(C) 2011 Michel P. Valim. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Two new species of Brueelia are described and illustrated. These new species and their type hosts are: Brueelia sueta ex Pharomachrus pavoninus (Spix, 1824), the Pavonine Quetzal and Brueelia cicchinoi ex Trogon viridis Linnaeus, the White-tailed Trogon. Both new species differ from the only Brueelia described on Trogon mexicanus by many morphological features, including those present in the male genitalia and female vulvar margin. Partial sequences of the mitochondrial cytochrome oxidase I (COI) gene for these two new species differ from one another by 13.6% uncorrected p-distance. Whereas Brueelia cicchinoi is only 0.3% divergent from previously published COI sequences identified as Brueelia sp. from the Mexican Trogon melanocephalus Gould, 1936 and Trogon massena Gould, 1938. We also found Brueelia cicchinoi on Trogon melanurus, Trogon collaris and Pharomachrus pavoninus. Thus Brueelia cicchinoi is found on multiple trogoniform hosts across an extremely large geographic distribution and has one of the largest number of host associations among Brueelia species.
Chewing lice, Brueelia, trogons, Philopteridae, new species
According to
The only Brueelia species parasitic on trogons (Aves, Trogoniformes), Brueelia insolita, was described by
Specimens used in this study have been collected by the
junior author and/or his colleagues using the Ethyl Acetate fumigation
technique as described in Bueter et al (2009) and were mounted on slides
following the procedures of
Using laboratory methods described by
We used PAUP* (version 4.0b10;
