(C) 2011 Anatoly B. Babenko. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The paper is devoted to a taxonomic revision of the genus Sensillonychiurus Pomorski & Sveenkova, 2006. Five new species of this genus, i.e. Sensillonychiurus mirus sp. n., Sensillonychiurus taimyrensis sp. n., Sensillonychiurus vegae sp. n., Sensillonychiurus vitimicus sp. n., and Sensillonychiurus amuricus sp. n., as well as three new species of the related genus Allonychiurus Yoshii, 1995, i.e. Allonychiurus subvolinensis sp. n., Allonychiurus elikonius sp. n., and Allonychiurus unisetosus sp. n. are being described from various regions of Eurasia. The diagnoses of both genera are amended to include described species. Two genera, Tantulonychiurus Pomorski, 1996 and Thibaudichiurus Weiner, 1996, are treated as junior synonyms of the genus Allonychiurus. Agraphorura eisi (Rusek, 1976) is transferred to Sensillonychiurus; Tantulonychiurus volinensis (Szeptycki, 1964) and Tantulonychiurus asiaticus Babenko, 2007 to Allonychiurus. A review of morphological peculiarities of Sensillonychiurus is performed, comparisons with the other genera of Thalassaphorurini given, and a key to the known species provided.
ά-taxonomy, morphological review, Sensillonychiurus, Allonychiurus, northern Asia, eastern Europe
This paper has been prompted through the discovery of a
new species on the Barents coast of Kola Peninsula. This species from
the tribe Thalassaphorurini
is characterized by the combination of morphological features that
fails to completely fit into any of the known genera of the tribe.
Unfortunately, the tribe’s generic classification, as well as that of
the whole subfamily Onychiurinae, is still far from perfect. Starting from the pioneering papers by
The only character uniting all of the members of the tribe Thalassaphorurini is the structure of the furcal remnant which forms a finely granulated area in mid-section of Abd.4
with 4 small setae arranged in two posterior rows. The second character
shared, i.e. distinct antennal and tergal sensilla, is probably
present in all genera, but not all species of the tribe. Taking this
into account, evidently the genus Uralaphorura is to be excluded from the tribe in having nothing in common with the other Thalassaphorurini,
being characterized by a quite different structure of the furcal
remnant with four posterior setae arranged in a line. Thus, Uralaphorura is probably closer to Onychiurini than to Thalassaphorurini (see also
According to R.J. Pomorski (personal communication), two of the remaining eight genera, i.e. Micronychiurus and Agraphorura
are to be considered as synonyms. Nevertheless we do not follow here
this suggestion as it was never officially published and a discussion on
the status of these genera is beyond the scope of our paper. In his
draft Synopsis on Palaearctic Onychiuridae, Pomorski also intended to synonymize the genus Tantulonychiurus (and also Thibaudichiurus) with Allonychiurus.
Most probably, this latter suggestion was dictated by its practical
usefulness, as well as by the impossibility to unity the known species
of all these “genera” into more or less natural groups, based only on
our present knowledge. For instance, according to
The new species mentioned in the beginning of Introduction appears to be especially similar to the known representatives of the small eastern Asiatic genus Sensillonychiurus. A study of the available material from M. Potapov’s and authors’ collections reveals a whole number of closely related forms and shows that the original diagnosis of the genus must be somewhat amended. Thus, the present paper includes a brief review of the morphological peculiarities of Sensillonychiurus as compared to the other genera of the tribe, a slightly changed diagnosis and a key to all of the known species of this genus, as well as descriptions of five new species. In addition, three further new species habitually similar but, according to the accepted system of Thalassaphorurini, assignable to the genus Allonychiurus, have also been described and used for comparative purposes. Types of all the new species are deposited in the collection of the Department of Zoology & Ecology, Moscow State Pedagogical University (MSPU).
Abbreviations
A–E papilla, a, b, d, e guards – main labial papillae and associated guard setae (
A, AB, AC and ABC – four types of labium in Onychiuridae in accordance with the presence of thickened and blunt-tipped setae on corresponding labial papillae (
Abd.1–6 – abdominal segments
A-B, T-setae, setae M and Y – tibiotarsal setae (
Ant.1–4 – antennal subsegments
AO – antennal organ on Ant.3
AS – anal spines on Abd.6
bl. f. – basolateral field of labium (mentum)
bm. f. – basomedial field of labium (submentum)
d0 – unpaired axial seta on area frontalis of the head
a0, m0 and p0 – unpaired axial setae on terga
Lg.1–3 – legs
ms – microsensillum
MSPU – Moscow State Pedagogical University
MVO – male ventral organ
PAO – postantennal organ
pso – pseudocellus
psx – parapseudocellus
px – proximal setae on labium
Th.1–3 – tergal segments
Ti.1–3 – tibiotarsi
U3 – inner edge of unguis on hind leg
VT – ventral tube
A review of the main morphological characters of Sensillonychiurus Pomorski & Sveenkova, 2006The present review is based on the morphological
peculiarities of five new species described in this paper, as well as
on published data on all four so far known species of the genus. Three
of them were described by the authors of the genus (
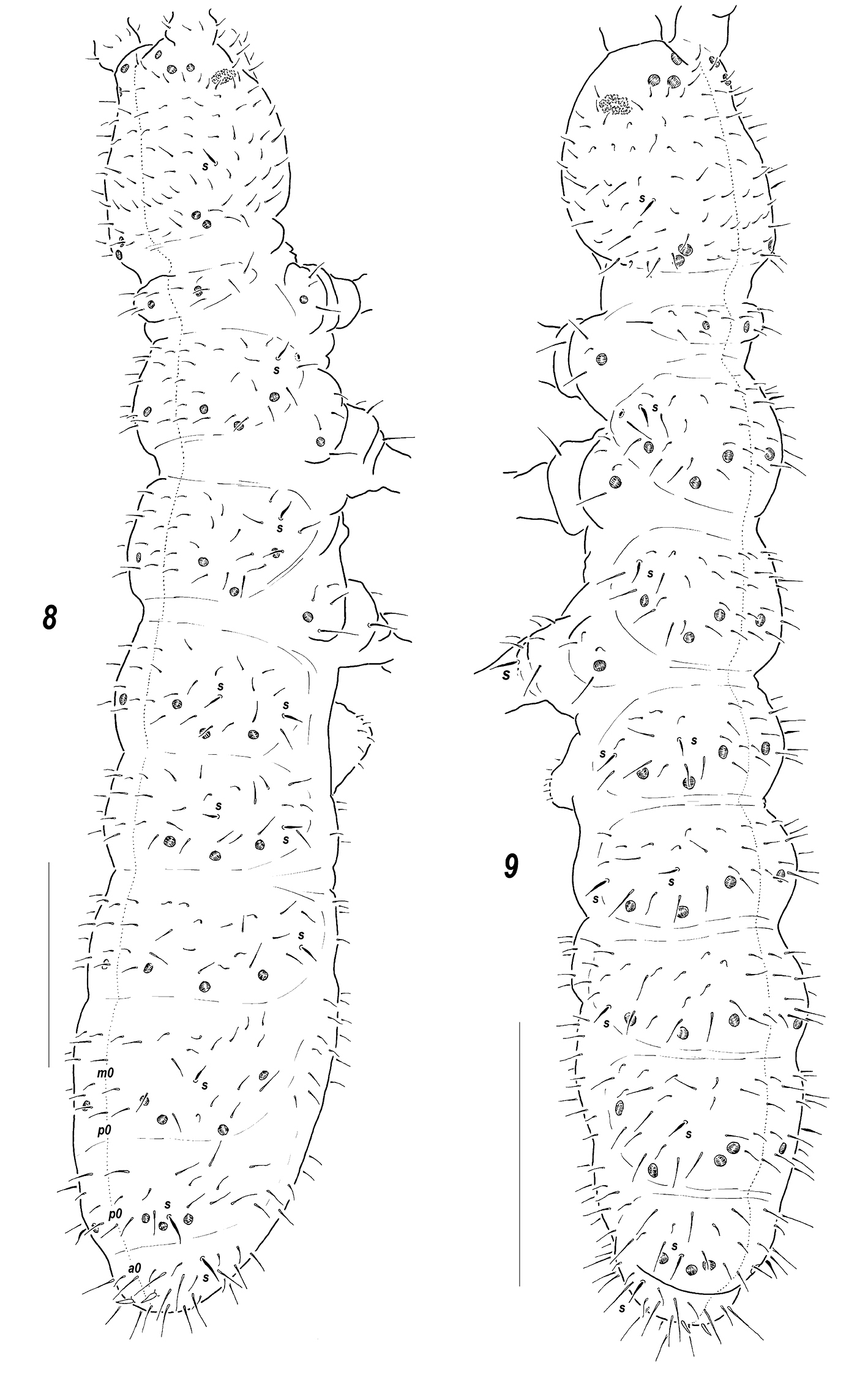
Body shape and size. All of the so far known species of Sensillonychiurus are among the smallest Onychiurinae, with body size ranging between 0.4 and 0.7 mm. The body is slender and elongated (Figs 8–9), with rather short antennae and clearly club-shaped Ant.4 (Fig. 10). Area antennalis is not distinctly demarcated.
Sensillar armature of the antennae.
Structure of the PAO. All species of Sensillonychiurus show a relatively wide PAO consisting of few (6–8) vesicles with numerous secondary lobes. As a whole, it usually looks like a single mass with only traces of vesicle divisions (Fig. 3).
Labrum. All congeners are characterized by a
constant number (7) of labral setae, four distal ones being longer and
clearly thicker, and two or four prelabral setae. The variant with two
prelabral setae seems to be more common (see Table 1), but this character is still unknown in Sensillonychiurus eisi, Sensillonychiurus virginis Pomorski & Sveenkova, 2006 and Sensillonychiurus geminus. Such a slightly reduced number of labral setae is also typical of all Thalassaphorura known for this character, as well as of the volinensis-group of Allonychiurus, but not of the flavescens-group, at least some of which showing nine labral setae (
Labium. The type of labium most frequently seen in the genus is AC, with the ABC-type is found only in two species, Sensillonychiurus mirus sp. n. and Sensillonychiurus vitimicus
sp. n. The number of setae on the proximal, basal and laterobasal
fields of the labium is more or less stable, although individual
variations and some asymmetry are visible in some specimens. The number
of distal guard setae of the labial palp corresponds to the most common
(and also complete) set found in Onychiurinae (
Dorsal and ventral pso. Contrary to the majority of Onychiurinae, the number of dorsal and ventral pso
does not significantly vary within the genus, being almost always as
following: 32/133/33343 (dorsal) and 1/000/0000 (ventral). There are
only two exceptions: Sensillonychiurus virginis, with a lesser number of pso on thoracic terga(32/022/33343 as a whole), and Sensillonychiurus geminus, with some pso on two abdominal sterna. The ventral pseudocellar formula of the latter species was given differently by
Parapseudocelli. The complete absence of parapseudocelli (psx) on the subcoxae, femora and abdominal sterna is characteristic of most of the studied species of the genus, except for Sensillonychiurus vegae sp. n. which sometimes possesses a pair of psx on Abd.4. Such a weak development of psx is rather frequent among Thalassaphorurini, also known in Micronychiurus, Agraphorura, Allonychiurus (in both flavescens- and volinensis-groups), and some Thalassaphorura. Probably it at least partly correlates with the small size of specimens. Some intraspecific variations of psx numbers are likely (see, for instance, description of Sensillonychiurus vegae sp.n.) and need further attention.
Dorsal chaetotaxy. The chaetotaxy in the genus was originally described as follows: “Seta d0 on the head absent. Abdominal terga of IV, V and VI with 2, 1 and 1 medial setae, respectively”. It can be added that these unpaired setae (m0 and p0 on Abd.4, p0 on Abd.5 and a0 on Abd.6)
are meso- or macrosetae probably belonging to the primary chaetotic
set, but not microsetae which can appear during ontogeny. Terga of Th.2-3 in adults with 3+3, of Abd.1-4 with 2+2 and of Abd.5
with 1+1, axial microsetae, additionally each tergum with 2+2
mesosetae in the axial group set out of line with microsetae (see, for
instance, Fig. 8).
The same pattern is found in all studied species which appear to have
an almost symmetrical (especially in the mid-section of terga) and
virtually identical dorsal chaetotaxy. This pattern seems to be unique
to Thalassaphorurini. Thus, Sensillonychiurus shares the absence d0 with only two genera of the tribe, Spinonychiurus and Detriturus. Known representatives of both these genera show different distributions of unpaired setae on the abdominal tip (
Tergal and sternal sensilla. The lateral microsensillum in all studied species is always present on Th.2, but usually absent from Th.3, except for two species, Sensillonychiurus minusculus Pomorski & Sveenkova, 2006 and Sensillonychiurus geminus. Several thickened macrosensilla in certain parts on terga and sterna are also very typical of Thalassaphorurini and of Sensillonychiurus as well. The most usual number of such sensilla in the studied species is as follows, 1/011/222111 from head to Abd.6 (Fig. 8), additionally two ventral sensilla are usually distinguishable on the anterolateral part of the head and one sensillum on each ventrolateral side of Abd.4 (Fig. 33). Variations are not frequent and somewhat obscure; the only clear exception being the European Sensillonychiurus mirus sp. n. which shows more dorsal sensilla (2/022/222221 as a whole). The described variability of the character in various genera of Thalassaphorurini permits to suggest that it can hardly be used in separating the genera. Moreover, the degree of sensillum differentiation varies widely both between and within species, being clearly age-dependent; sometimes the sensilla look like slightly thickened macrosetae distinguished only due to their positions. Some level of population variability of the character is not improbable either.
