(C) 2011 Denis J. Brothers. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The taxonomic placement of an enigmatic species of wasp known from two specimens in Late Cretaceous New Jersey amber is investigated through cladistic analyses of 90 morphological characters for 33 terminals ranging across non-Aculeata, non-Chrysidoidea, most subfamilies of Chrysidoidea and all genera of Plumariidae (the family to which the fossils were initially assigned), based on use of exemplars. The fossil taxon is apparently basal in Chrysidoidea, most likely sister to Plumariidae, but perhaps sister to the remaining chrysidoids, or even sister to Chrysidoidea as a whole. It is described as representing a new family, Plumalexiidae fam. n., containing a single species, Plumalexius rasnitsyni gen. et sp. n. Previous estimates of relationships for the genera of Plumariidae and for the higher taxa of Chrysidoidea are mostly confirmed. The importance of outgroup choice, and additivity and weighting of characters are demonstrated.
amber, fossil, Plumalexiidae, Plumalexius rasnitsyni, new genus, new species, classification
The phylogeny of the Hymenoptera, and particularly the Aculeata, has recently been investigated critically by several authors (
As chrysidoids, plumariids are very unusual morphologically, the males having broad wings with a relatively rich wing venation including well developed accessory veins in the apical membrane similar to those of many of the very distantly related Mutillidae (Vespoidea) and Heterogynaidae (Apoidea) (the latter consequently mistakenly assigned to Plumariidae by
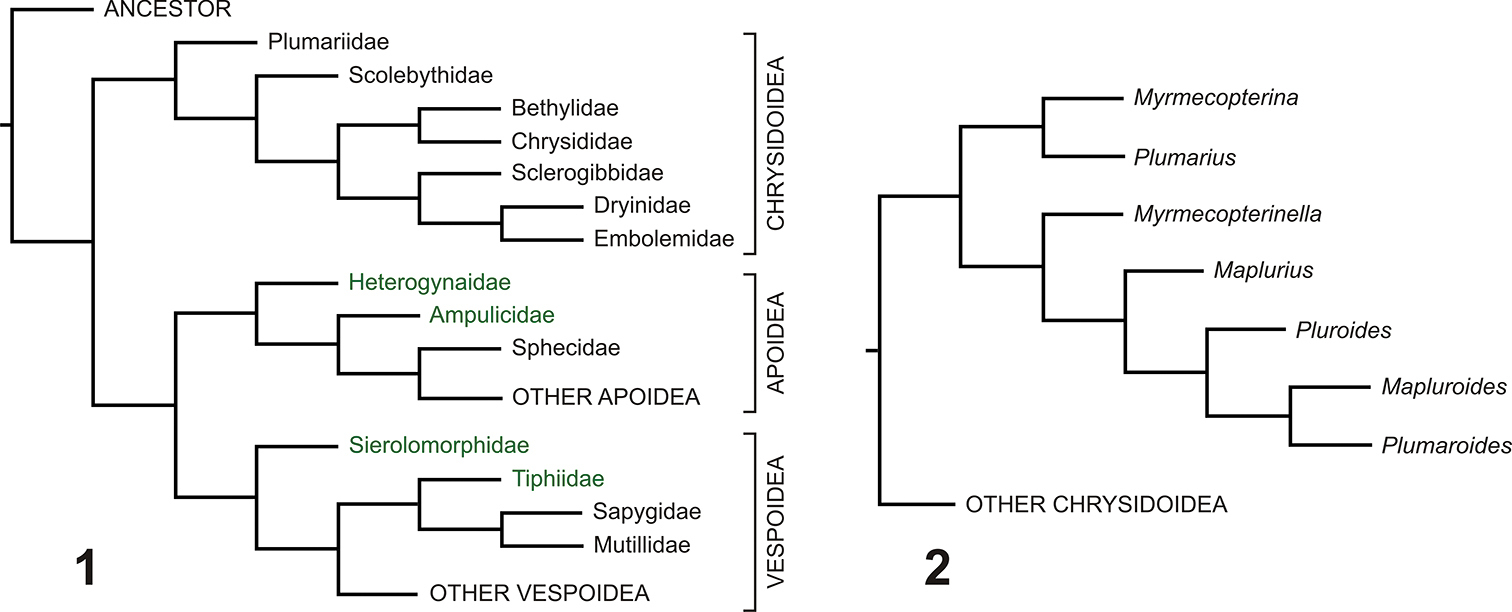
Previous estimates of relationships. 1 Aculeata, superfamilies, and families of Chrysidoidea (modified from
Within the Chrysidoidea, the family which apparently arose next (after the Plumariidae had diverged) is the Scolebythidae (see Figure 1). This also has a putatively Gondwanan distribution, based on modern representatives, with Pristapenesia Brues, 1933 in the neotropics, Clystopsenella Kieffer, 1911 in the neotropics and Australia, Ycaploca Nagy, 1975 in South Africa, Australia, Fiji, New Zealand and New Caledonia, and Scolebythus Evans, 1963 in Madagascar and South Africa (
No fossil Plumariidae have yet been described, but two conspecific male specimens from Late Cretaceous New Jersey (USA) amber were recently stated to be members of the family (Rasnitsyn in
The two amber pieces (Figures 3–4), each embedded in epoxy as described by
Previous cladistic analyses of the Chrysidoidea and Plumariidae (e.g. Brothers, 1999;
Parsimony analyses by TNT (
urn:lsid:zoobank.org:act:621025A6-DE9F-445E-8D8B-05194B6EECF7
Plumalexius Brothers, new genus.
Male. Pronotum forming a short convex band reaching tegula; propleura closely associated, anterodorsally exposed as a short neck, posteriorly swollen and transversely truncate; prosternum short and scarcely exposed medially; mesopleuron large and swollen; metasternum somewhat depressed. Forewing with pterostigma very large, seven closed cells (costal, basal, subbasal, marginal, first and second submarginals, first discal), second submarginal cell with long anterior margin, no accessory veins in apical membrane. Hind wing with closed cells (basal and subbasal at least), anal (vannal/plical) lobe well developed; jugal lobe absent. Coxae subglobose, trochanters inserted apically.
Female. Unknown.
Plumalexius rasnitsyni Brothers, new species
The genus name, which is masculine, is derived from “Plumariidae”, to which it was first assigned, and “Alexandr”, the first name of Professor Dr Rasnitsyn, honoured in this Festschrift.
Male. Compound eye oval with convex inner margin; antenna with many long fine erect setae (number of antennomeres unknown); mandible with four apical teeth along truncate apical margin; maxillary palp at least 5-segmented; labial palp at least 3-segmented. Pronotum much shorter than mesoscutum, with slight anterior collar (flange) and posteroventral angle rounded; mesoscutum transverse; notaulus distinct, complete; tegula small, convex; metapostnotum apparently about as long as metanotum; propodeum long, weakly constricted apically; meso-metapleural suture straight. Hind wing with two closed cells, vein C present only basally, anal (vannal/plical) lobe less than half length of wing. Tibiae without spines or strong setae; tibial spurs 1–2–2; basitarsomeres much longer than other tarsomeres; arolia large; claws simple. Metasoma ovoid, sessile basally, apical tergum apparently simple, seventh sternum reduced, hypopygium simple with convex apex.
Female. Unknown.
