(C) 2012 Ryouichi Higashi. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Two new species of the interstitial ostracod genus Parvocythere, Parvocythere gottwaldi sp. n. and Parvocythere gracilis sp. n., are herein described. Although these two new species are clearly distinguishable by certain morphological differences in elements of the male copulatory organ, and the carapace, they share the following simplified characters of the appendages and male copulatory organ: antennular fourth podomere with no suture; reduced claws on the distal end of antenna; and asymmetric male copulatory organ. The morphological differences among known and new Parvocythere species suggest that the species of this genus can be classified into two groups by the presence/absence of the suture on the antennular fourth podomere. The “Group S” is characterised by the presence of the antennular suture, and all species of this group have a two-clawed antenna and symmetric male copulatory organ, characters which are generally seen in cytheroid ostracods. The species belonging to “Group N” are characterised by the absence of the suture, regarded as a pedomorphic character, show the following characters: two clawed or one clawed antenna, and symmetric or asymmetric male copulatory organ. The morphological variation within Group N includes reductive characters regarded as an adaptation to the narrow spaces of the interstitial environment of a sandy beach. These intrageneric morphological variations of the exclusively interstitial genus Parvocythere suggest the possibilities that Group N might be derived from Group S, and that some adaptive characters to an interstitial environment could have developed after the colonisation of these environments.
Interstitial animal, Podocopa, taxonomy, antennular suture
The genus Parvocythere Hartmann, 1959 includes 14 species (type species: Parvocythere dentata Hartmann, 1959), all of which are known as interstitial dwellers, inhabiting sedimentary interstices. This genus is characterised by a small body size, only two pairs of walking legs (
Parvocythere japonica Watanabe, Tsukagoshi & Higashi, 2008 was so far known as the single species belonging to this genus in Japan. In the present paper, two new Parvocythere species from central Japan are described, and the evolutionary trend of the genus Parvocythere is discussed on the basis of the interspecific morphological variations among the Parvocythere species.
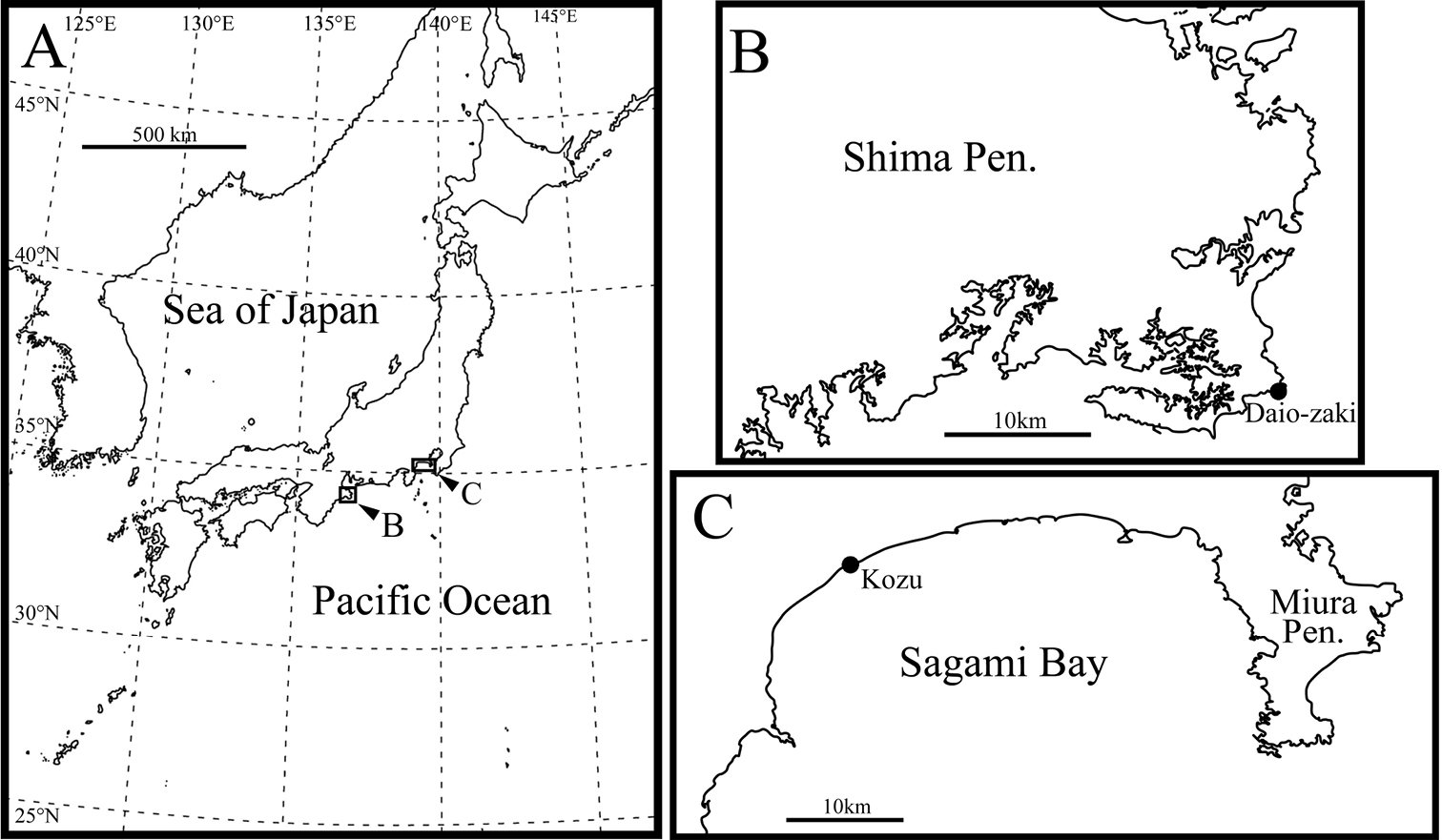
Materials and methodsSediment samples were collected from littoral beaches at two sites in central Japan (Fig. 1): Daio-zaki, Shima City, Mie Prefecture (34°16.59'N, 136°53.83'E), and Kozu, Odawara City, Kanagawa Prefecture (35°16.73'N, 139°12.75'E). The sediments were taken with a small scoop from the bottom of a hole dug with a shovel to depth of water table. The samples were washed five times in a bucket of fresh water in the laboratory, and the supernatant was then strained through a 25 µm mesh sieve. The specimens of Parvocythere were then picked out under a binocular microscope (SHZ-10, OLYMPUS), from the concentrate retained on the sieve.
Sampling localities. A map of Japan B and C sampling sites.
The specimens were dissected under a binocular microscope, chitinous parts mounted on glass slides and the valves air-dried. The chitinous parts and the valves were observed and drawn using a differential interference contrast microscope with a camera lucida (BX 50, Olympus). The valves were osmium-coated with an osmium plasma coater (OPC 40, Nippon Laser), and were then observed under a SEM (JSM-5600LV, JEOL).
The type series is deposited in the collection of the Shizuoka University Museum, identified by a number with the prefix SUM-CO.
Taxonomy Order Podocopida Sars, 1866Superfamily Cytheroidea Baird, 1850
Family Parvocytheridae Hartmann, 1959
Genus Parvocythere Hartmann, 1959
urn:lsid:zoobank.org:act:DA15A671-D4D4-427C-A92D-3AC27CE729D7
http://species-id.net/wiki/Parvocythere_gottwaldi
Figs 2–6Holotype: adult male (SUM-CO-2023), right valve length 173 µm, height 88 µm, left valve length 174 µm, height 84 µm, appendages mounted on slide and valves preserved in a cardboard cell slide, Paratypes: 14 adult males (SUM-CO-2024–2037) and 12 adult females (SUM-CO-2038–2049). All illustrated specimens were collected from interstitial pore-water at the type locality on October 10, 2008.
Daio-zaki, Shima City, Mie Prefecture, Pacific coast of central Japan, 34°16.59'N, 136°53.83'E (Fig. 1B), in sediments at approximately 20 cm depth. The sediment is mainly composed of clastic very coarse sand and granules.
This species is named in honour of Dr Jochen Gottwald, in recognition of his significant contribution to our knowledge of interstitial ostracods.
Carapace rounded trapezial in lateral view with very slight protrusion on antero-ventral area and sharp wrinkle-like groove running from middle to posterior in ventral area of both valves. Tapering anterior margin. Forty-two pore systems per valve. Seven and four marginal pores along anterior and posterior margins, respectively. Marginal infold broad in anterior but very narrow in posterior. Hingement modified pentodont type. No suture on middle of fourth podomere of antennula. Antenna with only one distal claw. Male copulatory organ asymmetric, with left organ (hemipenis) larger than right one. Left hemipenis bearing long copulatory duct single coiled in distal part, short and stout claw-like dorsal ramus, two-pronged ventral ramus, and well-developed crescent-shaped distal lobe with thick seta on centre of proximal part. Right organ reduced and bearing much reduced copulatory duct and crescent-shaped distal lobe with thick seta on proximal part.
