(C) 2011 Caio Antunes-Carvalho. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Two new Neotropical species of Ceracis Mellié are described: Ceracis cassumbensis Antunes-Carvalho & Lopes-Andrade, sp. n. from a single locality in northeastern Brazil and Ceracis navarretei Antunes-Carvalho & Lopes-Andrade, sp. n. from a single locality in southern Mexico. Scanning Electron Microscope images of adults and photographs of holotypes and male terminalia are provided for both species, their similarities and differences with other Ceracis are briefly discussed, and the cucullatus species-group is redefined for including the new species described herein.
Ciid, minute tree-fungus beetle, Ciinae, Brazil, Mexico
Ceracis Mellié (Coleoptera: Ciidae: Ciinae) encompasses 47 described species, being the second most speciose genus of the family. The genus was redefined by
Ceracis cucullatus (Mellié), which names the cucullatus group, has drawn the attention of ciidologists due to its broad and disjunct geographic distribution. It is widespread in the Neotropical region, also occurring in several localities of the Afrotropical and Afrotemperate regions (sensu
While conducting a survey on the morphology, life cycle and geographic distribution of Ceracis cucullatus, mainly to evaluate the conspecificity of disjunct populations under this name, we found two morphologically related new species. Here we describe Ceracis cassumbensis sp. n., a rare record of Ciidae in a Brazilian estuarine system, and Ceracis navarretei sp. n. from southern Mexico. We include them in the cucullatus species-group, which is redefined.
