(C) 2012 David Penney. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Spiders (Araneae) are one of the most species-rich orders on Earth today, and also have one of the longest geological records of any terrestrial animal groups, as demonstrated by their extensive fossil record. There are currently around 1150 described fossil spider species, representing 2.6% of all described spiders (i.e. extinct and extant). Data for numbers of fossil and living spider taxa described annually (and various other metrics for the fossil taxa) were compiled from current taxonomic catalogues. Data for extant taxa showed a steady linear increase of approximately 500 new species per year over the last decade, reflecting a rather constant research activity in this area by a large number of scientists, which can be expected to continue. The results for fossil species were very different, with peaks of new species descriptions followed by long troughs, indicating minimal new published research activity for most years. This pattern is indicative of short bursts of research by a limited number of authors. Given the frequent discovery of new fossil deposits containing spiders, a wealth of new material coming to light from previously worked deposits, and the application of new imaging techniques in palaeoarachnology that allow us to extract additional data from historical specimens, e.g. X-ray computed tomography, it is important not only to ensure a sustained research activity on fossil spiders (and other arachnids) through training and enthusing the next generation of palaeoarachnologists, but preferably to promote increased research and expertise in this field.
Arachnida, Araneae, palaeontology
With 42, 751 currently recognized extant species (
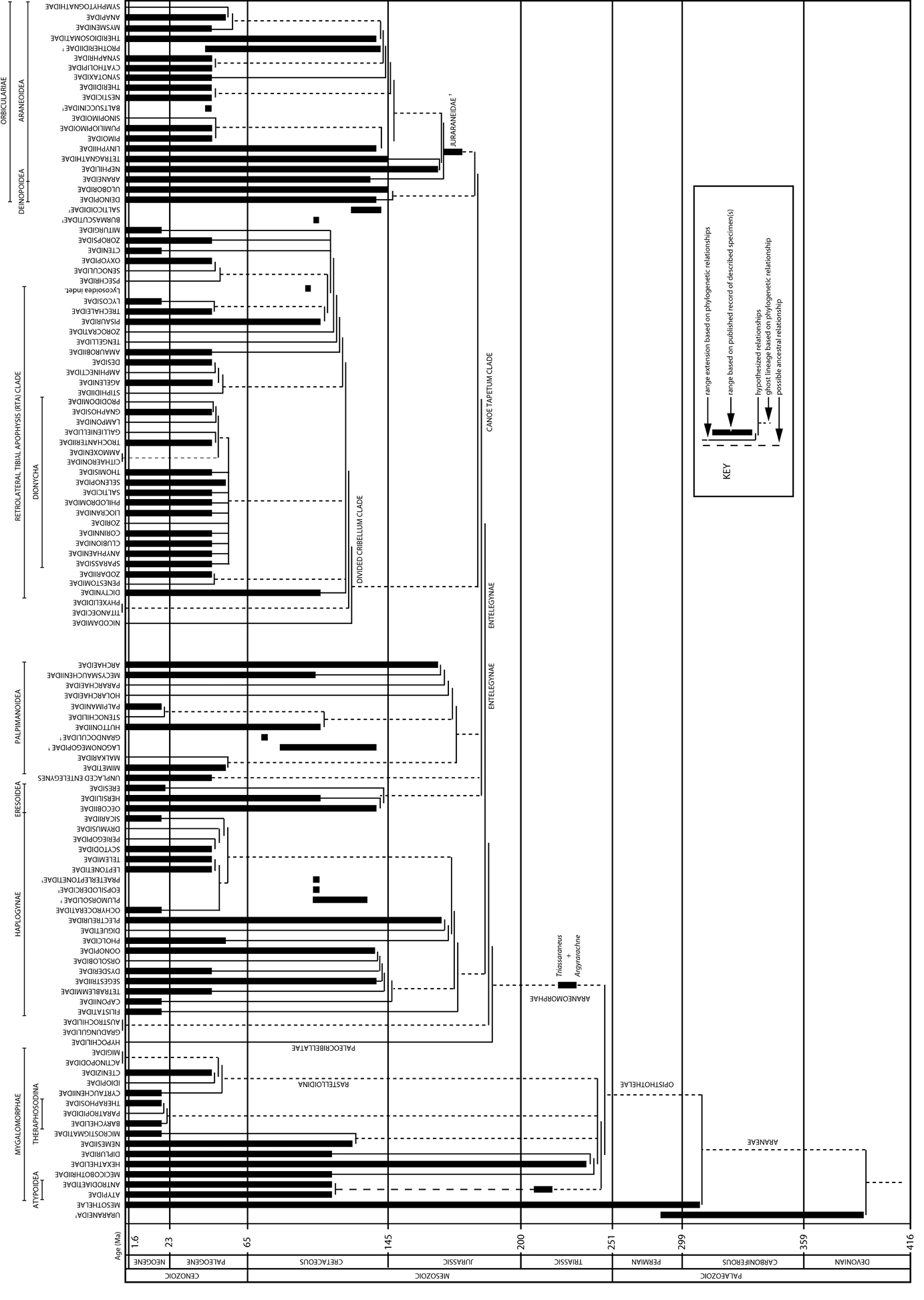
The evolutionary tree of spiders (updated from
One of the most valuable contributions that fossils can make towards modern studies of spider evolution is dating when groups or families first appeared. Fossils provide a minimum age for any given family (Figure 1) or genus and they have been used to calibrate molecular phylogenies (e.g.
New, significant amber deposits containing fossil spiders are being discovered frequently (e.g.
Data for numbers of fossil spider species (excluding subfossils in copal, peat cores and extant species collected from archaeological sites) were taken from