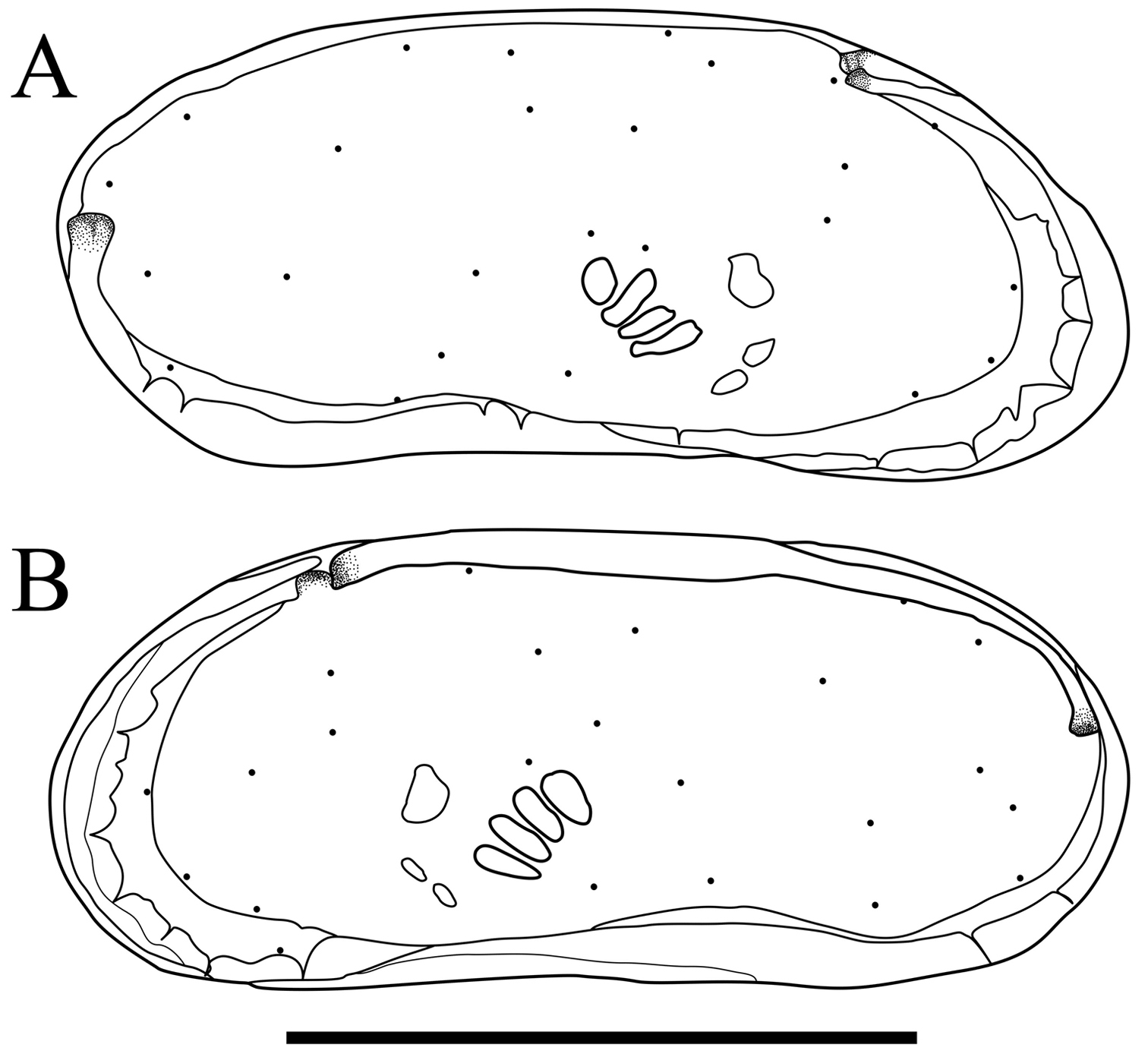
Carapace (Figs 2 and 3). Carapace rounded trapezial in lateral view with very slight protrusion on antero-ventral area and sharp wrinkle-like groove running from middle to posterior in ventral area of both valves. Anterior margin tapers more than posterior margin. Dorsal margin slightly rounded. Ventral margin almost straight. Carapace surface generally smooth. All pore systems of simple type and 42 per valve. Seven and four marginal pores along anterior and posterior margins, respectively. Marginal infold broad in anterior area but very narrow in posterior area. Vestibula occupying large part of marginal infold. Inner surface covered with numerous pits. Hingement modified pentodont type. Right valve slightly overlapping left valve along hinge line. Four adductor muscle scars in oblique row. Mandibular scar visible beneath frontal scar.
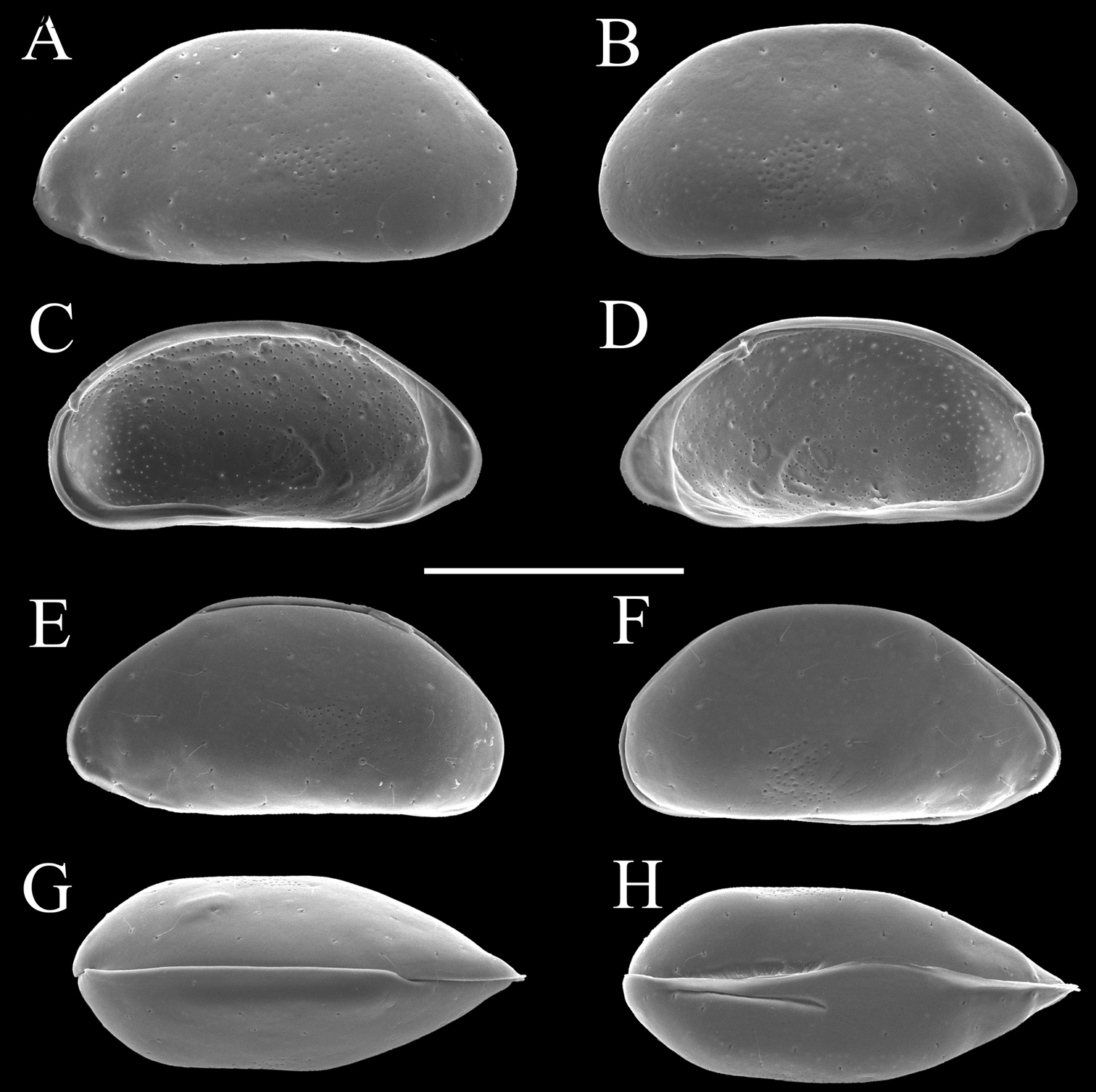
Carapaces of Parvocythere gottwaldi sp. n. Holotype (SUM-CO-2023). A right external view B left external view. Each of the carapace structures are transmitted images. Scale bar indicates 100 µm.