Holotypes of the new species are deposited in the Museu de Zoologia, University of São Paulo, São Paulo, Brazil (MZUSP) and paratypes are deposited in both MZUSP and the Field Museum of Natural History, Chicago, USA (FMNH). Other specimens studied are held in the Price Institute of Phthirapteran Research, University of Utah, Salt Lake City, USA (PIPeR). For material collected in 2005 and 2007 host specimen vouchers are deposited in the Museu Paraense Emilío Goeldi (MPEG) and FMNH and are indicated by field numbers and specimen numbers.
Taxonomic treatment Brueelia Kéler, 1936Type species Brueelia rossittensis Kéler, 1936 = Brueelia brachythorax (Giebel, 1874).
Type host: Bombycilla garrulus (Linnaeus, 1758) (Passeriformes, Bombycillidae).
urn:lsid:zoobank.org:act:476AC361-B912-4BDC-8373-483E37D76E49
http://species-id.net/wiki/Brueelia_sueta
Figs 1–6; 13–14Pharomachrus pavoninus (Spix, 1824) – Pavonine Quetzal
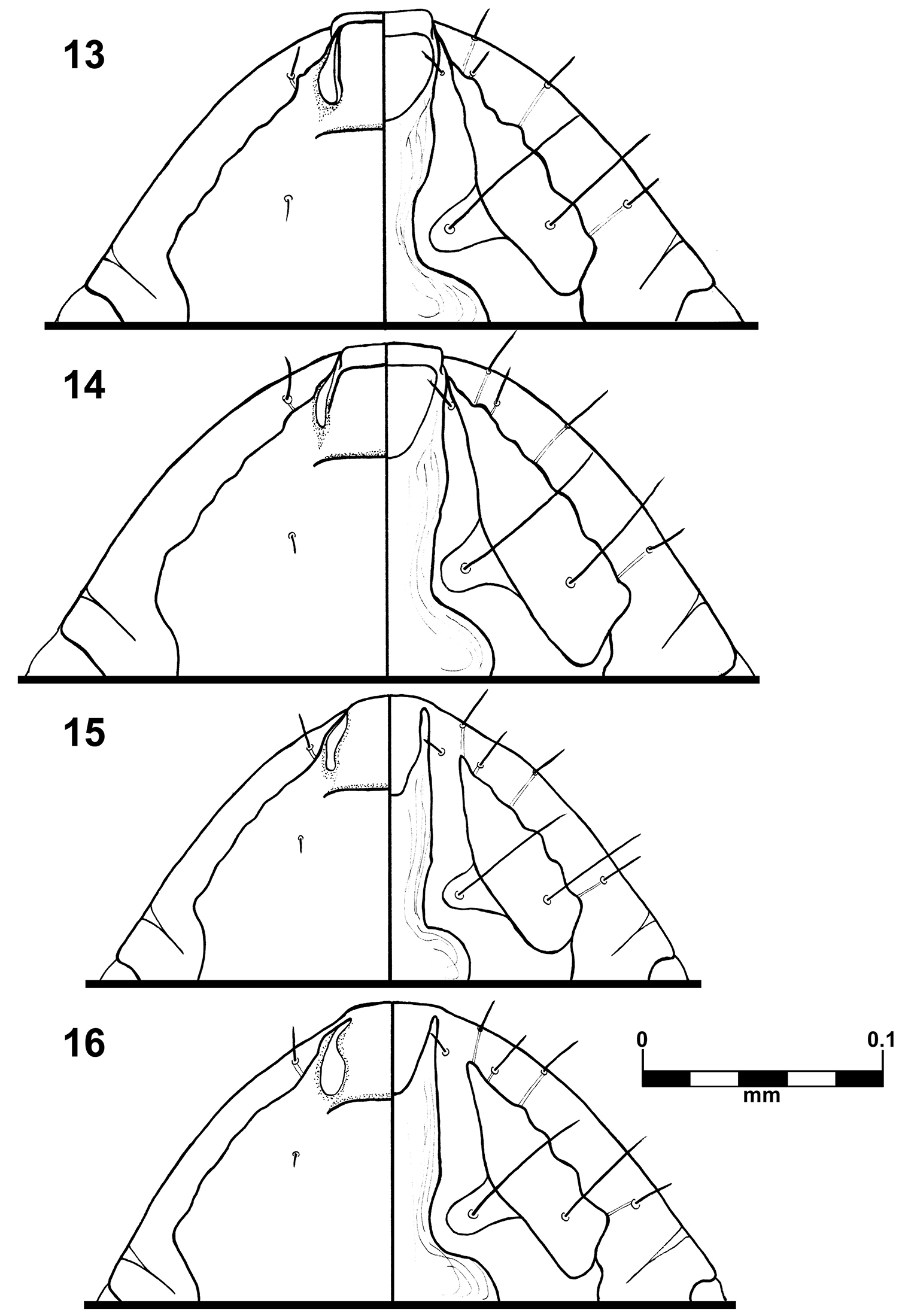
This species is unique in the thickness of the temporal carina (Fig. 3) and by the shape of the anterior ventral plate (Figs 13–14) in both sexes. It is morphologically close to Brueelia insolita due the absence of postspiracular setae on segment IV in females, but they differ significantly in characters such as shape of the vulvar margin (with a notch in Brueelia insolita); number of setae on gonapophysis (six in Brueelia insolita), and more spiniform setae on vulvar margin. The males of both species can be distinguished by the shape of genitalia and the tendency to have two setae postspiracular accessories on tergites V–VIII (whilst Brueelia insolita has only one).
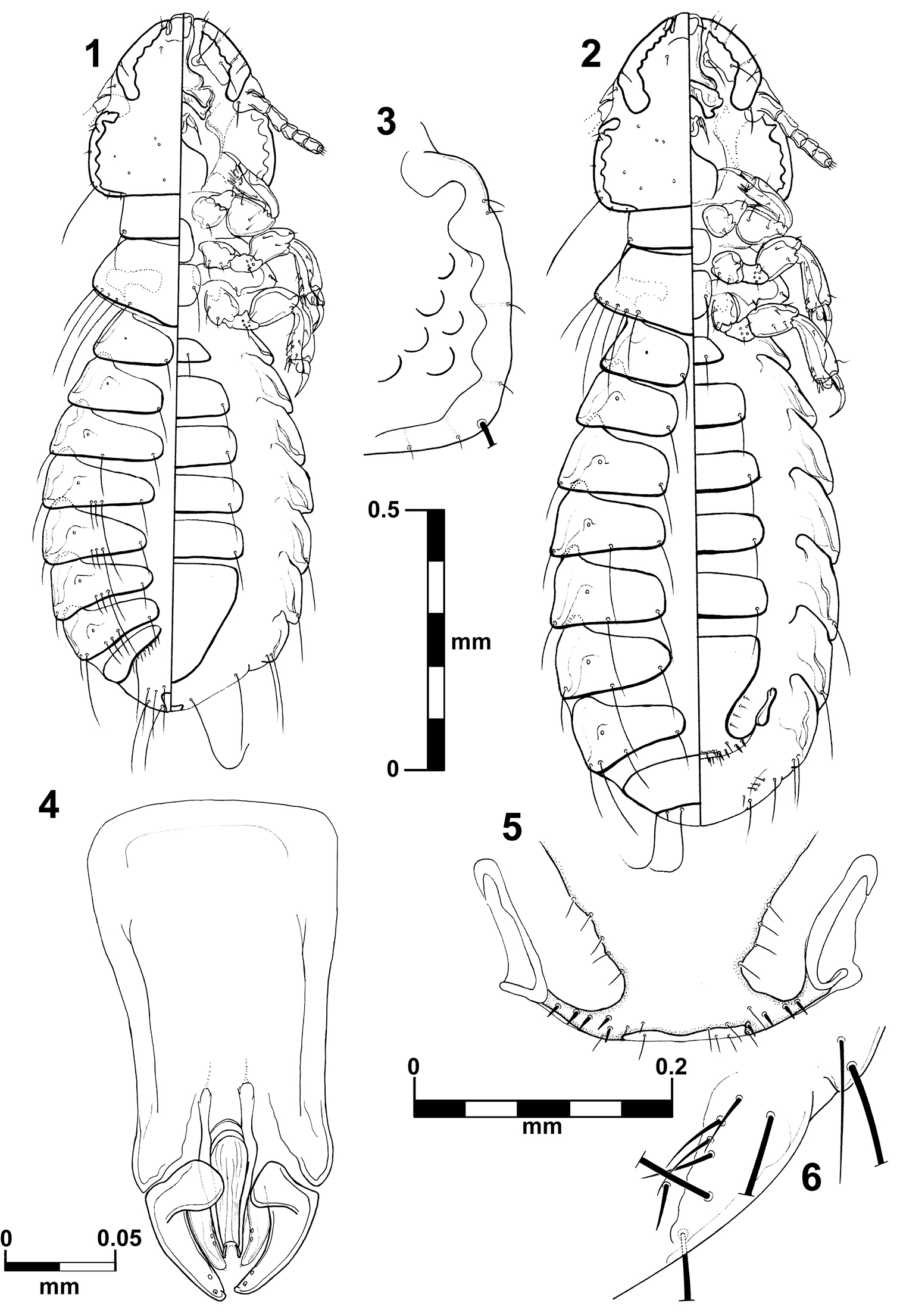