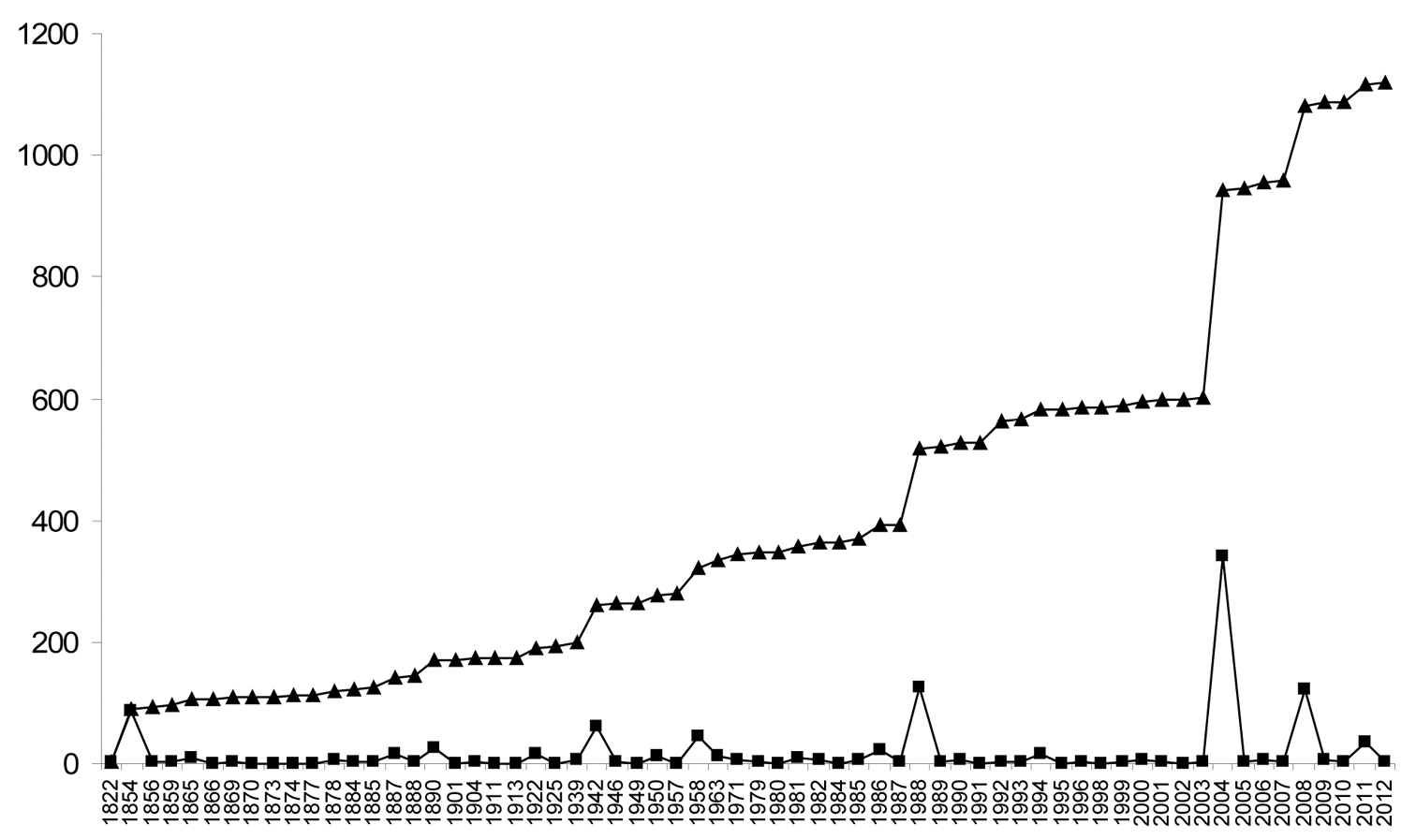
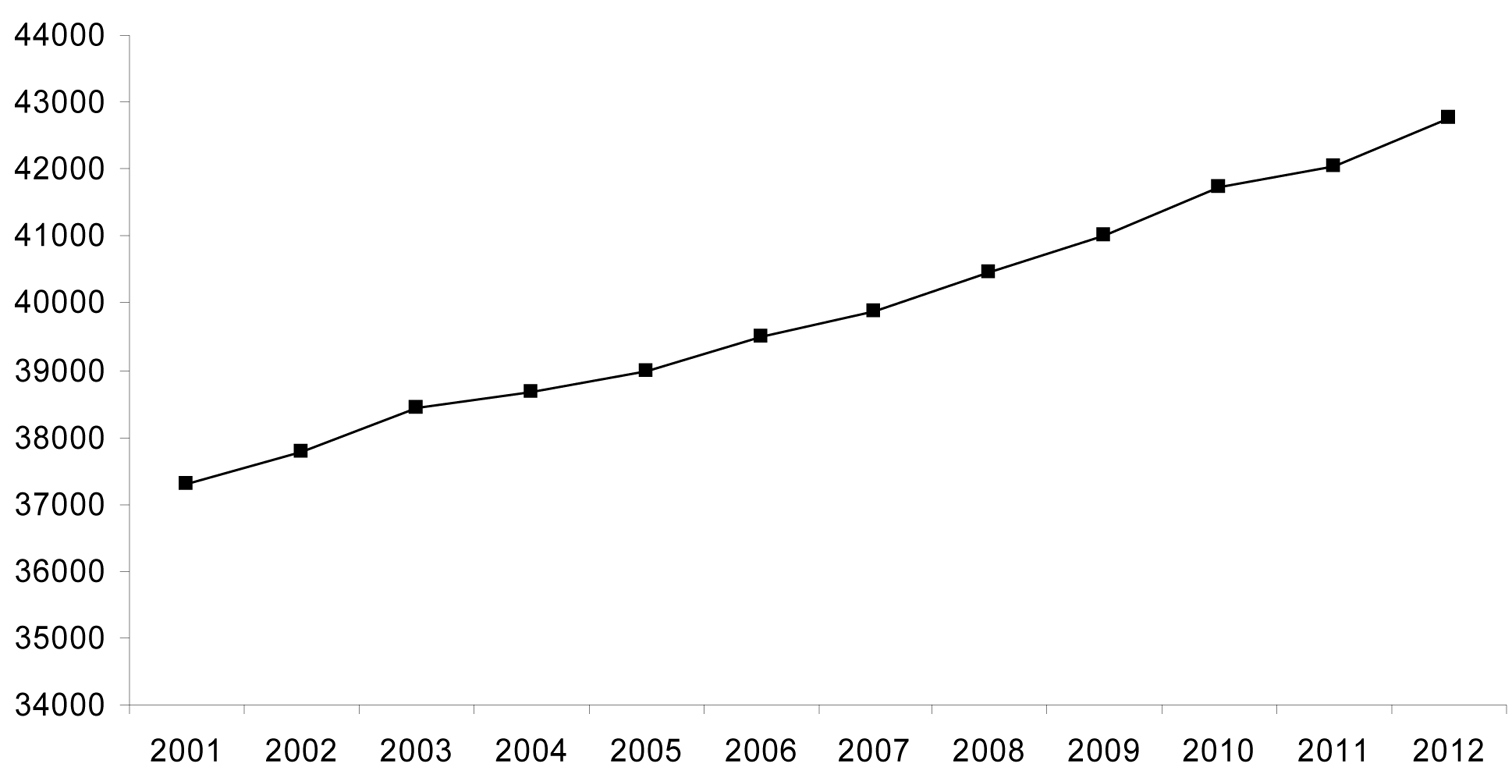
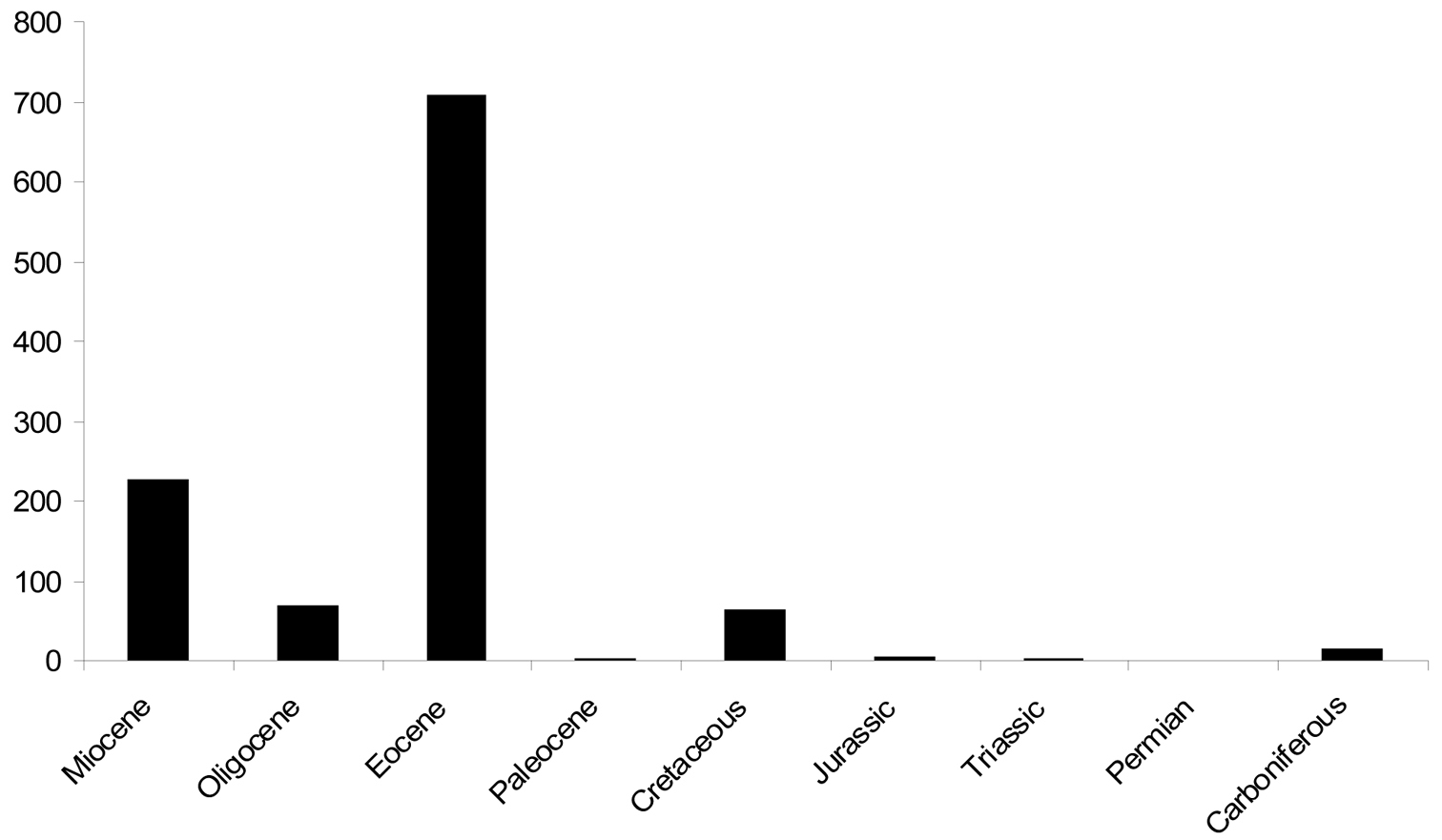
Data for numbers of fossil spider species (excluding subfossils) are plotted in Figure 2 and data for extant species are plotted in Figure 3. The line of best fit (not illustrated) for the extant species data has a formula of y = 482.81x + 36738 (R2 = 0.992), suggesting an annual increment in the number of described extant species of approximately 480; based on a calculated mean of the actual data the value is 496 ± 162. The plot of the palaeontological data does not show a linear increase, but rather sporadic peaks interspersed with periods of little activity. The classification of fossil species within families is shown in Figure 4 and the numbers of fossil spider species per geological time period are shown in Figure 5.
The numbers of described fossil spider species by year. Note that data for the 126 years where no fossil spider species were described are not included. Hence, the actual lull periods between peaks of activity are artificially shortened in this graph. For example, the period between the first