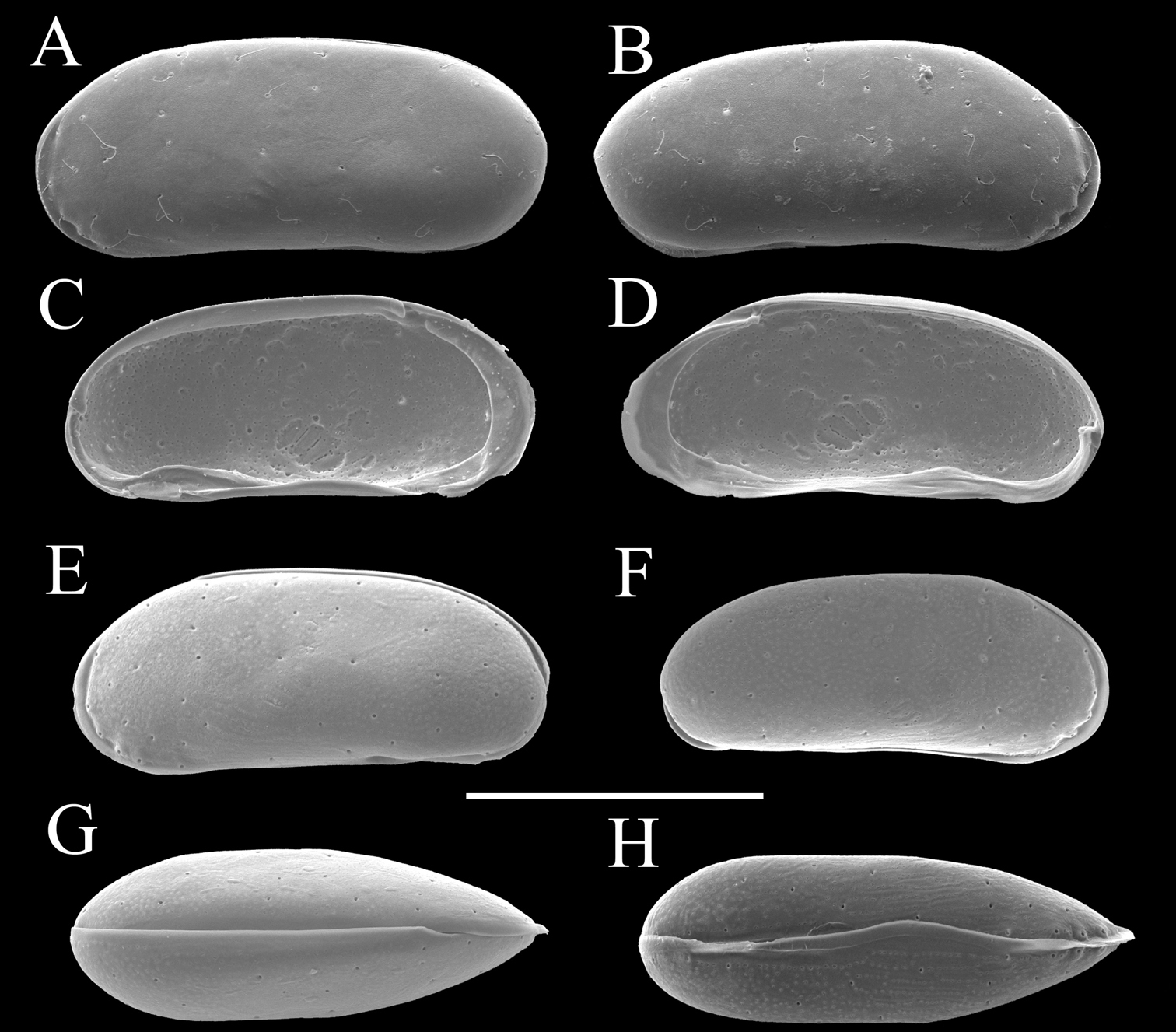
Carapaces of Parvocythere gottwaldi sp. n. A–D, G and H male specimens: A and B paratype (SUM-CO-2025) C and D paratype (SUM-CO-2026) G paratype (SUM-CO-2027) H paratype (SUM-CO-2028). A left external lateral view B right external lateral view C internal view of left valve D internal view of right valve G dorsal view H ventral view. E and F female specimens: E paratype (SUM-CO-2039) F paratype (SUM-CO-2040). E left external view F right external view. Scale bar indicates 100 μm.
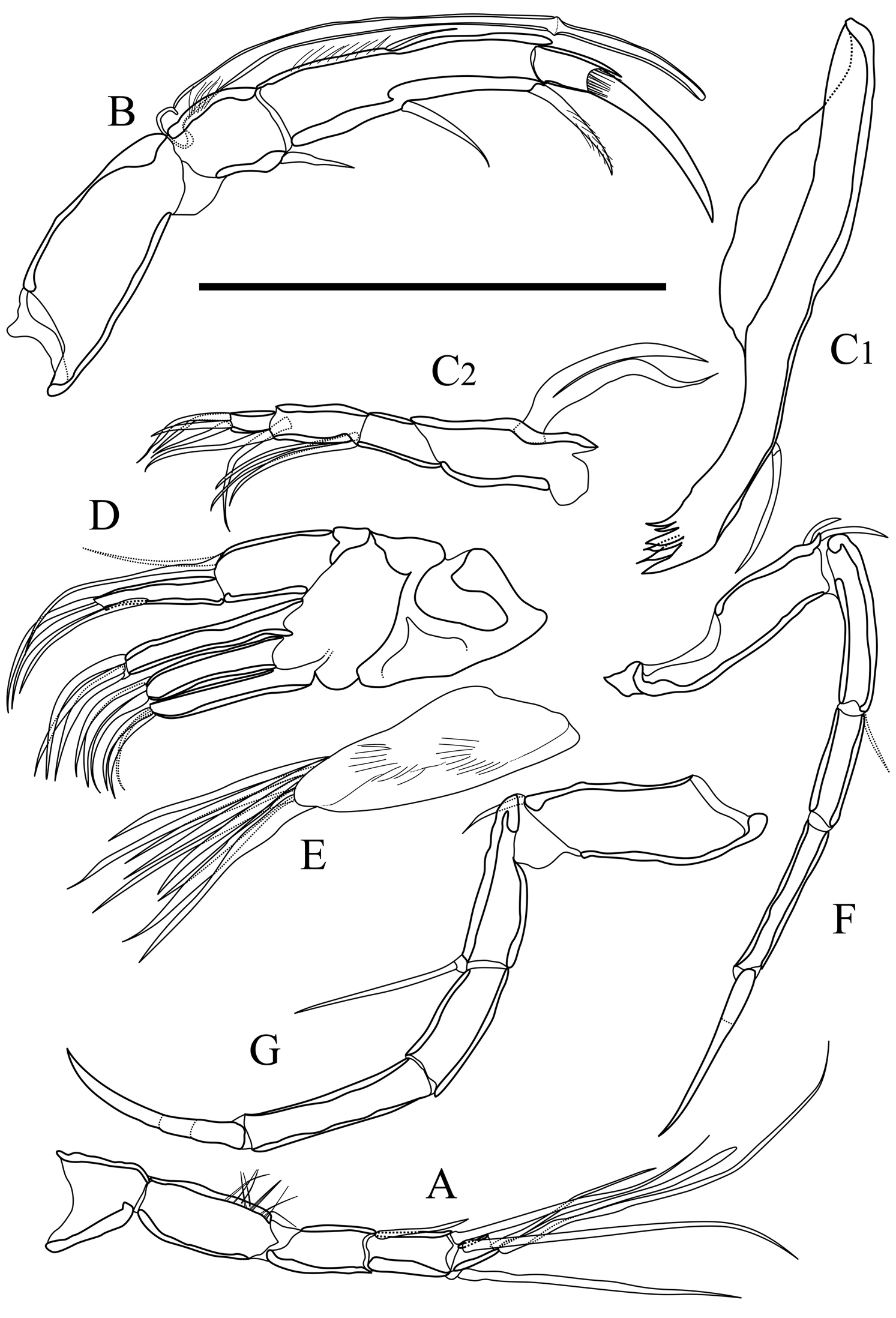
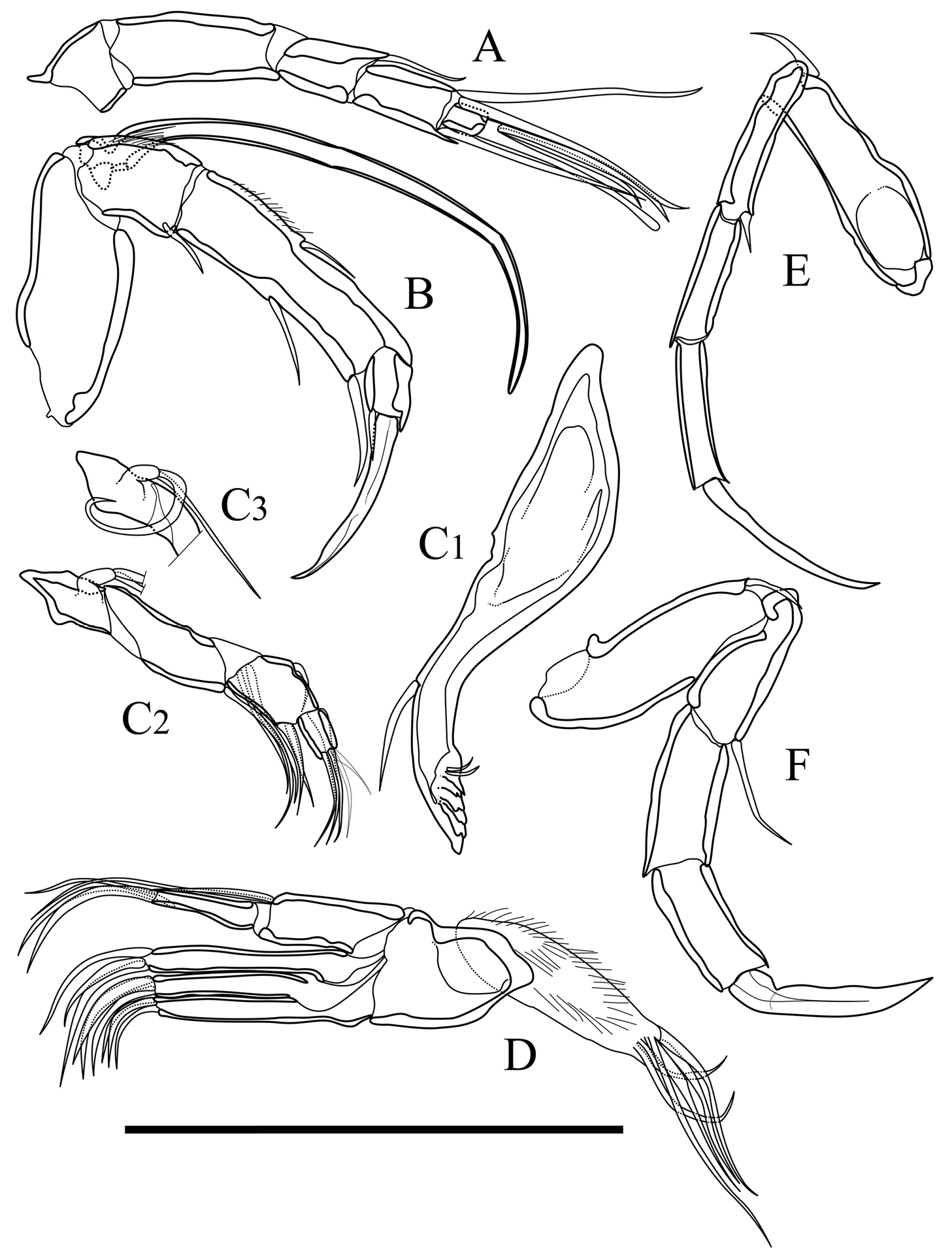
Antennula (Fig. 4A). Five articulated podomeres. First podomere bare and short. Second podomere nine quarters as long as first podomere, with fine setae along antero-distal margin. Third podomere half as long as second podomere, with one medium seta on antero-distal end. Fourth podomere as long as third podomere, with two long setae on antero-distal end and one long seta on postero-distal end. Fifth podomere half as long as fourth podomere, with one long and one very long simple setae and one spatula-like long seta on distal end.