Habitus as in fig. 1. Body pigmentation uniform, all plates barely yellowish slightly more pigmented on some details of the pleural areas. Head oval shaped, as long as wide. Small hyaline margin distinguishable; anterior dorsal head plate not completely surrounded by the dorsal preantennal suture. Preantennal margin slightly convex; marginal carina thickened with its inner margin sinuate, and completely pigmented (Figs 13–14). Tracks of cybarial muscles practically indistinct. Frontoclypeal suture with its nodal area well defined. Tracks of insertion of the mandibular adductor muscles well marked. Gular plate well pigmented with a broad rhombic silhouette. Temples forming an acute angle at level of the marginal temporal setae 3; temporal carina pigmented and thick, with its inner margin deeply sinuate (Fig. 3); eye imbedded within thickened carina making its distinction on margin of the head difficult (Fig. 3). Pterothorax with 5–7 marginal setae on each side; pterothoracic apodeme well developed, not reaching the lateral margin of the pterothorax. Mesosternal and metasternal plates not fused, both slightly longer than wide, only the metasternal plate bearing two long setae. Abdomen with tergites II–VIII lightly and uniformly pigmented. Tergal chaetotaxy: postspiracular long on IV–VIII; two small accessory setae on V–VIII (atypical specimens with only one seta in one side); and one sutural seta on II–VIII. Tergite IX+X (from the lateral to meson) with one short, one long and six (rarely seven) short setae. Paratergal chaetotaxy: II–III 0; IV–V 1; VI–VII 2; VIII 4. Sternal plates II–VI yellowish, typically with one pair of setae on each, subgenital plate uniformly pigmented. Genitalia (Fig. 4): basal plate wide, with sub-parallel lateral borders; straight and broad subtriangular paramera, with rhombic tips (Fig. 4); lateral sclerites of the endomeral complex long (2/3 of the paramera length) subtriangular with their posterior edge smooth, bearing 2 sensillae each.