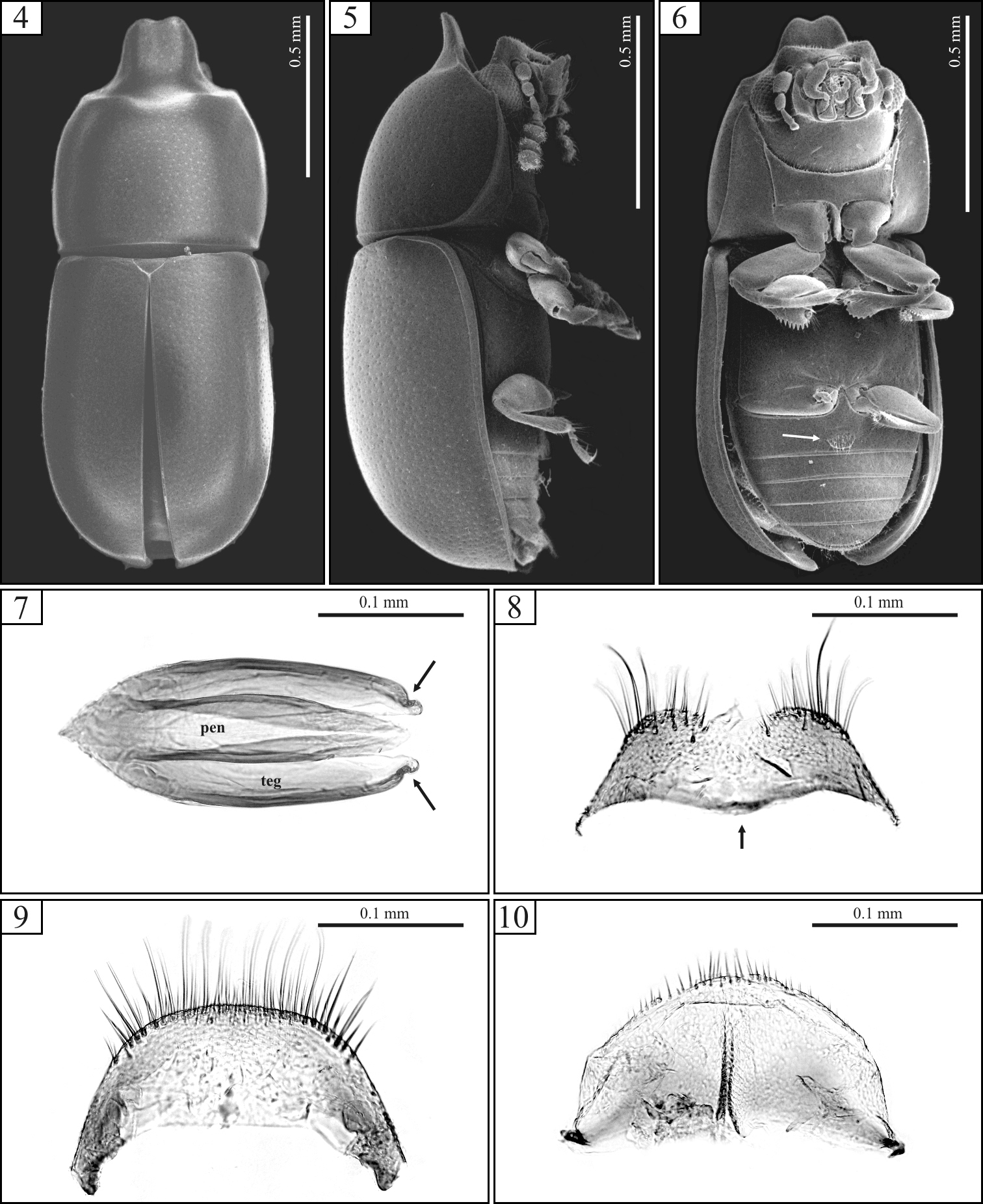
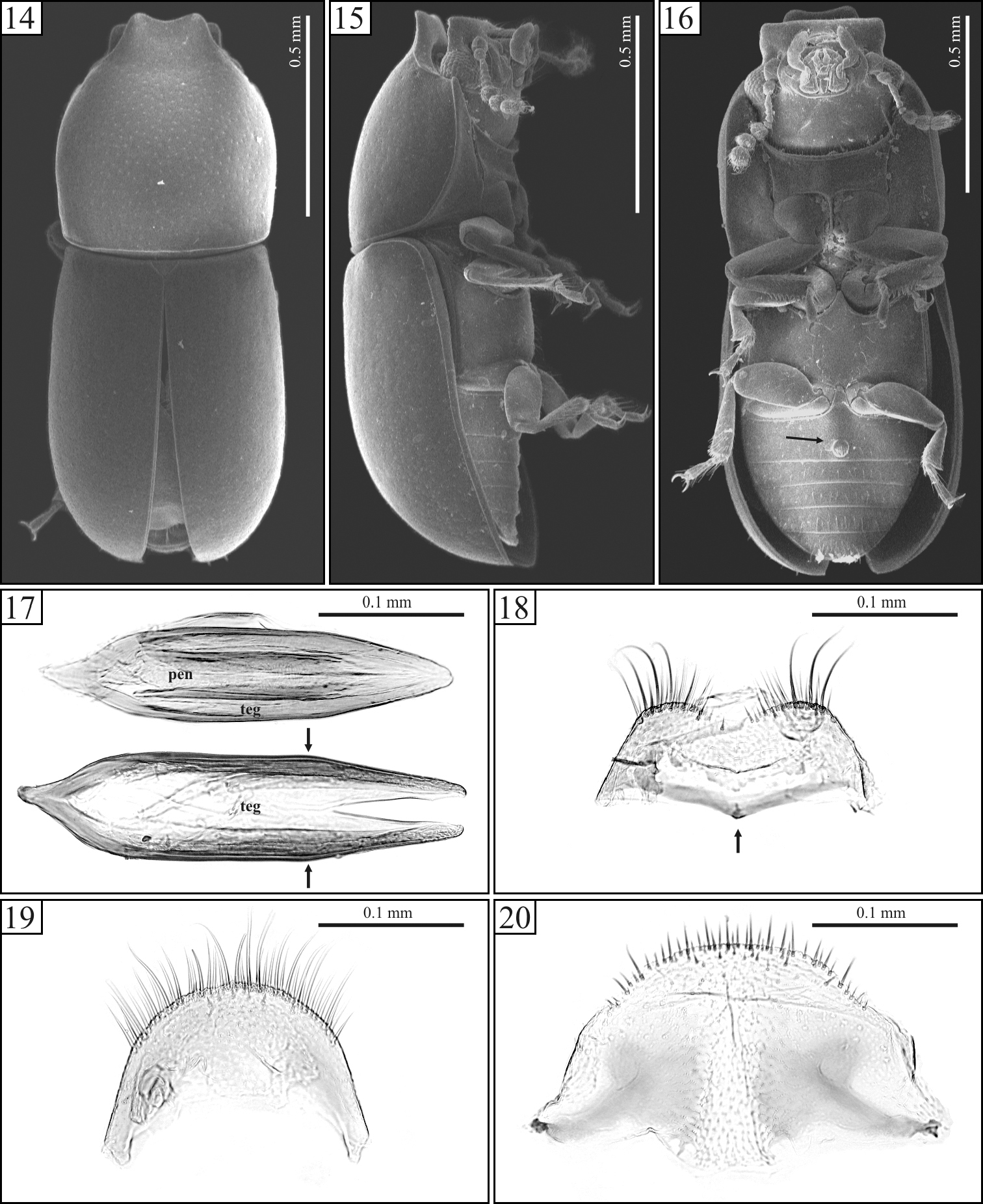
Material and methodsHolotypes were neither dissected nor examined under Scanning Electron Microscope (SEM). SEM images of whole specimens (Figs 4-6, 14-16) and photographs of dissected sclerites of male terminalia (Figs 7-10, 17-20) are from topotypes (specimens collected in the type locality but not labeled as paratypes; sensu
Examination of specimens, measurements and descriptions were made under a Zeiss Stemi 2000 stereomicroscope with a scale ocular. Holotypes were photographed with a Canon EOS 1000D digital camera attached to the same stereomicroscope. Digital photographs taken from different focus were processed and enhanced in the image stacking freeware CombineZP (
The following abbreviations are used for measurements and ratios: CL, length of the antennal club; EL, elytral length (taken from the base of scutellum to the elytral apex); EW, greatest elytral width; FL, length of the antennal funicle; GD, greatest depth of the body (taken from the elytra to the metaventrite); PL, pronotal length along midline; PW, greatest pronotal width; TL, total length (EL+PL; head not included). Range, mean and standard deviation are given for the abovementioned measurements and the following ratios: EL/EW; EL/PL; GD/EW; PL/PW; TL/EW. The ratio GD/EW was adopted as an indication of degree of