Antenna (Fig. 4B). Four articulated podomeres. First podomere with two segmented spinneret (exopodite) on distal end. Second podomere half as long as first podomere, with bunch of fine setae along middle of anterior margin and one medium seta on postero-distal end. Third podomere eight-thirds as long as second podomere, with numerous fine setae along antero-proximal margin, medium setae on middle of anterior margin and on middle of posterior margin, respectively, and one medium setulous seta on postero-distal end. Fifth podomere quarter length of fourth podomere, with numerous fine setae on distal margin and one stout claw on distal end.
Mandibula (Fig. 4C1, C2). Coxa (Fig. 4C1) elongated, with one medium seta on antero-ventral part. Six coxal endites. Palp (Fig. 4C2) consisting of four articulated podomeres. First podomere (basis) with bifurcated lamella (exopodite) on middle of dorsal margin. Second podomere two-thirds as long as first podomere, with three medium setae on ventro-distal end. Third podomere five-fourths as long as second podomere, with one stout medium seta on outside of distal end and one medium seta on ventro-distal end. Fourth podomere two-fifths as long as third podomere, with four setae on distal end.
Maxillula (Fig. 4D and E). Thin branchial plate (exopodite; Fig. 4E) with approximately nine plumose setae. Basal podomere with one palp and three endites (Fig. 4D). Palp consisting of two articulated podomeres: first podomere with one long and one medium seta on antero-distal end; second podomere eleven-tenth as long as first podomere, with one medium seta on middle of posterior margin and two medium setae on distal end. Endites with three setae, respectively.
Fifth limb (Fig. 4F). Four articulated podomeres. First podomere with two short setae on antero-distal end. Second podomere five-sixths as long as first podomere, with one medium seta on antero-distal end. Third podomere four-fifths as long as second podomere. Fourth podomere three-halves as long as third podomere, with stout distal claw.
Sixth limb (Fig. 4G). Four articulated podomeres. First podomere with one short seta on antero-distal end. Second podomere four-fifth as long as first podomere, with one long seta on antero-distal end. Third podomere as long as second podomere. Fourth podomere seven-fifth as long as third podomere with stout distal claw.
Appendages of Parvocythere gottwaldi sp. n. A–D, F and G holotype (SUM-CO-2023) E paratype (SUM-CO-2024). A antennula B antenna C1 coxa of mandibula C2 palp of mandibula D palp and endites of maxillula E branchial plate of maxillula F fifth limb G sixth limb. Scale bar indicates 50 µm.
Seventh limb. Absent.
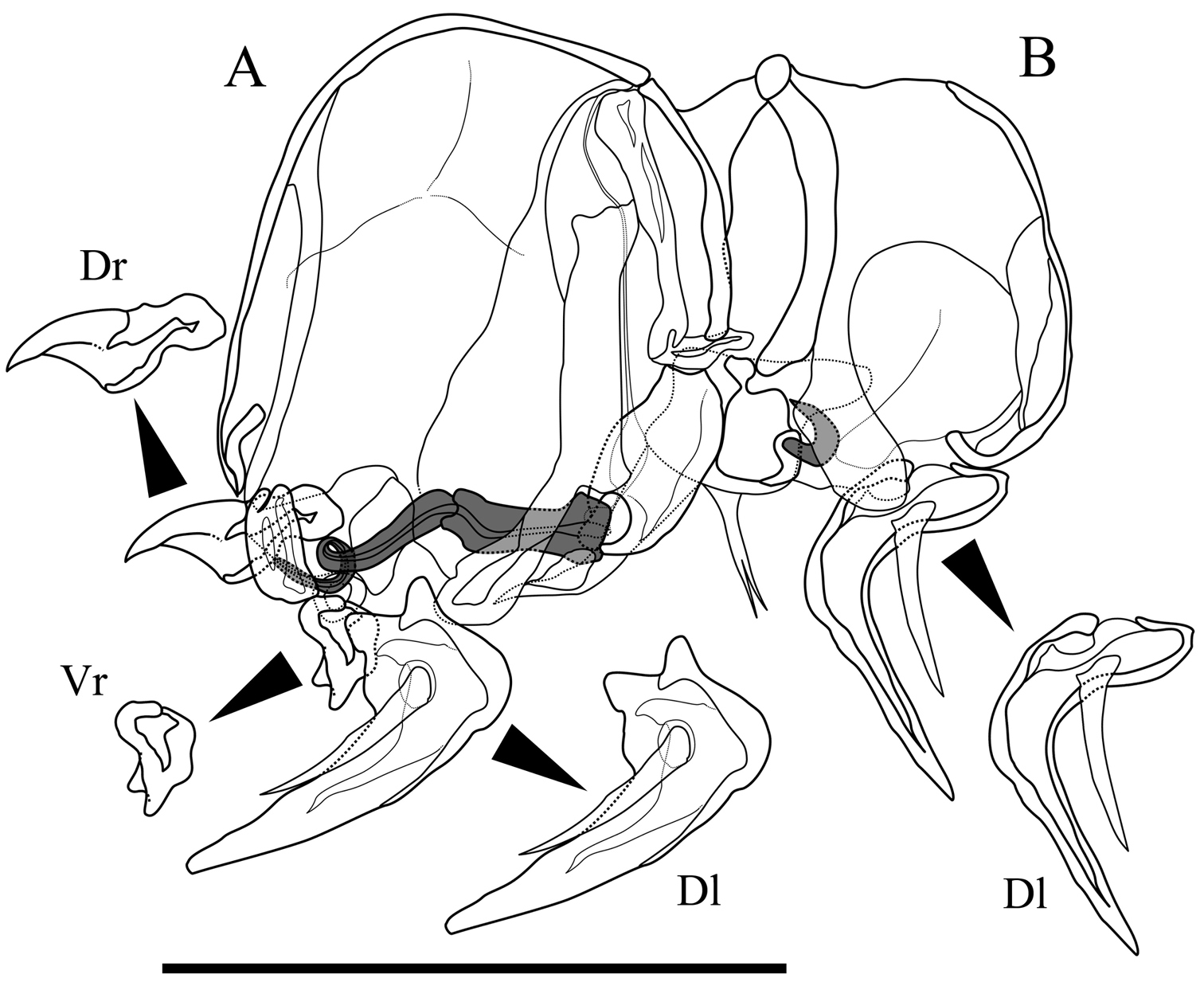
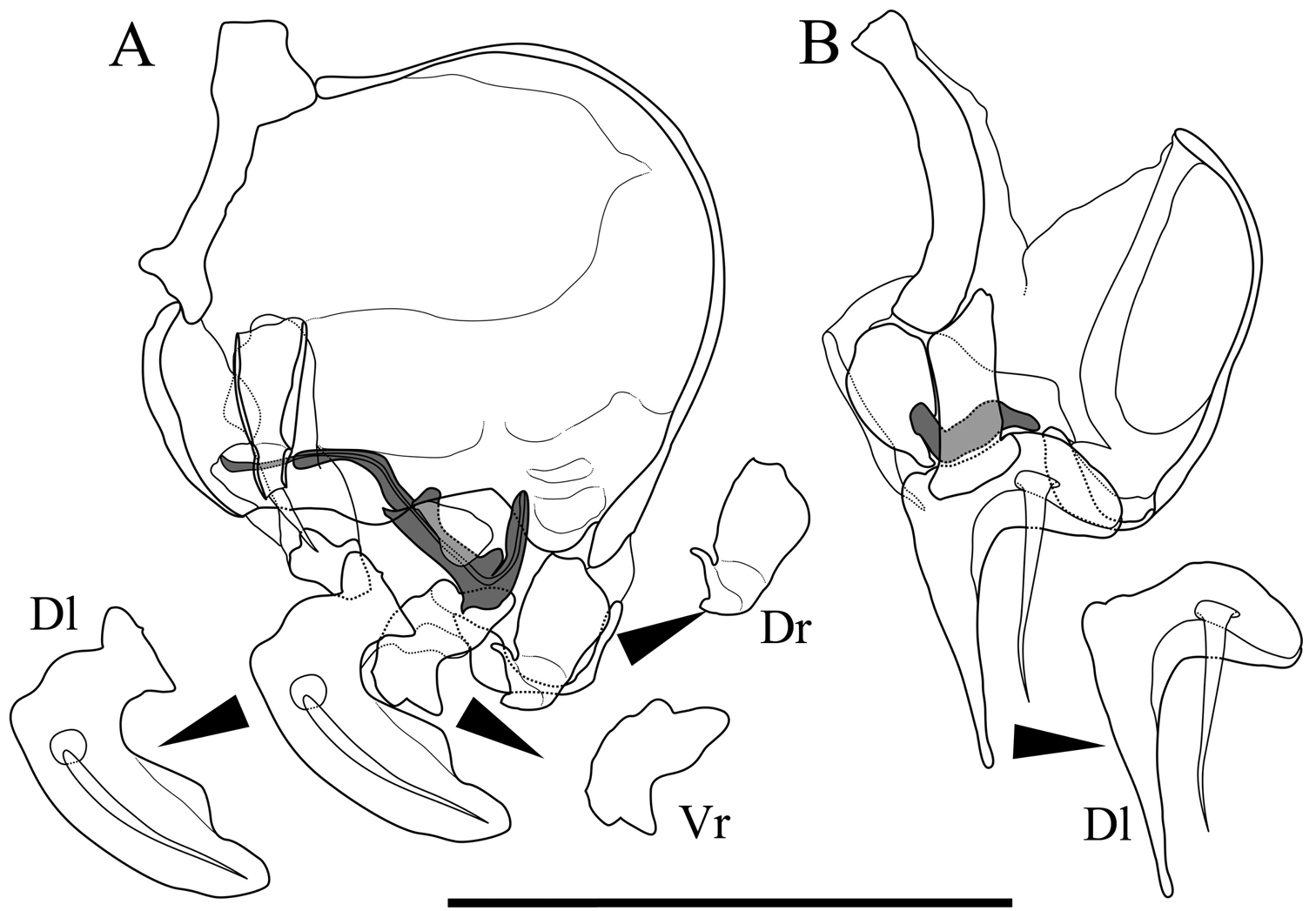
Male copulatory organ (Fig. 5). Asymmetric. Right organ (Fig. 5B) smaller than left (Fig. 5A), with reduced components: square capsule with weakened framework; copulatory duct (Cd) extremely reduced; distal lobe (Dl) thin and crescent-shaped with thick seta on proximal part. Left organ bearing well-developed long copulatory duct (Cd) single coiled in distal part; short and stout claw-like dorsal ramus (Dr); two-pronged ventral ramus (Vr); and crescent-shaped distal lobe (Dl) with thick seta on centre of proximal part.
