(C) 2011 Ronald Vonk. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Psammogammarus wallacei sp. n. is described from the shallow marine interstitial of a sand and coral rubble beach on the Gura Ici islands (North Moluccas; Indonesia). This is the first record of this circum-tropical genus from SE Asia, with the geographically closest relative inhabiting the Ryukyu archipelago in Japan. The new species is highly distinctive by the display of sexual dimorphism on pleopod II, with the medial margin of the male proximal article of exopod provided with a comb of short, blunt curved spinules; no other representative of the genus is known to display sexually-dimorphic appendages aside of the gnathopods. The new species is also noteworthy by the outline of the palm margin of male gnathopod II, hardly excavated, and by showing a carpus broader than long. An overview of the genus Psammogammarus with 14 species to date is provided.
Shallow marine interstitial, sandy beaches, genus review, Psammogammarus, sexual dimorphism
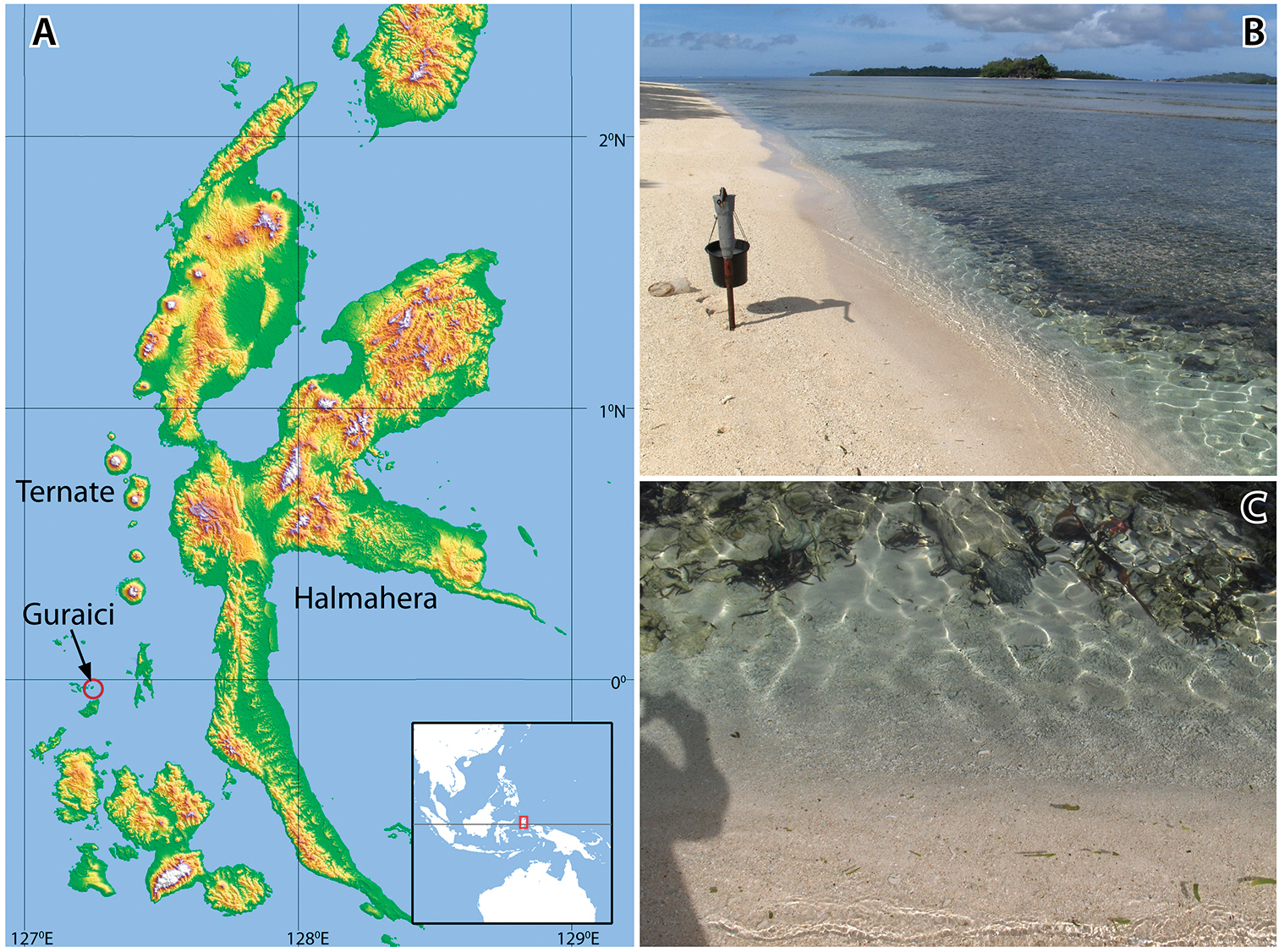
In October and November 2009, a marine expedition was organized by NCB Naturalis, the Research Centre for Oceanography of the Indonesian Institute of Sciences (RCOLIPI), and students of Universitas Khairun, Ternate, North Moluccas. The main goal of the expedition was to investigate the community structure and species composition of coral reef biotas off western Halmahera, in particular around the volcanic islands of Ternate and Tidore. In the wake of this survey a shore party sampled beaches and inland brackish wells for subterranean invertebrates. Several types of coastal sediments were probed, such as beaches of homogeneous black volcanic sand, coral rubble bars, fine silt between stones in bays, and very shallow coral reef flats filled with sand and thick packets of coral fragments. Although the research was based at LIPI’s field station at Ternate, a two day survey was held at the more southwardly located Gura Ici islands (Fig. 1).
Between the copepod and peracarid crustaceans encountered
in the thick layers of coarse beach sand in this island group a typical
shallow marine interstitial gammaridean amphipod was found, belonging
to the melitid genus Psammogammarus
S. Karaman, 1955. This group is widespread in tropical and subtropical
marine beach environments and can only be dug out or pumped from
groundwater. Its occurrence begins, from a landward view, in
anchialine cave waters in coastal localities, such as known from
Bonaire (
It is remarkable that Psammogammarus has not been found in Australia despite quite intensive sampling there in exactly the same shallow marine sediments in which it occurs in many other tropical seas of the world (Yerman and Krapp Schickel 2008).
Location and habitat of Psammogammarus wallacei sp. n. A Gura Ici islands (Guraici = local spelling), low islands consisting of calcareous coral rises and mangrove fringed sand flats B groundwater pump with perforated pipe at 50 cm in the sediment (coarse sand and degraded coral) C shoreline at low tide, leaving a permanent narrow channel between the beach and the reef flat.
The marine stygofaunal research carried out in the North
Moluccas concentrated on collecting subterranean species in diverse
habitats at different places on the islands. We sampled in coastal
areas, wells, small brackish lakes, beaches, and mangrove fringes of
Ternate, Hiri, Tidore, Maitara, Guraici Islands, and the west and
east side of northern Halmahera. Sampling gear consisted of a
biophreatical Bou-Rouch groundwater pump and steel pipes (see
Following
Suborder Gammaridea Latreille, 1802
Family Melitidae Bousfield, 1973
Genus Psammogammarus S. Karaman, 1955
urn:lsid:zoobank.org:act:5E679036-2477-4485-BAD0-6F6D452D2288
http://species-id.net/wiki/Psammogammarus_wallacei
Figs 2–8Collected by R. Vonk and Mr. Sumadijo, 9 November 2009.Gura Ici islands, north beach of Pulau Lelei, thick coral rubble bar at waterline bordering shallow reef flat (stn. 09–60; 0°01'38.64"N, 127°14'38.53"E); B-Rh pump placed on slope of coral bar, pipe 50 cm depth, 50 l filtered. Holotype: Adult male (with penile papillae) 2.65 mm retaining all limbs except U3, completely dissected and mounted on single slide [ZMA, amph. 206076]. Paratypes: five adult males of 2.53, 2.54, 2.55, 2.57 and 2.63 mm; two brooding females (oostegites developed, setose) 2.60 and 2.98 mm; two juveniles 1.79 and 1.87 mm. All in single vial [ZMA, amph. 206075].
