(C) 2011 Achille Casale. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Typhloreicheia monacha sp. n. and Typhloreicheia ilianae sp. n. are described from two caves of Central-Eastern Sardinia (Nuoro province): the Bue Marino cave and the Nurra ‘e Pradu cave, respectively. Both caves are located in the part of the island where many highly specialised subterranean carabid beetles are localised. Typhloreicheia monacha is apparently related to two other species of the same area, i.e. Typhloreicheia onnisiCasale & Magrini, 2004 and Typhloreicheia elegans (Dodero, 1916); Typhloreicheia ilianae is closely related to Typhloreicheia henroti Jeannel, 1957, known from a cave near Dorgali. Relationships and diagnostic features among these taxa are discussed and illustrated, and a key for identification of the specialised subterranean Typhloreicheia species of Sardinia is provided. The hypothesis of adaptive radiation of Reicheiina species in Sardinia, recently proposed by the senior author of this contribution, is further elaborated in light of new data.
Coleoptera, Carabidae, Scaritinae,Typhloreicheia, new species, Sardinia, adaptive radiation
The subtribe Reicheiina is a lineage of endogean and
hypogean carabid beetles, currently classified in the tribe Clivinini
of the subfamily Scaritinae of the family Carabidae (
Work with Reicheiina beetles is hampered by the small sizes of its representatives and by the scarcity of material, some taxa being known from one or a few specimens. Morphological characters currently employed in defining various members of Reicheiina are those of external body shape and male genitalia, while immature stages, for example, are still completely unknown.
Monophyly of Reicheiina has never been adequately
demonstrated and a distinct possibility exists that the group is an
artificial assemblage of unrelated subterranean scaritine clades (
However, the monophyly of the Euro-Mediterranean core of Reicheiina formed by the genus Typhloreicheia and its relatives is highly plausible. In Sardinia, the genus Typhloreicheia Holdhaus, 1924 is markedly diversified (
Newly designated Sardinian Typhloreicheia type specimens were collected during speleological or biospeleological investigations. All subsequent focused attempts to obtain further individuals by using pitfall traps or baits were unsuccessful.
Male genitalia were dissected, dehydrated in ethanol, cleared in cold KOH, examined and illustrated, using standard techniques before their definitive inclusion on microscope slides attached to the respective specimens. Line drawings were made using a camera lucida attached to stereomicroscopes Wild M-5, and a microscope Leitz Orthoplan.
Photographs of male genitalia were prepared by Paolo Magrini (Florence), with a Nikon D1 digital camera mounted on a Nikon Labophot II binocular microscope. P. Magrini also prepared distributional maps of Typhloreicheia in Sardinia.
The median lobe of aedeagus is synonym of phallus of
authors. The proximal gonocoxite 1, and the more distal gonocoxite 2
(in the sense of
Acronyms:
TL body Total length, from the anterior margin of clypeus to the apex of elytra, measured along the elytral suture.
L overall body length, from apex of mandibles to apex of elytra, measured along the suture.
PL/PW ratio length of Pronotum, as linear distance from the anterior to the posterior margin (peduncle included), measured along the midline to the maximum Width of Pronotum.
EL/EW ratio length of Elytra, as linear distance from the basal ridge to the apex, measured along the elytral suture to the maximum Width of Elytra.
EL/PW ratio maximum Width of Elytra to maximum Width of Pronotum
AL length of antenna.
Collections:
CCa all type specimens are preserved in Casale Collection (University of Sassari, Italy)
Resultsurn:lsid:zoobank.org:act:D1845710-A731-46D0-9904-65DD4662188E
Italy, Central-Eastern Sardinia: Dorgali (Nuoro province), Cala Gonone: Bue Marino cave (speleological inventory number: 12 Sa/NU), 0 m a.s.l., 40°14'51"N; 9°37'29"E
Holotype male with the following data: I – Sardegna, Dorgali (NU), Gr. Bue Marino 10.IX.2006 P. Marcia leg.; paratypes: female, same data as holotype; female: I – Sardegna, Urzulei (NU) Codula di Luna, Gr. Su Spiria 1988 Sa/NU 23.VII.1995 R. Loru leg. (CCa).
The Latin noun “monachus–a” (= monk) recalls themonk seal Monachus monachus (Hermann, 1779), the so-called “Bue Marino”, in the tradition of the Mediterranean languages, from which the type locality cave derives its name. Monk seal is presently one of the most endangered mammalian species of the Mediterranean fauna, although it was still present in the Eastern coast of Sardinia until the ’60s of the past century.
Typhloreicheia monacha new species, for both its external features and characteristics of male genitalia, seems to be related to Typhloreicheia onnisi Casale & Magrini, 2004, known from three caves in the Gairo region (Central-Eastern Sardinia), and, to a lesser extent, to Typhloreicheia elegans (Dodero, 1916) known from a cave of Arcuerì Mt. near Seui. The male features of the latter, however, are still unknown.