Male copulatory organs of Parvocythere gottwaldi sp. n. Holotype (SUM-CO-2023). A external view of left organ B external view of right organ. Copulatory ducts are shaded. Abbreviation: Dr dorsal ramus Vr ventral ramus Dl Distal lobe. Scale bar indicates 50 µm.
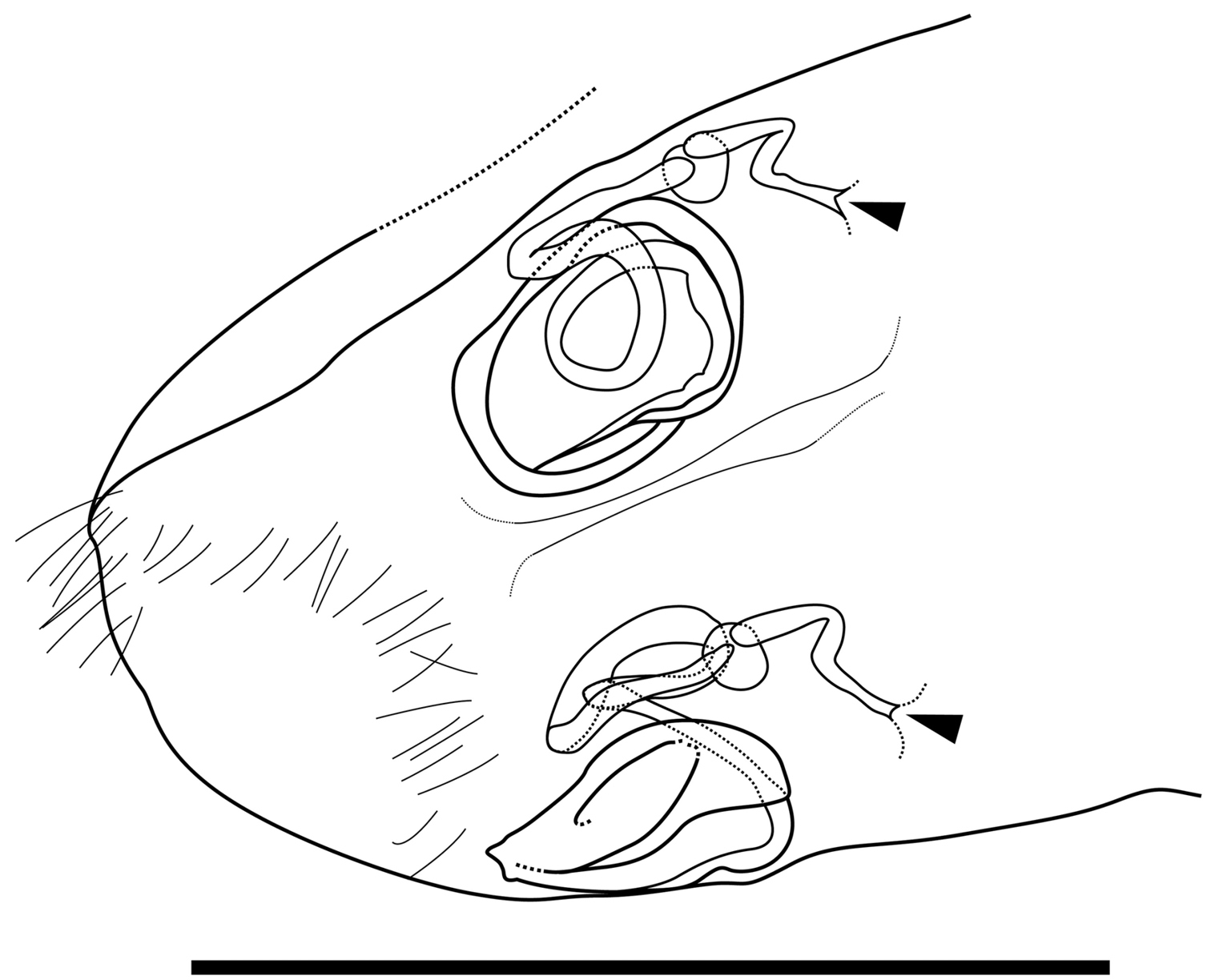
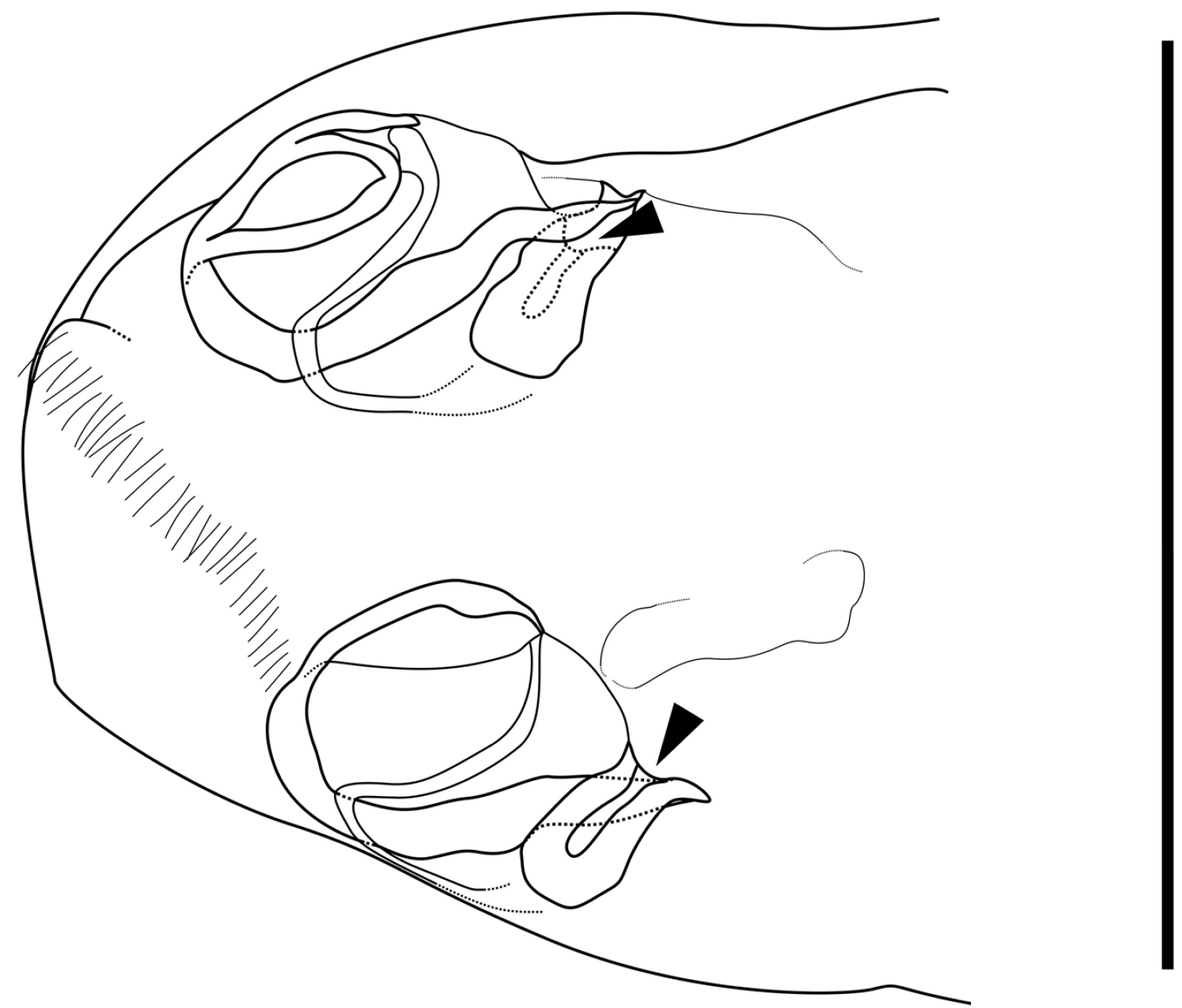
Genitalia of female (Fig. 6). Almost symmetric and consisting of rounded frame-work and winding duct with vesicle-like joint nearby opening. Duct opening on ventral side.
