de Moor and H.M. Barber-James] / ECR 124S” (AMGS); 2 female (pinned), KwaZulu-Natal: “Tugela river [30°6'0"S, 29°11'24"E], 15.XI.1959, [M. Chutter] / GEN 380H” (AMGS); 3 larvae (in alcohol), KwaZulu-Natal: “Mooi River [29°15'0"S, 29°58'12"E], 15.VI.1995, [C.W.S. Dickens] / MOI 52K” (AMGS); 4 larvae (in alcohol), KwaZulu-Natal: “Mooi River [29°21'36"S, 29°53'24"E], 4.I.1996, [F.C. de Moor, C.W.S. Dickens & R.S. Crass] / MOI 69H” (AMGS).
The specific epithet ‘necopinata’ is a feminine adjective in the second declension which refers to the Latin for unexpected or unforeseen, following the discovery of the adults strikingly different from Leptosialis africana and relating to the unforeseen occurrence of this second species in the relatively well-sampled waters of South Africa.
The adults of Leptosialis necopinata sp. n. may be easily distinguished from adults of Leptosialis africana in having broadly rounded to truncated wings compared to narrowly rounded wings in the latter species. In addition the forewing venation of Leptosialis necopinata sp. n. has MA and one or both branches of MP all forked distally, whereas those of Leptosialis africana are fused into three simple veins. The larvae of Leptosialis necopinata sp. n. can be distinguished from Leptosialis africana by the paler colouration of head and thorax, which lack distinct marks on the vertex and pronotum.
Forewing length 10.6 mm, hindwing length 9.8 mm (n = 2).
Head (Fig. 7) black, slightly pale brown surrounding posterior margin of compound eyes; vertex with a pair of raised black vittae medially, and several small raised black protrusions laterally Compound eyes blackish brown, strongly produced. Antennae blackish brown, pilose, approximately half the length of the forewing. Mouthparts blackish brown. Thorax (Fig. 8) entirely black. Legs dark brown throughout, bearing dense brown setae; tarsal claws reddish brown. Wings (Fig. 9) distally broadly rounded to truncate, brown, slightly darker proximally; veins brown. Forewing approximately 3.0 times as long as wide; proximally with five to eight distinct costal crossveins; sc-r absent; RS distally branched, MA bifurcated or trifurcated, MP with one or both main branches bifurcated, and CuA bifurcated, three or four crossveins between R and Rs. Hindwing as broad as forewing, about 3.0 times as long as wide; with two or three distinct costal crossveins proximally; venation similar to forewing, with three crossveins between R and Rs. Abdomen blackish brown. Terminalia (Figs 16–19) with 9th tergum transversely arched, with a pair of digitiform processes posterolaterally that curve slightly ventromedially; anterior and posterior margins slightly arcuately concave in dorsal view; 9th sternum slightly longer than 9th tergum, posterior margin moderately produced; 9th gonocoxite in lateral view nearly elliptical; ectoproct paired, small, roundly inflated ventrad; 11th gonocoxite broadly triangular dorsoventrally, dorsal margin slightly sinuous in dorsal view, ventrally with a pair of acute hook-like processes in caudal view, in lateral view ventral processes with an anteriorly directed, hook-shaped accessory protuberance one third along its length.
Adult female (Fig. 4).Forewing length 11.7 mm, range 11–12 mm, hindwing length 10.4 mm, range 10–11 mm (n = 5). Larger than male, but similar in colouration and wing venation. Head pale to dark brown, with frons and clypeus black; vertex with a pair of raised black vittae medially, and several small raised black markings laterally. Terminalia (Figs 20–21) with 7th sternum broad, with posterior margin feebly produced; 8th gonocoxite almost separated into a pair of subtriangular lobes, which are obtusely protruding posterolaterally; 9th gonocoxite broad, apex with small, stout gonostylus; ectoproct feebly sclerotized, small, suboval.
LarvaHead (Figs 7, 8, 23) yellow, slightly darkended medially on frons. Prothorax (Fig. 7) yellow to orange, dorsally with indistinct markings, meso- and metathorax castaneous with reticulated yellowish markings; Legs pale yellow, bearing dense setae, tarsal claws reddish brown; Abdomen dark purplish-brown dorsally with paired, pale, submedian, comma-shaped marks on each segment; lateral abdominal gills pale yellow.
South Africa: Eastern Cape and KwaZulu-Natal provinces (Fig. 24).
