(C) 2012 Diana Delicado. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Several Pseudamnicola (Corrosella) populations of the central and eastern Iberian Peninsula have been ascribed to Pseudamnicola (Corrosella) astieri (Dupuy, 1851), though recent evidence demonstrates the species could be endemic to the departments of Var and Alpes-Maritimes in France. Through the identification of cryptic species using a combined morphological and phylogenetic approach, this paper provides a detailed morphological description of Pseudamnicola (Corrosella) astieri, clarifying its taxonomic boundaries and confirming it as a French endemic. In parallel, by comparing Pseudamnicola (Corrosella) populations from the provinces of Castellón and Valencia in Eastern Spain, it was observed that rather than Pseudamnicola (Corrosella) astieri they represented a new species here described as Pseudamnicola (Corrosella) hauffei sp. n. Among other characters, the two species show marked differences in shell shape, male and female genital systems, radular formula and concentration of the nervous system. Pseudamnicola (Corrosella) hauffei sp. n. was also compared morphologically to another two Pseudamnicola (Corrosella) species living in nearby areas [Pseudamnicola (Corrosella) hinzi Boeters, 1986 and Pseudamnicola (Corrosella) navasiana (Fagot, 1907)], molecularly to Pseudamnicola (Corrosella) falkneri (Boeters, 1970), the type species of the subgenus, and to the rest of the Pseudamnicola (Corrosella) species described so far. Morphological differentiation between the species is supported by a genetic divergence of 7.4% inferred from a partial sequence (658 bp) of the mitochondrial gene cytochrome c oxidase subunit I (COI). On the basis of an average 8% (5.39 to 11.15%) divergence estimated for the COI gene in other Pseudamnicola (Corrosella) species reported in GenBank, the existence of two specific entities is here proposed, which will have impact on conservation policies both in France and in Spain.
Hydrobiidae, Pseudamnicola (Corrosella), Pseudamnicola (Corrosella) astieri (Dupuy 1851) , Pseudamnicola (Corrosella) hauffei sp. n. , France, Spain, Iberian Peninsula, taxonomy, COI, cryptic species, conservation
The Mediterranean basin, and within it the Iberian Peninsula, has been identified as a biodiversity hotspot for animal species including those of hydrobiid gastropods (
Two subgenera are currently recognized within the Pseudamnicola: Pseudamnicola (Corrosella), occurring in the Iberian Peninsula and one small area in the South of France; and Pseudamnicola (Pseudamnicola), widely distributed in freshwater ecosystems of the Mediterranean basin. The diversity of the subgenus Corrosella is much lower than that of Pseudamnicola and only 11 nominal species (described by:
One of these 11 species is Pseudamnicola (Corrosella) astieri (Dupuy, 1851), originally described from the surroundings of Grasse in the department of Alpes-Maritimes (France). Several other species were later cited from the neighbouring Var department (Bythinella anteisensis Berenguier 1882, Bythinella berenguieri Bourguignat in Berenguier 1882, Bythinella doumeti Bourguignat in
Our paper provides a wide conchological and anatomical description of a new species of Pseudamnicola (Corrosella) from eastern Spain (Iberian Peninsula), Pseudamnicola (Corrosella) hauffei sp. n., and, through its re-description, compares it with the species Pseudamnicola (Corrosella) astieri from Var (France)and with other Pseudamnicola (Corrosella) species with close-by distribution areas in the Iberian Peninsula. Morphological studies were combined with cytochrome c oxidase subunit I (COI) sequence analysis in the light of previously published molecular data (
The study area comprised the Departments of Alpes-Maritimes and Var in southeastern France and the provinces of Castellón and Valencia in eastern Spain. Specimens were collected from several sites in this area (see Figure 1) and deposited in the Collection of Molluscs of the Museo Nacional de Ciencias Naturales (MNCN), Madrid, Spain.
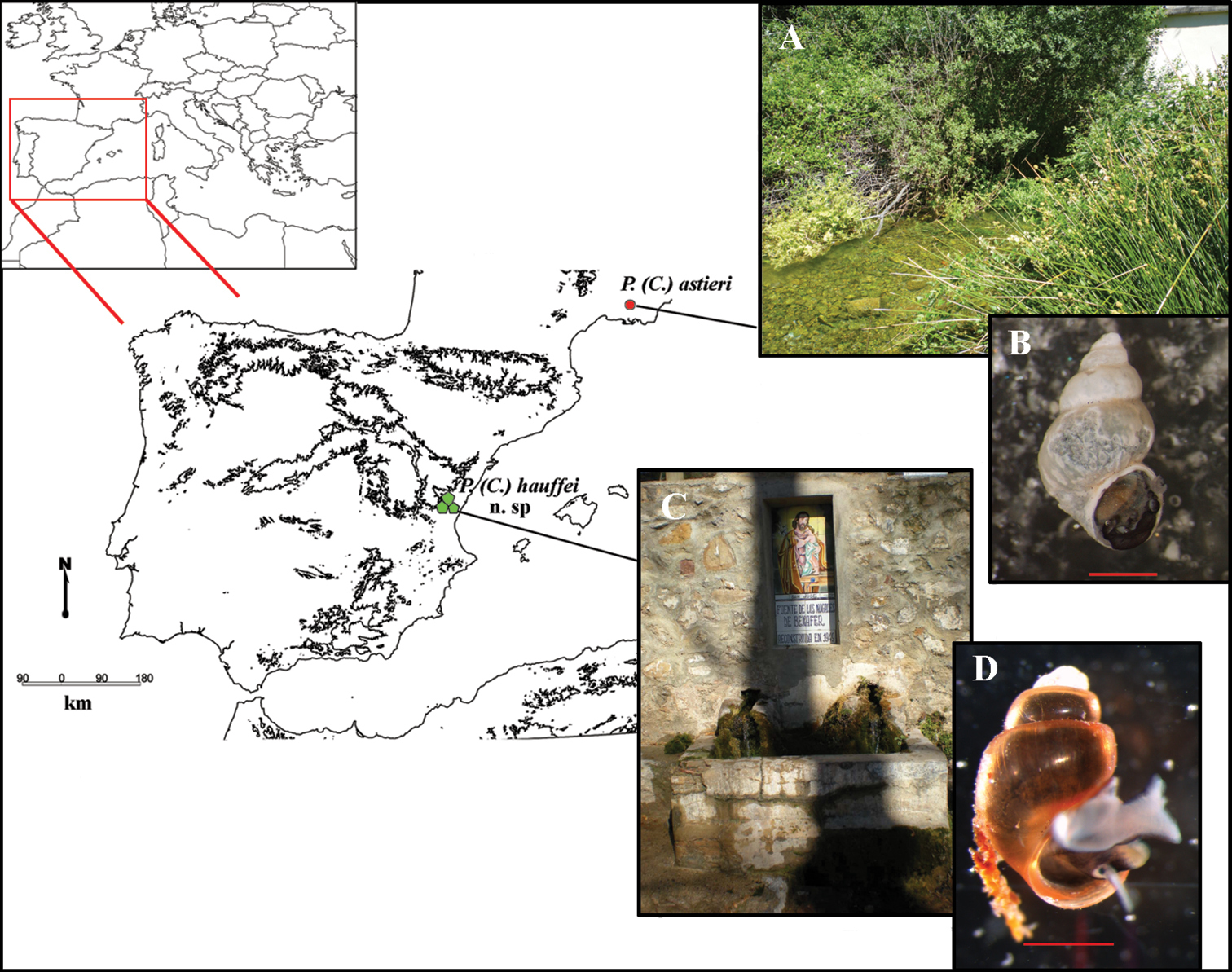
Map of localities of Pseudamnicola (Corrosella) astieri and Pseudamnicola (Corrosella) hauffei sp. n. A Photograph of Source d’Argens, Brue-Aurillac, Var, France B Preserved specimen of Pseudamnicola (Corrosella) astieri C Photograph of Nogales spring, Benafer, Castellón, Spain (type locality of Pseudamnicola (Corrosella) hauffei sp. n.) D Alive specimen of Pseudamnicola (Corrosella) hauffei sp. n. Scale bar in B and D represents 1 mm.
Anatomical observations and morphometric measurements were made on specimens relaxed with menthol crystals and fixed in ethanol following the procedures described in
Specimens were dissected under a Leica MZ 16 A stereomicroscope and photographed using a Nikon ds fi1 camera. All measurements were made using Nis-Elements V. 2.2. software. Anatomical illustrations were prepared from camera lucida drawings. Environmental scanning electron microscope (ESEM) images of shells were captured using a Philips Quanta 200 in low-vacuum mode, after removal of the periostracum by immersion in 5% sodium hypochlorite and then cleaning by ultrasonication. The radula and operculum were cleaned by immersion in KOH solution (10g/l) at room temperature. Both structures were then rinsed in distilled water and air-dried before mounting on stubs and coating with a thin (10–20 nm) gold layer in an Emitech K550X sputter coating unit followed by observation in high-vacuum mode.
