(C) 2012 Jason E. Bond. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The trapdoor spider genus Myrmekiaphila currently comprises 11 nominal species. A recent molecular phylogenetic evaluation of the group identified a number of problems with respect to how species and species groups were delineated by Bond and Platnick in their 2007 taxonomic revision of the genus. We report herein the discovery of a new species, Myrmekiaphila tigris sp. n. The phylogenetic position of the species is evaluated using a molecular phylogenetic approach based on a set of mtDNA markers. Our preferred phylogenetic hypothesis supports the recognition of a new species and further highlights the need to more carefully investigate species boundaries within the genus. These results further indicate that palpal bulb morphology is rapidly evolving and has likely been a contributing factor in rendering a number of species paraphyletic with respect to the molecular data.
New species, Integrative taxonomy, Molecular taxonomy, Southeastern United States, Myrmekiaphila, Cyrtaucheniidae, Euctenizinae
The genus Myrmekiaphila Atkinson, 1886 is a moderately diverse assemblage of trapdoor spiders distributed predominantly throughout the southeastern United States. Revised recently by
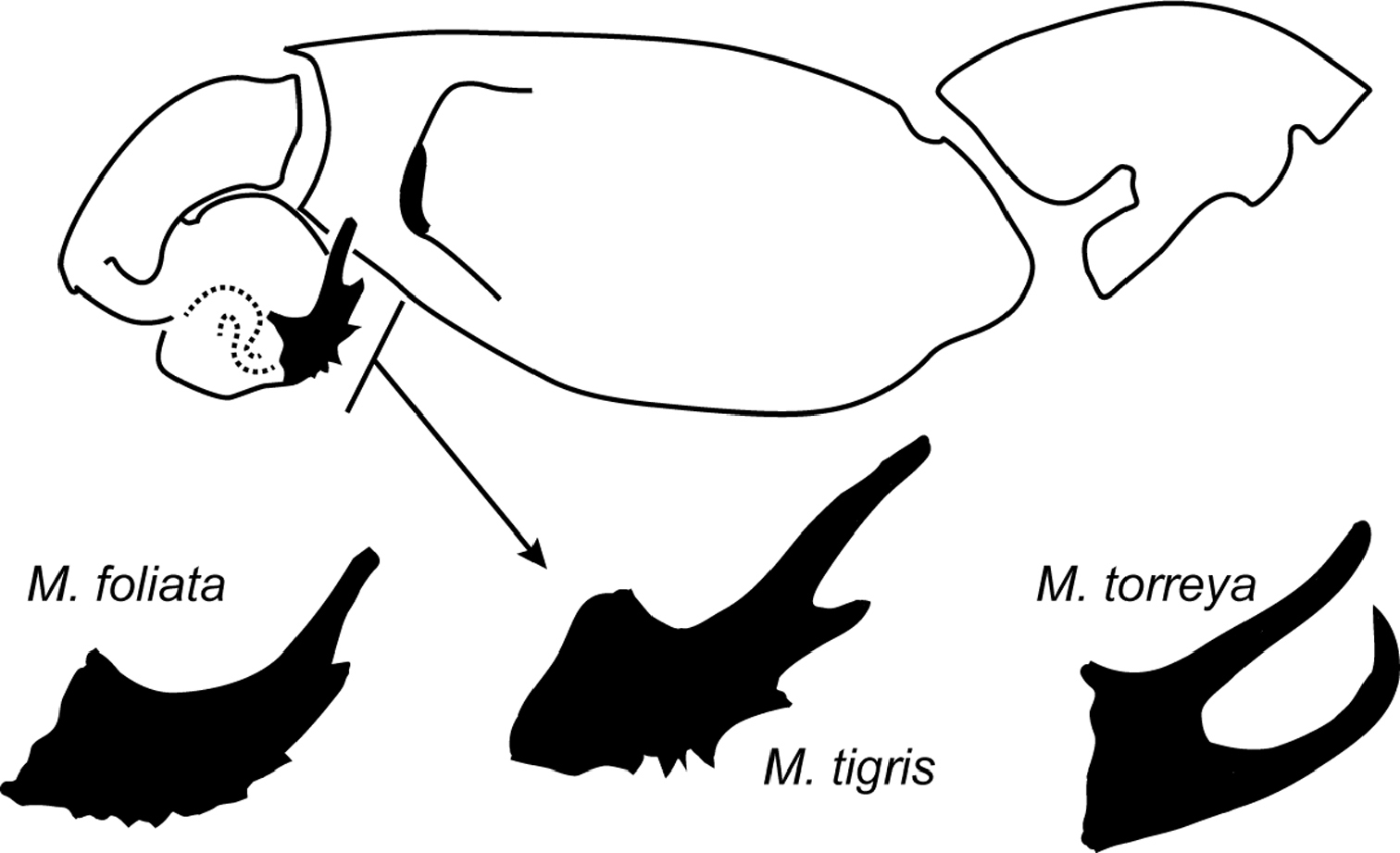
Myrmekiaphila species delimitation and diagnosis relies heavily on modifications of the male first walking leg – often termed the mating clasper – and the morphology of the palpal bulb. Mating clasper morphology is typically discriminated on the basis of unique spination patterns whereas palpal bulb modifications are often quite dramatic comprising relatively pronounced branched and serrated structures (Fig. 1). Such major differences in palpal bulb morphology stand in relatively stark contrast to the paucity of differentiation observed for palpal bulb structure in other related mygalomorph taxa (see
