(C) 2011 Carol Castillo. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of Acaenitinae, Arotes ucumari Castillo & Sääksjärvi, sp. n., is described and illustrated representing the first record of the subfamily from South America. The new species was collected from a premontane tropical rain forest in the Peruvian Andes at 1500 m. A key to the world species of Arotes Gravenhorst, 1829 is provided. The subspecies Arotes albicinctus moiwanus (Matsumura, 1912)is raised to species rank, Arotes moiwanus stat. n.
Amazonia, Arotes, Neotropics, parasitoid wasp, Peru, rainforest
The Acaenitinae is one of the most conspicuous
subfamilies of Ichneumonidae (Hymenoptera). It is clearly monophyletic
as defined by at least one striking synapomorphy: the very long and
triangular hypopygium of the female, although less developed in some
species of the Coleocentrus group. The subfamily was traditionally classified in two tribes, the Acaenitini and Coleocentrini (
Many acaenitines are large in size, vividly coloured and
possess long ovipositors. Despite this, little is known about their
biology and specimens are rare in entomological collections. Many
species of the subfamily live in ancient forests and in the Neotropics
have been found in highlands (
The aim of the present paper is to describe a new species of Arotes from the tropical Peruvian Andes. Also, we raise to species rank Arotes moiwanus stat. n. and provide a key to the world species of the genus.
Material and methodsThe only known specimen of the new species is deposited in The Natural History Museum, University of San Marcos, Lima, Peru (UNSM). The specimen is currently on loan to the Zoological Museum, University of Turku, Finland (ZMUT). We searched for more Neotropical specimens of Arotes at the Natural History Museum, London (NHM), the Canadian National Collection of Insects, Ottawa (CNC) and the American Entomological Institute, Gainesville, Florida (AEI).
To verify the new species status of our Peruvian specimen, we examined specimens of 10 of the 15 previously described Arotes
species in CNC and NHM and also compared it to descriptions of all the
described species. We were not able to examine specimens of Arotes annulicornis Kriechbaumer, Arotes flaviscutatus Wang & Huang, Arotes odontus Uchida, Arotes nigricoxis (Förster) and Arotes sugiharai Uchida. All species except for Arotes nigricoxis have been included in the key on the basis of original descriptions, online images of Japanese specimens (
Observations were made using Olympus SZX10 and SZ40
stereomicroscopes. Layer photos of the holotype were taken using an
Olympus SZX16 with motorized focus drive attached to an Olympus E520
digital camera. Digital photos were combined by using the programmes
Deep Focus 3.1 and Quick PHOTO CAMERA 2.3. Images of specimens in BMNH
were taken with a Canon EOS 450D digital camera attached to a Leica MZ12
stereomicroscope and with a Canon EOS 450D with a Pentax 50 mm macro
lens. Several partially focused images were combined using Helicon Focus
v. 4.80 software. Digital photos at CNC were made using a Leica MZ16
stereomicroscope with motorized focus drive attached to a Leica DFC420
digital camera. Photos were combined using Leica Application Suites
Montage Multifocus software. Morphological terminology and forms of
description follow those of
http://species-id.net/wiki/Arotes
Arotes can be distinguished from other genera of Acaenitinae by combination of both of the following characters: hind tarsal claws with a sharp, accessory tooth near apex of claw and areolet of fore wing open with intercubitus distal to vein 2m-cu.
Description. Moderately large wasps, mostly black, black and white or black and yellow; legs may be reddish or yellowish in part; antennae with or without white band; fore wing with or without dark spots. Mandible with dorsal tooth equal to or slightly shorter than ventral tooth; clypeus with a pre-apical transverse ridge, the apical edge with medial tubercle in most species; subocular sulcus complete; face centrally swollen, with weak transverse ridges or weak central rugose ridges, and with median vertical ridge which extends between antennae and onto frons as distinct carina; occipital carina complete dorsally. Notaulus strong, reaching posteriorly to centre of mesoscutum; scutellum flattened, laterally carinate at least at anterior end; submetapleural carinae more or less complete, not expanded anteriorly; propodeum quite long, with more or less clearly defined area superomedia; propodeal spiracle elliptical. Fore wing without areolet, intercubitus distal to 2m-cu; vein 2m-cu with two well-separated bullae. All tarsal claws with sharp, accessory tooth near apex of claw. Ventral swelling of 1st sternite from acute to smoothly rounded, bearing numerous, long, erect hairs; ovipositor projecting beyond apex of metasoma by about 1.8-2.7 times length of hind tibia; ovipositor with ventral ridges apically that are vertical and widely spaced.