urn:lsid:zoobank.org:act:20DEDD72-2F53-43D6-B455-E987604C0265
http://species-id.net/wiki/Plumalexius_rasnitsyni
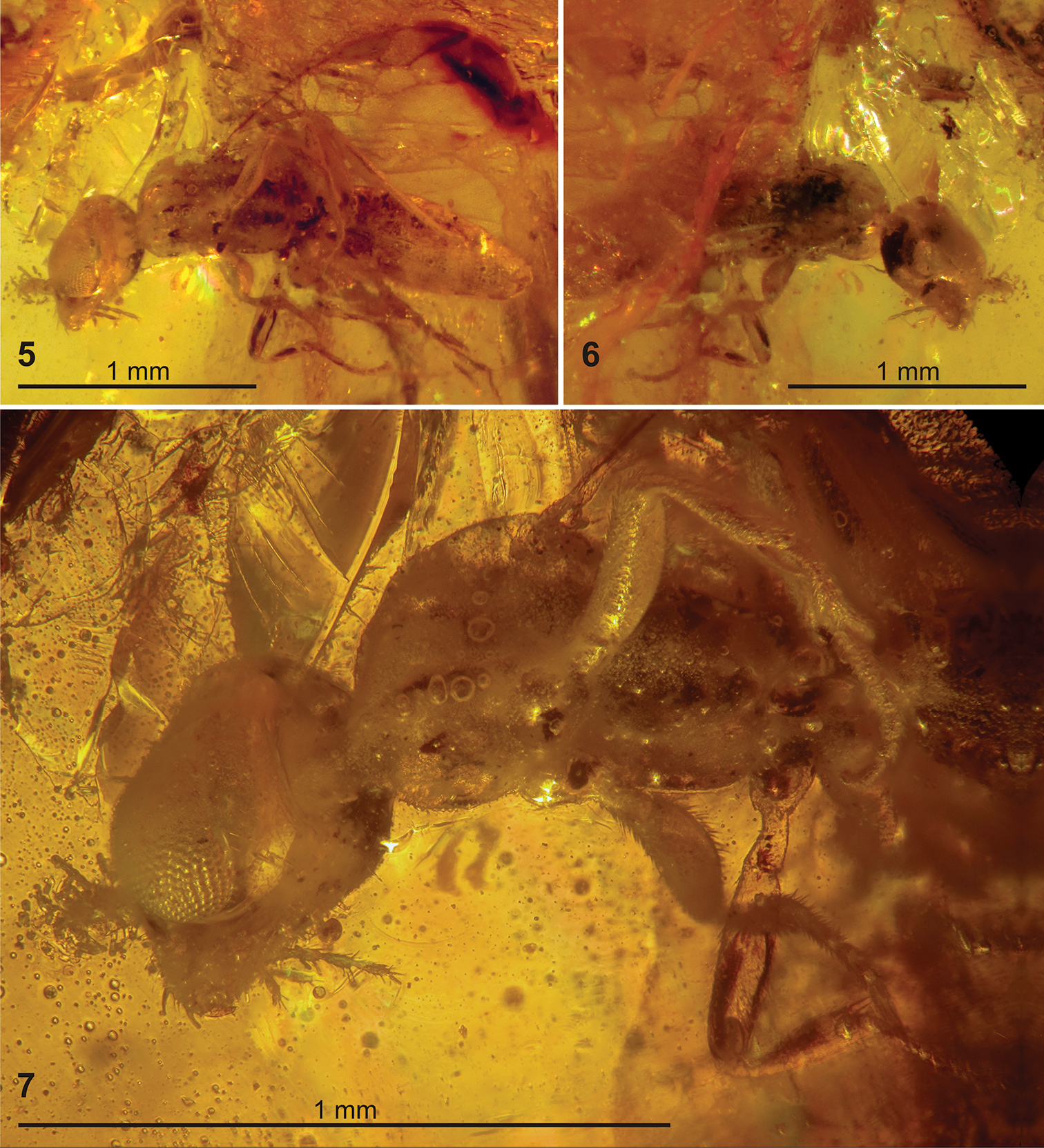
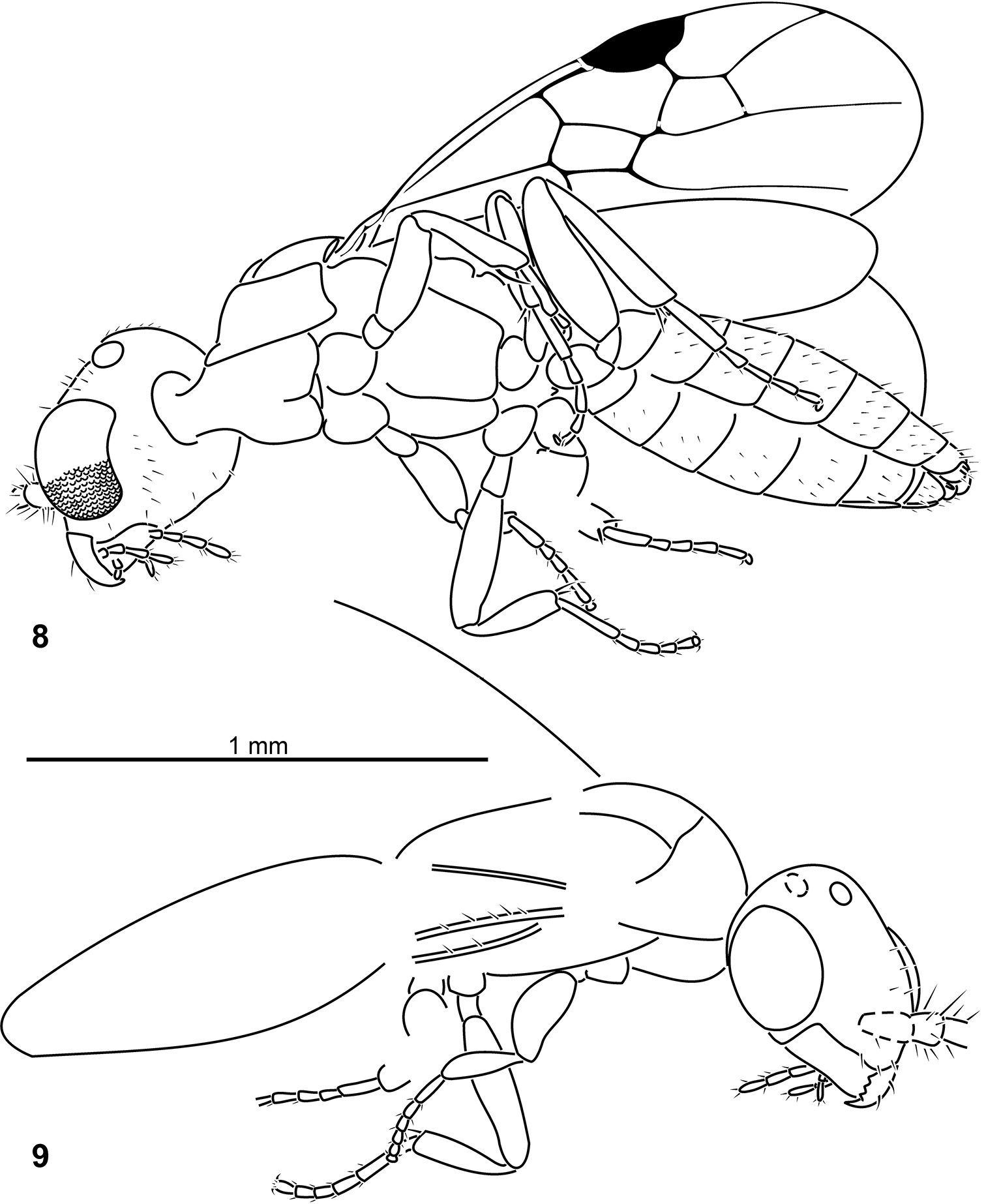
Figs 3 –13Holotype male (Figures 3, 5–9), in heavily fractured block of yellowish amber embedded in a trapezoidal epoxy matrix about 22 × 10 × 7 mm, with labels as follows: “NEW JERSEY Amber: / Late Cretaceous / NEW JERSEY: Middlesex Co / Sayreville, White Oaks Pit / 1995, coll.Paul Nascimbene / AMNH no. NJ-695”, “NEW JERSEY Amber: / Late Cretaceous / AMNH no. NJ-695 / HYMENOPTERA:”, “Plumariidae” [Rasnitsyn’s handwriting], “HOLOTYPE / Plumalexius / rasnitsyni ♂ / D.J. Brothers, 2011” [red label, printed].
Paratype male (Figures 4, 10–13), in heavily fractured block of yellowish amber embedded in a rectangular epoxy matrix about 18.5 × 13.5 × 9 mm, with labels as follows: “NEW JERSEY Amber: / Late Cretaceous / NEW JERSEY: Middlesex Co / Sayreville, White Oaks Pit / 1995, coll.Paul Nascimbene / AMNH no. NJ-175”, “NEW JERSEY Amber: / Late Cretaceous / AMNH no. NJ-175 / HYMENOPTERA: / Family? (PN-2a) / Plumariidae” [Rasnitsyn’s handwriting], “?Family / Det. L. Masner 1996”, “PARATYPE / Plumalexius / rasnitsyni ♂ / D.J. Brothers, 2011” [yellow label, printed]. (This specimen is presumed to be a male because of its similarity to the holotype even though the metasoma is mostly not visible.)
The species name, a noun in the genitive case, honours Professor Dr Alexandr Rasnitsyn, who first recognised the significance of the specimens.
(based on holotype, paratype data in parentheses where different or feature not visible in holotype): Male. Entirely pale yellowish (reddish) brown with venation slightly darker. Head and body length as preserved 2.03 (2.37) mm; estimated head length 0.24 (0.29) mm; estimated mesosoma length 0.80 (0.77) mm; estimated metasoma length 0.90 (0.89) mm; approximate forewing length 1.32 (1.46) mm; approximate hindwing length 1.03 (1.17) mm. Head and metasoma with scattered fine short erect setae, mesosoma almost glabrous, antennal pedicel and flagellomeres with fine long erect setae; legs with dense recumbent setae and scattered semi-erect setae.
Head: Hypognathous; about as wide as high; vertex evenly rounded. Eye ovate with convex inner margin, moderately protuberant, apparently glabrous, ommatidia distinct. Ocelli ovate, large. Occipital carina distinct. Frons and clypeus weakly convex; clypeus transverse with convex anterior/apical margin. Gena simple. Antennal sockets simple, apparently about as close to eyes as to each other, apparently close to posterior/dorsal margin of clypeus. Antennal scape about as long as wide (distinctly flattened posterolaterally and broadened towards apex), with several erect setae; pedicel (about half length of scape and of first flagellomere), with many fine long erect setae; (flagellomeres 1–4 becoming slightly longer sequentially, with many fine long erect setae). Mandible long, evenly broad and curved; two prominent short curved setae on lateral surface; apex truncate with four similar sharp teeth, apical tooth the longest. Maxillary palp at least 5-segmented; labial palp at least 3-segmented [palp bases concealed by foam but segmentation inferred from assumed points of origin].
