(C) 2011 Lars Vilhelmsen. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The head capsule of a taxon sample of three outgroup and 86 ingroup taxa is examined for characters of possible phylogenetic significance within Hymenoptera. 21 morphological characters are illustrated and scored, and their character evolution explored by mapping them onto a phylogeny recently produced from a large morphological data set. Many of the characters are informative and display unambiguous changes. Most of the character support demonstrated is supportive at the superfamily or family level. In contrast, only few characters corroborate deeper nodes in the phylogeny of Hymenoptera.
Hymenoptera, morphology, phylogeny, head anatomy
Phylogenetic research on Hymenoptera has been pursued for more than a century (see
Characters from the head capsule have long been included in attempts to make phylogenetic inferences across the Hymenoptera.
In the present paper, I review characters from the head capsule from previous treatments as well as introducing some new ones. The characters are scored from a taxon sample closely matching that of
Heads of the taxa examined were detached from the rest of the body and the antennae and mouthparts were removed. The heads were examined in a dissection microscope and bisected in a parasagittal plane to allow the observation of internal features. In addition, SEM images of anterior and posterior views of the head of a number of taxa (see below) were downloaded from MorphBank (http://www.morphbank.net/) for a number of taxa and used to score as well as illustrate characters (see Figs 1-6). The data set was assembled in Mesquite (
Taxa for which Morphbank SEM images were available are indicated with ‘MB’ in the list below, followed by the image numbers.
OutgroupNeuroptera, Chrysopidae: Chrysopa perla (Linnaeus, 1758). Mecoptera, Panorpidae: Panorpa communis (Linnaeus, 1758). Lepidoptera, Micropterigidae: Micropterix calthella (Linnaeus, 1761).
HymenopteraXyeloidea, Xyelidae: Macroxyela ferruginea (Say, 1824) MB 102882, 102884, 102885.
Tenthredinoidea, Tenthredinidae: Athalia rosae (Linnaeus, 1758) MB 102486-88; Notofenusa surosa (Konow, 1905). Diprionidae: Monoctenus juniperi (Linnaeus, 1758). Pergidae: Heteroperreyia hubrichi Malaise, 1955.
Pamphilioidea, Pamphiliidae: Onycholyda amplecta (Fabricius, 1804) MB 102834, 102837.
Cephoidea, Cephidae: Cephus pygmeus (Linnaeus, 1767) MB 102672, 102946.
Siricoidea, Anaxyelidae: Syntexis libocedrii Rohwer, 1915. Siricidae: Tremex columba (Linnaeus, 1763) MB 102749, 134704; Urocerus gigas (Linnaeus, 1758).
Xiphydrioidea, Xiphydriidae: Xiphydria camelus (Linnaeus, 1758); Xiphydria prolongata (Geoffroy, 1758) MB 102708-9, 134719.
Orussoidea, Orussidae: Orussobaius minutus Benson, 1938; Orussus abietinus (Scopoli, 1763) MB 134672, 134675.
Ceraphronoidea, Ceraphronidae: Ceraphron sp. MB 78643, 78654. Megaspilidae: Lagynodes sp. MB 79001, 79018; Megaspilus fuscipennis (Ashmead, 1888); Megaspilus sp. MB 134646, 134648.
Chalcidoidea, Aphelinidae: Cales noacki Howard, 1907 MB 101253, 101256, 101328, 101330; Coccophagus rusti Compere, 1928 MB 101454, 101458. Chalcididae: Acanthochalcis nigricans Cameron, 1884; Acanthochalcis sp. MB 134808, 134812. Eulophidae: Cirrospilus coachellae Gates, 2000 MB 101190, 101192. Eurytomidae: Eurytoma gigantea Walsh, 1870 MB 101509, 101511. Mymaridae: Gonatocerus ashmeadi (Girault, 1915) MB 101615, 101617; Gonatocerus morrilli (Howard, 1908). Pteromalidae: Cleonymus sp. MB 101374-75, 101380-81; Nasonia vitripennis (Walker, 1836) MB 101734, 101736, 101738; Spalangia nigripes Curtis, 1839. Torymidae: Megastigmus transvaalensis (Hussey, 1956) MB 101672, 101672, 101709.
Cynipoidea, Cynipidae: Diplolepis rosae (Linnaeus, 1758) MB 71826-27; Periclistus brandtii (Ratzeburg, 1832) MB 72041-42. Figitidae: Anacharis sp.; Melanips opacus (Hartig, 1840) MB 75299; Melanips sp. MB 75329; Parnips nigripes (Barbotin, 1964) MB 80037, 80053. Ibaliidae: Ibalia leucospoides (Hochenwarth, 1785); Ibalia rufipes Cresson, 1879 MB 77314-15.
Evanioidea, Aulacidae: Aulacus impolitus Smith, 1991 MB 103222, 103243; Pristaulacus strangaliae Rohwer, 1917 MB 103739, 103747. Evaniidae: Brachygaster minuta (Olivier, 1792) MB 103249, 103257; Evania albofascialis Cameron, 1887 MB 134630, 134632; Evaniella semaeoda Bradley, 1908 MB 103427, 103443. Gasteruptiidae: Gasteruption spp. MB 103453; Pseudofoenus spp. MB 103788, 103810.
Ichneumonoidea, Braconidae: Aleiodes terminalis Cresson, 1869 MB 103161, 103186; Doryctes erythromelas (Brullé, 1846) MB 103274, 103330; Orgilus gracilis (Brues, 1908) MB 103649, 103655; Rhysipolis sp. MB 103818, 103843; Urosigalphus sp.; Wroughtonia ligator (Say, 1824) MB 103109, 103126, 134714, 134716. Ichneumonidae: Dusona egregia (Viereck, 1916) MB 103363, 103366, 134625, 134627; Labena grallator (Say, 1836) MB 103512, 103524; Lymeon orbum (Say, 1835) MB 103559, 103562; Pimpla aequalis (Provancher, 1880) MB 103694, 103718; Zagryphus nasutus (Cresson, 1868) MB 103134, 103149.
Megalyroidea, Megalyridae: Dinapsis spp.; Megalyra fasciipennis Westwood, 1832 MB 103568, 103589, 134657, 134660.
Mymarommatoidea, Mymarommatidae: Mymaromma anomalum (Blood & Kryger, 1922) MB 101797-98, 101800.