Total DNA was isolated from the foot tissue of the snails using the ChargeSwitch gDNA Micro Tissue (Invitrogen, Paisley, UK) extraction kit. Partial COI sequences were amplified by polymerase chain reaction (PCR) using LCO1490 (
Species name, locality details, Genbank accession numbers and publication references for mtCOI sequences.
| Species name | Locality | Genbank accession number | Reference |
|---|---|---|---|
| Pseudamnicola (Corrosella) luisi | La Gitana spring, La Peza, Granada, Spain. | JF312220 |
|
| Pseudamnicola (Corrosella) falkneri | La Armada spring, Orce, Granada, Spain. | JF312224 |
|
| Pseudamnicola (Corrosella) manueli | La Garganta stream, Nava de San Pedro, Jaén, Spain. |
JF312227 JF312228 |
|
| Pseudamnicola (Corrosella) bareai | Spring in Ermita de las Santas, Granada, Spain. |
JF312225 JF312226 |
|
| Pseudamnicola (Corrosella) marisolae | Pilar del Mono spring, Dúrcal, Granada, Spain. |
JF312218 JF312219 |
|
| Pseudamnicola (Corrosella) iruritai | Don Pedro spring, Loja, Granada, Spain. |
JF312221 JF312222 |
|
| Pseudamnicola (Corrosella) andalusica | La Salud spring, Toscarejo, Jaén, Spain. | JF312223 |
|
| Pseudamnicola (Pseudamnicola) lucensis | Thermal spring in Bagni di Lucca, Tuscany, Italy. | AF367651 |
|
| Pseudamnicola (Pseudamnicola) macrostoma negropontina | Artificial pond in Marmaris, Evvoia island, Greece. | EF061915 |
|
| Hydrobia acuta acuta | Lac de Tunis, Tunisia. | AF278804 |
|
| Pyrgula annulata | Lake Garda, Brescia, Italy. | AY341258 |
|
Shell and operculum characters:AH: aperture height; AL: aperture length; AW: aperture width; LBW: length of body whorl; NL: length of opercular nucleus; NW: width of opercular nucleus; NSW: number of spire whorls; OL: operculum length; OLWL: length of the last whorl of the operculum; OLWW: width of the last whorl of the operculum; OW: operculum width; SL: shell length; SW: shell width; WAW: width of the antepenultimate whorl; WBW: width of the body whorl; WPW: width of the penultimate whorl.