Mesosoma ovate, about twice as long as wide/high. Pronotum forming a curved oblique ribbon anterolateral to mesoscutum, broader medially than posterolaterally; posterodorsal margin evenly concave; posterolateral margin strongly emarginate and approaching tegula dorsally; posteroventral angle broadly rounded; anteroventrally with slight collar (flange) but leaving propleura exposed anteriorly. Propleura closely associated or fused; anteriorly produced as a short neck; swollen posteriorly; posterior margin apparently almost straight but exposing small part of prosternum medially; forecoxae approximated. Mesoscutum shorter than wide, moderately convex; notaulus distinct and complete, weakly diverging anteriorly; tegula small and convex. (Mesoscutellum apparently almost as long as scutum, weakly convex.) Mesopleuron large and convex; meso-metapleural suture distinct, almost straight. Mesosternum with posteromedial margin almost straight and apparently slightly overhanging mesocoxal base; mesocoxae slightly separated. (Metanotum short and transverse, flattened, not constricted medially. Metapostnotum apparently as long as metanotum, slightly depressed.) Metasternum apparently somewhat depressed. Metacoxae slightly separated. Propodeum slightly longer than mesoscutum, weakly convex but more strongly so posteriorly although without any defined posterior declivity; incision between mesosoma and metasoma weak.
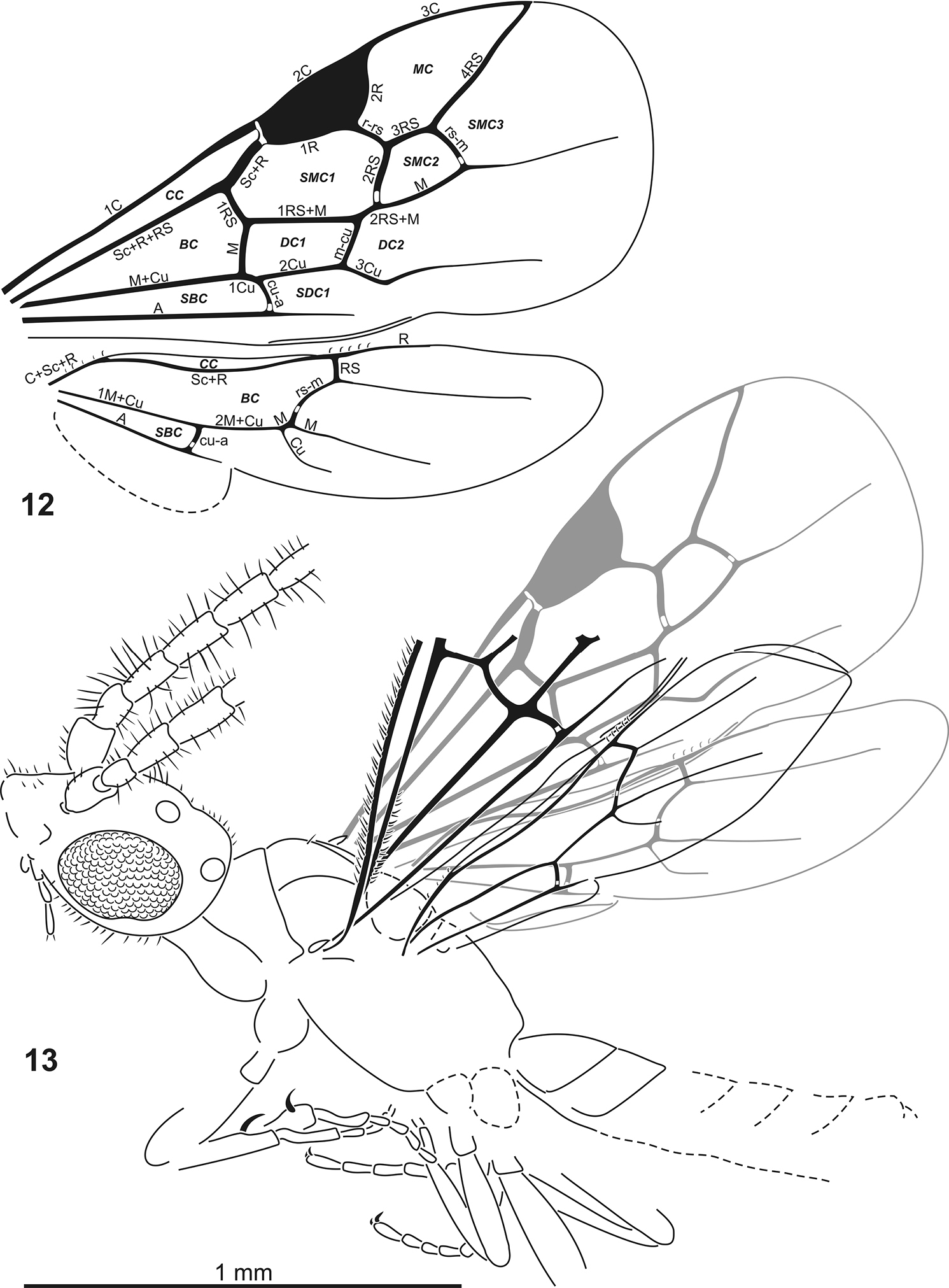
Forewing broad, about 2.2 × as long as wide, about 1.7 (1.8) × as long as mesosoma, with seven closed cells, veins approaching but not reaching margin. Costal cell well developed, broad. Pterostigma large, about 0.19 × as long and 0.17 (0.20) × as wide as wing, entirely sclerotised. Marginal cell about 2.38 (2.17) × as long as wide, 1.41 (1.52) × as long as pterostigma, apex acute. First submarginal cell about 2.08 (2.61) × as long as wide, 0.81 (0.80) × as long as marginal cell. Second submarginal cell almost as large as pterostigma, broadly sessile anteriorly, about 0.64 (0.57) × as long as first submarginal cell. Veins tubular except for nebulous free apical sections of M, Cu and A. No trace of any accessory vein(s) in apical membrane. Prestigmal vein (Sc+R) scarcely swollen, about 1.33 (1.37) × as long as vein 1Rs. Crossvein cu-a distinctly postfurcal, about 1.33 (1.75) × as long as 1Cu. Vein Cu2 absent, first subdiscal cell broadly open apically.
Hind wing about 0.8 × as long as forewing. (Basal and subbasal cells closed by tubular veins; costal cell open anteriorly, vein C present only basally. Veins tubular except for nebulous free apical sections of Rs, M, Cu and A. A few basal hamuli present in a cluster; about five apical hamuli. Crossvein rs-m long, about 2.67 × as long as 1Rs. Vein 1M very short, about 0.13 × as long as 2M+Cu. Crossvein cu-a distantly antefurcal, 2M+Cu about 0.57 × as long as 1M+Cu. Anal (vannal/plical) lobe apically delimited by moderate incision, lobe about 1.19 × as long as submedian cell, about 0.4 × as long as wing.) Jugal lobe absent.
Legs well developed, moderate in size; trochanters well developed and cylindrical; no trochantelli; tibiae without any spines or strong setae; basitarsomeres long, about as long as next three tarsomeres combined; all arolia large and flattened; claws simple ventrally. Foreleg with coxa subglobose, trochanter inserted apically; femur slightly swollen, with inner/anterior surface flattened; tibia with simple, weakly curved, bladelike calcar subapically. Mid- and hind legs with coxae globose, hind coxa somewhat larger than mid-coxa; tibiae each with two straight simple apical spurs, inner spur somewhat longer than outer.
Metasoma elongate oval, about 2.6 (indeterminable in paratype) × as long as wide/high; terga subequal in length. First tergum broad, weakly contracted toward base, profile evenly merging with second. First sternum apparently simple and evenly overlapping second. Seventh tergum apparently simple with apical margin convex. Seventh sternum apparently reduced and mostly concealed. Hypopygium simple, weakly convex, with narrowly rounded apical margin. Cercus apparently present, cylindrical. Genitalia with paramere apparently broadly rounded apically.
Female. Unknown.
New Jersey amber containing specimens of Plumalexius rasnitsyni sp. nov. 3 Specimen NJ-695, holotype (circled) 4 Specimen NJ-175, paratype (circled) 5–7 Specimen NJ-695, holotype 5 Ventrolateral view 6 Dorsolateral view 7 Detail, ventrolateral view.
Plumalexius rasnitsyni sp. nov., holotype. 8 Ventrolateral view 9 Dorsolateral view.
Plumalexius rasnitsyni sp. nov., specimen NJ-175, paratype. 10 Dorsolateral view 11 Details, dorsolateral view.
Plumalexius rasnitsyni sp. nov. 12 Wings, based on both specimens 13 Paratype, dorsolateral view, right wings in grey. Abbreviations. Wing veins: A = anal, C = costa, Cu = cubitus, M = media, R = radius, RS = radial sector, Sc = subcosta (numerals indicate abscissae, all lower-case indicates crossveins); cells: BC = basal cell (cell R), CC = costal cell (cell C), DC = discal cell (cells 1M, 2M), MC = marginal cell (cell 2R1), SBC = subbasal cell (cell 1Cu), SDC = subdiscal cell (cell 2Cu), SMC = submarginal cell (cells 1R1, 1Rs, 2Rs).
Table 1 shows the distribution of character states across the taxa.