Body measurements (n = 4): HL, 0.33–0.35; PAW, 0.27–0.28; TW, 0.35–0.36; PL, 0.13–0.14; PW, 0.24–0.25; PTL, 0.15–0.16; PTW, 0.34–0.35; AL, 0.71–0.80; AW 0.48–0.52; GL, 0.23; and TL, 1.25–1.35.
Habitus as in figure 2. Pigmentation of the head, thorax and abdomen much as for male, differing in body size, terminalia and tergal chaetotaxy (one long postspiracular seta on V–VIII). Pterothorax with 4–6 marginal setae on each side. Tergites II–VIII divided medially, IX+X entire and uniformly pigmented. Subgenital plate uniformly pigmented, lacking posterior notch, with 3–5 small setae each side (Fig. 5). Gonapophysis commonly with 4 setae (Figs 6). Vulva with 4–5 short and spiniform setae, and 3–6 (rarely 2) long and thin setae on each side (Fig. 5).
Body measurements (n = 4): HL, 0.37–0.38; PAW, 0.30–0.31; TW, 0.38–0.39; PL, 0.13–0.16; PW, 0.26–0.27; PTL, 0.14–0.16; PTW, 0.37–0.38; AL, 0.94–1.10; AW 0.50–0.59; and TL, 1.52–1.67.
Brueelia sueta sp. n.: male, dorso-ventral views (1); female, dorso-ventral views (2); temporal carina (3); male genitalia (4); female vulvar margin (5); female gonapophysis (6).
Male holotype, ex Pharomachrus pavoninus, JAP766 MPEG 62493; BRAZIL: Amazonas, Maraã, Lago Cumapi (01°43'48.6"S; 65°52'45.5"W ), 31.VII.2007, J.D. Weckstein col., at MZUSP. Paratypes: 3 males and 4 females (one female DNA voucher Brsp.Phpa.1.4.2011.19), same data as holotype. 1 male and 1 female (DNA voucher) paratypes at FMNH.
The epithet derives from suetus (L.), which means: wont; accustomed; usual. It makes reference to the fact of the genus Brueelia is a common parasite on trogons, rather than insolitus (L., unusual) as believed by
urn:lsid:zoobank.org:act:5EF8725E-FAE3-4B43-B4F0-2EC82E090B62
http://species-id.net/wiki/Brueelia_cicchinoi
Figs 7–12; 15–16Trogon viridis Linnaeus, 1766 – White-tailed Trogon
The shape of the anterior ventral plate is unique in this species (Figs 15–16). It is morphologically close to Brueelia insolita by the thickness of the temporal carina and by the presence of setae on the mesosternal plate; but they can be distinguished by the shape of the anterior ventral plate. The males of this species can be distinguished by the distinct genital architecture and by the presence of two long setae on tergite IX+X (only one in Brueelia insolita and Brueelia sueta sp. n.). In females, the presence of postspiracular setae on segment IV; the chaetotaxy of vulvar margin; and lacking of the notch on vulvar margin are the most distinctive characters. This species can be promptly distinguished from the Brueelia sueta sp. n. by the thickness of the temporal carina, genitalia and tergal chaetotaxy in males; and by the presence of postspiracular setae on tergite IV and vulvar chaetotaxy in females.