Anatomical characters: Ag: albumen gland; Bc: bursa copulatrix; CC: cerebral commissure; Cg: capsule gland; Ct: ctenidium; dBc: duct of the bursa copulatrix; LCG: left cerebral ganglion; LPG: left pleural ganglion; Os: osphradium; P: penis; Po: pallial oviduct; Pr: prostate gland; RCG: right cerebral ganglion; Ro: renal oviduct; RPG: right pleural ganglion; SR: seminal receptacle; Ss: style sac; St: stomach; SubC: suboesophageal connective; SubG: suboesophageal ganglion; SupC: supraoesophageal connective; SupG: supraoesophageal ganglion; L: length; W: width. The concentration of the nervous system was measured as the “RPG” ratio (
Collections.BOE, Boeters, München, Bundesrepublik Deutschland; MHNG, Muséum d’histoire naturelle de la Ville de Genève, Switzerland; MNCN, Museo Nacional de Ciencias Naturales, Madrid, Spain.
Collectors. B.A., B. Arconada; C.N., C. Noreña; D.D., D. Delicado; D.M., D. Moreno; J.M.R, J.M. Remón; R.A., R. Araujo.
Results Systematic descriptionshttp://species-id.net/wiki/Pseudamnicola_astieri
Figs 2 –4Surroundings of Grasse, France (Dupuy, 1851).
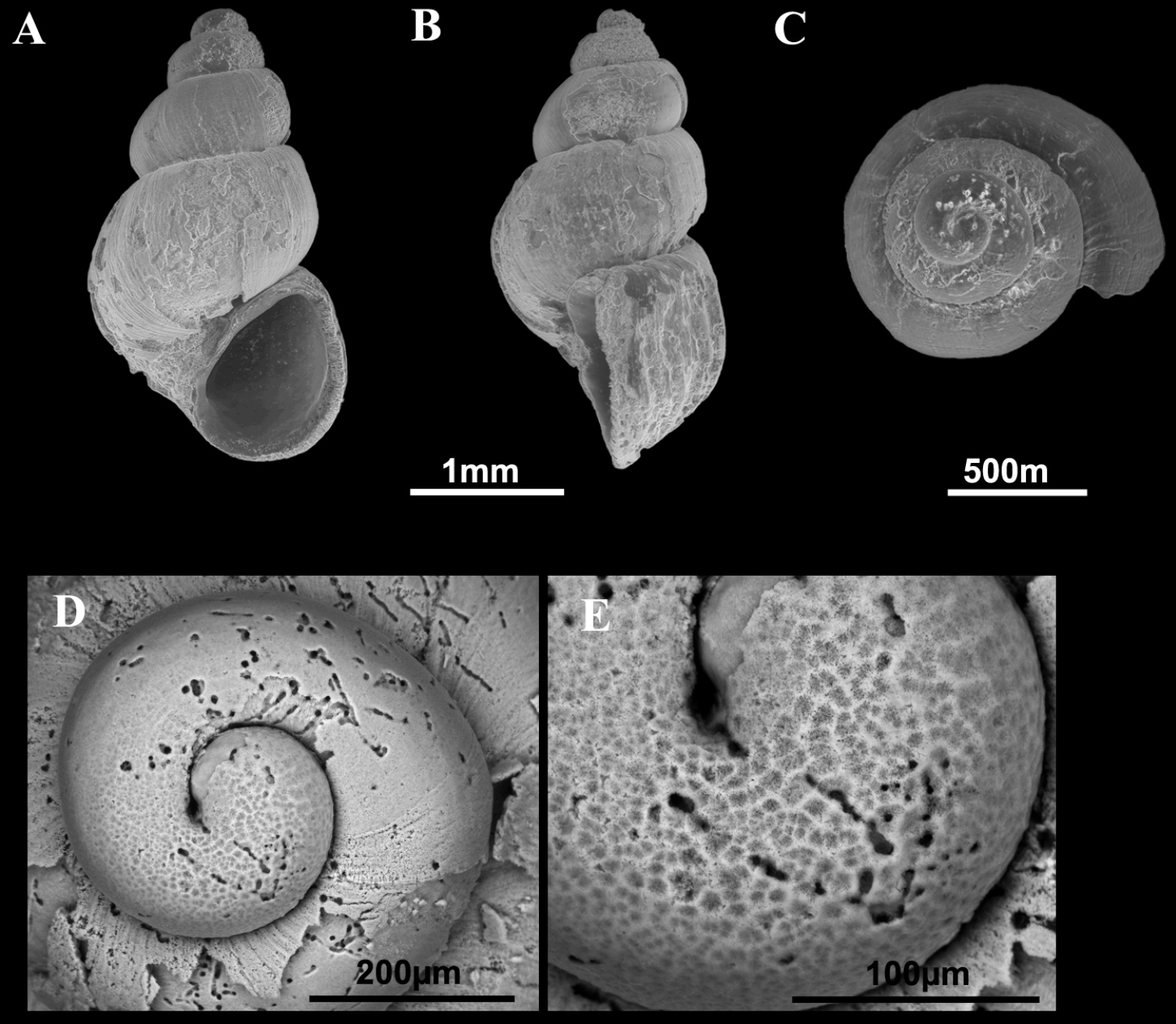
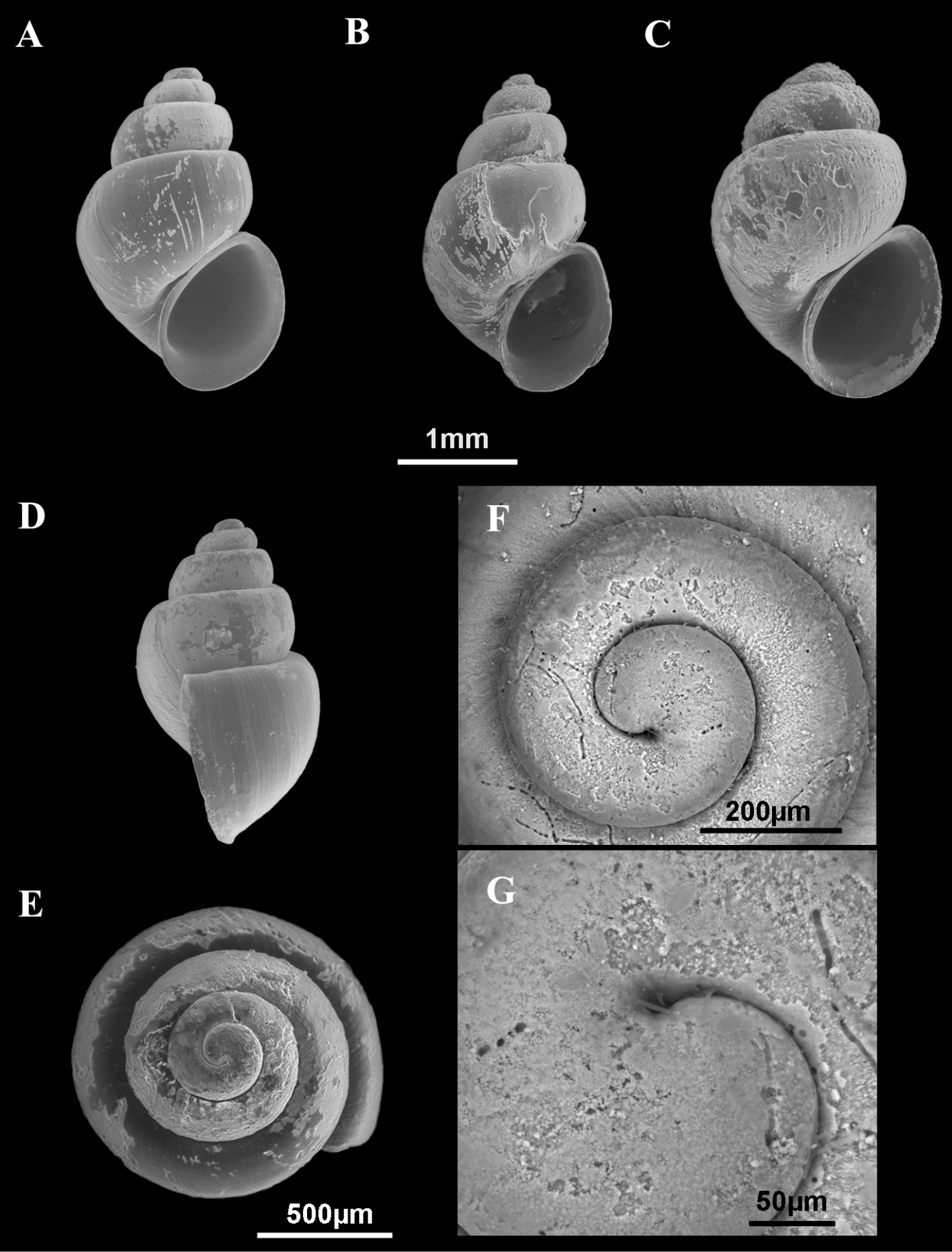
Shells of Pseudamnicola (Corrosella) astieri. A–E Shells from Source d’Argens, Brue-Aurillac, Var, France D–E Protoconch and detail of the microsculpture from the protoconch.
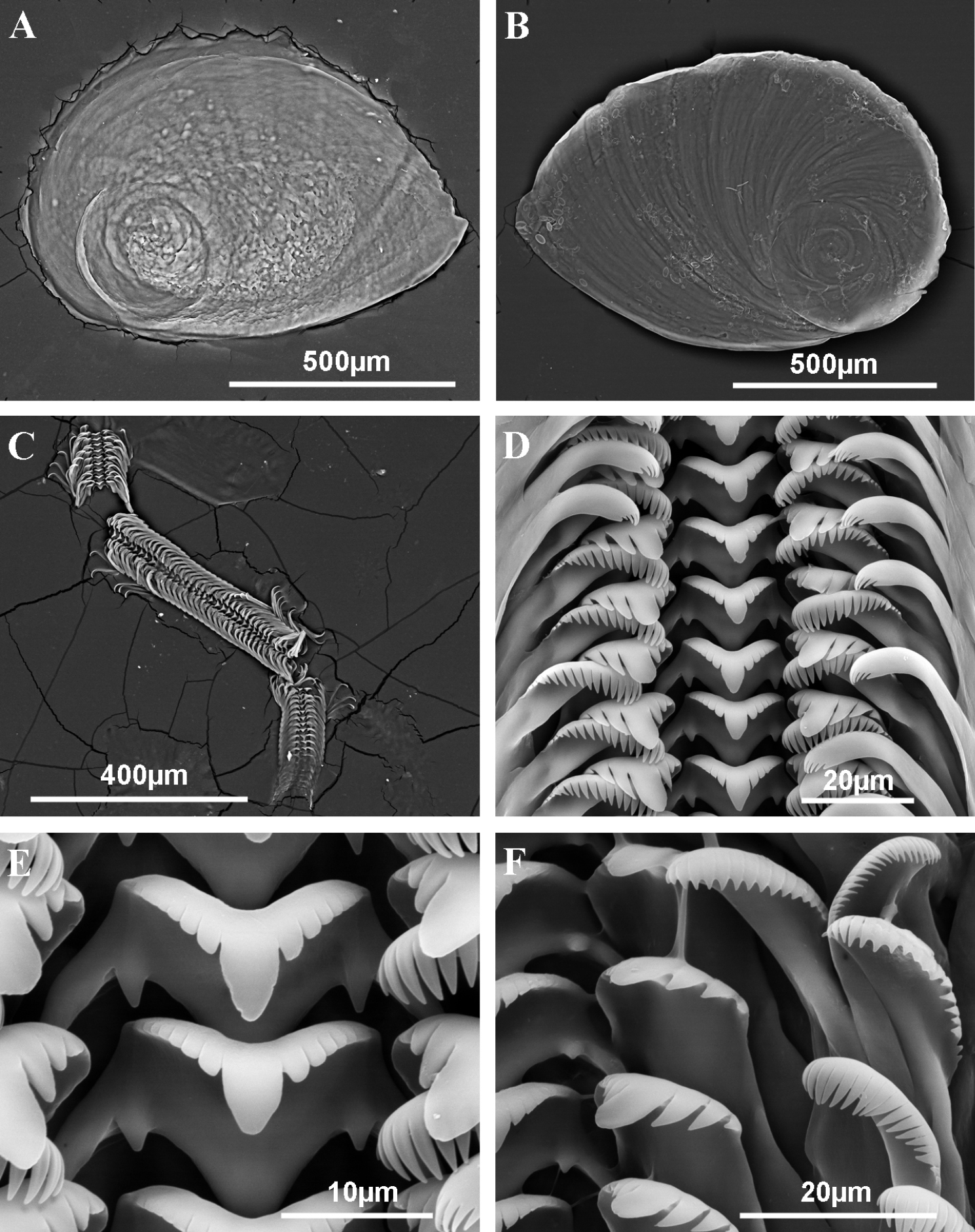
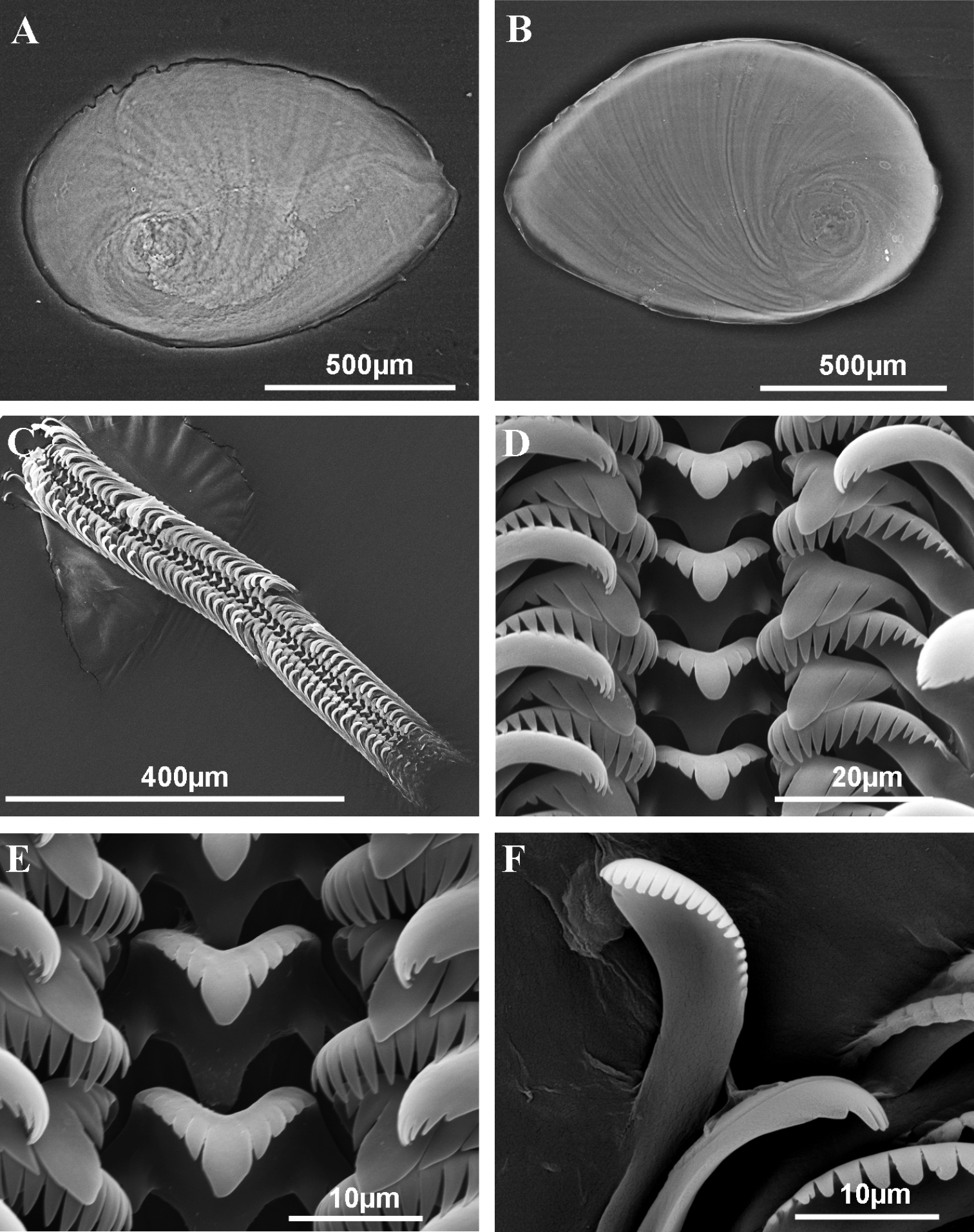
Operculum and radula of Pseudamnicola (Corrosella) astieri from Source d’Argens, Brue-Aurillac, Var, France. A Internal side of the operculum B External side of the operculum C Radula D Rows of teeth of the radula E Central tooth F Lateral, internal and external marginal teeth.