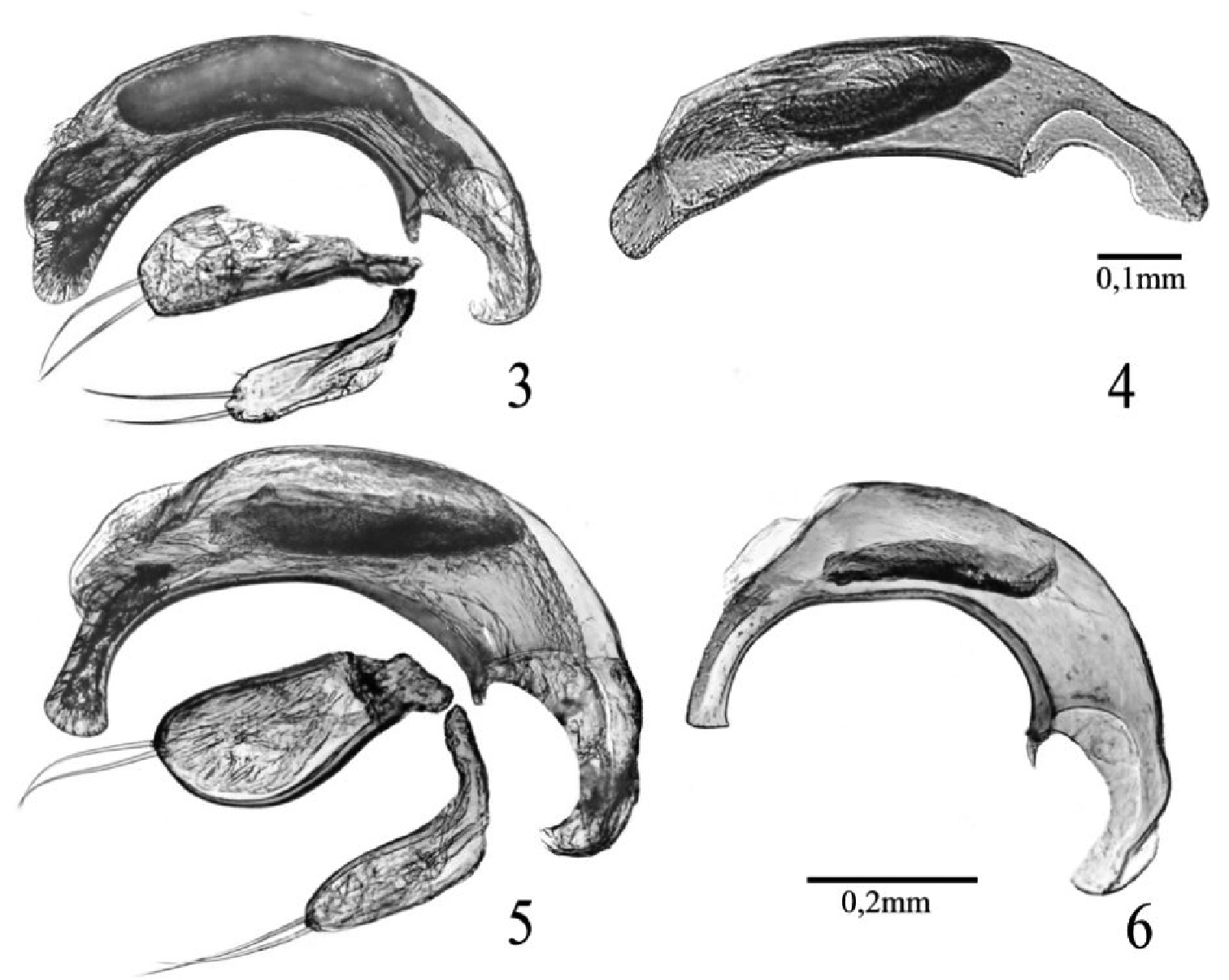
From Typhloreicheia onnisi the new species can be distinguished mainly by its stouter and wider head, with more convex genae; by its wider pronotum, with anterior angles larger and more prominent, and lateral margins more rounded and less constricted toward the base; by the elytra shorter, with lateral margins with more numerous (23–25) (14–16 in Typhloreicheia onnisi) and more prominent marginal teeth; and by the different shape of the aedeagus (see Figures 3–4).
A medium-large sized Typhloreicheia species (TL: 2.95–3.08 mm; L: 3.28–3.40 mm).
