(C) 2012 Rolando Bastida-Zavala. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Six species of Serpula and Spiraserpula were identified, mainly, from the material of the expeditions of the Rosenstiel School of Marine and Atmospheric Science, University of Miami, including two new species of Serpula. Serpula madrigalae sp. n. from the Turks and Caicos has a tube with five longitudinal ridges, four rows of alveoli and a medium-sized shallow symmetrical opercular funnel with 17 radii, and an inner surface with opercular tubercles. Serpula vossae sp. n. from the Western Caribbean and Bahamas has a tube with 6–8 longitudinal ridges, and a large, deep symmetrical opercular funnel, with 21–33 radii, and a smooth inner surface. Serpula cf. vermicularis, recorded from the Gulf of Guinea (tropical eastern Atlantic), is distinguished from the nominal species in possessing fewer opercular radii (33–39) and the lack of a proximal rasp in the bayonet chaetae; tubes are missing. The distribution range is extended for the three known Spiraserpula species found in the collections, Spiraserpula caribensis, Spiraserpula karpatensis and Spiraserpula ypsilon.
ResumenSeis especies de Serpula y Spiraserpula fueron identificadas, principalmente del material de las expediciones de la Rosenstiel School of Marine and Atmospheric Science, University of Miami, incluyendo dos nuevas especies de Serpula. Serpula madrigalae sp. n. es descrita de las Turks y Caicos, se caracteriza por tener un tubo con cinco costillas longitudinales, con cuatro hileras de alvéolos y un embudo simétrico, mediano y somero, con 17 radios, y la superficie opercular interna con tubérculos. Serpula vossae sp. n. es descrita del Caribe occidental y Bahamas; su tubo tiene 6–8 costillas longitudinales, un embudo opercular simétrico, largo y profundo, con 21–33 radios, y la superficie opercular interna lisa. Serpula cf. vermicularis es registrada del golfo de Guinea (Atlántico oriental tropical); se distingue de la especie nominal por tener menos radios operculares (33–39) y le falta la denticulación fina proximal en las setas bayoneta, los tubos se perdieron. Para las tres especies de Spiraserpula halladas, Spiraserpula caribensis, Spiraserpula karpatensis y Spiraserpula ypsilon, se amplió el ámbito de distribución.
Annelida, Bahamas, Caribbean, new records, new species, taxonomy, Turks and Caicos
Serpula Linnaeus, 1758 the type genus of the polychaete family Serpulidae Rafinesque, 1815, has 31 species (
In the Western Atlantic, the genus Serpula is very poorly known, as only two species have been recorded: Serpula vermicularis granulosa by
The current state of our knowledge on Serpula species, compared to that of almost 100 species of Hydroides (
Spiraserpula Regenhardt, 1961 was established initially for fossil serpulids.
This work is part of a larger study examining subtidal and deep sea serpulids from the Grand Caribbean region and from the Gulf of Guinea, tropical eastern Atlantic.
Materials and methodsBetween 1963 and 1975, the Rosenstiel School of Marine and Atmospheric Science (RSMAS) conducted the University of Miami Deep Sea Expeditions aboard of R/V Gerda, John Elliot Pillsbury, James M. Gillis and Columbus Iselin, and sampled more than 3, 350 stations from the Gulf of Panama, throughout the Caribbean to the Gulf of Guinea, the Straits of Florida, the Bahamas, the area northward to the Bermudas and the deep basins and the deep waters, from the intertidal to 8, 650 m in the Puerto Rico Trench (
Additionally, two specimens of Serpula (recorded by
The specimens of Serpula and Spiraserpula were fixed with 10% formalin and preserved with 70% alcohol. They were studied in a standardized way (
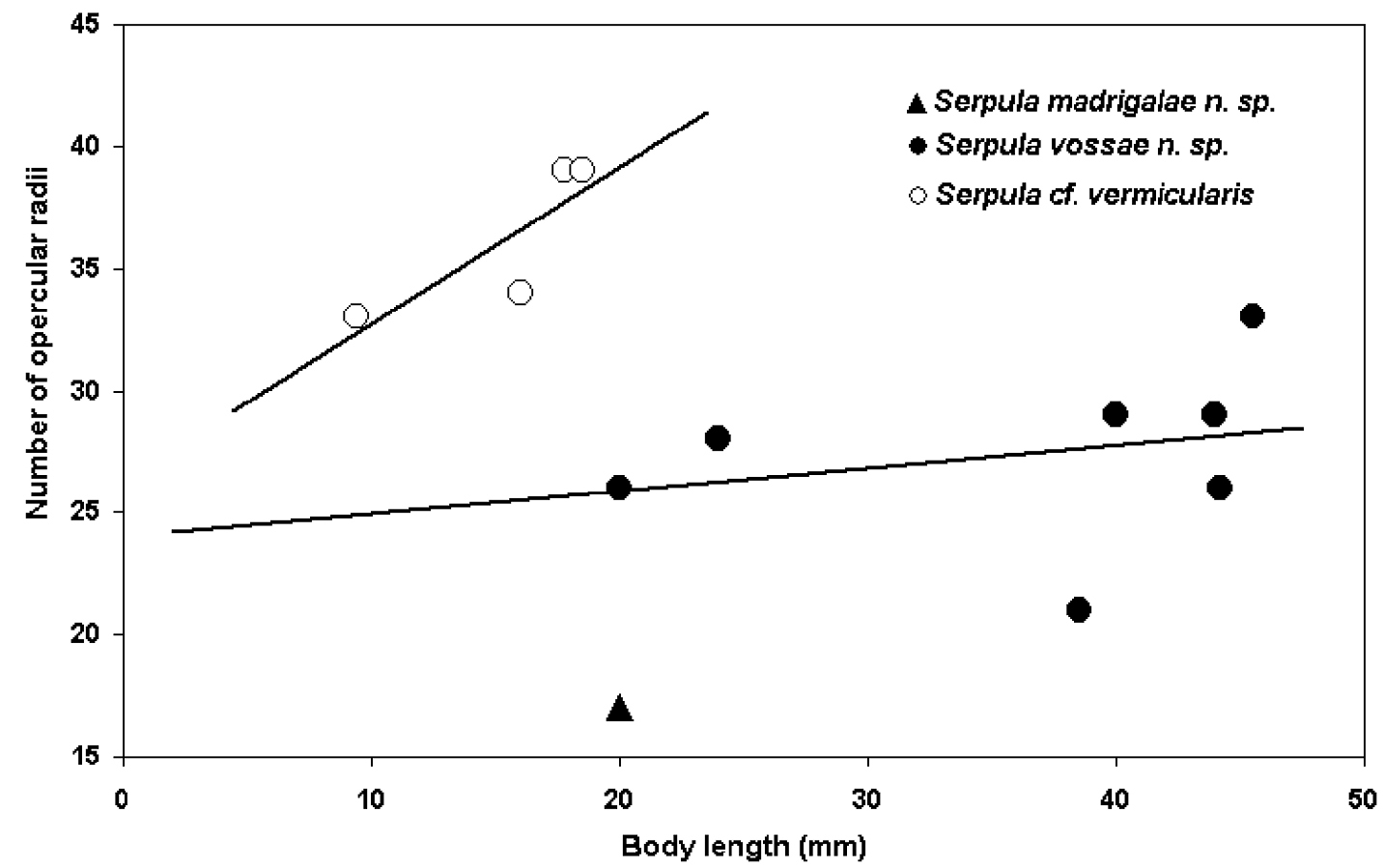
The main standard measurements and observations on Serpula were: total length (measured from most distal part of the operculum to the pygidium), thoracic width (measured from the collar region level), number of thoracic chaetigers, number of radioles in each lobe of the branchial crown, number of longitudinal ridges on the tube (not counting basal ridges attached to the substratum), presence or absence of peristomes, transverse ridges or alveoli on the tube, opercular length (measured from the base of funnel, or constriction, if present, to the tips of the radii), opercular diameter (measured across the distal part of the funnel), number of funnel radii, number of teeth on bayonet chaetae and the presence or absence of a proximal rasp in these chaetae. An exploratory analysis of the number of opercular radii and body length ratio of the Serpula species is included. Scales of figures and photographs are in millimeters.