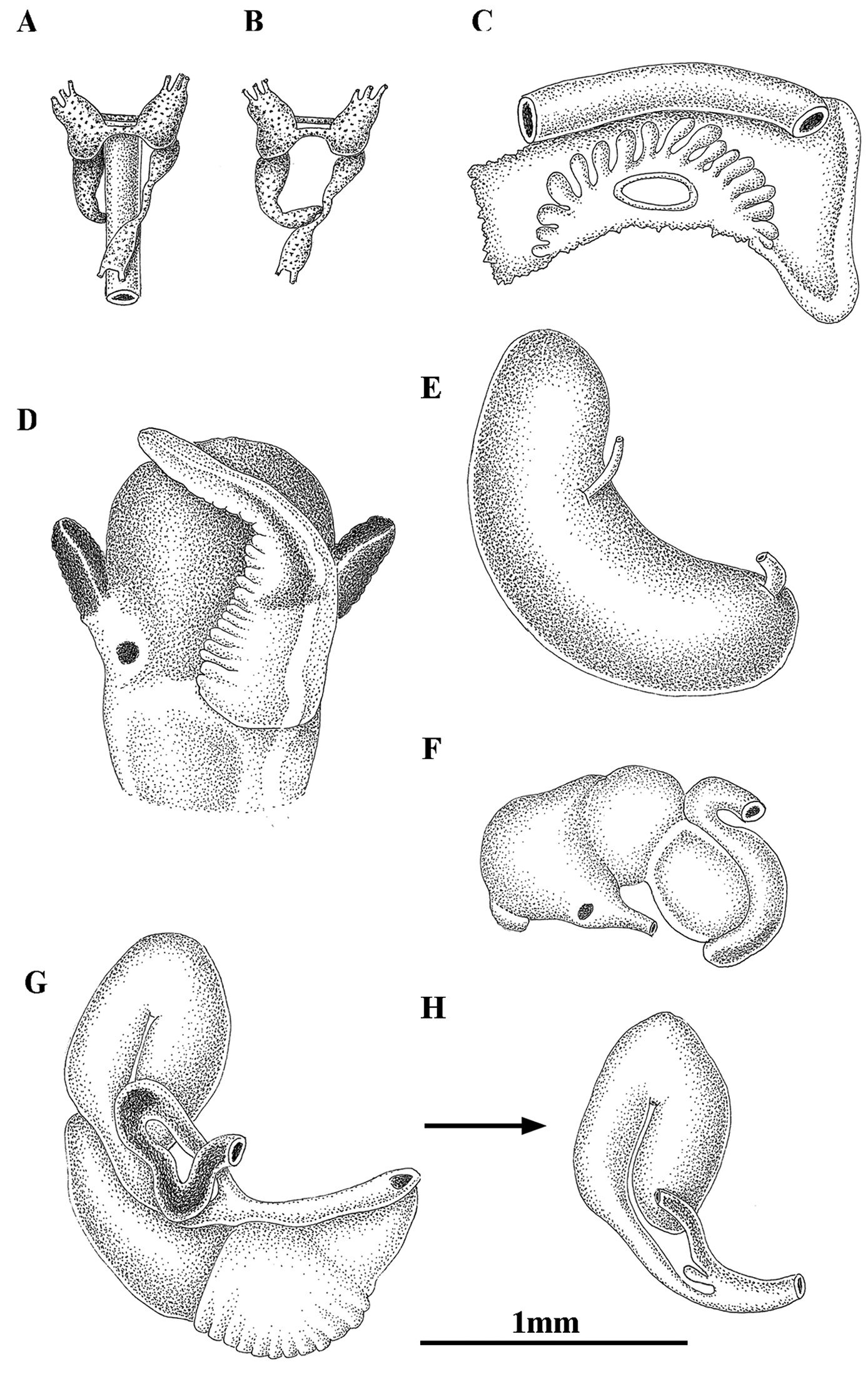
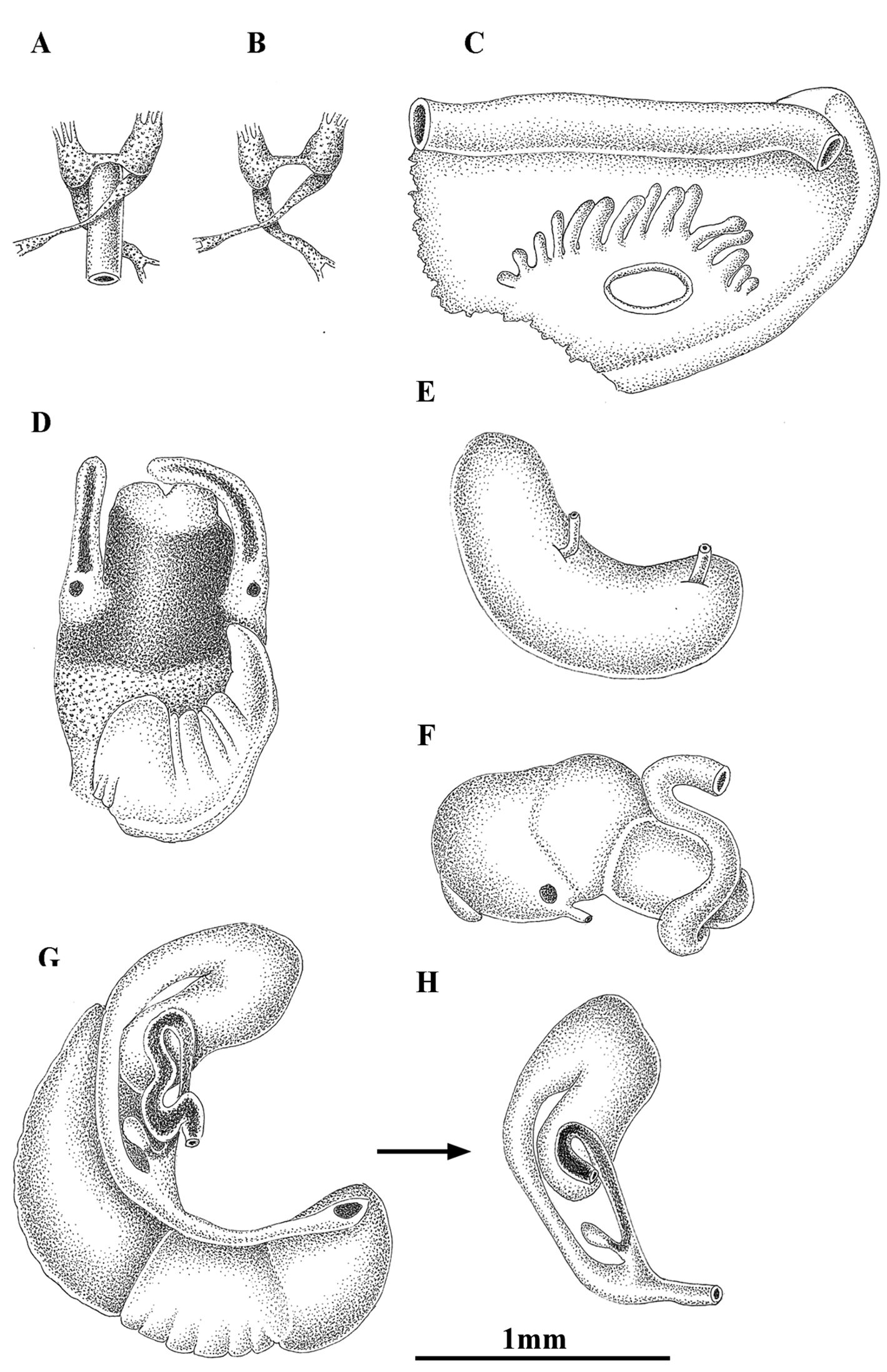
Anatomy of Pseudamnicola (Corrosella) astieri from Source d’Argens, Brue-Aurillac, Var, France. A, B Partial nervous system C Ctenidium and osphradium D Head of a male and penis E Prostate gland F Stomach G Female genitalia H Bursa copulatrix and seminal receptacle.