convexity, and TL/EW indicates degree of body elongation. These measurements and ratios were taken from the whole type series. Measurements of antennomeres, eyes, scutellum and abdominal ventrites were taken only from holotypes. Morphological variations between specimens of the type series (males and females) are given in the section on “Variation”, together with measurements and ratios (accompanied by mean + standard deviation). Specimens selected as holotypes are fully pigmented males.
We compared specimens of Ceracis cassumbensis sp. n.and Ceracis navarretei sp. n. withnamed specimens of Ceracis cucullatus from Brazil, Galapagos and several localities from Africa. Dissected terminalia of males from these localities were also carefully compared. The terminology adopted for external morphology and male terminalia's sclerites are explained by
The following acronyms are used in this paper:
ANIC Australian National Insect Collection, CSIRO Ecosystem Sciences (Canberra, Australia)
CZUG Colección Entomológica del Centro de Estudios en Zoología, Universidad de Guadalajara (Zapopan, Jalisco, Mexico)
LAPC Cristiano Lopes-Andrade Private Collection (Viçosa, MG, Brazil)
Descriptionsurn:lsid:zoobank.org:act:26A7C976-D6E8-44C2-AC4A-A795421CAE24
http://species-id.net/wiki/Ceracis_cassumbensis
Figs 1–10“Ilha da Cassumba” (Cassumba island) in Caravelas, southern portion of the state of Bahia, northeastern Brazil (17°46'S, 39°17'W).
The specific epithet refers to the terra typica of the species.