Eye. Absent.
Caudal part of female of Parvocythere gottwaldi sp. n. Dorsal view (paratype, SUM-CO-2038). Arrows indicate openings. Scale bar indicates 50 µm.
See Table 1.
Dimensions of valves of Parvocythere gottwaldi sp. n. from type locality.
| Length (µm) | Height (µm) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | Observed range | N | Mean | Observed range | N | ||
| Male | Right valve | 170 | 165–175 | 11 | 87 | 85–92 | 11 |
| Left valve | 173 | 169–176 | 11 | 85 | 82–87 | 11 | |
| Female | Right valve | 167 | 164–174 | 9 | 88 | 84–95 | 9 |
| Left valve | 171 | 168–176 | 7 | 86 | 83–91 | 7 | |
Type locality and Kozu, Odawara City, Kanagawa Prefecture, Pacific coast of central Japan (35°16.73'N, 139°12.75'E).
Parvocythere gottwaldi sp. n. resembles Parvocythere mauiensis Hartmann, 1991 in the outline of carapace. The new species, however, can be distinguished from the other species by its antenna with only one distal claw and the asymmetric male copulatory organ. Although this new species is also similar to Parvocythere spec. A, as referred to in
urn:lsid:zoobank.org:act:8F4E53A3-FACF-48D2-8862-CC4634E2503D
http://species-id.net/wiki/Parvocythere_gracilis
Figs 7–11Holotype: adult male (SUM-CO-2050), right valve length 170 µm, height 75 µm, left valve length 169 µm, height 73 µm, appendages mounted on slide and valves preserved in a cardboard cell slide, Paratypes: 7 adult males (SUM-CO-2051–2057) and 8 adult females (SUM-CO-2058–2065). All illustrated specimens were collected from interstitial pore-water at the type locality on April 16, 2010.
Kozu, Odawara City, Kanagawa Prefecture, Pacific coast of central Japan, 35°16.73'N, 139°12.75'E in sediments at approximately 20 cm depth. The sediment is mainly composed of clastic granules and pebbles.
The Latin gracilis (slender) refers to the slender aspect of the carapace of this species.
Carapace elongated and bean-shaped in lateral view. Anterior margin slightly extending like thin plate in ventral and dorsal views. Middle of posterior margin of right valve slightly pointed in lateral view. Forty-two pore systems per valve. Six and two marginal pores along anterior and posterior margins, respectively. Marginal infold narrow in anterior and ventral, and very narrow in posterior. Hingement modified pentodont type. No suture on middle of fourth podomere of antennula. Antenna with only one stout claw and one tiny seta on distal end. Male copulatory organ asymmetry: left organ larger than right one. Left organ bearing long L-shaped copulatory duct, dorsal ramus with two small projections, blunt two-pronged ventral ramus, and well-developed crescent-shaped distal lobe with one conspicuous seta on its centre; right organ reduced and bearing extremely reduced copulatory duct and well-developed crescent-shaped distal lobe with thick seta on proximal part.
Carapace (Figs 7, 8). Carapace elongated and bean-shaped in lateral view. Anterior margin gently rounded in lateral view. Anterior part slightly extending like thin plate in ventral and dorsal views. Posterior margin gently rounded in left valve and slightly pointed at middle height of right valve. Dorsal margin slightly rounded. Ventral margin almost straight. Carapace surface smooth. All pore-systems of simple type and 42 per valve. Six and two marginal pores along anterior and posterior margins, respectively. Marginal infold narrow in anterior and ventral, and very narrow in posterior area. Vestibula occupying large part of marginal infold. Inner surface covered with numerous pits. Hingement modified pentodont type. Right valve slightly overlapping left valve along hinge line. Four adductor muscle scars in oblique row. Two separated mandibular scars visible beneath frontal scar.
Carapaces of Parvocythere gracilis sp. n. Holotype (SUM-CO-2050). A right external view B left external view. The carapace structures are transmitted images. Scale bar indicates 100 µm.
Carapaces of Parvocythere gracilis sp. n. A–D and H male specimens: A paratype (SUM-CO-2051) B paratype (SUM-CO-2052) C and D paratype (SUM-CO-2053) H paratype (SUM-CO-2054). A left external lateral view B right external lateral view C internal view of left valve D internal view of right valve H ventral view. E–G female specimens: E paratype (SUM-CO-2059) F paratype (SUM-CO-2059) G paratype (SUM-CO-2060). E left external view F right external view G dorsal view. Scale bar indicates 100 μm.
Antennula (Fig. 9A). Five articulated podomeres. First podomere bare and short. Second podomere three times as long as first podomere and bare. Third podomere half as long as second podomere, with one medium seta on antero-distal end. Fourth podomere eleven-tenths as long as third podomere, with two very long setae on antero-distal end and one long seta on postero-distal end. Fifth podomere three-eighths as long as fourth podomere, with two long and one long spatula-like setae on distal end.
Antenna (Fig. 9B). Four articulated podomeres. First podomere with indistinct two-segmented spinneret (exopodite) on distal end. Second podomere half as long as first podomere, with bunch of fine setae at middle of anterior margin and one short seta on postero-distal end. Third podomere five-halves as long as second podomere, with numerous fine setae along antero-proximal margin, one short seta on middle of anterior margin, one medium seta on middle of posterior margin, and one short and thick seta on postero-distal end. Fifth podomere two-seventh as long as fourth podomere, with one stout claw and one very short seta on distal end.
Mandibula (Fig. 9C1, C2, C3). Coxa (Fig. 9C1) elongated, with one seta on antero-ventral part, two very short setae on postero-ventral part, and six coxal endites. Palp (Fig. 9C2) consisting of four articulated podomere. First podomere (basis) with bifurcated lamella (exopodite; Fig. 9C3) on middle of dorsal margin. Second podomere twice as long as first podomere, with three long setae on outside of distal margin. Third podomere two-thirds as long as second podomere, with one short seta on middle of dorsal margin, one medium seta on outside of distal end and two short setae on ventro-distal end. Fourth podomere four-sevenths as long as third podomere, with one short and three medium setae on distal end.
Maxillula (Fig. 9D). Thin branchial plate (exopodite) with approximately six plumose setae. Basal podomere with one palp and three endites. Palp consisting of two articulated podomeres: first podomere with one long and one medium setae on antero-distal end; second podomere as long as first podomere, with one medium seta on middle of posterior margin and two medium setae on distal end. Endites with three setae at the distal end.
Fifth limb (Fig. 9E). Four articulated podomeres. First podomere with one short seta on antero-distal end. Second podomere four-thirds as long as first podomere, with one very short seta on antero-distal end. Third podomere nine-tenths as long as second podomere. Fourth podomere eleven-tenths as long as third podomere, with long distal claw.
Sixth limb (Fig. 9F). Four articulated podomeres. First podomere with one short seta on antero-distal end. Second podomere two-thirds as long as first podomere, with one medium seta on antero-distal end. Third podomere nine-tenths as long as second podomere. Fourth podomere as long as third podomere with well-developed stout distal claw.
Appendages of Parvocythere gracilis sp. n. Holotype (SUM-CO-2050). A antennula B antenna C1 coxa of mandibula C2 palp of mandibula C3 proximal part of mandibular palp D maxillula E fifth limb F sixth limb. Scale bar indicates 50 µm.
Seventh limb. Absent.
Male copulatory organ (Fig. 10). Asymmetric. Right organ smaller than left, with reduced components: square capsule with weakened framework; copulatory duct (Cd) extremely reduced; and distal lobe (Dl) thin and crescent-shaped with thick seta on proximal part. Left male copulatory organ bearing L-shaped long copulatory duct (Cd), dorsal ramus (Dr) with two projections, two-pronged blunt ventral ramus (Vr), and crescent-shaped and well-developed blunt-tipped distal lobe (Dl) with thick seta on centre.
Male copulatory organs of Parvocythere gracilis sp. n. Holotype (SUM-CO-2050). A internal view of left organ B external view of right organ. Copulatory ducts are shaded. Abbreviation: Dr dorsal ramus Vr ventral ramus Dl Distal lobe. Scale bar indicates 50 µm.
Genitalia of female (Fig. 11). Symmetric and consisting of rounded frame-work, sigmoid duct connected with strongly sclerotised opening. Duct opening on ventral side.
Eye. Absent.
Caudal part of female of Parvocythere gracilis sp. n. Dorsal view (paratype, SUM-CO-2058). Arrows indicate openings. Scale bar indicates 50 µm.
See Table 2.