Male G2 palm margin only slightly excavated, devoid of mid-palmar strong robust setae; carpus broader-than-long. Uropod III exp2 longer than exp1; endopod elongated, more than 50% length of exp1. Protopod of U1 with one basofacial robust seta. Distomedial angle of U2 protopod provided with transverse comb of 4 robust setae. Telson with two lateral and one distal robust setae. Posteroventral angle of epimeral plate III pointed but weakly produced. Armature (robust setae) on ventral margins of epimeral plates as 1-(2 or 3)-3. Coxal endite (= inner plate) of maxillule provided with 6 setae; basal endite (= outer plate) with 9 robust setae. Oblique row on inner plate of maxilla composed of 4 setae. Basal endite (= inner plate) of maxilliped provided with three robust setae; ischial endite (= outer plate) with 4. Basis of P7 weakly expanded, with sub-parallel anterior and posterior margins. Pleopod II sexually-dimorphic.
Species name after the 19th century British naturalist Alfred R. Wallace, who was based in Ternate during his explorations of the Moluccas.
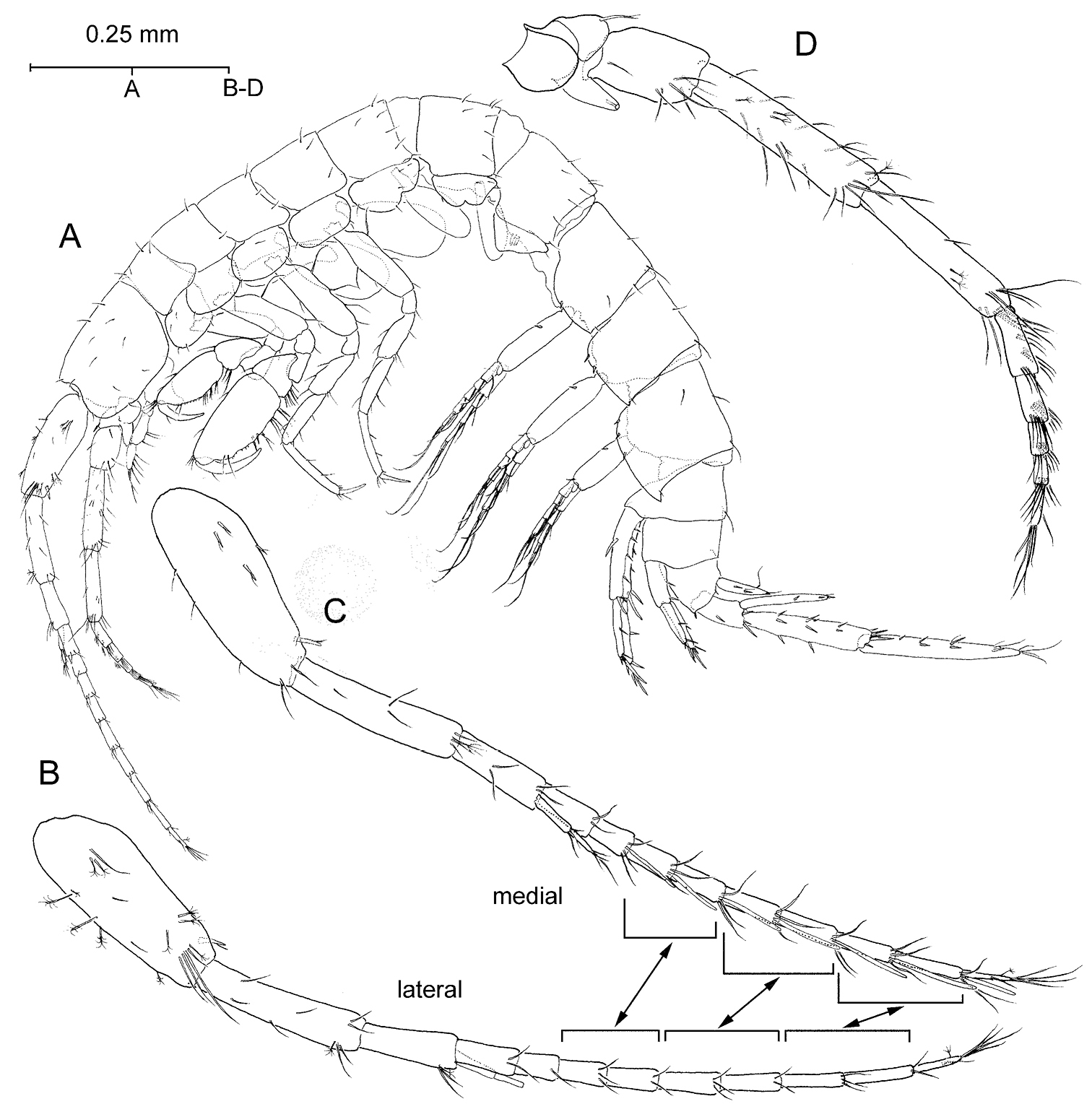
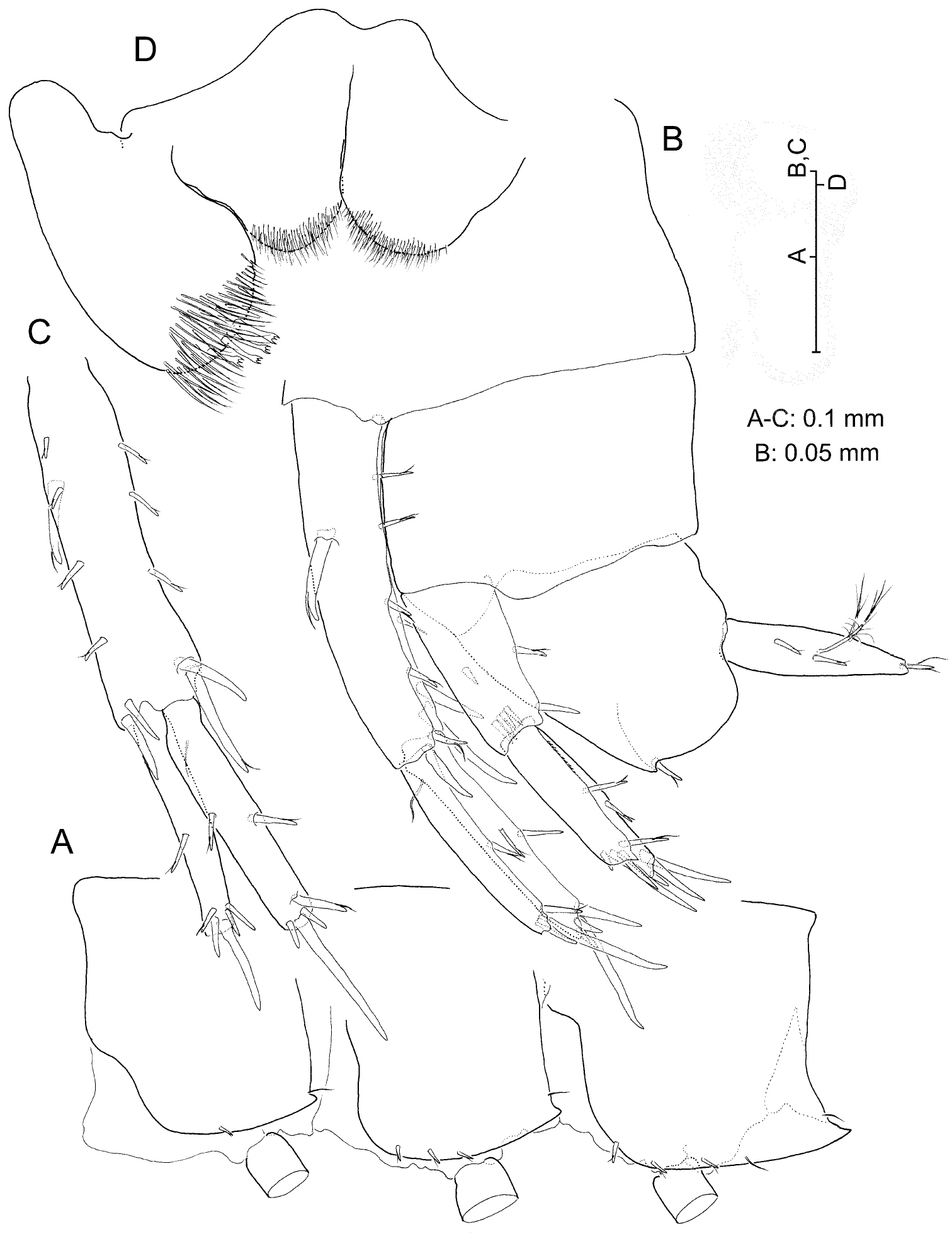
Adult male. Eyeless. Body (Fig. 2A) elongate and slender, unpigmented, somites devoid of relevant armature or sculpturing except for robust seta present on posteroventral angle of urosomite III (Fig. 8B). Head lacking rostrum; lateral lobes evenly rounded; antennal sinus hardly indicated, unnotched. Pereiopodal coxae narrow, hardly overlapping. Epimeral plates (Fig. 8A) with acute posteroventral angles, that of epimeral plate III more produced than rest; armature of ventral margin of plates (flagellate robust setae) as 1-3-3 or 1-2-3; posterior margin of plates each with single simple seta implanted adjacent to posterodistal angle.
Antennule short, about half as long as body length (Fig. 2A). Peduncle segments relative length as 100: 77: 43; proximal segment provided with stout flagellate robust seta subdistally on ventromedial margin (Fig. 2B, C). Main flagellum about as long as peduncle, with armature on medial and lateral margins exactly as depicted; armature of two most proximal articles and terminal article differing from rest as figured. Accessory flagellum 2-articulate, overreaching distal margin of proximal article of main flagellum.
Antenna (Fig. 2A, D) shorter, about three-quarters length of antennule. Gland cone slender, straight, pointing anteriorly; relative length of three distal segments of peduncle as 48: 100: 88. Flagellum short, slightly longer than distal segment of peduncle.