The following abbreviations are used in the text:Collections
ECOSUR Colección de Referencia. El Colegio de la Frontera Sur, Chetumal, Quintana Roo, México.
UMML Marine Invertebrate Museum, Rosenstiel School of Marine and Atmospheric Science, University of Miami, Miami, Florida, USA.
UMAR Colección de Invertebrados Marinos, Universidad del Mar, Puerto Ángel, Oaxaca, México.
USNM National Museum of Natural History, Washington D.C., USA.
Characters
OL Opercular length
OD Opercular diameter
THW Thoracic width
TL Total length of the body
Statistical terms
n sample size
r: range of data
µ mean
± standard deviation
SystematicsClass Polychaeta Grube, 1850
Family Serpulidae Rafinesque, 1815
Genus Serpula Linnaeus, 1758Type species. Serpula vermicularis Linnaeus, 1767 by subsequent designation (
urn:lsid:zoobank.org:act:1EACCEA3-111D-4F4A-AF5B-CD03E15FBF6C
http://species-id.net/wiki/Serpula_madrigalae
Figs 1A–D, 2A–G, 5, 6Turks and Caicos. East of Caicos Island.
Turks and Caicos. Holotype (USNM 1157006), RV Pillsbury, cruise 7106, sta. 1423, 21°41'N, 71°23'W, 10-feet otter trawl, 18 m, July 19, 1971 (ex UMML 22.1054).
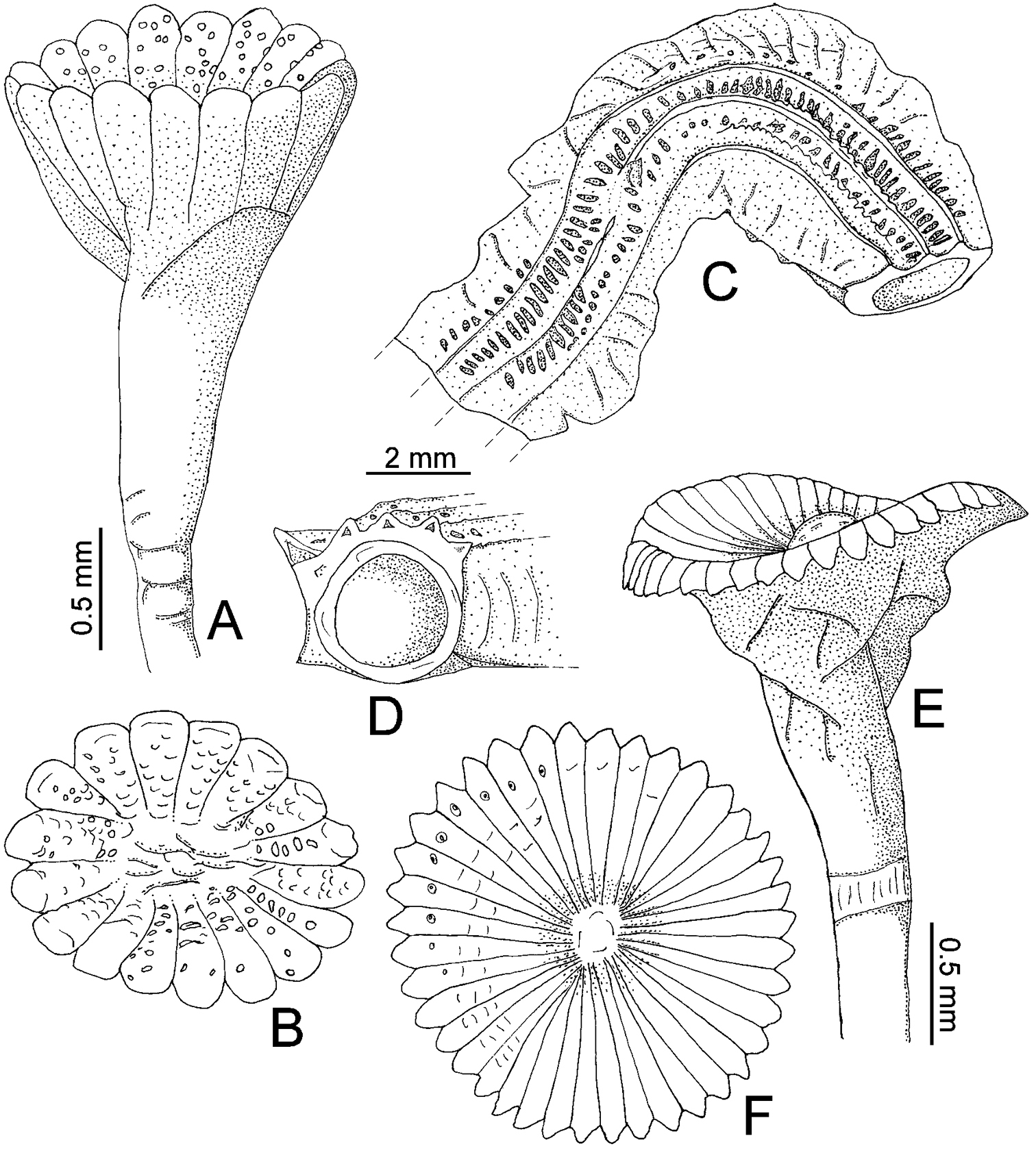
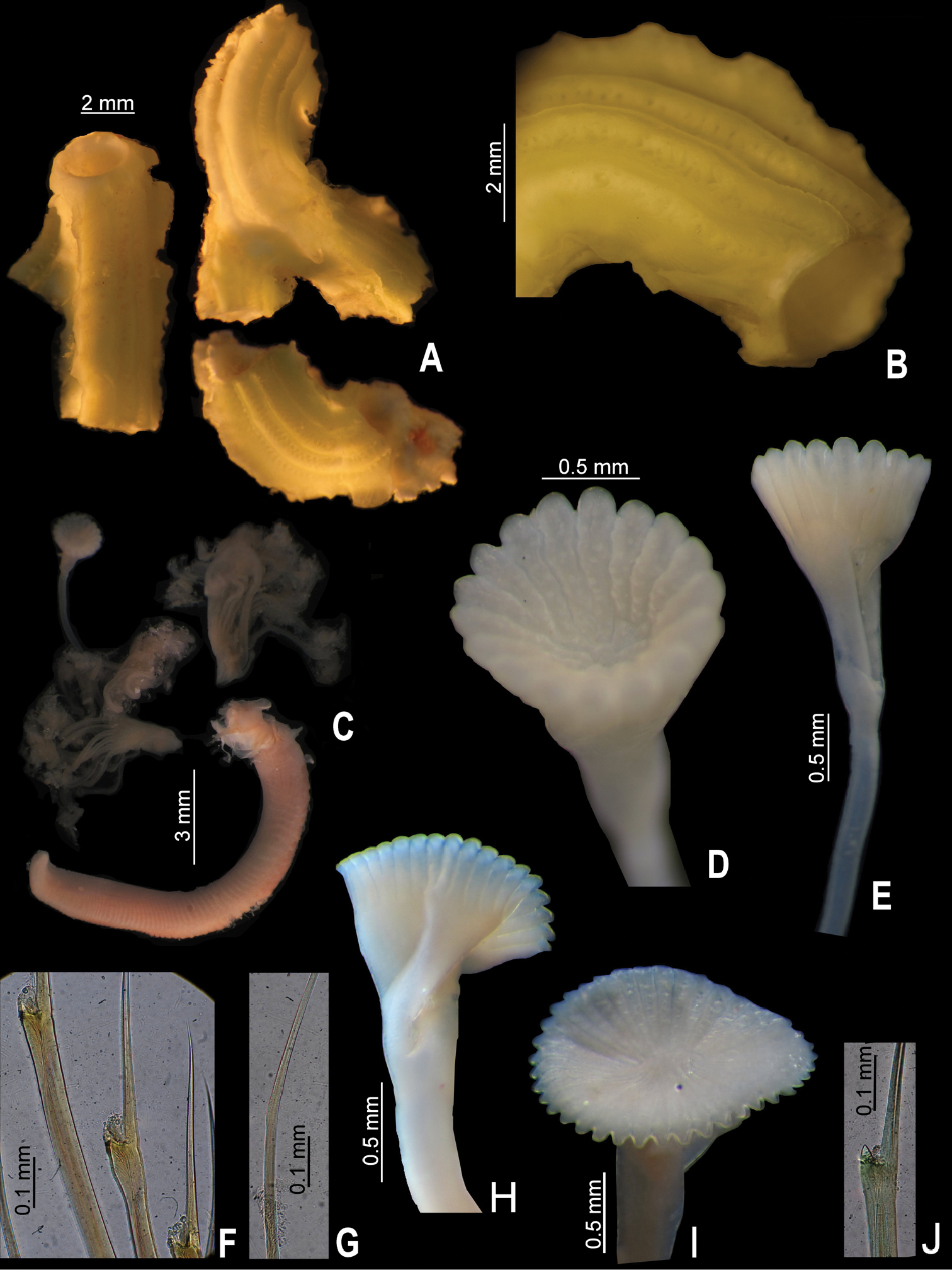
Tube color greenish yellow (Fig. 2A–B); with five longitudinal ridges, lateral-most ridges larger than middle ones (Figs 1C–D, 2A–B); lacking transverse ridges and peristomes; with four rows of alveoli, more evident between dorsal-most longitudinal ridges (Figs 1C, 2A–B).