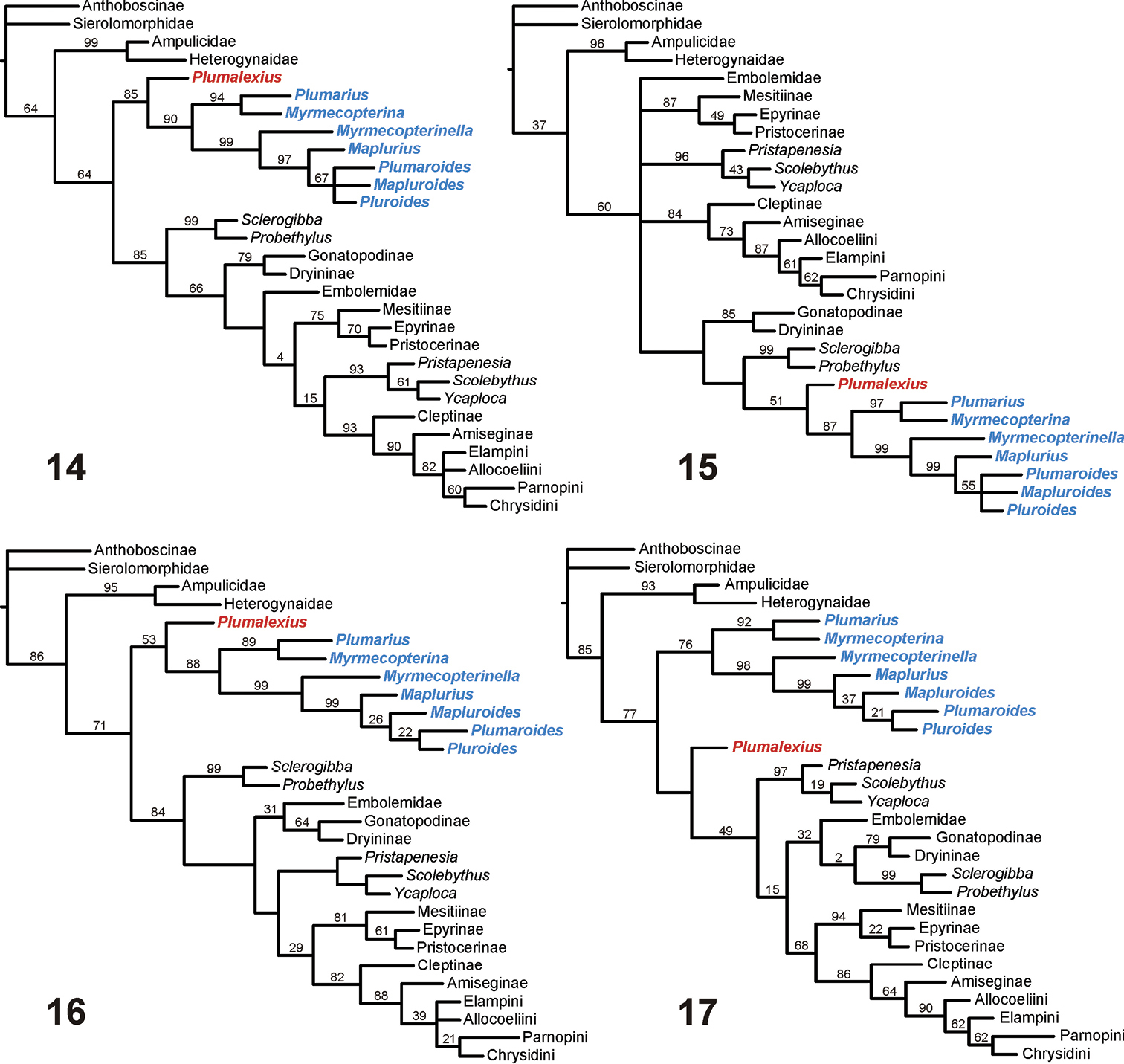
The cladograms resulting from the analyses using an aculeate outgroup (illustrated using Anthoboscinae, but the relationships within the Chrysidoidea were not affected by changing this to any of the other three aculeates) are shown in Figures 14–17. The consensus tree from the “equally weighted additive” analysis (Figure 14) shows Chrysidoidea as monophyletic, all chrysidoid families also as monophyletic (although with their relationships often not convincingly resolved, as shown by several apparent clades having no or very low relative resampling support), Plumalexius sister to Plumariidae (this clade sister to the remaining chrysidoids), and the plumariid genera with similar relationships to those found earlier (see Figure 2). In contrast, although the consensus tree from the “equally weighted non-additive” analysis (Figure 15) also shows Chrysidoidea as monophyletic, all chrysidoid families also as monophyletic (with their relationships even less resolved), and the same relationships for the plumariid genera, (Plumalexius + Plumariidae) now groups with Sclerogibbidae and Dryinidae, although without relative support. The consensus tree from the “implicitly weighted additive” analysis (Figure 16) shows similar relationships as the equally weighted version (Figure 14), except that there is slightly greater resolution for the families of Chrysidoidea and Scolebythidae is no longer sister to Chrysididae which is now monophyletic with Bethylidae, although some branches lack positive relative support values; there is also greater resolution for the plumariid genera. Similarly, the single MPC from the “implicitly weighted non-additive” analysis (Figure 17) produced improved resolution, showing (Bethylidae + Chrysididae) as monophyletic, but Plumalexius is grouped with the chrysidoids other than Plumariidae (although without positive relative branch support). The differences from previous analyses, most strikingly involving Scolebythidae and Sclerogibbidae (see Figure 1), are probably due to two factors: the use of exemplars instead of groundplans (introducing polymorphisms), and the position of the Aculeata s.str. outgroup taxa as relatively more derived than the Chrysidoidea.
Chrysidoidea relationships using Aculeata s.str.(Anthoboscinae) as outgroup 14 Characters equally weighted, some characters additive (strict consensus of 4 MPCs, raw lengths 383, CI = 0.41, RI = 0.71) 15 Characters equally weighted, all characters non-additive (strict consensus of 4 MPCs, raw lengths 349, CI = 0.45, RI = 0.71) 16 Characters implicitly weighted (k = 2.5), some characters additive (strict consensus of 2 MPCs, raw lengths 386, CI = 0.41, RI = 0.71) 17 Characters implicitly weighted (k = 2.5), all characters non-additive (1 MPC, raw length 352, CI = 0.44, RI = 0.70). Notes: Plumalexius shown in red, genera of Plumariidae shown in blue. Numbers are estimated GC branch-support values (see text); branches without numbers showed no positive support under the resampling protocol used.
Data matrix for analyses of relationships of members of Chrysidoidea. (Specimens used shown in Appendix A, characters in Appendix B. Values between square brackets indicate polymorphisms.)
