(C) 2011 ChungKun Shih. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
We describe a new genus and species of Mecoptera with siphonate mouthparts, Sinopolycentropus rasnitsyni gen. et sp. n., assigned to the family Pseudopolycentropodidae Handlirsch, 1925. The specimen was collected from late Middle Jurassic nonmarine strata of the Jiulongshan Formation in Inner Mongolia, northeastern China. The new material provides additional evidence for an early diversification of pseudopolycentropodids that was ongoing during the Middle Jurassic. This diversity also adds to the variety of known pseudopolycentropodids with tubular proboscides that apparently fed on ovulate fluids produced by Mesozoic gymnosperms.
Pseudopolycentropodidae, fossil scorpionfly, new taxon, Jiulongshan Formation, proboscis, insect-plant associations, gymnosperms
The Pseudopolycentropodidae is an extinct and relatively nonspeciose family considered as phylogenetically basal to recent Mecoptera (
Currently, the Pseudopolycentropodidae consists of thirteen described species assigned to three genera from the mid-Triassic to the mid-Cretaceous (
Mouthpart, wing and antennal features of Mid-Mesozoic long-proboscid scorpionflies
| Taxon | Localities and age | Body Length (mm) | Forewing | Proboscis Features | Clypeal area (mm2) | Antenna type | Sex | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Length (mm) | Width (mm) | Length (mm) | Width (mm) | Food tube dia. (mm) | Surface | Terminus | ||||||
| Pseudopolycentropodidae | ||||||||||||
| Sinopolycentropus rasnitsyni gen. et sp. n.1 | Daohugou, Inner Mongolia, China; Middle Jurassic (Bathonian–Callovian boundary) | 5.5 | 6.1 | 2.4 | 1.9 | 0.1 | 0.027 | Fine setae | Absent | ?? | Moniliform – compact with annulate hairs | ♀ |
| Parapolycentropus burmiticus Grimaldi & Rasnitsyn 20051 | Tanai, Kachin, Myanmar; Early Cretaceous (Albian) | 3.0 | 4.0 | – | 1.3 | 0.121 | 0.014 | Setate, annulated | Lobate (Type 4) | — | Moniliform– aristate | ♀ |
|
Pseudopolycentropus latipennis |
Aulie, Chimkent, Kazakhstan; Late Jurassic (Kimme-ridgian) | 6.5 | ~9.8 | ~4.3 | 1.85 | 0.085 | ?? | Fine setae | Tip broken | — | Filiform– compact | ♀ |
| Pseudopolycentropus daohugouensis Zhang 2005 | Daohugou, Inner Mongolia, China; Middle Jurassic (Bathonian–Callovian boundary) | 7.5 | ~7.0 | ~3.7 | 1.82 | 0.146 | 0.048 | ? | Tip broken | — | Moniliform | ? |
|
Pseudopolycentropus janeannae Ren, Shih & |
Daohugou, Inner Mongolia, China; Middle Jurassic (Bathonian–Callovian boundary) | 7.0 7.0 |
7.5 7.0 |
3.8 3.0 |
1.7 1.75 |
0.130 0.125 |
0.038 0.038 |
Fine setae | Absent | — | Filiform– compact | ♀, ♂ |
|
Pseudopolycentropus novokshonovi Ren, Shih & |
Daohugou, Inner Mongolia, China; Middle Jurassic (Bathonian–Callo vian boundary) | 7.0 | 8.0 | 3.9 | 1.5 | 0.13 | 0.038 | Transversely ridged? | Tip broken | 0.468 | Filiform– compact | ? |
| Mesopsychidae | ||||||||||||
| Lichnomesopsyche gloriae Ren, Labandeira & Shih 2010 | Daohougou, Inner Mongolia, China; Middle Jurassic (Bathonian–Callo vian boundary) | 28.0 23.0 23.0 – |
25.0 24.0 27.0 24.0 |
7.0 8.0 8.0 8.0 |
9.9 8.9 8.9 10.1 >8.0 9.0 |
0.24 0.28 0.18 0.25 0.24 0.30 |
0.111 0.130 0.060 0.138 0.137 0.132 |
Coarse setae | Pseudo- labellum (Type 1) | 0.387 (n = 7) | Filiform –broad | ♀, ♂ |
| Lichnomesopsyche daohugouensis Ren, Labandeira & Shih 2010 | Daohougou, Inner Mongolia, China; Middle Jurassic (Bathonian–Callo vian boundary) | >14 | 22.0 | 6.5 | 8.8 | 0.34 | 0.094 | Coarse setae | Pseudo- labellum (Type 1) | 0.361 | Filiform broad | – |
| Vitimopsyche kozlovi Ren, Labandeira & Shih 2010 | Pingquam, Hebei China, Early Creta- ceous (Barremian) | – | 24.0 | 8.0 | 9.0 | 0.58 | 0.14 | Smooth | Absent | 0.436 | – | – |
| Aneuretopsychidae | ||||||||||||