Body yellowish-brown, branchial crown and operculum yellow pale (preserved material only, Fig. 2C). TL= 20 mm; THW= 1.6 mm. Branchial crown with 18 radioles in each lobe; lacking branchial membrane.
Peduncle smooth, with well-defined constriction (Fig. 2D); inserted in left lobe. Club-shaped pseudoperculum present.
Operculum with moderately long, shallow, symmetrical funnel; lacking bulbous basal part (Figs 1A, 2D–E). OL= 2.3 mm, OD= 1.4 mm. Interradial grooves 1/3 of funnel length (Figs 1A, 2E). Funnel has 17 radii with rounded tips. Opercular inner surface with irregular tubercles (Figs 1B, 2D).
Collar thick, with short ventral and dorsal lobes. Thorax consists of seven chaetigers. Collar chaetal fascicles symmetrical with regard to size and composition, unlike in some specimens of Serpula vossae sp. n. Bayonet chaetae with two blunt-elongate teeth, distal blade smooth, lacking proximal rasp (Figs 2F); hooded (capillary) chaetae present (Fig. 2G).
Thoracic membranes well developed, narrowing toward to last thoracic chaetigers, fused ventrally, forming a short apron. Remaining six thoracic chaetigers with hooded (limbate) chaetae of two sizes; saw-shaped uncini.
Anterior part of abdomen lacking distinct achaetous region. Anterior and middle abdominal chaetigers with flat-trumpet chaetae. Posterior chaetigers with ‘capillary’ chaetae. Anterior and posterior uncini saw-shaped.
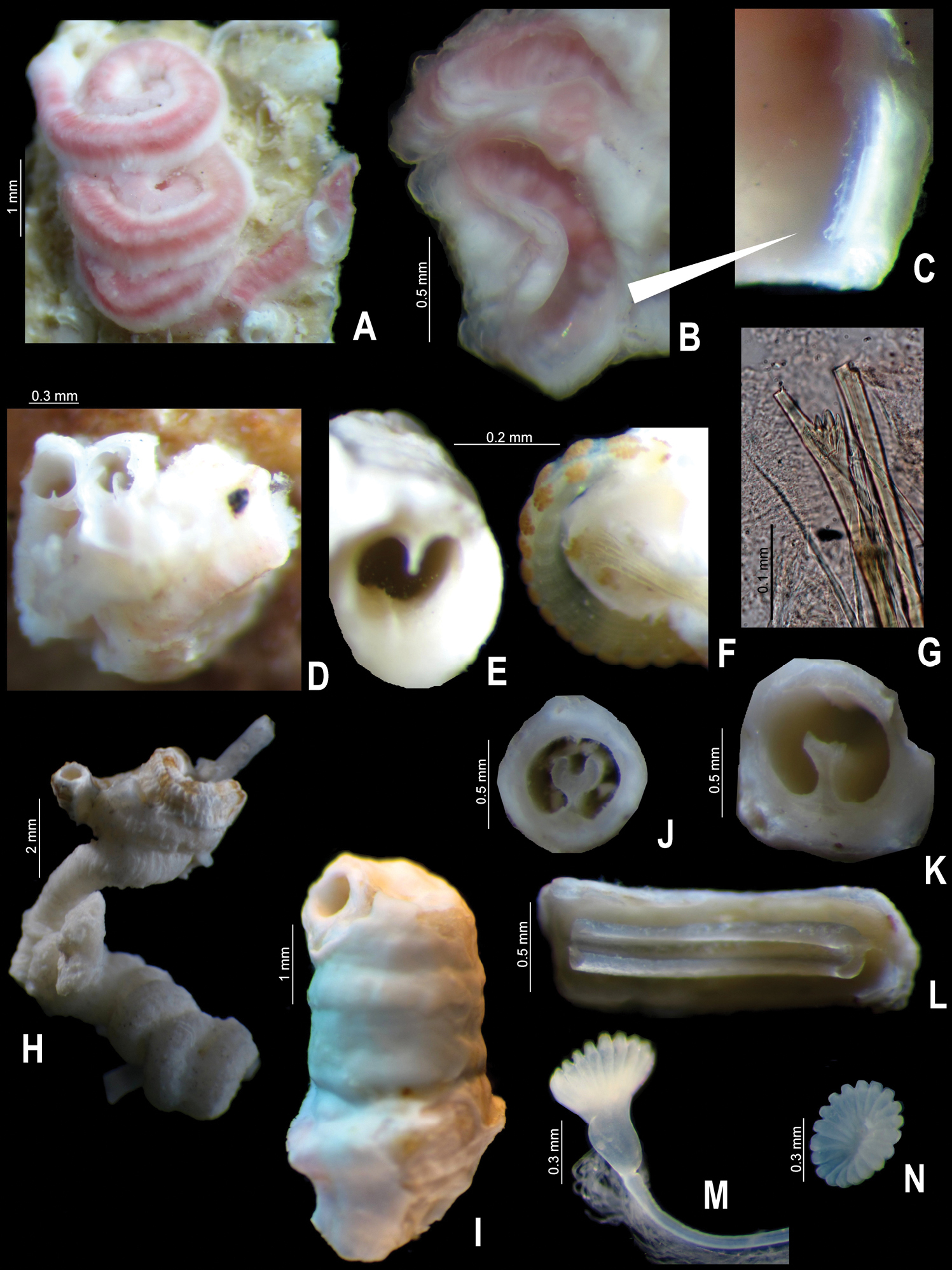
A–D Serpula madrigalae sp. n., from Turks and Caicos Islands, USNM 1157006, holotype A–B operculum in lateral and aboral views C–D tube in dorsal and frontal views E–F Serpula cf. vermicularis, from Nigeria, UMML 22.545 E–F operculum in lateral and aboral views.
A–G Serpula madrigalae sp. n., from Turks and Caicos, USNM 1157006, holotype A–B tube and detail C entire body D–E operculum, in aboral and lateral views F bayonet chaetae G hooded (capillary) chaetae H–J Serpula cf. vermicularis, from Nigeria, UMML 22.545 H–I two distinct opercula in lateral and aboral views J bayonet chaetae.