Key to the world species of ArotesNote. Asthenomeris nigricoxis Förster is a species of Arotes according to
Specimens at CNC with intermediate colour patterns indicate that Arotes albicinctus (Gravenhorst) and Arotes annulicornis Kriechbaumer may be synonyms.
| 1 | Propodeum completely black (Fig. 1) | 2 |
| – | Propodeum with at least some light colour (Figs 2-3) | 8 |
| 2(1) | Fore wing with one or two discrete, dark spots (Figs 4-5) or, if male and spot indistinct, antennal flagellum apically broadly yellow-white | 3 |
| – | Fore wing lacking discrete, dark spots, at most vaguely infuscate on apical margin; male antenna not broadly yellow-white apically | 7 |
| 3(2) | Fore wing with two discrete dark marks, one at apex of wing and one adjacent to pterostigma | Arotes sugiharai Uchida (eastern Palaearctic: Japan) |
| – | Fore wing with only apical dark mark | 4 |
| 4(3) | Flagellum entirely dark | Arotes facialis (Cameron) (Neotropical: Guatemala) |
| – | Flagellum not entirely dark: lighter ventrally than dorsally and/ or with a medial light band | 5 |
| 5(4) | Hind femurpredominantly orange-red | Arotes ustulatus Kriechbaumer (western Palaearctic) |
| – | Hind femur predominantly black or dark brown | 6 |
| 6(5) | Hind tibia orange to orange-brown in basal half | Arotes odontus Uchida (eastern Palaearctic: Russia – Sakhalin Oblast) |
| – | Hind tibia pale yellow or ivory in basal half | Arotes maurus Rohwer (some specimens) (western and central Nearctic) |
| 7(2) | First and second tergites with light-coloured posterior margins | Arotes albicinctus (Gravenhorst) (trans-Palaearctic and Oriental) |
| – | First and second tergites completely black | Arotes annulicornis Kriechbaumer (western and central Palaearctic) |
| 8(1) | Hind tibia entirely black except may be narrowly light coloured at extreme base | 9 |
| – | Hind tibia broadly yellow/white at base, can be black apically, or mostly yellow or red | 10 |
| 9(8) | Antennal flagellum without white band (Fig. 4); first sternite sub-basally with smoothly rounded projection | Arotes ucumari sp. n. (Neotropical: Peru) |
| – | Antennal flagellum with white band (Fig. 5); first sternite sub-basally with acutely angled, sharp projection | Arotes pammae Gauld (Neotropical: Costa Rica) |
| 10(8) | Hind tibia completely yellow or orange, at most, slightly darker orange at apex | 11 |
| – | Hind tibia with some dark colour (black or brown) | 13 |