Platygastroidea, Platygastridae: Archaeoteleia mellea Masner, 1968 MB 101920, 101922, 103191, 103211; Isostasius sp. MB 103486, 103503; Proplatygaster sp. MB 103760, 103776, 134687, 134690; Sparasion formosum Kieffer, 1910 MB 103049, 103068; Telenomus podisi Ashmead, 1893 MB 103079, 103096.
Proctotrupoidea, Diapriidae: Belyta sp. MB 101879-80, 101882; Pantolytomyia ferruginea (Dodd, 1915) MB 79029; Poecilopsilus sp. MB 79100. Heloridae: Helorus anomalipes (Panzer, 1798); Helorus sp. MB 78665, 104116, 134637, 134639. Maamingidae: Maaminga rangi Early et al., 2001. Monomachidae: Monomachus antipodalis Westwood, 1874 MB 80081-82. Pelecinidae: Pelecinus polyturator (Drury, 1773) MB 79061, 134682-85. Proctotrupidae: Austroserphus albofasciatus Dodd, 1933 MB 78510, 103974, 103978; Phaenoserphus sp. MB 80116, 80121, 80147; Proctotrupes sp. Roproniidae: Ropronia garmani (Ashmead, 1898) MB 78872, 134697, 134700. Vanhorniidae: Vanhornia eucnemidarum Crawford, 1909 MB 78898, 134709, 134711.
Stephanoidea, Stephanidae: Megischus spp. MB 103607, 103620, 134650, 134653; Schlettererius cinctipes (Cresson, 1880).
Trigonaloidea, Trigonalidae: Orthogonalys pulchella (Cresson, 1867) MB 103661, 103682, 134678, 134680; Taeniogonalos gundlachii (Cresson, 1865) MB 103032, 103043.