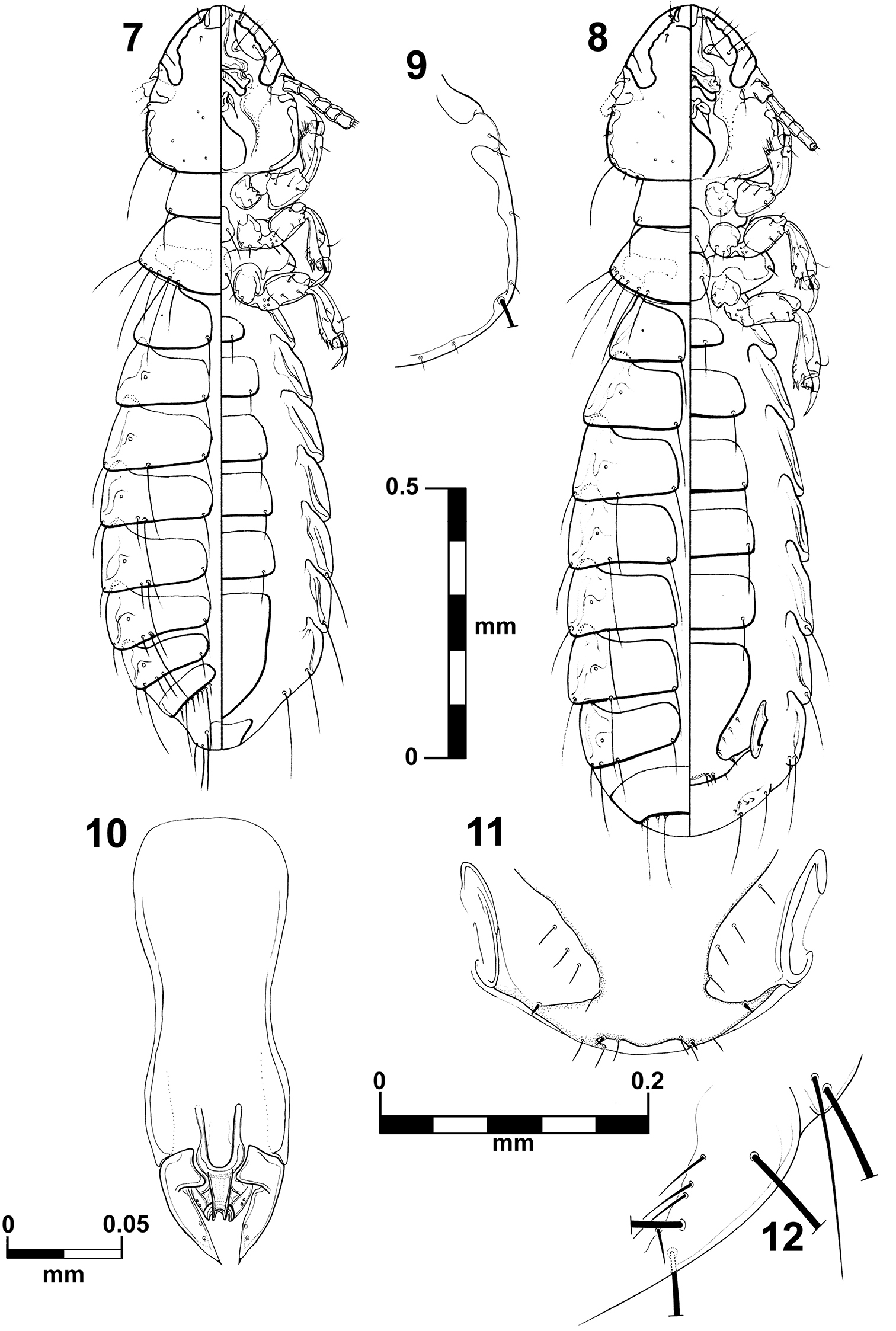
Habitus as in fig. 7. Body pigmentation uniform, all plates barely yellowish in color. Head oval shaped, slightly longer than wide. Hyaline margin indistinguishable; anterior dorsal head plate not completely surrounded by the dorsal preantennal suture. Preantennal margin slightly convex; marginal carina thickened with its inner margin sinuate (Figs 15–16). Tracks of cybarial muscles practically indistinct. Frontoclypeal suture with its nodal area well defined. Tracks of insertion of the mandibular adductor muscles faintly marked. Gular plate well pigmented with a broad rhombic silhouette. Temples more rounded; temporal carina pigmented and thinner, with its inner margin only superficially sinuate (Fig. 9); eye distinct from the temporal carina (Fig. 9). Pterothorax with 5–6 marginal setae on each side; pterothoracic apodeme well developed, not reaching the lateral margin of the pterothorax. Mesosternal and metasternal plates not fused, both slightly longer than wide and bearing two long setae each. Abdomen with tergites II–VIII lightly and uniformly pigmented. Tergal chaetotaxy: postspiracular long on IV–VIII; one small accessory setae on V–VIII (atypical specimens lack this seta on one side); and one sutural seta on II–VIII. Tergite IX+X (from the lateral to meson) with one short, one long, three short, one long, and one short setae. Paratergal chaetotaxy: II–III 0; IV–V 1; VI–VII 2; VIII 3. Sternal plates II–VI yellowish, typically with one pair of setae on each, subgenital plate uniformly pigmented. Genitalia (Fig. 10): basal plate wide, with concavity on lateral borders; straight and broad subtriangular paramera, with pointed tips (Fig. 10); lateral sclerites of the endomeral complex short (1/3 of the paramera length) and subtriangular with their posterior edge smooth, bearing 2 sensillae each.