Ventral chaetotaxy. Most of the species of the genus lack setae on thoracic sterna. The only exception is Sensillonychiurus vitimicus sp. n., with 0-1-1 setae on each side of the linea ventralis on the thorax (Fig. 33). Among Thalassaphorurini, the complete absence of ventral setae on the thorax is only observed insome species of the genus Micronychiurus (Pomorski, pers. communication) and Agraphorura (
Tibiotarsal chaetotaxy
The pattern characteristic of all studied species of the genus can be
described as follows: seven or nine setae in the distal whorl (all or
two T-setae absent), 7-7-6 setae in B-whorl, Y-seta present, but M-seta absent (Figs 20, 29–30). The same pattern with 9 distal setae was previously found in Sensillonychiurus eisi by
Subdivision of sterna. Among Thalassaphorurini there is a genus, Spinonychiurus, characterized by such a unique feature as a secondary division of Abd.3 sternum. Some traces of such division can also be seen in all well preserved specimens of Sensillonychiurus (Fig. 6), as well as in some other small-sized species of various group of Onychiurinae. Nevertheless, the anterior subsegment in Sensillonychiurus is narrow and, contrary to Spinonychiurus, lacks setae.
Furcal remnant position. In complete agreement with the main diagnostic character of Thalassaphorurini, the furcal remnant in all studied Sensillonychiurus is in the form of a finely granulated area in the mid-section of Abd.4,
with four small setae arranged in two posterior rows. Individual
variations in number and position of these setae are not frequent, but
have been noted. The number of setal rows on manubrial area is also more
or less stable: usually two rows (mm and mp according to
Anal spines. A full spectrum from complete absence to strong spines set on low papillae is found among the studied species, but an intermediary situation is most frequent. The same is characteristic of Spinonychiurus and Micronychiurus, but not of Detriturus and Agraphorura (complete absence of spines), Thalassaphorura (AS absent as an exception) and Allonychiurus (spines always present).
Based on this review of the morphological features, the following can be concluded:
Regardless of one’s opinion on the status of the genus Sensillonychiurus, all studied species represent a rather homogeneous group of closely related forms, characterized by many common morphological features and seemingly congruent distributions mainly covering the northern parts of eastern Asia with insulated records from North America and Eastern Europe.
The genus Sensillonychiurus shares many characters with representatives of other genera of Thalassaphorurini, but a combination of characters seems to be unique for the tribe. The only features, which set the genus apart from all other Thalassaphorurini, appear to be not the number of guard setae in AO but dorsal chaetotaxy and anterior position of furcal remnant at a contact with border between Abd.3 and 4 although the data concerning other genera is still rather limited for a final decision.
Briefly, the genus can be defined as Thalassaphorurini featuring compound vesicles in the PAO, a partial reduction of guard setae in the AO and on the tibiotarsi, the absence of d0 on the head, anterior position of furca remnant and a clearly demarcated dorsal border between Abd.5 and 6.
Sensillonychiurus minusculus Pomorski & Sveenkova, 2006: 191, by original designation.
Small-sized Thalassaphorurini with low number of compound vesicles in PAO; labrum with 7 setae, labium of AC or ABC-type; AO with 4–5 papillae and 3–4 guard setae, smooth sensory clubs; distinct antennal, tergal and sternal sensilla, without d0 on head, Abd.4 with m0 and p0, Abd.5 with p0, Abd.6 dorsally with 1+1 prespinal microsetae and 1 medial macroseta; distal whorl of setae on Ti.1-3 with 7 or 9 setae, both M seta on all legs and B6 on Ti.3 absent; pso on Th.1 usually present, no tendency to dorsal pso multiplication, low number of sternal pso; psx usually absent; sternum of Abd.3 not clearly divided, furcal remnant situated at contact with border between Abd.3-4 sterna with two regular rows of manubrial setae set posteriorly to 4 dental setae; AS present or absent.
urn:lsid:zoobank.org:act:E9A79C2A-7B38-405D-8C45-7B8E67C0C22C
http://species-id.net/wiki/Sensillonychiurus_mirus
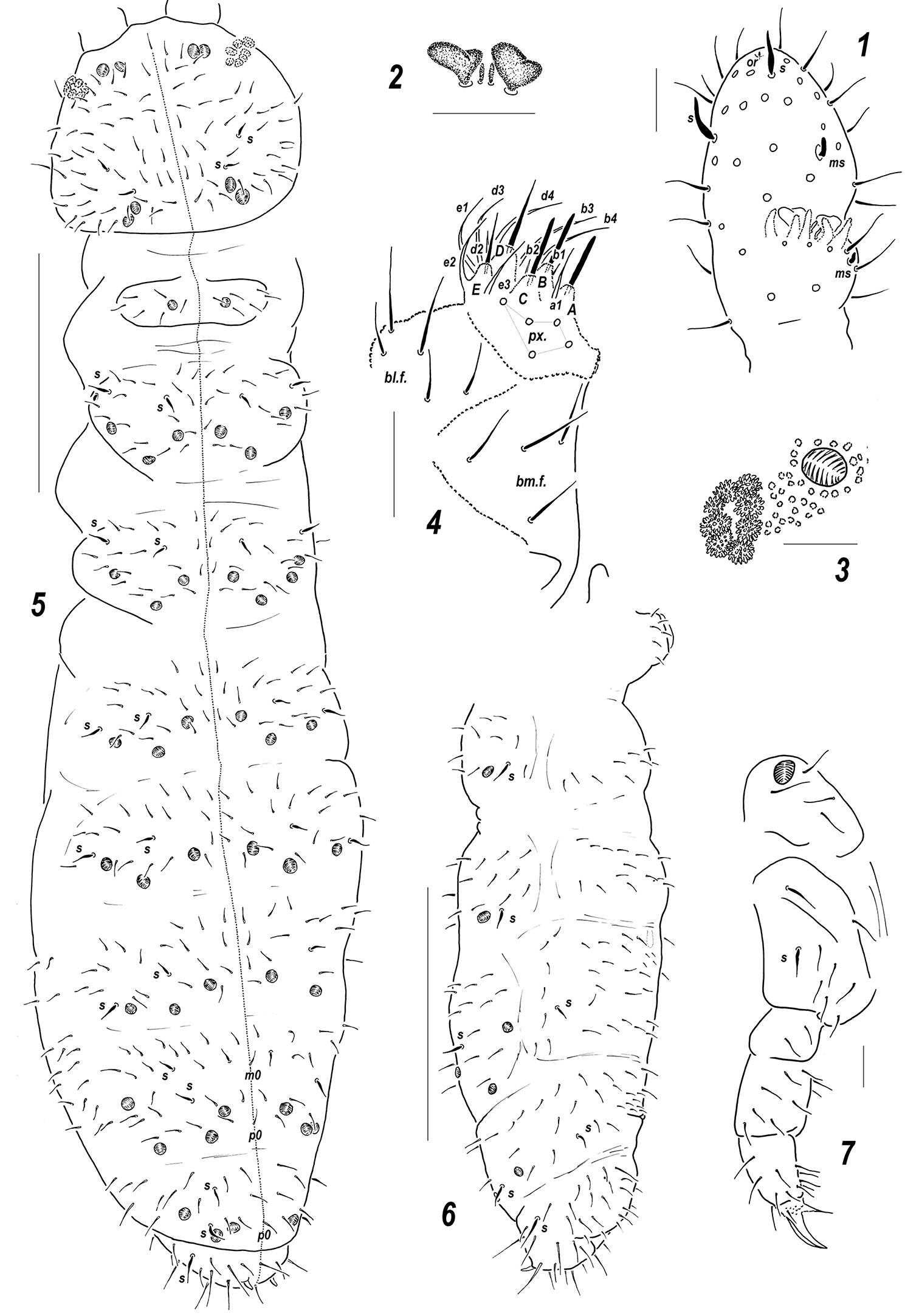
Figs 1–7Holotype ♀, Russia, NW of European part, Kola Peninsula, Dalnie Zelentsy [69°07'N, 36°03'E ], coastal sandy steep with sparse vegetation (flotation), 19.vii.2009, leg. A. Babenko (MSPU).
Paratypes 5 ♀, same data as holotype (MSPU).
Colour white. Size 0.56–0.60 mm. Body slender and elongated. Antennae about as long as head, antennal area not clearly demarcated. Ant.4 with two distinct thickened sensilla, subapical organite and basal microsensillum present (Fig. 1). Ant.3 organ consisting of 5 papillae, 2 sensory rods, 2 smooth and usually slightly bilobed sensory clubs (Fig. 2), 4 guard setae, and a lateral microsensillum (Fig. 1). Ant.1 and 2 with 7–8 and 12–13 setae, respectively. PAO with 7–8 composed vesicles (Fig. 3). Labrum with 7 setae and 4 prelabral ones. Apical part of labium with thick terminal setae on papillae A, B and C (ABC-type), 11 guard setae, a1 clearly longer and thicker than other spiniformguard setae, i.e. b1-2 and d2 (Fig. 4), and 5 proximal setae. Basal fields of labium (mentum and submentum) with 4 and 5 setae, hypostomal complex reduced to one long seta and a minute projection. Maxillary palp simple, with 2 sublobal setae.
Pseudocellar formula (pso) as follows, dorsal: 2(3)2/133/33343 (rarely some pso duplicated), ventral: 1/000/0000, parapseudocelli (psx) invisible. Each upper subcoxa with one pso. Localization of pso as in Fig. 5. Granulation fine and uniform, without areas of enlarged granules. Dorsal chaetotaxy almost symmetrical, setae smooth and clearly differentiated only on abdominal tip, in more anterior parts of body setae differing in shape but not in size: some of them straight, thick and blunt, others curved and pointed, sensilla distinct: 2/022/222221 (dorsal) and 2/000/00011 (ventral) (Figs 5–6), occasionally some additional mesosetae can be thickened and look like other sensilla, thickened sensillum present on coxae Lg.3 (Fig. 7). Th.1 with 6+6 setae as a rule. Lateral microsensilla present only on Th.2. Unpaired dorsal seta d0 on head absent, Abd.4 with m0 and p0, Abd.5 with p0, Abd.6 dorsally with one axial macroseta and 1+1 prespinal microsetae (Fig. 5). Thoracic sterna without setae along linea ventralis, ventral chaetotaxy of abdomen as in Fig. 6. Abd.3 sternum unclearly divided, anterior subsegment without setae. Furca reduced to a small area of fine granulation situated at contact with border between Abd.3-4 sterna, with 2+2 small posterior setae arranged in 2 rows, manubrial area with 4+4 setae set in two rows (Fig. 6). Ventral tube with 6+6 distal setae, proximal ones at corpus base absent. Upper subcoxae usually with 3-3-4, tibiotarsi with 17-17-16, setae: distal whorl with 9 setae (7 A and two T-setae), row B with 7-7-6 setae, setae M absent but Y present. Unguis simple, with neither inner nor lateral tooth, unguiculus with an indistinct basal lamella, shorter than unguis (Fig. 7). Anal spine short but rather strong, set on unclear papillae.
Sensillonychiurus mirus sp. n. 1 Ant.3–4; 2 sensorial elements of Ant.3 organ 3 PAO and adjacent pso 4 labium 5 dorsal chaetotaxy and pso distribution 6 abdomen, lateral view 7 Lg.3. Scales: 5–6 – 0.1 mm, 1–4, 7 – 0.01 mm.
Sensillonychiurus mirus sp. n. clearly differs from the all previously described species of the genus first of all in having not three but four guard setae in AO. Nevertheless it is not a unique character for the group. The same structure of AO (5 papillae and 4 guards) as in Sensillonychiurus mirus sp. n. is known in two other species of the genus, Sensillonychiurus vitimicus sp. n. and Sensillonychiurus amuricus sp. n. (see descriptions below). All these species which are characterized by only a weak reduction of AO with a highest possible number of papillae and 4 guard setae have many other characteristics in common (see Table 1.). Nonetheless, Sensillonychiurus mirus sp. n. can easily be distinguished from Sensillonychiurus vitimicus sp. n. by the complete absence of setae on thoracic sterna, from Sensillonychiurus amuricus sp. n. in the different type of labium (ABC in Sensillonychiurus mirus sp. n. versus AC in Sensillonychiurus amuricus sp. n.), and in four prelabral setae (Sensillonychiurus amuricus sp. n. possesses only two prelabral setae which are more common in the genus).