Apoidea, Ampulicidae: Ampulex compressa (Fabricius, 1781) MB 134606, 134608; Crabronidae: Pison chilense Spinola, 1851 MB 102586-88; Sphecidae: Stangeella cyaniventris (Guérin-Méneville, 1831).
Chrysidoidea, Bethylidae: Cephalonomia stephanoderis Betrem, 1961 MB 102530-31, 102534. Chrysididae: Chrysis angolensis Radoszkowski, 1881. Plumaridae: Plumarius sp. Scolebythidae: Ycaploca evansi Nagy, 1975.
Vespoidea, Pompilidae: Aporus niger (Cresson, 1867). Rhopalosomatidae: Rhopalosoma nearcticum Brues, 1943. Sapygidae: Sapyga pumila Cresson, 1880 MB 102635, 102637. Vespidae: Metapolybia cingulata (Fabricius, 1804).
Results and discussion: annotated character list1. Ocellar corona
(0) absent (e.g., Figs 1A-C, 2B-D)
(1) present (Figs 1D, 2A)
The presence of a circlet or semicirclet of cuticular teeth around the median ocellus is a ground plan feature of both Orussidae (
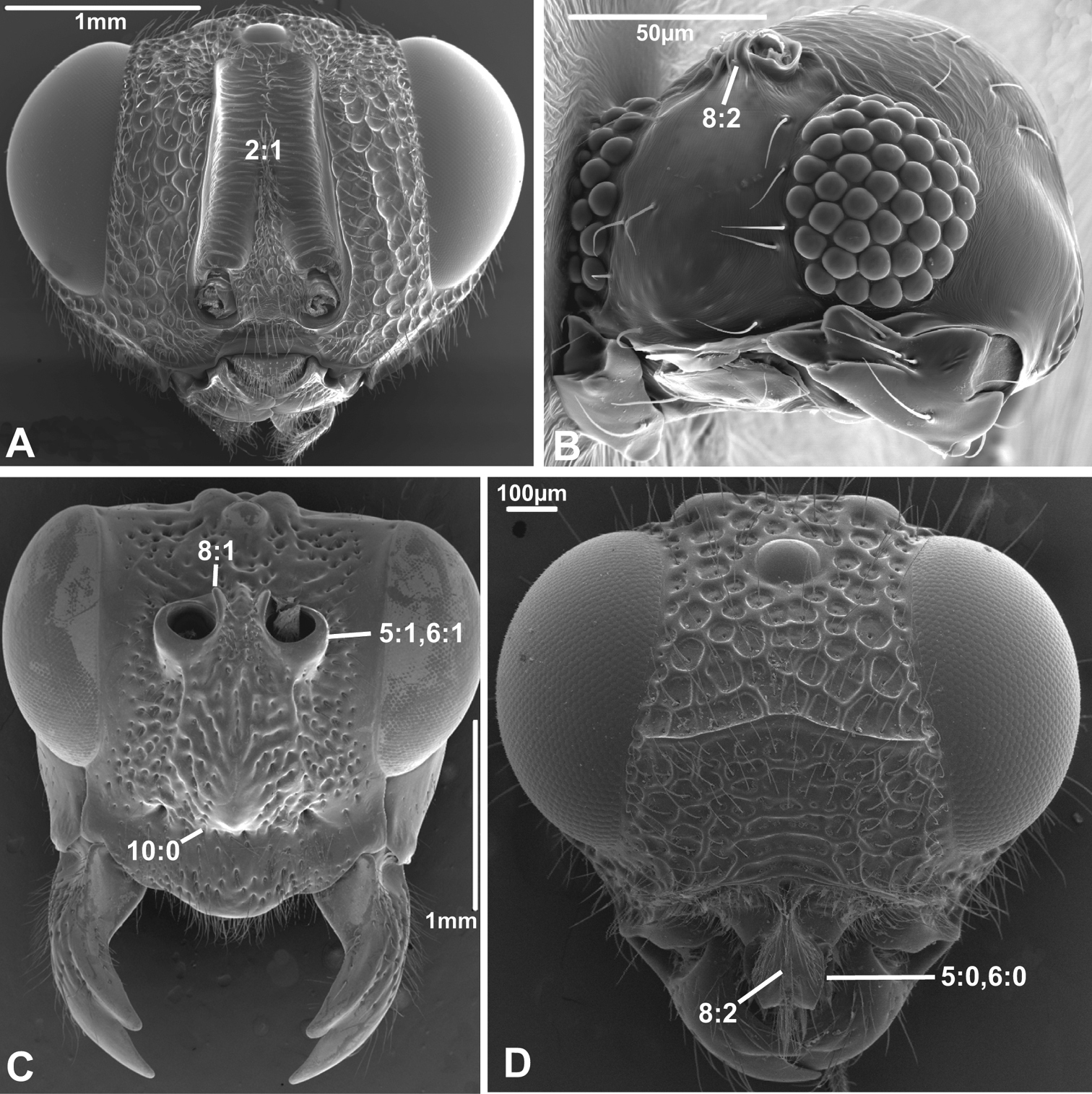
2. Supraantennal grooves or depressions
(0) absent (e.g., Figs 3A-B, 4B, D)
(1) present (Figs 3C, 4A)
Grooves or depressions, also called scrobes, are prominent above the antennal foramina in many Chalcidoidea and apparently belong to the ground plan of the superfamily. However, they were not observed in Gonatoceros (Mymaridae). The Mymaridae have been suggested to be the sister group of the remainder of the Chalcidoidea (e.g.,