Named after my wife, Dr Socorro García-Madrigal, a specialist on crustaceans, who gave me the necessary encouragement and time to undertake this research.
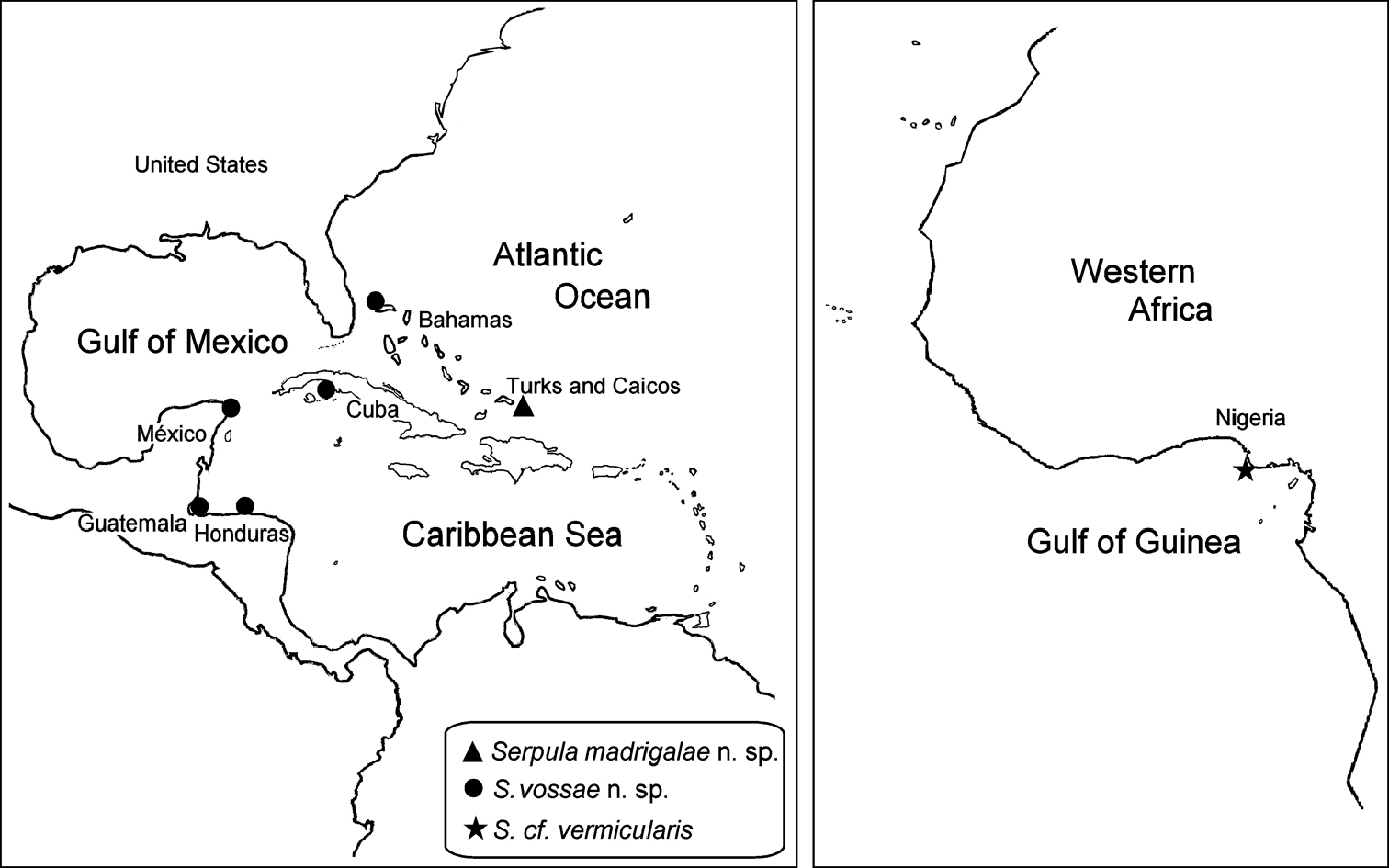
Only recorded from the vicinity of Caicos Island, Turks and Caicos Islands (Fig. 6).
Sublittoral, 18 m. In the same sample there were other serpulids: Pomatostegus stellatus, Pseudovermilia multispinosa, Spirobranchus giganteus, and Vermiliopsis annulituba.
Serpula madrigalae sp. n. resembles other Serpula species with symmetrical, moderately long and shallow funnels, such as Serpula cavernicola, Serpula granulosa Marenzeller, 1884, Serpula israelitica Amoureux, 1976, Serpula jukesii Baird, 1865, Serpula narconensis Baird, 1865, Serpula oshimae Imajima & ten Hove, 1984, Serpula tetratropia Imajima & ten Hove, 1984, Serpula vermicularis Linnaeus, 1767, and Serpula zelandica Baird, 1865. However, Serpula madrigalae sp. n. differs from all other Serpula species with regard to its characteristic tube which has five longitudinal ridges and four rows of alveoli (Figs 1C–D, 2A–B).
Serpula madrigalae sp. n. resembles Serpula vermicularis granulosa, in having tubercles on the internal surface of the operculum; however, the diagnosis of the latter species was brief (
Serpula madrigalae sp. n. also resembles Serpula sp. A, from the northeastern part of the Gulf of Mexico, with regard to the shape of the operculum, the number of radii and the depths from which they were collected. However, they differ with regards to other features: Serpula madrigalae sp. n. has irregular tubercles on the internal surface of the operculum (Figs 1B, 2D) and lacks a proximal rasp in the bayonet chaetae (Fig. 2F), while Serpula sp. A lacks tubercles (
urn:lsid:zoobank.org:act:3165E4EF-A4B8-47B2-B500-D5C8C1557646
http://species-id.net/wiki/Serpula_vossae
Figs 3A–D, 4A–J, 5, 6Honduras. Southwest of Honduras.
Holotype (USNM 1157004), RV Pillsbury, cruise 6802, sta. 629, 15°58'N, 86°09'W, 40 m, March 21, 1968 (ex UMML 22.611); paratype (USNM 1157005), RV Pillsbury, cruise 6802, sta. 628, Honduras, East of Cayos Cochinos, 15°57'N, 86°15'W, 47 m, March 21, 1968 (ex UMML 22.610).
Guatemala. One complete specimen (UMML 22.1053) RV Pillsbury, cruise 6802, sta. 613, West of Punta Cortes, 15°58'N, 88°20'W, 10-feet otter trawl, 39 m, March 19, 1968. México. One complete specimen (ECOSUR s.n.) RV Edwin Link sta. 2792, 13 km from East of Isla Mujeres, Quintana Roo, 21°14'N, 86°36'W, 130 m, August 28, 1990, E. Escobar and L. Soto leg. Cuba. One complete specimen (Instituto de Oceanología de Cuba) Cayo Diego Pérez, Golfo de Batabanó, 15 m, July 20, 1988, D. Ibarzábal leg. Bahamas. Two complete specimens (UMML 22.435) RV Gerda, cruise 6433, sta. 391, North of Bahamas, 27°20'N, 79°11'W, screen dredge, 68 m, September 19, 1964.