A few specimens collected from Source d’Argens , Brue-Aurillac, Var, France after finding the type area and other localities in Alpes-Maritimes and Var practically destroyed by severe storms. A total of two females and four males have been examined for anatomical descriptions.
Source d’Argens, Brue-Aurillac, Var, France, 43°30.24'N, 5°54.43'E, D.D., 21 June 2010, MNCN 15.05/60025 (70° ethanol, Figures 2–4) and MNCN/ADN 54949–54951 (absolute ethanol). For more localities, see
Shell yellowish or whitish with a body whorl occupying 2/3 shell length and a deep suture between whorls; protoconch microsculpture granulated; central radular tooth formula 7-C-7; style sac surrounded by a black pigmented intestine; elongate bursa copulatrix U-shaped; elongate seminal receptacle without duct; penis slender with a black patch of pigmentation and some folds in its middle region; nervous system brown pigmented with supraoesophageal connective about two times longer than suboesophageal.
Shellovate-conic with 4–4.75 spire whorls, height 2.5–3.5 mm (Figure 2A–C; Table 2); periostracum yellowish or whitish; protoconch approximately 370 µm wide with 1.5 whorls and a nucleus around 150 µm long (Figure 2D, E); protoconch microsculpture granulated, more intense on apex (Figure 2E); body whorl about 2/3 total length; teleoconch whorls convex with a deep suture; peristome orthocline; aperture complete, oval, with an inner lip thicker than outer lip; peristome margin simple, straight (Figure 2B).
Shell measurements (in mm) of Pseudamnicola (Corrosella) astieri from d’Argens spring, Seillons, France and Pseudamnicola (Corrosella) hauffei sp. n. from Los Nogales spring, Benafer, Castellón, Spain. Probability p values of T-test are provided for each variable, n.s. = no significant.
|
Pseudamnicola (Corrosella) astieri (n=11) |
Pseudamnicola (Corrosella) hauffei sp. n. (n=18) |
T-test (p values) |
|||||
|---|---|---|---|---|---|---|---|
|
Mean (Max-Min) |
SDN | CV |
Mean (Max-Min) |
SDN | CV | ||
| SL | 3.11 (3.56–2.58) |
0.25 | 0.08 | 2.50 (2.85–2.17) |
0.18 | 0.07 | p < 0.001 |
| SW | 1.92 (2.28–1.74) |
0.15 | 0.08 | 1.59 (1.78–1.44) |
0.10 | 0.06 | p < 0.001 |
| SL/SW | 1.62 (1.76–1.45) |
0.10 | 0.06 | 1.57 (1.73–1.46) |
0.07 | 0.04 | n.s. |
| AH | 1.37 (1.78–1.26) |
0.14 | 0.10 | 1.20 (1.36–1.04) |
0.08 | 0.07 | p < 0.001 |
| SL–LBW | 1.10 (1.39–0.76) |
0.16 | 0.15 | 0.69 (1.00–0.49) |
0.13 | 0.19 | p < 0.001 |
| WBW | 1.76 (1.89–1.59) |
0.09 | 0.05 | 1.42 (1.57–1.29) |
0.07 | 0.05 | p < 0.001 |
| AL | 1.39 (1.72–1.28) |
0.12 | 0.08 | 1.26 (1.41–1.11) |
0.09 | 0.07 | p < 0.01 |
| AW | 1.01 (1.33–0.85) |
0.14 | 0.14 | 0.89 (1.11–0.78) |
0.08 | 0.09 | p < 0.01 |
| WPW | 1.23 (1.33–1.11) |
0.08 | 0.07 | 0.95 (1.12–0.80) |
0.08 | 0.08 | p < 0.001 |
| WAW | 0.19 (0.32–0.15) |
0.06 | 0.32 | 0.28 (0.37–0.19) |
0.04 | 0.14 | p < 0.001 |
| NSW | 4.30 (4.75–4.00) |
0.31 | 0.07 | 4.18 (4.50–4.00) |
0.21 | 0.05 | n.s. |
SDN, Unbiased estimate for Standard Deviation, CV, Coefficient of Variation.
Operculum corneous, yellowish, thin, pliable, ellipsoidal, paucispiral with nucleus submarginal (Figure 3A, B; Table 3); muscle attachment area oval, located near the nucleus.
Operculum measurements (in mm) of Pseudamnicola (Corrosella) astieri from d’Argens spring, Seillons, France and Pseudamnicola (Corrosella) hauffei sp. n. from Los Nogales spring, Benafer, Castellón, Spain.
| Pseudamnicola (Corrosella) astieri (n=5) | Pseudamnicola (Corrosella) hauffei sp. n. (n=7) | |||||
|---|---|---|---|---|---|---|
| Mean (Max-Min) | SDN | CV | Mean (Max-Min) | SDN | CV | |
| OL | 1.10 (1.18–0.96) | 0.05 | 0.05 | 1.08 (1.18–0.96) | 0.08 | 0.08 |
| OW | 0.79 (0.84–0.73) | 0.04 | 0.05 | 0.74 (0.84–0.66) | 0.06 | 0.08 |
| OLWL | 0.48 (0.56–0.40) | 0.05 | 0.11 | 0.49 (0.63–0.36) | 0.08 | 0.17 |
| OLWW | 0.34 (0.40–0.25) | 0.05 | 0.16 | 0.33 (0.38–0.29) | 0.03 | 0.09 |
| NL | 0.47 (0.52–0.39) | 0.04 | 0.09 | 0.36 (0.44–0.26) | 0.06 | 0.17 |
| NW | 0.36 (0.42–0.31) | 0.04 | 0.12 | 0.29 (0.45–0.21) | 0.06 | 0.22 |
SDN, Unbiased estimate for Standard Deviation, CV, Coefficient of Variation.
Radula intermediate length (20% total shell length) bearing some 50 rows of teeth (Figure 3C, Table 4); central tooth has a tongue-shaped median cusp and seven blunt lateral cusps (Figure 3D, E); lateral teeth with three tapered cusps on each side of a long central tongue-shaped cusp; inner marginal teeth have 18 sharp cusps, shortening towards the tooth base; outer marginal teeth with 19 sharp cusps (Figure 3D, F).
Radula formulae and measurements (in mm) of three radulae of Pseudamnicola (Corrosella) astieri from d’Argens spring, Seillons, France and three of Pseudamnicola (Corrosella) hauffei sp. n. from Los Nogales spring, Benafer, Castellón, Spain.
| Pseudamnicola (Corrosella) astieri | Pseudamnicola (Corrosella) hauffei sp. n. | |
|---|---|---|
| Central teeth | 7+C+7/1–1 | 5+C+5/1–1 |
| Central teeth width | ~ 20 µm | ~ 15 µm |
| Lateral teeth | 3–C–3 | 3–C–3 |
| Inner marginal teeth | ≥ 18 cusps | ≥ 15 cusps |
| Outer marginal teeth | ≥ 19 cusps | ≥ 19 cusps |
| Radula length | ~ 700 µm | ~ 600 µm |
| Radula width | ~ 90 µm | ~ 95 µm |
| Number of rows | ~ 55 | ~ 50 |
Pigmentation and anatomy: Head dark brown pigmented from snout to neck (Figure 4D); pigmentation clearer on neck; tentacles also brown pigmented except for a narrow band on these and on ocular lobes; snout long as wide, with medial lobation; foot intermediate length and pigmented in dorsal region. Ctenidium in middle region of pallial cavity filling ca. 70% of its length with 17–18 gill filaments; osphradium intermediate width under central gill filaments (Figure 4C, Table 5). Stomach slightly longer than wide with a small posterior caecum; style sac shorter than stomach and surrounded by intestine black pigmented (Figure 4F, Table 5).