Dimensions of valves of Parvocythere gracilis sp. n. from type locality.
| Length (µm) | Height (µm) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | Observed range | N | Mean | Observed range | N | ||
| Male | Right valve | 166 | 163–170 | 4 | 73 | 71–75 | 4 |
| Left valve | 164 | 162–169 | 5 | 71 | 70–73 | 5 | |
| Female | Right valve | 158 | 156–159 | 4 | 70 | 69–71 | 4 |
| Left valve | 154 | 152–157 | 6 | 68 | 67–68 | 6 | |
Type locality and Daio-zaki, Shima City, Mie Prefecture, Pacific coast of central Japan, (34°16.59'N, 136°53.83'E).
Parvocythere gracilis sp. n. resembles Parvocythere galapagoensis Gottwald, 1983, Parvocythere schmidti Gottwald, 1983 and Parvocythere subterranea Gottwald, 1983 in the shape of carapace. The new species, however, is slenderer than the other species, and can be distinguished from them by the following reduced characters: no rib posterior to adductor muscle scars; no suture on middle of antennular fourth podomere; only one developed distal claw of antenna; and asymmetric male copulatory organ. Although Parvocythere gracilis sp. n. also resembles Parvocythere elongata Hartmann, 1959 and Parvocythere supralitoralis Gottwald, 1983 in the shape of carapace and the reductive characters of antennula and antenna, only the new species has asymmetric male copulatory organ. Moreover, Parvocythere gracilis sp. n. is also similar to Parvocythere dimorpha Hartmann, 1974 in the shape of the carapace, the many characters of appendages and an asymmetric male copulatory organ but the new species differs from other species in the number of antennal distal claw, the thickness of sixth limb and the form of copulatory duct.
The Parvocythere species, including the new species Parvocythere gottwaldi sp. n. and Parvocythere gracilis sp. n., are remarkably varied in the characters of antennula, antenna and male copulatory organ (Table 3).
Character states of Parvocythere species. Asterisked species have remarkably small length (<160 µm). Abbreviations: S, Group S; N, Group N; 1+1, one claw and one seta on distal end of antenna; sym, symmetric; asym, asymmetric; D, dentata group; E, elongata group.
| Species | Body length (µm) | Suture on antennular 4th podomere | Grouping in this study | Number of antennal distal claw | Male copulatory organ |
Grouping in |
|---|---|---|---|---|---|---|
| Parvocythere directocostata | 220 | divided | S? | 2 | sym | D |
| Parvocythere dentata | 190–210 | present | S | 2 | sym | D |
| Parvocythere fernandinensis | 185–193 | present | S | 2 | sym | E |
| Parvocythere galapagoensis | 197–220 | present | S | 2 | sym | D |
| Parvocythere schmidti | 168–193 | present | S | 2 | sym | D |
| Parvocythere subterranea | 160–181 | present | S | 2 | sym | D |
| Parvocythere psammophila | 168 | present | S | 2 | sym | D |
| Parvocythere mauiensis | 217 | present | S | 2 | - | - |
| Parvocythere japonica | 170–190 | present | S | 2 | sym | D |
| Parvocythere marginocostata | 190–240 | absent | N | 2 | sym | E |
| Parvocythere dimorpha | 170–180 | absent | N | 2 | asym | D |
| Parvocythere hartmanni | 150–160 | absent | N | 1+1 | sym | D |
| Parvocythere supralitoralis* | 126–139 | absent | N | 1+1 | sym | D |
| Parvocythere gracilis sp. n. | 156–170 | absent | N | 1+1 | asym | D |
| Parvocythere elongata | 150–180 | absent | N | 1 | asym | E |
|
Parvocythere spec. A in |
155 | absent | N | 1 | asym | D |
| Parvocythere gottwaldi sp. n. | 168–176 | absent | N | 1 | asym | D |
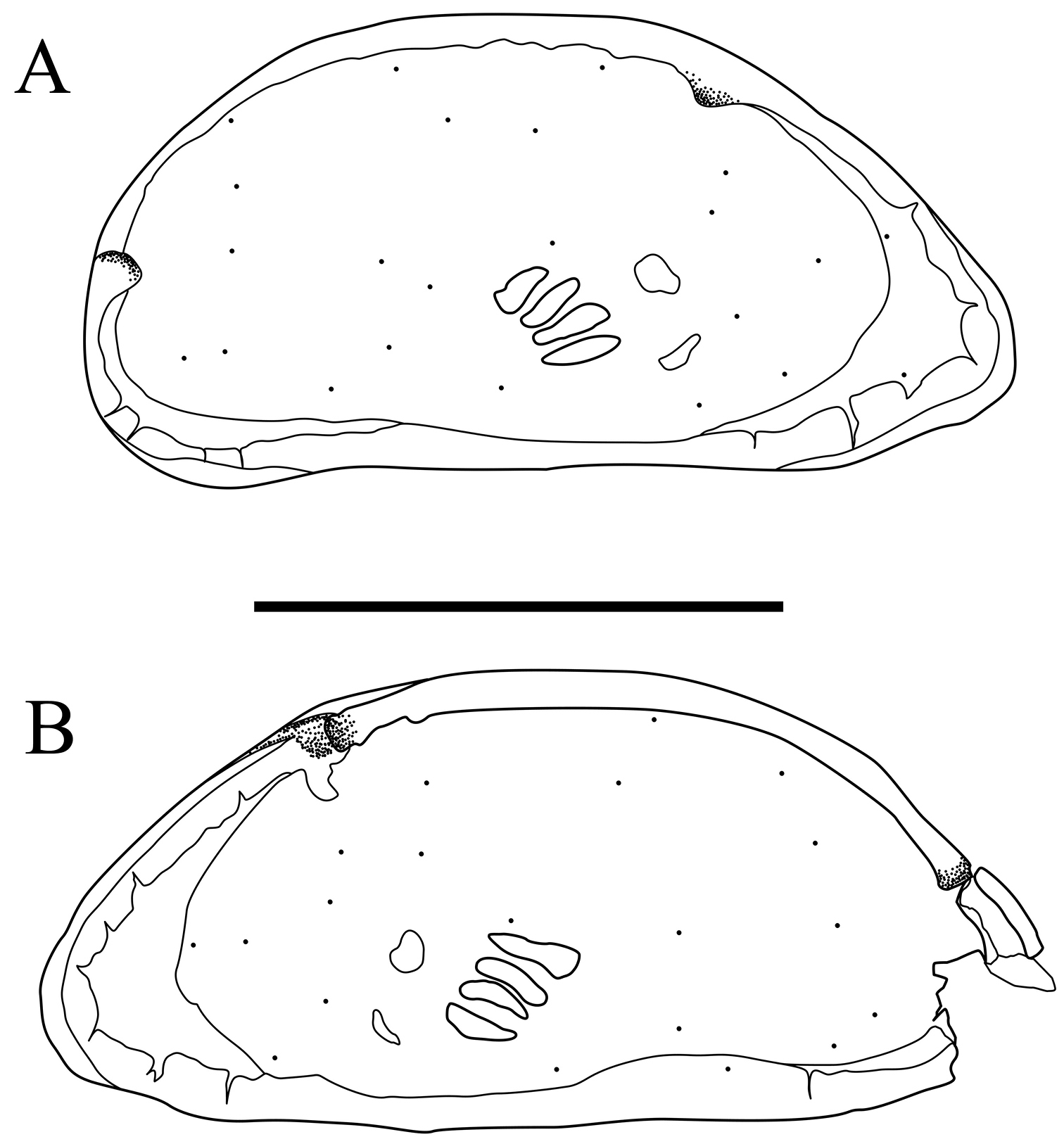
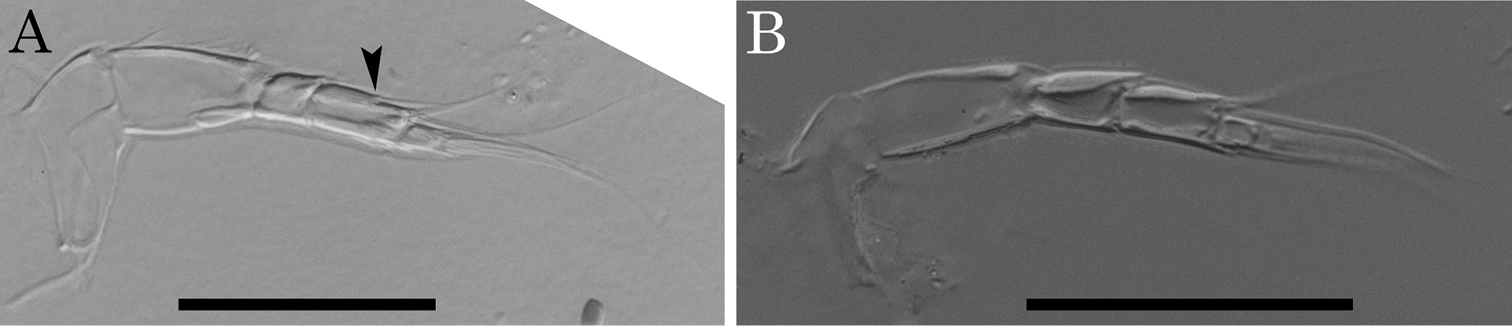
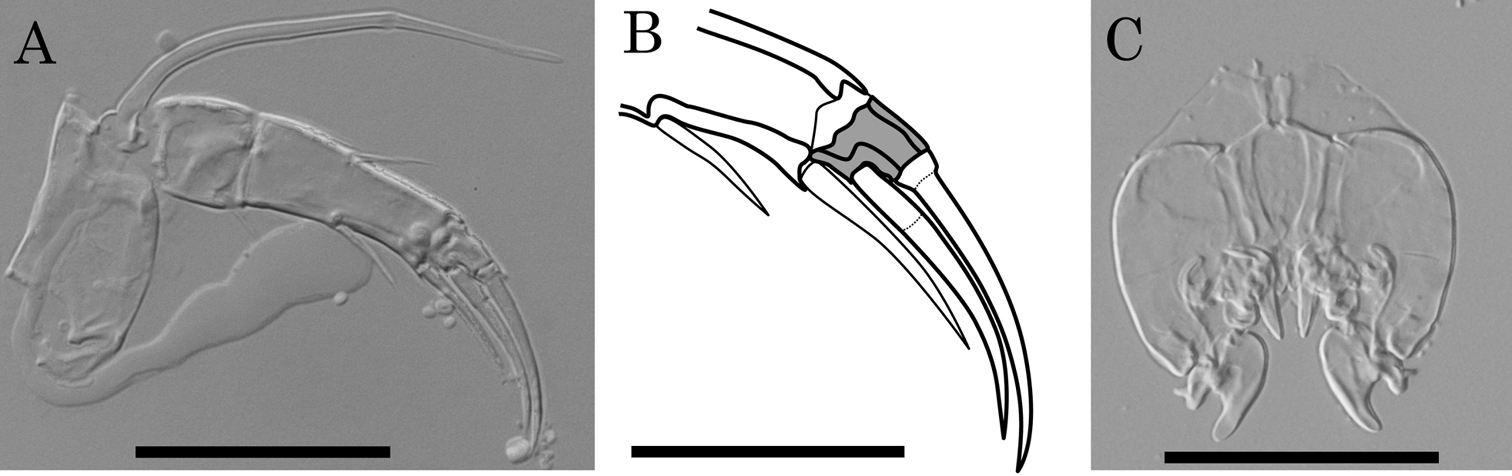