| Jeholopsyche liaoningensis Ren, Shih & Labandeira 2011 | Huangbanjigou, Liaoning, China, Early Cretaceous (Barremian) | 23.0 | 21.5 | 6.0 | 6.8 | 0.34 | 0.10 | Smooth, annulated | V-shaped pseudolabellum (Type 2) | 0.493 | Filiform– compact | ♂ |
| Aneuretopsyche minima Rasnitsyn & Kozlov 1990 | Aulie, Chimkent, Kazakhstan; Late Jurassic (Kimme-ridgian) | — | ~10.5 | — | 4.7 | 0.18 | 0.060? | Fine setae, transversely ridged | Absent | — | Filiform– compact | ? |
| Aneuretopsyche rostrata Rasnitsyn & Kozlov 1990 | Aulie, Chimkent, Kazakhstan; Late Jurassic (Kimme-ridgian) | 21.0 | 25.0 | ~7.1 | 7.3 | 0.21 | 0.075? | Fine setae, transversely ridged | Faint pseudo-labellum (Type 3) | – | Filiform–compact | ♀ |
| Nedubroviidae | ||||||||||||
|
Nedubrovia shcherbakovi |
Isady, Vologda, Russia (Late Permian (Wuchiapingian) | ~3.0 | 3.4 | ~1.3 | >.035 | NR2 | NR2? | Fine setae, | ?? | – | ??– | ? |
1 Parapolycentropus and Sinopolycentropus are the only two genera of Pseudopolycentropodidae known to have labial palps, albeit they are diminutive (Figs 2B, 2E,
2 NR: not reported.
These five scorpionfly taxa are highly significant because Pseudopolycentropus possessed distinctive, elongate tubular, or siphonate, proboscides for surface fluid feeding on exposed plant fluids, such as the pollination drops of seed plants (
Ren and colleagues (
The long proboscides of Mecoptera, in addition to other clades such as nemestrinid flies, seem to have originated during a 15 million-year interval during the mid Jurassic from a 170 to 155 Ma (
Adding to this inventory of long-proboscid scorpionflies, we recently collected a well-preserved fossil pseudopolycentropodid from the Middle Jurassic Jiulongshan Formation in Daohugou Village, Ningcheng County, Inner Mongolia, China. Based on its unique combination of antennae, mouthparts, and wing venation, a new genus and species is erected herein.
Geological and paleobiological contextThe Jiulongshan Formation is a lacustrine sequence that crops out near Daohugou Village, Shantou Township, Ningcheng County, in Inner Mongolia of northeastern China (41°19.532' N, 119°14.589' E) (
There is considerable evidence for a diverse local flora at Daohugou, which was important for plant interacting insects, such as pseudopolycentropodid scorpionflies. The Jiulongshan Formation has provided evidence for the floral composition of the surrounding forests. These forests were dominated by arborescent seed plants, principally Coniferopsida (Pityophyllum, Rhipidiocladus, Elatocladus, Schizolepis, Podozamites), Ginkgopsida (Ginkgoites, Ginkgo, Baiera, Czekanowskia, Phoenicopsis), Cycadopsida (Pseudoctenis, Zamites), and Bennettitopsida (Anomozamites) (
This study is based on a fossil specimen (CNU-MEC-NN-2010044 p/c), with part and counterpart, housed in the fossil insect collection of the Key Lab of Insect Evolution and Environmental Changes, College of Life Sciences, Capital Normal University, Beijing, China (CNUB; Dong Ren, Curator). The specimen was examined dry or under alcohol using a Leica M165 C dissecting microscope, and illustrated with the aid of a drawing tube attachment. Photographs of specimens were taken by Leica dfc500 and line drawings in Figure 1 were made by CorelDraw 12. The drawing in Figure 2 was done as a camera lucida tracing that subsequently was inked on polyester film and then reduced in size. Illumination for the drawing of this specimen consisted of three types of light and variation in light angle and origin for accentuation of morphological features, such as wing venation, that normally were difficult to observe and typically unavailable at lower magnification microscopes. The morphological terminology used herein is that of
urn:lsid:zoobank.org:act:2C136D49-76A0-4BA4-8991-445366C376E5
Sinopolycentropus rasnitsyni Shih, Yang, Labandeira & Ren, sp. n.
