(C) 2011 G.Yu. Lyubarsky. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Cryptophagus alexagrestis Lyubarsky & Perkovsky, sp. n. is described based on a fossil inclusion in Late Eocene Rovno amber (Ukraine). The new species is similar to the extant Cryptophagus skalitzkyi Reitter and Cryptophagus dilutus Reitter, differing from the latter by having a very transverse, short and dilated 10th antennal segment, and from the former by the very elongate segments of the flagellum.
Cryptophagidae, Cryptophagus, Late Eocene, Rovno amber, Ukraine
The family Cryptophagidae is a group of small beetles with about 800 described species placed in approximately 50 genera and represented in all biogeographic realms. Most members of the family are free-living and mycophagous.
Silken fungus beetles are very common in the litter of forests in temperate climatic regions, where only Staphylinidae, Curculionidae and Carabidae are more abundant (
Discoveries of cryptophagids in fossil resins (see
The tarsal formula 5–5–5, 3-segmented antennal club,
and closed procoxal cavities of the new species are quite characteristic
of the family Cryptophagidae.
The new species has antennal insertions exposed in dorsal view;
pronotum with a well-developed marginal callosity; mesocoxal cavity
closed laterally by the sternum; ventrite 1 longer than the remaining
ventrites; and confused elytral punctation. These characters are
indicative of the genus Cryptophagus (Cryptophaginae). Representatives of Cryptophagus are found in all biogeographic realms; the genus includes 137 species from the Palaearctic Region (
Photographs were taken at the Paleontological Institute, Russian Academy of Sciences (Moscow) by V.A. Kolyada and the second author using a Leica M 165 microscope. To create diffused illumination, a cup of white styrofoam was placed between an object and a light source. The captured images were assembled with Helicon Focus 5.01 software.
TaxonomyFamily Cryptophagidae Kirby, 1837
Subfamily Cryptophaginae Kirby, 1837
Cryptophagus Herbst, 1863
urn:lsid:zoobank.org:act:DB75F93F-056F-4B85-8505-5F255C694388
http://species-id.net/wiki/Cryptophagus_alexagrestis
Figs 1–2Holotype, SIZK K-24572, Klesov, Rovno amber, Late Eocene. Syninclusion: Chironomidae. Sex of the holotype unknown.
From Alex, in honour of Prof. Alexandr Rasnitsyn, and “agrestis”from Latin ager for field, farm.
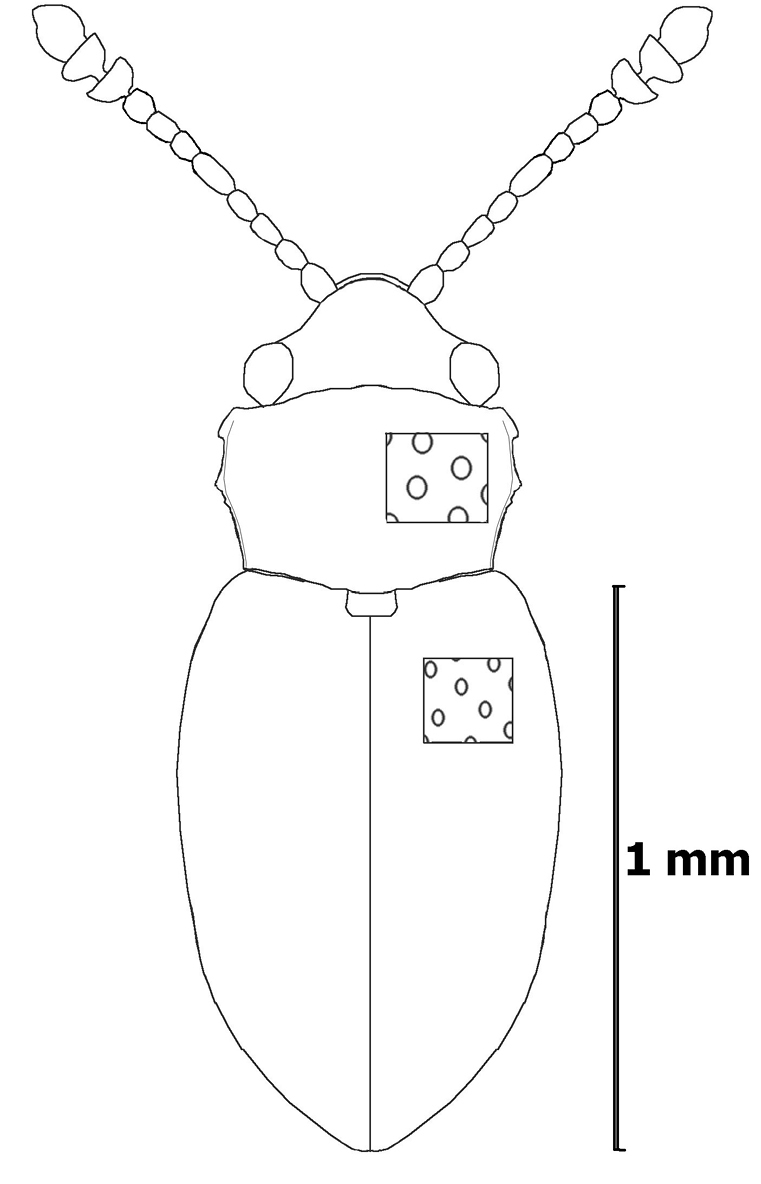
Body broadly elongate, slightly convex; head, pronotum, and elytra brown. Elytra slightly convex, covered with elevated pubescence.