On the basis of palpal differences, presence or absence of a second prong and serration patterns (Fig. 1 but also see
The focus of this paper is the description of a new species, Myrmekiaphila tigris Bond & Ray. This species was brought to the attention of the first author by C. Ray who collected numerous specimens during the winter months (Dec-Jan) of 2011/2012 from sidewalks and swimming pools located in the town of Auburn, Alabama in a moderately populated housing subdivision.
Line drawing of Myrmekiaphila tigris male pedipalp; distal aspect of palpal bulb shown for Myrmekiaphila foliata, Myrmekiaphila tigris, and Myrmekiaphila torreya for comparative purposes.
Unique voucher numbers were assigned to all specimens (AUMS) and corresponding labels added to each vial. Collecting locality latitude/longitude data was georeferenced as described in
Species descriptions are formatted similarly to
Protocols for sample, tissue, DNA extraction, polymerase chain reaction (PCR), and sequencing follow those outlined in
Tree searches on the partitioned data set using Maximum Likelihood and Bayesian inference were conducted using RAxML ver. 7.2.8 (
Phylogenetic data sets (NEXUS and PHYLIP format) for all specimens evaluated in this study; locality data for Myrmekiaphila tigris.
The data underpinning the analysis reported in this paper were deposited on 25 April 2012 in the Dryad Data Repository at doi: 10.5061/dryad.pk24v (Bond et al. 2012) and at GBIF, the Global Biodiversity Information Facility, http://ipt.pensoft.net/ipt/resource.do?r=myr_dataset.
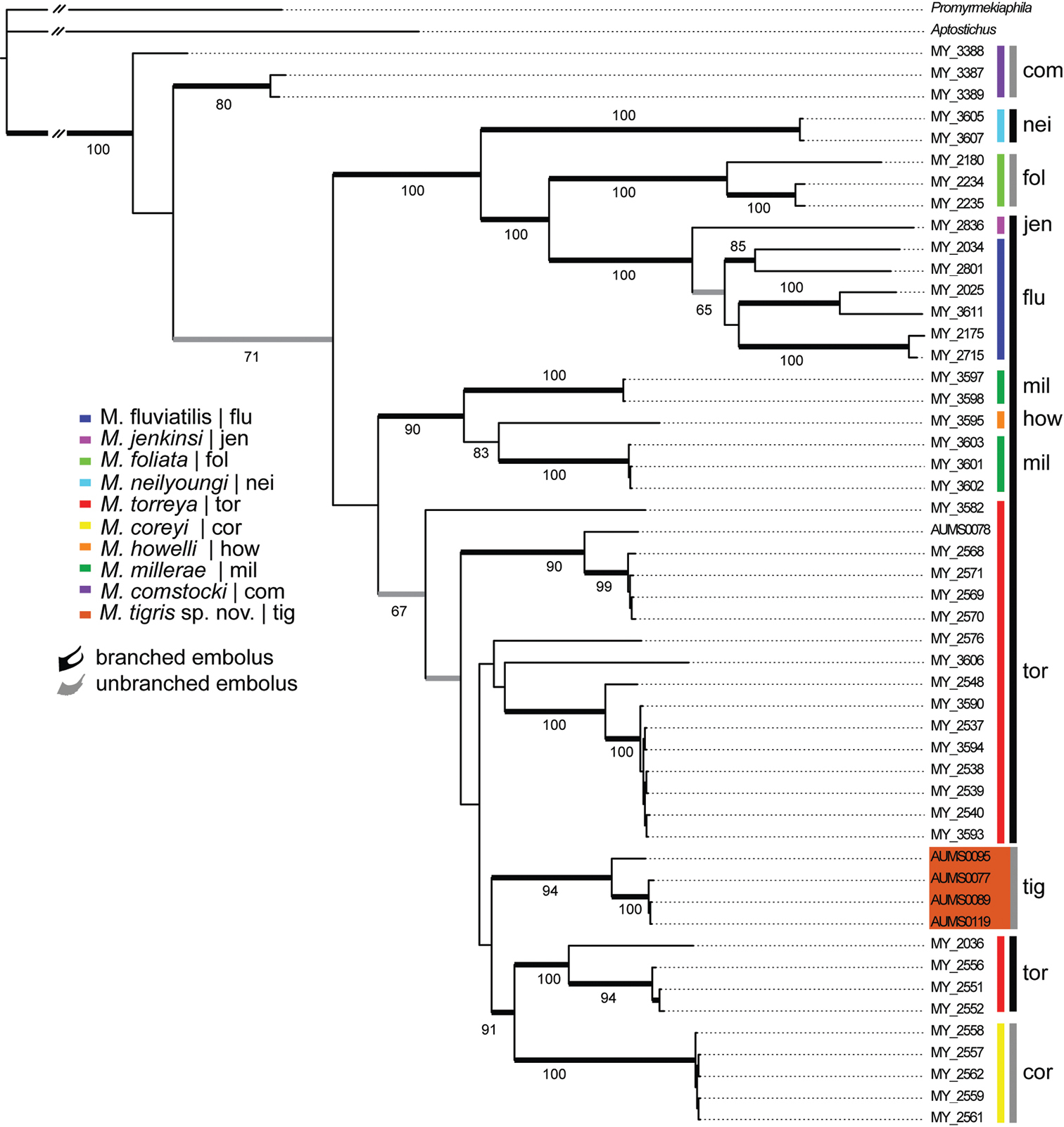