Ctenidium, osphradium and digestive system measurements (in mm) of Pseudamnicola (Corrosella) astieri from d’Argens spring, Seillons, France and Pseudamnicola (Corrosella) hauffei sp. n. from Los Nogales spring, Benafer, Castellón, Spain.
|
Pseudamnicola (Corrosella) astieri (n=5) |
Pseudamnicola (Corrosella) hauffei sp. n. (n=7) |
|||||
|---|---|---|---|---|---|---|
|
Mean (Max-Min) |
SDN | CV |
Mean (Max-Min) |
SDN | CV | |
| Ct L | 1.06 (1.25–0.90) |
0.15 | 0.14 | 1.11 (1.27–0.92) |
0.16 | 0.14 |
| Os L | 0.32 (0.43–0.20) |
0.09 | 0.27 | 0.33 (0.45–0.24) |
0.07 | 0.22 |
| Os W | 0.13 (0.15–0.11) |
0.02 | 0.16 | 0.21 (0.25–0.15) |
0.03 | 0.15 |
| Ss L | 0.59 (0.67–0.50) |
0.06 | 0.11 | 0.62 (0.66–0.58) |
0.03 | 0.05 |
| Ss W | 0.35 (0.37–0.31) |
0.02 | 0.06 | 0.37 (0.41–0.33) |
0.03 | 0.08 |
| St L | 0.71 (0.74–0.66) |
0.04 | 0.06 | 0.73 (0.84–0.66) |
0.06 | 0.09 |
| St W | 0.61 (0.67–0.56) |
0.04 | 0.07 | 0.69 (0.78–0.61) |
0.06 | 0.09 |
SDN, Unbiased estimate for Standard Deviation, CV, Coefficient of Variation.
Female genitalia with a slender pallial oviduct (Figure 4G; Table 6); capsule gland longer than albumen gland and consisting of two regions, the posterior one being more transparent; elongate bursa copulatrix, long, folded and U-shaped with a duct about 70% of bursa length; renal oviduct straight and less pigmented from the insertion point of the bursal duct to where it begins to fold and black pigmented making one or two loops; elongate seminal receptacle without duct (Figure 4H) joining renal oviduct just before the point where the bursal duct joins the renal oviduct.
Female and male genitalia measurements (in mm) of two females and four males of Pseudamnicola (Corrosella) astieri from d’Argens spring, Seillons, France and four females and four males of Pseudamnicola (Corrosella) hauffei sp. n. from Los Nogales spring, Benafer, Castellón, Spain.
| Pseudamnicola (Corrosella) astieri | Pseudamnicola (Corrosella) hauffei sp. n. | |||||
|---|---|---|---|---|---|---|
|
Mean (Max-Min) |
SDN | CV |
Mean (Max-Min) |
SDN | CV | |
| Po L | 2.22 (2.35–2.08) |
0.24 | 0.11 | 2.04 (2.32–1.75) |
0.26 | 0.13 |
| Po W | 0.58 (0.59–0.56) |
0.03 | 0.04 | 0.45 (0.49–0.41) |
0.04 | 0.10 |
| Ag. L | 0.95 (1.04–0.84) |
0.18 | 0.18 | 0.81 (1.00–0.71) |
0.14 | 0.17 |
| Cg. L | 1.01 (1.11–0.91) |
0.18 | 0.17 | 1.00 (1.11–0.93) |
0.09 | 0.09 |
| SR1 L | 0.14 (0.15–0.14) |
0.01 | 0.09 | 0.18 (0.22–0.15) |
0.03 | 0.18 |
| BC L | 1.24 (1.32–1.15) |
0.15 | 0.12 | 1.37 (1.56–0.95) |
0.30 | 0.22 |
| BC W | 0.30 (0.35–0.25) |
0.09 | 0.29 | 0.31 (0.36–0.25) |
0.05 | 0.18 |
| dBC L | 0.60 (0.75–0.65) |
0.09 | 0.15 | 0.62 (0.72–0.46) |
0.13 | 0.21 |
| Pr L | 1.67 (1.86–1.55) |
0.14 | 0.08 | 1.37 (1.46–1.26) |
0.09 | 0.06 |
| Pr W | 0.58 (0.69–0.52) |
0.09 | 0.15 | 0.45 (0.51–0.41) |
0.05 | 0.12 |
| P L | 1.26 (1.35–1.15) |
0.09 | 0.07 | 1.28 (1.60–1.12) |
0.24 | 0.19 |
| P W | 0.37 (0.40–0.33) |
0.03 | 0.09 | 0.66 (0.75–0.50) |
0.12 | 0.18 |
| PL/Head length | 1.02 (1.16–0.86) |
0.16 | 0.16 | 0.91 (1.07–0.82) |
0.15 | 0.17 |
SDN, Unbiased estimate for Standard Deviation, CV, Coefficient of Variation.
Male genitalia bear a bean-shaped prostate gland about three times longer than wide (Figure 4E, Table 6); penis long, slender, with a black patch of pigmentation and some folds in its middle region; attachment area behind right eye (Figure 4D); penial duct scarcely visible running straight close to the outer penis margin.
Nervous system brown pigmented, consisting of disperse points of pigmentation; cerebral ganglia equal in size; supraoesophageal connective more than two times longer than suboesophageal (Figure 4A, B; Table 7). Mean RPG ratio 0.42 (moderately concentrated).
Nervous system measurements (in mm) of Pseudamnicola (Corrosella) astieri from d’Argens spring, Seillons, France and Pseudamnicola (Corrosella) hauffei sp. n. from Los Nogales spring, Benafer, Castellón, Spain.
|
Pseudamnicola (Corrosella) astieri (n=5) |
Pseudamnicola (Corrosella) hauffei sp. n. (n=5) |
|||||
|---|---|---|---|---|---|---|
|
Mean (Max-Min) |
SDN | CV |
Mean (Max-Min) |
SDN | CV | |
| LRCG | 0.22 (0.24–0.20) |
0.02 | 0.10 | 0.21 (0.23–0.18) |
0.02 | 0.10 |
| LLCG | 0.22 (0.25–0.18) |
0.03 | 0.15 | 0.22 (0.24–0.19) |
0.02 | 0.10 |
| LCC | 0.11 (0.12–0.07) |
0.02 | 0.19 | 0.15 (0.19–0.12) |
0.02 | 0.14 |
| LRPG | 0.13 (0.18–0.10) |
0.03 | 0.25 | 0.15 (0.16–0.12) |
0.02 | 0.14 |
| LLPG | 0.17 (0.20–0.13) |
0.03 | 0.19 | 0.23 (0.28–0.20) |
0.03 | 0.14 |
| LsupG | 0.14 (0.17–0.11) |
0.03 | 0.23 | 0.12 (0.15–0.10) |
0.02 | 0.18 |
| LsubG | 0.11 (0.13–0.09) |
0.01 | 0.10 | 0.12 (0.14–0.09) |
0.02 | 0.18 |
| LPsupC | 0.18 (0.24–0.11) |
0.05 | 0.30 | 0.29 (0.39–0.21) |
0.07 | 0.26 |
| LPsubC | 0.07 (0.09–0.06) |
0.01 | 0.15 | 0.10 (0.13–0.05) |
0.03 | 0.32 |
| RPG | 0.40 (0.49–0.38) |
0.05 | 0.13 | 0.51 (0.58–0.46) |
0.05 | 0.10 |
SDN, Unbiased estimate for Standard Deviation, CV, Coefficient of Variation.
The only available information on the anatomy of this species in the literature corresponded to populations from Foux à Draguignan (figure 2, 4, 7, 9 in
Comparingshell sizes among the Pseudamnicola (Corrosella) species from the northern half of Iberian Peninsula, the shells of Pseudamnicola (Corrosella) astieri are larger (2.5–3.5 mm) than those of Pseudamnicola (Corrosella) hauffei sp. n. (2.20–2.90 mm) (see statistically significant differences in shell measurements in Table 2) and Pseudamnicola (Corrosella) hinzi
urn:lsid:zoobank.org:act:4DC6D03C-09B8-4E52-924C-39D6B01BBF42
Los Nogales spring, Benafer, Castellón, Spain, 30°55.80'N, 0°34.34'W.