Head transverse, of normal size, with hemispherical, somewhat coarsely facetted eyes, strongly and sparsely punctured. Antennae long, slender, with club reaching beyond base of pronotum, joints of flagellum elongate, 4th, 6th segment more than 1.5 times as long as broad, 5th 2 times as long as broad, 9th and 10th transverse, 11th obliquely oval, joints 9–11 equal in width.
Pronotum flat, not very strongly narrowed basally, distinctly transverse, barely 1.6 times broader than long, moderately not strongly and sparsely punctured (distance between punctures more than their diameter), an individual puncture less than the diameter of a facet. Pronotum without sublateral line, somewhat convex, sides narrowed basally and apically, with a single lateral tooth. Sides finely margined, anterior edge weakly sinuate. Callosity occupies at most one-seventh of side margin, with a small, elongate-oval patch of bare surface invisible from above; caudolateral corner obtuse angular, callosity without point. Lateral tooth far before middle of lateral margin. Posterior corners obtuse, base round, slightly sinuate, basal groove narrow.
Scutellum small, transverse. Elytra oval, humeral corners rounded, shoulders a little broader than maximum breadth of pronotum, 1.7 times as long as wide and 3.0 times as long as thorax, moderately convex, slightly flattened behind scutellum, with slightly rounded sides and a narrowly rounded apex, punctuation less strong and more sparse than that on pronotum.
Length of body 1.8 mm.
Cryptophagus alexagrestis sp. n., holotype (SIZK K-24572, Schmalhausen Institute of Zoology, Kiev) a body, dorsal b body, lateral c front part, dorsal.
Dorsal view, Cryptophagus alexagrestis sp. n.
Cryptophagus alexagrestis sp. n.is most similar to the modern Cryptophagus laterangulus Reitter (Caucasus, Iraq, Iran, Turkmenistan, Kazakhstan), Cryptophagus pseudoschmidti Woodroffe (Eastern Europe, Siberia, Mongolia), Cryptophagus dilutus Reitter (Holarctic: North Africa, Europe, Caucasus, Middle Asia, Iran, Iraq, India, China, Siberia, North America), Cryptophagus skalitzkyi
Reitter (Europe, Caucasus, Turkey, Iran, Turkmenistan, Uzbekistan,
Tajikistan, Afghanistan, Pakistan, India, Kyrgyzstan, Kazakhstan,
Eastern Siberia) with elevated elytral pubescence, bare surface of
callosity not visible from above, 4th segment of antenna elongate,
nearly 1.5 times as long as broad, lateral tooth far before middle of
pronotum, small length of callosity (see
| 1 | Punctation of prothorax distinctly dense. Eyes often strongly prominent, hemispherical, with large facets. Lateral tooth extremely strongly prominent, but very small. Length 2.3–2.5 mm | Cryptophagus laterangulus |
| – | Punctation of prothorax sparse. Lateral tooth not extremely prominent | 2 |
| 2 | Eyes large, length greater than half the length of the head, asymmetrical conical, prominent, with large facets, diameter of facet more than 11 µm. 5th segment of antenna weakly elongated, 1.5 times as long as broad. Callosity occupies from 1/5 to 1/4 of length of lateral margin. Lateral tooth of prothorax normal or small, nearly reduced. Length 2.6–3.4 mm | Cryptophagus pseudoschmidti |
| – | Eyes normal in size, length less than half the length of the head, slightly prominent, with small facets, diameter of facet less than 11 µm. Lateral tooth of prothorax normal. Callosity occupies from 1/8 to 1/5 of length of lateral margin | 3 |
| 3 | 10th segment of antenna rounded, about 1.5 times as broad as long. Callosity with point. Callosity occupies 1/6–1/5 length of lateral margin of pronotum. Length 2.1–2.7 mm | Cryptophagus dilutus |
| – | 10th segment of antenna short and dilated, very transverse, as least twice as broad as long. Callosity short, its length occupying 1/8–1/6 length of lateral margin of pronotum | 4 |
| 4 | Facets of eyes small, diameter of facet less than 8 µm. Segments of flagellum weakly elongate, 4th, 5th, 6th segment 1.5 times as long as broad. Prothorax convex, very strongly narrowed basally. Punctuation of prothorax moderately dense, distance between neighbouring punctures equal to diameter of puncture or slightly less. Length 1.9–2.3 mm | Cryptophagus skalitzkyi |
| – | Facets of eyes large, diameter of facet more than 11 µm. Segments of flagellum strongly elongate, 4th, 6th segment more than 1.5 times as long as broad, 5th 2 times as long as broad. Prothorax flat, not very strongly narrowed basally. Length 1.8 mm | Cryptophagus alexagrestis sp. n. |
We are grateful to Alexandr Rasnitsyn for providing access to facilities and continuous help; Victor Kolyada for kindly taking photographs of the specimen; Victor Fursov for checking the first draft of manuscript; David Penney for quickly checking the English.