Labrum trapezoidal. Paragnaths (Fig. 8D) with distinct, well developed inner lobes; outer lobes each with 4 stout tricuspidate setae on tip.
Left mandible (Fig. 3A) incisor with 5 rounded teeth, lacinia with 4 teeth; spine row composed of 7 elements; molar columnar, molar seta shorter than right mandible counterpart. Palp 3-segmented, distal segment shorter than middle segment, provided with 6 stiff simple setae along medial margin but not in a regular row; middle segment with three flagellate stiff setae on medial margin. Right mandible (Fig. 3B) differing from left counterpart in almost quadrate lacinia with distal margin finely denticulated except for two larger rounded denticles at one angle; spine row comprising 5+3 elements.
Maxillules (Fig. 3C) symmetrical, coxal endite (= inner plate) with 4+2 marginal setae; basal endite (= outer plate) with 9 robust setae distally, 4 of which bicuspid and placed conforming an inner row, rest denticulated and conforming outer row. Endopod (= palp) faintly 2-segmented, distal segment slightly expanded distally, with 4 broad, short denticulated robust setae on distal margin, and 1–2 denticulated setae subdistally on outer surface of segment.
Maxilla (Fig. 3D) inner plate with oblique row of 4 plumose setae; distal margin with 4 pinnate setae and 4 simple setae with blunt tip provided with pore; two pinnate setae subdistally on medial margin of plate as figured. Outer plate distal margin with 10 simple setae with blunt tip, two shorter, ordinary simple setae, plus seta with a few pinnules proximally as figured.
Maxilliped (Fig. 3E) basal endite (= inner plate) subrectangular, straight distal margin provided with three short subtriangular robust setae, two of which smooth, third with two rounded denticles; other armature on endite comprising subterminal oblique row of 5 pinnate setae on anterior surface, and three simple setae subterminally on posterior surface. Ischial endite (= outer plate) with convex outer margin; straight inner margin with 6 simple setae with blunt tip; distal margin oblique, with 4 pectinate robust setae. Other relevant armature on endite comprising submarginal row of 4 simple setae with blunt tip running subparallel to inner margin on posterior surface. Merus-dactylus (= palp) as in Fig. 3E, F, with claw (= dactylus+unguis) as long as propodus.
Coxal gills on gnathopod II and pereiopods III-VI (Fig. 2A); gills II (Fig. 4D) and III-IV (Fig. 5B) each longer than basis of corresponding pereiopod, gills V-VI (Fig. 6A, B) shorter than basis; gill VI reduced. Gill II narrow, sausage-shaped, rest ovoid; all provided with short stalk.