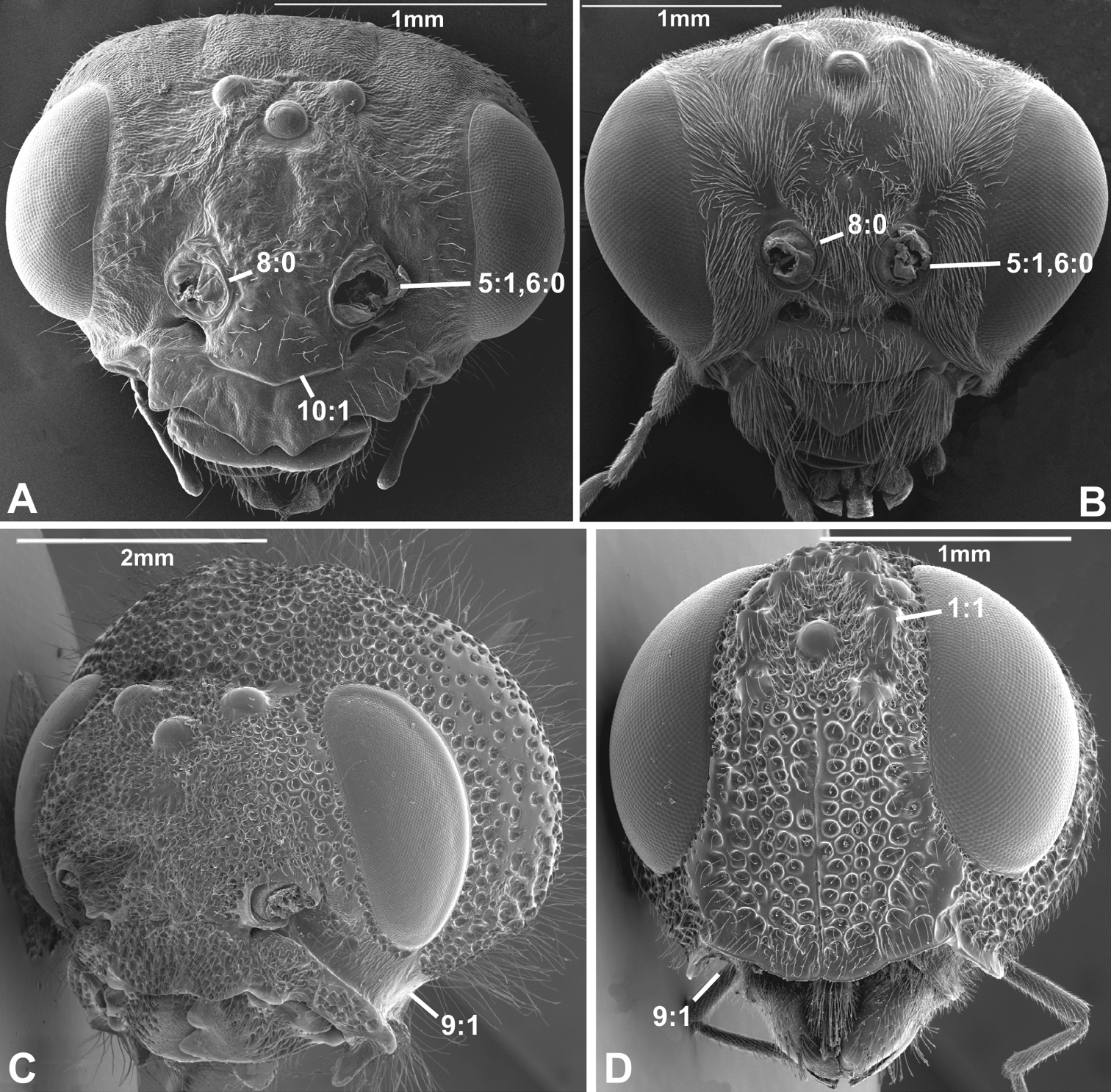
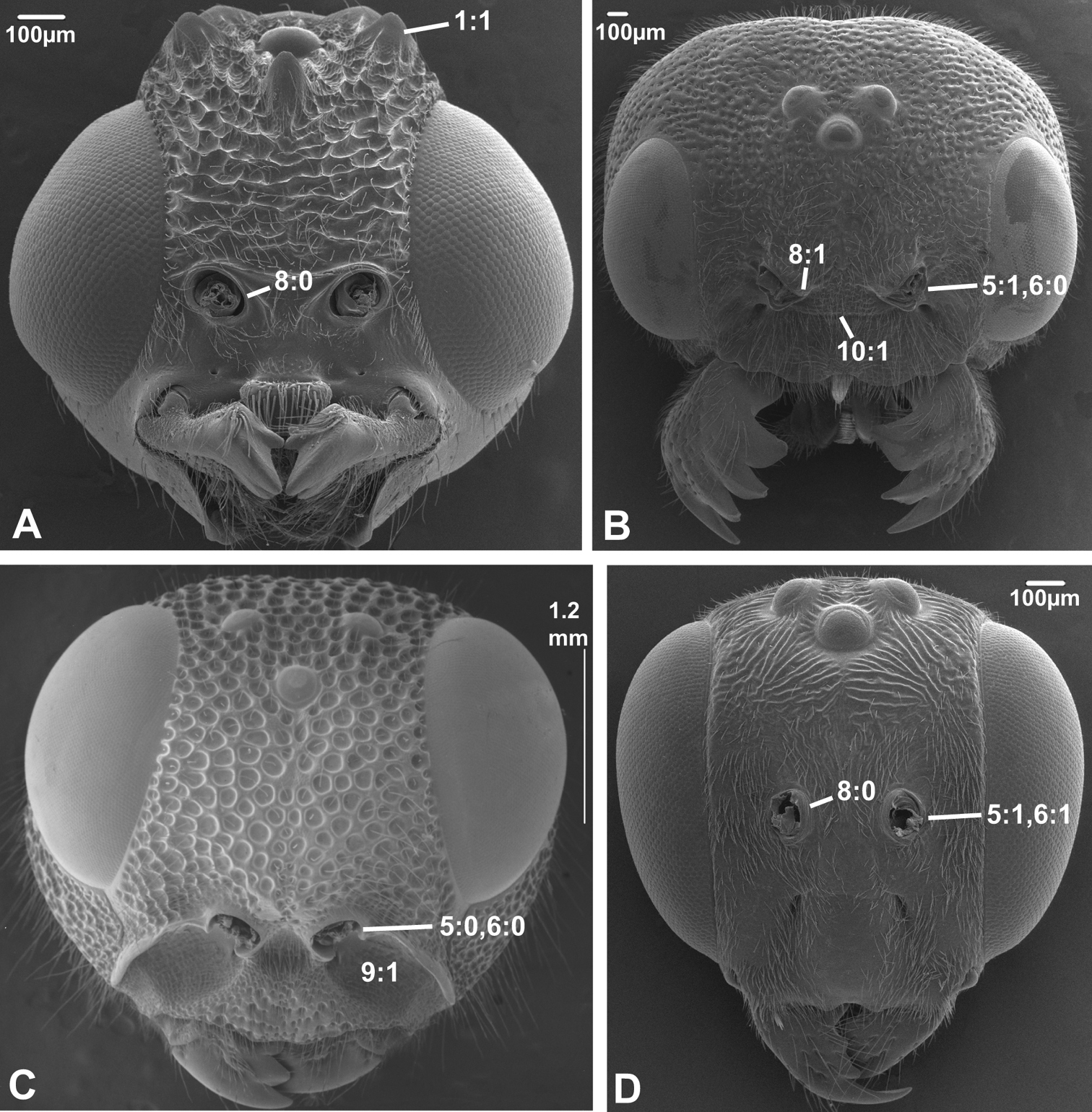
Anterior view of head capsule of A Macroxyela ferruginea (Xyeloidea, Xyelidae); modified from MB 102882 B Athalia rosae (Tenthredinoidea, Tenthredinidae); modified from MB 102486 C Tremex columba (Siricoidea, Siricidae); modified from MB 134704 D Orussus abietinus (Orussoidea, Orussidae); modified from MB 134672. Numbers indicate character numbers:character states.
Anterior view of head capsule of A Megischus sp. (Stephanoidea, Stephanidae), modified from MB 103607 B Taeniogonalos gundlachii (Trigonaloidea, Trigonalidae); modified from MB 103032 C Megalyra fasciipennis (Megalyroidea, Megalyridae); modified from MB 103568 D Pseudofoenus sp. (Evanioidea, Gasteruptiidae); modified from MB 103788. Numbers indicate character numbers:character states.
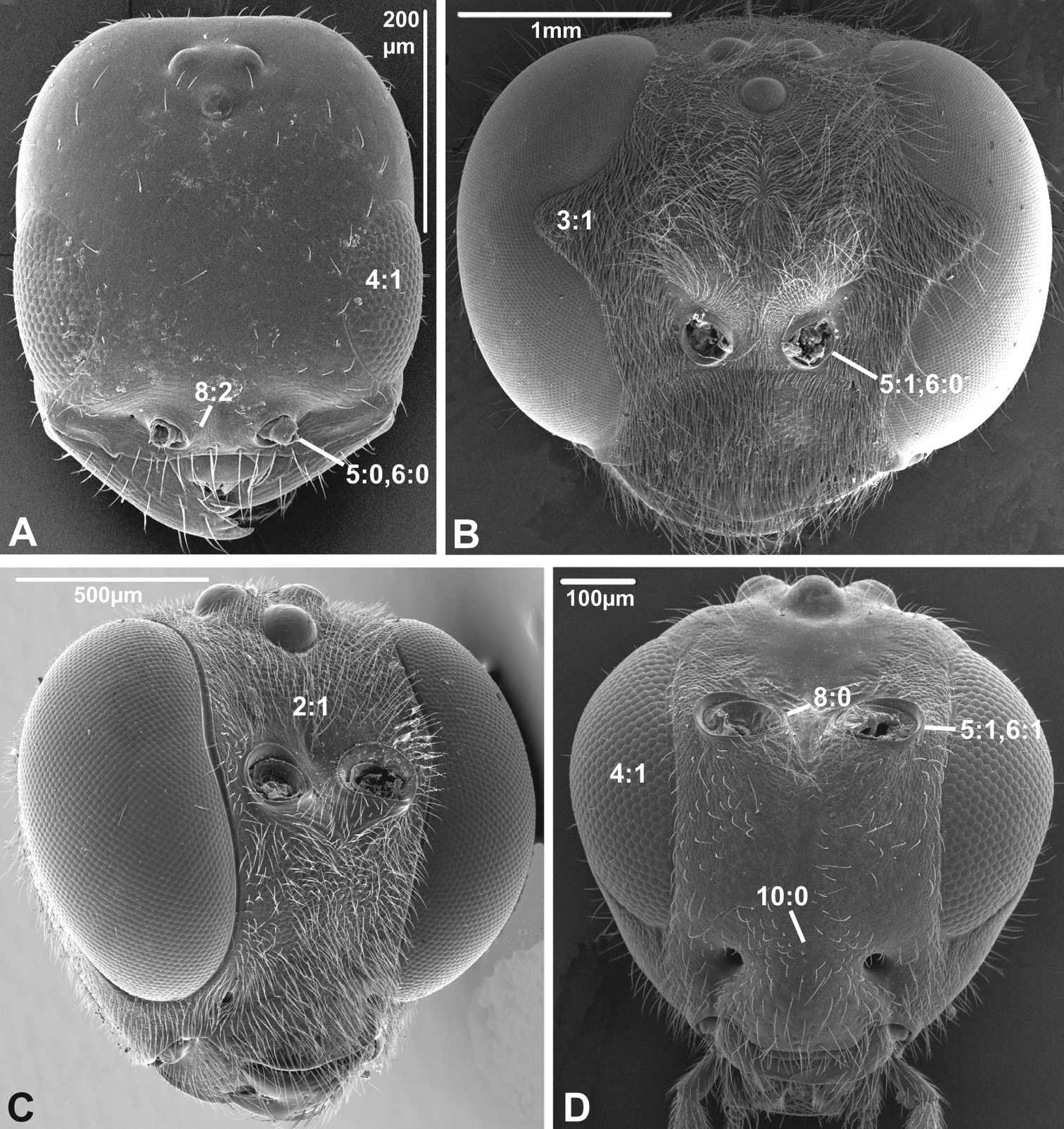
3. Notch on the inner margin of the eye
(0) absent (e.g., Figs 3A, C-D)
(1) present (Fig. 3B)
A prominent notch is present on the inner margin of the eye in some Aculeata (especially Vespoidea) and Ichneumonoidea, but does not seem to be a ground plan feature of either of these taxa.