The generic name is a combination of the prefix “Sino” for China, and a shortened version, with the infix removed, of the type genus of its referred family, “Pseudopolycentropus”. The gender is masculine.
Forewing broad and rounded, triangular in overall shape, with base of Sc merging with R; R2+R3 forking earlier than R4+R5 forking. Antennae moniliform, compact, robust and thick, multiarticulate with annulate hairs. Distinct, multiarticulate labial palps and long occipital bristles also distinguish this taxon from all previously described Pseudopolycentropodidae except for Parapolycentropus Grimaldi & Rasnitsyn 2005.
This genus can be assigned to the Pseudopolycentropodidae by a short Sc, simple R1, Rs with four branches, M with five branches, a dc cell present, and a simple CuA. It can be differentiated readily from all other genera of Pseudopolycentropodidae by the base of Sc merging with R, R2+R3 forking earlier than R4+R5, and moniliform antennae consisting of compact, robust, relatively short, articles with annulate hairs. Distinctive labial palps and long occipital bristles also distinguish this taxon from all previously described Pseudopolycentropodidae except for Parapolycentropus. In addition, body length of Sinopolycentropus (5.5 mm) is shorter than that of Pseudopolycentropus (6.5 to 7.5 mm), but longer than that of Parapolycentropus (3.0 mm).