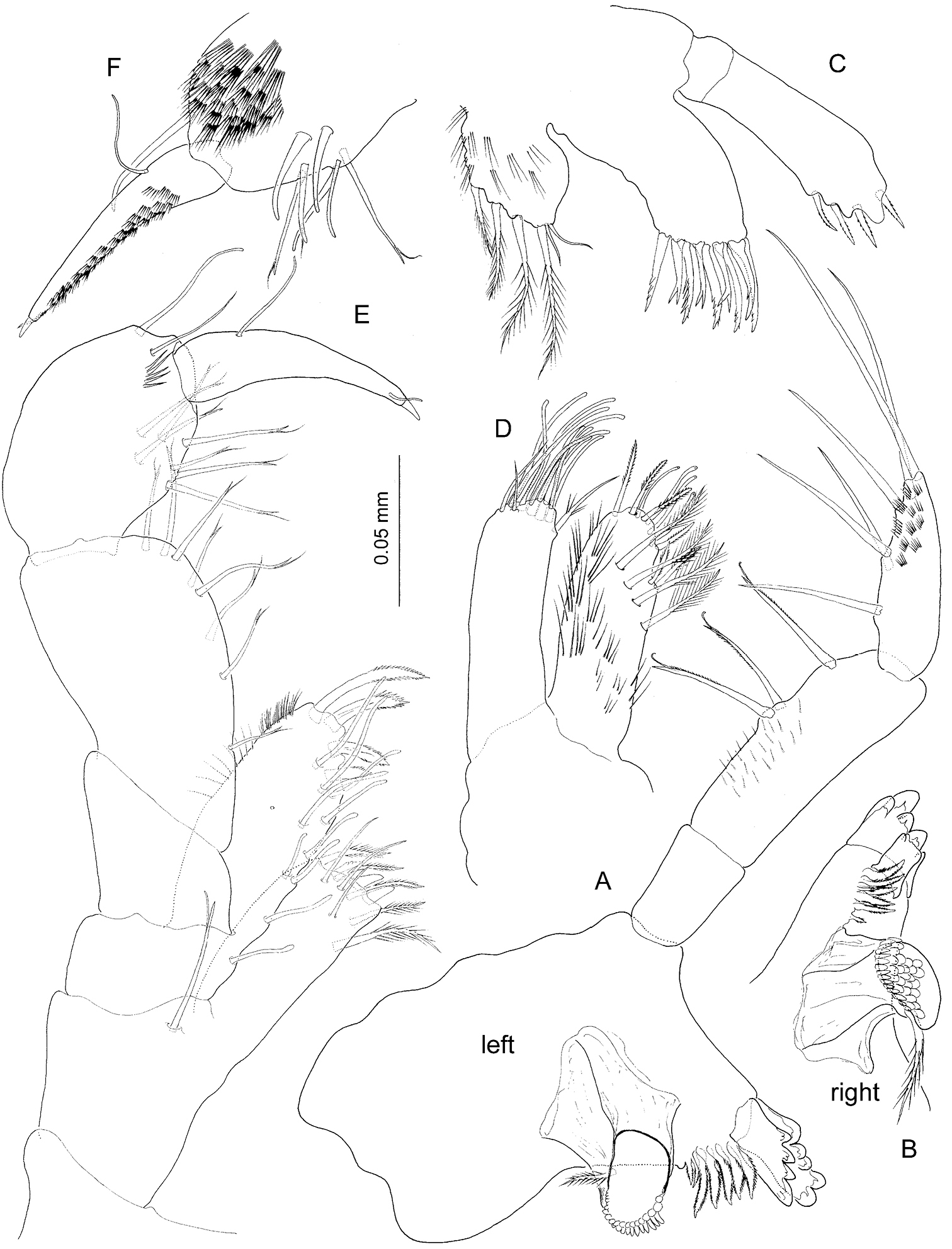
Gnathopod I (Fig. 4A-C) propodus clearly longer than carpus, elongate (1.9 times as long as broad), with parallel anterior and posterior (= lateral and medial) margins. Palm margin oblique, convex, finely serrated, with submarginal row of about 10 short flagellate robust setae along medial side. Palm angle not produced, ordinarily with two unequal flagellate robust setae, but extraordinarily with 3–4 (Fig. 4C). Coxa (Fig. 4B) slightly broader than long, with evenly rounded anterior margin; posterior margin slightly excavated.
Gnathopod II (Fig. 4D) propodus elongate (1.9 times as long as broad) with subparallel anterior and posterior margins; palm angle hardly produced, with two unequal flagellate robust setae; palm margin (Fig. 4E) finely serrated, half adjacent to palm angle hardly excavated, with submarginal row of ca. 9 short flagellate robust setae along medial side. Merus posterodistal angle slightly produced, but lacking pointed tip. Carpus triangular, short, less than half length of propodus.
Pereiopods III-IV (Fig. 5A, B) subsimilar, with coxae much broader than long, subrectangular, expanded anteriorly into evenly rounded lobe and posterior margin not excavated, straight.
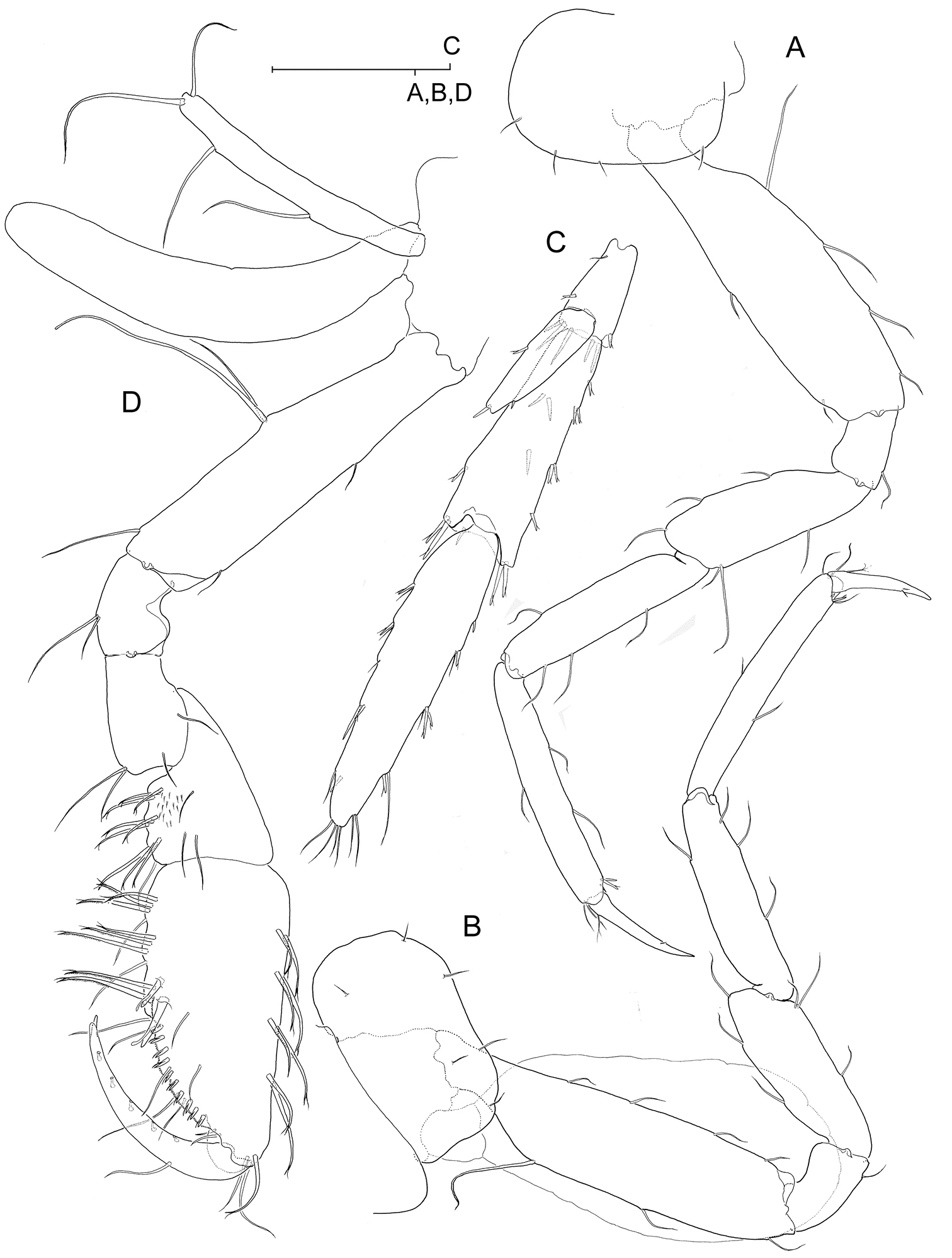
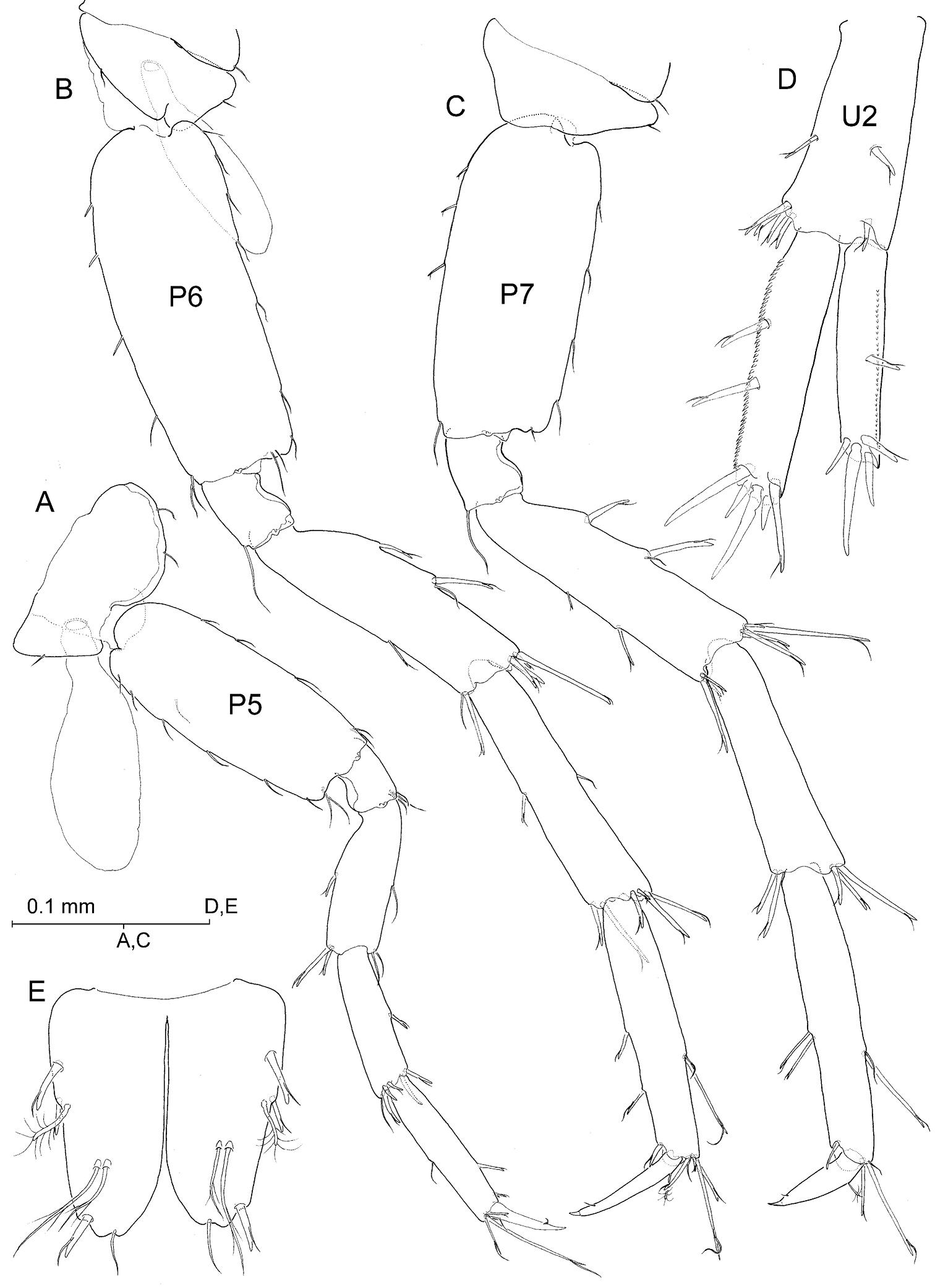
Pereiopods V-VII unequal in length, P5 (Fig. 6A) shortest, P6 (Fig. 6B) longer than P7 (Fig. 6C). Basis of each pereiopod only moderately expanded, with subparallel margins and with posterodistal angle distinct but not overhanging. Nail (dactylus + unguis) of P6 more slender and clearly longer than those of P5 and P7. Unguis reduced in all limbs. Coxa V with expanded, evenly rounded anterior lobe.
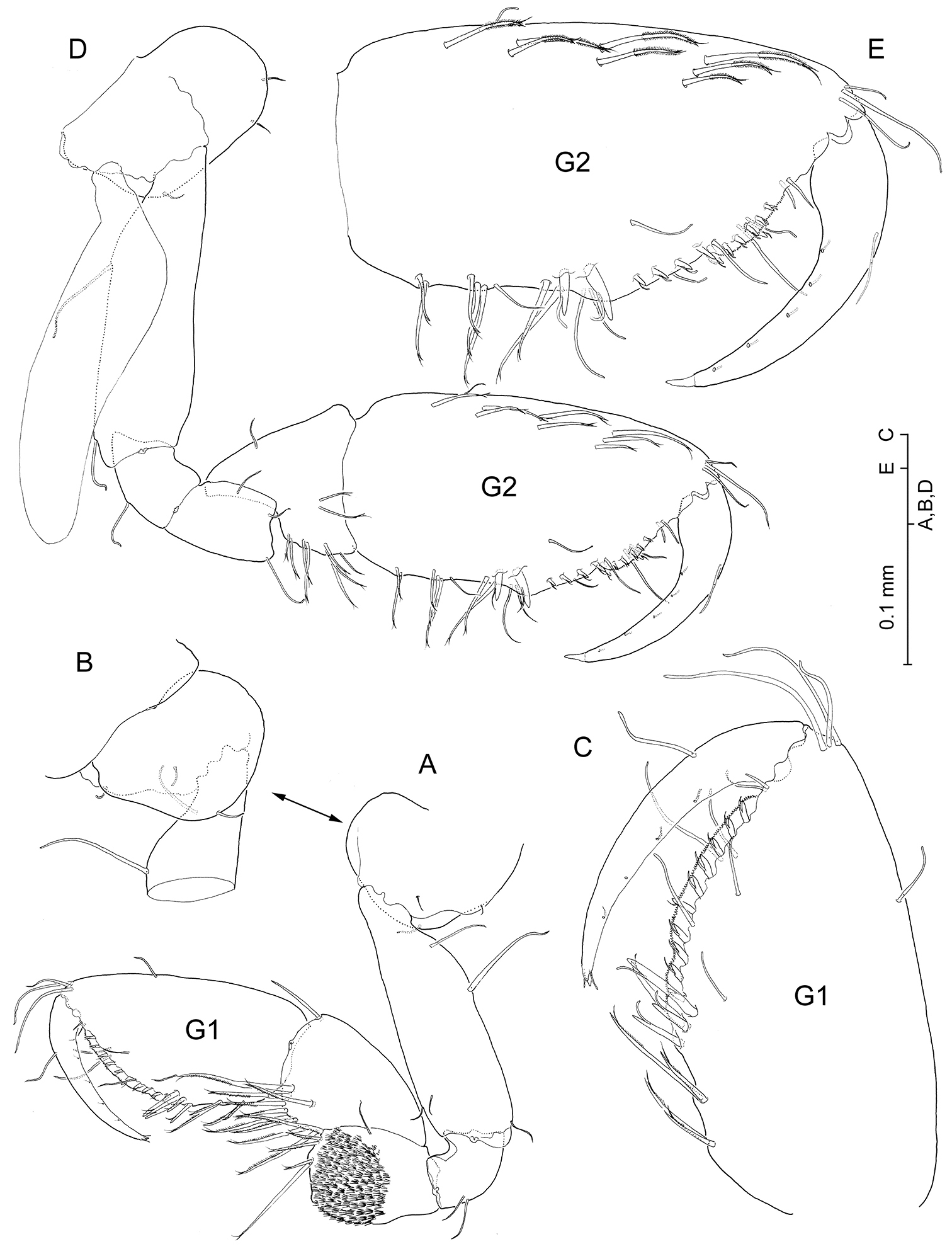
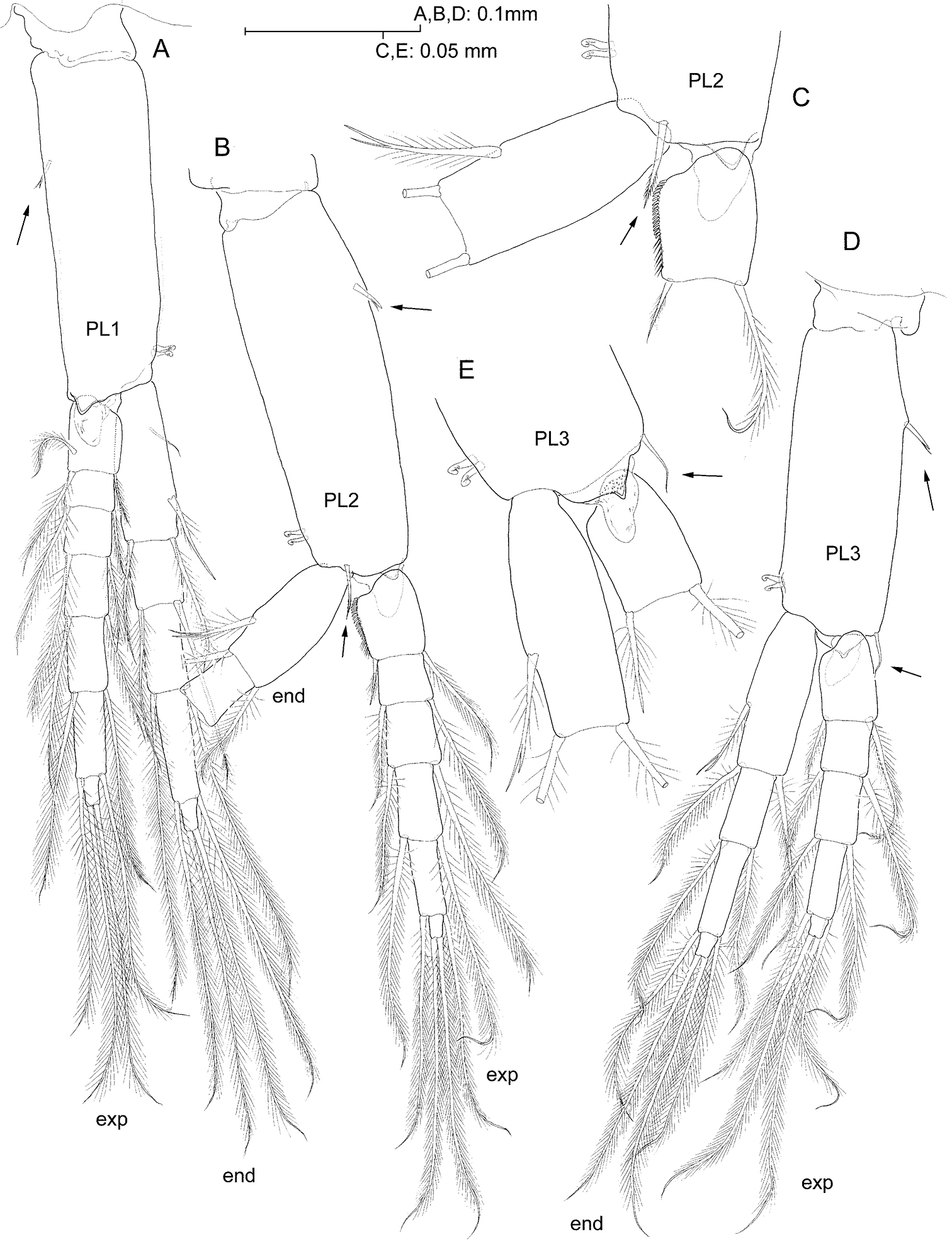