4. Hairs on eyes
(0) absent or indistinct, as most as long as diameter of ommatidium (e.g., Figs 3B, C)
(1) distinct setae longer than ommatidium present on at least part of eye (Figs 3A, D)
Among the non-apocritan lineages, distinct hairs on the eyes (between the ommatidia) were only observed in Cephus (Cephidae). In contrast, hairs are present in many apocritan taxa, especially among the Chalcidoidea and the Proctotrupomorpha s.str. (
Anterior view of head capsule of A Cephalonomia stephanoderis (Chrysidoidea, Bethylidae); modified from MB 102531 B Pison chilense (Apoidea, Crabronidae); modified from MB 102586 C Dusona egregia (Ichneumonoidea, Ichneumonidae); modified from MB 134625 D Orgilus gracilis (Ichneumonoidea, Braconidae); modified from MB 103649. Numbers indicate character numbers:character states.
5. Position of antennal foramina relative to eyes
(0) below or level with ventral margin of eyes (Figs 2C, 3A, 4D)
(1) above ventral margin of eyes (Figs 1A, B, 2B, D, 3B, D, 4C)
Anterior view of head capsule of A Acanthochalcis sp. (Chalcidoidea, Chalcididae), modified from MB 134808 B Mymaromma anomalum (Mymarommatoidea, Mymarommatidae); modified from MB 101797 C Pelecinus polyturator (Pelecinidae); modified from MB 79061 D Sparasion formosum (Platygastridae); modified from MB 103049. Numbers indicate character numbers:character states.
6. Position of antennal foramina relative to clypeus
(0) equal or closer to clypeus than its own diameter (Figs 1A, B, 2B, C, 3A, B, 4D)
(1) further from clypeus than its own diameter (Figs 2D, 3D, 4C)
These two characters are partly overlapping, but it was decided score them separately to partition the information as finely as possible. Both characters are quite variable across the Hymenoptera, also within many superfamilies. Having the antennal foramina below the ventral margins of the eyes is apparently apomorphic for Orussidae and Platygastroidea, and possibly synapomorphic for Megalyridae + Ceraphronoidea.
7. Frontal shelf
(0) absent
(1) present, antennal foramina facing upwards (
8. Inner margin of antennal foramen (ordered)
(0) not distinctly raised compared to outer margin (Figs 1A, B, 2A, D, 3D)
(1) with distinct projection, raised compared to outer margin (Figs 2B, 4C)
(2) area between projections raised as well forming interantennal process (Figs 3A, 4B, D)
The presence of raised inner margins on the antennal foramina that might or might not be connected medially (states 1&2) are of scattered occurrence throughout the Hymenoptera. Having just the margins raised might be apomorphies of the Orussidae and Trigonalidae, as well as occurring in the groundplan of the Platygastroidea; within the latter, the projections have fused medially in Archaeoteleia, Sparasion and Telenomus (formerly placed in a separate family, the Scelionidae; see
9. Subantennal groove
(0) absent or weakly developed (e.g., Figs 1A, B, 2A, D)
(1) present, distinct (Figs 1C, D, 2C)
The presence of a well developed groove extending from the antennal foramen and ventrally of the eye for accommodating the proximal part of the antenna is a putative apomorphy of Siricidae (
10. Epistomal sulcus (unordered)
(0) absent or reduced, internal ridge absent (Figs 3D, 4C))
(1) present, distinct, usually with internal ridge (e.g., Figs 1A, 2B)
(2) present, not continuous medially, extends dorsally to antennal foramen
The epistomal sulcus and corresponding internal ridge separates the clypeus from the frons. Having these structures well developed and continuous medially is apparently a hymenopteran ground plan feature. The epistomal sulcus has been reduced a number of times within the Hymenoptera, notably in many Chalcidoidea and Cynipoidea, although it is uncertain whether the absence is a ground plan feature of either of these superfamilies. Having the sulcus well developed laterally, but discontinuous medially (state 2) is a putative apomorphy of Evaniidae.
11. Clypeus
(0) not inflected
(1) inflected, covering base of labrum (Figs 5A, C;
In all Hymenoptera where this feature could be observed, the ventral margin of the clypeus is sclerotised also on its posterior, internal side, and the labrum is situated slightly posterior to it (Fig. 1A).