Main diagnostic characters of the known species of Sensillonychiurus
| Dorsal pso | Dorsal sensilla | AO papillae/guards | Position of ms on Ant.4 | Number of prelabral setae | Type of labium | ms on Th.3 | Number of setae on Th.1 | Ventral setae on thorax | Number of distal tibiotarsal setae | pso/psx on Abd.4 | Unguiculus / unguis ratio | Anal spines | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensillonychiurus eisi | 32/133/33343 | 1/011/22211 | 4/3 | low | ? | AC | ? | 5+5 | ? | 9 | ? | 0.3 | – |
| Sensillonychiurus minusculus | 32/133/33343 | 1/011/222111 | 4/3 | low | 2 | AC | + | 5+5 | – | 7 | – | 0.5 | – |
| Sensillonychiurus virginis | 32/022/33343 | 1/011/222111 | 4/3 | low | ? | AC | – | 5+5 | – | 9 | – | 0.33 | – |
| Sensillonychiurus geminus | 32/133/33343 | 1/011/222111 | 5/3 | low | ? | AC | + | 5+5 | – | 9 | pso | 0.75 | + |
| Sensillonychiurus mirus sp.n. | 2(3)2/133/33343 | 2/022/222221 | 5/4 | upper | 4 | ABC | – | 6+6 | – | 9 | – | ~0.5 | + |
| Sensillonychiurus taimyrensis sp.n. | 32/133/33343 | 1/011/221111 | 4/3 | upper | 2 | AC | – | 6+6 | – | 7 | – | ~0.6 | + |
| Sensillonychiurus vegae sp.n. | 32/133/33343 | 1/011/221111 | 4/3 | low | 2 | AC | – | 6+6 | – | 7 | –/psx | ~0.6 | + |
| Sensillonychiurus vitimicus sp.n. | 32/133/33343 | 1/011/221111 | 5/4 | upper | 4 | ABC | – | 6+6 | + | 9 | – | ~0.6 | + |
| Sensillonychiurus amuricus sp.n. | 32/133/33343 | 1/011/221111 | 5/4 | upper | 2 | AC | – | 6+6 | – | 9 | – | ~0.7 | + |
| Sensillonychiurus sp. | 32/133/33343 | 1/011/2222?11 | 4/3 | low | 2 | ? | – | 6+6 | – | 9 | – | ? | + |
Initially, the name mirus (odd, strange, unusual in Latin) reflects both an isolated position of the new species within the genus and the gap between its type-locality and the distributions of the other known species of the genus which are pure Asiatic or American. The level of morphological uncommonness has lowered after the performed survey of all available material, but the geographical isolation still exists.
Known only from the type locality.
urn:lsid:zoobank.org:act:AC031C4C-13EA-45F9-9DD5-575BD8287653
http://species-id.net/wiki/Sensillonychiurus_taimyrensis
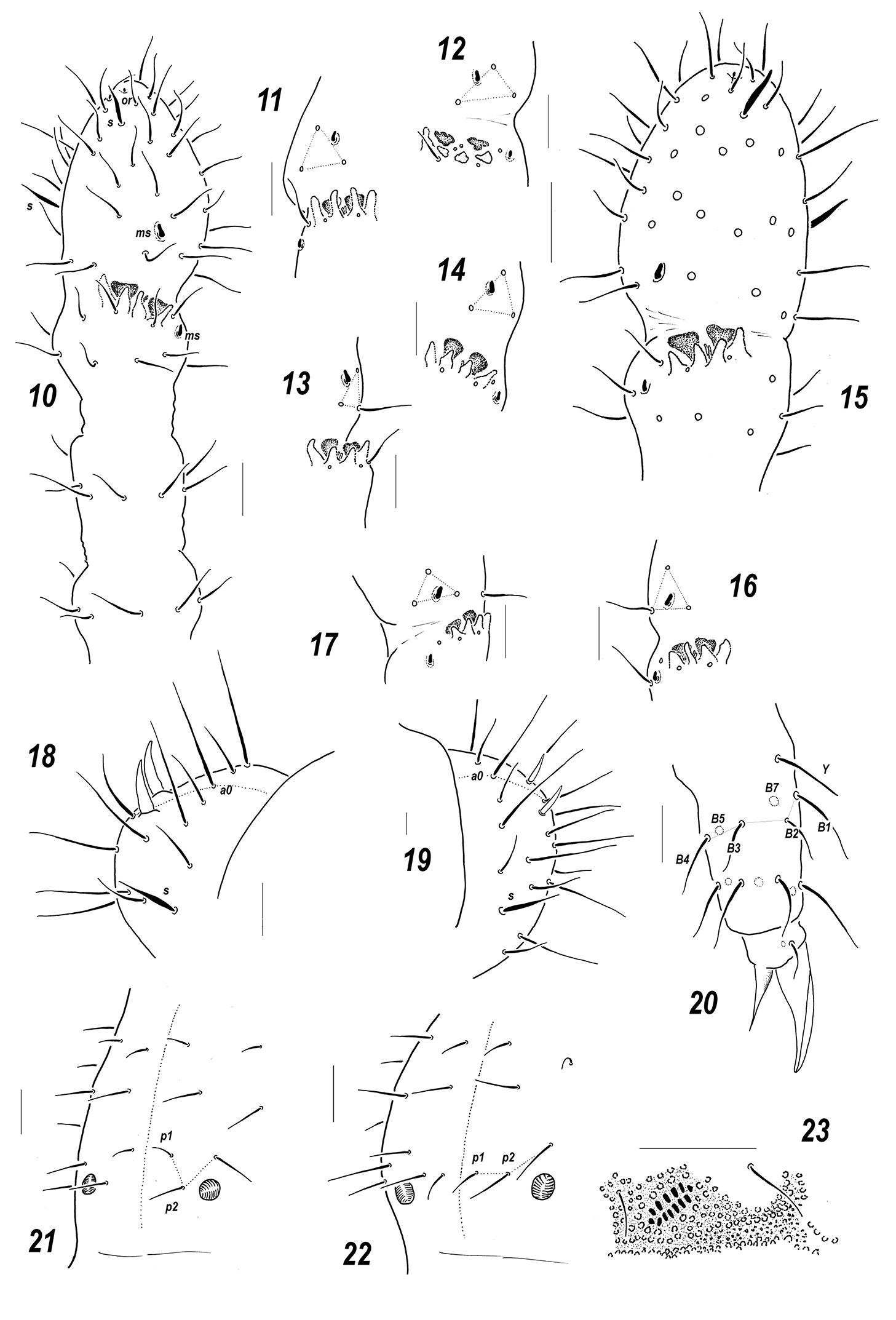
Figs 8, 10–14, 18, 21Holotype ♀, Russia, Taimyr Peninsula, northern coast of Taimyr Lake, Postoyannaya River [74°38'N, 101°55'E ], low river terrace, mosses, Dryas sp., Astragalus spp., 02.viii.1993, leg. A. Babenko (MSPU).
Paratypes 5 ♀ and 4 ♂, same data as holotype; 2 ♀ and 1 ♂, Taimyr Peninsula, northwestern coast of Lake Pyasino [70°04'N, 87°39'E ], herbaceous meadow on south-facing slope, sand, 03.viii.2001; 16 ♀, 10 ♂ and 6 juv., Taimyr Peninsula, middle reaches of Pyasina River, Ust-Tareya [73°15'N, 90°35'E ], herbaceous meadow on south-facing slope, 22.vii.2010, leg. A. Babenko (MSPU).
Other material. 1 ♀, Russia, Siberia, northwestern Buryatia, Ust’-Barguzin [53°25'N, 109°01'E ], Lake Baikal shore, sandy beach (ca 5 m from water edge, flotation), 21.viii.2008, leg. M. Potapov; 1 ♀, Russia, Siberia, Buryatia, Vitim Plateau, vicinity of Eravna (Sosnovo-Ozerskoe) [52°27'N, 111°09'E ], dry birch forest, 21.viii.2009, leg. A. Chimitova.
Colour white. Size 0.56–0.62 mm. Body slender and elongated. Antennae about as long as head, antennal area not clearly demarcated. Ant.4 with a subapical organite, two distinct thickened sensilla, and a subbasal microsensillum set well above proximal row of setae (Figs 10–14). Ant.3 organ consisting of 4 papillae, 2 sensory rods, 2 smooth sensory clubs, 3 guard setae, and a lateral microsensillum (Fig. 10). Ant.1 and 2 usually with 8 and 13 setae, respectively. PAO with 7(8) composed vesicles. Labrum with 7 setae and 2 prelabral ones (2/3-4). Apical part of labium of AC-type, with (5)6 proximal setae and usually with a complete set of guard setae (11), although asymmetrical absence of one of e-guard setae also visible, a1-guard long. Basal fields of labium (mentum and submentum) with 4 and 5 setae. Hypostomal complex with one long and one shorter projection. Maxillary palp simple, with 2 sublobal setae.
Pseudocellar formula (pso) as follows, dorsal: 32/133/33343, ventral: 1/000/0000, parapseudocelli (psx) invisible. Each upper subcoxa with one pso. Localization of pso as in Fig. 8. Granulation rather fine and uniform, without areas of clearly enlarged granules. Dorsal chaetotaxy almost symmetrical, setae smooth and clearly differentiated, especially on last abdominal terga, in anterior parts of body meso and microsetae similar in size but differing in shape: mesosetae straight and blunt, microsetae curved and pointed, sensilla more or less distinct on terga and less evident on sterna: 1/011/221-2111 (dorsal) (Fig. 8) and 2/000/0000-1 (ventral), sensillum on coxae of Lg.3 present but not distinct. Th.1 with 6+6 setae. Lateral microsensilla present only on Th.2. Unpaired dorsal seta d0 on head absent, Abd.4 with m0 and p0, Abd.5 with p0, Abd.6 with one axial macroseta (Figs 8, 18). Axial microsetae p1 set anteriorly to mesosetae p2 on Abd.1-3 (Fig. 21). Thoracic sterna without setae along linea ventralis. Abd.3 sternum unclearly divided, anterior subsegment without setae. Furca reduced to a small area of fine granulation situated at contact with border between Abd.3-4 sterna, with 2+2 small posterior setae arranged in two rows, manubrial area with 4+4 setae set in two rows. Ventral tube with 6+6 distal setae, proximal ones at corpus base absent. Upper subcoxae usually with 3-4-4, tibiotarsi with 15-15-14, setae: distal rows with 7 setae (all T-setae absent), row B with 7-7-6 setae, setae M absent but Y present. Unguis simple, with neither inner nor lateral tooth, unguiculus with an indistinct basal lamella, clearly shorter than unguis (about 0.6–0.65 U3).Anal spines short (0.7–0.75 U3) but rather thick (thickness/length 0.23–0.28), set on low papillae.
Dorsal chaetotaxy and pso distribution, Sensillonychiurus taimyrensis sp. n. 8 and Sensillonychiurus vegae sp. n. 9 Scale: 0.01 mm.
Sensillonychiurus taimyrensis sp. n. (10–14, 18, 21) and Sensillonychiurus vegae sp. n. (15–17, 19–20, 22–23) 10 antenna 11–17 position of ms on Ant.4, different views 18–19 Abd.6 20 tibiotarsus of Lg.3; 21–22 axial chaetotaxy of Abd.3 23 ventral psx on posterolateral part of Abd.4 (specimen from Vitim Plateau). Scale: 0.01 mm.
Apart from Sensillonychiurus taimyrensis sp. n., only two known species of the genus, i.e. Sensillonychiurus minusculus and Sensillonychiurus vegae sp. n., completely lack all T-setae on tibiotarsi (distal whorl with 7 setae). Sensillonychiurus minusculus clearly differs in having lateral ms on Th.3 and Abd.6 without AS. Two other species, Sensillonychiurus vegae sp. n. and Sensillonychiurus taimyrensis sp. n. are very similar, sharing many common characteristics (see Table 1). Nonetheless Sensillonychiurus taimyrensis sp. n. can be easily distinguished due to stronger AS set on low papillae (cf. Figs 18 and 19), more distal position of ms on Ant.4 (cf. Figs 10–14 and 15–17) and clear differences in the mutual position of microsetae p1 and mesosetae p2 on Abd.3 (cf. Figs 21 and 22).
The new species was named after its terra typica.
Despite a few records the new species is
probably widespread in eastern Siberia being found in such remote
regions as Taimyr’s tundras and mountainous Buryatia. Previously the
species was erroneously listed for Taimyr as Tantulonychiurus volinensis (Szeptycki, 1964) by
urn:lsid:zoobank.org:act:0086C2ED-D20C-45F3-A220-4F4D9383558B
http://species-id.net/wiki/Sensillonychiurus_vegae
Figs 9, 15–17, 19–20, 22–23Holotype ♂, Russia, eastern Siberia, Yakutia (Sakha Republic), mouth of Yana River, Shirokostan Peninsula, vicinity of Lake Ledyanoe [72°25'N, 141°00'E ], Dryas association on steep slope, 04.viii.1994, leg. A. Babenko (MSPU).