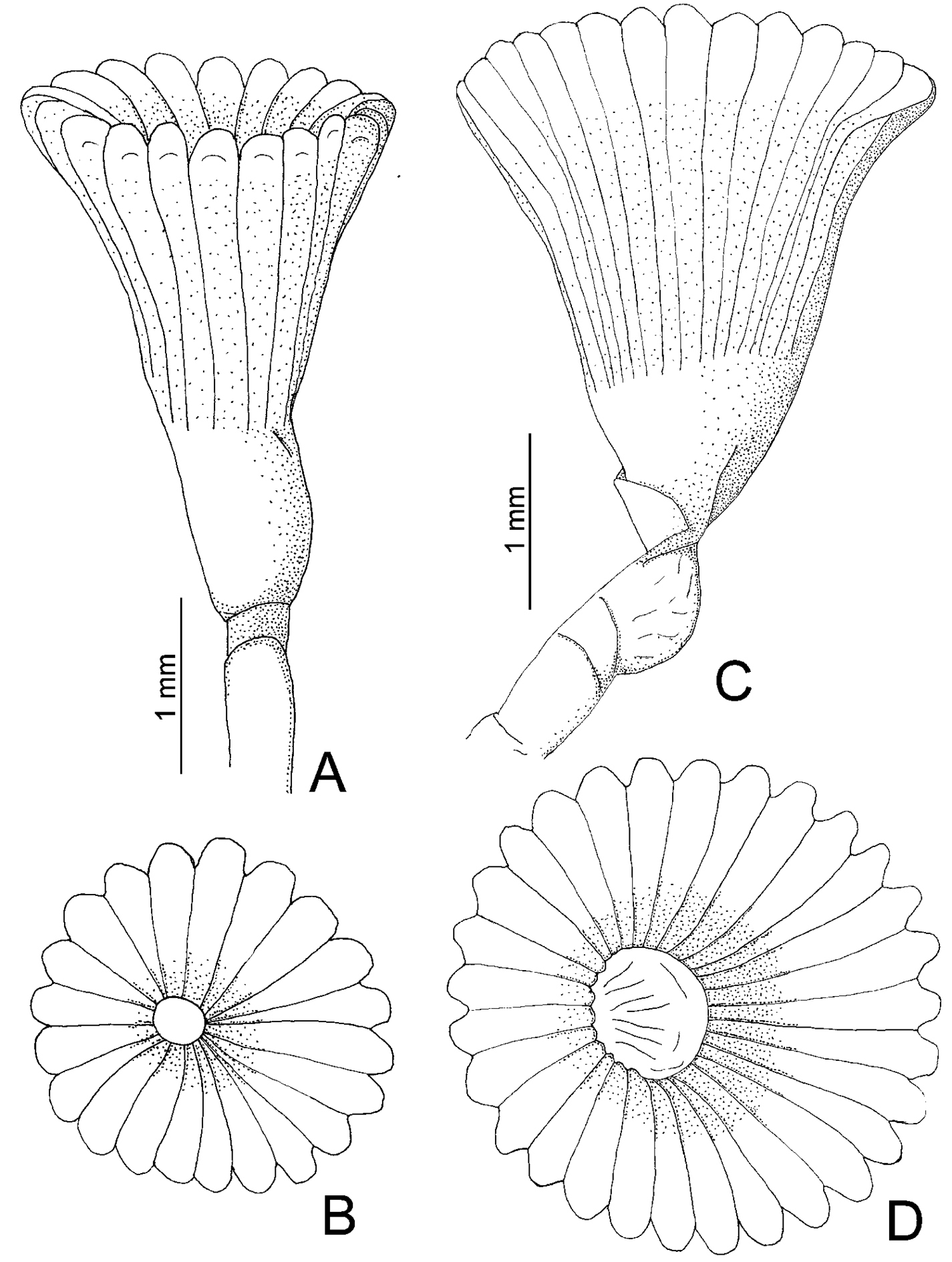
Tube color brownish, or light brown to white; with 6–8 longitudinal ridges, all similar in size; some tubes with shallow transverse ridges, forming a rugged surface, other tubes lacking transverse ridges; most tubes lacking peristomes, two have only one peristome with appearance of a groove with shallow growth lines. Tubes lacking alveoli (Fig. 4A, C–D).
Body pale yellow (preserved material only, Fig. 4B). TL= 38.5 mm (n=7, r:20–45.5, µ=36.6 ±10.3); THW= 2 mm (n=7, r:1.5–3.4, µ=2.3 ±0.6). Branchial crown with 29 radioles (n=7, r:19–37, µ=30.9 ±6.4) left, and 29 right (n=7, r:12–35, µ=30.9 ±8.8); lacking branchial membrane (Fig. 4E).
Peduncle smooth with insertion on left (n=2) or right (n=5); with shallow (n= 4) to well-defined constriction (n= 3) (Figs 3A, C, 4H–J). Pseudoperculum club-shaped, present in all specimens.
Operculum with long, deep symmetrical funnel; with a slightly bulbous basal part above constriction (Figs 3A, C, 4H–J). OL= 3.2 mm (n=7, r:2–4.5, µ=3.3 ±0.8), OD= 2 mm (n=7, r:1.4–2.8, µ=2.3 ±0.5). Interradial grooves 2/3 of funnel length. Funnel with 21 radii (n=7, r:21–33, µ=27.4 ±3.7) with rounded tips (Figs 3A, C, 4H–J). Opercular inner surface lacking tubercles (Fig. 3A–D).
Collar thick, with short ventral and dorsal lobes. Thorax consists of seven chaetigers. Collar fascicles in three specimens asymmetrical with regard to sizes and number of chaetae; right fascicle with larger and more chaetae than left fascicle (Fig. 4E). Bayonet chaetae with two blunt-elongate teeth, distal blade smooth, lacking proximal rasp (Fig. 4G); hooded (capillary) chaetae present.
Thoracic membranes well developed, narrowing toward last thoracic chaetigers, fused ventrally, forming a short apron. Remaining six thoracic chaetigers with hooded (limbate) chaetae of two sizes; saw-shaped uncini.
Anterior part of abdomen lacks a distinct achaetous region. Anterior and middle abdominal chaetigers with flat-trumpet chaetae. Posterior chaetigers with ‘capillary’ chaetae. Anterior and posterior uncini saw-shaped.
A–D Serpula vossae sp. n., from Honduras, USNM 1157004, holotype A–B operculum in lateral and aboral views; from Bahamas, UMML 22.435 C–D operculum in lateral and aboral views.
A–J Serpula vossae sp. n., from Honduras, USNM 1157004, holotype A tube B entire body; from Guatemala, UMML 22.1053 C–D tube and detail of peristome; from Bahamas, UMML 22.435 E collar region; from Cuba, IO F radiole with eggs; from Honduras, USNM 1157004, holotype G bayonet chaetae H operculum; from Guatemala, UMML 22.1053 I operculum; from Bahamas, UMML 22.435 J operculum.
Operculum of holotype (USNM 1157004) has roseate radial tips (Fig. 4H); the rest of specimens are yellow to white (Fig. 4I–J). Operculum and radioles of specimen from Guatemala (UMML 22.1053) have hard particles adhered, possibly salt concretions (Fig. 4I); operculum more rigid compared with the other specimens.
Named after Professor Nancy Voss, a distinguished cephalopod specialist and Director of the Marine Invertebrate Museum, who generously loaned the serpulid samples from the oceanographic expeditions of the University of Miami.
Tropical Caribbean. Bahamas, Cuba, Mexican Caribbean, Guatemala and Honduran Caribbean (Fig. 6).
Sublittoral, 15 to 130 m. On rocky and sandy bottoms, and associated with siliceous sponges and several syllid polychaetes specimens. In the same samples, there were other serpulids: Hyalopomatus sp., Hydroides parvus, Pomatostegus stellatus, Pseudovermilia fuscostriata, Pseudovermilia occidentalis, Spiraserpula ypsilon, and a vermetid shell.
The specimen from Cayo Diego Pérez, Cuba, has eggs adhering to the pinnules of the radioles. The eggs, circular to slightly oval, are 55–68 µm (Fig. 4F).