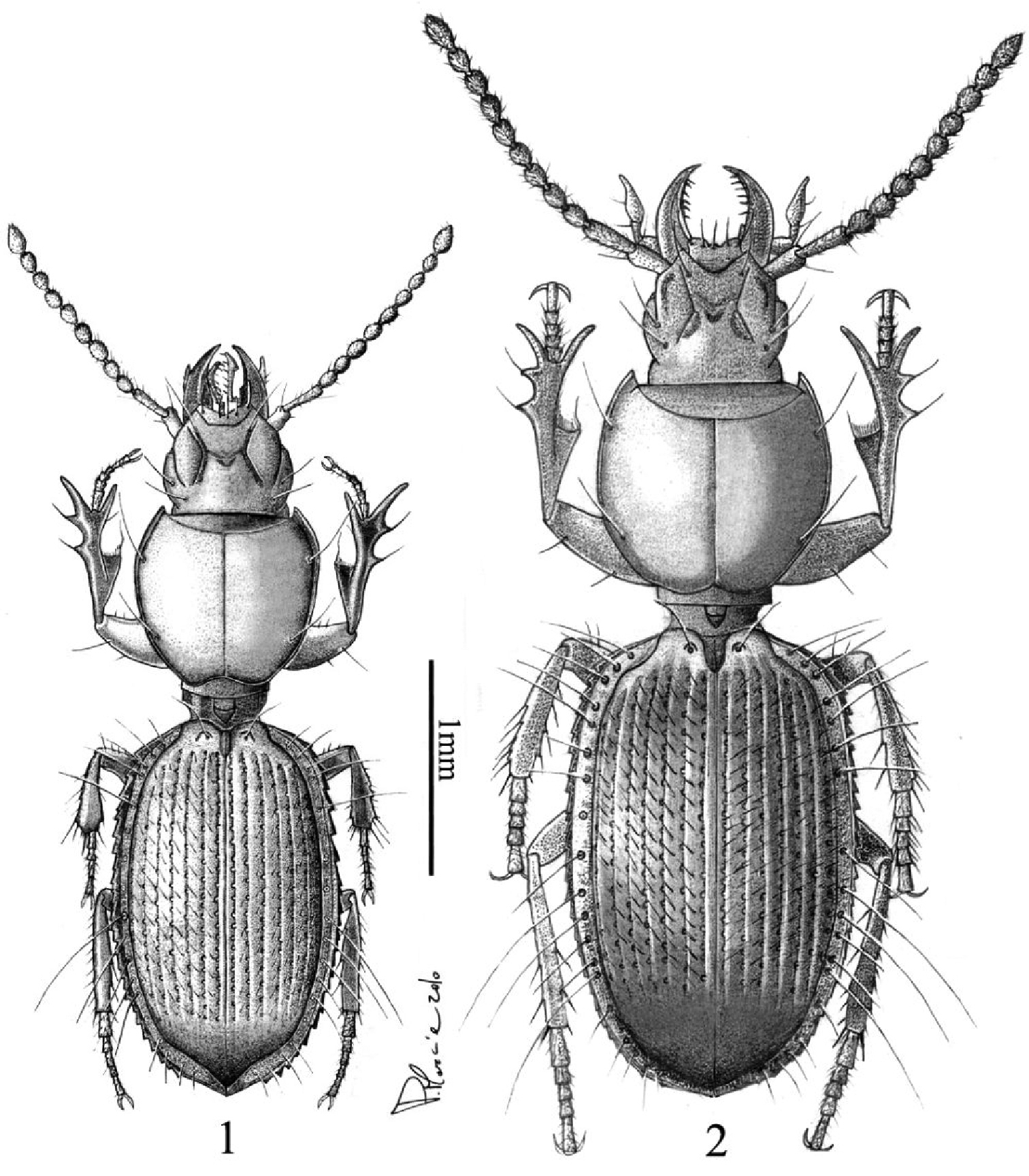
Body elongate, convex (Figure 1). Colour testaceous reddish, antennae and mouthparts slightly paler. Integument shiny, polished; microsculpure with fine, hardly visible microlines in form of isodiametric mesh pattern on head and elytra, almost vanished on pronotum.
Head with ocular part of genae regularly convex, constricted toward the neck. Eyes absent. Supra-antennal plates separated from genae by deep and broad furrow; frontal furrows very deep, transversally wrinkled; vertex with an evident, convex tubercle in the middle; antennae moderately elongate (AL: 1.31 mm in male holotype), antennomeres 3–10 slightly longer than wide.
Pronotummoderately convex, elongate (PL/PW: 1.0), with its maximum width at the anterior third; sides moderately rounded, slightly attenuated in front, markedly constricted to the basal peduncle; anterior angles acutely prominent; median furrow narrow and deeply impressed; lateral furrows very narrow and superficial.
Elytraelongate-ovate (EL/EW: 1.6), distinctly wider than pronotum (EW/PW: 1.21), convex, with their maximum width in the middle; humeri broadly rounded; lateral furrows wide, flattened, not narrowed at apex; lateral margin reflexed, with numerous (23–25), small but acutely prominent marginal teeth; striae all evident, deeply punctuate, gradually disappearing at apex; elytral intervals moderately convex, intervals 2–7 each bearing a series of short, erected setae; umbilicate series of 16–19 punctures along stria 8.
Male genitalia as in Figure 3. Median lobe of aedeagus markedly curved, with short, rounded and flattened apex. Endophallus with developed, apical copulatory piece and an elongate packet of serrate scales in the middle. Parameres each with two apical setae.
Female genitalia (examined, not illustrated) without any peculiar characteristic: gonocoxites 2 rather short, regularly curved outwards, each with two moderately elongate, robust spiniform setae on the outer side, the distal of them being distinctly longer and thickener than the proximal one.
Two specimens of Typhloreicheia monacha
sp. n. were collected during one of bio-speleological expeditions
organized in the last years by the authors. The specimens were walking
on sandy, humid soil on the banks of the subterranean lakes in the
inner parts of the Bue marino cave, in the same habitat as those noted
for the molopine carabidbeetle Speomolops sardous Patrizi, 1955 and its larva (
In spite of many subsequent investigations in
both these caves, including setting pitfall traps, no further
individuals of this species have been obtained. Typhloreicheia monacha, like other species of the same genus in Sardinia (and most so-called troglobiont species:
The following features of Typhloreicheia monacha sp. n. suggest its affinities with the elegans species group (
Typhloreicheia monacha sp. n., male holotype, dorsal aspect 2 Typhloreicheia ilianae sp. n., male holotype, dorsal aspect.
urn:lsid:zoobank.org:act:B5A1C382-9E73-4F50-B15A-611522B1F9A7
Italy, Central-Eastern Sardinia: Oliena (Nuoro province), Corrasi Mt.: Nurra ‘e Pradu cave (speleological inventory number: 3083 Sa/NU), 1220 m a.s.l. , 40°15'27.0"N, 009°25'45.3"E
Holotype male with the following data: I – Sardegna, Oliena (NU), M. Corrasi 26.I.2008 P. Marcia”, “Gr. Nurra ‘e Pradu” (CCa).