Paratypes 6 ♀, 1 ♂, and 1 juv., Russia, eastern Siberia, Yakutia (Sakha Republic), left bank of Kolyma River [69°32'N, 160°44'E ], grass (Elymus sibiricus) association on a polar fox hill, 19.viii.1994, leg. A. Babenko (MSPU).
Other material: 1♀ and 2♂, Russia, Siberia, northwestern Buryatia, Ust’-Barguzin [53°25'N, 109°01'E ], shore of Lake Baikal, pine forest on sandy dunes (flotation), 21.viii 2008, leg. M. Potapov; 2♀, 6♂ and 6 juv., Russia, Siberia, Buryatia, Vitim Plateau, vicinity of Eravna (Sosnovo-Ozerskoe) [52°27'N, 111°09'E ], pine forest with Rhododendron dauricum, 08.ix.2008, leg. A. Chimitova; 2 ♂, same region, but birch forest, 25.viii.2009, leg. A. Chimitova (MSPU).
Colour white. Size 0.40–0.52 mm, holotype 0.47 mm long. Body slender and elongated. Antennae about as long as head, antennal area not clearly demarcated. Sensillar armature of Ant.4 as usual: two distinct thickened sensilla, a subapical organite and a basal microsensillum set almost in line with proximal row of setae (Figs 15–17). Ant.3 organ consisting of 4 papillae, 2 sensory rods, 2 smooth sensory clubs, 3 guard setae, and a lateral microsensillum (Fig. 15). Ant.1 and 2 usually with 8 and 13(14) setae, respectively. PAO with 6–7(8) composed vesicles. Labrum with 7 setae and 2 prelabral ones (2/3–4), four setae of apical row thicker. Apical part of labium with thick terminal setae on papillae A and C (AC – type), (5)6 proximal setae and a complete set (11) of guard setae: 7 long [b3-4, d3-4, e1-3] and 4 spiniform [a1, b1-2 and d2] ones set on papillae, a1 clearly longer than others. Basal fields (mentum and submentum) with 4 and 5 setae. Maxillary palp simple, with two sublobal setae.
Pseudocellar formula (pso) as follows, dorsal: 32/133/33343, ventral: 1/000/0000, Abd.4 sterna with or without 1+1 parapseudocelli laterally (see Variability). Each upper subcoxa with one pso. Granulation fine and uniform, slightly enlarged granules rarely present around medial pso on abdominal tip and on head. Dorsal chaetotaxy almost symmetrical (Fig. 9), setae smooth and clearly differentiated, especially on last abdominal terga, in anterior parts of body meso and microsetae only slightly differing in size but different in shape: mesosetae straight and blunt, microsetae curved and pointed. Tergal sensilla (1/011/221111 in number) distinct, sternal ones (2/000/0000-1) hardly distinguished, sensillum on coxae of Lg.3 evident. Th.1 usually with 6+6 setae. Lateral microsensilla present only on Th.2. Unpaired dorsal seta d0 on head absent, Abd.4 with m0 and p0, Abd.5 with p0, Abd.6 with one axial macroseta (Figs 9, 18). Axial microsetae p1 lying almost in line with mesosetae p2 on Abd.3 (Fig. 22) and sometimes also on Abd.2. Thoracic sterna without setae along linea ventralis. Abd.3 sternum unclearly divided, anterior subsegment narrow and without setae. Furca reduced to a small area of fine granulation situated at contact with border between Abd.3-4 sterna, with 2+2 small posterior setae arranged in two rows, manubrial area usually with 4+4 setae set in two rows. Ventral tube with 6+6 distal setae, proximal ones at corpus base absent. Upper subcoxae usually with 3-4-4, tibiotarsi with 15-15-14, setae: distal rows with 7 setae (all T-setae absent), row B with 7-7-6 setae, setae M absent but Y present (Fig. 20). Unguis simple, with neither inner nor lateral tooth, unguiculus with an indistinct basal lamella, about 0.6 times as long as inner edge of U3.Anal spine rather long (0.6–0.7 U3) but thin (thickness/length 0.13–0.23) (Fig. 19), set without papillae.
The types of Sensillonychiurus vegae sp.n. completely lack psx as well as all so far studied species of the genus. Nonetheless, at least some of the specimens collected on Vitim Plateau possess 1+1 ventral parapseudocelli on Abd.4 (Fig. 23) being otherwise identical to the types. This population may represent a separate species, but its reliable distinction is hardly possible. Anyway, more material from different points of the distributional range is needed to evaluate the constancy and significance of this character.
Virtually all of the main morphological characteristics of Sensillonychiurus vegae sp. n. (structure of AO and PAO, labrum and labium, dorsal and ventral chaetotaxy, number and distribution of pso, presence of ms only on Th.2, number of setae on subcoxae, tibiotarsi and VT) are identical to those of sympatric Sensillonychiurus taimyrensis sp. n. Concerning the differences of Sensillonychiurus vegae sp. n. from Sensillonychiurus taimyrensis sp. n. see description of the latter.
The new species was initially collected during the joint Swedish-Russian expedition arranged in 1994 in order to commemorate A.E. Nordenskiöld’s first trip on “Vega” board along the Northern Sea Route (1878–1879). That is why it is named after Nordenskiöld’s famous steamship “Vega”.
Known from several remote areas of eastern Siberia. Previously, it was erroneously listed for Yakutia as Tantulonychiurus volinensis (Szeptycki, 1964) by
urn:lsid:zoobank.org:act:E99FF1BA-7739-4019-A89B-7F2E998A520F
http://species-id.net/wiki/Sensillonychiurus_amuricus
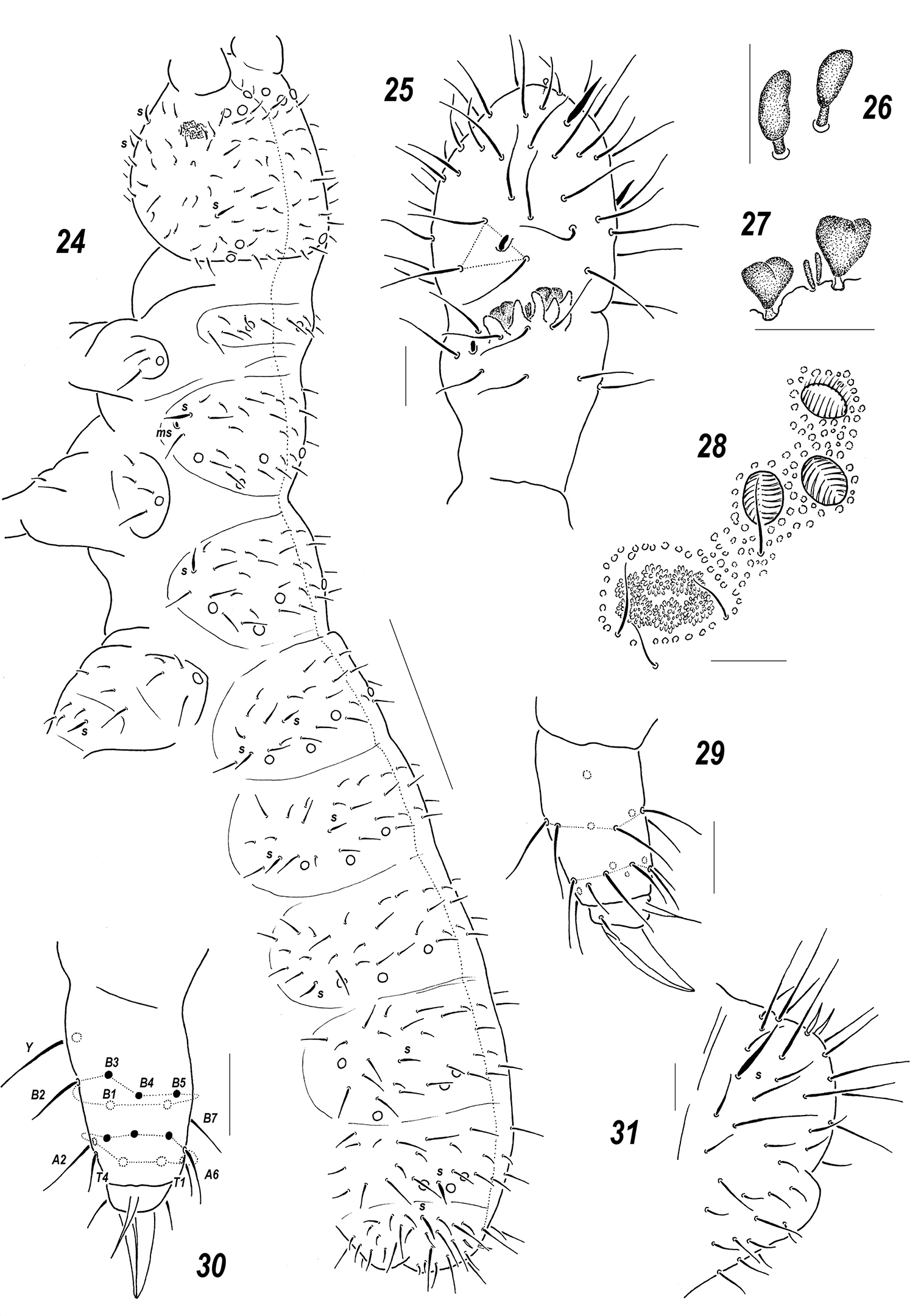
Figs 24–31Holotype ♀, Russia, Asiatic part, Khabarovsk suburbs, right bank of Amur river [48°33'N, 135°01'E ], upper part of sandy beach (flotation), 26 iv 2010, M. Potapov leg (MSPU).
Paratypes 3 ♀, 4 ♂ and 1 juv., same data as holotype (MSPU).
Colour white. Size of mature specimens 0.62–0.72 mm. Body slender and elongated. Antennae about as long as head, antennal area not clearly demarcated. Ant.4 with a subapical organite, two distinct thickened sensilla, and a subbasal microsensillum set well above proximal row of setae (Fig. 25). Ant.3 organ consisting of 5 papillae, 2 sensory rods, 2 smooth sensory clubs (Figs 26–27), 4 guard setae, and a lateral microsensillum (Fig. 25). Ant.1 and 2 usually with 8 and 13(14) setae, respectively. PAO with 6–7 composed vesicles (Fig. 28). Labrum with 7 setae and 2 prelabral ones (2/3–4). Apical part of labium with thick terminal setae on papillae A and C (AC – type), 7 long guard setae [b3-4, d3-4, e1-3] and 4 spiniform ones [a1, b1-2 and d2] set on low papillae, a1 clearly longer and thicker than b1. Proximal part of labium as usual, with 6 setae, basal fields (mentum and submentum) with 4 and 5 setae. Maxillary palp simple, with 2 sublobal setae.
Pseudocellar formula (pso) as follows, dorsal: 32/133/33343, ventral: 1/000/0000, parapseudocelli (psx) invisible. Each upper subcoxa with one pso. Localization of pso as in Fig. 24. Granulation rather fine and uniform, without areas of clearly enlarged granules. Dorsal chaetotaxy almost symmetrical, setae smooth and clearly differentiated, especially on last abdominal terga, differences between macro- and microsetae in anterior parts of body not so pronounced but visible: macrosetae more straight and blunt, microsetae curved and pointed. Dorsal sensilla distinct, flame-like, 1/011/221111 in number (Fig. 24), ventral ones (2/000/0001) slightly thickened and sometimes hard to detect, sensillum on coxae of Lg.3 distinct. Th.1 with 6+6 setae. Lateral microsensilla present only on Th.2. Unpaired dorsal seta d0 on head absent, Abd.4 with m0 and p0, Abd.5 with p0, Abd.6 with one axial macroseta (Fig. 24). Thoracic sterna without setae along linea ventralis. Abd.3 sternum unclearly divided, anterior subsegment without setae. Furca reduced to a small area of fine granulation situated at contact with border between Abd.3-4 sterna, with 2+2 small posterior setae arranged in two rows, manubrial area with 4+4 setae set in two rows. Ventral tube with 6+6 distal setae, proximal ones at corpus base absent. Upper subcoxae usually with 3-4-4, tibiotarsi with 17-17-16, setae: distal rows with 9 setae (2 T-setae absent), row B with 7-7-6 setae, setae M absent but Y present (Figs 29–30). Unguis simple, with neither inner nor lateral tooth, unguiculus with an indistinct basal lamella, shorter than unguis (ca 0.7 U3).Anal spines short (0.7–0.75 U3) and thin, set without papillae (Fig. 31).
