(C) 2011 Alexander F. Emeljanov. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Alicodoxa rasnitsyni gen. et sp. n. (Dictyopharinae: Orthopagini) is described based on a nymph from Rovno amber; it also occurs in Baltic amber. A small additional wax plate dorsal to the large wax plate of abdominal tergites VI–VIII is first reported in this and other genera of Dictyopharidae. A lectotype is designated for Pseudophana reticulata Germar & Berendt, 1856 transferred to Protepiptera (Achilidae): Protepiptera reticulata (Germar & Berendt, 1856), comb. n.
Fulgoroidea, planthoppers, Dictyopharinae, Orthopagini, Achilidae, fossil, Baltic amber, Eocene, morphology, wax plates
The family Dictyopharidae
is poorly represented in the fossil record. Its oldest member,
described from the Upper Cretaceous (Santonian) of Taimyr, has been
assigned to the extinct tribe Netutelini (
Two nymphs from Late Eocene Baltic amber were described by
The Rovno amber from NW Ukraine is roughly
contemporaneous to Baltic amber, both Late Eocene in age and containing
similar but distinct insect faunas with many species in common (
The specimen was collected in Klesov, Rovno Region,
Ukraine, and deposited in the amber collection of the Schmalhausen
Institute of Zoology, National Academy of Sciencesof Ukraine, Kiev
(SIZK). Morphological terminology follows (
Family Achilidae Stål, 1866
Tribe Achilini Stål, 1866, s.l.
Genus Protepiptera Usinger, 1939
http://species-id.net/wiki/Protepiptera_reticulata
Late instar nymph (“pupa”) in Baltic amber, former East Prussia (
Of the two syntype nymphs, the late instar is selected as a lectotype. The 3rd instar nymph, belonging to Dictyopharinae, is discussed under the new genus and species below. According to
This species is possibly a senior synonym of Protepiptera kaweckii Usinger, 1939 based on adults and very common in Baltic amber. Other possible senior synonyms of Protepiptera kaweckii are “Cixius” longirostris Germar et Berendt, 1856 and “Oliarus” oligocenus Cockerell, 1910 (
Subfamily Dictyopharinae Spinola, 1839
Tribe Orthopagini Emeljanov, 1983
Alicodoxa rasnitsyni sp. n.
The genus and the type species are named in honour of our friend and colleague Prof. Alexandr Rasnitsyn. The grammatical gender is feminine.
Metope not visible in dorsal aspect. Coryphe 1/3 longer than pronotal disc along midline. Pronotum deeply angulately emarginate posteriorly. Lateral carinae of mesonotal disc anteriorly converging at acute angle. Fore femur without subapical tooth. Abdominal tergites IV–V with 1–2 sensory pits displaced forwards from the row of pits. Tergites VI–VII with 2 medial and 2–3 lateral pits. Tergites VI–VIII with large lower and small upper wax plates, upper plate of tergite VII subdivided.
Similar to the extant genera Orthopagus Uhler and Saigona Matsumura, but in the nymphs of these latter the metope is visible from above, coryphe longer relative to pronotal disc, pronotum less emarginate posteriorly, and tergites VI–VII with 4 medial and 1–0 lateral pits. Subdivided upper wax plate of tergite VII is unknown in other Dictyopharidae. Other characters listed under Diagnosis assign the new genus to Orthopagini within Dictyopharinae (see Discussion).