The name derives from the Oliena village at the base of the Corrasi mountain in which this new species, and many other hypogean organisms were discovered. An ancient legend says that the inhabitants (Ilienses in Latin) of Troy (Ilios in ancient Greek), after the fall of the town to the Greeks, reached Sardinia and founded a village named Iliana, which subsequently became Oliena.
Typhloreicheia ilianae is the largest in size among the Sardinian congeners known so far, with its TL: 3.64 and L: 4.05 (male holotype). It is evidently related to Typhloreicheia henroti Jeannel, 1957 (see features of the median lobes of aedeagus in respective species: Figures 5–6), but can be distinguished from the latter mainly by its larger size (TL: 3.10 – 3.65, L: 3.40 - 3.86 in Typhloreicheia henroti); by wider and more robust head, with supra-antennal plates more prominent laterally, by more convex genae markedly constricted to the neck, by antennomeres 3–11 more thickened, and by anterior angles of both clypeus and labrum more acutely prominent; and by the different shape of the median lobe of aedeagus (Figures 5–6).
A large sized Typhloreicheia species (TL: 3.64; L: 4.05 mm, in male holotype).
Body elongate but robust, convex (Figure 2). Colour testaceous reddish, antennae and mouthparts slightly paler.
Integument shiny, highly polished; microsculpure with fine, hardly visible microlines in form of isodiametric mesh pattern on head and elytra, almost vanished on pronotum.
Head robust, with ocular part of genae markedly convex, constricted toward the neck. Eyes absent. Anterior angles of clypeus acutely prominent. Supra-antennal plates prominent laterally, with outer margin beaded, separated from genae by deep and broad furrow; frontal furrows very deep, with shallow wrinkles in the posterior tract; vertex with an evident, convex tubercle in the middle; antennae elongate (AL: 1.58 mm in male holotype) but robust, thickened; antennomeres 6–10 slightly longer than wide.
Pronotum markedly convex, relatively wide (PL/PW: 0.95), with its maximum width at the basal third; sides moderately rounded, slightly narrowed in front, markedly constricted to the basal peduncle; anterior angles acutely prominent; median furrow very shallow; lateral furrows very narrow and superficial.
Elytraelongate-ovate (EL/EW: 1.62), distinctly wider than pronotum (EW/PW: 1.24), convex, with their maximum width in the middle; humeri broadly rounded; lateral furrows wide, flattened, not narrowed at apex; lateral margin reflexed, elytra with lateral margins with numerous (24) prominent marginal teeth; striae deep, deeply punctuate, all evident, gradually disappearing et apex; elytral intervals convex, intervals 2–7 each bearing a series of short, erected setae; umbilicate series of 16–19 punctures along stria 8.
Male genitalia as in Figure 5. Median lobe of aedeagus markedly curved, with long apical lamina, which is widened, hatched-like distally. Endophallus with a reduced, inconspicuous apical copulatory piece and an elongate packet of serrate scales in the middle. Parameres each with two apical setae.
Female genitalia: unknown.
Typhloreicheia ilianae sp. n. is known only from the type locality. The holotype was sampled in wet soil under big stones in a small pit (- 3.7 m), which represents the entrance of a large hypogean system, reaching the depth of 101 m. The associated subterranean fauna includes some of the most specialised troglomorphic endemic Sardinian elements, such as Sardaphaenops supramontanus supramontanus Cerruti & Henrot, 1956 (Coleoptera, Carabidae, Trechini) and Patriziella sardoa Jeannel, 1956 (Coleoptera, Cholevidae, Leptodirini), both described from a nearby cave Nurra ‘e Sas Palumbas. The Nurra ‘e Pradu cave is also one of the few localities of Sardinia from which the large sized, trogloxenic centipedePlutonium zwierleini Cavanna, 1881 is reported (Zapparoli 2009). We sampled in this cave the following taxa, all endemic to central-eastern Sardinia and representing new faunal records: the orthopteran Acroneuroptila cf. sardoa Baccetti, 1960, the sphodrine carabid beetle Laemostenus pippiai (G. Fiori, 1961) (A. Casale det.), the cholevid beetle Ovobathysciola majori (Reitter, 1885) (A. Casale det.), and the terrestrial snail Tacheocampylaea carotii (Paulucci, 1882) (S. Birindelli det.). Subsequent trapping in this cave using pitfall traps, did not produce additional individuals of Typhloreicheia ilianae.
Typhloreicheia ilianae is the largest among the Sardinian species known so far, exceeding the large size of Typhloreicheia kraussei (Reitter, 1914), a typical endogean, not hypogean species reaching 3.69 mm in length (
The new taxon appears related to Typhloreicheia henroti Jeannel, 1957, known from the Gurennoro cave (= Pisanu cave, 215 Sa/NU, near Dorgali). Both species are similar in having large-sized body and elytral lateral margins bearing numerous (21–24) teeth extending all the way from the humeral angle to elytral apex. Also both species share the peculiar shape of the median lobe of aedeagus, which is unique among the Sardinian species (Figures 5–6). These species form a pair of adelphotaxa very isolated from the rest of species known so far on the island, here indicated as henroti species group, excluding the other specialised hypogean species of Sardinia known so far (Typhloreicheia elegans, Typhloreicheia onnisi, and Typhloreicheia monacha sp. n.) treated above aselegans species group in the narrow sense.