Serpula vossae sp. n. resembles other Serpula species with long and deep symmetrical funnels, as in Serpula columbiana Johnson, 1901, Serpula concharum Langerhans, 1880, Serpula longituba Imajima, 1979, Serpula sinica Wu & Chen, 1979, Serpula uschakovi Kupriyanova, 1999, Serpula vittata Augener, 1914, and Serpula watsoni Willey, 1905. However, Serpula vossae sp. n. differs in having an operculum with a smooth inner surface, while Serpula watsoni has tubercles(
Comparison between Serpula vossae sp. n. and other similar species (AD) = according to published illustrations.
| Species: | Serpula columbiana | Serpula concharum | Serpula longituba | Serpula sinica | Serpula uschakovi | Serpula vittata | Serpula watsoni | Serpula vossae |
|---|---|---|---|---|---|---|---|---|
| Localities | Puget Sound | Atlantic of Spain and Mediterranean | Kushimoto Harbour, South Japan | South China Sea | Sea of Japan | Micronesia, Melanesia, West Australia | Sri Lanka, Japan, Micronesia, Melanesia, South China Sea, Australia | West Caribbean and Bahamas |
| References |
|
|
|
|
|
|
|
This work |
| Depth (m) | 15–60 | 0–500 | 30–40 | 202–219 | 15 | 7–11 | shallow | 15–130 |
| Tube color | white | white | white | violet-red | white | brownish | white | white to brownish |
| Longitudinal ridges | absent | 3–5, smooth | absent | ? | ? | 5 | 5 | 6–8 |
| Transverse ridges | present | absent (AD) | absent | ? | ? | present | ? | present |
| Peristomes | absent | absent (AD) | absent | ? | absent | absent | absent | absent to one |
| Alveoli | absent | absent (AD) | absent | ? | ? | absent | absent | absent |
| TL (mm) | 56 | (13–25) 15–20 | 29 | 17 | 120 | 31 | 24 | 20–45.5 |
| TW (mm) | 6 | (1–1.5) | 0.8 | ? | 11 | 1.5 | 1.8 | 1.5–3.4 |
| Opercular radii | 55–160 | 15–25 | 32 | 32 | 62–136 | 18–23 | 25–55 | 21–33 |
| Opercular tubercles on inner surface | present | ? | absent (AD) | ? | present | absent | present | absent |
| Constriction | well defined | well defined | well defined | well defined (AD) | absent | well defined | well defined | well defined |
| Sides of thoracic membrane | not fused | apron | apron | ? | short apron | not fused | apron | short apron |
| Number of radioles per branchial lobe | 10–35 | 6–15 | 9–10 | 13 | 43–61 | 16–30 | 22–30 | 12–37 |
| Teeth of bayonet chaetae | 2 | 2–4 | not applicable | 0 | 2 | 10 | 5 | 2 |
| Proximal rasp of bayonet chaetae | absent | ? | not applicable | present | absent | present | absent | absent |
Regarding the Serpula species recorded in the Western Atlantic, Serpula vossae sp. n. differs from Serpula vermicularis granulosa Day, 1973, from Beaufort, North Carolina, because the former has a longer, deeper operculum, and lacks tubercles on the internal funnel surface (Figs 3A–D, 4H–J); while Serpula vossae sp. n. differs from Serpula sp. A (
Serpula vossae sp. n. differs from Serpula cf. vermicularis, recorded here from Nigeria, in the same characters mentioned for Serpula vermicularis granulosa, and, additionally in having fewer opercular radii in relation to the body length than the latter (Fig. 5).
Figs 1E–F, 2H–J, 5, 6
Nigeria. Five specimens (UMML 22.545), RV Pillsbury, sta. 248, Southeast of Lagos, 4°05’N, 5°40’E, 10-foot try-net, 33 m, May 13, 1965.
Tubes missing. Body light brown (preserved material only). TL= 18.5 mm (n=4, r:9.4–18.5, µ=36.6 ±10.3); THW= 1.7 mm (n=5, r:1.2–1.7, µ=1.6 ±0.2). Thoracic membranes and opercular peduncles of all the specimens damaged. Branchial crown with 27 radioles (n=4, r:19–27, µ=23.8 ±3.4) left, and 25 right (n=4, r:17–25, µ=22.5 ±3.7); lacking inter-radiolar membrane.
Peduncle smooth, with insertion on left (n=2) or right (n=2); lacking constriction between it and operculum, its position represented only by a slight change in color (Figs 1E, 2I). Club-shaped pseudoperculum present in all specimens.
Operculum with short, shallow symmetrical funnel; lacking bulbous basal part (Figs 1E, 2I). OL= 2 mm (n=4, r:1.3–2.1, µ=1.9 ±0.4), OD= 1.8 mm (n=4, r:1.1–1.8, µ=1.6 ±0.3). Interradial grooves 1/4 of funnel length (Fig. 2H). Funnel with up to 39 radii (n=4, r:33–39, µ=36.3 ±3.2) with blunt tips (Fig. 1E–F). Opercular inner surface lacking tubercles (Figs 1E–F, 2I).
Collar thick, with ventral and dorsal lobes short. Thorax consists of seven chaetigers. Collar chaetal fascicles symmetrical with regard to size and composition unlike in Serpula vossae sp. n. Bayonet chaetae with two blunt-elongate teeth, distal blade smooth, lacking proximal rasp (Fig. 2J); hooded (capillary) chaetae present.
Thoracic membranes apparently well developed (membranes damaged), narrowing toward posterior thorax, fused ventrally, forming a short apron. Remaining six thoracic chaetigers with hooded (limbate) chaetae of two sizes; saw-shaped uncini.
Abdomen with anterior achaetous region. Anterior and middle abdominal chaetigers with flat-trumpet chaetae. Posterior chaetigers with ‘capillary’ chaetae. Anterior and posterior uncini saw-shaped.
Exploratory analysis of the number of opercular radii and body length ratio: Serpula madrigalae sp. n. (n= 1, only for reference), Serpula vossae sp. n. (n= 7), and Serpula cf. vermicularis (n= 4).
Distribution of Serpula madrigalae sp. n., Serpula vossae sp. n., and Serpula cf. vermicularis.
Two specimens with a hyaline circle in radii tip (Fig. 1F). One specimen with few inconspicuous tubercles in interior funnel surface.
Nigeria, Gulf of Guinea (Fig. 6).
Sublittoral, 33 m.
Serpula cf. vermicularis resembles the nominal species; unfortunately, the tubes of all the specimens are missing. There are some differences with the nominal species, particularly with regard to the number of radii: Serpula cf. vermicularis has 33–39 opercular radii (Fig. 1F, 2I, 5), while
Type species. Spiraserpula spiraserpula Regenhardt, 1961 (fossil), by original designation.
http://species-id.net/wiki/Spiraserpula_caribensis
Figs 7A–D, 8Awa Blancu, Curaçao.
Panama Caribbean. One specimen (ECOSUR P0615) Colon, Club Náutico, fouling prospection, June 3, 2002, S.I. Salazar-Vallejo leg. Mexican Caribbean. Nine specimens (ECOSUR P0614, P0616), two specimens (UMML 22.1061), two specimens (UMAR-Poly 110), Playa Azul, Cozumel, coral rock, 10 m, March 25, 2001, leg. H.A. ten Hove.