Pleopods progressively shorter towards posterior, biramous, rami multi-articulate, apparently similar at first sight, but differing remarkably in minute details as follows. Protopods each with two retinacles, and with flagellate robust seta placed proximo-laterally on anterior surface of segment (pleopods I-II; Fig. 7A, B), or on lateral margin (pleopod III; Fig. 7D). Protopod of pleopod I devoid of any other armature; protopod of pleopod II with seta on anterodistal margin (Fig. 7B, C); protopod of pleopod III with seta on distolateral angle (Fig. 7D, E). Short, subtriangular process protruding posterodistally on each protopod, those on pleopods I-II with smooth surface (Fig. 7A, C), that on pleopod III microspinulate (Fig. 7E). Proximal article of endopod of pleopod I with one reduced smooth seta proximally on anterior surface (Fig. 7A); seta absent from rest of pleopods (Fig. 7B, D); proximal seta on medial margin of article apparently unicuspid (Fig. 7A), vs. seta bifid on pleopods II-III (Fig. 7C, E). Proximal article of exopod of pleopod II sexually-dimorphic: proximal two-thirds of medial margin with row of short, curved denticles with rounded tip, and row of ordinary setules along distal third of margin (Fig. 7B, C).
Uropods I-II strongly dissimilar in length (Fig. 8B), biramous, exopod shorter than endopod, and with margins of both protopod and rami provided with flagellate robust setae. Uropod I (Fig. 8C) protopod much longer than exopod, provided with stout basofacial robust seta and with pair of robust setae on each posterodistal angle, medial pair appreciably longer than lateral counterpart. Posterolateral margin of segment provided with 4 short robust setae, posteromedial margin with three. Exopod with 5 distal robust setae and with robust seta on posterolateral margin; endopod with 4 distal setae and with robust seta about midway on both