Typhloreicheia spp., male genitalia, right lateral aspect: 3 Typhloreicheia monacha sp. n., median lobe f aedeagus and parameres 4 Typhloreicheia onnisi Casale & Magrini, median lobe of aedeagus 5 Typhloreicheia ilianae sp. n., median lobe of aedeagus and parameres 6 Typhloreicheia henroti Jeannel, median lobe of aedeagus.
The following operative and provisional key is provided to distinguish the cave dweller (deep hypogean, or troglophilic) Typhloreicheia species known so far in Central Eastern Sardinia:
| 1 | Larger in size (TL: mm 2.9–3.6; L: 3.0–4.0); elytra with intervals 2–7 all having setiferous punctures, and with lateral margins serrate from the humeral angle to apex. Deep hypogean species, known from caves only | 2 |
| – | Smaller in size (TL less than 3 mm); elytra with only intervals 2–3-5–7 having setiferous punctures. Endogean but troglophilic species, occasionally found in caves | 6 |
| 2 | Larger in size (TL: 3.10–3.65; L: 3.40–4.05). Median lobe of aedeagus with apical lamina very elongate, spatulate or axe-shaped distally (Typhloreicheia henroti species group in new sense) | 3 |
| – | Smaller in size (TL: 2.90–3.10; L: 3.00–3.40). Median lobe of aedeagus (in the two species in which it is known) with apical lamina short, rounded or sub-truncate distally (Typhloreicheia elegans species group in new sense) | 4 |
| 3 | Larger in size (TL: 3.64 mm; L: 4.05 mm, in male holotype). Median lobe of aedeagus larger, with apical lamina wider distally (Figure 5) (Central Eastern Sardinia, Corrasi Mt.: Nurra ‘e Pradu cave) | Typhloreicheia ilianae sp. n. |
| – | Smaller in size (TL: 3.10 – 3.65 mm; L: 3.40 – 3.86 mm). median lobe of aedeagus smaller, with apical lamina more elongate and narrower distally (Figure 6) (Central Eastern Sardinia, Dorgali: Gurennoro or Pisanu cave) | Typhloreicheia henroti Jeannel, 1957 |
| 4 | Anterior angles of both clypeus and pronotum rounded, slighly prominent in front; genae slightly convex (Central Eastern Sardinia, Seui: Is Diavolus cave) | Typhloreicheia elegans (Dodero, 1916) |
| – | Anterior angles of both clypeus and pronotum very prominent in front; genae very convex, inflate | 5 |
| 5 | Elytra shorter, ovate, with lateral sides rounded and lateral margins with markedly prominent and numerous (23–25) teeth. Median lobe of aedeagus markedly curved, with apical lamina shorter and rounded distally (Figure 3) (Central Eastern Sardinia, Dorgali: Bue Marino cave) | Typhloreicheia monacha sp. n. |
| – | Elytra elongate, sub-parallel sided; lateral margins with slightly prominent and less numerous (14–16) teeth. Median lobe of aedeagus slightly curved, with apical lamina more developed and sub-truncate distally (Figure 4) (Central Eastern Sardinia: caves in the Gairo region) | Typhloreicheia onnisi Casale & Magrini, 2004 |
| 6 | Larger in size (TL: 2.31–2.50; L: 2.58–2.99). Elytra with lateral margins serrate in the basal third only. Median lobe of aedeagus with apical lamina short; endophallus with copulatory piece in the shape of twisted lamina (Central Eastern Sardinia: Sadali, Is Janas cave; Nurallao [Nuoro]) | Typhloreicheia jana Leo, Magrini & Fancello, 2005 |
| – | Smaller in size (TL: 1.99–2.22; L: 2.11–2.50). Elytra with lateral margins serrate from the humeral angle to apex. Median lobe of aedeagus with apical lamina markedly elongate and curved on the ventral side; endophallus with copulatory piece in the shape of triangular lamina, rounded distally and hollow at base. Central Eastern Sardinia, near Dorgali and Galtellì, in deep soil and caves | Typhloreicheia pandora (Holdhaus, 1924) |
As recently recalled (
Furthermore, these authors do not consider members of the subtribe Reicheiina some taxa cited as either potentially belonging, or closely related, to the subtribe Reicheiina: the monotypic genus Italodytes Müller, 1938 from Apulian caves, Syleter Andrewes, 1941 (including Afrotropical and Oriental species), Psilidius Jeannel, 1957 (including Afrotropical species), Leleuporella Basilewsky, 1956 (including Afrotropical species and one from Sri Lanka), Trilophus Andrewes, 1927 (including Oriental species) and Trilophidius Jeannel, 1957 (including Afrotropical and Oriental species).
2. Biogeographical remarks at local scaleTwo hypotheses have been proposed to explain the origin and the exceptional specific diversity of the genus Typhloreicheia in Sardinia.
The first hypothesis proposes at least two different,
heterochronic colonisation events. The older one is represented by the
common ancestor of the subgenus Sardoreicheia Jeannel, 1957. This subgenus was treated as synonym of Typhloreicheia by
This scenario should be similar to the process recently proposed by
The second hypothesis proposes that all Sardinian Typhloreicheia species are derived from a common Tyrrhenian, Miocene ancestor.
In other words, the question is: how many times have Reicheiina colonized the island of Sardinia? An adequate solution of this question based exclusively on morphological features is hardly possible. Involvement of molecular data might eventually help to generate complete phylogenetic hypotheses for Typhloreicheia and related genera and thus assist with detecting the number of colonisation events.