Results and discussionThe –ln likelihood value for the best scoring tree recovered from the RAxML analysis was -11, 248.44. The harmonic and arithmetic means of the log likelihood values for all post burn-in topologies following MrBayes analyses were -11, 307.70 and -11, 364.56, respectively. Figure 2 summarizes the results of the phylogenetic analysis. The trees inferred from the both the Bayesian and likelihood analyses were largely congruent with respect to tree topology; bootstrap support values were less than posterior probabilities at some nodes.
The inferred phylogeny clearly indicates the hypothesized new species is not closely related to Myrmekiaphila foliata and is placed within the somewhat enigmatic torreya species group (sensu
The discovery of a second species with an unbranched embolus phylogenetically “embedded” within the torreya species group further complicates the systematics of the genus and generates more questions regarding species delimitation and the nature of the evolution of these somewhat enigmatic palpal bulb modifications. As discussed by
Preferred tree topology based on Bayesian analysis of the 12S/16S mitochondrial DNA data set. Color key and three-letter identifiers (inset) refer to species defined by
urn:lsid:zoobank.org:act:1879EB33-3133-4A1A-96E5-5C2176A1AFB7
http://species-id.net/wiki/Myrmekiaphila_tigris
Common name: The Auburn Tiger Trapdoor SpiderMap 1; Figs 3–11
Male holotype (AUMS090), female paratype (AUMS089, from Alabama, Lee County, Auburn, along Grove Hill Road, forested area across from intersection with High Point Drive, 32.5786, -85.4543, 180m, coll. J. Bond 25.i.2012; additional male paratypes (AUMS077, 081–084) from same vicinity, coll. C. Ray i.2012. Male holotype and female paratype deposited in AUMNH; additional male paratypes deposited in AUMNH, AMNH and FMNH.