described fossil spider in 1822 and the next data plot is actually 32 years. Squares = newly described fossil spider species, triangles = cumulative number of described fossil spider species. Data derived from
The cumulative number of newly described extant spider species this century. Data from
Number of fossil spider species per family (as currently assigned).
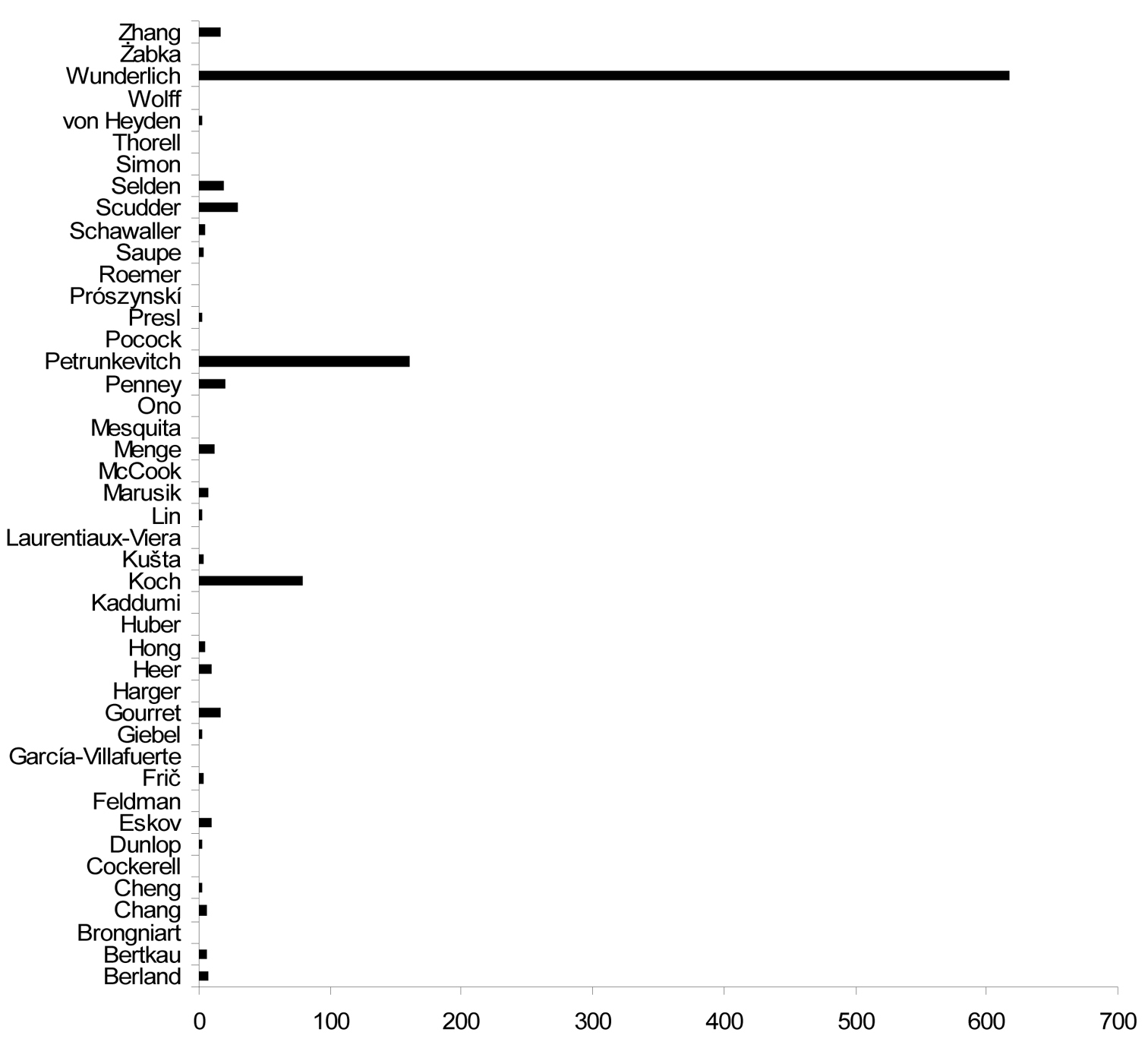
Number of fossil spider species described by different arachnologists. Only first authorship data are considered, so in reality some authors will have described more species than the value indicated.
Our research has focused on species as this tends to be the most informative unit of bio/palaeodiversity data; families are too few to allow any informative analysis on a broad scale, and genera are too idiosyncratically defined. Nonetheless, it is interesting to note that 70 (= 63%) extant spider families (including Comaromidae sensu
It is evident from Figure 4 that some families are much more common as fossils than others, for example Theridiidae, Salticidae, Linyphiidae and Araneidae, and it is noteworthy that these represent four of the five most diverse spider families on the planet today. The fifth family is Lycosidae, which are ground dwellers and so are unlikely to be preserved in amber and most probably only evolved in the Miocene (