The similarities between the male genitalia of some
Sardinian species and those of species of the Apennine chain, the Elba
island and Sicily, supports the hypothesis of multiple colonisations of
the island by different ancestors. This is further strengthened by the
remarkable absence of Typhloreicheia species in Corsica and Baleares, where the genus is replaced by Reicheia Saulcy, 1863. Furthermore, the scenario in Sardinia has been complicated by the recent description of the genus Dimorphoreicheia
Magrini, Fancello & Leo, 2003 (with two species known so far),
characterised by the presence of setiferous pores on the disc of the
pronotum, a feature previously thought to be peculiar to the genus Reicheadella Reitter, 1913, with some species distributed in the southern Balkans (
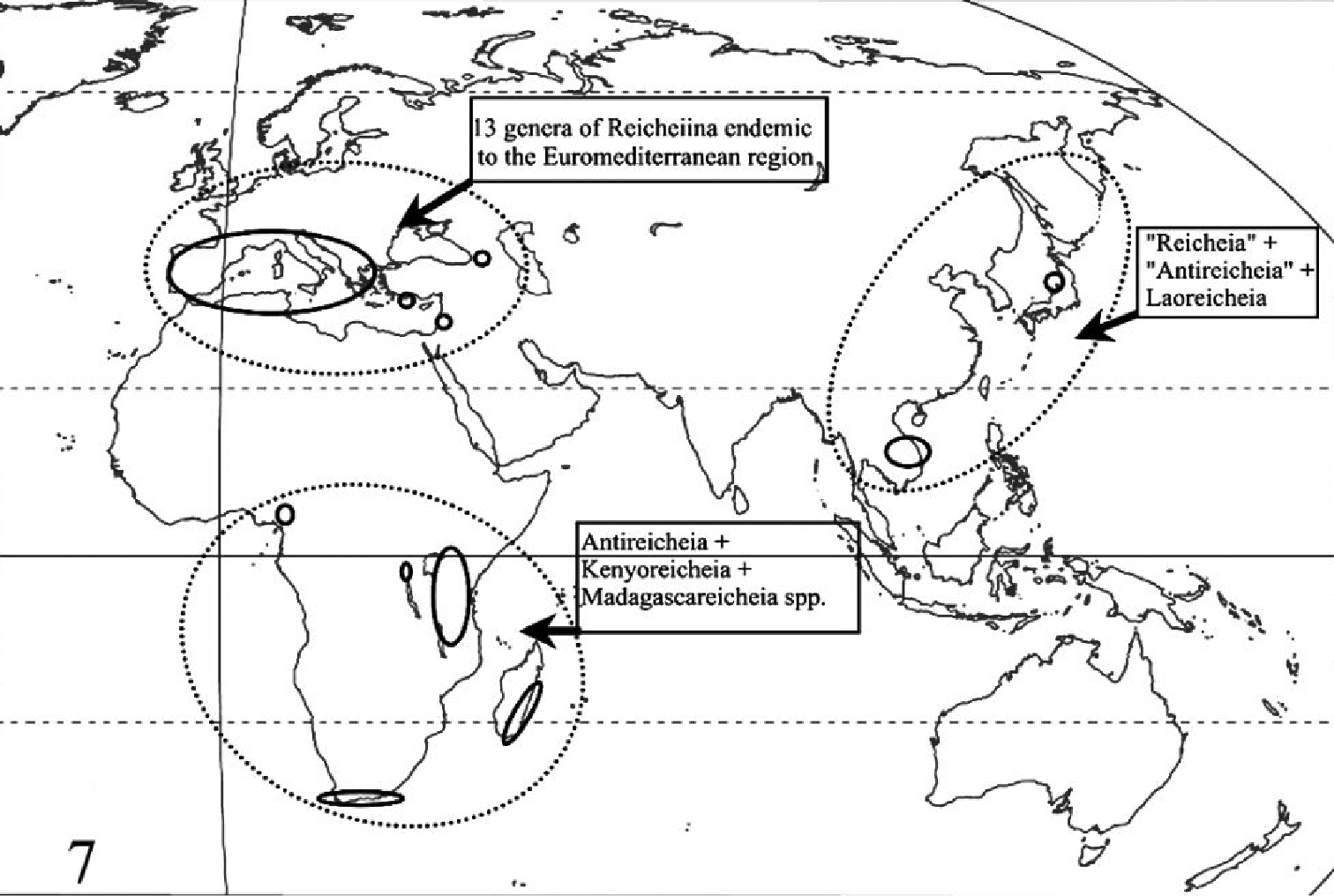
World distribution of the subtribe Reicheiina in the current sense.
Adaptive radiation might work very rapidly. Classic examples include Hawaiian silverswords and Drosophila, Darwin’s finches on the Galápagos islands, Anolis lizards on Caribbean islands, and cichlids of the East African Great Lakes, among others (
The genus Typhloreicheia
in Sardinia shows a spectacular diversity much exceeding that in all
other Tyrrhenian areas (Iberian Peninsula, Baleares, Apennines,
Sicily) where the genus is represented. This fact already induced
Recent research in the field shows a scenario in which two, three or more sympatric Typhloreicheia species are present in every micro-sector of the island. Additionally, if they are living in the same area, they show different habitat choice (soil litter, deep soil, crevices, or caves, respectively), and different adaptive features to a subterranean way of life. A good example is offered by two cave-dwelling species, i.e. Typhloreicheia henroti Jeannel, 1957 and Typhloreicheia monacha sp. n., which in the Dorgali area (Central-Eastern Sardinia) co-occur with the endogean Typhloreicheia doderoi (Holdhaus, 1924) and Typhloreicheia pandora (Holdhaus, 1924). In close areas, other so-called “troglobitic” species (sampled in caves only) are reported from the Jurassic massifs of the central-eastern part of the island, where many highly specialized subterranean taxa are localized: Typhloreicheia elegans (Dodero, 1916), Typhloreicheia onnisi Casale & Magrini, 2004, and Typhloreicheia ilianae sp. n. described above (Figure 9).