Body measurements (n = 5), ex Trogon viridis: HL, 0.30–0.31; PAW, 0.23–0.24; TW, 0.28–0.30; PL, 0.11–0.14; PW, 0.20; PTL, 0.12–0.16; PTW, 0.27–0.28; AL, 0.77–0.87; AW 0.37–0.42; GL, 0.19–0.21; and TL, 1.27–1.38.
Body measurements (n = 3), ex Trogon massena: HL, 0.31–0.33; PAW, 0.24–0.25; TW, 0.29–0.30; PL, 0.12–0.13; PW, 0.20–0.21; PTL, 0.13; PTW, 0.29–0.31; AL, 0.83–0.93; AW 0.39–0.47; GL, 0.20–0.21; and TL, 1.34–1.48.
Body measurements (n = 1), ex Trogon melanocephalus: HL, 0.33; PAW, 0.26; TW, 0.31; PL, 0.13; PW, 0.21; PTL, 0.14; PTW, 0.32; AL, 0.91; AW 0.50; GL, 0.19; and TL, 1.46.
Body measurements (n = 2), ex Trogon collaris: HL, 0.30–0.31; PAW, 0.23–0.24; TW, 0.29–0.30; PL, 0.11–0.13; PW, 0.19–0.21; PTL, 0.15; PTW, 0.28–0.30; AL, 0.83–0.84; AW 0.39–0.43; GL, 0.18–0.20; and TL, 1.37–1.38.
Habitus as in figure 8. Pigmentation of the head, thorax and abdomen much as for male, differing in body size, terminalia and tergal chaetotaxy. Pterothorax with 5–6 marginal setae on each side. Tergites II–VIII divided medially, IX+X entire and uniformly pigmented. Subgenital plate uniformly pigmented, lacking posterior notch, with 3–4 small setae each side (Fig. 11). Gonapophysis commonly with 3 setae (Figs 12). Vulva with 2–3 short and spiniform setae and 2–3 long and thin setae on each side (Fig. 11).
Body measurements (n = 3), ex Trogon viridis: HL, 0.32–0.33; PAW, 0.26; TW, 0.32; PL, 0.12–0.13; PW, 0.21–0.22; PTL, 0.13–0.15; PTW, 0.30–0.31; AL, 0.98–1.07; AW 0.44–0.46; and TL, 1.53–1.61.
Body measurements (n = 2), ex Trogon melanurus: HL, 0.34–0.36; PAW, 0.26–0.27; TW, 0.33–0.34; PL, 0.15–0.16; PW, 0.23; PTL, 0.15; PTW, 0.33; AL, 0.98–1.05; AW 0.46–0.47; and TL, 1.56–1.60.
Body measurements (n = 3), ex Trogon massena: HL, 0.34; PAW, 0.26–0.28; TW, 0.32–0.33; PL, 0.13; PW, 0.21–0.23; PTL, 0.15–0.16; PTW, 0.32–0.33; AL, 1.01–1.10; AW 0.46–0.47; and TL, 1.59–1.69.
Body measurements (n = 1), ex Trogon melanocephalus: HL, 0.35; PAW, 0.27; TW, 0.33; PL, 0.14; PW, 0.22; PTL, 0.14; PTW, 0.34; AL, 1.09; AW 0.49; and TL, 1.68.