urn:lsid:zoobank.org:act:F0581436-F029-46E3-9229-29D20AFF5140
http://species-id.net/wiki/Alicodoxa_rasnitsyni
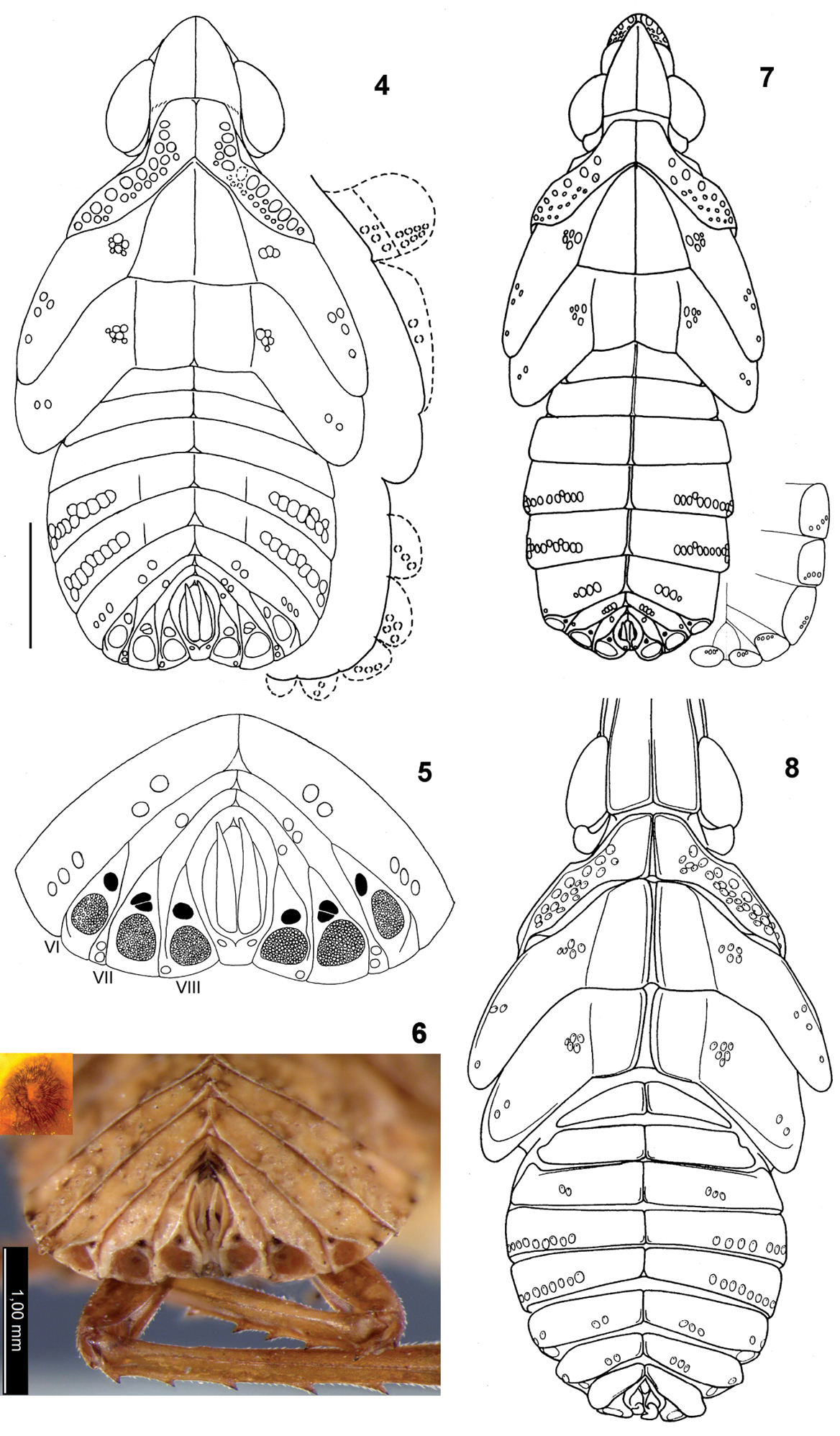
Figs 1–5Holotype, 4th instar nymph, SIZK K-3719, Klesov, Rovno amber, Ukraine; Late Eocene. Syninclusions: Mycetophilidae, Sciaroidea, Symphypleona, Acari, stellate hairs. Petaloid blind fissures arising all around lateral margins of the nymph and directed nearly laterad completely separate its dorsal and ventral sides so that only parts of its mid and hind legs are visible from above. The ventral aspect is mostly masked with variously directed fissures and in some places with milky impurities.
Nymph dark brown, ovoid, moderately elongate, 4.1 mm long, 2.2 mm wide; head projecting forwards beyond oval contour; dorsum finely transversely shagreened. Coryphe somewhat longer than wide; its lateral and anterior margins forming regular parabola; its posterior margin very shallowly concave, situated at about eye midlength in dorsal aspect. All carinae of coryphe, including posterior one and medial one, well developed. Metope (only partly visible) with medial areas somewhat widened at head apex; lateral areas with two rows of sensory pits, with an additional row of four smaller pits in between the two rows. Rostrum reaching beyond hind coxae, apical segment shorter than subapical one.
