(C) 2011 Victor H. Gonzalez. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new subgenus of Protandrena Cockerell (Panurginae: Protandrenini) from South America, Andinopanurgus Gonzalez & Engel, subgen. n., is described and figured for distinctive species of the genus occurring at mid- and high elevations in the Andes from Venezuela to Peru (1100–3400 m). In addition to the distribution, the subgenus is easily distinguished from other subgenera by a unique combination of morphological characters in both sexes, especially in the hidden sterna and genitalia of the male. Protandrena amyae sp. n., and Protandrena femoralis sp. n., are also described and figured from the Ecuadorian and Peruvian Andes. New geographical records and a key to the species are also provided.
Anthophila, Apoidea, Panurginae, Heterosarus, Rhophitulus, South America, taxonomy
Panurgine bees of the tribe Protandrenini (sensu
Although depauperate in andrenid bees by comparison to
the temperate Andes, during the last decade several species of
panurginae have been identified from the tropical Andes, from Bolivia
to Venezuela (
Summary of generic and subgeneric classification of Protandrenini (sensu
| Taxa | Species | Distribution |
|---|---|---|
| Genus Anthemurgus Robertson | 1 | NA (USA) |
| Genus Anthrenoides Ducke | 65 | SA (Argentina, Brazil, Chile, Paraguay) |
| Genus Chaeturginus Lucas de Oliveira & Moure | 2 | SA (Brazil) |
| Genus Liphanthus Reed | ||
| Subgenus Leptophanthus Ruz & Toro | 9 | SA (Argentina, Chile) |
| Subgenus Liphanthus Reed | 9 | SA (Chile) |
| Subgenus Melaliphanthus Ruz & Toro | 3 | SA (Chile) |
| Subgenus Neoliphanthus Ruz & Toro | 1 | SA (Chile) |
| Subgenus Pseudoliphanthus Ruz & Toro | 4 | SA (Argentina, Chile) |
| Subgenus Tricholiphanthus Ruz & Toro | 3 | SA (Chile) |
| Subgenus Xenoliphanthus Ruz & Toro | 4 | SA (Chile) |
| Genus Neffapis Ruz | 1 | SA (Chile) |
| Genus Parapsaenythia Friese | 7 | SA (Argentina, Bolivia, Brazil, Paraguay) |
| Genus Protandrena Cockerell | ||
| Subgenus Andinopanurgus subgen. n. | 7 | SA (Colombia, Ecuador, Peru, Venezuela) |
| Subgenus Austropanurgus Toro* | 1 | SA (Chile) |
| Subgenus Heterosarus Robertson* | 41 | NA, CA, SA |
| Subgenus Metapsaenythia Timberlake | 1 | NA (Mexico, USA) |
| Subgenus Parasarus Ruz* | 1 | SA (Argentina, Chile) |
| Subgenus Protandrena Cockerell* | ~50 | NA, CA, SA |
| Subgenus Pterosarus Timberlake* | ~40 | NA, CA (Canada to Guatemala) |
| Genus Psaenythia Gerstaecker | ~80 | SA (Argentina, Brazil, Chile) |
| Genus Pseudopanurgus Cockerell | 33 | NA, CA (USA to Costa Rica) |
| Genus Rhophitulus Ducke | ||
| Subgenus Cephalurgus Moure & Lucas de Oliveira* | 5 | SA (Brazil, Paraguay) |
| Subgenus Panurgillus Moure | 21 | SA (Brazil, Argentina) |
| Subgenus Rhophitulus Ducke | 3 | SA (Brazil, Argentina) |
| Incertae sedis | ||
| Genus Stenocolletes Schrottky | 1 | SA (Argentina) |
Morphological terminology follows that of
Genus Protandrena Cockerell, 1896
urn:lsid:zoobank.org:act:1F38EF71-936F-4F45-9A56-F84107823BE9
Protandrena bachue Gonzalez & Ruz, 2007.