Each antenna with eight antennomeres. Pronotum with relatively fine punctation; its anterior edge projected for- and upward forming a raised plate, slightly concave, with a short emargination at apex. Elytral punctation relatively dense. First abdominal ventrite with a broad transversely oval, setose sex patch (Fig. 6, arrow). Tegmen with lateral edges bearing a small excavation near apex (Fig. 7, arrows).
Male holotype (Figs 1-3), measurements in mm: TL 1.56; PL 0.60; PW 0.64; EL 0.96; EW 0.64; GD 0.56. Ratios: PL/PW 0.94; EL/EW 1.50; EL/PL 1.60; GD/EW 0.88; TL/EW 2.44. Body elongate, robust; dorsal and ventral surfaces dark brown, almost black; basal antennomeres and funicle, mouthparts and legs mostly yellowish brown; antennal club blackish and terminal palpomere of the maxillary palp yellowish black. Head barely visible from above; dorsal surface subglabrous, sparsely punctate, bearing a transverse impression at disc, preceded by a weak protuberance (seen in the dissected topotype); frontoclypeal ridge produced forward, transversely concave, with anterior margin emarginate at middle forming two subtriangular plates visible from below (Fig. 6), the anterior edge with a row of setae along it. Each eye with a widest diameter of 0.14 mm; some short slender yellowish setae emerging from the intersection between ommatidia. Each antenna with eight antennomeres (FL 0.09, CL 0.17, CL/FL 1.89); length of antennomeres (in mm) as follows (from base to apex): 0.07, 0.05, 0.05, 0.03, 0.02, 0.05, 0.05, 0.07; each antennomere of the club bearing several sparse slender setae, and four conspicuous sensillifers symmetrically positioned at its upper portion. Pronotum with sides reasonably rounded, widest at middle; lateral margins narrow, not visible from above, except for the most posterior corners; anterior edge projected for- and upward, forming a curved raised plate, slightly concave, with a short emargination at apex (Figs 1, 4); disc impressed in the area surrounding pronotal projection; anterolateral angles inconspicuously produced, relatively obtuse; punctation relatively fine, single, uniformly distributed, the posterior half of the median longitudinal surface devoid of punctures; distance between punctures from 1.75 to 2.25 puncture-widths, being greater at the anterior half of pronotum (including pronotal projection); each puncture bearing a fine yellowish decumbent minute seta; in between punctures shiny, microreticulate. Scutellum small, triangular, with few punctures, each one bearing a short, fine, decumbent bristle; basal width 0.11mm and length along the longitudinal midline 0.05 mm. Hind wings developed. Elytra with sides subparallel at the basal two-thirds, then abruptly converging toward apex; punctation single, confused, denser than pronotal punctation; punctures irregular, but ever finer than those on pronotum; vestiture similar to that of pronotum, but in between punctures smooth and shiny. Ventral sclerites microreticulate. Prosternum in front of coxae shallowly concave longitudinally, and a bit transversely convex; surface beside coxae weakly concave; prosternal process laminate, reasonably elevated, almost as long as coxae. Metaventrite moderately convex, bearing sparse slender setae; punctation shallow, consisting mostly of few punctures close to the lateral edges; median suture (discrimen) obscurely indicated posteriorly (see section on “variation”). Abdominal ventrites bearing sparse slender decumbent yellowish setae, longer than those on the dorsal surface; punctation shallow and sparse; lengths of abdominal ventrites (from base to apex, at the longitudinal midline) as follows (in mm): 0.19; 0.08; 0.08; 0.06; 0.06; length of abdominal ventrites together 0.46 mm; abdominal width (basal width of the first abdominal ventrite) 0.63 mm; first abdominal ventrite bearing a broadly transverse margined setose sex patch (Fig. 6, arrow), located postered of center, with a transverse diameter of 0.06 mm. Apex of each protibia expanded; outer apical angle rounded and bearing a row of spines.