| Characters | 1 - 10 | 11 - 20 | 21 - 30 | 31 - 40 | 41 - 50 | 51 - 60 | 61 - 70 | 71 - 80 | 81 - 90 |
| Taxa | |||||||||
| *Ichneumonidae | 010100000[012] | 000021000[01] | 001[01]000202 | 000[01]1000[03]0 | 001[01]21[01]00[12] | [45]02?00010[12] | 0[34]00000100 | 002001000[02] | 0000000000 |
| *Trigonalidae | 1100010012 | 0000[01]01000 | 0010000202 | 0011100030 | 1000201001 | 000000000[01] | 0301000100 | 0000000000 | 0000022200 |
| *Evaniidae | 0001100100 | 0000000001 | 0010100202 | 0011101033 | 5021011001 | 200-001103 | 1402100000 | 00000[01]0000 | 1000001020 |
| *Gasteruptiidae | 0211000100 | 0000100101 | 1100000202 | 0011211033 | 0011011001 | 620-000103 | 1412100100 | 0001000000 | 1000001020 |
| Anthoboscinae | 0110001112 | 0001200000 | 1002100100 | 1002100030 | 3300000001 | 0000000000 | 0400010000 | 0001020000 | 0000121001 |
| Sierolomorphidae | 111001111[02] | 0001100000 | 1002100100 | 0001200012 | 3112000010 | 100-001001 | 1401000100 | 0011020000 | 0000002301 |
| Ampulicidae | [01]110000110 | 0001200001 | 0000100011 | 1011120043 | 221[12]0101[01]1 | 0000000000 | [01][04]01000[01]00 | 0[01]2[23]0[12]0004 | 010002[12]021 |
| Heterogynaidae | 0110000100 | 0001200001 | 0000100011 | 2011121043 | 2211010111 | 300-110100 | 1101010100 | 0023000004 | 0000020221 |
| Plumalexius | 1??0000?02 | 20100??100 | 1?0?1?0??? | 00001?001? | ????1?0010 | 6100000011 | 1401010110 | 1023000000 | 0??001000? |
| Plumarius | 0001100111 | 2131101101 | 1100100000 | 0000100111 | 4412211000 | 6101010011 | 0001010100 | 002201[01][01]03 | 0100010000 |
| Plumaroides | 0000011102 | 0001112220 | 1001111200 | 1000100120 | 3422211000 | 6101111010 | 1001011110 | 1123020000 | 011211100? |
| Myrmecopterina | 0[12]10100111 | 2011101101 | 1100100000 | 0000100102 | 44[12][12]211000 | 6102110011 | 1001010110 | 1022011003 | 0101011000 |
| Myrmecopterinella | 0000001202 | 2001213200 | 0001111200 | 11001?1132 | ?411211010 | 620-011011 | 1301010110 | 0013220000 | 010001010? |
| Maplurius | 0000011102 | 2121113220 | 1001111200 | 1000100120 | 3412211010 | 6101011011 | 1001011110 | 1122020010 | 010111020? |
| Mapluroides | 0000011102 | 0001112220 | 1001121200 | 10000?0120 | ?411211000 | 6101011012 | 0001011110 | 1122020000 | 010111020? |
| Pluroides | 0000011102 | 0001113210 | 1001111200 | 10001?0120 | ?411211000 | 6101011011 | 0001011110 | 1122020000 | 010211100? |
| Scolebythus | 1110000100 | 2000000100 | 0020001000 | 1000101020 | 3301000011 | 620-001012 | 2222110111 | 0002000000 | 0100021020 |
| Pristapenesia | 1??0000100 | 0000[02]00100 | 00200010?? | [12]0001?10?? | ?322000111 | 65[01]-00101? | 2222110111 | 0002100000 | 0100010020 |
| Ycaploca | 1110000100 | 1000000100 | 0020001000 | 1000101020 | 3312000010 | 620-001012 | 2222110111 | 0012100000 | 0100021020 |
| Mesitiinae | 1130000122 | 20[01]0001000 | 0000100010 | 0011011032 | 4122010111 | 651-000012 | 2522110100 | 0021000004 | 0100020210 |
| Epyrinae | 111000012[02] | 0000001000 | 0000100000 | 1011011032 | 4122010111 | 651-001111 | 2522110100 | 0021020004 | 0100010010 |
| Pristocerinae | 1[01][012]0000120 | [02]0[01]0[02]01000 | 0000100000 | 101101[01]032 | 4122010111 | 651-001111 | 2522110100 | 0021020004 | 0100020210 |
| Cleptinae | 1000000100 | 0000111000 | 1010001000 | 0011110133 | 3122001111 | 651-001111 | 2211110100 | 0012000004 | 0100022020 |
| Amiseginae | 1110001100 | 0000221000 | 1010000010 | 0011210[01]33 | 4122001011 | 651-001011 | 1222110100 | 0013000003 | 0100022020 |
| Elampini | 1110001100 | 0001011001 | 1010000000 | 00111?0033 | ?222001011 | 65[01]-001110 | 1322110100 | 0013000004 | 0100022??0 |
| Allocoeliini | 111000?100 | 0001211001 | 1010000000 | 00111?0033 | ?222001011 | 651-001110 | 1222110100 | 0013000001 | 0?00022??0 |
| Parnopini | 111000110[02] | 0001244001 | 1010001000 | 00111?0033 | ?[02]22001012 | 651-000112 | 1322110110 | 0113010004 | 0100022??0 |
| Chrysidini | 1110001100 | 0001211001 | 1010000000 | 0011[12]10[01]33 | 4[02]12001011 | 651-00111[013] | 13[12][12]110110 | 0013000004 | 0100022020 |
| Sclerogibba | 0220001020 | 1001111100 | 1000100010 | 0011100022 | 5121110012 | 630-000012 | 2223110100 | 1022010002 | 0100010010 |
| Probethylus | 0220001020 | 1001132100 | 1000110010 | 0011100012 | 5121110012 | 630-000012 | 2223110100 | 1022010002 | 0100010010 |
| Gonatopodinae | 1220001310 | 10001[02][12]201 | 0010000000 | 0001100132 | 5022100010 | 650-001112 | 1423110110 | 1022100002 | 0100022020 |
| Dryininae | 1[12][12]0000310 | 1000101[12]01 | 0000100000 | 0011100132 | 5022[01]00010 | 650-001112 | 2523110100 | 1022100002 | 0100022020 |
| Embolemidae | [01]1[12]1000300 | [01]0000[123][12]001 | 0000120012 | 0011101[01]32 | 5[23]12100111 | 6[45][01]-00[01][01]1[012] | 1423110110 | 0022000002 | 01000[12]1020 |
*Non-aculeate outgroup taxa excluded from some analyses (see text)
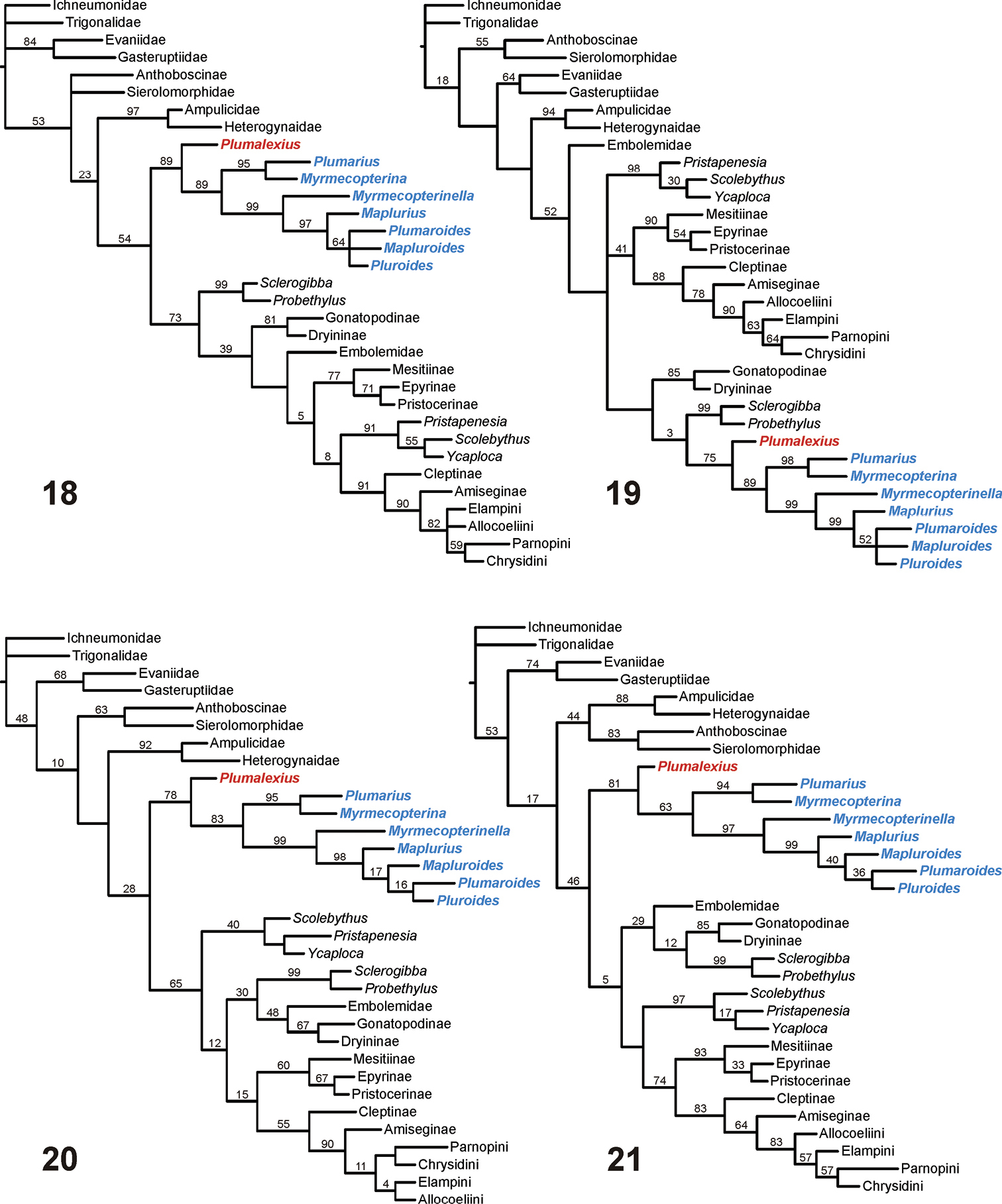
To try to address the second of the above concerns, analyses were done using four non-Aculeata as outgroup. The resulting cladograms (Figures 18–21) are presented with Ichneumonidae as outgroup, but using any of the other three non-aculeates made no difference to the relationships shown for the Chrysidoidea. The consensus tree from the “equally weighted additive” analysis (Figure 18) shows Chrysidoidea as monophyletic, all chrysidoid families also as monophyletic (but their relationships still sometimes unconventional), Plumalexius basal to Plumariidae (with high support), and the plumariid genera with similar relationships to those found earlier. Although the study was not intended to reflect the relationships amongst the outgroup taxa, it is interesting that Aculeata s.str. (Vespoidea and Apoidea) appears as paraphyletic in this analysis. The consensus tree from the “equally weighted non-additive” analysis (Figure 19) also shows Chrysidoidea as monophyletic (but with Embolemidae as basal), all chrysidoid families also as monophyletic but with most relationships very different from previous findings (except that Bethylidae and Chrysididae are well supported as monophyletic, and many of the family relationships have no positive relative branch support), with Plumariidae (and Plumalexius sister to it) appearing as most closely related to Sclerogibbidae and Dryinidae; the relationships for the plumariid genera remain consistent. Aculeata s.str. now appears as polyphyletic, with Evanioidea interpolated between Vespoidea and Apoidea. The single MPC resulting from the “implicitly weighted additive” analysis (Figure 20) is fully resolved, shows Chrysidoidea as monophyletic, all chrysidoid families as monophyletic, Plumariidae (and Plumalexius sister to it) as sister to the remaining chrysidoids, and the relationships of those families as found by previous analyses (see Figure 1); the relationships of the plumariid genera also agree with previous analyses (see Figure 2), except that Plumaroides appears as sister to Pluroides rather than Mapluroides. Aculeata s.str. is paraphyletic but with the apparent sister-group relationship of Apoidea to Chrysidoidea not supported (the resampling analysis instead showed a monophyletic Aculeata s.str. as supported with a value of 12). The “implicitly weighted non-additive” analysis also produced a single MPC (Figure 21) with a monophyletic Chrysidoidea, monophyletic chrysidoid families, Plumalexius sister to Plumariidae, and Bethylidae and Chrysididae forming a monophyletic group but apparently sister to Scolebythidae (but without relative branch support); relationships for the plumariid genera are the same as for the “additive” analysis. Aculeata s.str. is now monophyletic (with good support) and sister to Chrysidoidea (with some positive support). It is notable that the chrysidoid relationships shown are more similar to those for the “additive” analyses than those seen in the “unweighted non-additive” analysis.