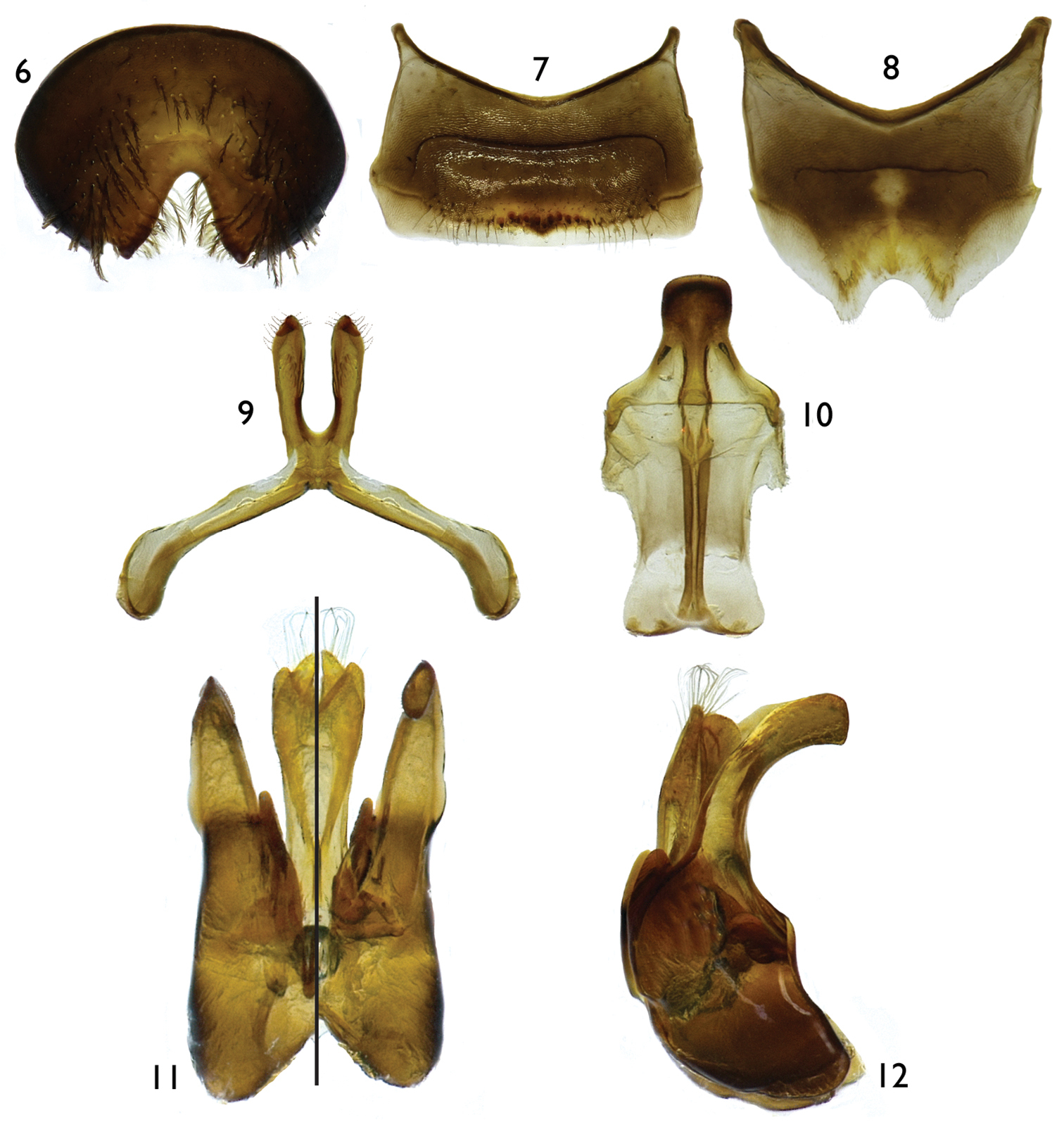
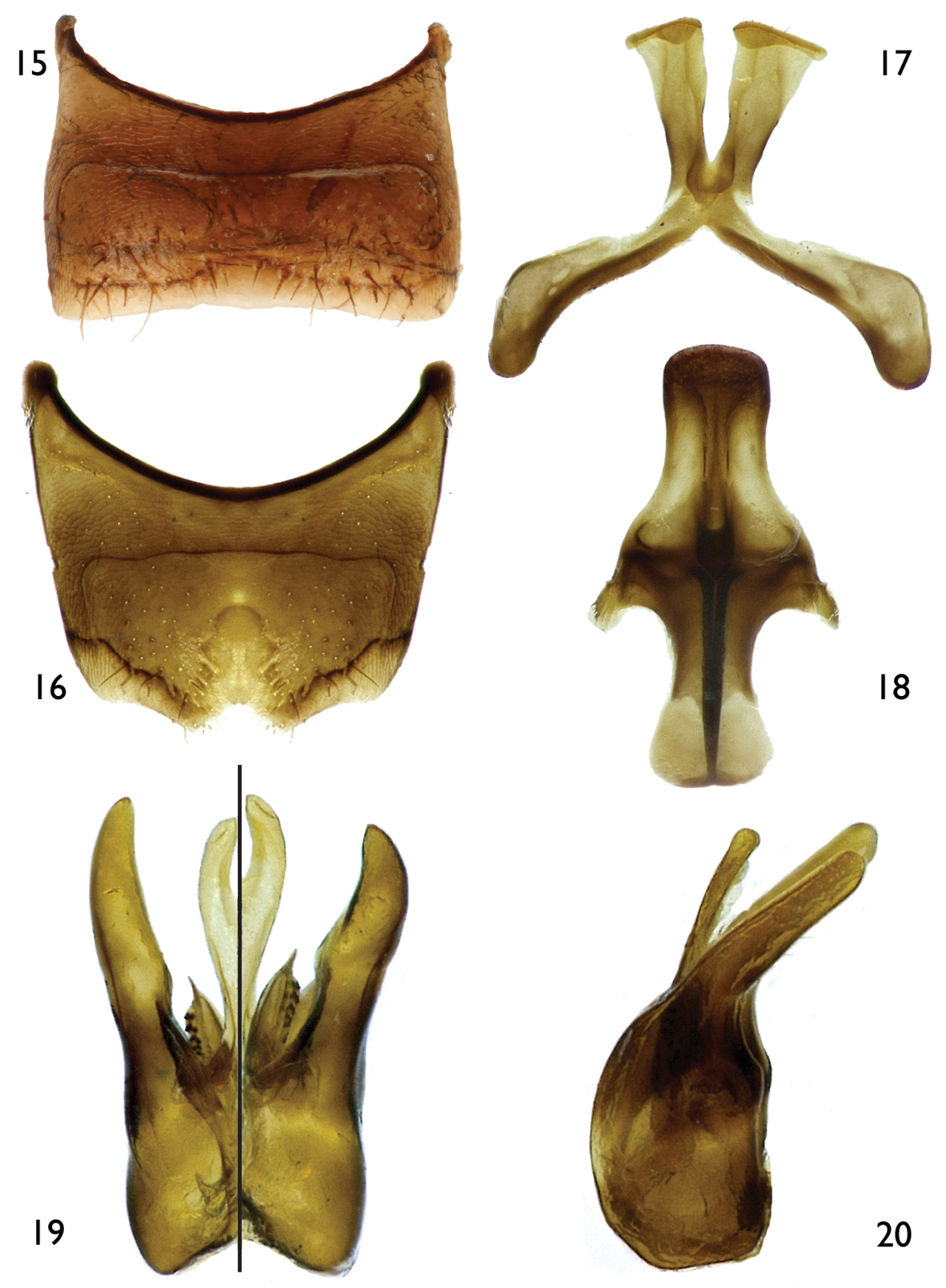
The new subgenus can be recognized easily by the following combination of characters: body predominantly dark brown to black with reduced yellow maculations; forewing with two submarginal cells (e.g., Figs 1, 13); propodeum glabrous basally; mesoscutum finely punctate; dorsal surface of propodeum longer than metanotum; anterior tentorial pit at outer subantennal sulcus, just above intersection between outer subantennal and epistomal sulci; female metatibial scopa with sparse, mostly simple setae; and male SVI with broad U- or V-shaped midapical emargination (Figs 8, 16); TVII with distal margin straight or medially emarginate (Fig. 6); and gonostylus simple, without apical lobes or projections, without long apical setae, and completely fused to gonocoxite (Figs 11, 12, 19, 20).
Female:Small to moderate-sized bees
(4–12 mm in length); color mainly dark brown to black, nonmetallic,
without yellow maculations except on pronotal lip; integument dull to
weakly shiny, distinctly imbricate to granular between punctures,
especially on dorsal surface of mesosoma and posterior surface of
mesofemur; punctures coarser, denser on head than on meso- and
metasoma; pubescence predominantly dark brown to black, short, and
sparse; pubescence longer and denser on head and mesosoma; metatibial
scopa consisting of sparse, long, mostly simple setae; metasomal terga
and sterna, except on apical segments, with distal margins glabrous;
SVI with dense patch of branched setae laterally (cf.