urn:lsid:zoobank.org:act:03B528B3-C5B9-45AB-9CF9-D046DD5028A6
http://species-id.net/wiki/Sinopolycentropus_rasnitsyni
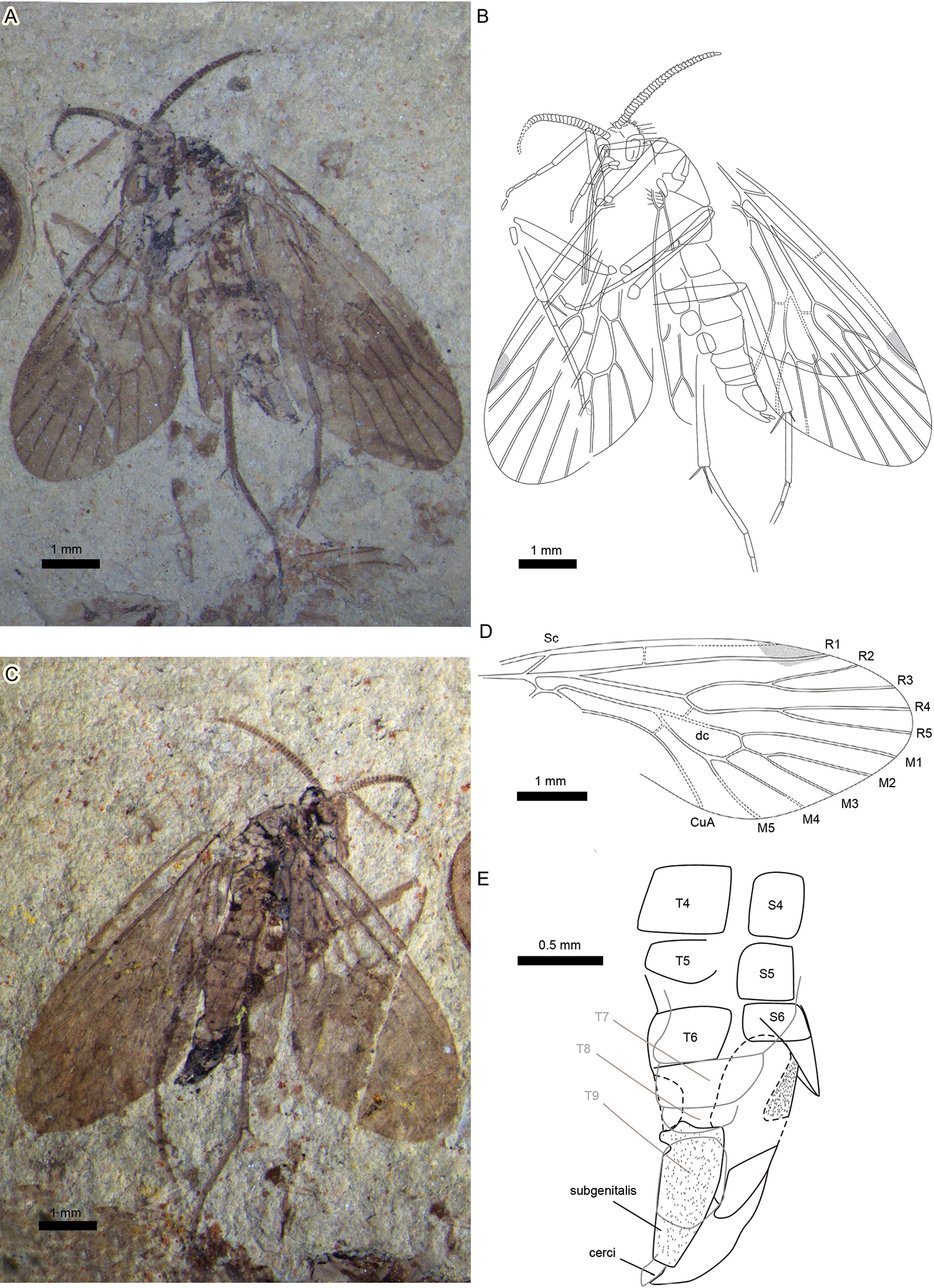
Figs 1 , 2Holotype, an almost complete specimen with well-preserved body and wings, female, part and counterpart, No.CNU-MEC-NN-2010044 p/c, is housed in the fossil insect collection of the Key Lab of Insect Evolution and Environmental Changes, College of Life Sciences, Capital Normal University, Beijing, China.
The specific name is dedicated to Dr. Alexandr Rasnitsyn for his contribution to paleoentomology and his recognition, with M. V. Kozlov, of the first fossil scorpionfly (Aneuretopsyche rostrata) with a documented long proboscis in 1990 (
As for the genus by monotypy.
A complete, small, female insect (Figs 1A-C); body length (excluding antennae and proboscis) 5.5 mm. Both forewings well-preserved, but hindwings only partially preserved, obscured due to overlap with forewings, thorax and abdomen.
Photographs and line drawings of holotype Sinopolycentropus rasnitsyni gen. etsp. n. (specimen no. CNU-MEC- NN2010044 p/c) A Digital image of part, no.CNU-MEC-NN2010044p B Line drawing of part, no.CNU-MEC-NN2010044p C Digital image of counterpart, no.CNU-MEC-NN2010044c D Line drawing of forewing venation, no.CNU-MEC-NN2010044p E Line drawing of abdomen and terminalia, no.CNU-MEC-NN2010044c. Scale bars: 1.0 mm or 0.5 mm as shown in figures..