Chrysidoidea relationships using non-Aculeata (Ichneumonidae) as outgroup 18 Characters equally weighted, some characters additive (strict consensus of 12 MPCs, raw lengths 468, CI = 0.35, RI = 0.68) 19 Characters equally weighted, all characters non-additive (strict consensus of 4 MPCs, raw lengths 423, CI = 0.39, RI = 0.67) 20 Characters implicitly weighted (k = 2.5), some characters additive (1 MPC, raw length 473, CI = 0.35, RI = 0.68) 21 Characters implicitly weighted (k = 2.5), all characters non-additive (1 MPC, raw length 426, CI = 0.38, RI = 0.67). Note: Plumalexius shown in red, genera of Plumariidae shown in blue. Numbers are estimated GC branch-support values (see text); branches without numbers showed no positive support under the resampling protocol used.
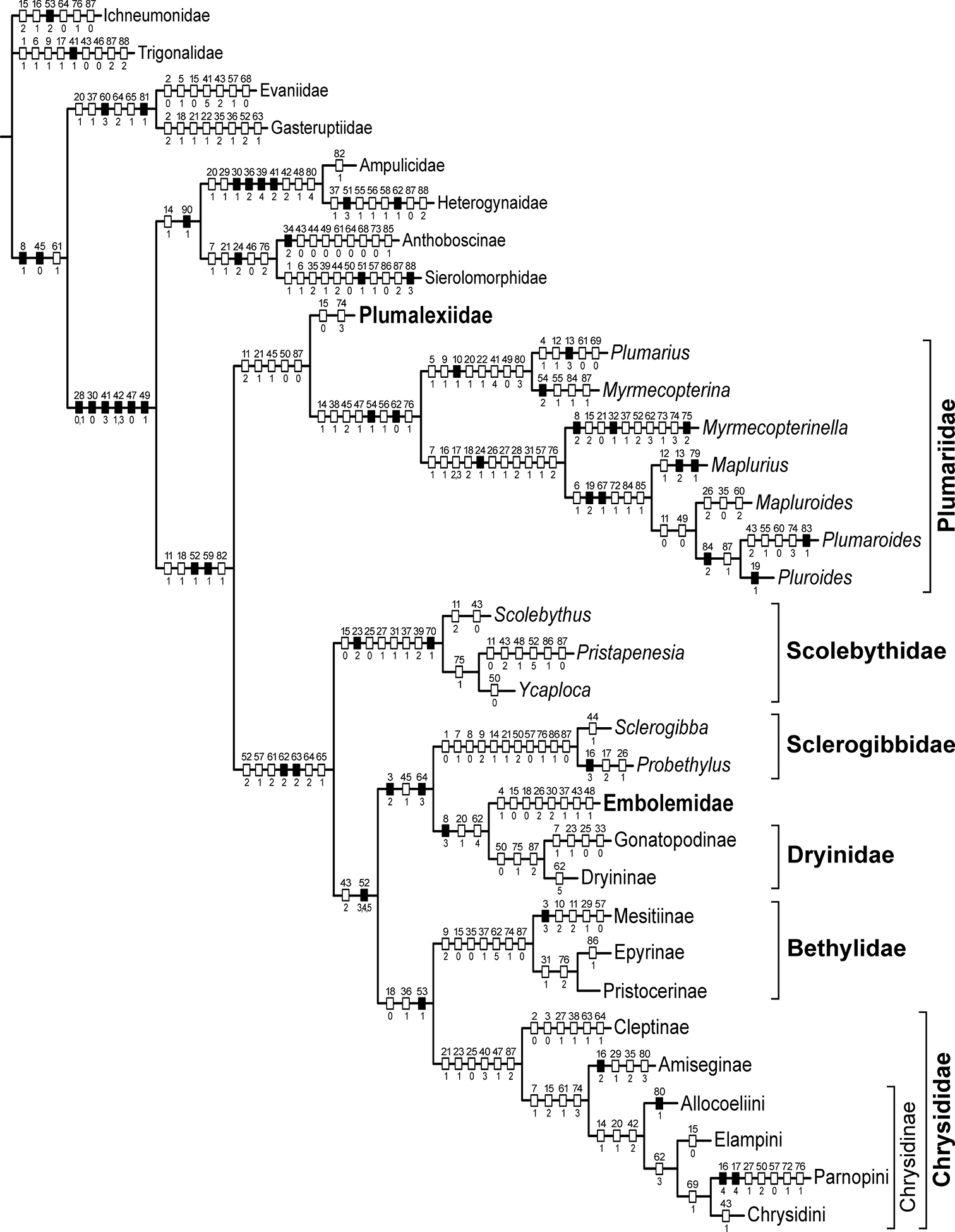
At first sight, consideration of all of the above results, involving not only the placement of Plumalexius but even more the relationships amongst the other chrysidoids, has produced a slightly confused picture, perhaps not unexpected for a set of analyses using exemplars and considerable polymorphism, and also based on characters which have previously been used at very different levels. The limitation of having to exclude all characters restricted to females (many of which have proved extremely informative in previous analyses, and one of which, the presence of an articulation within gonocoxite IX, is probably the most significant unique synapomorphy for Chrysidoidea) has also had an effect. Nevertheless, it is gratifying that the results of most previous studies have been confirmed, or at least not convincingly contradicted. Accordingly, I consider that the cladogram which agrees best with those results, one using an expanded outgroup and additive characters, and derived using implied weighting (an approach advocated by
Preferred cladogram of families of Chrysidoidea and genera of Plumariidae (raw length 474, CI = 0.35, RI = 0.68) showing only unambiguous character-state changes. Notes: open hashmarks indicate homoplasious states, black hatchmarks indicate unique states; character numbers above, state numbers below (polymorphisms separated by commas).
Plumalexius seems convincingly indicated as sister to the Plumariidae, although one analysis was ambiguous about this; trees with it placed as sister to the remaining chrysidoids or as sister to the Chrysidoidea as a whole differ in length from that shown in Figure 22 by only 4 and 5 steps respectively (lengths 478 and 479 compared with 474), emphasising its relatively basal position. It does not share any unique synapomorphies with Plumariidae, however (the five unambiguous states supporting the sister relationship to Plumariidae are 11-2: flagellomere setae conspicuous and erect; 21-1: pronotal posteroventral margin strongly concave; 45-1: metasternum weakly depressed anteromedially; 50-0: pterostigma large and prominent; and 87-0: hypopygium completely exposed or almost so, all states found elsewhere in relatively distantly related taxa). The long erect flagellar setae of Plumalexius and some Plumariidae have been indicated as a putative synapomorphy for the family (Rasnitsyn 2002: Fig. 331). The arrangement of the setae in Plumalexius is most similar to that in Myrmecopterina, although the setae are less dense and considerably longer in Plumalexius, but the present analysis has shown that other plumariid genera lack such setae and, conversely, they are also present in some Scolebythidae and Bethylidae at least; prominent flagellomere setae are actually found widely in the Chrysidoidea. The other most obvious similarity, more extensive venation in both wings than in other chrysidoids, is a symplesiomorphy. It is thus evident that there is no key apomorphy associating Plumalexius with the Plumariidae sufficient to assign it to that family. Were that to be done, the expanded family would lose its present defining features, such as the presence of apical accessory veins in the wing membrane, the reduced second submarginal cell and the tapered mandibles with few apical teeth. In view of this, I conclude that the best solution is to propose a new family for it, as has been done above, something which also emphasises its distinctiveness. In contrast to the specialised morphology of Scolebythidae, showing several adaptations enabling the effective parasitisation of wood-boring beetle larvae, the morphology of Plumalexius provides little clue as to its biology, specially since the female is unknown. The male looks like a very generalised wasp, probably very similar to the form ancestral to Chrysidoidea as a whole.
Apart from the above results, the variety of analyses performed has shown that the use only of an outgroup which is sister to the ingroup, and which may have many characters with relatively more-derived states than the ingroup, may produce misleading or ambiguous results (Figs 14–17 all show different relationships from the preferred result). Instead, the outgroup should be expanded to include taxa similarly related to both the ingroup and its sister group. Furthermore, the use of additive characters where reasonable inferences of additivity can be made is likely to produce better-resolved cladograms than if all characters are considered non-additive, and it seems that using implied weighting not only improves the results obtained under both scenarios, but also reduces the uncertainty induced by considering all characters non-additive. The results obtained here, therefore, indicate that wherever possible additive characters and a method (such as implied weighting) which gives greater weight to the more reliable characters should be used.