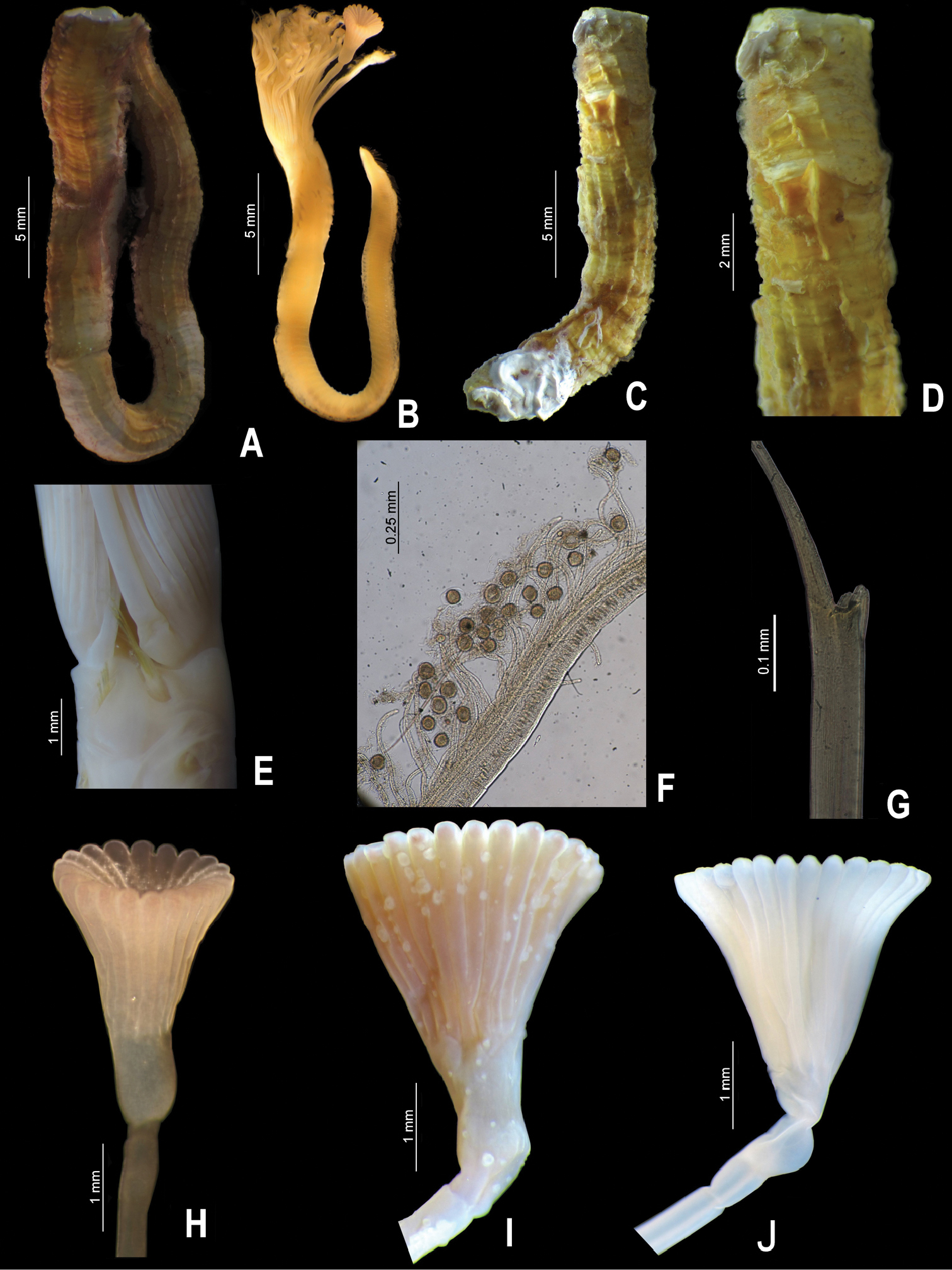
Some specimens forming tube aggregations; others were found isolated. Tubes sinuous or spiraled (Fig. 7A), with two internal ridges: mid-dorsal one smooth, mid-ventral one serrated (Fig. 7B–C), occasionally with two internal lateral ridges (Fig. 7D). Some tubes externally pinkish, others with two dorsal pink bands (Fig. 7A–B). Body brown to dark brown (preserved material only). The worms are damaged. Branchial crowns lost. Thorax with eight chaetigers, including collar fascicles. Abdomen damaged.
A-D: Spiraserpula caribensis, from Cozumel, UMAR-Poly 110 A complete tube B–C internal surface of the tube and detail of ventral internal ridge D other specimen with lateral internal ridges E–F Spiraserpula karpatensis, from Los Roques Islands, UMML 22.1055 E detail of the mouth tube F abdomen with gametes G–L Spiraserpula ypsilon, from Trinidad and Tobago, UMML 22.1059 G collar chaetae; from Bahamas, UMML 22.1056 H tube attached to Pseudovermilia fuscostriata; from Trinidad and Tobago, UMML 22.1059 I tube; from Honduras, UMML 22.1057 J–K tube in cross section L tube in longitudinal section M–N Spiraserpula sp., from Los Roques Islands, UMML 22.1060 M–N operculum, lateral and aboral views.
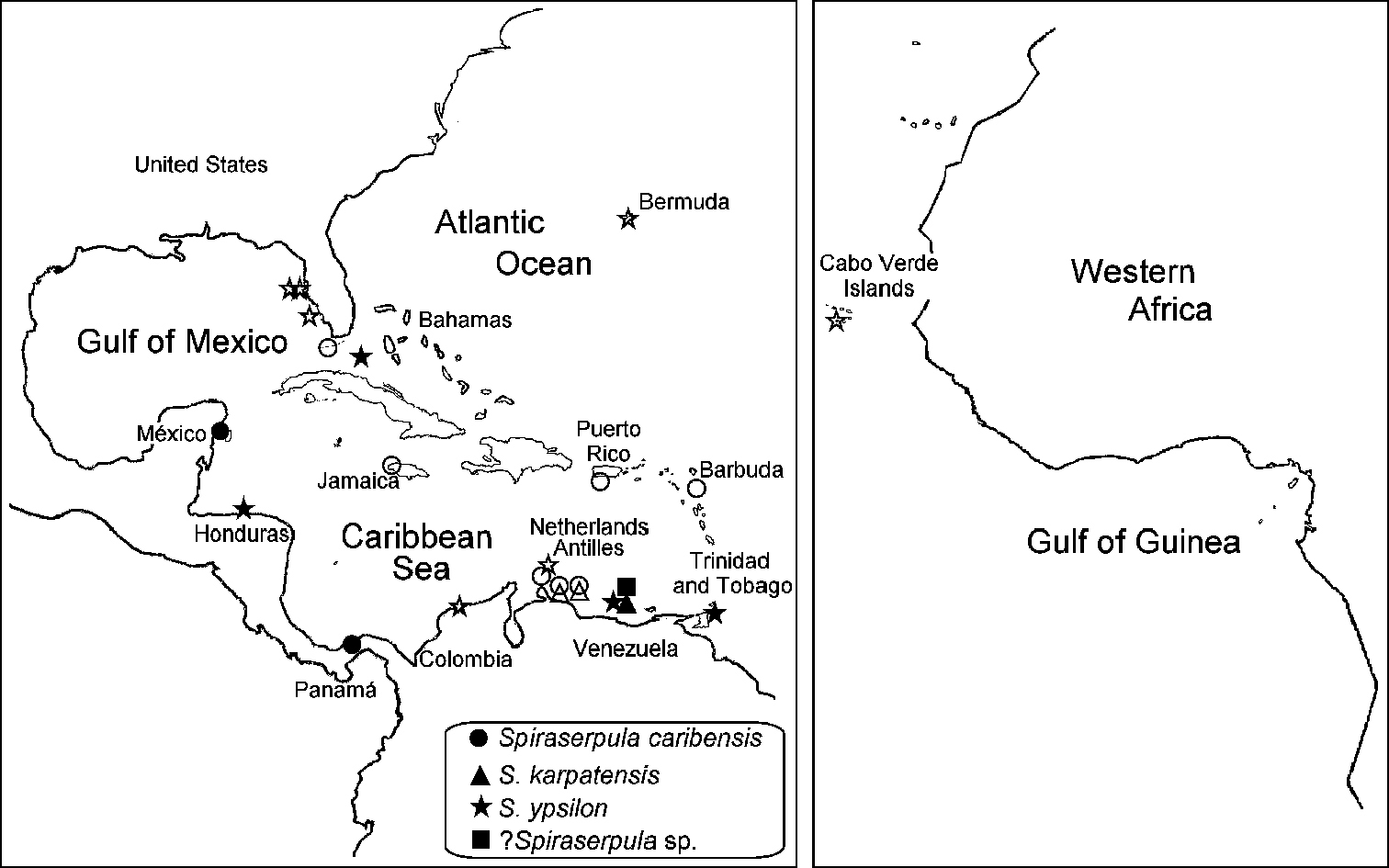
Distribution of Spiraserpula caribensis, Spiraserpula karpatensis, Spiraserpula ypsilon and ?Spiraserpula sp. Closed symbols denote examined material, open symbols literature records.