| 11(10) | First sternite sub-basally strongly convex, like a tubercle | Arotes flaviscutatus Wang & Huang (Oriental: China – Fujian Province) |
| – | First sternite sub-basally weakly convex, not tuberculate | 12 |
| 12(11) | Mesoscutum black or orange, sometimes with restricted lighter coloured areas, but not a continuous lighter coloured stripe along notaulus; middle of pronotum just dorsoposterior to pronotal trough almost impunctate, punctures separated by much more than their diameter | Arotes melleus (Say) (some specimens) (central and eastern Nearctic) |
| – | Mesoscutum black with extensive yellow or white regions, notaulus completely encompassed by a wide yellow or white stripe; middle of pronotum punctate, punctures separated by their own diameter or less | Arotes decorus Say (southcentral and eastern Nearctic) |
| 13(10) | Mesoscutum black or orange, notaulus not completely encompassed by a wide yellow or white stripe | 14 |
| – | Mesoscutum black with extensive yellow or white regions, notaulus completely encompassed by a wide yellow or white stripe | 16 |
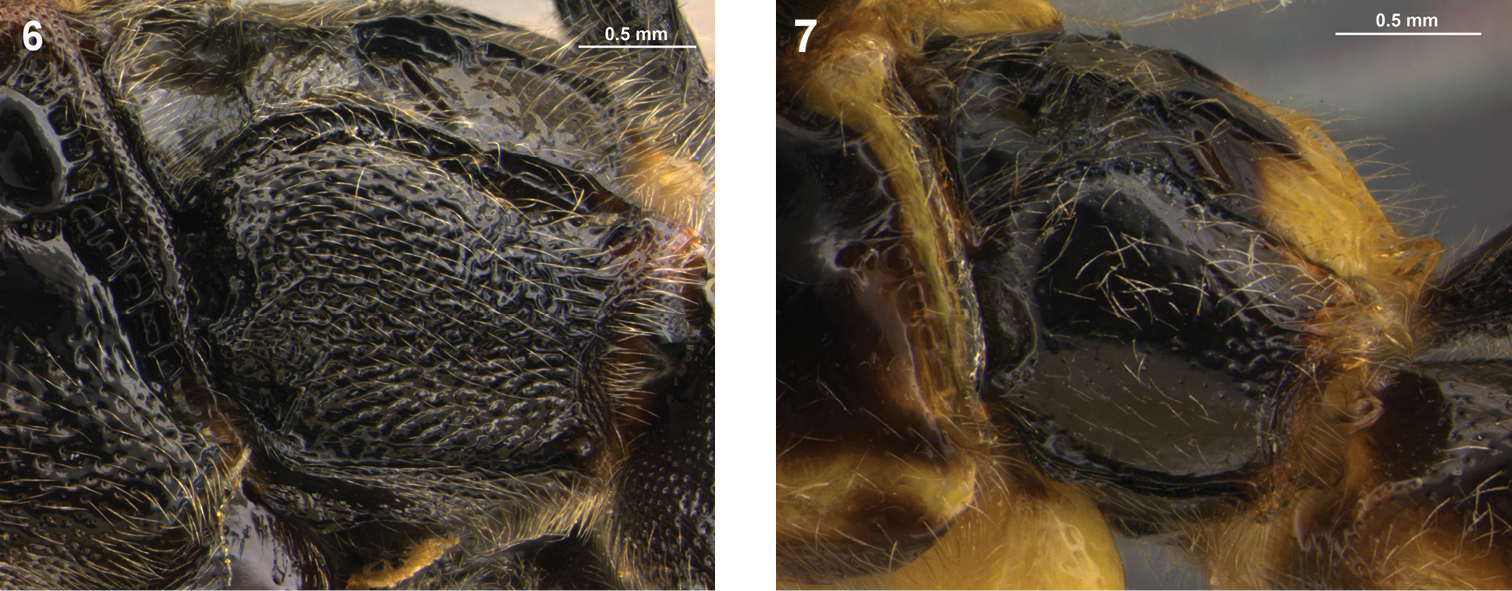
| 14(13) | Metapleuron extensively rugose to rugoso-punctate (Fig. 6) | Arotes maurus Rohwer (some specimens) (western and central Nearctic) |
| – | Metapleuron finely to densely punctate without rugosity | 15 |
| 15(14) | Metapleuron polished with fine punctures separated by much more than their diameter (Fig. 7); hind femur ventrally evenly narrowing subapically towards apex; female with mesoscutum ranging from completely fulvous (most specimens) to completely black | Arotes melleus (Say) (some specimens) (central and eastern Nearctic) |
| - | Metapleuron sub-polished with coarser punctures separated by their own diameter or less; hind femur ventrally with a strong, subapical swelling that narrows abruptly towards apex; female with mesoscutum completely black | Arotes moiwanus (Matsumura) (eastern Palaearctic and Oriental) |
| 16(13) | Hind tibia with at least basal 0.4 light coloured (basal 0.6 light in some specimens) | Arotes amoenus Cresson (southcentral and eastern Nearctic) |
| – | Hind tibia with no more than basal 0.2 light coloured | Arotes maculatus Sheng & Sun (eastern Palaearctic: China – Henan Province) |
urn:lsid:zoobank.org:act:6F98E674-ED64-4EEA-9DD9-3DAD72D6C30F
Type locality. Peru, Dept. of Cusco, Manu National Park, Cosñipata valley, San Pedro, 13°02'58"S, 71°32'13"W, 1500 m elev., C. Castillo leg., 20 September 2007.