Male terminalia. (Figs 7–10) Ninth segment (=genital ring) V-shaped. Fused ninth and tenth tergites (Fig. 10) with posterior margin rounded and bearing small suberect bristles at middle; sides slightly diverging, almost subparallel. Eighth sternite (Fig. 8) with posterior margin shallowly emarginate at middle; posterior angles rounded and bearing some bristles; lateral margins diverging; anterior margin biconcave, rounded and slightly sclerotized at middle but not forming a strut (Fig. 8, arrow). Eighth tergite (Fig. 9) with posterior margin almost straight, bearing long and short bristles along it; lateral margins diverging; anterior margin concave. Aedeagus (Fig. 7) around twice as long as wide; basal piece not observed, possibly membranous. Tegmen slightly longer than and twice as wide as penis; posterior portion subtriangular, then subparallel sided at most of its length, lateral edges slightly curved inward to apex; both sides bearing a small excavation near apex (Fig. 7, arrows). Penis elongate, subcylindrical; sides subparallel at the basal three-fourths, with apical one-fourth subtriangular and weakly sclerotized.
Females. Differing from males in the following features: frontoclypeal ridge rounded, not produced. Lateral margins of pronotum rounded; anterior margin rounded, not produced, bearing small yellowish setae along it; pronotal and elytral punctation slightly finer than in males. Abdominal sex patch absent.
Habitus of Ceracis cassumbensis Antunes-Carvalho & Lopes-Andrade, sp. n., holotype. 1 Dorsal view 2 Lateral view 3 Ventral view.
Ceracis cassumbensis Antunes-Carvalho & Lopes-Andrade, sp. n., SEM of male topotypes (4–6) and slide preparations of male terminalia of a topotype (7–10). 4 Dorsal view 5 Lateral view 6 Ventral view, showing the transversely oval sex patch at the first abdominal ventrite (arrow) 7 Aedeagus showing penis (pen) and tegmen (teg). Note the conspicuous excavation in either side of tegmen (arrows) 8 Eighth sternite with anterior margin rounded at middle (arrow) 9 Eighth tergite 10 Fused ninth and tenth tergites.
Males, measurements in mm (n=21, including holotype): TL 1.12–1.80 (1.46 + 0.18); PL 0.44–0.84 (0.66 + 0.11); PW 0.48–0.76 (0.63 + 0.07); EL 0.68–0.96 (0.80 + 0.07); EW 0.52–0.76 (0.64 + 0.07); GD 0.44–0.68 (0.55 + 0.06). Ratios: PL/PW 0.92–1.19 (1.04 + 0.07); EL/EW 1.12–1.33 (1.25 + 0.06); EL/PL 1–1.55 (1.23 + 0.14); GD/EW 0.76–0.92 (0.86 + 0.04); TL/EW 2.15–2.50 (2.28 + 0.09). Body varying from dark reddish brown to dark brown (almost black). Frontoclypeal ridge and apex of pronotum weakly developed in the smallest males and strongly projected in the largest ones. Discrimen indiscernible to barely discernible in most individuals.
Females, measurements in mm (n=10): TL 1.32–1.56 (1.45 + 0.09); PL 0.56–0.68 (0.62 + 0.05); PW 0.56–0.68 (0.61 + 0.05); EL 0.76–0.92 (0.84 + 0.05); EW 0.6–0.72 (0.66 + 0.04); GD 0.52–0.6 (0.56 + 0.04). Ratios: PL/PW 1–1.07 (1.01 + 0.02); EL/EW 1.17–1.44 (1.28 + 0.09); EL/PL 1.24–1.44 (1.36 + 0.08); GD/EW 0.81–0.94 (0.85 + 0.05); TL/EW 2.06–2.44 (2.22 + 0.12).
Male holotype (LAPC) “BRASIL: BA Caravelas; Ilha da Cassumba 30.ii.2006 leg. K.S. Furieri, F.C.C. Barreto, E.S. Rediguieri” “Ceracis cassumbensis Antunes-Carvalho & Lopes-Andrade HOLOTYPUS” [printed on red paper]. Paratypes: 20 males, 10 females (LAPC), same data as holotype. All paratypes distinguished labeled “Ceracis cassumbensis Antunes-Carvalho & Lopes-Andrade PARATYPUS” [printed on yellow paper].