margins, aside of reduced simple seta on anteroproximal surface of segment. Uropod II (Fig. 6D) protopod about as long as exopod, with one robust seta on distolateral angle and with transverse row of 4 robust setae on distomedial angle (see Fig. 8B). Margins of segment each with single robust seta about midway. Exopod with 4 distal robust setae and robust seta about midway of posterolateral margin; endopod with 5 distal robust setae and two robust setae along posteromedial margin. Lateral margin of exopod and medial margin of endopod each minutely serrated (Fig. 6D). Uropod III (Figs 2A; 5C) strongly elongated, all segments somewhat flattened, foliaceous, provided with numerous flagellate robust setae as figured. Protopod short; proximal segment of exopod about 2.2 times as long as protopod, distal segment exceedingly longer (1.5×) than proximal segment. Endopod elongate, pointed, much longer than protopod and attaining about 57% length of proximal exopodal segment; one robust seta on tip.
Telson (Fig. 6E) cleft almost until base, slightly longer than broad, tip of each lobe shallowly excavated, provided with simple seta. Three flagellate robust setae on each lobe, one placed subdistally while other two proximally on lateral margin as figured. One penicillate seta disposed adjacent to one of lateral robust setae as in Figs 8B and 6E. Pair of long setae on dorsal surface of each lobe as figured.
Adult female. As male except for gnathopod II, which displays more elongated carpus and evenly convex palm margin of protopod (compare Figs 4D, E and 5D). In addition, proximal article of exopod of pleopod II unmodified. Oostegites present on gnatopod II and pereiopods III-V, linear, provided with few sparsely set marginal setae (Fig. 5D). Condition of uropod III unresolved since none of two females collected retained it.