ALABAMA: Choctaw Co.: Silas [31.7654, -88.3290, MYR013], 19.ii.1912 (H. Smith, CUC), 1♂. Lee Co.: Opelika [32.6454. -85.3783, MYR133], 1.i.1985 (D. Folkerts, CDF), 1♂; 3.2km S Auburn along Wire Road [32.5776, -85.5246, MYR135], 24.ii.1974 (R. Skinner, CAU), 1♂; Auburn [32.6099, -85.4808, MYR124], (AMNH), 2♀, 1 juv., [MYR127], (N. Banks, C. Baker, MCZ), 1♀, [MYR132], 10.iv.1941 (AMNH), 3♀, [MYR137], 3.iii.1968 (W. Ivey, CAU), 1♂, [MYR139], 10.v.1975 (B. Muse, CAU), 1♀, [MYR288], 1.vi.1986 (G. Mullen, CAU), 1♀; Auburn, Grove Hill subdivision, 32.5786, -85.4543 (AUMS086–088, 091, 096–115, 117–119, 121, 123–127, 130–138), 180m, xii.2011-i.2012 (C. Ray, D. Held, J. Bond, N. Garrison, AUMNH), 41♂ 2♀, 5 juv. Macon Co.: Tuskegee National Forest, Wire Road, S Interstate 85 [32.4577, –85.6576, MYR138], 12.xii.1975 (Weatherby, Brooks, CAU), 1♀; Tuskegee National Forest, 32.4522, -85.6378 [AUMS094–095], 30.i.2012 (C. Hamilton, AUMNH), 2 juv. Montgomery Co.: McGus Station [MYR140], 24.x.1915 (H. Smith, CUC), 1♂. GEORGIA: Putnam Co.: no specific locality [33.3335, -83.3499, MYR040], 23.iv.1974 (W. Merrill, FSCA) 1♂; Eatonton [33.3268, -83.3885, MYR131], 18.iv.1974 (W. Merrill, FSCA), 1♂.
The specific epithet, the Latin name for tiger, is a noun taken in apposition and refers to the mascot of Auburn University.
Male palpal bulb morphology (Figs 7, 8) is similar to Myrmekiaphila foliata (Fig. 1, inset) but Myrmekiaphila tigris specimens have a longer sinuous embolus with an elongate sub distal tooth. Potentially sympatric Myrmekiaphila torreya males have two-pronged bulb whereas Myrmekiaphila tigris males have only a single prong. Males also appear to have a more robust palpal tibia with a larger retro-distal lateral ledge than in other species (Fig. 7). Females are much more difficult to definitively recognize from other species on the basis of morphological differences, however, the spermathecal base is considerably less wide than noted for Myrmekiaphila torreya (