Holotype female, pinned. Original label: “Peru, CU, San Pedro, 13°02'58"S, 71°32'13"W, 1500 m, Malaise trap, 20.ix.2007, C. Castillo". UNSM.
Arotes ucumari sp. n. (Fig. 4) can be distinguished from all other described Arotes spp. by combination of all the following characters: 1) hind tibia black; 2) scutellum yellow; 3) antenna without a medial light coloured band; 4) hind femur ventrally not or only slightly swollen subapically.
Arotes ucumari sp. n. is readily distinguished from other New World species of Arotes (except Arotes facialis) on account of its totally black antennae (character 3). It differs from Arotes facialis in that it has more extensive yellow colouration on the meso- and metasoma (Arotes facialis is almost completely black). In addition, the propodeal carination of the three neotropical species is different (Figs 1-3). The area superomedia of Arotes facialis is hexagonal to subcircular whereas that of Arotes ucumari is irregularly octagonal (Fig. 2). In coloration, Arotes ucumari is similar to Arotes pammae Gauld (Fig. 5) but may be separated from that species by the black antennae, smoothly rounded first sternite of metasoma and the propodeal carination (in Arotes ucumari, the anterior transverse carina joins the area superomedia at its upper half and the shape of the area superomedia is irregularly octagonal).
Female. Habitus in Figure 4. Lower face broad, inner margins of eyes ventrally divergent; frons concave, smooth; antenna with 34 flagellomeres; antenna about as long as fore wing. Pronotum with striae directed to hind corner of pronotum, middle of pronotum just dorsoposterior to pronotal trough impunctate; mesoscutum with lobes sparsely, coarsely punctate, closely punctate on front side; scutellum more closely punctate than mesoscutum; mesopleurum anteriorly, ventrally coarsely punctate; metapleurum coarsely, closely punctate but, above submetapleural carina punctures are separated by more than their diameter; propodeum with area superomedia clearly delineated, almost hexagonal anteriorly, posteriorly narrowed, so that it is irregularly octagonal (Fig. 2), posterior border of area superomedia concave; anterior transverse carina joining area superomedia at its upper half; lateral longitudinal carina only delineating area externa and area posteroexterna; area petiolaris confluent with area posteroexterna. Fore wing length 14 mm, wing without areolet, with cross vein 2rs-m (or 3rs-m, depending on interpretation) distal to 2m-cu. Hind femur ventrally with a slight subapical swelling. First metasomal sternite with projection smoothly rounded; ovipositor projecting beyond apex of metasoma by about 1.9 times length of hind tibia.
Yellowish species with black marks. Head light yellow with temple, frons and inner margin of occiput black; antenna black except infuscate tip on last flagellomere. Mesosoma mostly light yellow with dorsal and hind margins of pronotum black, mesoscutum black with yellow marks on lateral and hind regions of central lobe, U-shaped mark in dorsal view, lateral sides of mesoscutum, scutellum and metanotum also yellow, hind margin of mesopleurum, mesosternum and anterior half of propodeum black. Wings slightly yellowish, with apex broadly infumate, pterostigma and veins black. Fore and mid legs with light yellow on dorsal surfaces of trochanters and femora, most of tibiae and all tarsi infuscate; hind leg black with yellow marks on lower half of coxa, two oval yellow marks on dorsal and lateral sides of coxa, most of trochanter and ventral half of femur light yellow. Metasoma black, tergites 1-2 with broad yellowish marks close to hind margin, tergite 3 almost entirely black, tergites 4+ with hind margins and lateral spots light yellow; subgenital plate infuscate with upper margin yellowish; ovipositor orange, ovipositor sheaths black with dull yellow tip.