Cassumba is a continental island at the Caravelas-Peruípe estuarine system, with around 120Km2. It is located at the northern portion of the Atlantic Forest and encompasses forest remnants and large mangrove areas mixed in a landscape apparently well preserved. It is the first record of Ciidae from the island and a rare record of the family from a Brazilian estuarine system. However, we do not know either the host-fungus of this single collection of Ceracis cassumbensis sp. n. or whether it was caught close to a mangrove or a forest remnant at the island.
urn:lsid:zoobank.org:act:63754F8E-972F-418F-988A-FA24504AFAA9
http://species-id.net/wiki/Ceracis_navarretei
Figs 11–20Dos Amates, southern portion of the state of Veracruz, southern Mexico (17°24'N, 94°35'W).
The specific epithet is in honor of José Luis Navarrete Heredia, who made available to us the majority of the specimens included in the type series.
Body with very fine, sparse punctation. Each antenna with nine antennomeres. Pronotum mostly black; elytra and apex of pronotum reddish brown. Pronotal apex projected for- and upward, forming a curve, raised foursquare plate, weakly emarginated at the anterior edge. Elytra with lateral margins subparallel at the basal half, then gradually converging toward the apex. Aedeagus 4× longer than wide (Fig. 17); tegmen with parallel sides at most of their lengths, lateral edges angulate at the beginning of the apical third (Fig. 17, arrows) and then converging in straight line toward the apex.
Male holotype (Figs 11-13), measurements in mm: TL 1.60; PL 0.72; PW 0.64; EL 0.88; EW 0.62; GD 0.56; TL/EW 2.58; PL/PW 1.13; EL/EW 1.42; EL/PL 1.22; GD/EW 0.90. Body subcylindrical, moderately convex; elytra and apex of pronotum reddish brown, remainder of pronotum black; ventral surface reddish brown; legs, mouthparts, basal antennomeres and funicle yellowish brown; antennal club dark brown. Head barely visible from above; dorsal surface flattened, subglabrous, bearing minute, sparsely decumbent fine setae, almost indiscernible; punctation sparse, consisting of shallow coarse punctures; frontoclypeal ridge produced forward, transversely concave, with its anterior margin slightly emarginate at middle, the anterior edge with a row of setae along it. Each eye with a widest diameter of 0.13 mm; some short slender yellowish setae emerging from the intersection between ommatidia. Each antenna with nine antennomeres (FL 0.09, CL 0.15, CL/FL 1.67); length of the antennomeres (in mm) as follows (from base to apex): 0.06, 0.04, 0.04, 0.02, 0.02, 0.02, 0.04, 0.04, 0.06; each antennomere of the club bearing several sparse slender setae, and four conspicuous sensillifers symmetrically positioned at its upper portion. Pronotum with subparallel sides, widest at middle; lateral margins narrow, being a bit thicker at the anterior portion; only the anterior and posterior corners can be seen from above, but the latter is weakly visible; anterior edge projected for- and upward, forming a curve, raised foursquare plate, slightly emarginated at apex (Figs 11, 14); raised plate transversely concave; anterolateral angles slightly produced, moderately obtuse; punctation fine, single, relatively uniform; distance between punctures from 2.5 to 3 puncture-widths; vestiture of yellowish decumbent seta; in between punctures shiny, microreticulate. Scutellum small, triangular, glabrous, with few fine punctures; basal width 0.11mm; length along the longitudinal midline 0.05 mm. Hind wings developed. Elytra with sides subparallel at basal half, then gradually converging to apex; only the most anterior corners visible from above; punctation single, confused, finer and denser than that of pronotum; vestiture consisting of minute, fine, decumbent yellowish setae; in between punctures smooth and shiny. Ventral sclerites microreticulate. Prosternum in front of coxae shallowly concave longitudinally and transversely convex; surface beside coxae weakly concave; prosternal process laminate, almost as long as coxae. Metaventrite moderately convex, bearing sparse slender setae; punctation shallow and sparse, almost indiscernible; discrimen indiscernible. Abdominal ventrites bearing sparse slender decumbent yellowish setae, longer than those on the dorsal surface; punctation shallow and sparse; lengths of abdominal ventrites (from base to apex, at the longitudinal midline) as follows (in mm): 0.19; 0.07; 0.07; 0.07; 0.08; abdominal length 0.50 mm, abdominal width (basal width of the first abdominal ventrite) 0.55 mm; first abdominal ventrite bearing a circular margined sex patch (Fig. 16, arrow), located postered of center, with a transverse diameter of 0.04 mm. Apex of each protibia expanded; outer apical angle rounded and bearing a row of spines.