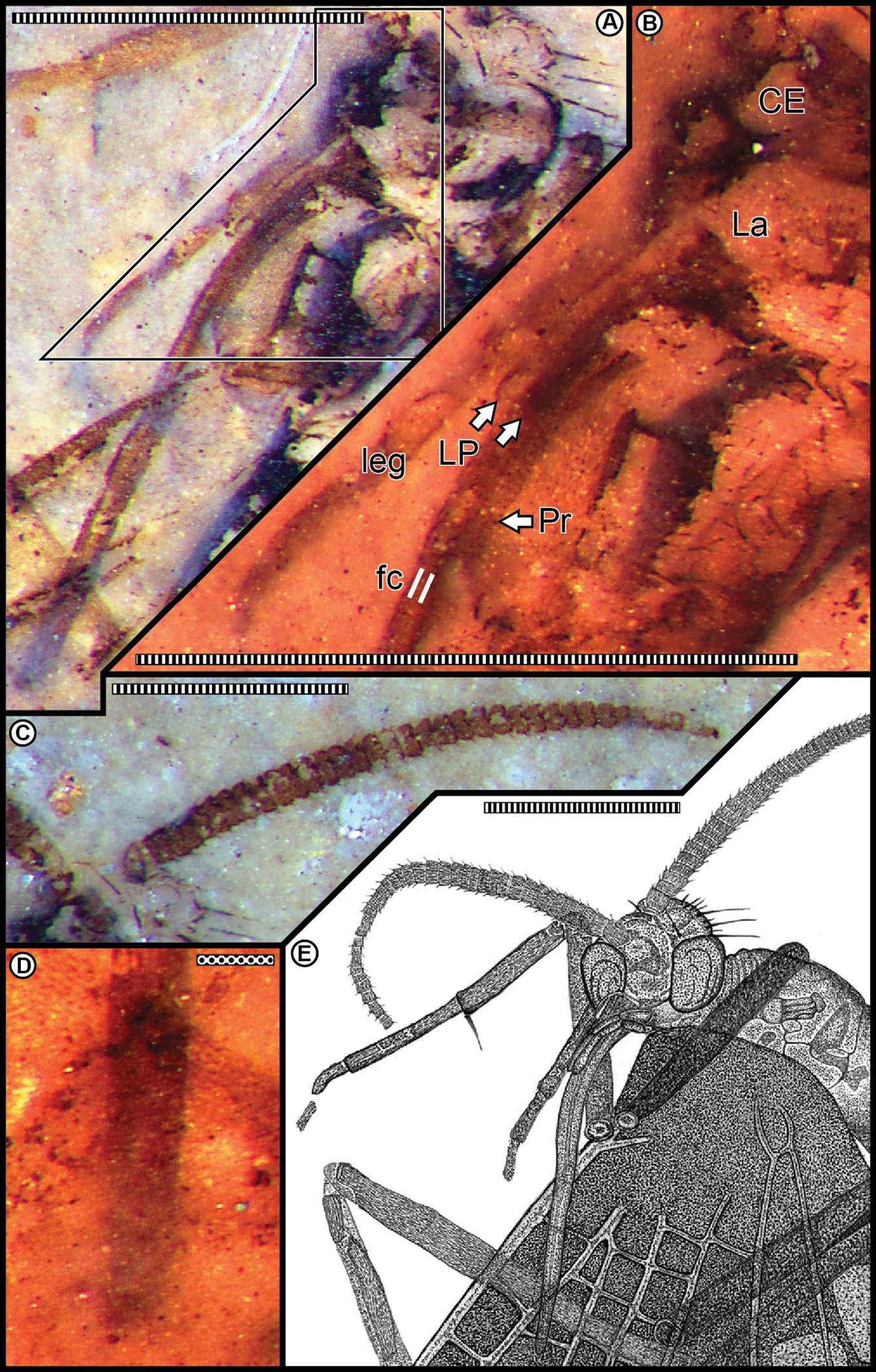
Head and mouthparts. Head capsule spheroidal–prolate, prolonged anteriorly, housing prominent, hemispheroidal, and bulbous compound eyes (Figs 2A, E). Occipital region invested with conspicuously projecting, long bristles and smaller setae. Antennae 2.0 mm long, moniliform, compact and thick, with annulate hairs (Fig. 2C); about same length as proboscis. Each antenna consists of a basal scape and ca. 40 articles; each article bears hairs especially noticeable in profile along its distal annulus; proximal articles about twice as wide as long, distal articles equant. Undefined clypeal region evident below antennal base insertions and above the labrum (Fig. 2B). Mouthparts consistent with previously documented combination for pseudopolycentropodids (but see comments in discussion section below). There is the typical absence of mandibles and maxillary region, and presence of labral and labial elements; in part represented by discernable palps consisting of three articles (Figs 2B, E). Labrum triangular and inconspicuous. A long, decurved, siphonate proboscis 2.0 mm long, labially derived, occurring in an anatomically downturned position that lacks external cuticular ornamentation but bears very fine setae. Proboscis siphon diameter ca. 0.10 mm; housing an inner, eccentrically positioned food canal ca. 0.027 mm in diameter. Proboscis terminus lacks absorptive structures, such as pseudolabellae, related to feeding (Fig. 2D). Two, short labial palps present, adjacent and lateral to the proboscis base, each 0.5 mm long; about one-fourth proboscis length (Fig. 2B). Labial palps composed of three articles, the distal article slightly clavate, with a smooth, rounded terminus, the proximal articles thinner, the proximal-most attached to an enlarged labial area at the ventral base of the head capsule.