Body measurements (n = 3), ex Trogon collaris: HL, 0.33–0.34; PAW, 0.26–0.27; TW, 0.32–0.33; PL, 0.13; PW, 0.20–0.22; PTL, 0.14–0.17; PTW, 0.32–0.33; AL, 1.01–1.08; AW 0.48–0.54; and TL, 1.58–1.66.
Male holotype, ex Trogon viridis, JAP765 FMNH 456563; BRAZIL: Amazonas, Maraã, Lago Cumapi (01°43'48.6"S; 65°52'45.5"W ), 31.VII.2007, J.D. Weckstein col., at MZUSP. Paratypes: 4 males and 3 females (one female DNA voucher Brsp.Trvi.1.4.2011.20), same data as holotype. 2 males and 1 female (DNA voucher) paratypes at FMNH.
Brueelia cicchinoi sp. n.: male, dorso-ventral views (7); female, dorso-ventral views (8); temporal carina (9); male genitalia (10); female vulvar margin (11); female gonapophysis (12).
Brueelia sueta sp. n.: male preantenal region, dorso-ventral views (13); female preantenal region, dorso-ventral views (14); Brueelia cicchinoi sp. n.: male preantenal region, dorso-ventral views (15); female preantenal region, dorso-ventral views (16).
1 male and 1 female (female DNA voucher Brsp.Phpa.4.4.2011.16), ex Pharomachrus pavoninus, JAP315 MPEG 62491; BRAZIL: Amazonas, Japurá, Rio Mapari (02°02'31.5"S; 67°17'16.6"W ), 17.VII.2007, J.D. Weckstein col., at FMNH; 2 females (one female DNA voucher Brsp.Trme.4.4.2011.13), ex Trogon melanurus, AMZ415 MPEG 59344; BRAZIL: Amazonas, Barcelos, Rio Aracá (0°25'12"S; 62°56'13"W ), 4.XII.2005, C.C. Ribas col., at MZUSP; 4 males and 4 females (one pair of specimens DNA vouchers Trsp.Trmas.3.29.1999.4 and Trsp.Trmas.4.7.1999.9), ex Trogon massena; MÉXICO: Campeche, 24 km S Sivituc (18°14'N; 90°12'W ), 6.III.1998, D.H. Clayton col., at PIPeR; 1 male and 2 females (one female DNA voucher Trsp.Trmel.5.4.1999.5); ex Trogon melanocephalus; MÉXICO: Campeche, 24 km S Sivituc (18°14'N; 90°12'W ), 9.III.1998, D.H. Clayton col., at PIPeR; 2 males and 3 females (FMNH-INS 28922, 28923); ex Trogon collaris; PERU: Madre de Dios, Hacienda Amazonia, near Atalaya ridged, 330m above hacienda, 5.VIII.1985, D.H. Clayton col. (#85-076 and 85-077), at FMNH.
This species is named after Armando C. Cicchino (Universidad Nacional de Mar del Plata, Mar del Plata, Argentina) in recognition of his more than thirty years of contributions to the taxonomy and systematics of the genus Brueelia and chewing lice in general.
Although we described herein a new Brueelia species from Pharomachrus pavoninus, this same host species, from a different locality, also harbored Brueelia cicchinoi sp. n. Nevertheless, we are certain that this record of Brueelia cicchinoi on the host Pharomachrus pavoninus is a reliable host-parasite association because: (1) the pair of Brueelia specimens collected from Pharomachrus pavoninus are morphologically identical with those collected from Trogon viridis; (2) both individuals of Pharomachrus pavoninus were collected on different days and at different localities; (3) no Trogon spp. were deloused or collected on the day that JDW collected Brueelia cicchinoi sp. n. from Pharomachrus pavoninus; (4) specimens of Brueelia cicchinoi sp. n. collected from Pharomachrus pavoninus and Trogon viridis are genetically identical (see below); (5) our specimens are only 1 bp different and thus nearly genetically identical to Brueelia sp. collected from two other Trogon species (see below) collected in México. Lastly, although the type host of Brueelia cicchinoi sp. n., Trogon viridis MPEG 62484, was collected in the Amazonian Imerí area of endemism between the Rio Japurá and Rio Negro, we also found Brueelia cicchinoi sp. n. on Trogon melanurus MPEG 59344) from the Imerí area of endemism between the Rio Branco and the Rio Negro and on Pharomachrus pavoninus MPEG 62491 from the Napo area of endemism south of the Rio Japurá. Although we do not have a male specimen of Brueelia cicchinoi sp. n. from Trogon melanurus we are also certain of this host association because the two specimens studied from Trogon melanurus are morphologically and genetically indistinguishable from those collected from Trogon viridis. Thus, Brueelia cicchinoi sp. n. is apparently a relatively widespread trogon parasite found on at least six species of trogons: Trogon viridis, Trogon melanurus, Trogon collaris, Trogon massena (see below), Trogon melanocephalus (see below), and Pharomachrus pavoninus.