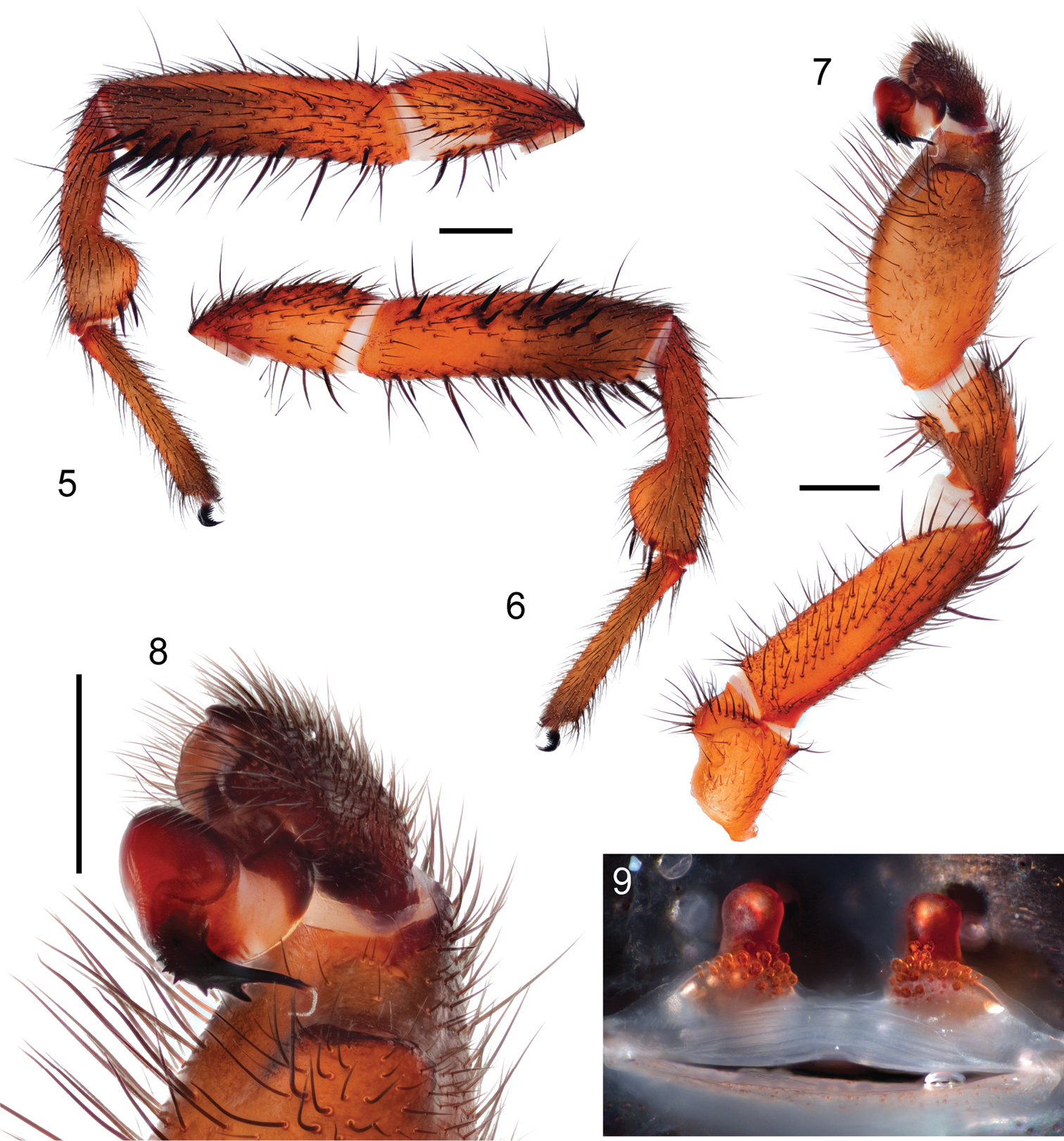
Specimen preparation and condition. Specimen collected live from burrow, preserved in 80%. Pedipalp, leg I left side removed, stored in vial with specimen. General coloration. Carapace dark red 2.5YR 3/6; legs, chelicerae darker in color, dusky red 10R 3/4. Abdomen dark brown 7.5YR 3/2 with broad faint dusky stripes posteriorly dorsal (Figs 3, 4), ventrum spinnerets pale yellow. Cephalothorax. Carapace 6.56 long, 5.63 wide, hirsute with thin black short setae, stout black bristles along fringe; surface smooth, pars cephalica elevated. Fringe, posterior margin with black bristles. Foveal groove deep, straight. Eyes only slight elevated. AER slightly procurved, PER slightly recurved. PME slightly larger in diameter than AME. Sternum moderately setose, STRl 3.72, STRw 3.35. Posterior sternal sigilla large, irregularly shaped, nearly contiguous, anterior sigilla pairs small, oval, marginal. Chelicerae with distinct anterior tooth row comprising 11 teeth, posterior margin with single row small denticles. Palpal endites with patch of small cuspules on proximal, inner margin, labium lacks cuspules, LBw 1.19, LBl 0.70. Rastellum consists of 4 stout spines on distinct mound. Abdomen. Setose, heavy black setae intermingled with fine black setae. Legs. Leg I: 5.85, 2.88, 4.25, 3.41, 2.84; leg IV: 6.00, 3.25. Light scopulae on tarsi, metatarsi legs I, II. Tarsus I with single, slightly staggered row of 9 trichobothria. Leg I spination pattern illustrated in Figures 4, 5; TSp 12, TSr 10, TSrd 2. Pedipalp. Articles stout, lacking distinct spines (figs 6, 7). PTw 1.50, PTl 3.00, Bl 1.24. Distinct, elongate ledge on distal-retrolateral aspect tibia. Embolus stout, tapering sharply towards tip, with serrations, elongate distal tooth (Fig. 8).