New extant spider species are described every year, but this is not so for fossils. In our palaeodata there are 126 years in which no fossil spider species were described. These are omitted from the graphs, so the actual lull periods between peaks of activity are artificially shortened in Figure 3. For example, the period between the first described fossil spider by
Number of fossil spider species described from each geological period. The Paleogene Period has been broken down into its various Epochs (Paleocene, Eocene and Oligocene) in order to show the spread of data; the Neogene Period is represented only by the Miocene Epoch because Pleistocene sub-fossils have not been included.
It should be noted that the holotypes of many of the older species names – e.g. the Florissant specimens described by
Data for extant taxa showed a steady linear increase of approximately 500 new taxa per year over the last decade, reflecting a rather constant research activity in extant spider taxonomy by a large number of scientists, which can be expected to continue. The results for the description of fossil species were very different, with peaks of new species descriptions followed by long troughs indicating short bursts of research by only a few authors, often with a long hiatus in between. Were these data to represent patterns within natural populations, one would consider the latter to be at considerable risk of extinction. Given the frequent discovery of new fossil deposits containing spiders, a wealth of new material coming to light from previously worked deposits, and the application of new imaging techniques in palaeoarachnology that allow us to extract additional data from historical specimens, e.g. X-ray computed tomography, it is important not only to ensure a sustained research activity on fossil spiders (and other arachnids) through training and enthusing the next generation of palaeoarachnologists, but preferably to promote increased research and expertise in this field.
DP acknowledges financial support from Siri Scientific Press, YMMs contribution to this work was supported in part by the Russian Foundation for Basic Research (grant № 11–0401716).