12. Mandibular foramen
(0) oral and mandibular foramen continuous (Fig. 5A)
(1) mandibular and oral foramina separated by subgenal sclerotisation (Fig. 5B)
The separation of the mandibular and oral foramina by sclerotisations continuous with the head capsule is a long established apomorphy of the Pamphilioidea (e.g.,
13. Occipital sulcus and ridge
(0) absent (e.g., Figs 5C, 6B, D)
(1) present (Figs 5A, B)
The occipital sulci are present above the occipital foramen in the basalmost lineages of Hymenoptera and is definitely a ground plan feature of Hymenoptera (
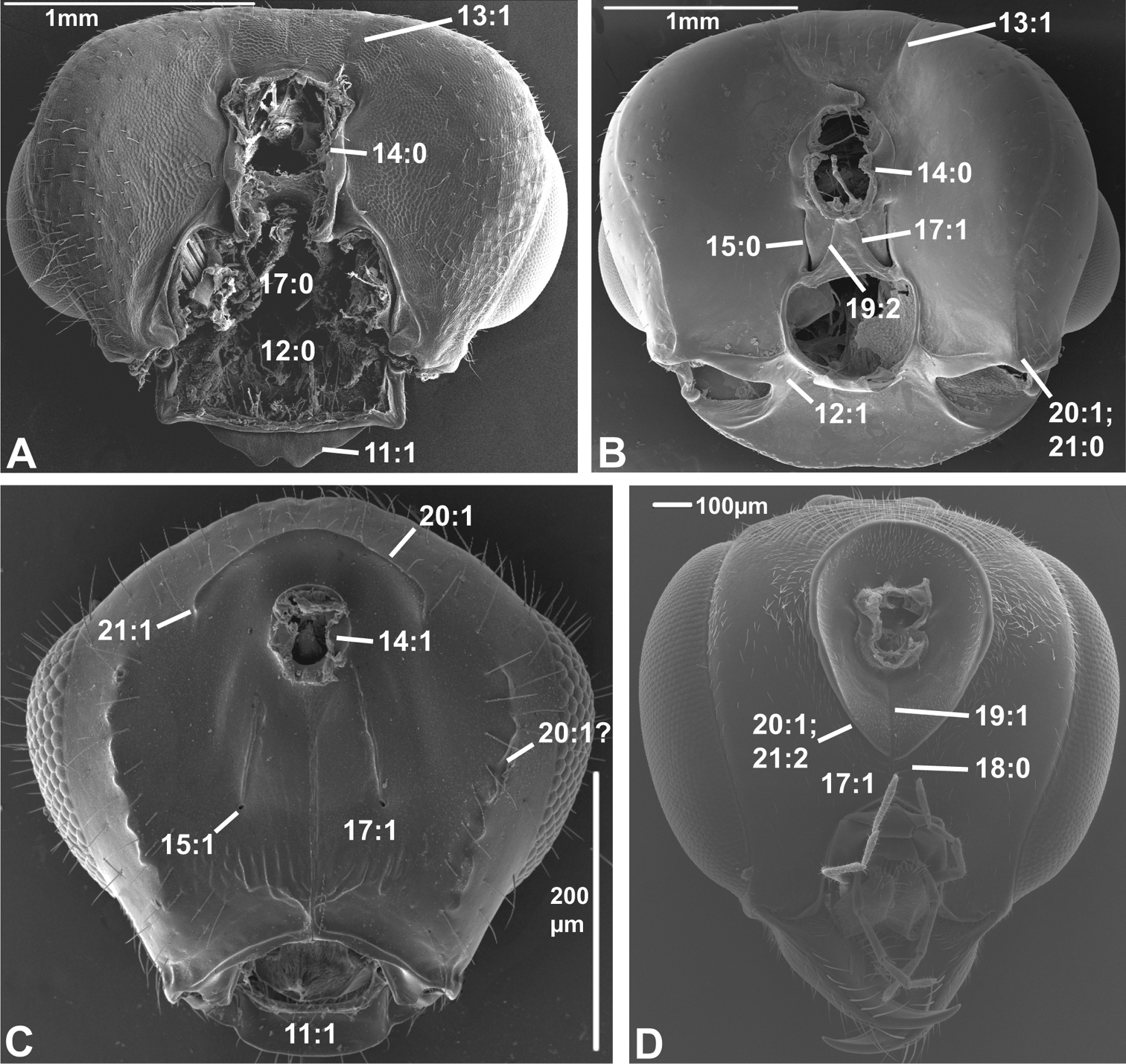
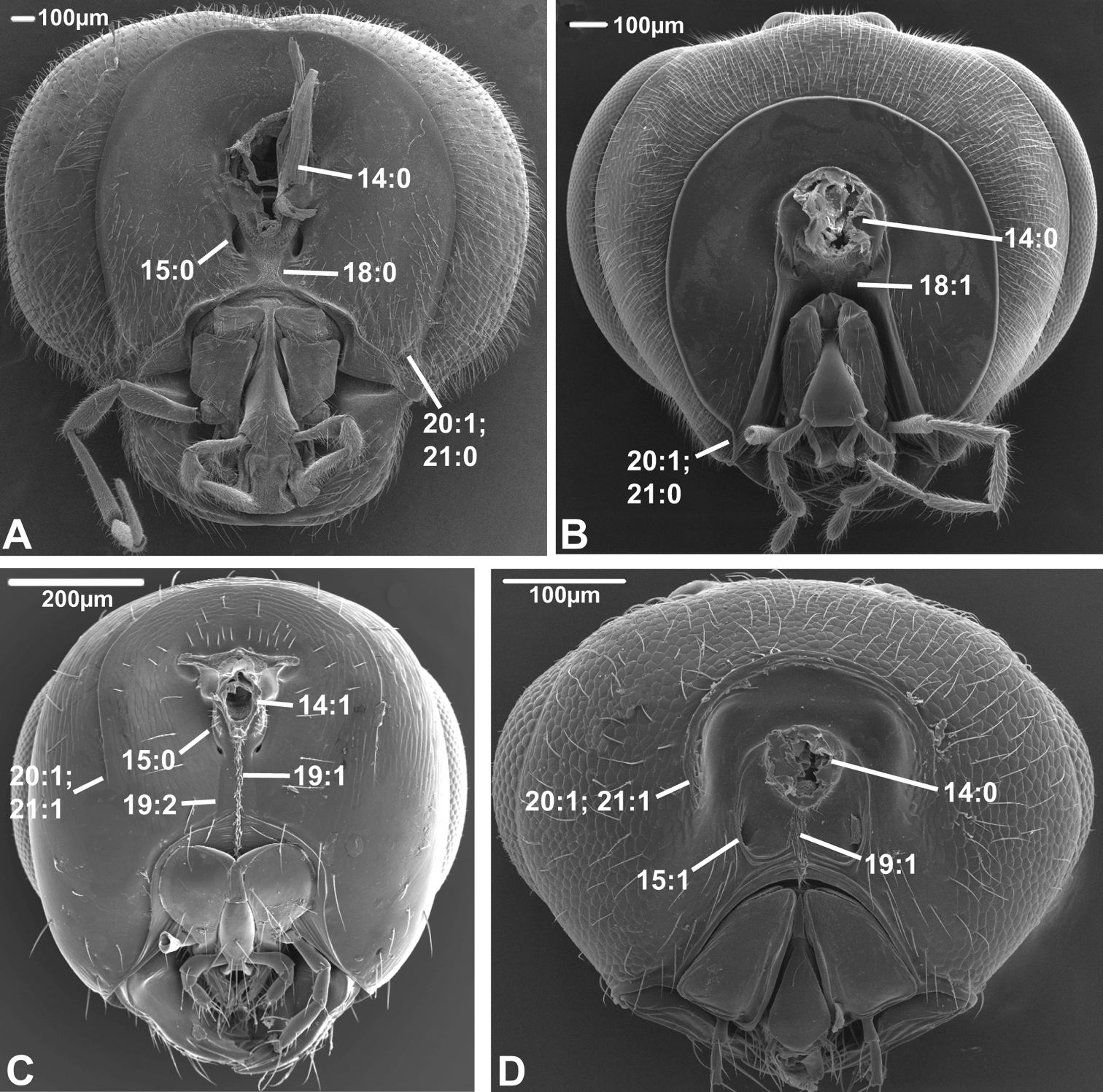
Posterior view of head capsule of A Macroxyela ferruginea (Xyeloidea, Xyelidae)¸ modified from MB 102885 B Onycholyda amplecta (Pamphilioidea, Pamphiliidae); modified from MB 102837 C Ceraphron sp. (Ceraphronoidea, Ceraphronidae); modified from MB 78643 D Pseudofoenus sp. (Evanioidea, Gasteruptiidae); modified from MB 103810. Numbers indicate character numbers:character states.