Holotype MNCN 15.05/60026a (SEM preparation, Figure 5A) and paratypes (Figures 5D–G, 6, 7) MNCN 15.05/60026b (SEM preparation, Figures 5D–G, 6, and 70° ethanol, Figure 7) and MNCN/ADN 54952–54969 (frozen material and 70° ethanol), D.D. & C.N., 19 March 2009; MNCN 15.05/60027 (70° ethanol), 26 May 1998, B.A.
Shells of Pseudamnicola (Corrosella) hauffei sp. n. A, D–G Shells from Nogales spring, Benafer, Castellón, Spain B Shell from San Miguel spring, Viver, Castellón, Spain C Shell from Agadín spring, Benafer, Castellón, Spain E–G Protoconch and microsculpture.
Four males and four females from type locality were examined for anatomical study. In addition, some populations from provinces of Castellón and Valencia (Spain) were also found and studied, dissecting likewise two males and two females from each for their identification.
Los Nogales spring, Benafer, Castellón, Spain (type locality), 30°55.80'N, 0°34.34'W, B.A., 26 May 1998, MNCN 15.05/60027 (70° ethanol); D.D. & C.N., 19 March 2009, MNCN 15.05/60026 (70° ethanol and ESEM preparation, Figures 5A, D–G) and MNCN/ADN 54952–54969 (frozen material); Agadín spring, Benafer, Castellón, Spain, 39°56.38'N, 0°34.54'W, D.D. & C.N., 19 March 2009, MNCN 15.05/60028 (70° ethanol and ESEM preparation, Figure 5C) and MNCN/ADN 54970–54974 (frozen material); irrigation ditch in Navajas, Castellón, Spain, 39°52.09'N, 0°30.37'W, R.A., D.M. & J.M.R 7 March 1990, MNCN 15.05/60029 (70° ethanol); Curso spring, Navajas, Castellón, Spain, 39°52.43'N, 0°30'W, B.A., 25 May 1998, MNCN 15.05/60030 (70° ethanol) and MNCN/ADN 54975–54989 (frozen material); La Peña spring, Navajas, Castellón, Spain, 39°52.77'N, 0°30.03'W, R.A., D.M. & J.M.R, 7 March 1990, MNCN 15.05/60031 (70° ethanol); La Esperanza spring, Navajas, Castellón, Spain, 39°52.19'N, 0°30.43'W, R.A., D.M. & J.M.R, 7 March 1990, MNCN 15.05/60032 (70° ethanol); Del Prado spring, Viver, Castellón, Spain, 39°56.23'N, 0°36.81'W, D.D. & C.N., 19 March 2009, MNCN 15.05/60033 (70° ethanol) and MNCN/ADN 54990–54992 (frozen material); San Miguel spring, Viver, Castellón, Spain, 39°55.68'N, 0°36.64'W, B.A., 25 May 1998, MNCN 15.05/60034 (70° ethanol); D.D. & C.N., 19 March 2009, MNCN 15.05/60035 (70° ethanol and ESEM preparation, Figure 5B) and MNCN/ADN 54997–54999 (70° ethanol); San Miguel ditch, Viver, Castellón, Spain, 39°55.68'N, 0°36.64'W, D.D. & C.N., 19 March 2009, MNCN 15.05/60036 (70° ethanol) and MNCN/ADN 54993–54996 (frozen material); Font Nova, Benifaió, Valencia, Spain, 39°0.55'N, 0°5.87'W, B.A., 26 May 1998, MNCN 15.05/60037 (70° ethanol); Cortés de Pallás, Valencia, Spain, 39°14.61'N, 0°26.01'W, B.A., 26 May 1998, MNCN 15.05/60038 (70° ethanol).
Shell, anatomical, operculum and radular measurements (Tables 2–7) were made on specimens from the type locality, Los Nogales spring in Benafer, Castellón.
Dedicated to the malacologist and ecologist Torsten Hauffe, for his help and support during the stay of the first author in Germany.
Shell yellowish with body whorl occupying 2/3 shell length; umbilicus slightly visible; protoconch microsculpture grooved; central radular tooth formula 5-C-5; style sac protruding below non-pigmented intestine; elongate bursa copulatrix J-shaped; renal oviduct pigmented until seminal receptacle, which has a pigmented short duct; penis triangular with a wide base attached to central area of head; nervous system brown pigmented with supraoesophageal connective about three times longer than suboesophageal.
Shellovate-conic (Figure 5A–C), yellowish periostracum with 4–4.5 spire whorls, height around 2.0–3.0 mm (Table 2); protoconch approximately 450 µm wide with 1.5 whorls and a nucleus around 200 µm long (Figure 5E, F); protoconch microsculpture grooved (Figure 5G); body whorl about 2/3 total length; whorls convex with deep suture; peristome frontal, complete, oval, with thick inner lip partly hiding umbilicus; outer peristome simple, straight (Figure 5D).
Operculum corneous, yellowish, thin, pliable, ellipsoidal, paucispiral, with nucleus submarginal (Figure 6A, B; Table 3); oval muscle attachment near nucleus.
Operculum and radula of Pseudamnicola (Corrosella) hauffei sp. n. from Nogales spring, Benafer, Castellón, Spain A Internal side of the operculum B External side of the operculum C Radula D Rows of teeth of the radula E Central tooth F External marginal teeth.
Radula with around 50 rows of teeth, medium in size (25% total shell length) (Figure 6C, Table 4); central tooth with a tongue-shaped median cusp and five lateral cusps, slightly sharpening towards central one (Figure 6D, E); lateral teeth with a long tongue-shaped median cusp and three tapered laterals; inner and outer marginal teeth bear 15 and 19 sharp cusps respectively (Figures 6D, F).
Pigmentation and anatomy: Head intensely brown pigmented from snout to neck (Figure 7D); pigment on neck clearer than on head; brown band of pigment also on tentacles, but not on ocular lobes; snout as long as wide, with medial lobation; foot intermediate length, pigmented on dorsal region. Ctenidium in the anterior region of pallial cavity with about 15 gill filaments; osphradium ellipsoidal under central gill filaments (Figure 7C, Table 5). Stomach slightly longer than wide (Figure 7F); style sac barely shorter than stomach, protruding below intestine (Table 5).
Anatomy of Pseudamnicola (Corrosella) hauffei sp. n. from Nogales spring, Benafer, Castellón, Spain. A, B Partial nervous system C Ctenidium and osphradium D Head of a male and penis E Prostate gland F Stomach G Female genitalia H Bursa copulatrix and seminal receptacle.