Thorax and legs. In lateral aspect pronotum short and neck-like; mesonotum broad, scutellum narrow, metanotum slightly shorter than scutum. Legs entirely covered with pubescence. Right foreleg originating from small, round coxa; long and slender femur (overlapping with thorax) and tibia (overlapping with head); left foreleg (overlapping with mouthparts) intersecting basitarsus of right foreleg and touching left antenna; tibia with at least two apical spurs. Midleg originating from small, round coxa; long and slender femur and tibia; tibia with at least 1 apical spur, tarsi of midleg 5-segmented, basitarsus longest, pretarsus with 1 evident claw. Hindleg originating from round coxa; long and slender femur and tibia; tibia with at least two, long, apical spurs. Tarsi of hindleg 5-segmented, basitarsus longest; length ratio of tibia and basitarsus 1:0.54 for left hind leg. Right hindleg disarticulated between femur and tibia.
Wings. Forewing broad, 6.1 mm long by 2.4 mm wide; length/width ratio 2.5; apical margin rounded (Figs 1A-D). (By comparison, the forewing length/width ratio is 2.0–2.3 for Pseudopolycentropus janeannae and 2.05 for Pseudopolycentropus novokshonovi.) Membrane covered in macrotrichia. Sc short, without anterior branches; base of Sc merging with R apex; Sc reaching C considerably before than Rs origin. Humeral vein absent. Crossvein c–r perpendicular to both R1 and C, just before wing midsection. R1 rectilinear at base, slightly arched toward C near wing midsection, coursing into the distinct pterostigma. Rs stem rectilinear. R2+R3 stem abruptly bent at crossvein r–m, then slightly arched toward C, with 2 long branches, R2 and R3. R2+R3 stem forking earlier than R4+R5; R4 longer than R2; R5 longer than R3. M forking slightly before that of Rs. Thyridium untraceable. M with 5 branches; M4+5 forking somewhat before the anterior M1+3 branch; M2+3 forking at about the same level as R4+R5 forking; M2+3 stem short and distinct. A crossvein between M4+5 stem and CuA, m–cua, near basal dc cell but present after M forking. M+CuA stem distinctly arched. M+CuA forking before R forking into R1 and Rs. Posterior wing margin almost rectilinear. Hindwing much smaller than forewing, but of similar shape. Right hindwing with only part of R2+R3 forking to R2 and R3; distal part of R4 and R5 preserved and left hindwing with a very short, terminal R1; basal Rs and part of R2+R3 forking to a preserved R2 and R3. Distal halves of fore- and hindwings suffused, pterostigma darkened (Figs 1A, 1B, 1D).
Abdomen. Abdomen elongate, tapering apically, with 9 visible segments. Basitergum (T1) fused to metathorax, segments 2-5 distinctly broad. Subgenitalis rectangular in shape and cerci visible (Figs 1B, 1C, 1E).
A Head, proboscis, and associated mouthparts B Mouthpart detail enlarged from template in A, showing base of proboscis (Pr), the tips of both labial palps (LP) at white arrows, labrum (La), and compound eye region (CE) C Right antenna D Proboscis tip, observed through the wing membrane E Camera lucida drawing of head, proboscis and associated mouthparts in A using a variety of light sources and angles. Scale bars: stippled, 0.1 mm; striped, 1.0 mm.