A similar process – e.g. the colonization of Sardinia by a continental Reicheia-like ancestor, maybe of Iberian origin through the rotation of the Corsica-Sardinia microplate (
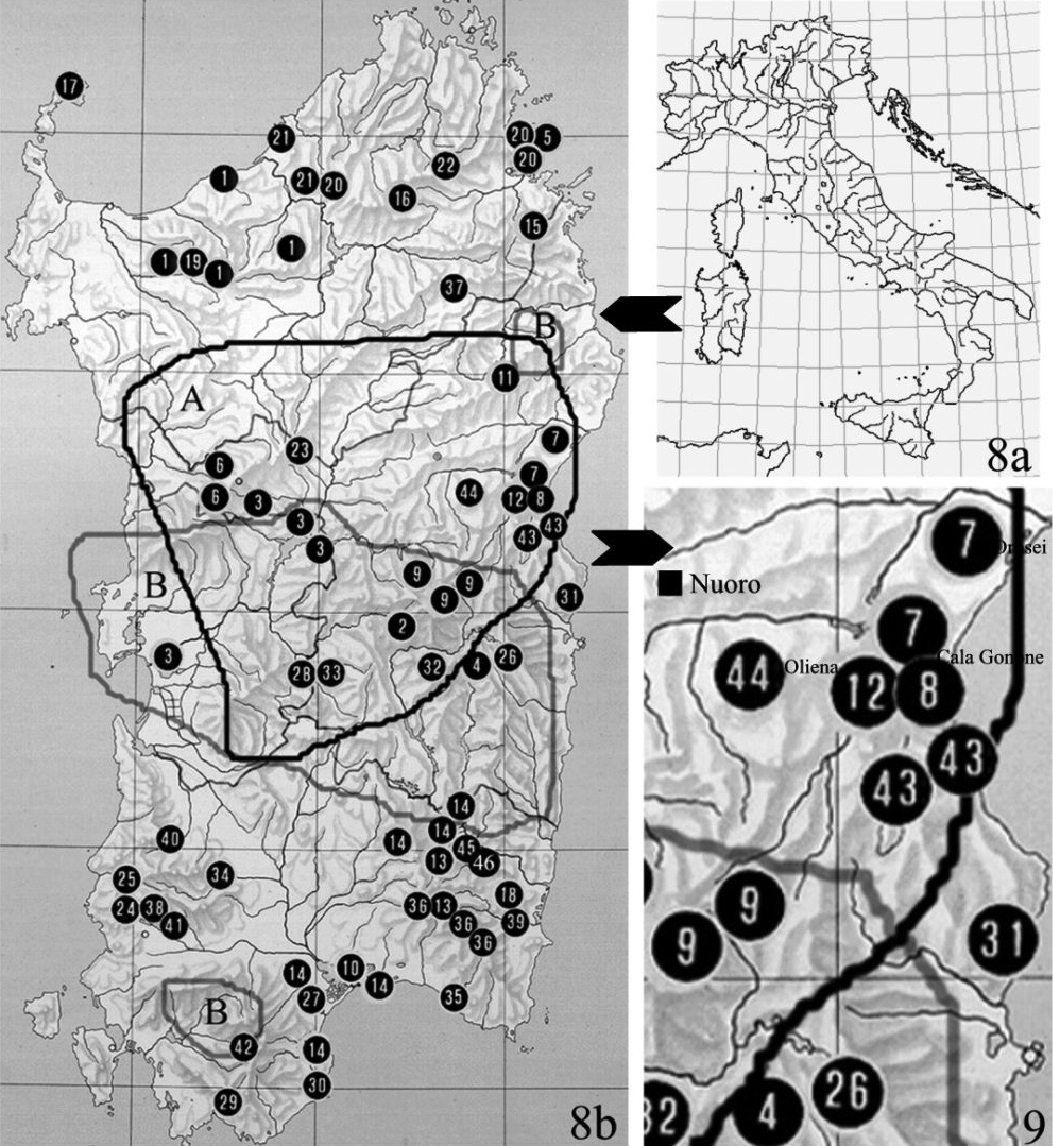
8 Geographical distribution of Typhloreicheia species known so far in Sardinia.Numbers in the map indicate the locality of each species, in chronological order of description. A and B, and related lines, indicate the range of the only two species with wider distribution in the island. A – Typhloreicheia denticulata (Holdhaus, 1924) sensu lato B – Typhloreicheia jucunda (Holdhaus, 1924) sensu lato; 1 – Typhloreicheia raymondi (Putzeys, 1869); 2 – Typhloreicheia sardoa (Baudi, 1891); 3 – Typhloreicheia kraussei (Reitter, 1914); 4 – Typhloreicheia elegans (Dodero, 1916); 5 – Typhloreicheia parallela (Holdhaus, 1924); 6 – Typhloreicheia manto (Holdhaus, 1924); 7 – Typhloreicheia pandora (Holdhaus, 1924); 8 – Typhloreicheia doderoi (Holdhaus, 1924); 9 – Typhloreicheia monticola (Holdhaus, 1924); 10 – Typhloreicheia occulta (Holdhaus, 1924); 11 – Typhloreicheia minima (Binaghi, 1936); 12 – Typhloreicheia henroti Jeannel, 1957; 13 – Typhloreicheia fausti Fancello, 1988; 14 – Typhloreicheia valeriae Fancello, 1988; 15 – Typhloreicheia fancelloi Magrini, 2000; 16 – Typhloreicheia melonii Magrini, 2001; 17 – Typhloreicheia arganoi Vigna Taglianti, 2001; 18 – Typhloreicheia viti Magrini & Bulirsch, 2002; 19 – Typhloreicheia vignai Magrini, 2003; 20 – Typhloreicheia consortii Magrini, 2003; 21 – Typhloreicheia degiovannii Magrini, 2003; 22 – Typhloreicheia nadiae Magrini, 2003; 23 – Typhloreicheia cirocchii Magrini, 2003; 24 – Typhloreicheia angelae Magrini, 2003; 25 – Typhloreicheia leoi leoi Magrini, 2003; 26 – Typhloreicheia onnisi Casale & Magrini, 2004; 27 – Typhloreicheia laurentii Magrini, 2004; 28 – Typhloreicheia medusa Magrini & Fancello, 2005; 29 – Typhloreicheia tegulae Leo, Magrini & Fancello, 2005; 30 – Typhloreicheia exilis Leo, Magrini & Fancello, 2005; 31 – Typhloreicheia supramontis Leo, Magrini & Fancello, 2005; 32 – Typhloreicheia jana Leo, Magrini & Fancello, 2005; 33 – Typhloreicheia eleonorae Leo, Magrini & Fancello, 2005; 34 – Typhloreicheia tanit Leo, Magrini & Fancello, 2005; 35 – Typhloreicheia regina Leo, Magrini & Fancello, 2005; 36 – Typhloreicheia pellita Leo, Magrini & Fancello, 2005; 37 – Typhloreicheia rocchii Magrini & Degiovanni, 2006; 38 – Typhloreicheia holdhausi Magrini, Fancello & Casale, 2006; 39 – Typhloreicheia petriolii Magrini & Fancello, 2007; 40 – Typhloreicheia abbazzii Magrini & Fancello, 2007; 41 – Typhloreicheia leoi pilosa Magrini & Fancello, 2007; 42 – T. sebera Magrini & Fancello, 2009; 43- Typhloreicheia monacha sp. n. Casale & Marcia; 44 – Typhloreicheia ilianae sp. n. Casale & Marcia; 45 – T. sp. n. Magrini, Marcia & Casale in litteris; 46 – T. sp. n. Magrini, Marcia & Casale in litteris (original by P. Magrini, updated with unpublished data). 9 Detail of the map of geographical distribution of Typhloreicheia species in Sardinia, showing the high concentration of sympatric species in the central-eastern part of the island.