Whether Plumalexius is sister to Plumariidae or not affects the estimated minimum age of Plumariidae: if it is, then Rasnitsyn’s (2002, 2010) estimate remains reasonable (after all, the common ancestor of two lineages must be at least as old as either lineage), but if it is sister to the remaining chrysidoids or to Chrysidoidea as a whole, then that estimate for Plumariidae is poorly founded. Since all other chrysidoid lineages date from the Early Cretaceous (
I am most grateful to Alex Rasnitsyn for bringing the New Jersey fossils to my attention; it is with the greatest pleasure that I dedicate this paper to him in commemoration of his 75th birthday. I am also grateful to David Grimaldi (American Museum of Natural History, New York) for lending the specimens to me and for comments on some characters; to Arturo Roig-Alsina (Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires) for the donation of specimens of Maplurius, Mapluroides, Plumaroides and Pluroides; to Simon van Noort (Iziko South African Museum, Cape Town) for the loan of a specimen of Myrmecopterinella; and to several other colleagues who have donated specimens of wasps over the years or provided useful insights. Doug Lundberg of Ambericawest (USA) very kindly gave me a piece of Dominican amber containing a specimen of Pristapenesia inopinata. Jim Carpenter (American Museum of Natural History) provided useful advice on some procedures for the analyses. Financial support from the University of KwaZulu-Natal Research Office is gratefully acknowledged.
All specimens are in D.J. Brothers’ collection (to be deposited in Iziko South African Museum, Cape Town (SAM), in due course) unless otherwise stated.
Ichneumonoidea:
Ichneumonidae: Cryptinae sp. (S. Africa), Pimplinae spp. (2, Malawi, S. Africa)
Trigonaloidea:
Trigonalidae: Taeniogonalos maculata (Smith), Trigonalys ?micanticeps (Strand)
Evanioidea:
Evaniidae: Evania sp. (S. Africa), Acanthinevania sp. (Australia)
Gasteruptiidae: Gasteruption spp. (2, Botswana, S. Africa)
Vespoidea:
Tiphiidae, Anthoboscinae: Anthobosca spp. (2, S. Africa)
Sierolomorphidae: Sierolomorpha bicolor Evans, Sierolomorpha canadensis (Provancher)
Apoidea:
Ampulicidae: Ampulex spp. (2, S. Africa), Dolichurus carbonarius Smith
Heterogynaidae: Heterogyna protea Nagy, Heterogyna nocticola Ohl, Heterogyna madecassa Day
Chrysidoidea:
Plumalexiidae: Plumalexius rasnitsyni sp. nov. (AMNH)
Plumariidae: Plumarius striaticeps (André), Plumarius spp. (2, Argentina); Plumaroides andalgalensis Brothers, Plumaroides brothersi Diez and Roig-Alsina; Myrmecopterina spp. (3, Botswana, Namibia, S. Africa); Myrmecopterinella sp. (S. Africa) (SAM); Maplurius spatulifer Roig-Alsina; Mapluroides ogloblini Diez, Hidalgo and Roig-Alsina; Pluroides porteri Diez, Roig-Alsina and Hidalgo
Scolebythidae: Scolebythus madecassus Evans; Pristapenesia primaeva Brues, Pristapenesia inopinata (Prentice and Poinar in Prentice, Poinar and Milki); Ycaploca evansi Nagy
Bethylidae: Mesitiinae: Pilomesitius ?madagascarensis Moczar, Sulcomesitius ?pondo (Benoit), Sulcomesitius ?schoutedeni (Benoit); Epyrinae: Epyris spp. (2, S. Africa); Pristocerinae: Acrepyris fraterna (Evans), ?Pristocera sp. (S. Africa), Apenesia sp. (S. Africa)
Chrysididae: Cleptinae: Cleptes alienus Patton; Amiseginae: Amisega similis Kimsey, Amisega flavicrus Kimsey, Bupon pashoanus Kimsey; Chrysidinae: Elampini: Hedychrum nobile (Scopoli), Hedychrum sp. (S. Africa); Allocoeliini: Allocoelia capensis (Smith); Parnopini: Parnopes fischeri Spinola, Parnopes edwardsii (Cresson); Chrysidini: Chrysis ignita Linnaeus, Chrysura pacifica (Say), Stilbum cyanurum (Förster)
Sclerogibbidae: Sclerogibba africana Kieffer, Sclerogibba berlandi Benoit, Sclerogibba?magrettii (Kieffer), Probethylus schwarzi Ashmead
Dryinidae: Gonatopodinae spp. (2, S. Africa); Dryininae spp. (2, S. Africa)
Embolemidae: Embolemus collinsi (Olmi), Embolemus confusa (Ashmead), Embolemus magna (Olmi), Embolemus sp. (New Caledonia), Embolemus brothersi Olmi
1. Compound eye, inner margin: sinuate = 0, convex = 1;
2. Compound eye, pores and setae: no pores or setae = 0, scattered pores and/or setae = 1, dense pores and/or setae = 2;
3. Compound eye, setae: absent = 0, minute = 1, short = 2, long = 3;
4. Antennal socket, distance from epistomal suture: less than socket width = 0, more than 1.5 × socket width = 1;
5. Clypeus, shape: transverse, without prominent median lobe = 0; with long median lobe narrower than intermandibular area = 1;
6. Clypeus, form: platelike, apical margin not deflexed = 0, convex, thickened, apical margin deflexed medially = 1;
7. Occipital carina: present = 0, absent = 1;
8. Antenna, antennomere number: more than fourteen = 0, thirteen = 1, twelve = 2, ten = 3;
9. Antenna, radicle-scape axis and insertion: axis nearly straight, simple annular constriction = 0, axis angled, simple annular constriction = 1, axis angled, radicle under flangelike expansion of scape = 2;
10. Antenna, scape form: simple, more or less cylindrical = 0, basally expanded ventromesally = 1, apically expanded ventromesally = 2 (NON-ADDITIVE);
11. Antenna, flagellomere setae orientation: inconspicuous and decumbent = 0, conspicuous and semi-decumbent, some erect = 1, conspicuous and erect = 2;
12. Antenna, flagellomere setae distribution: evenly developed, scattered = 0, better developed ventrally, in irregular transverse rows = 1;
13. Antenna, flagellomere setae length: much less than flagellomere width = 0, about 0.5–1 × flagellomere width = 1, about 1.5–2 × flagellomere width = 2, about 3–4 × flagellomere width = 3;
14. Mandible, form: apical margin truncate (chewing type) = 0, apical margin strongly tapering (cutting type) = 1;
15. Mandible, apical teeth: four or more = 0, three = 1, two or fewer = 2;
16. Maxillary palp, segments: six = 0, five = 1, four = 2, three = 3, one = 4;
17. Labial palp, segments: four = 0, three = 1, two = 2, one = 3, absent = 4;
18. Pronotum, anterior collar (flange): present, covering propleura = 0, present but reduced and exposing propleura = 1, extremely reduced to slight ridge, effectively absent = 2;
19. Pronotum, posterolateral lobe: simple = 0, with preapical vertical blunt carina, posteriorly depressed = 1, with preapical vertical lamella, posteriorly concave = 2;
20. Pronotum, posteroventral angle: rounded = 0, narrowly acute = 1;
21. Pronotum, posteroventral margin: straightish = 0, strongly concave = 1;
22. Propleura, dorsally: separated by membranous region = 0, closely approximated although not fused = 1;
23. Propleura, posterior margin: almost straight, propleura almost entirely contiguous mesad = 0, indented on medial halves, propleura partially separated = 1, mostly indented, propleura almost entirely separated = 2;
24. Proepimeron: clearly distinguishable at outer angle and along posterior margin = 0, reduced, discernible only at outer angle = 1, indistinguishable = 2;
25. Prosternum, form: mostly in a single plane = 0, distinctly biplanar, mostly depressed posteriorly = 1;
26. Prosternum, ventral view: well developed with apophyseal pit(s) = 0, visible as triangular sclerite without apophyseal pit = 1, reduced, scarcely visible = 2;
27. Procoxae, contiguity: contiguous basally = 0, well separated basally = 1;
28. Prepectus, form: very well developed, long (nearing midline) and broad = 0, well developed, short (far from midline) and broad = 1, reduced, short (far from midline) and narrow = 2;
29. Prepectus, midventrally: halves divided, free from each other = 0, halves fused midventrally = 1;
30. Prepectus, fusion: entirely articulating or surrounded by membrane = 0, fused with mesopleuron = 1, fused with pronotum = 2 (NON-ADDITIVE);
31. Notauli: diverging anteriorly = 0, parallel = 1, absent = 2 (NON-ADDITIVE);
32. Scutellum: simple = 0, produced posterodorsally as sharp-edged flange = 1;
33. Mesepimeron, development: well developed, flangelike, overlapping metepisternum = 0, much reduced, abutting metepisternum = 1;