Caribbean, Florida and Pacific of Panama.
Intertidal to sublittoral, 10 m. On coral debris.
Spiraserpula caribensisis easily distinguishable from the other Caribbean species by their pink tubes (Fig. 7A).
http://species-id.net/wiki/Spiraserpula_karpatensis
Figs 7E–F, 8Karpata, Bonaire.
Venezuela. One incomplete specimen and one empty tube (UMML 22.1055), RV Pillsbury, cruise 6806, sta. 745, North of Los Roques Islands, 11°58'N, 66°50'W, 10-feet otter trawl, 65 m, July 24, 1968.
Empty tube larger (Fig. 7E) than occupied one attached to empty tubes of Spiraserpula ypsilon. Tubes sinuous or spiraled, with two internal ridges: mid-dorsal one smooth, mid-ventral one serrated (Fig. 7E). Both tubes white, internal and externally (Fig. 7E). The branchial crown and thorax of incomplete specimen is missing. Abdomen partially transparent, with double packets of gametes in each segment (Fig. 7F).
Eastern Caribbean. Bonaire, Curaçao and Los Roques Islands.
Sublittoral, 65 m. On coral debris.
Spiraserpula karpatensis resembles Spiraserpula caribensis with regard to the dorsal and ventral ridges (Fig. 7C, E); however, Spiraserpula karpatensis does not possess pinkish tubes unlike Spiraserpula caribensis.
http://species-id.net/wiki/Spiraserpula_ypsilon
Figs 7G–L, 8Brava, Cape Verde Islands.
Bahamas. Two empty tubes (UMML 22.1056), RV Gerda, cruise 6804, sta. 983, North of Elbow Cay, Bahamas, 24°05'N, 80°20'W, triangle dredge, 216 m, March 5, 1968). Honduras. One specimen (UMML 22.1057), RV Pillsbury, cruise 6802, sta. 629, Southwest of Honduras Cape, 15°58'N, 86°09'W, 41-feet otter trawl, 40 m, March 21, 1968. Venezuela. Two empty tubes (UMML 22.1058), RV Pillsbury, cruise 6806, sta. 745, North of Los Roques Islands, 11°58'N, 66°50'W, 10-feet otter trawl, 65 m, July 24, 1968. Trinidad and Tobago. One specimen (UMML 22.1059), one specimen (UMAR-Poly 111), RV Pillsbury, cruise 6907, sta. 840, East of Trinidad Island, 10°40'N, 60°37'W, 10-feet otter trawl, 33 m, sponges, July 1, 1969.
One specimen (UMML 22.1058) attached to tube of Spiraserpula karpatensis and another (UMML 22.1056) attached to Pseudovermilia fuscostriata tube (Fig. 7H). Tubes sinuous or strongly spiraled (Fig. 7H); in another the tube forms a very tight cylindrical spiral (Fig. 7I). Tubes with two internal longitudinal ridges: mid-dorsal one serrated, mid-ventral one Y-shaped (Fig. 7J–L); sometimes, along length of tube, Y-shaped ridge changes to smooth ridge. Tubes white (Fig. 7H–I). Body pale to dark brown (preserved material only). Worms damaged. Branchial crown with 5–6 radioles by branchial lobe. Collar damaged, lobes could not be observed. Bayonet chaetae with 3–4 blunt teeth (Fig. 7G); hooded (capillary) chaetae present. Thorax with seven chaetigers, including collar chaetae. Abdomen damaged.
Caribbean, Florida and Pacific of Panama.
Sublittoral, 33–216 m. On coral debris.
Spiraserpula ypsilonis very similar to Spiraserpula paraypsilon Pillai & ten Hove, 1994, described from the Netherlands Antilles, mainly with regard to the internal ridges of the tube. However, some differences separate both species, mainly the absence of lateral tubercles in the thoracic uncini in Spiraserpula ypsilon, characteristic of Spiraserpula paraypsilon; additionally, Spiraserpula ypsilonhas fewer radioles (6–7) than Spiraserpula paraypsilon (11).
Figs 7M–N, 8
Venezuela. One specimen (UMML 22.1060), RV Pillsbury, cruise 6806, sta. 745, North of Los Roques Islands, 11°58'N, 66°50'W, 10-feet otter trawl, 65 m, July 24, 1968.
Tube attached to a chaetopterid tube, is white and lacks any internal ridges characteristic of Spiraserpula. External surface with granular appearance, internally smooth. Body white, fragmented and damaged but complete. Branchial crown with nine radioles per lobe; lacking inter-radiolar membrane.
Peduncle smooth, inserted in right lobe, with well-defined constriction between it and operculum (Figs 7M). Pseudoperculum club-shaped. Operculum is zygomorphic; with a conspicuous bulbous basal part above constriction (Fig. 7M). Interradial grooves 1/3 of funnel length; 19 radii with rounded tips (Fig. 7N); inner surface smooth (Fig. 7N).
Collar damaged, lobes could not be observed. Bayonet chaetae with 2–3 sharp-elongate teeth; hooded (capillary) chaetae present. Thorax with eight chaetigers, including collar chaetae. Abdomen damaged, with approximately 61 segments, a distinct achaetous region absent between the thorax and abdomen.
Only recorded from Los Roques Islands, Venezuela.
Sublittoral, 65 m. The same sample contained other serpulids: Spiraserpula karpatensis, Spiraserpula ypsilon, several empty tubes of serpulids resembling Protula and Vermiliopsis, a chaetopterid tube, and a lumbrinerid.
Most of the tube belonging to this specimen is missing and the remaining fragments lacked the internal ridges characteristic of Spiraserpula. The operculum of this Spiraserpula sp. resembles that of Spiraserpula karpatensis, Spiraserpula plaiae Pillai & ten Hove, 1994 and Spiraserpula sumbensis Pillai & ten Hove, 1994; the former two are from Caribbean and the latter is from Indonesia. Due to the loss of the rest of the tube the present specimen cannot be assigned to species. It may be a juvenile stage of another genus, such as Crucigera or Serpula.
Despite having reviewed 158 lots of serpulids from the same number of stations collected during past deep sea expeditions, specimens of Serpula were found only at six stations (3.8%), which combined with the fact that there were only two previous records of Serpula (
However, the original descriptions of the species mentioned in the remarks, indicate that many are incomplete, unclear or contradictory with respect to the figures provided. Descriptions need to be standardized and include as many characters as possible, as argued extensively by
As regards Spiraserpula, another little-known serpulid genus in the Caribbean closely similar to Serpula, complete descriptions of species were made in an important and recent revision of the genus, including most species from the Caribbean (
The serpulid collections of the oceanographic expeditions of the Rosenstiel School of Marine and Atmospheric Science, University of Miami were made available by Nancy Voss (UMML-RSMAS, Miami) and the specimen from Cuba was loaned by Diana Ibarzábal (IO-Cuba). Generous accommodation and laboratory space were made available by Sergio Salazar-Vallejo and Emilia González (ECOSUR, Chetumal) besides their time, to study these interesting serpulids. Harry ten Hove (NCB Naturalis, Leiden) and Luis Carrera-Parra (ECOSUR, Chetumal) provided some critically important papers. Nancy Voss and Sergio Salazar-Vallejo made corrections and comments to an earlier draft. Two anonymous referees and Chris Glasby (as editor) made careful corrections and comments to the latest version. This work was partially supported by the project PROMEP/103–5/09/1353.