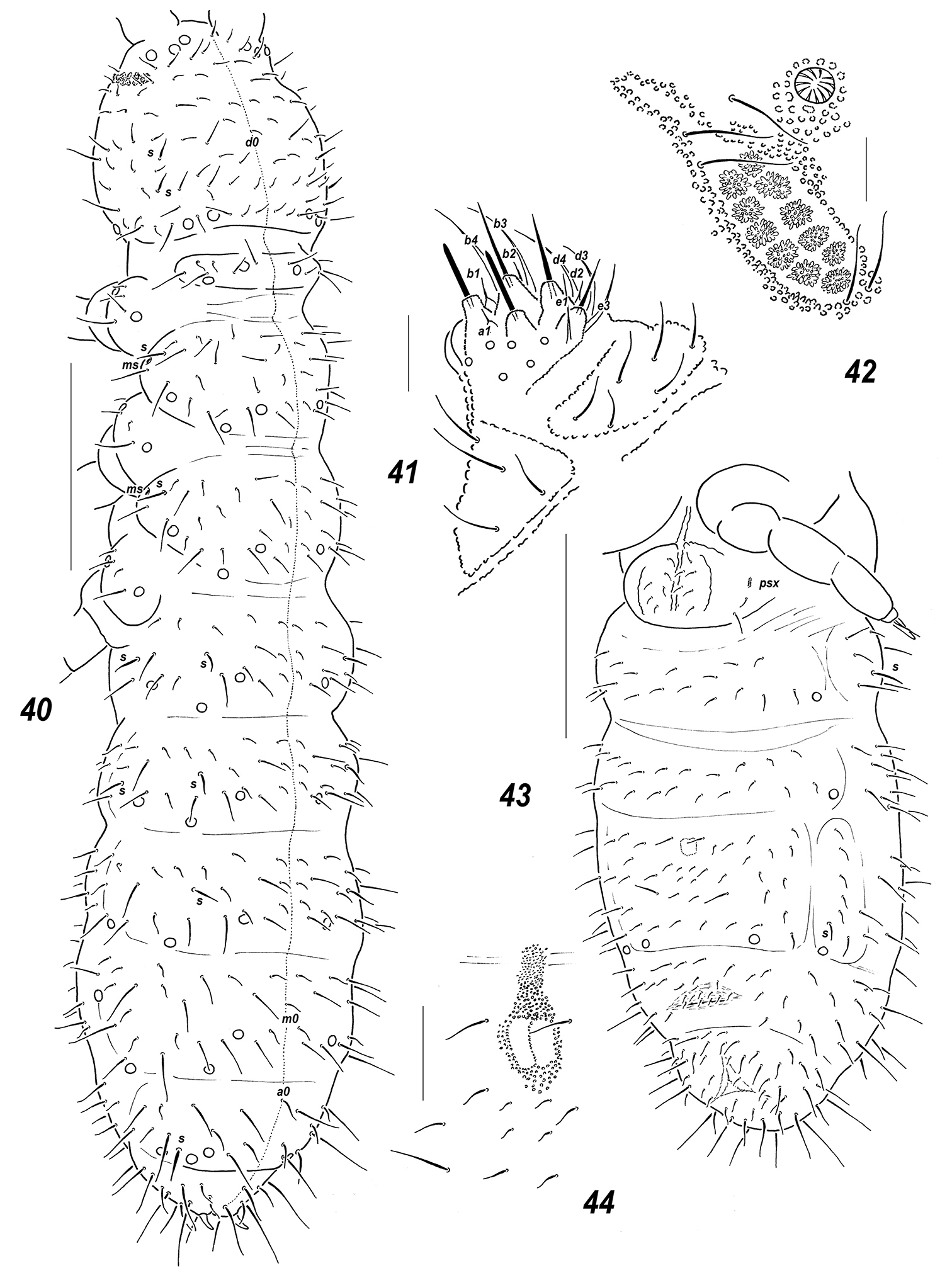
Sensillonychiurus amuricus sp. n. 24 dorsal chaetotaxy 25 Ant.3–4 26–27 sensorial elements of Ant.3 organ, different view 28 PAO and adjacent pso 29–30 tibiotarsus of Lg.3, different views 31 Abd.6. Scales: 24 – 0.1 mm, 25–31 – 0.01 mm.
The same structure of AO (five papillae and four guard setae) as in Sensillonychiurus amuricus sp. n. is only known in two species of the genus, Sensillonychiurus mirus sp. n. and Sensillonychiurus vitimicus sp. n. All these species which are characterized by only a weak reduction of AO with a full number of papillae and 4 guard setae also show the highest number of setae (9) in the distal tibiotarsal whorl. Both can easily be distinguished from Sensillonychiurus amuricus sp. n. in having a different type of the labium (ABC versus AC in Sensillonychiurus amuricus sp. n.) and four prelabral setae (Sensillonychiurus amuricus sp. n. possesses only two prelabral setae, which occurs more commonly in the genus). Apart from this, Sensillonychiurus amuricus sp. n. is the largest congener.
Two other species of the genus, Sensillonychiurus virginis and Sensillonychiurus geminus, are characterized by the most complete set of tibiotarsal setae (17-17-16) but against the background of a pronounced reduction of AO.
The new species was named after its terra typica.
Known only from the type locality.
urn:lsid:zoobank.org:act:1EB550A9-8192-4BEF-8826-93D1ABB96418
ßhttp://species-id.net/wiki/Sensillonychiurus_vitimicus
Figs 32–36Holotype ♂, Russia, Siberia, Buryatia, Vitim Plateau, vicinity of Telemba [52°44'N, 113°16'E ], larch forest with Betula fruticosa, 23.viii.2009, leg. A. Chimitova (MSPU).
Paratypes 7 ♀ and 3 ♂, same data as holotype; 1♀ same region but… larch forest with rich herbaceous cover, 04.x.2009, leg. A. Chimitova (MSPU).
Colour white. Size 0.58–0.68 mm (females), 0.50–0.58 (males). Body slender and elongated. Antennae about as long as head, antennal area not clearly demarcated. Ant.4 with 2 distinct thickened sensilla, a subapical organite and a basal microsensillum present, the latter set well above proximal row of setae (Fig. 34). Ant.3 organ consisting of 5 papillae, 2 sensory rods, 2 smooth sensory clubs, 4 guard setae, and a lateral microsensillum (Fig. 34). Ant.1 and 2 with 8 and (12)13 setae, respectively. PAO with 7–8 composed vesicles. Labrum with 7 setae and 4 prelabral ones. Labium of AC-type, but terminal setae on papillae C slightly thinner, guard setae as usual for genus: 7(6) long (b3-4, d3-4, e1-3) and 4 spiniform (a1, b1-2 and d2) ones, a1 clearly longer and thicker than others. Proximal part of labium with (5)6 setae, mentum and submentum with 4 and 5 setae, respectively. Maxillary palp simple, with 2 sublobal setae.
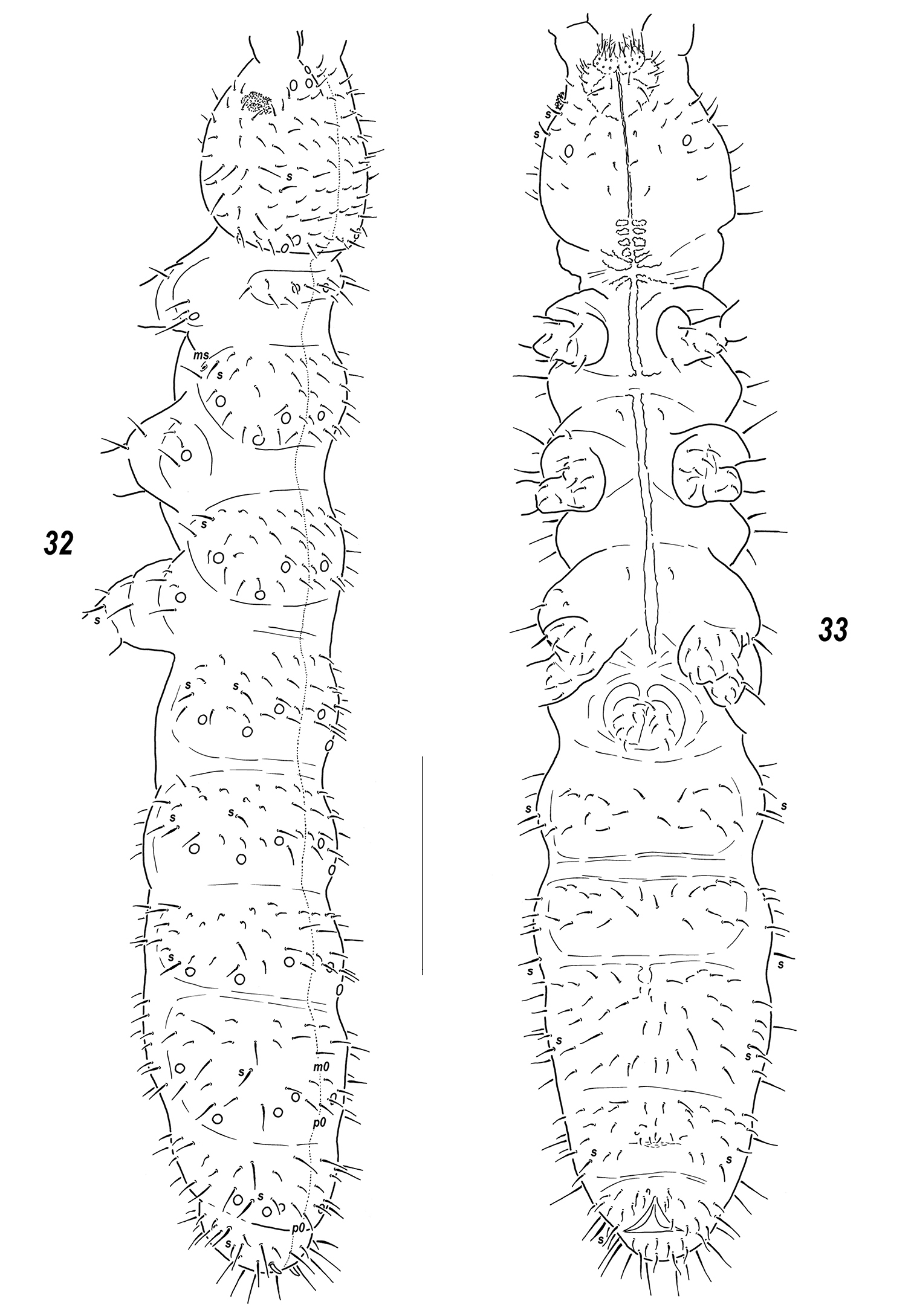
Pseudocellar formula (pso) as follows, dorsal: 32/133/33343, ventral: 1/000/0000, parapseudocelli (psx) invisible. Each upper subcoxa with one pso. Localization of pso as in Fig. 32. Granulation fine and uniform, slightly enlarged granules often present around pso on last abdominal terga. Dorsal chaetotaxy almost symmetrical, setae smooth and clearly differentiated especially on abdominal tip, in more anterior parts of body macro and microsetae mainly differing in shape, sensilla distinct on terga and less evident on sterna: 1/022/221111 (dorsal) and 2/000/00011 (ventral) (Figs 32–33), thickened sensillum present also on coxae of Lg.3. Th.1 with 6+6 setae. Lateral microsensilla present only on Th.2. Unpaired dorsal seta d0 on head absent, Abd.4 with m0 and p0, Abd.5 with p0, Abd.6 dorsally with one axial macroseta and 1+1 prespinal microsetae (Fig. 32). Thoracic sterna with 0-1-1 setae on each side of linea ventralis, ventral chaetotaxy of abdomen as in Fig. 33. Abd.3 sternum unclearly divided, anterior subsegment without setae. Furca reduced to a small area of fine granulation situated at contact with border between Abd.3-4, with 2+2 small posterior setae arranged in two rows, manubrial area with 4+4 setae set in two rows (Fig. 33). Ventral tube with 6+6(7) distal setae, proximal ones at corpus base absent. Upper subcoxae usually with 3-(3)4-4, tibiotarsi with 17-17-16 setae: distal rows with 9 setae (7 A and two T-setae), row B with 7-7-6 setae, setae M absent but Y present (Fig. 36). Unguis simple, with neither inner nor lateral tooth, unguiculus with indistinct basal lamella, clearly shorter than unguis (Fig. 36).Anal spine rather strong (about as long as 0.6–0.7 U3), set on unclear papillae (Fig. 35).
Sensillonychiurus vitimicus sp. n. 32 dorsal chaetotaxy 33 ventral chaetotaxy. Scale: 0.1 mm.