34. Mesosternum, form posteriorly: smoothly truncate = 0, mesad with short transverse carina or weak tooth = 1, mesad with lamella projecting over mesocoxal base = 2;
35. Mesocoxae, contiguity: broadly separated = 0, slightly separated = 1, contiguous = 2;
36. Mesosoma, second dorsal phragma: strongly oblique, muscles 2ph-3ph anterior = 0, scarcely oblique posteriorly, muscles 2ph-3ph anterior = 1, scarcely oblique posteriorly, muscles 2ph-3ph posterior = 2 (NON-ADDITIVE);
37. Metanotum, shape: mesally about as long as laterally or longer = 0, mesally half as long as laterally or shorter = 1;
38. Metanotum, lateral length: less than half medial length of scutellum = 0, more than two-thirds medial length of scutellum = 1;
39. Metapostnotum, development: distinctly lengthened laterally = 0, evenly long throughout = 1, distinctly shortened laterally = 2, shortened throughout = 3, greatly expanded posteromedially = 4 (NON-ADDITIVE);
40. Metapostnotum, hind margin: entirely distinct = 0, distinct medially, indistinct laterally = 1, obliterated medially, indistinct laterally = 2, entirely obliterated = 3 (NON-ADDITIVE);
41. Mesosoma, third dorsal phragma: forming distinct even flange = 0, forming narrow even flange = 1, forming narrow even flange laterally only = 2, medially reduced but distinguishable = 3, absent medially = 4, entirely absent = 5 (NON-ADDITIVE);
42. Metapleuron, endophragmal pit and sulcus: pit posteriorly placed, sulcus angled or rounded ventral to pit = 0, pit posteriorly placed, sulcus acutely extended ventral to pit = 1, pit posteriorly placed, sulcus entirely dorsal to pit and curved = 2, pit anteriorly placed, sulcus entirely dorsal to pit and straight = 3, pit anteriorly placed, sulcus acutely extended ventral to pit = 4 (NON-ADDITIVE);
43. Metathoracic-propodeal pleural suture, dorsally: distinct and complete = 0, reduced but partly discernible = 1, obliterated = 2;
44. Metathoracic-propodeal pleural suture, ventrally: distinct and complete = 0, reduced but partly discernible = 1, obliterated = 2;
45. Metasternum, form anteromedially: not depressed = 0, weakly depressed = 1, entirely depressed = 2;
46. Metacoxal cavities: open = 0, closed = 1;
47. Propodeum, length: longer than high = 0, shorter than high = 1;
48. Propodeum, declivity: imperceptibly merging with disk, not identifiable = 0, distinct from disk = 1;
49. Forewing, tubular/nebulous veins: reaching apical margin = 0, ending before apical margin = 1;
50. Forewing, pterostigma: large and prominent = 0, medium to small but distinct = 1, very small, indistinct or absent = 2;
51. Forewing, closed cells and cell 2Cu (variable 1): ten (C, R, 1Cu, 1R1, 1M, 2Cu, 2R1, 1Rs, 2M, 2Rs) = 0, seven (C, R, 1Cu, 1R1+1Rs, 1M, 2Cu, 2R1) = 1, seven (C, R, 1Cu, 1R1, 1M, 2Cu, 2R1)= 2, six (C, R, 1Cu, 1R1, 2Cu, 2R1) = 3, seven (R, 1Cu, 1R1+1M+1Rs, 2Cu, 2R1, 2Rs, 2M) = 4, six (R, 1Cu, 1R1+1M+1Rs, 2Cu, 2R1, 2M) = 5, seven or fewer (cells 2Cu, 2M and 2Rs open or lost) = 6 (NON-ADDITIVE);
52. Forewing, closed cells and cell 2Cu (variable 2): six or more (2Cu present and closed) = 0, seven (C, R, 1Cu, 1R1, 1M, 2R1, 1Rs) = 1, six (C, R, 1Cu, 1R1, 1M, 2R1) = 2, five (C, R, 1Cu, 1R1, 2R1) = 3, four (C, R, 1Cu, 1M) = 4, three (C, R, 1Cu) = 5;
53. Forewing, costal cell: broad, wider than thickness of bounding veins = 0, narrow, as wide as thickness of bounding veins or less = 1, eliminated, veins C and Sc+R+RS fused = 2;
54. Forewing, 2nd submarginal cell (1Rs) shape (absent coded “-”): anteriorly broadly sessile = 0, shortened and anteriorly briefly sessile to briefly petiolate = 1, much reduced and anteriorly strongly petiolate = 2;
55. Forewing, marginal cell anterior margin: more than 0.7 × pterostigma width = 0, less than 0.5 × pterostigma width = 1;
56. Forewing, apical accessory vein (“RS2”): absent, not even spectral = 0, present, nebulous or spectral = 1;
57. Forewing, prestigma (vein Sc+R) form: narrow, apically less than 1.5 × width of 1Sc+R = 0, broad, apically more than 1.8 × width of 1Sc+R = 1;
58. Forewing, prestigma (vein Sc+R) length: at least as long as 1RS = 0, less than 0.5 X length of 1RS = 1;
59. Forewing, vein Cu2: present = 0, absent = 1;
60. Hind wing, basal hamuli: several, dispersed along costal margin = 0, few, concentrated into cluster = 1, one = 2, absent = 3;
61. Hind wing, tubular/nebulous veins: reaching apical margin = 0, into apical half of wing but not reaching apical margin = 1, restricted to basal half of membrane = 2;
62. Hind wing, veins C and Sc+R: both long and separated, cell C closed = 0, both long and fused = 1, C short, Sc+R absent except at base = 2, C short but distinct, Sc+R long = 3, C absent except at base, Sc+R long = 4, C absent except at base, Sc+R short = 5 (NON-ADDITIVE);
63. Hind wing, vein M+Cu: well developed, tubular = 0, distinguishable but nebulous = 1, absent = 2;
64. Hind wing, anal veins: A1 well developed, A2 present = 0, A1 well developed (more than half length anal lobe), A2 absent = 1, A1 short (less than half length anal lobe), A2 absent = 2, A1 minute, A2 absent = 3;
65. Hind wing, veins rs-m and cu-a: both present = 0, both absent = 1;
66. Hind wing, incised anal (vannal/plical) lobe: absent, at most indicated by slight notch = 0, present, distinct acute incision = 1;
67. Hind wing, anal (vannal/plical) lobe length: short, less than 0.5 × length of wing = 0, long, more than 0.6 × length of wing = 1;
68. Hind wing, jugal lobe: present, delimited apically by incision = 0, absent = 1;
69. Tarsal claws, form ventrally: toothed = 0, simple = 1;
70. Protrochanter, basal insertion: apical on coxa = 0, basolateral on coxa = 1;
71. Foretarsus, arolium: narrower than tarsal apex and shorter than claws = 0, at least as broad as tarsal apex and at least as long as claws = 1;
72. Meso- and metatarsi, arolia: well developed = 0, vestigial = 1;
73. Mesocoxa, subdivision and insertion: large basi- and disticoxites, cavities large = 0, reduced basicoxite and large disticoxite, cavities moderate = 1, much-reduced basicoxite and large disticoxite, cavities small = 2;
74. Mesotrochantellus: distinctly present = 0, reduced but discernible as complete ring = 1, much reduced, almost indiscernable, ventrally only = 2, absent = 3;
75. Mesotibia, spurs: two = 0, one = 1, none = 2;
76. Mesotibia, scattered spines: absent = 0, weak = 1, moderately strong = 2;
77. Metacoxa, specialised area of setae on ventral surface: absent = 0, present = 1;
78. Metatrochanter, specialised area of setae on ventral surface: absent = 0, present = 1;
79. Metafemur, apex: simple = 0, with toothlike projection on each side at tibial articulation = 1;
80. Metatibia, inner apical spur: simple, similar to outer spur = 0, forming calcar with weak simple dorsal carina = 1, forming calcar with dorsal setose carina = 2, forming calcar with dorsal setal fringe only = 3, forming calcar with fine dorsal pectination = 4 (NON-ADDITIVE);
81. Metasoma-propodeum attachment: ventral, between hind coxae = 0, dorsal, distant from hind coxae = 1;
82. Metasoma, T1 articulation with S1: overlapping and articulating with base of S1 = 0, narrowed and fused with base of S1 dorsolaterally = 1;
83. Metasoma, T7 surface: simple, ecarinate = 0, longitudinally carinate = 1;
84. Metasoma, T7 apical flange: absent = 0, present, narrow = 1, present, broad, 0.33 × as long as tergum = 2;
85. Metasoma, S1: ecarinate = 0, with strong median longitudinal carina = 1;
86. Metasoma, S7: well developed and exposed = 0, reduced and partly exposed = 1, much reduced and concealed = 2;
87. Metasoma, hypopygium (S8) concealment: completely exposed or almost so = 0, partially concealed = 1, completely concealed or almost so = 2;
88. Metasoma, hypopygium (S8) shape: simple with blunt to rounded apex = 0, triangular with pointed apex = 1, truncate with weakly emarginate apex = 2, peglike = 3 (NON-ADDITIVE);
89. Metasoma, cercus: present, well developed, cylindrical = 0, present but much reduced, cylindrical = 1, absent or vestigial flattened setose disk = 2;
90. Sexual dimorphism, antennomere number: absent = 0, present (♂ 13, ♀ 12) = 1.