Pronotal disc strongly projecting forwards (2/3 of its median length situated anterior to level of posterior eye margins), its anterior margin truncate, almost straight, anterolateral angles rounded, lateral margins moderately diverging backwards. Posterior margin of pronotum with deep right-angled emargination reaching almost 1/3 of pronotal disc length. Pronotal disc slightly narrower and 1/4 shorter along midline than coryphe, bordered with distinct carinae along all free margins, medial carina also distinct, but posterolateral carinae undeveloped, and boundary between disc and lateral lobes traceable only as flexure of surface plane (posterior ends of these flexures close to points where lateral carinae of mesonotal disc approach pronotal margin). Sensory pits of pronotal disc and paradiscal areas forming one entity: disc with row of 4 large pits along lateral margin and 3 smaller pits in second, more medial row; paradiscal area with 6 large pits in marginal row (indistinctly subdivided into two groups, each with 3 pits) and 8–10 smaller pits in second row. Humeral area with 3 pits in main row and 1 additional pit; pectoral group of pits (about 7 in two tangled rows) situated as is usual in the family near posterior margin of paranotal lobe. Lateral and collateral carinae distinct. Mesonotal disc arrow-shaped anteriorly (its lateral carinae anteriorly converging at nearly acute angle, running parallel to posterior pronotal margin). Group of 6–7 pits (3 medial pits larger) situated laterad of lateral carinae. Fore wing pads mediad of subcostal carina with 3 pits (in triangle) in middle part and 1 pit near apex, and in costal area with 2 pits in middle part (against group of 3 pits). Posterior margins of fore and hind wing pads subparallel, directed obliquely posterolaterad. Metanotal disc rectangular, about 1.3 times as wide as long, with all carinae distinct. Medial group of pits laterad of disc similar to that on mesonotum; hind wing pad subapically with 2 pits in oblique row subparallel to posterior pad margin. Fore legs slightly widened, as long as mid legs. Fore femora without subapical tooth on posteroventral carina. Fore tibiae slightly flattened, lanceolate, widest about midlength. Mid tibiae not widened, relatively slender. Hind tibiae with 5 lateral spines including knee spine; apical teeth not possible to count. Hind tarsus three-segmented.
Abdomen with well developed middorsal carina, indistinct intermediate carinae on tergites IV–V, without sublateral carinae. Tergites I–III without pits; tergites IV–V with long (complete) rows of 8–10 pits, 5th or 6th pit (from body midline) displaced anteriad (sometimes there are 2 such pits, forming rudimentary second row anteriorly – see Fig. 1). Lateral areas of tergites IV–V with 3–4 pits in row or group. Tergites VI–VIII with several pits displaced medially (2 pits on VI, 2 pits on VII, 0–1 pit on VIII – absent at one side) and several pits laterally (ventrally: 3 pits on VI, 2 pits on VII, 1 pit on VIII). Lateral area with 3 pits on tergite VI, 2 pits on tergite VII, and 1 pit on tergite VIII. Tergite IX ventrally with pair of pits (1 pit at each side).
Alicodoxa rasnitsyni gen. et sp. n. (Orthopagini), 4th instar nymph, Rovno amber: 1 dorsal view 2 anteroventral view 3 lateral view (all to same scale); inset, wax plates of the right side, enlarged.
Wax plates situated in subtriangular posterolateral areas of tergites VI–VIII, separated from rest of tergite by carina and facing posteriad. Wax plates of uniform structure: large rounded lower (lateral) plate with discernible circular wax gland pores and small adjacent upper (medial) plate. Small upper wax plate on tergite VII subdivided, crossed by narrow chitinous bridge nearly perpendicular to body sagittal plane.
The three-segmented hind tarsus in the holotype of Alicodoxa rasnitsyni sp. n. indicates the 4th or 5th instar, while the fore wing pads not nearly reaching the apices of hind wing pads suggest the 4th instar (or 5th instar of a brachypterous planthopper, but the latter is not consistent with the presence of well developed abdominal wax plates).
In the nymphs of Dictyopharinae posterolateral carinae of the pronotal disc are usually absent, as in the new genus, and sensory pits of the second row are arranged more or less evenly across the imaginary boundary between pronotal disc and paradiscal area, though their number is not the same at the left and right sides.