14. Position, occipital foramen
(0) approx. halfway between top of head and oral foramen (Figs 5A, B, 6A, B, D)
(1) distance from top of head to occipital foramen half or less than distance to oral foramen (Figs 5C, 6C)
The dorsally displaced occipital foramen is apparently an apomorphy of the Ceraphronoidea (Fig. 5C). It is also observed in a few other apocritan taxa (e.g., some Chalcidoidea).
Posterior view of head capsule of A Taeniogonalos gundlachii (Trigonaloidea, Trigonalidae); modified from MB 103043 B Dusona egregia (Ichneumonoidea, Ichneumonidae); modified from MB 103363 C Megastigmus transvaalensis (Chalcidoidea, Torymidae); modified from MB 101672 D Isostasius sp. (Platygastroidea, Platygastridae); modified from MB 103503. Numbers indicate character numbers:characters states.
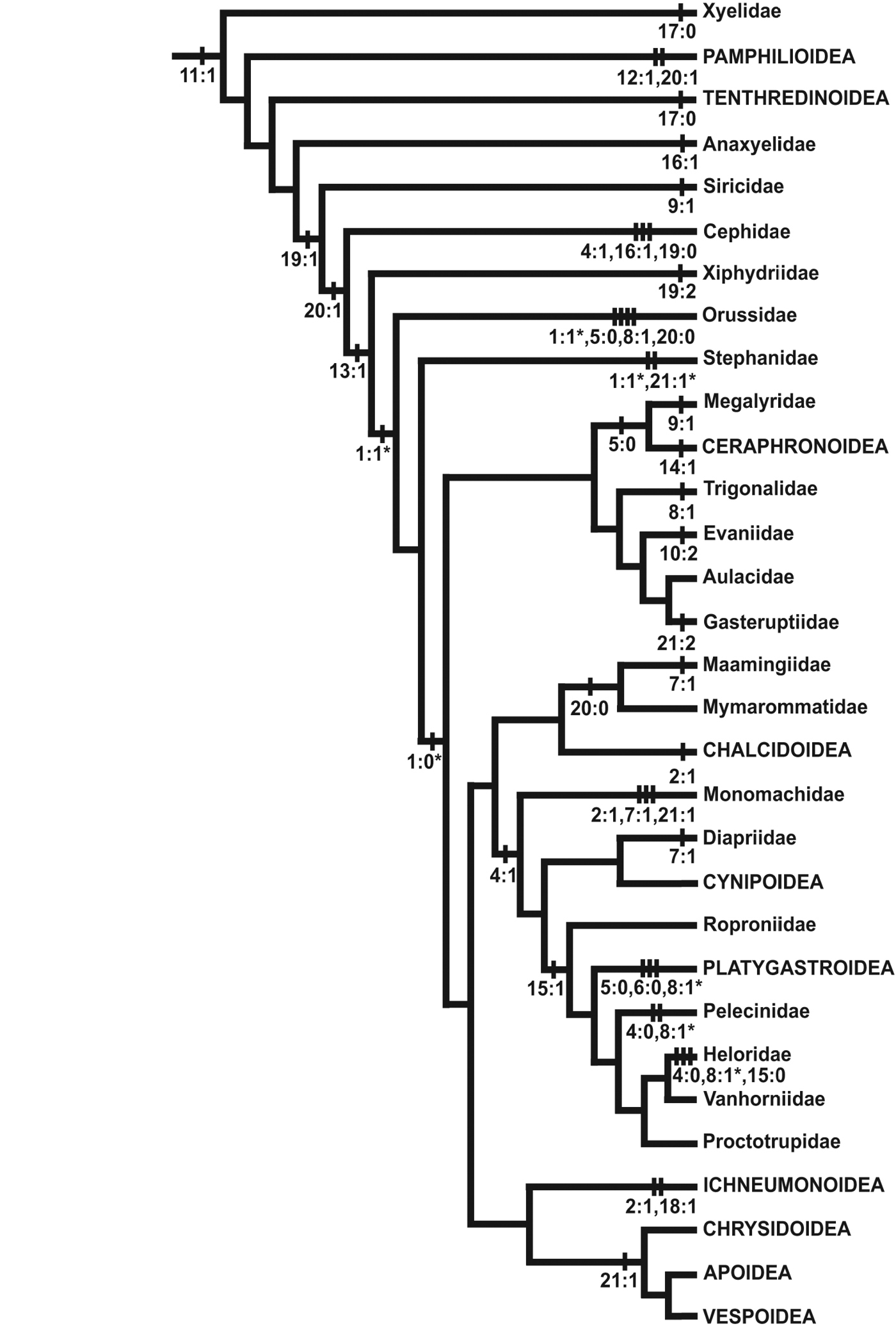
Selected character state changes mapped onto a phylogeny of Hymenoptera. Tree modified from Vilhelmsen et al. 2010a, fig. 69. Outgroup taxa have been removed and terminals have been collapsed to the family or superfamily level; Aulacidae, Diapriidae, Chrysidoidea and Vespoidea are treated as monophyletic. Not all character state changes shown; changes marked with a * have equally parsimonious alternative optimisations.