Male terminalia. (Figs 17–20) Ninth segment (=genital ring) V-shaped. Fused ninth and tenth tergites (Fig. 20) with posterior margin reasonably straight, with small suberect bristles along it; sides diverging, each bearing a small protuberance at middle. Eighth sternite (Fig. 18) with posterior margin weakly emarginate at middle; posterior angles rounded and bearing some bristles; lateral margins diverging; anterior margin biconcave, sclerotized and forming a short median strut (Fig. 18, arrow). Eighth tergite (Fig. 19) with posterior margin rounded, bearing long and medium size bristles along it; lateral margins diverging; anterior margin concave. Aedeagus (Fig. 17) 4× longer than wide; basal piece not observed, possibly membranous. Tegmen slightly longer than and twice as wide as penis; posterior portion subtriangular and then parallel sided at most of its length, either side angulate at the beginning of the apical third (Fig. 17, arrows) and converging in straight line toward apex. Penis elongate, subcylindrical; sides subparallel at the basal three-fourths, with apical one-fourth subtriangular and weakly sclerotized.
Females. Differing from males in the following features: frontoclypeal ridge rounded, not produced. Head with dorsal surface usually convex. Lateral margins of pronotum rounded; anterior margin rounded, not produced; pronotal and elytral punctation slightly finer than in males. Abdominal sex patch absent.
Habitus of Ceracis navarretei Antunes-Carvalho & Lopes-Andrade, sp. n., holotype. 11 Dorsal view 12 Lateral view 13 Ventral view.
Ceracis navarretei Antunes-Carvalho & Lopes-Andrade, sp. n., SEM of male topotypes (14–16) and slide preparations of male terminalia of topotypes (17–20). 14 Dorsal view 15 Lateral view 16 Ventral view, showing the circular margined sex patch at the first abdominal ventrite (arrow) 17 Above, aedeagus showing penis (pen) and tegmen (teg). Below, a tegmen alone. Arrows indicate the angulation point from which the sides of tegmen converge in straight line toward the apex 18 Eighth sternite, showing the anterior margin weakly produced at middle (arrow) 19 Eighth tergite 20 Fused ninth and tenth tergites.
Males, measurements in mm (n=22, including holotype): TL 1.22–1.74 (1.53 + 0.13); PL 0.46–0.78 (0.67 + 0.08); PW 0.50–0.68 (0.61 + 0.05); EL 0.76–0.96 (0.86 + 0.05); EW 0.50–0.68 (0.60 + 0.05); GD 0.40–0.60 (0.53 + 0.04). Ratios: PL/PW 0.92–1.27 (1.09 + 0.08); EL/EW 1.38–1.57 (1.44 + 0.05); EL/PL 1.14–1.65 (1.31 + 0.12); GD/EW 0.80–0.94 (0.88 + 0.04); TL/EW 2.43–2.73 (2.56 + 0.09). Color of pronotum varying from black to reddish brown, usually reddish; elytra dark reddish to reddish brown. Anterior edge of pronotum weakly developed in the smallest males and strongly projected in the largest ones. In some cases the anterior and posterior corners of the lateral margins of pronotum are not visible from above. Surface of pronotum weakly to distinctly microreticulate. Eighth sternite with anterior margin completely rounded to weakly produced at middle.