Two character states are observed in the antennular fourth podomere of this genus: one is the presence of a suture on the middle of the podomere with two setae on its anterior and posterior sides; the other is the absence of the suture and setae (Fig. 12). As an exception, Parvocythere directocostata Hartmann, 1974 has a six-segmented antennula in which the fourth and fifth podomeres correspond to the sutured fourth podomere. The suture on antennular fourth podomere is observed in the following eight species: Parvocythere dentata, Parvocythere fernandinensis Gottwald, 1983, Parvocythere galapagoensis, Parvocythere schmidti, Parvocythere subterranea, Parvocythere psammophila Gottwald, 1983, Parvocythere mauiensis and Parvocythere japonica. On the other hand, no suture on the antennular fourth podomere is observed in the other eight species: Parvocythere elongata, Parvocythere hartmanni Marinov, 1962, Parvocythere dimorpha, Parvocythere marginocostata Hartmann, 1974, Parvocythere supralitoralis, Parvocythere gottwaldi sp. n., Parvocythere gracilis sp. n. and Parvocythere spec. A, referred in
Morphological variations of antennule of Parvocythere species. A antennule of Parvocythere japonica (holotype) B antennule of Parvocythere gottwaldi sp. n. Arrow indicates suture on the middle of fourth podomere. Scale bars indicate 30 µm, respectively.
Three states of antennal distal end are observed among the Parvocythere species (
Antenna and male copulatory organ of Parvocythere japonica (holotype) as a representative of the Group S. A antenna B sketch of distal region of antenna C male copulatory organ. Shaded podomere is distal fourth podomere. Scale bars indicate 30 µm, 20 µm and 50 µm for A, B and C respectively.
One of the paired male copulatory organs (hemipenes) is strongly reduced in Parvocythere elongata, Parvocythere dimorpha and Parvocythere spec. A referred in
Regarding the carapace, the two groups do not show any remarkable character differences. The many species of Group N, however, are relatively small (< 160 µm of the carapace length) (see Table 3). Actually, the carapace length is within the range of 160–220 µm in Group S but of 126–180 µm in Group N, except for the large Parvocythere marginocostata (190–240 µm), so most species of Group N have smaller carapaces. The smaller body size can be regarded as an adaptive character to the interstitial life (e.g.
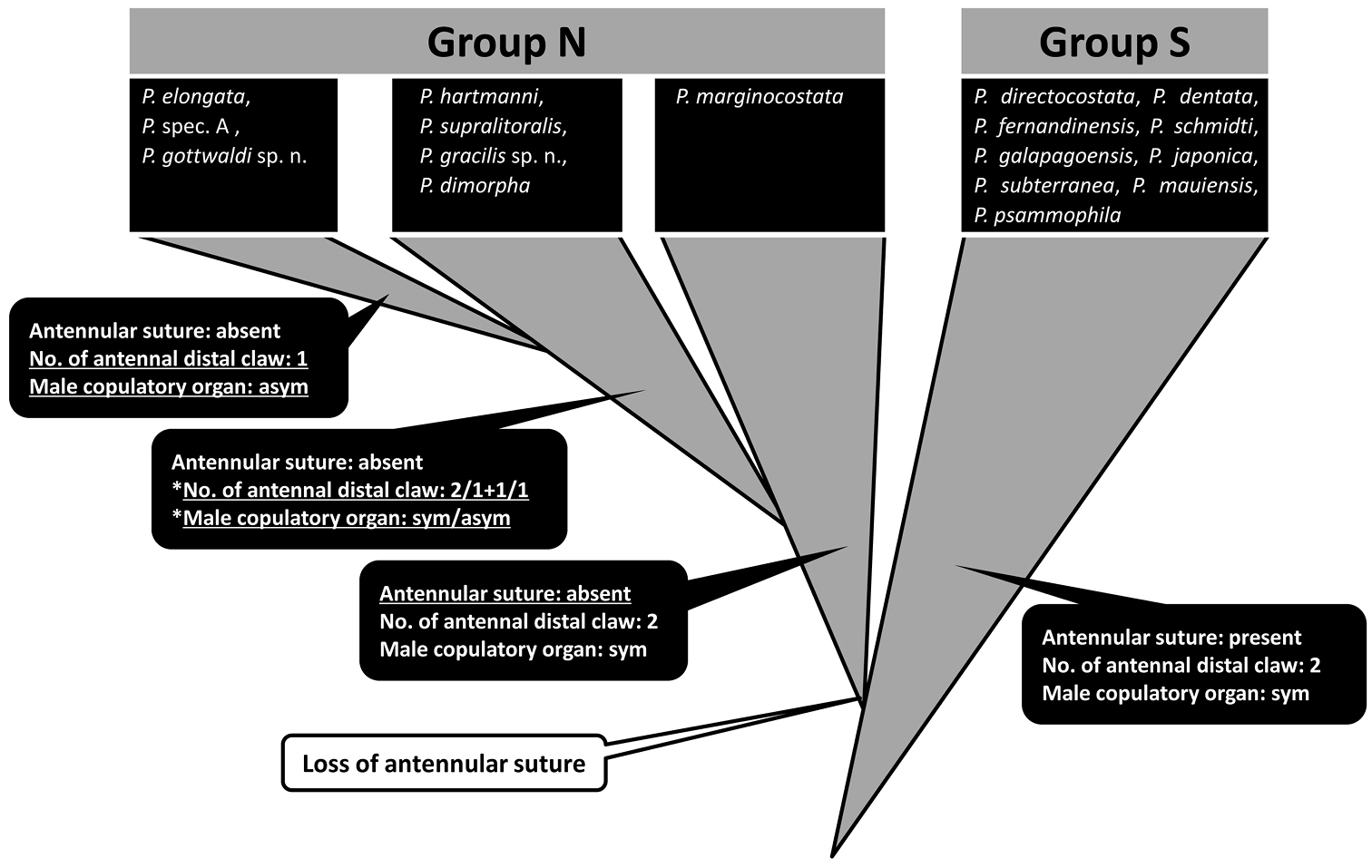
The Parvocythere species can be divided into Group S, maintaining plesiomorphies, and Group N which includes derived species developing apomorphies as reductive appendages, male copulatory organs, and smaller carapace (Fig. 14). Although
Schematic of inferred evolutionary relationships of the Parvocythere species. Triangles coloured in gray indicate each group of species. Species in blackly closed squares indicate them classified into each group. The characters of antennula, antenna and male copulatory organ are represented in the blackly closed balloons and under-lined characters are novel ones. Evolutionary change is represented in an open balloon. The species in the second step of Group N have only one of the reductive states: reductive one of two antennal distal claws or one of pared male copulatory organ (with an asterisk “*”).
The genus Parvocythere is composed of only interstitial species and characterised by some reductive characters (e.g. small body size less than 250 μm of the length, only two paired walking legs and the absence of eyes), regarded as the result of an adaptation to the interstitial environment (
The morphological comparison among Parvocythere species, including the two new species, suggests that Group N derived after Group S through the heterochronic change of antennula, and that the reduction of antennal distal claw and/or the forming of the asymmetric male copulatory organ occurred as an evolutionary tendency within Group N (Fig. 14). These morphological variations among the exclusively interstitial Parvocythere species suggest that animal taxa specialised for the interstitial life could also evolve further characters related to their adaptation.
Our thanks go to members of the Ostracod Research Team of Shizuoka University for their valuable suggestions and continuous support, and to the staff members of the Misaki Marine Biological Station, the University of Tokyo. This study was partly funded by the Japan Society for the Promotion of Science: a Research Fellowship for Young Scientist (No. 216862) and the Grant-in-Aid for Scientific Research (No. 19370034, 23370042).