http://species-id.net/wiki/Brueelia_insolita
This species is readily distinguished from the other species herein described by the size and shape of the male genitalia and the conspicuous central notch present on the vulvar margin in females. Its head carina and body chaetotaxy are close to that found on Brueelia cicchinoi sp. n.
(n=1): HL, 0.34 (0.32); PAW, 0.28 (0.25); TW, 0.33 (0.31); PL, 0.13 (0.12); PW, 0.23 (0.21); PTL, 0.15; PTW, 0.34 (0.33); AL, 0.78 (0.70); AW 0.48 (0.46); GL, 0.26; and TL, 1.34 (1.26).
(n=6): HL, 0.37–0.38 (0.34); PAW, 0.29–0.31 (0.27); TW, 0.36–0.37 (0.33); PL, 0.13–0.15 (0.12); PW, 0.23–0.25 (0.22); PTL, 0.15–0.18 (0.15); PTW, 0.34–0.36 (0.32); AL, 0.99–1.12 (0.94); AW 0.54–0.57 (0.52); and TL, 1.58–1.69 (1.54).
1 male and 6 females, ex Trogon mexicanus, DB703; GUATEMALA: Huehuetenango, Soloma, 12.IX.1958, D. Baepler col., at PIPeR.
Unexpectedly we had the opportunity to discover and analyze specimens from the same lot as those used by
Based on a MP bootstrap analysis of the partial mitochondrial COI sequences, support for the monophyly of trogon Brueelia was only 56%. However, all 48 of the MP trees (TL=913, CI=0.36, RI=0.70) indicated that Brueelia parasitizing trogons were monophyletic. Furthermore, uncorrected p-distances indicate that the morphologically diagnosable Brueelia species, Brueelia sueta sp. n. and Brueelia cicchinoi
sp. n., are differentiated genetically as well. Uncorrected
p-distances between them range from 13.4–13.6%, whereas uncorrected
p-distances within Brueelia cicchinoi sp. n. from the five different host taxa Trogon viridis, Trogon melanurus, Trogon massena, Trogon melanocephalus, and Pharomachrus pavoninus are extremely small and range from 0–0.3%. Brueelia cicchinoi sp. n. from Amazonian Trogon viridis, Trogon melanurus, and Pharomachrus pavoninus are identical across 382 bp of COI. However, these three specimens differ by a single bp from the Mexican Brueelia sp. from Trogon massena and Trogon melanocephalus sequenced by
Our data in combination with data published by
We thank Alexandre Aleixo (Museu Paraense Emílio Goeldi, Belém, Brazil), Shannon Hackett and John Bates (Field Museum of Natural History, Chicago, USA) for their support and assistance during fieldwork and/or collection of the data presented herein. We also thank Camila Ribas for collecting specimens used in this work and the Field Museum Insect Division including Margaret Thayer, Daniel Summers and James Boone for their advice and access to collections. Petra Sierwald made her NSF PEET funded microscope available for our use. Dale Clayton and Sarah Bush provided access to mounted specimens at the University of Utah, PiPeR collection. This work was supported in part by the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil) with a postdoctoral fellowship 200453/2010-6 to MPV, NSF DEB-0515672 and DEB-1120054 to JDW, and the Field Museum’s Emerging Pathogens Project, funded by the Davee Foundation and the Dr. Ralph and Marian Falk Medical Research Trust. DNA sequencing was carried out at the Field Museum’s Pritzker Laboratory for Molecular Systematics and Evolution, operated with support of the Pritzker Foundation.