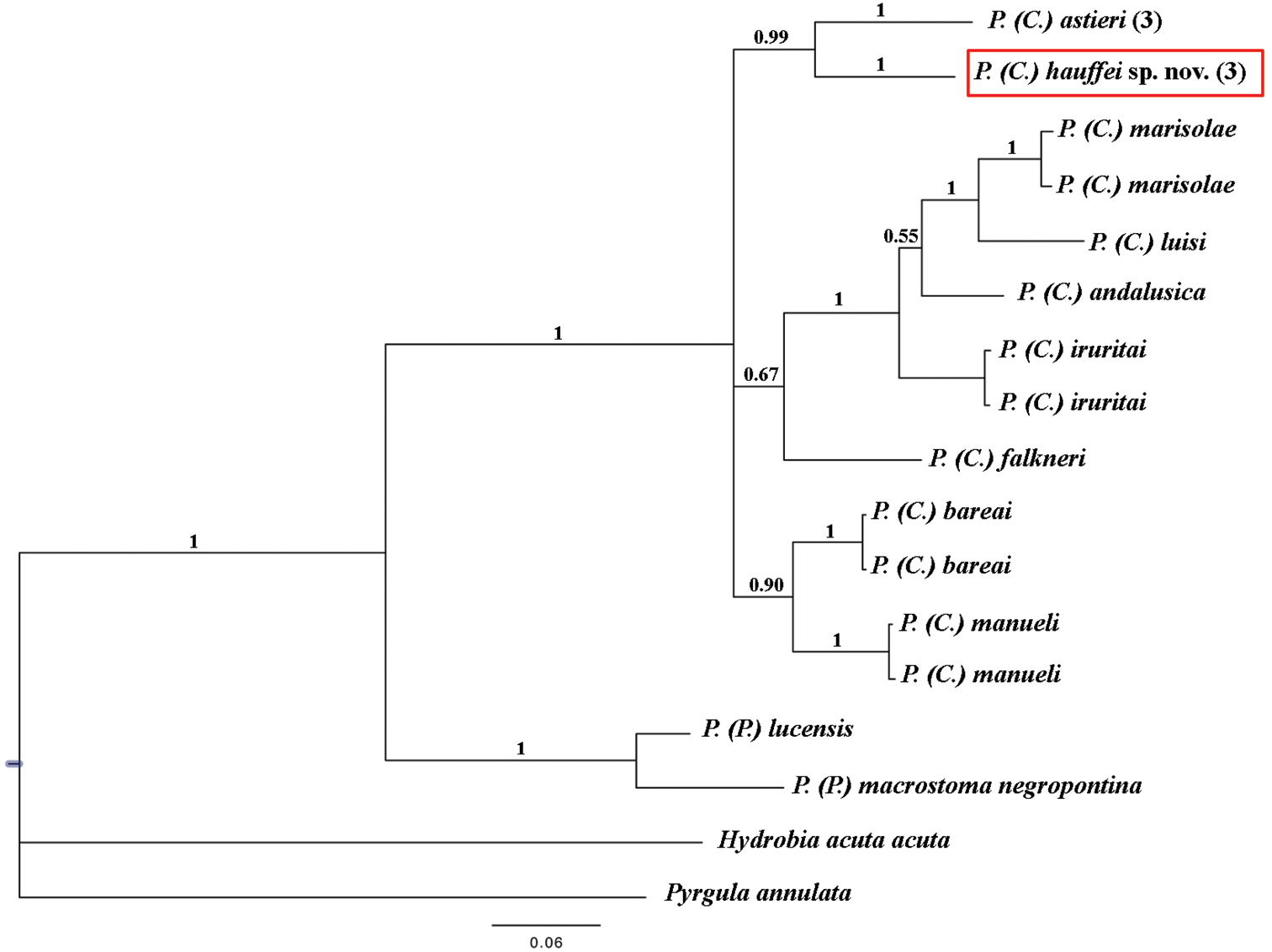
Bayesian 50% majority rule consensus tree inferred employing COI mitochondrial gene partial sequence. The numbers above branches represent Bayesian posterior probabilities. The numbers between brackets symbolize specimens with identical haplotypes. Scale bar: expected changed per site.
Female genitalia with a pallial oviduct about four times longer than wide (Figure 7G; Table 6); capsule gland slightly longer than albumen gland and denser in posterior region; genital aperture in the anterior extreme of pallial oviduct; elongate bursa copulatrix, J-shaped folded with a duct less than 50% bursa length; renal oviduct scarcely pigmented from the insertion point of bursal duct to where it begins to fold and black pigmented, making two or three loops; elongate seminal receptacle with pigmented short duct (Figure 7H) joining renal oviduct slightly above the point where the bursal duct joins the renal oviduct.
Male genitalia bearing a bean-like prostate gland about three times longer than wide (Figure 7E, Table 6); penis triangular with a wide base attached to central area of head with some folds in middle section and a narrow patch of black pigment on distal surface (Figure 7D); vas deferens uncoiled in penis running straight close to the external margin.
Nervous system brown pigmented, but ganglia darker than connectives and commissures; cerebral ganglia equal in size; supraoesophageal and suboesophageal ganglia similar in shape and size; supraoesophageal connective around three times longer than suboesophageal (Figure 7A, B; Table 7). Mean RPG ratio 0.51 (elongated).
Some of the localities where this species was found were cited by
Compared to the other Pseudamnicola (Corrosella) species living in nearby areas, Pseudamnicola (Corrosella) hinzi and Pseudamnicola (Corrosella) navasiana, Pseudamnicola (Corrosella) hauffei sp. n. has a shorter and more ovate shell shape, a longer bursa copulatrix, bursa duct and seminal receptacle, and a more triangular wider-based penis.
The data set analysed included data for 11 Pseudamnicola species and 658 characters of the COI gene. New sequences for both species were deposited in Genbank under accession numbers JQ067672 – JQ067677, while the rest of the sequences were obtained from this same database (see Table 1). Hydrobia acuta acuta (Draparnaud, 1805) and Pyrgula annulata (Linnæus, 1758) were used as outgroups.
Pseudamnicola (Corrosella) hauffei sp. n. differed 7.44% with respect to Pseudamnicola (Corrosella) astieri specimens and moreover, both were clustered as sister species (Table 8 and Figure 8). Through Bayesian analysis, the subgenus Corrosella was found to be well supported and divided into three clades, whose phylogenetic relationships are still unclear. The clades comprising Pseudamnicola (Corrosella) hauffei sp. n. and Pseudamnicola (Corrosella) astieri, or Pseudamnicola (Corrosella) manueli and Pseudamnicola (Corrosella) bareai were well supported (posterior probabilities over 0.90). However the clade including Pseudamnicola (Corrosella) marisolae, Pseudamnicola (Corrosella) luisi, Pseudamnicola (Corrosella) andalusica, Pseudamnicola (Corrosella) iruritai and Pseudamnicola (Corrosella) falkneri was not well supported.
Genetic divergence matrix for the species examined based on the COI gene sequence.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Pseudamnicola (Corrosella) astieri | - | ||||||||||||
| 2. Pseudamnicola (Corrosella) hauffei sp. n. | 7.44 | - | |||||||||||
| 3. Pseudamnicola (Corrosella) marisolae | 10.26 | 10.41 | - | ||||||||||
| 4. Pseudamnicola (Corrosella) luisi | 10.79 | 12.16 | 6.00 | - | |||||||||
| 5. Pseudamnicola (Corrosella) iruritai | 8.13 | 9.42 | 6.38 | 6.69 | - | ||||||||
| 6. Pseudamnicola (Corrosella) andalusica | 9.11 | 10.64 | 6.46 | 6.54 | 5.39 | - | |||||||
| 7. Pseudamnicola (Corrosella) falkneri | 8.97 | 8.81 | 9.19 | 10.33 | 7.83 | 8.05 | - | ||||||
| 8. Pseudamnicola (Corrosella) bareai | 7.86 | 7.65 | 9.14 | 9.59 | 7.87 | 8.50 | 8.42 | - | |||||
| 9. Pseudamnicola (Corrosella) manueli | 8.73 | 8.74 | 9.65 | 11.15 | 8.81 | 9.03 | 7.67 | 5.46 | - | ||||
| 10. Pseudamnicola (Pseudamnicola) lucensis | 15.06 | 14.78 | 15.15 | 15.21 | 13.69 | 14.31 | 13.66 | 12.90 | 14.22 | - | |||
| 11. Pseudamnicola (Pseudamnicola) macrostoma negropontina | 14.45 | 14.47 | 14.85 | 14.89 | 14.16 | 14.32 | 13.67 | 14.42 | 14.54 | 7.68 | - | ||
| 12. Hydrobia acuta acuta | 17.91 | 19.49 | 17.60 | 17.26 | 15.78 | 16.35 | 16.62 | 17.12 | 18.29 | 18.65 | 18.97 | - | |
| 13. Pyrgula annulata | 16.67 | 17.44 | 17.55 | 16.67 | 14.99 | 16.82 | 17.58 | 16.09 | 17.05 | 16.90 | 18.14 | 17.38 | - |
Based on this wide morphological study and our molecular data, we were able to delimit both species and clearly rule out the hypothesis of the presence of Pseudamnicola (Corrosella) astieri in the Iberian Peninsula, identifying it as an endemism of the Alpes-Maritimes and Var departments of France, as proposed by
Through a phylogenetic approach based on partial sequence data for the COI gene provided in GenBank for other Pseudamnicola (Corrosella) species, we were able to estimate a mean genetic divergence of about 8% (5.39 to 11.15%) (
Besides clarifying the taxonomic status of these two species and their phylogenetic relationship as sister species, our findings point to a greater diversity of Pseudamnicola (Corrosella) than previously thought, with implications for the protection of this poorly known group of molluscs. Indeed, their fragile ecosystems susceptible to the effects of human activities, altered water regimes, pollution, etc. means that most of these hydrobiid species are seriously threatened or even endangered (see Hydrobiidae spp. by Arconada et al. in
The authors thank Dr. M. Haase and Dr. E. Gittenberger for their constructive comments and suggestions. A.L. Tormo, M. Furio and A. Jorge from the MNCN assisted with the ESEM study and photomicrographs. Drawings were redone by I. Díaz Cortaberría. Dr. M.A. Alonso-Zarazaga provided advice on nomenclature. The English was reviewed by A. Burton. The manuscript was also improved by the comments and suggestions of Dr. R. Hershler. This work has been financed by the MICINN projects Fauna Ibérica IX (CGL2007-66786-C8-01) and FaIb X (CGL2010-22267-C07-01) and the support of a JAE Predoctoral fellowship (JAEPre047) to DD.