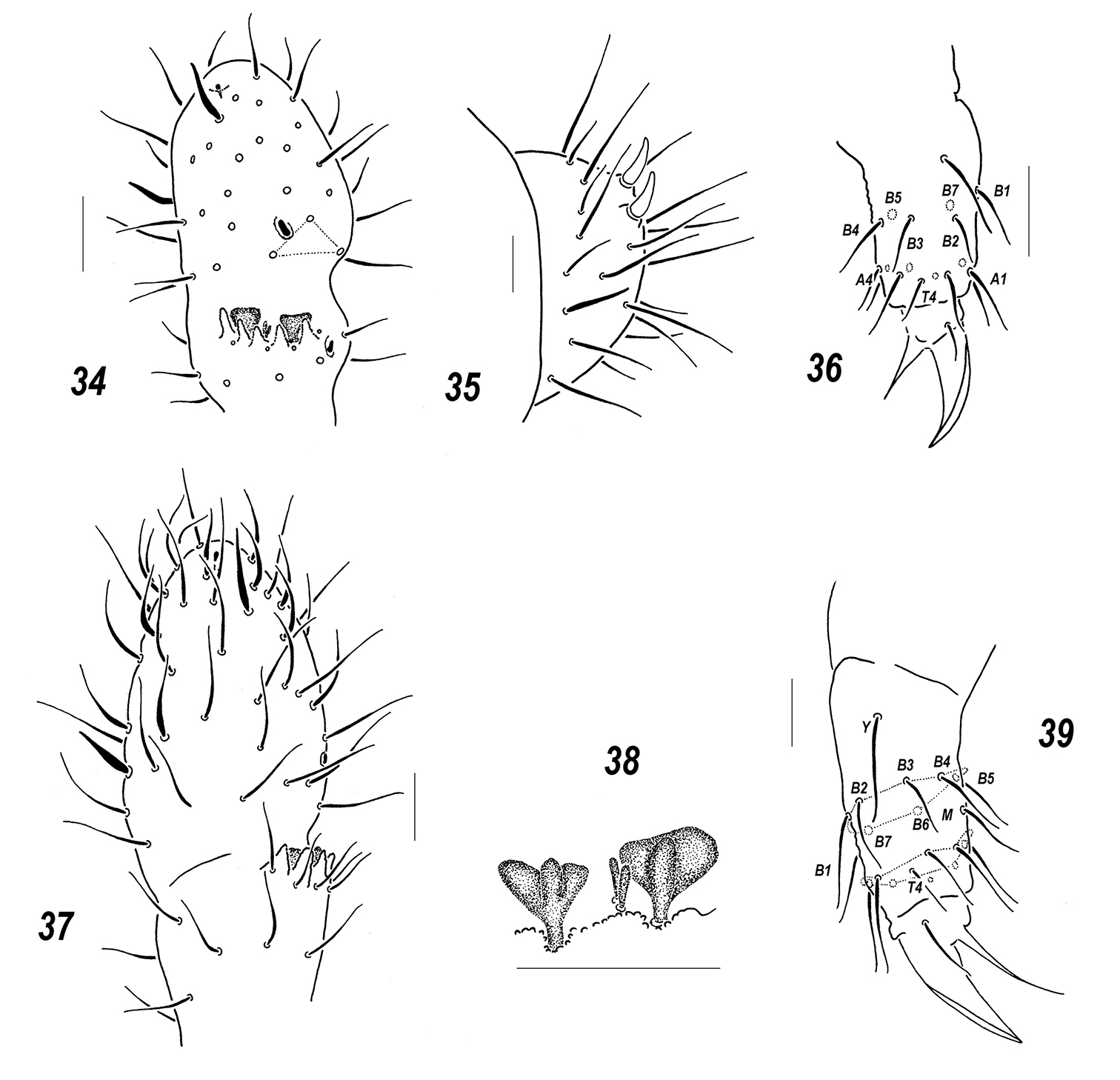
Sensillonychiurus vitimicus sp. n. (34–36) and Allonychiurus elikonius sp. n. (37–39) 34, 37 Ant.3-4 35 Abd.6 36, 39 tibiotarsus of Lg. 3, different views 38 sensorial elements of Ant.3 organ. Scale: 0.01 mm.
Due to the presence of four guard setae in AO, Sensillonychiurus vitimicus sp. n. is the most similar to Sensillonychiurus mirus sp. n. and Sensillonychiurus amuricus sp. n. All these three species have many other characteristics in common (see Table 1), but Sensillonychiurus vitimicus sp. n. can easily be distinguished by the presence of setae on thoracic sterna (a presumed apomorphic condition within Onychiuridae according to
The new species was named after its terra typica.
Known from several biotopes in vicinity of the type locality.
One more species of the genus Sensillonychiurus was found on Kamchatka (vicinity of Petropavlovsk, sandy sea beach with weed debris, leg. L. Lobkova). It differs from Sensillonychiurus virginis in having setiform anal spines, from Sensillonychiurus geminus by the absence of lateral ms on Th.3. The lack of material (only a single female is available) did not allow us to describe it, but it is listed in the key and in Table 1 as Sensillonychiurus sp.
Key to the known species of Sensillonychiurus Pomorski & Sveenkova, 2006
| 1 | AS not differentiated | 2 |
| – | AS present | 4 |
| 2 | Tibiotarsi with 7 distal setae | Sensillonychiurus minusculus Pomorski & Sveenkova, 2006 |
| – | Tibiotarsi with 9 distal setae | 3 |
| 3 | Dorsal pso as 32/022/33343 | Sensillonychiurus virginis Pomorski & Sveenkova, 2006 |
| – | Dorsal pso as 32/133/33343 | Sensillonychiurus eisi (Rusek, 1976), comb. n. |
| 4 | Tibiotarsi with 9 distal setae | 5 |
| – | Tibiotarsi with 7 distal setae | 9 |
| 5 | Both Th.2-3 with lateral ms, ventral pso on Abd.4 present [1/000/0101 as a whole] | Sensillonychiurus geminus Pomorski & Sveenkova, 2006 |
| – | Only Th.2 with lateral ms, Abd.4 without ventral pso [1/000/0000 as a whole] | 6 |
| 6 | AO with 5 papillae and 4 guard setae (Figs 1, 25) | 7 |
| – | AO with 4 papillae and 3 guard setae (as in Fig. 10) | Sensillonychiurus sp. |
| 7 | Thorax with ventral setae | Sensillonychiurus vitimicus sp. n. |
| – | Thorax without ventral setae | 8 |
| 8 | Labium of the ABC type (Fig. 4), 4 prelabral setae present | Sensillonychiurus mirus sp. n. |
| – | Labium of the AC type, only two prelabral setae present | Sensillonychiurus amuricus sp. n. |
| 9 | AS strong, set on low papillae (Fig. 18), ms on Ant.4 clearly above proximal setae (Figs 10–14), microsetae p1 set anteriorly to mesosetae p2 on all terga from Abd.1 to Abd.3 (Fig. 21) | Sensillonychiurus taimyrensis sp. n. |
| – | AS as thick short setae (Fig. 19), ms on Ant.4 almost in line with proximal setae (Figs 15–17), microsetae p1 set in line with p2 on Abd.3 (Fig. 22) | Sensillonychiurus vegae sp. n. |
http://species-id.net/wiki/Allonychiurus
Onychiurus flavescens Kinoshita, 1916: 458, by original designation.
Small- or medium-sized Thalassaphorurini with compound vesicles in PAO; labrum with 7 or 9 setae, labium of AC or ABC-type; AO with 4–5 papillae and 5 guard setae, smooth or granulated sensory clubs; antennal and tergal sensilla usually distinct, d0 on head present, Abd.4 and 5 usually with some axial microsetae, Abd.6 dorsally with 2+2 prespinal microsetae and 1–2 medial macrosetae; distal whorl on Ti.1-3 with 7, 9 or 11 setae, B-whorlusually complete on all tibiotarsi, M seta present; no tendency to dorsal pso multiplication, head and abdominal sterna with ventral pso, dorsal pso on Th.1 usually present; psx not numerous or absent; sternum of Abd.3 not subdivided, furcal remnant situated at some distance from border between Abd.3-4 sterna, with one or several rows of manubrial setae posterior to dental setae; MVO present or absent; AS present.
As it was already stressed in Introduction the genus is accepted here in a wider scope than it was proposed by
urn:lsid:zoobank.org:act:6843EC79-00D9-4039-96E1-D5088E2ACA99
http://species-id.net/wiki/Allonychiurus_elikonius
Figs 37–44Holotype ♀, Russia, Yakutia (Sakha Republic), Suntar-Khayata Mt Range, upper reaches of Kyubyume River [63°13'N, 139°32'E ], 1, 300 m alt., sandbank in Elikon River bed (flotation), 06.vii.2002, leg. O. Makarova (MSPU).
Paratypes: 22 females on slides and more than 300 specimens in alcohol, same data as holotype; 11 females, same region, 1, 480 m alt., plant community with predominance of Dryas sp. on slope, 07.vii.2002; 7 females, same region, 1, 430 m alt., herbaceous meadow on south-facing slope, 07.vii.2002; 14 females on slides and more than 800 specimens in alcohol, same region, greenhouse of “Vostochnaya” Meteorological Station, 1, 287 m alt., 24.vii.2002, leg. O. Makarova (MSPU).
Colour white. Size 0.72–0.84 mm. Body slender and elongated. Antennae about as long as head, antennal area not clearly demarcated. Ant.4 rather long and narrow, with several curved and slightly thickened sensilla, 2 of which (dorso-subapical and inner-subbasal) straighter and especially distinct, a subapical organite small, usually spherical, a basal microsensillum present (Fig. 37). Ant.3 organ consisting of 4 (or rarely 4+5) low papillae, 2 sensory rods, 2 smooth sensory clubs with ribs (Fig. 38), 5 guard setae, and a lateral microsensillum (Fig. 37). Ant.1 and 2 as a rule with 9 and 12–13 setae. PAO with 10–12 composed vesicles set at some distance from each other (Fig. 42). Labrum with 7 setae and 4 prelabral ones. Apical part of labium with thick terminal setae on papillae A and C (AC-type), 6 long (b3-4, d3-4, e1, 3; e2 absent) and 4 spiniform (a1, b1-2 and d2), guard setae, a1shorter than others (Fig. 41). Proximal field of labium usually with 6 setae, basal fields (mentum and submentum) with 4 and 6 setae. Maxillary palp simple, with 2 sublobal setae.
Pseudocellar formula (pso) as follows, dorsal: 32/233/33343, ventral: 11/000/0112, additionally one parapseudocellus (psx) present on each side of VT anteriorly to basal setae (Fig. 43). Each upper subcoxa with two pso. Localization of pso as in Figs 40, 43. Granulation fine and uniform, without areas of enlarged granules. Dorsal chaetotaxy almost symmetrical, setae smooth and clearly differentiated, especially on abdominal tip, sensilla not always distinct, sometimes hard to detect, particularly so on sterna and medially on Abd.1-3: 2/011/222010 (dorsal) and 2/000/00010 (ventral) (Fig. 40), a thickened sensillum on coxae of Lg.3 present. Th.1 with 5-6(7) setae on each side. Terga of Th.2-3 and Abd.1-3 with 3+3, Abd.4 with 2+2 and Abd.5 with 1+1, axial microsetae. Lateral microsensilla present on both Th.2-3. Unpaired dorsal setae: d0 on head, microseta m0 on Abd.4, microseta a0 on Abd.5, and 2 macrosetae a0 and m0 on Abd.6, supplemented by 2+2 prespinal microsetae (Fig. 40).
Sterna of Th. 2-3 with 1+1 setae along linea ventralis, ventral chaetotaxy of abdomen as in Fig. 43. Abd.3 sternum unclearly divided, anterior subsegment without setae. Furca reduced to a small area of fine granulation situated at some distance from border between Abd.3-4, with 2+2 small posterior setae arranged in 2 rows (Fig. 44), manubrial area with 4+4 setae arrange in 2 rows but only one of them set posteriorly to small dental setae (Fig. 43). Ventral tube with (5)6+6 distal setae and 2 proximal ones at corpus base. Upper subcoxae with (3)4-4-4, tibiotarsi with 18-18-18, setae: distal whorl with 9 setae (7 A and 2 T-setae), 7 setae in row B on each leg, setae M and Y present (Fig. 39). Unguis simple, with neither inner nor lateral tooth, unguiculus narrow with a long apical filament, latter usually reaching slightly beyond unguis (Fig. 39).Anal spine thick and slightly curved, set on unclear papillae.
Allonychiurus elikonius sp. n. 40 dorsal chaetotaxy 41 labium 42 PAO and adjacent pso 43 ventral chaetotaxy 44 furcal remnant. Scales: 40, 43 – 0.1 mm, 41-42, 44 – 0.01 mm.
The main morphological features of Agraphorura elikonius sp. n. are similar to those of Agraphorura volinensis, Agraphorura subvolinensis sp. n. and Agraphorura asiaticus (Babenko, 2007), comb. n. (see Table 2). Thus, all four species are characterized by virtually identical dorsal chaetotaxy and similar numbers of pso on all terga, sterna and subcoxae. The presence of a complete set of B-setae and M-seta on all tibiotarsi is also shared by them. Agraphorura elikonius sp. n. has a different type of the labium (AC in Agraphorura elikonius sp. n. versus ABC in three other species) and differs from Agraphorura volinensis and Agraphorura subvolinensis in the mutual position of antennal pso (cf. Figs 40 and 45). There are also some variations of the number of distal setae on the tibiotarsi in these four species (7 setae in Agraphorura volinensis and Agraphorura asiaticus, 9 in Agraphorura elikonius and Agraphorura subvolinensis). Agraphorura asiaticus is the only species in the group showing five papillae in AO (found in elikonius only in exceptional cases), and only Agraphorura subvolinensis is characterized by the presence of setae on all thoracic sterna (absent from Th.1 in all other species).