Male: As in female except longer, sparser body pubescence, clypeus often maculate, metabasitibial plate glabrous, and the following: antennal flagellum unmodified (Figs 13, 27) to weakly (Fig. 2) or strongly (Fig. 26) crenulate on posterior surface; compound eyes subparallel to slightly convergent ventrally. Outer surfaces of pro- and mesotibiae apically with small spine; metatibia with posterior marginal carina weakly toothed basally; metabasitarsus with posterodistal margin not distinctly projecting as in female; pretarsal claws symmetrical or with inner ramus shorter than outer. Metasoma usually more elongate than in female, sometimes petiolate; TVII without pygidial plate, distal margin straight or with V-shaped median emargination (Fig. 6); sterna with distal margins straight or convex, except SVI with distinct U- or V-shaped median emargination (Figs 8, 16); SVII with apical lobes attached to small discal area, not distinctly constricted basally, distally retrorse (Figs 9, 17, 30); SVIII longer than broad, midapical projection rather short (about one-half length of disc body), not distinctly constricted basally, broadly rounded apically (Figs 10, 18); genital capsule slightly longer than broad, gonobase absent; gonostylus about as long as penis valves or slightly longer, simple, fully fused to gonocoxite, gently or strongly curved in profile, without long, branched setae on apex; volsella clearly differentiated in medial digitus and lateral cuspis, denticulate, digitus elongate; penis valves simple, narrow, basally fused; penis membranous, bilobed, apically wide, about as long as penis valves (Figs 11, 12, 19, 20).