15. Position, posterior tentorial pits
(0) adjacent/at level with occipital foramen/condyles (Figs 5B, 6A, B)
(1) considerably ventral to occipital foramen (Figs 5C, 6D)
The posterior tentorial pits are considered level with the occipital foramen when at least their dorsal ends reach the level of the ventral margin of the foramen (e.g., Fig. 5B). The ventrally displaced posterior tentorial pits are of scattered occurrence across the Apocrita. The trait is prominent in some Ceraphronoidea (Fig. 5C), where it is perhaps correlated with the dorsal position of the occipital foramen (see previous character). The ventrally placed tentorial pits also occur in the Pelecinidae, Platygastridae, Proctotrupidae, Ropronidae and Vanhornidae and might be interpreted as a ground plan feature/synapomorphy of a larger clade within the Proctotrupomorpha (see Fig. 7), but then the condition has to have reversed within the Heloridae and Archaeoteleia, Sparasion and Telenomus (Platygastroidea).
16. Postoccipital bridge
(0) absent
(1) present (
In contrast to the ventral sclerotisation (see character 17), this is an internal structure, formed by a sclerotisation between the insertion points of the ventral profurco-postoccipital muscles ventrally of the tentorial bridge (see
17. Sclerotisation between occipital and oral foramina
(0) absent, foramina confluent (Fig. 5A)
(1) present, foramina separate (Figs 5B-D)
The various configurations of the ventral head sclerotisations in basal Hymenoptera were discussed at length in
As it is scored here, the presence of a ventral head sclerotisation is optimized as a ground plan feature of the Hymenoptera, contrary to the inference of
18. Ventral sclerotisation configuration
(0) at most oblique ventral to tentorial pits, not extending anteriorly (Figs 5D, 6A)
(1) horizontal ventral to pits and extending anteriorly (Fig. 6B)
This character has been scored inapplicable when the ventral sclerotisation (see previous character) is absent. The sclerotisation is inflected just below the tentorial pits in some apocritan taxa, forming the dorsal and anterior parts of a trough that accommodates the labiomaxillary complex. This condition was already noticed by
19. Longitudinal sulci on ventral head sclerotization (ordered)
(0) none
(1) one median sulcus or hair line present, at least ventrally (Figs 5D, 6C, D)
(2) two sublateral sulci present, not merging ventrally (Figs 5B, 6C)
This character has been scored inapplicable when the ventral sclerotisation (see character 17) is absent. When a narrow median hair line is present, it has been scored state 1. In some cases (e.g., Megastigmus (Torymidae) Fig. 6C) both a median hair line and two sublateral sulci are present; these instances were scored as polymorphic (1&2). These longitudinal sulci and hair lines are some of the anatomical landmarks used to differentiate between the different types of ventral sclerotisations (see character 17), state 1 being indicative of a postgenal bridge, state 2 of a hypostomal bridge. This character is highly variable throughout the Hymenoptera. If treated as ordered, there is an unambiguous change from state 2 to state 1 in the common ancestor of Siricidae, Cephidae (which has state 0), Xiphydriidae (which has state 2 derived secondarily), Orussidae, and Apocrita (in which this character is very variable and displays few unambiguous changes). This corroborates the suggestion of
20. Occipital carina
(0) absent (Fig. 5A)
(1) present (Figs 5B-D, 6A-D)
The occipital carina extends dorsally and laterally of the occipital foramen, usually reaching the ventral margin of the head capsule close to the mandibular bases (but see following character). The presence of the occipital carina is not a ground plan character of the Hymenoptera but apparently evolved at least twice among the basal Hymenoptera, in the Pamphilioidea and (with reversals, e.g., in Maamingidae + Mymarommatidae) in the common ancestor of Cephidae, Xiphydriidae, Orussidae (where it is not a ground plan character, at least for the extant members of the family, see
21. Occipital carina configuration (unordered)
(0) reaching ventral margin of head capsule (Figs 5B, 6A, B)
(1) not reaching ventral margin and not continuous medially (Figs 5C, 6C, D)
(2) continuous ventrally of occipital foramen (Fig. 5D)
This character has been scored inapplicable when the occipital carina is absent. In most Hymenoptera that have an occipital carina it reaches the ventral margin of the head capsule. Some taxa have the ventral ends of the occipital carina terminating before this, notably the Aculeata and Stephanidae. In a few apocritans, the ventral ends of the occipital carina joins medially below the occipital foramen, forming a continuous rim around the latter; this condition is a putative apomorphy of the Gasteruptiidae (Fig. 5D).
ConclusionThe characters explored in the present paper show considerable variation across the Hymenoptera. For many of the characters, there is considerable variation within as well as between the families and superfamilies. The most unequivocal character support is usually displayed toward the distal ends of the tree as autapomorphies of families or superfamilies (the presence of a medially interrupted epistomal sulcus (char. 10:2) in Evaniidae; the presence of a dorsally displayed occipital foramen (char. 14:1) in Ceraphronoidea; the impression of the ventral sclerotisation below the occipital foramen to form a cavity for the labiomaxillary complex (char. 18:1) in Ichneumonoidea; having the occipital carina continuous ventrally of the occipital foramen (char. 21:2) in Gasteruptiidae); or autapomorphies of the Hymenoptera (the inflection of the clypeus; char. 11:1). Characters that corroborate more inclusive clades above the superfamily level are much rarer. An example of one such character is the absence of the occipital sulcus and ridge (char. 13:1) which has been lost in Xiphydriidae, Orussidae and Apocrita.
This pattern was even more obvious in the analyses of the much more comprehensive mesosomal data set assembled by
I thank the organizers of this special volume of ZooKeys for inviting me to contribute to it. Mike Sharkey and an anonymous referee provided constructive criticism that helped improve the paper. Denis Brothers, Matt Buffington, Jim Carpenter, Andy Deans, Gary Gibson, John Heraty, John Huber, Fredrik Ronquist, Susanne Schulmeister, Mike Sharkey, Dave Smith, and Matt Yoder all provided specimens for the present study. Andy Boring, Andy Deans, Johan Liljeblad and Susanne Schulmeister submitted some images to MorphBank reproduced here. Financial support was provided by NSF AToL grant EF-0337220.