Psammogammarus wallacei sp. n., male paratype 2.53 mm, P5-P7 wanting. A body, lateral B left antennule, lateral C same showing medial armature D right antenna, lateral. Arrows pointing at serially-homologous pairs of articles of main flagellum of antennule.
Psammogammarus wallacei sp. n., male holotype. A left mandible B right mandible C maxillule D maxilla E maxilliped, posterior F inset of distal segments of palp of latter, anterior.
Psammogammarus wallacei sp. n., male holotype. A right gnathopod I, medial B inset of coxa, lateral C palm, medial D left gnathopod II, medial; palm, medial. Notice that C and E are not at same scale.
Psammogammarus wallacei sp. nov., male holotype. A left pereiopod III with coxal gill omitted, lateral B right pereiopod IV, lateral C right uropod III, dorsal D left gnathopod II of female paratype 2.98 mm showing coxal gill and oostegite, coxal plate omitted, medial. [Scale bars: 0.25 mm (C); 0.1 mm (A, B, D)]
Psammogammarus wallacei sp. n., male holotype. A right pereiopod V, lateral B left pereiopod VI, lateral C left pereiopod VII, lateral D right uropod II, posterior E telson, dorsal.
Psammogammarus wallacei sp. n., male holotype. A left pleopod I, posterior B left pleopod II with distal portion of endopod omitted, endopod unnaturally bent to expose medial margin of proximal article of exopod, anterior view C detail of latter, anterior D left pleopod III, anterior E detail of distal portion of protopod of right pleopod III, posterior. Arrows pointing at armature elements of protopod.
Psammogammarus wallacei sp. n., male holotype. A left epimeral plates, lateral B urosome, lateral (uropod III wanting) C left uropod I, posterior D paragnaths.
The combined display of a basic gammaridean
body plan, linear oostegites, antennae without calceoli, no sternal
gills, G1 smaller than G2, both lacking integumentary rugosities, G1
of melitoid type, dispariramous uropod III with a reduced endopod and a
2-segmented exopod with both segments highly elongated and about the
same length, and paragnaths with distinct inner lobes, relates the new
taxon from Indonesia with a reduced cluster of melitid genera of mostly
stygobytic habits known as the Eriopisa-group. The discrimination
between several of the components in this complex proved to be
controversial (
The new taxon differs markedly from Victoriopisa
Karaman & Barnard, 1979, a genus from coastal lagoons and marine
shallow waters comprising 9 species, in displaying the proximal
articles of the antennary flagellum not fused; the ventral margin of
epimeral plate II devoid of a row of long setae; the P7 basis not
broadly expanded, similar to P6; and the propodus of G1 longer than
carpus (vs. carpus equal or longer than propodus in Victoriopisa) (see
Eriopisa Stebbing, 1890, comprising two species, viz. Eriopisa elongata (Bruzelius, 1859) from the deep Atlanto-Mediterranean and Eriopisa incisa
McKinney, Kalke and Holland 1978 from shallow muddy bottoms on the
Gulf of Mexico, differs in the notched head lobe; the forwardly pointed
coxal plate of G1; the broadly expanded basis of P7, dissimilar to P6;
the carpus of G1 as long as or longer than propodus; and the epimeral
plate II bearing a row of long setae along ventral margin (
Flagitopisa
Karaman, 1984, comprising two species from wells and river alluvia in
the Philippines, differs in the presence of sternal gills on the first
pleonite; the uniarticulate condition of the antennulary accessory
flagellum; the high number of robust setae on the basal endite of
maxilliped (11 vs. 3 in the new taxon, and up to 6 in the rest of taxa
of the Eriopisa-group); and the G1 carpus, which is longer than propodus (
Nedsia
Barnard & Williams, 1995, including 11 species from Australian
inland groundwaters, displays a G1 carpus longer than propodus; a
broadly expanded, foliaceous U3 exopod; and a 2-segmented mandibular
palp (see
Norcapensis Bradbury & Williams, 1997, a monotypic genus from subterranean waters of W Australia, differs in the fusion of the proximal articles of the antennal flagellum; the carpus of G1 longer than propodus; and the huge G2 propodus, with a short posterior margin and a strongly oblique palm margin.
Tunisopisa
Stock, 1980, a monotypic genus from wells in Tunisia, displays a
non-elongated exp2 of U3 but is traditionally included in the Eriopisa-group.
This taxon shows a presumed unsegmented maxillulary endopod; a broadly
expanded, foliaceous U3 exopod; a G1 propodus longer than carpus; and a
peculiar palm of G1, provided with a series of transverse
integumentary ridges (
The new Indonesian species is assigned to Psammogammarus after its P7 basis, similar to P6, and the proportions of the two distal segments of the mandibular palp, with segment 3 much shorter than segment 2. Only some species in Nedsia among members of the Eriopisa-group show a slender or weakly broadened basis of P7 approaching the nearly linear basis of P7 of the new taxon, but all Nedsia members display a 2-segmented mandibular palp.
Psammogammarus wallacei sp. n. differs at first glance from a highly distinctive group of three species of non-interstitial habits, inhabitants of anchialine caves and wells, namely Psammogammarus burri Jaume & Garcia, 1992 from the Balearic Is., Psammogammarus longidactylus Vonk & Stock, 1987 from Bonaire (Netherlands Antilles), and Psammogammarus longiramus (Stock & Nijssen, 1965) from Entedebir Is. (Red Sea). This cluster is characterised by the common display of a male G2 with non-excavated, evenly convex palm margin and an elongated, longer-than-broad carpus.
Psammogammarus gracilis (Ruffo & Schiecke, 1975), from the shallow infralittoral interstitial medium of Malta (Mediterranean), shows a highly characteristic bisinusoid conformation of the male G2 palm margin. This feature differs markedly from the condition displayed in the rest of Psammogammarus, including the new species, where the palm margin is either excavated or evenly convex.
A third cluster of Psammogammarus, of interstitial habits, displays a male G2 palm margin strongly excavated, namely: Psammogammarus coecus S. Karaman, 1955 from the western Mediterranean and Adriatic shallow infralittoral; Psammogammarus garthi (Barnard, 1952) from a tidal pool at Baja California (Mexico); Psammogammarus initialis Stock & Sánchez, 1987 and Psammogammarus stocki Vonk, 1990, both from beaches and tidal pools at Tenerife (Canary Is.); Psammogammarus mawatarii Tomikawa, Kakui and Yamasaki 2010 from a tidal pool in southern Japan; and Psammogammarus spinosus Stock & Vonk, 1992 from a beach at the Cape Verde Islands. Additional remarkable differences between these and other Psammogammarus species vs. Psammogammarus wallacei sp. n. are shown in Table 1. Intraspecific variation has been accounted for. For instance Psammogammarus burri possesses the rather high number of 19 setae on the coxal endite (=outer plate) of the maxillule (character 13, Table 1).
However, this number is fixed within the other 6 specimens used for
this study. Another research that focussed on variation within
bogidiellid amphipods also confirmed only small variation is present in
this feature – in one case out of seven 6 spines present instead of 7 (
Only Psammogammarus caesicolus Stock, 1980 from Curaçao and Bonaire displays a shallowly excavated male G2 palm margin approaching the condition found in the new species. This species shares also with the new taxon the display of a reduced armature on both the coxal endite of the maxillule and the inner plate of the maxilla, a comb of 4 robust setae on the distomedial angle of U2 protopod, an elongate U3 endopod, and a robust seta on tip of telson (see Table 1). But they differ markedly in the exp2 of U3, which is shorter than exp1 in Psammogammarus caesicolus vs. longer in the new species; in the presence of 2–3 basofacial spines on the protopod of U1 in Psammogammarus caesicolus vs. only one in the new species; in the armature of epimeral plates, devoid of robust setae in Psammogammarus caesicolus vs. a 1-(2 or 3)-3 arrangement in the new species; and in the armature of palm angle of G2 in both sexes, with 4 robust setae in male and three in female Psammogammarus caesicolus, vs. two robust setae in both sexes in the new species.