Variation (8). Cl 6.81–10.30, 7.56±0.41; Cw 5.40–8.08, 6.08±0.30; STRl 3.80–5.25, 4.14±0.17; STRw 3.38–4.85, 3.71±0.17; LBw 1.02–1.57, 1.21±0.07; LBl 0.56–0.70, 0.64±0.02; leg I: 5.35–8.32, 6.29±0.31; 4.60–5.75, 4.73±0.16; 3.25–4.85, 3.75±0.18; 2.88–4.20, 3.14±0.16; leg IV: 5.75–8.88, 6.52±0.35; 3.09–4.45, 3.48±0.16; PTl 3.00–4.32, 3.27±0.15; PTw 1.56–2.13, 1.68±0.07; Bl 1.20–1.60, 1.30±0.04; TSp 12–21, 15.25±1.01; TSr 10–14, 11.75±0.41; TSrd 1–2, 1.88±0.13.
Specimen preparation and condition. Female collected live from burrow, preserved in same manner as male holotype. Genital plate removed, cleared in trypsin, stored in microvial with specimen. Color. Carapace dark reddish gray 2.5YR 3/1; legs, chelicerae, dark reddish brown 2.5YR 3/4. Abdomen reddish black dorsally 2.5YR 2.5/1 faint dusky bands dorsally; ventrum, spinnerets pale yellow (figs 10, 11). Cephalothorax. Carapace 7.36 long, 6.38 wide, generally glabrous, few thin setae, pars cephalica elevated. Fringe lacks setae. Foveal groove deep, slightly procurved. Eye group slightly elevated on very low mound. AER slightly procurved, PER slightly recurved. PME-AME subequal in diameter. Sternum widest at coxae II/III, moderately setose, STRl 4.60, STRw 4.08. Three pairs of sternal sigilla anterior pairs moderate size, oval, positioned marginally, posterior pair larger, irregularly shaped, nearly contiguous. Chelicerae anterior tooth row armed with 10 teeth with single posterior margin denticle row. Palpal endites with 47 cuspules concentrated at the inner promargin posterior heel; labium with 5 cuspules, LBw 1.46, LBl 0.97. Rastellum consist of 10 very stout spines positioned on distinct mound. Abdomen. Moderately setose, posterior median spinnerets reduced in size. Walking legs. Anterior two pairs noticeably more slender than posterior pairs. Leg I 16.39 long. Tarsus I with single staggered row of 10 trichobothria. Legs I, II with moderately heavy scopulae on tarsi, metatarsi. PT3s 14, TB3s 9. Rudimentary preening comb on retrolateral distal surface, tarsus - metatarsus joint metatarsus III, IV. Spermathecae. Two simple spermathecal bulbs, moderately elongate neck, arranged on low subtriangular base (fig. 9).