Male. Unknown.
Biology. The host of Arotes ucumari sp. n. is not known. North American Arotes species have been reared from Melandrya (Melandryidae), Leptura (Cerambycidae) and Tomoxia (Mordellidae) (
Ecology. The type locality is in a primary forest
at the south east limit of Manu National Park. On the eastern slopes of
the Andes, this altitude (1500 m) is considered as a major ecotone
between the humid montane forest and the premontane forest belt (
Etymology. Ucumari is the quechuan name for the only South American species of bear, Tremarctos ornatus, the Spectacled Bear. Just as is possible in the case of the Acaenitinae parasitoid wasps, the tremarctine bears reached the New World via the Bering Strait, and expanded their range southwards into North and South America. By naming the new species as Arotes ucumari we hope to draw attention to the conservation of both of these rare tropical Andean species.
The status of Arotes (Albicinctus) moiwanus Matsumura, 1912.
During the process of comparing specimens to verify the new species status of Arotes ucumari, examination of material of Arotes albicinctus and its subspecies Arotes albicinctus moiwanus at NHM and CNC revealed differences that could indicate that these two forms may represent two distinct species. Since
In most ichneumonid species in which
subspecies are recognized, the only indicator of subspecies is colour,
not sculpture. For example, Campoplex sugiharai sugiharai (Uchida), Campoplex sugiharai australis Momoi and Campoplex sugiharai okinawensis Momoi (see
Propodeum of neotropical species of Arotes, posterodorsal view. 1. Arotes facialis, 2. Arotes ucumari sp. n., 3. Arotes pammae.
Habitus, lateral view. 4. Arotes ucumari sp. n., holotype female (Peru). 5. Arotes pammae.
Metapleuron, lateral view. 6. Arotes maurus, 7. Arotes melleus.
Whereas description of a species based on a single
specimen is not ideal, we are confident that the species is distinct
based on the unique combination of characters listed in the diagnosis
and our assessment of species-specific characters within the genus Arotes. For example, the presence of a completely black flagellum in Arotes ucumari is very rare for the genus (in the previously described species only Arotes facialis from
Guatemala has this character, all other species have either a medial
pale band or are lighter on the ventral surface of the flagellum than
the dorsal surface). It is highly unlikely that Arotes ucumari and Arotes facialis are conspecific because the body of Arotes facialis is almost completely black (
In our opinion, it is important to describe the new
species now, rather than wait for additional material to be collected.
Considering the extensive collecting done in this region, for
example, the Colombian Arthropod Project (CAP) from 2001 to 2003, 188
Malaise trap months in Peru from 1998-2001 (
Key morphological differences between Arotes albicinctus and Arotes moiwanus
| Arotes albicinctus | Arotes moiwanus |
| Frons with strong oblique striations extending from medial ocellus towards eye. Orbit with dense, coarse punctures near antenna (Fig. 8). | Frons with weak striations or striations absent between medial ocellus and eye. Orbit with moderately fine, sparse punctures near antenna (Fig. 9). |
| Scutellum and propodeum black. | Scutellum and propodeum marked with light colour (creamy-white to yellow). |
Head, dorsal view. 8. Arotes albicinctus, 9. Arotes moiwanus.
Personal funding for Carol Castillo was provided by the Turku University Foundation and the Kone Foundation, Finland (research grants to the research team of Ilari E. Sääksjärvi). The Amazon Conservation Association (Peru) awarded financial support for the field work. Research permits were issued by the Ministry of Environment (Peru). Diana Barnes (CNC) produced the images of A. albicinctus, A. moiwanus, A. maurus and A. melleus, and Anu Veijalainen (ZMUT) helped with the edition of the A. ucumari layered image. Rikio Matsumoto kindly donated a specimen of Arotes moiwanus to BMNH.