Two species of Psammogammarus are known only from a single female, but their morphology enables an easy separation from the new taxon. Thus, Psammogammarus scopulorum
Stock, 1983, a coarse sand bar inhabitant of Los Roques archipelago
in Venezuela differs markedly from the new species in the telson
armature, devoid of lateral robust setae; the reduced marginal armature
on both protopod and rami of U1 and U2; the comparatively longer U3
endopod (attaining 96% length of proximal segment of exopod, vs. 57% in
the new species); the relative length of the U3 exopodal segments,
with exp1 longer than exp2 (vs. the reverse in the new species); and the
armature of epimeral plates, with 0-1-1 robust setae compared to 1-(2
or 3-3) in the new species (see Table 1;
Psammogammarus bluefieldensis Ortiz, Lalana & Beltrán, 1993, from shallow muddy bottoms of the Caribbean coast of Nicaragua, differs from the rest of members of the genus in the display of a comparatively shallowly excavated telson (cleft only to almost midway compared to almost at base in the rest of species) and a 3-articulate accessory flagellum of antennule. In addition, it differs from the new species in the U1 and U2 rami, devoid of marginal armature; the shorter U3 endopod (attaining only 34% length of exp1, vs. 57% in the new species); and the U3 exp2, much shorter than exp1 (63% length of exp1, vs. exp2 longer than exp1 in the new species), among other distinctive features (see Table 1).
The new species from Indonesia displays a
faint sexual dimorphism on pleopod II, where the male displays a rake
conformed of short and blunt curved spinules along the medial margin of
the proximal article of the exopod (Fig. 7B, C).
Main diagnostic features of Psammogammarus species.
| Psammogammarus wallacei sp. n. | Psammogammarus bluefieldensis | Psammogammarus burri | Psammogammarus coecus | Psammogammarus caesicolus | Psammogammarus garthi | Psammogammarus gracilis | Psammogammarus initialis | Psammogammarus longidactylus | Psammogammarus longiramus | Psammogammarus mawatarii | Psammogammarus scopulorum | Psammogammarus spinosus | Psammogammarus stocki | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | male G2, outline of palm margin | excavated | ? | convex | excavated | excavated | excavated | excavated | excavated | convex | convex | excavated | ? | excavated | excavated |
| 2 | male G2, mid-palmar strong robust setae | 0 | ? | 3 | 1? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 |
| 3 | male G2, carpus | broader>long | ? | longer>broad | broader>long | broader>long | broader>long | broader>long | broader>long | longer>broad | longer>broad | broader>long | ? | broader>long | broader>long |
| 4 | U3 exopod, relative length of segments | exp2 > exp1 | exp2 < exp 1 | exp2 < exp1 | exp2 > exp1 | exp2 < exp1 | exp2 = exp1 | exp2 = exp1 | exp2 > exp1 | exp2 < exp1 | exp2 < exp1 | exp2 > exp1 | exp2 < exp1 | exp2 < exp1 | exp2 = exp1 |
| 5 | U3 end, % length of exp1 | 57 | 34 | 23 | 54 | 72 | 20 | 19 | 89 | 117 | 110 | 27 | 96 | 36 | 19 |
| 6 | U1 protopod, basofacial robust setae | 1 | 2 | 1 | 2 | 3 | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 7 | U2 protopod, robust setae on distolateral angle | 1 | 1 | 1 | 1 | 3 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| 8 | U2 protopod, robust setae on distomed. angle | 4 | 2 | 3 | 2 | 4 | 2? | 1 | 4 | 2 | 2 | 2 | 2 | 2 | 1 |
| 9 | telson, lateral robust setae | 2 | 0 | 3 | 2 or 3 | 3 | 1–2 setae | 1 | 3 | 2 | 4 | 1 | 2 | 2 | 3 |
| 10 | telson, distal robust setae | 1 | ? | 0 | 2 or 3 | 1 | 2 setae | 2 | 3–4 spines | 2 | 2 | 1 | 0 | 2 | 0 |
| 11 | epimeral plate III, posteroventral angle | weakly produced | strongly produced | quadrate | strongly produced | quadrate | strongly produced | rounded | weakly produced | weakly produced | weakly produced | rounded | strongly produced | rounded | rounded |
| 12 | epimeral plates, ventral margin (robust setae) | 1-(2 or 3)-3 | ? | 2-4-4 | 0-0-0 | 0-1-1 | ? | 0-0-0 | 0-1-2 | 0-1-2 | ? | 0-0-0 | 0-1-1 | 0-0-0 | 1-1-1 |
| 13 | maxillule, setae on coxal endite | 6 | 4 | 19 | 7 | 6 | 5 | 5 | 14 | 14 | 15 | 5 | 7 | 5 | 4 |
| 14 | maxillule, basal endite (robust setae) | 9 | 7 | 16 | 9 | 9 | 9 | 7 | 9 | 9 | 9 | 9 | 9 | 7 | 7 |
| 15 | maxilla inner lobe, no. setae on oblique row | 4 | 3 | 16 | 6 | 5 | 5 | 3 | 15 | 12 | 12 | 4 | 5 | 4 | 4 |
| 16 | maxilliped, basal endite (robust setae) | 3 | 3 | 3 | 3 | 6 | 3 | 0 | 3 | 3 | 3 | 3 | 3 | 3 | 0 |
| 17 | maxilliped, ischial endite (robust setae) | 4 | ? | 7 | 7 | 4 | 0? | ? | 11 | 6 | 8 | 4 | 4 | 4 | 0 |
| 18 | P7 basis, outline | weakly expanded | weakly expanded | weakly expanded | weakly expanded | weakly expanded | weakly expanded | weakly expanded | moderately expanded | broadly expanded | moderately expanded | weakly expanded | weakly expanded | weakly expanded | weakly expanded |
| 19 | P7 basis, margins | subparallel | subparallel | subparallel | subparallel | subparallel | subparallel | subparallel | convex | convex | subparallel | subparallel | subparallel | subparallel | subparallel |
| 20 | pleopod II, sexual dimorphism | yes | ? | No | No | no | ? | ? | no | no | ? | ? | ? | ? | yes? |
Ternate is situated in the centre of maximum marine species diversity, the Coral Triangle (
Because subterranean amphipods are known to be poor
dispersers, their distribution patterns are expected to depend more on
plate tectonics than on oceanic currents (
We are grateful to Prof. Dr. Suharsono, director of RCO-LIPI, for sponsoring the research. Mrs. Yosephine Tuti (co-expedition leader) assisted in the preparations and logistics of the expedition. We thank Mr. Fasmi Ahmad, head of the LIPI research station in Ternate for his assistance. Mr. Sumadijo (RCO-LIPI, Jakarta) and students Samar Ishak and Dodi Kahar assisted in the fieldwork and were guides and translators; their help and creativity were indispensable. The research permit was issued by the Indonesian State Ministry of Research and Technology (RISTEK). Contribution to Spanish MCINN project CGL2009-08256, partially financed with FEDER funds.