The 3rd instar nymph from Baltic amber illustrated by
Alicodoxa gen. n. is a typical member of Dictyopharinae in its general habitus and basic structural features. The subfamily assignment is confirmed by the presence of typical wax plates on the VI–VIII abdominal tergites (wax plates are undeveloped in another subfamily, Orgeriinae).
Nymphs of Dictyopharinae
are rather uniform and difficult to identify, except for some aberrant
forms. Nymphal characters to diagnose all the tribes have not been
revealed yet, but there is a certain amount of morphological
descriptions of varying accuracy and credibility (
The descriptions scattered in the literature include those of nymphs (mainly of the last, 5th instar) from the tribes Dictyopharini, Orthopagini, Nersiini, Taosini, Phylloscelini, and Scoloptini. With the exception of Phylloscelini (genus Phylloscelis Germar), we examined nymphs from all these tribes plus the nymphs (yet undescribed) of the tribe Aluntiini, available in the collection of the Zoological Institute RAS. The nymphs of Lappidini, Capenini, Cleotychini, and Hastini remain unknown.
The wax plates of planthopper nymphs are originally
confined to the abdominal tergites VI–VIII. The presence of 5 primary
wax plates at each side of the tergites VI–VIII is characteristic of all
Fulgoroidea nymphs except the primitive family Cixiidae (
Within Dictyopharidae (Dictyopharinae) the structure of wax plates is quite uniform, except for the wax plates of segment VI often being small (Dictyophara multireticulata Mulsant & Rey) or absent (Nersia Stål, Scolops Schaum), but apparently only in the late instars (traced through developmental stages in Nersia:
4–5 Alicodoxa rasnitsyni gen. et sp. n., 4th instar nymph, Rovno amber: 4
dorsal view, slightly corrected and schematized; on the right,
arrangement of sensory pits on the lateral body parts facing ventrad
(scale bar, 1 mm) 5 posterior part of the abdomen, arrangement of wax plates and sensory pits; 6–7 Saigona ussuriensis (Lethierry) (Orthopagini), nymphs, recent: 6 5th instar, posterior part of the abdomen with wax plates; inset, upper wax plate of segment VIII, enlarged 7 4th instar, dorsal view; on the right, arrangement of sensory pits on ventrolateral parts of the tergites; 8 Dictyophara pannonica (Germar), 4th instar nymph, recent, dorsal view (apical part of the head not shown; after
In Zanna tenebrosa (Fabricius) of the subfamily Zanninae, considered the least advanced in the family Fulgoridae (
The new genus is assigned to the tribe Orthopagini based on the arrow-shaped anterior convergence of the lateral carinae of the mesonotal disc, combined with one or two sensory pits being displaced forwards from rows on the abdominal tergites IV–V; the latter character is found only in Orthopagini, the former also in Nersiini. The subapical tooth at the fore femur is characteristic of most Orthopagini but absent in several species of Centromeria Stål and in the new genus.
The tribe Orthopagini is presently distributed mainly in the Oriental and eastern Palearctic regions, plus three or four genera in the tropical Africa. The tribe Nersiini is Neotropical by origin and is confined to the New World (with only one subendemic genus, Rhynchomitra Fennah, in the Nearctic). Only the tribe Dictyopharini occurs now in Europe; in the nymphs of this tribe lateral carinae of the mesonotal disc are straight, not curved mediad, terminating separately at the anterior segment margin, and several pits on the abdominal tergites are displaced posteriad (not anteriad) from the row (Fig. 8).
The nymphs of Worskaitini are unknown; the adult of Worskaito stenexi Szwedo from Baltic amber differs from Alicodoxa gen. n. by the narrower, more elongate head. Because elongation of the head in Dictyopharinae, if it occurs, proceeds gradually from instar to instar, the head proportions of the 4th instar suggest that the head of adult Alicodoxa rasnitsyni sp. n. must be short and broad.
We are deeply grateful to Dr. Evgeny Perkovsky (SIZK) for providing the specimen for study, to Dr. Roman Rakitov (PIN) for checking the English of the manuscript, and to two anonymous reviewers for valuable comments. The study was supported by the Russian Foundation for Basic Research grant 08-04-00134 and the Presidium of RAS program “Biosphere origin and evolution” (subprogram II).