Daohugou Village, Shantou Township, Ningcheng County, Inner Mongolia, China; Jiulongshan Formation, Middle Jurassic (Bathonian–Callovian boundary interval).
Three aspects of this discovery are significant for understanding the ecological roles of Pseudopolycentropodidae with plants in the local ecosystem at Daohugou. First is recognition of the distinct morphological features that separate Sinopolycentropus gen. n. from all other coexisting pseudopolycentropodid taxa. Second are the implications that the unique antennal and especially mouthpart modifications have for host-plant use. Last is the importance of rarity in understanding the pollinator associations in an increasingly well-documented, preangiospermous ecosystem from the Middle Jurassic.
Distinctiveness of Sinopolycentropus from other PseudopolycentropodidaeThis new, long-proboscid species is distinct from all other members of Pseudopolycentropodidae by several differentiating features. These differences are the base of the Sc vein merging with R vein; the R2+R3 vein forking earlier than that of the R4+R5 vein; and relatively short, moniliform, and robust antennae bearing hairs on their annulae. To date, all described pseudopolycentropodids have their R4+R5 vein forking earlier than that of their R2+R3 vein, except for Pseudopolycentropus triasicus Papier, Nel & Grauvogel-Stamm, 1996, which has its R4+R5 vein forking at variable levels vs. its R2+R3 vein (
The presence of small, distinctive, three-segmented labial palps is only shared with Parapolycentropus burmiticus (
Typically, pseudopolycentropodids have filiform or moniliform antennae with a relatively high ratio of antenna length to unappendiculate body length. For example, Pseudopolycentropus daohugouensis was reported to have an antenna/body length ratio somewhat greater than 0.37 (2.8/7.5); Pseudopolycentropus janeannae with greater ratios of 0.5 (3.5/7) and 0.57 (4/7); and Pseudopolycentropus novokshonovi with an intermediate ratio of 0.43 (3/7). However, Parapolycentropus burmiticus has a distinctively different type of antennae, characterized by a funnel-shaped scape, a scoop-shaped pedicel, the basal five flagellomeres tapered in size and the apical eleven segments significantly more diminutive, forming an arista (
Structural features of the antennae and mouthparts may be relevant for seeking conspecifics and host plants. The unique characteristics of antennal shape, structure and length in Sinopolycentropus rasnitsyni might be associated with the sensory detection of mates or food sources. The presence of prominent hairs encircling the annular area of each antennal article may be involved in detection of conspecific pheromonal cues or specific chemicals from particular plant hosts. Currently, we cannot draw any conclusions regarding these suggestions, pending scanning electron or high-resolution light microscopy of the antennal hair setal bases and other features. Of more importance are the structure, size, and shape of the proboscis, which indicate that it would have been used for access and imbibition of pollination drops or similar ovulate fluids from a variety of smaller gymnospermous fructifications (
In the extensive collection of more than 250, 000 fossil insect specimens from Daohugou at Capital Normal University, we currently have collected seven specimens of Pseudopolycentropus janeannae, one specimen of Pseudopolycentropus novokshonovi, and a single specimen of Sinopolycentropus rasnitsyni. This suggests that these three species are extremely rare compared to other, co-occurring insect taxa. Significantly, approximately 20 specimens of small, nonglossate moths also have been found in this collection, of similar size and ecological relationships with plants as the pseudopolycentropodids. This comparison indicates that the ecologically equivalent pseudopolycentropodids are considerably rarer, and by inference, may have had more specialized associations with host plants than those of moths. These low abundances indicate that rarity, indeed, was an important feature of the Middle Jurassic Jiulongshan insect fauna and flora, contrary to the viewpoint that rarity was only a feature of angiosperm-dominated biotas from the mid Cretaceous and younger (
We appreciate valuable comments and suggestion by Dmitry Shcherbakov, Alexey Bashkuev, and an anonymous reviewer. This research was supported by the National Natural Science Foundation of China grants 31071964 and 40872022, Beijing Natural Science Foundation Program grant 5082002, and the Key Project of Beijing Municipal Commission of Education. Thanks are extended to Finnegan Marsh for producing Figure 2. This is contribution 151 of the Evolution of Terrestrial Ecosystems Consortium at the National Museum of Natural History, in Washington, D.C.