Available data, and discoveries in progress (including two further species not yet described, but indicated in the map of Figure 8), suggest several reasons to hypothesize that Reicheiina in Sardinia formed a remarkably diversified clade through the process of adaptive radiation.
The speciation events that produced these taxa should
have been originated by isolation of small populations in
micro-geographic areas, in deep soils and caves, and an exceptional
extent of adaptive colonisation and diversification into a variety of
soil and underground compartments and ecological niches induced by the
Plio-Pleistocene climatic changes, with some cases of subsequent
overlap of distributions in wet, forested phases (
The hypothesis here proposed, that Typhloreicheia (in the current sense) radiated in Sardinia, and then re-colonized the close, circum-Mediterranean islands and mainland, remains of course a mere hypothesis, that should be tested. Unfortunately, as recalled in Introduction, the study of this group is difficult owing both to the small sizes of its representatives, and particularly the scarcity of material, some taxa being known from a few or only one specimens, so that molecular data on them – like fossils, or larval stages - are so far fully absent. Therefore, future investigations will need further, strong efforts both in the field and laboratory.
We are particularly indebted to our good friend Paolo Magrini (Florence), for providing some of the illustrations to this paper, and for data, discussion, and pleasant days in the field in Sardinia. For the loan of material, the assistance both in the field and in laboratory, and information on which the present contribution is based, we thank all those who helped us in several years of study of the subterranean Sardinian fauna: in particular, concerning the present contribution, Giuseppe Grafitti, Enrico Lana, Roberto Loru, Alessandro Molinu, Carlo Onnis, Laura Sanna and Fabio Stoch. A great acknowledgement is due to Vasily Grebennikov (Ottawa), for suggestions, corrections and improvements to the original manuscript. We thank also Stefano Birindelli for identification of terrestrial snails.
Research was in part supported by grants INTERREG 3 (Sardinia-Corsica-Tuscany), PRIN projects of the Italian Ministry of the University and Scientific Research (“Zoogeography of Mediterranean - Southern African disjoint distributions by a multimethod approach” and “The endemism in Italy”). P. Marcia has received support from Regione Sardegna through a grant co-financed with funding from the PO Sardegna FSE 2007–201 L.R.7/2007 “Promozione della ricerca scientifica e dell’innovazione tecnologica in Sardegna”.