Main diagnostic characters of the known species of the volinensis-group of Allonychiurus
| Dorsal pso | Ventral pso | pso on upper subcoxae | AO papillae/ guards | Ventral setae on thorax | Dorsal sensilla | Type of labium | Number of prelabral setae | Number of distal setae on tibiotarsi | Unguiculus / unguis ratio | MVO position | Number of setae on VT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allonychiurus volinensis | 32/233/33343 | 11/000/1112‡ | 2-2-2 | 4/5 | 0-1-1 | 1/011/222121 | ABC | 4 | 7 | 0.9-1.1 | Abd.4 | 1/6/2 |
| Allonychiurus foliatus | 32/233/33323† | 01/000/0000 [?] | 1-2-2 | 4/5 | ? | ? | ? | ? | ? | 0.75 | VT + genital plate | ?/6/? |
| Allonychiurus mariangeae | 32/233/33343 | 11/000/1112 | 2-2-2 | 4/5 | 1-1-1 | 2/011/111111 | ? | ? | 9 | 0.75 | genital plate | 0/6/2 |
| Allonychiurus donjiensis | 22/222/22222 [?] | 11/200/0011 [?] | 1-1-1 | 4/5 | ? | ? | ? | ? | ? | 0.75 | ? | ?/6/? |
| Allonychiurus jindoensis | 32/233/33333 | 10/000/0102 | 1-1-1 | 4/5 | ? | ? | ? | ? | ? | 0.75 | ? | ?/6/? |
| Allonychiurus asiaticus | 32/233/33343 | 11/000/0112 | 2-2-2 | 5/5 | 1-1-1 | 1/011/222221 | ABC | 4 | 7 | 0.7-0.8 | absent | 1/6/2 |
| Allonychiurus elikonius sp. n. | 32/233/33343 | 11/000/0112 | 2-2-2 | 4(5)/5 | 0-1-1 | 2/011/222010 | AC | 4 | 9 | 0.9-1.1 | males unknown | 0/6/2 |
| Allonychiurus subvolinensis sp. n. | 32/233/33343 | 11/000/1112 | 2-2-2 | 4/5 | 1-1-1 | 1/011/222111 | ABC | 4 | 9 | ~0.9 | Abd.4 | 1/6/2 |
| Allonychiurus unisetosus sp.n. | 32/233/33343 | 11/000/0111 | 2-2-2 | 4/5 | 0-1-1 | 1/011/222121 | ABC | 2 | 9 | 0.9-1.1 | Abd.4 [?] | 1/6/2 |
† According to the original description, the species is characterized by 33/233/33323 dorsal pso and complete absence of ventral pso; most lateral pso on posterior side of a head are considered here as being ventral.‡ Slightly different formula of ventral pso, i.e. 11/000/0112, is given by
It is more difficult to distinguish Allonychiurus elikonius sp. n. from three Korean and one Chinese species of the group, namely Allonychiurus mariangeae (Thibaud & Lee, 1994), Allonychiurus donjiensis (Lee & Kim, 1994), Allonychiurus jindoensis (Lee & Kim, 1994), and Allonychiurus foliatus (Rusek, 1967), because their descriptions are incomplete and probably not fully correct in certain details. The most complete description is that of Allonychiurus mariangeae. It is rather similar to Allonychiurus elikonius sp. n. in having an almost identical chaetotaxy, the same number of dorsal pso and tibiotarsal setae (see Table 2). The only difference of the sternal pso formula is the presence of true pseudocellus on Abd.1 in Allonychiurus mariangeae instead of an elongated parapseudocellus without clear cuticular ring in Allonychiurus elikonius sp. n. However, these organs are homologous and sometimes difficult to distinguish. The most characteristic feature of Allonychiurus mariangeae is the presence of MVO in mature males. Unfortunately, Allonychiurus elikonius sp. n. in the region under study is only represented by parthenogenetic populations: among more than 100 specimens checked, all were females. Formally, these species differ in size (0.75–0.83 mm in Allonychiurus elikonius sp. n. versus 0.5–0.65 mm in Allonychiurus mariangeae), in the absence of ventral setae on Th.1 in elikonius, in the different number of setae on Ant.1 (9 in Allonychiurus elikonius versus 8 in Allonychiurus mariangeae), by unguiculus length (equal to or slightly longer than unguis in Allonychiurus elikonius versus 0.75 of U3 in Allonychiurus mariangeae), and by the absence of a0 on Abd.5 in Allonychiurus mariangeae, but all these characters are probably variable.
Three remaining species of the volinensis-group were described as having a lesser number of dorsal and ventral pso (see Table 2). Yet this probably needs verification. In any case, clear differences in the ecological preferences of compared species confirm the specificity of Allonychiurus elikonius sp. n. The monsoon subtropical climate of southern Korea (the habitats of Allonychiurus mariangeae, Allonychiurus donjiensis, and Allonychiurus jindoensis are sand beaches) and central China (vicinity of Shanghai, the only known locality of Allonychiurus foliatus) has nothing to do with the extremely continental conditions of mountainous Yakutia (about 160 km from Oymyakon, one of the coldest places on Earth), where Allonychiurus elikonius sp. n. was found. Nevertheless, the probability that some of these nominate species can probe to be conspecific with Allonychiurus elikonius sp. n. cannot be completely ruled out until their adequate redescriptions.
The new species was named after its type-locality, Elikon River.
Still known only from the region of the type-locality, where it inhabits a number of different communities up to 1, 500 m alt.
urn:lsid:zoobank.org:act:2400049E-3FC5-4642-AD7D-05683CA7F275
http://species-id.net/wiki/Allonychiurus_subvolinensis
Figs 45–49Holotype ♂, Russia, Tuva Republic, northern macroslope of Eastern Tannu-Ola Mt Range, 5 km S of Lake Chagytai [51°00'N, 94°43'E ], larch forest belt, 1, 300 m alt., under larch (Larix sibirica), 16.vi.2003, leg. S.K. Stebaeva (MSPU).
Paratypes ♂, same region and locality, ca 1, 400 m, 17.vi.2003; ♀, same region, meadow steppe, ca 1, 200 m alt., under Dracocephalum ruyschiana, 17.vi.2003; 6♀ and 3♂, Russia, Tuva Republic, southern macroslope of Eastern Tannu-Ola Mt Range, 20 km N of Khol’-Oozhu [50°44'N, 94°23'E ], 1, 600 m alt., meadow steppe, under Spiraea sp., 16.vii.1993; 7 specimens, Russia, Tuva Republic, foothills of southern macroslope of Eastern Tannu-Ola Mt Range, basin of Aryskannyg-Khem River, 15 km E of Khol’-Oozhu [50°41'N, 94°35'E ], ca 1, 100-1, 250 m alt., dry steppe, under Nanophyton grubovii, 17.vii.1993; 12♀ and 10♂, Russia, Tuva Republic, Sangelen Plateau, 25–30 km NE of Erzin [50°15'N, 95°09'E ], ca 1, 000 m alt., upper terrace of Erzin River, steppe with Caragana spinosa, 03viii.1995, all leg. S.K. Stebaeva (MSPU).
Colour white. Size 0.55–0.62 mm. Body slender and elongated, slightly wider in region of Abd.4. Antennae about as long as head, antennal area not clearly demarcated. Ant.4 rather short and wide, 2 usual sensilla not especially thickened but distinct, a subapical organite and a basal microsensillum present. Ant.3 organ consisting of 4 low papillae, 2 sensory rods, 2 smooth sensory clubs without clear ribs, 5 guard setae, and a lateral microsensillum. Ant.1 and 2 with 8 and (12)13 setae, respectively. PAO wide (length/width ratio ca 1.5), with about 7–10 composed vesicles set close together. Labrum as a rule with 7 setae and 4 prelabral ones, but holotype with an abnormal number of setae set asymmetrically. Apical part of labium with thick terminal setae on papillae A, B and C (ABC – type), seta A clearly thicker, 6 long (e2 absent) and four spiniform (a1, b1-2 and d2), guard setae, a1shorter than others. Proximal field of labium with 5 setae, basal fields (mentum and submentum) with 4 and 5 setae. Maxillary palp simple, with 2 sublobal setae.
Pseudocellar formula (pso) as follows, dorsal: 32/233/33343 (Fig. 45), ventral: 11/000/1112. Each upper subcoxa with two pso. Granulation fine and uniform, without areas of enlarged granules. Dorsal chaetotaxy more or less symmetrical, setae smooth and rather thick, clearly differentiated only on abdominal tip, sensilla: 1/011/222111 (dorsal) and 2/000/00010 (ventral), but distinguishable mainly because of their stable positions, only lateral ones on Th.2-Abd.1 and posterior one on Abd.5 always distinct (Fig. 45), as well as a sensillum on coxae of Lg.3. Th.1 with 5+5(6) setae. Terga of Th.2-Abd.1 with 3, Abd.2-3 with 3(4), Abd.4 with 2-3 and Abd. 5 with 1, pairs of axial microsetae, additionally each tergum with 1+1 posterior axial mesosetae set slightly out of line with microsetae. Some unpaired dorsal setae also present: d0 on head, microseta m0 on Abd.4, microseta a0 on Abd.5, and two macrosetae a0 and m0 on Abd.6, supplemented by 2+2 prespinal microsetae (Fig. 45, 49). Lateral microsensilla present on both Th.2-3.
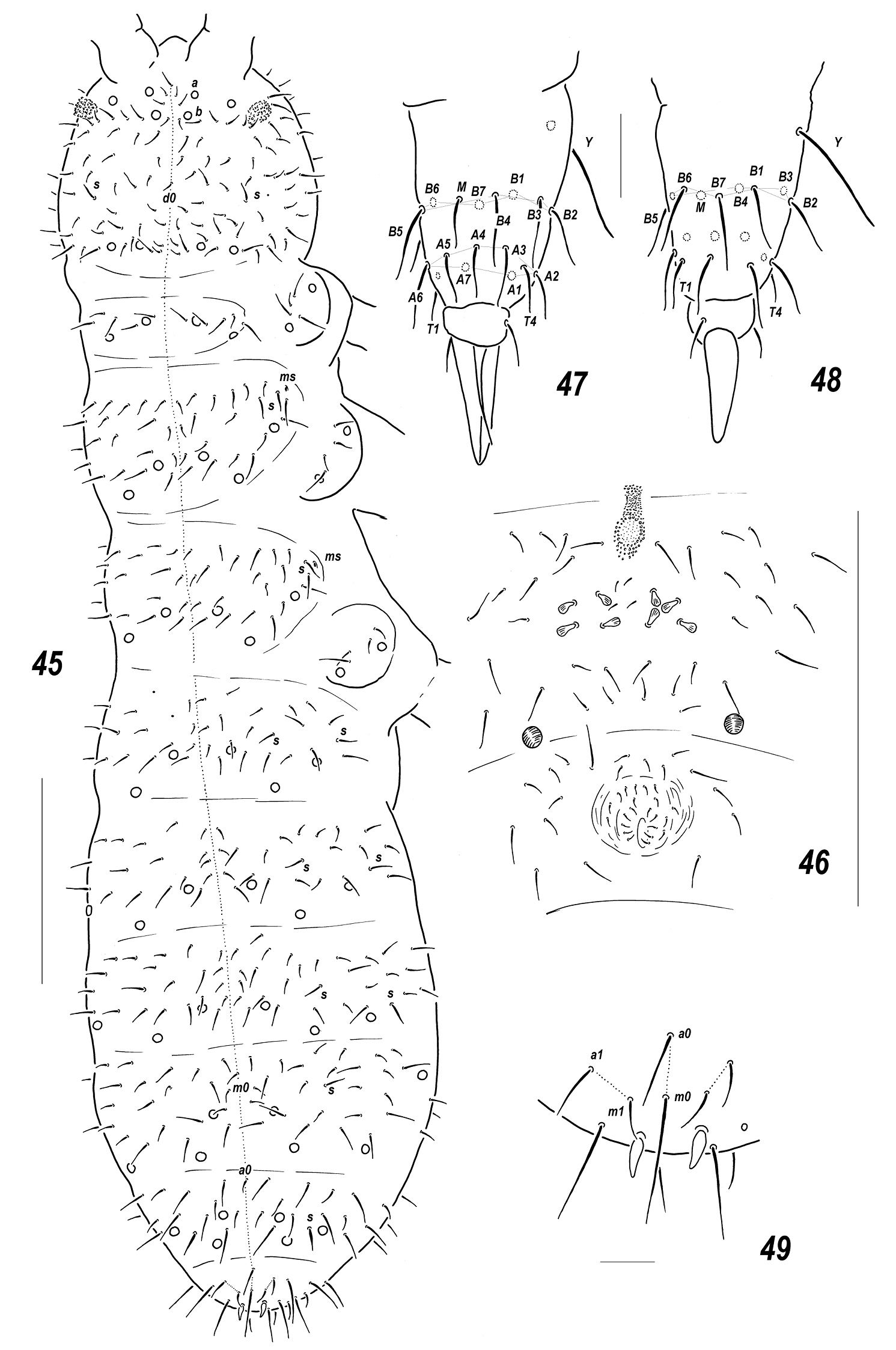
Each sternum of Th. 1-3 with 1+1 setae along linea ventralis. Secondary division of Abd.3 sternum unclear because of bad preservation. Furca reduced to a small area of fine granulation situated at some distance from border between Abd.3-4 sterna with 2+2 small posterior setae arranged in 2 rows (Fig. 46), manubrial area with 4+4 setae arrange in 2 rows but only one of them set posteriorly to dental setae (Fig. 46). Ventral tube usually with 1+1 frontal, 6+6(5–7) distal and 2 proximal setae at corpus base. Upper subcoxae usually with 4-4-4, tibiotarsi with 18-18-18. setae: distal row on each leg with 9 setae (7 A and 2 T-setae), 7 setae in row B, setae M and Y present (Figs 47–48). Unguis simple, with neither inner nor lateral tooth, unguiculus narrow, almost as long as unguis (ca 0.9 U3).Anal spines thick and slightly curved, set without clear papillae. Reproductive males with MVO identical to that in Allonychiurus volinensis with 4+4 modified club-like setae in mid-ventral section of Abd.4 behind furcal remnant (Fig. 46), in not reproductive males these setae spiniform.