Females, measurements in mm (n=18): TL 1.16–1.50 (1.35 + 0.09); PL 0.44–0.58 (0.51 + 0.04); PW 0.44–0.60 (0.54 + 0.04); EL 0.72–0.92 (0.84 + 0.05); EW 0.46–0.62 (0.55 + 0.04); GD 0.42 + 0.54 (0.49 + 0.04). Ratios: PL/PW 0.88–1.00 (0.95 + 0.04); EL/EW 1.43–1.59 (1.51 + 0.05); EL/PL 1.54–1.79 (1.64 + 0.07); GD/EW 0.81–0.96 (0.89 + 0.05); TL/EW 2.31–2.57 (2.43 + 0.08).
Male holotype (CZUG) “MEXICO: Veracruz Dos Amates 03.vi.1988 S.L. Álavez leg.” “Ceracis navarretei Antunes-Carvalho & Lopes-Andrade HOLOTYPUS” [printed on red paper]. Paratypes: 19 males, 16 females (11 males and 12 females at CZUG, 8 males and 4 females at LAPC), same data as holotype; 2 females and 2 males (2 females and 1 male at ANIC, 1 male at LAPC) “MEXICO: Veracruz Dos Amates 28/2/1987 polypore 0114 J. Navarrete”. All paratypes distinguished labeled “Ceracis navarretei Antunes-Carvalho & Lopes-Andrade PARATYPUS” [printed on yellow paper].
Dos Amates is surrounded by small villages, being a mosaic of forest remnants and deforested areas apparently far from major urban areas. We have no information on the host-fungus of this new species. We only know that a few specimens were collected in a polypore (see “Type series” above).
Organizing morphologically similar species of Ciidae into species-groups has been an useful taxonomic tool, especially in speciose genera as Ceracis, Cis Latreille and Scolytocis Blair (
Among the species in the cucullatus group, as proposed here, Ceracis navarretei sp. n. is possibly the most similar to Ceracis cucullatus, mainly to its African populations. Differences are notable especially on male terminalia: The tegmen of Ceracis navarretei sp. n. has the lateral edges parallel at most of their lengths, the apical third converging in straight line toward the apex.In named specimens of Ceracis cucullatus examined by us, the sides of tegmen are either subparallel or weakly curved. Moreover, the aedeagus in Ceracis navarretei sp. n.is 4× longer than wide, while in Ceracis cucullatus it is around 3×. Ceracis cassumbensis sp. n. may be distinguished from Ceracis cucullatus by its greater depth of the body (most evident when comparing females), antennae with eight antennomeres, elytral punctation denser and abdominal sex patch larger and transversely oval (Fig. 6, arrow). Moreover, either lateral edge of the tegmen in Ceracis cassumbensis sp. n. has a peculiar excavation near apex (Fig. 7, arrows). This characteristic is also observed in Ceracis similis Horn, although this species is distinguishable from Ceracis cassumbensis sp. n. by its reddish body, punctation comparatively coarser and denser and relatively wider pronotal lamina.
The morphological limits of both Ceracis cucullatus and Ceracis bicornis were not satisfactorily established. The former is one of the most widely distributed ciid species in the tropics and the latter is widespread in the Neotropical region, having been reported in Mexico, Guatemala, Costa Rica, Peru (
We thank José Luis Navarrete Heredia (Universidad de Guadalajara, Mexico) and John F. Lawrence (CSIRO Ecosystem Sciences, Australia) for the loan of all specimens of Ceracis navarretei, and Karina Schmidt Furieri for collecting and donating to us the known specimens of Ceracis cassumbensis. Electron microscopy facilities were provided by Núcleo de Microscopia e Microanálise (NMM) of Universidade Federal de Viçosa (UFV). Photographs of male terminalia were made at Laboratório de Acarologia (Departamento de Entomologia, UFV). Financial supports were provided by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG: PPP 21/2008, APQ-00049-09; PPM 03/2010, PPM-00017-10), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq: PROTAX 52/2010 nº 562229/2010-8; Grant nº131453/2010-6 to the senior author) and the Graduate Program in Entomology of UFV.