Females known only from three specimens.
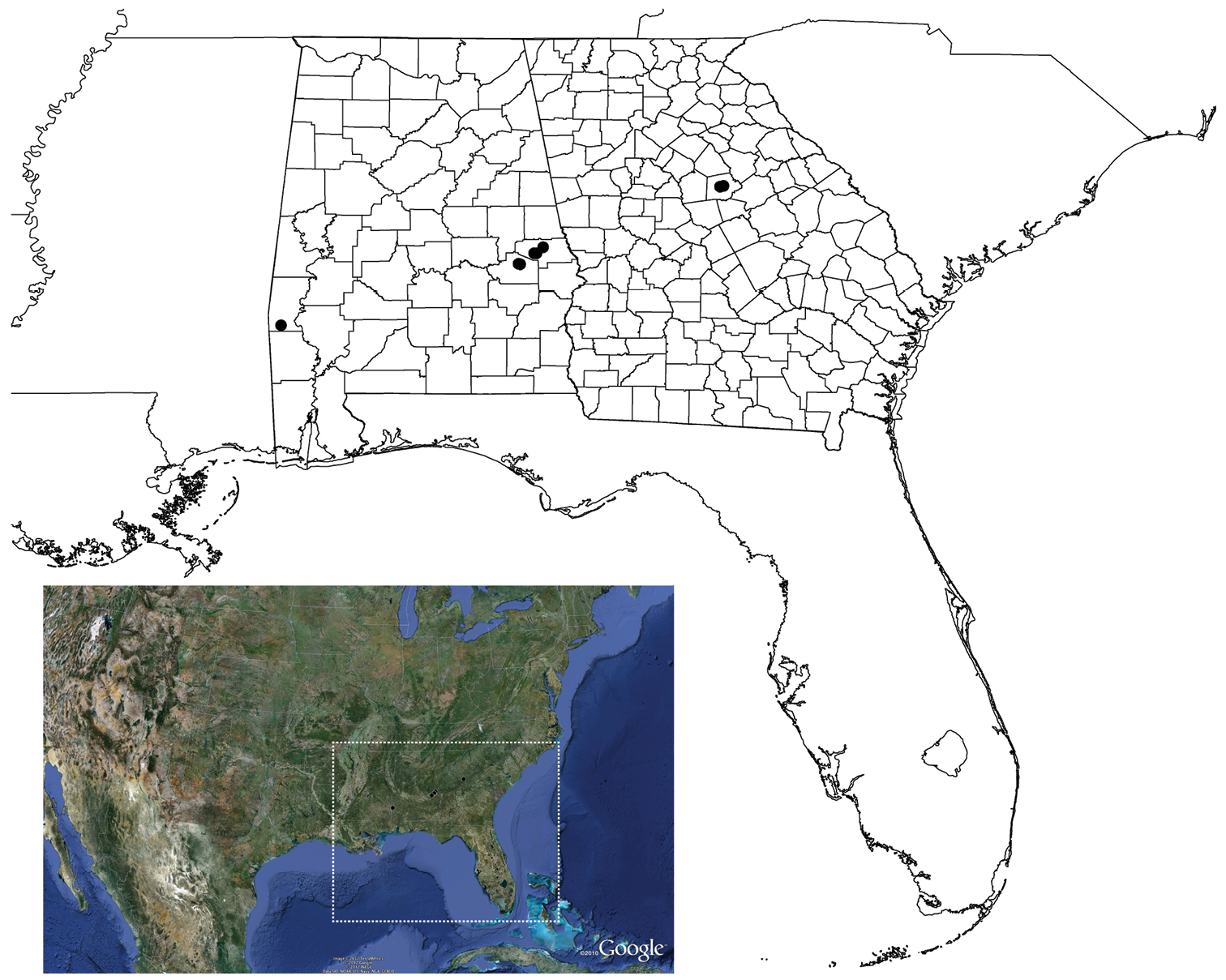
Known from central Alabama, counties of Choctaw, Lee, Macon, and Montgomery, and the piedmont region of Georgia, Putnam County (Map 1). The type locality comprises primarily young second growth mixed deciduous forest located at the transition from the Piedmont to Coastal Plan physiographic region. The population known from the additional material in western Alabama is located in Coastal Plain Province. Specimens were found to be presumably syntopic with Cyclocosmia, Antrodiaetus, Ummidia, and possibly Myrmekiaphila torreya (collected from the region). Male specimens were collected from swimming pools and wandering on warm, damp mornings during the months of December and January. Females were collected from 6–8 cm deep burrow, some with below-ground side chambers with a trapdoor.
JQ708212–JQ708215
Geographic distribution of Myrmekiaphila tigris. County outlines for Alabama and Georgia are shown. Lower color inset, dotted line, shows extent of distribution map.
Myrmekiaphila tigris sp. n. male holotype specimen in life. 3 oblique view 4 dorsal view. Scale bar = 5mm.
Myrmekiaphila tigris sp. n. male holotype and female paratype. 5, 6 1st walking leg of male, left side retrolateral and prolateral view 7 pedipalp, retrolateral view 8 palpal bulb 9 cleared spermathecae. Scale bars = 1mm.
Myrmekiaphila tigris sp. n. female paratype specimen in life. 10 oblique view 11 dorsal view. Scale bar = 5mm.
This project was supported by NSF grant DEB 0315160 and Auburn University. We are grateful to Dr. David Held for access to his property and specimens from his swimming pool. Rebecca Godwin assisted in taking measurements. Les Goertzen suggested the specific epithet tigris.
Locality data for Myrmekiaphila tigris.
Explanation note: Locality data (CSV format) for Myrmekiaphila tigris specimens (doi: 10.3897/zookeys.190.3011.app1). File format: Microsoft Excel comma delimited.