Allonychiurus subvolinensis sp. n. 45 dorsal chaetotaxy 46 MVO on Abd.4 47–48 tibiotarsus of Lg.3, different views 49 Abd.6, dorsal chaetotaxy. Scales: 45–46 – 0.1 mm, 47–49 – 0.01 mm.
Allonychiurus subvolinensis sp. n. is very similar to the European Allonychiurus volinensis (Szeptycki, 1964), comb. n. in many features. Both have a somewhat isolated position within the volinensis-group of Allonychiurus due to the wide PAO, the presence of MVO on Abd.4 and the different positions of pso at the antennal base, with b-pseudocelli set closer to the mid-line than a-pseudocelli. They can easily be distinguished from each other due to the different number of tibiotarsal setae (9 setae in distal whorl in Allonychiurus subvolinensis sp. n. versus 7 setae in Allonychiurus volinensis) and by the presence of ventral setae on all thoracic sterna in Allonychiurus subvolinensis sp. n. (Allonychiurus volinensis lacks setae on Th.1). The third very similar species of the same group, Allonychiurus unisetosus sp. n., is described below. For differences with Allonychiurus volinensis and Allonychiurus subvolinensis sp. n. see the description of Allonychiurus unisetosus sp. n.
The name reflects the general similarity to Allonychiurus volinensis.
The new species was previously listed for Tuva as Onychiurus s.str. by
urn:lsid:zoobank.org:act:613014D9-6782-4406-B0C6-CEC3DEEDB568
http://species-id.net/wiki/Allonychiurus_unisetosus
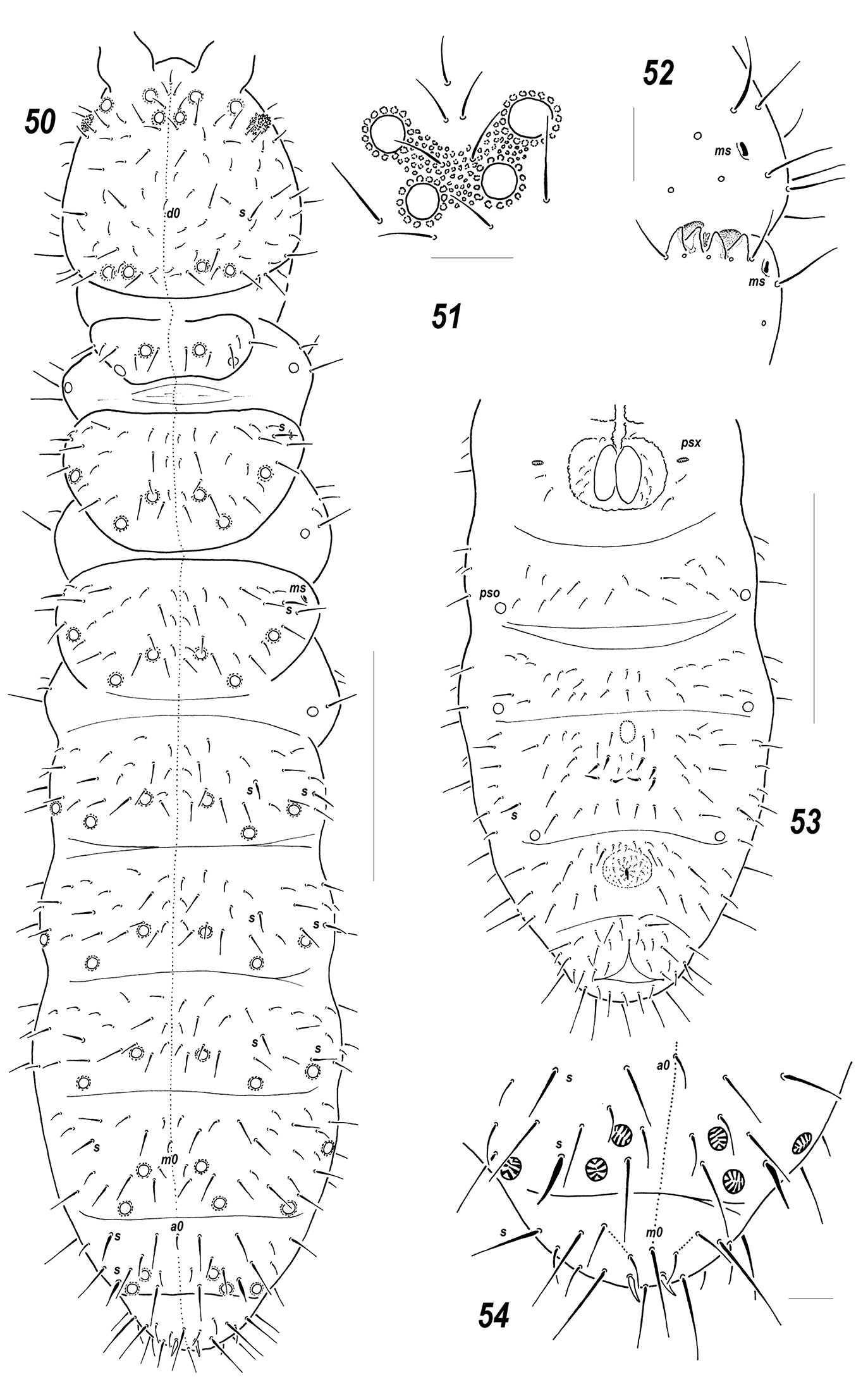
Figs 50–54Holotype ♂, Russia, Tuva Republic, northern macroslope of Eastern Tannu-Ola Mt Range, vicinity Shuurmak [50°38'N, 95°18'E ], spruce-larch (Picea obovata, Larix sibirica) forest, on larch stump under Cladonia chlorophaea, 1, 450 m alt., 12.viii.1997, leg. N.V. Sedel'nikova (MSPU).
Paratypes 8♀ and ♂, same sample as holotype; 1♀, same region, stony outcrops in mountain steppe, under Xanthoparmelia somloёnsis and Parmelia saxatilis, 1, 450 m alt., 12.viii.1997, leg. N.V. Sedel'nikova (MSPU).
Colour white. Size 0.55–0.65 mm. Body elongated, wider in region of Abd.4. Antennae about as long as head, antennal area not clearly demarcated. Ant.4 rather short and wide, 2 usual sensilla not especially thickened but distinct, a subapical organite and a basal microsensillum present. Ant.3 organ consisting of 4 low papillae, 2 sensory rods, 2 smooth sensory clubs without clear ribs, 5 guard setae, and a lateral microsensillum (Fig. 52). Ant.1 and 2 usually with 8 and 13 setae, respectively. PAO wide (length/width ratio ca 1.5), with (7)8–10 composed vesicles set close together. Labrum with 7 setae and 2 prelabral ones. Apical part of labium with thick terminal setae on papillae A, B and C (ABC – type), seta A clearly thicker, 6 long (e2 absent) and four spiniform (a1, b1-2 and d2), guard setae, a1shorter than others. Proximal field of labium with 5 setae, basal fields (mentum and submentum) with 4 and 5 setae. Maxillary palp simple, with 2 sublobal setae.
Pseudocellar formula (pso) as follows, dorsal: 32/233/33343 (Fig. 50), ventral: 11/000/0111 (one specimen with 1+2 ventral pso on Abd.4 also visible), sternum of Abd.1 with 1+1 psx on each side of VT (Fig. 53). Upper subcoxae with two pso and (2)3-(3)4-(3)4 setae, respectively. Generally granulation rather fine, but areas of clearly enlarged granules usually present around some pso and in mid and lateral parts of thorax. Dorsal chaetotaxy almost symmetrical, setae smooth and rather thick, clearly differentiated into macro and microsetae, sensilla poorly distinguishable, 1/011/222121 (dorsal) and 2/000/00010 (ventral), only lateral ones on Th.2-Abd.1 and posterior one on Abd.5 always distinct (Figs 50, 54). Sensillum on coxae of Lg.3 present. Th.1 with (4)5+5 setae. Terga of Th.2-Abd.3 with 3, Abd.4 with 2 and Abd. 5 with 1, pairs of axial microsetae, additionally each tergum with 1+1 posterior axial mesosetae set slightly out of line with microsetae. Unpaired dorsal setae: d0 on head, microseta m0 on Abd.4, microseta a0 on Abd.5, and only one macrosetae (m0) on Abd.6, supplemented by 2+2 prespinal microsetae (Figs 50, 54). Lateral microsensilla present on both Th.2-3.
Sterna of Th. 1-3 with 0-1-1 setae on each side of linea ventralis. Furca reduced to a small area of fine granulation situated at some distance from border between Abd.3-4 sterna with 2+2 small posterior setae arranged in 2 rows, manubrial area with 4+4 setae arrange in 2 rows but only one row set posteriorly to small dental setae (Fig. 53). Ventral tube usually with 1+1 frontal, 6+6 distal and 2(3) proximal setae at corpus base. Tibiotarsi with 18-18-18 setae: distal row on each leg with 9 setae (7 A and 2 T-setae), 7 setae in row B, setae M and Y present. Unguis simple, without teeth, unguiculus narrow, gradually tapering, with fine filament reaching tip of unguis. Anal spines curved and rather thin, set without papillae. MVO in reproductive males probably identical to that in Allonychiurus volinensis but in both available mature males only thickened setae present in mid-ventral section of Abd.4 (Fig. 53).
Allonychiurus unisetosus sp. n. 50 dorsal chaetotaxy 51 position of anteromedial pso on head 52 AO 53 ventral chaetotaxy of abdomen 54 Abd.6, dorsal chaetotaxy. Scales: 50, 53 – 0.1 mm, 51–52, 54 – 0.01 mm.
Allonychiurus unisetosus sp. n., Allonychiurus volinensis and Allonychiurus subvolinensis sp. n. constitutes a rather homogeneous subgroup among the known species of the volinensis-group of Allonychiurus. All of them are characterized by identical position of antennal pso with b-pseudocellus set close to midline and out of antennal area (cf. Figs 50–51 and 40). Such a position is unique for the group. Allonychiurus unisetosus sp. n. shares equal number of tibiotarsal setae (9) with Allonychiurus subvolinensis sp. n. and identical ventral chaetotaxy of thorax (0-1-1) with Allonychiurus volinensis (see Table 2) but differs from both species in having only two prelabral setae, one ventral pso on Abd.4 as a rule, only one axial macroseta on dorsal side of Abd.6 (cf. Figs 54 and 49), and clearly thinner AS.
The name reflects the presence of only one axial macroseta on Abd.6 in the new species separating it from similar congeners.
Known from several nearby localities of mountain Tuva, previously listed for the same region as Onychiurus s.str. sp. by
http://species-id.net/wiki/Allonychiurus_asiaticus
15 specimens, Russia, Siberia, Krasnoyarsk Territory, Achinsk Region, 7 km from Nazarovo [57°02'N, 90°39'E ], ca 400 m alt., meadows of various types, 1987–88; 9 specimens, Russia, West Siberia, 25 km S of Novosibirsk, Academgorodok [54°49'N, 83°08'E ], wet grass-herbaceous meadow, 02.X.1994, all leg. S. Stebaeva.
The above new material collected from an area lying far south (more than 1, 000 km) of the terra typica of the species differs from the original description in having more clearly differentiated tergal sensilla, but otherwise being very similar. These specimens may even represent a separate species, but material from intermediate areas is needed to evaluate the significance of these differences.
We express our sincere thanks to M. Potapov, O. Makarova, I. Kaprus’ and Yu. Sveenkova for the loan of valuable material and for their friendly and fruitful comments. We are also much indebted to S. Golovatch for his kindly editing the English of an advanced draft, as well as to anonymous reviewers and Academic Editor, Louis Deharveng for critical remarks and constructive comments.
The paper has been supported through grants of the Russian Foundation for Basic Research (projects 11-04-00941, 11-04-01725, 11-04-01655) and through several scientific programmes of the Russian Academy of Sciences.