The larvae of Leptosialis necopinata sp. n. have been described in detail by
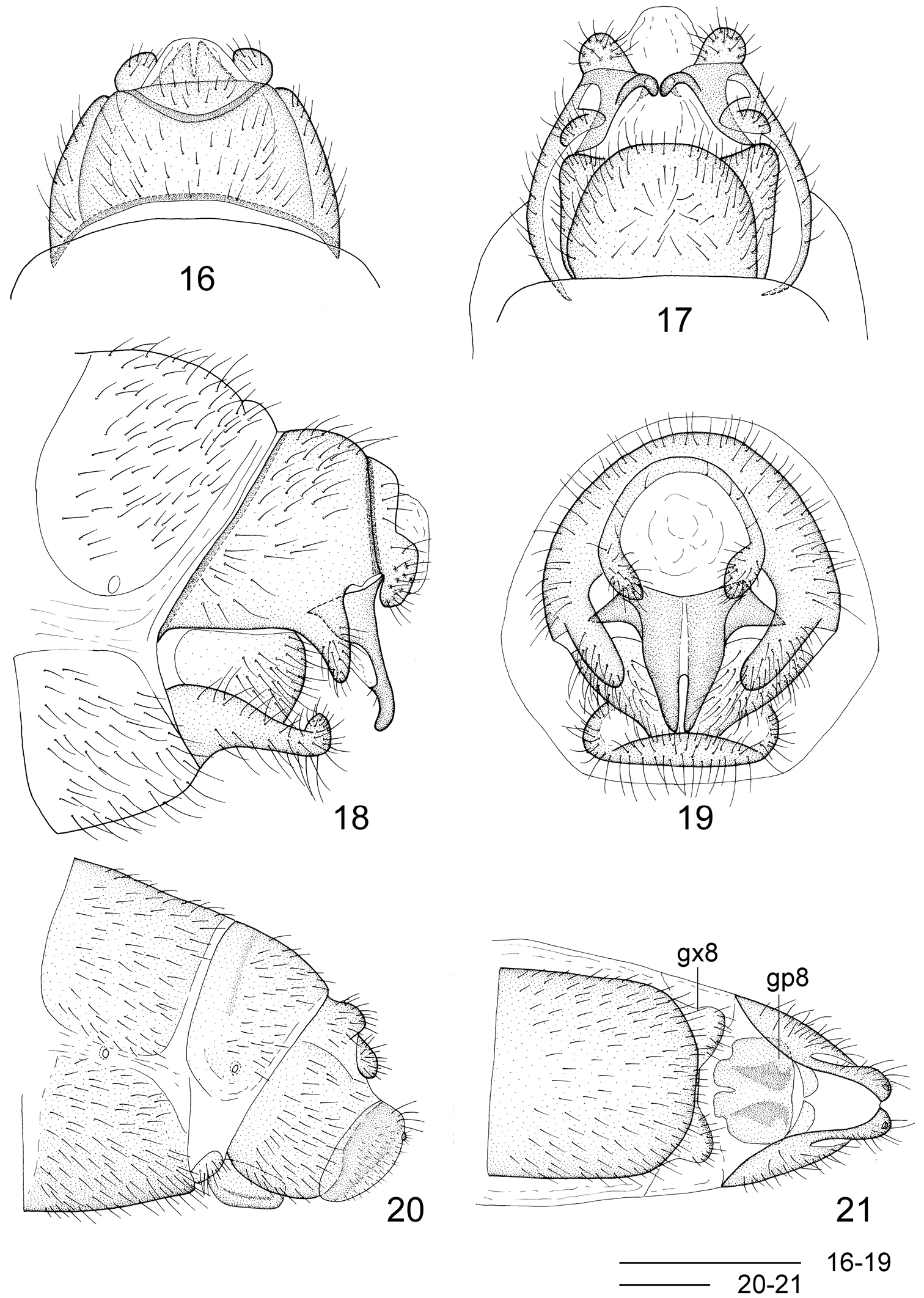
Leptosialis necopinata sp. n. 16 male terminalia, dorsal view 17 male terminalia, ventral view 18 male terminalia, lateral view; 19 male terminalia, caudal view 20 female terminalia, lateral view 21 female terminalia, ventral view. gx8: eighth gonocoxite; gp8: eighth gonapophyses. Scale bars = 0.5 mm.
Leptosialis larvae, showing color patterns on head and thorax 22 Leptosialis africana 23 Leptosialis necopinata sp. n. Scale bar = 1.0 mm.
Distribution map of specimens housed in the Iziko and Albany Museums in South Africa. Leptosialis africana: open squares, Leptosialis necopinatasp. n.: open diamonds. Type localities for each species indicated with “+”. Shading indicates elevation.
At first glance Leptosialis necopinata sp. n. appears to belong to the Australian endemic genus Stenosialis (
The authors thank Ms. Margie Cochrane (SAMC, Cape Town) and Mr. David Goodger (NHM, London) for assistance in accessing the specimens in the Iziko South African Museum and the Natural History Museum collections; the Directorate of Museums and Heritage, Eastern Cape, for providing research facilities and encouraging this research. We also thank Dr. Fumio Hayashi (Tokyo) and Dr. Yukimasa Kobayashi (Tokyo) for their kind help during the guest research of XL at Tokyo Metropolitan University. The authors thank the two anonymous reviewers for feedback that improved the manuscript. This research was supported by the National Natural Science Foundation of China (No. 31000973 to XL), the Foundation for the Author of National Excellent Doctoral Dissertation of PR China (No. 201178 to XL), and the National Research Foundation of South Africa (South African Biosystematics Initiative Grant No. 71139 to BWP). Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NRF.
Localities of specimens housed in the Iziko and Albany Museums in South Africa. (doi: 10.3897/zookeys.201.2623.app) File format: Comma Separates List (csv).