The new genus-group name is a combination of Andes, referring to the Andean distribution of this group of bees, and Panurgus, type genus of the Panurginae. The name is masculine.
In addition to the type species, Protandrena bachue Gonzalez & Ruz, the subgenus includes the following taxa: Protandrena amyae sp. n., Protandrena femoralis sp. n., Protandrena guarnensis Gonzalez & Ruz, Protandrena maximina Gonzalez & Ruz, Protandrena rangeli Gonzalez & Ruz, and Protandrena wayruronga Gonzalez & Ruz.
The subgenus occurs at mid- and high elevations (1100–3400 m) in the Andes from Venezuela to Peru. Two species groups (one consisting of Protandrena guarnensis and Protandrena femoralis, the other including the remaining species) can be recognized within Andinopanurgus by the characters indicated in the key to species (infra) (Table 2).
The general habitus of Andinopanurgus as well as the shape of the male sixth and seventh sterna suggest species of Rhophitulus Ducke and Heterosarus Robertson but the propodeum is basally pubescent in Rhophitulus and, in both taxa, TVII is gently or strongly projected medially on the distal margin, SVII has lobes with much broader apex, and the gonostylus has long branched setae apically and is partially fused to the gonocoxite, at least ventrally. In addition, Andinopanurgus lacks the distinctive dorsal remnant of the gonobase of Rhophitulus. The rather narrow and distally retrorse apical lobes of SVII of Andinopanurgus (Figs 9, 17) resemble those of Protandrena s.str. and Metapsaenythia Timberlake, but the apex of these lobes lack the distinctive spatulate setae present in the latter. Metapsaenythia has also a propodeum basally pubescent and a metasoma that is frequently red. If future studies demonstrate that Protandrena s.l. is paraphylectic, perhaps some of its subgenera, including Andinopanurgus, may well be recognized at the generic level.
Summary of currently included species in Andinopanurgus with information on the known sexes, distribution, and some morphological characters. Plus (+) and dash (–) symbols indicate presence and absence of a particular character, ? = unknown.
| Taxon | Sexes known | Distribution | Elevation (m.s.l.) | Male | Female | |
|---|---|---|---|---|---|---|
| Antennal flagellum | Spines of SV | Antennal flagellum | ||||
| “bachue species group” | ||||||
| Protandrena amyae sp. n. | ♂ | Ecuador: Napo | 2438 | weakly crenulate | + | ? |
| Protandrena bachue Gonzalez & Ruz | ♂♀ | Colombia: Boyacá, Cundinamarca | 2830–3380 | strongly crenulate | + | weakly crenulate |
| Protandrena maximina Gonzalez & Ruz | ♀ | Venezuela: Mérida | 2360 | ? | ? | unmodified |
| Protandrena rangeli Gonzalez & Ruz | ♂♀ | Colombia: Boyacá, Cundinamarca | 2600–2830 | unmodified | + | unmodified |
| Protandrena wayruronga Gonzalez & Ruz | ♂ | Ecuador: Napo, Pichincha | 3150 | strongly crenulate | + | ? |
| “guarnensis species group” | ||||||
| Protandrena guarnensis Gonzalez & Ruz | ♂♀ | Colombia: Antioquia | 2000 | unmodified | – | unmodified |
| Protandrena femoralis sp. n. | ♂♀ | Peru: Pasco | 1100–1780 | unmodified | – | unmodified |
urn:lsid:zoobank.org:act:BBCDA150-6DF7-4F65-824C-638DA480DF87
http://species-id.net/wiki/Protandrena_(Andinopanurgus)_amyae
Figs 1–12♂ (Fig. 1), Ecuador: Napo. Past. Road from Baeza to Papallacta, km. 188, 13-IV-1977 [13 April 1977], Elaine R. Hodges (USNM).
♂, Ecuador: Napo, Baeza (22 Kms. W.), 15 May 1975, elev. 8000ft. / Collected by sweeping net above damp road bed / Collected by Ashley B. Gurney (SEMC).
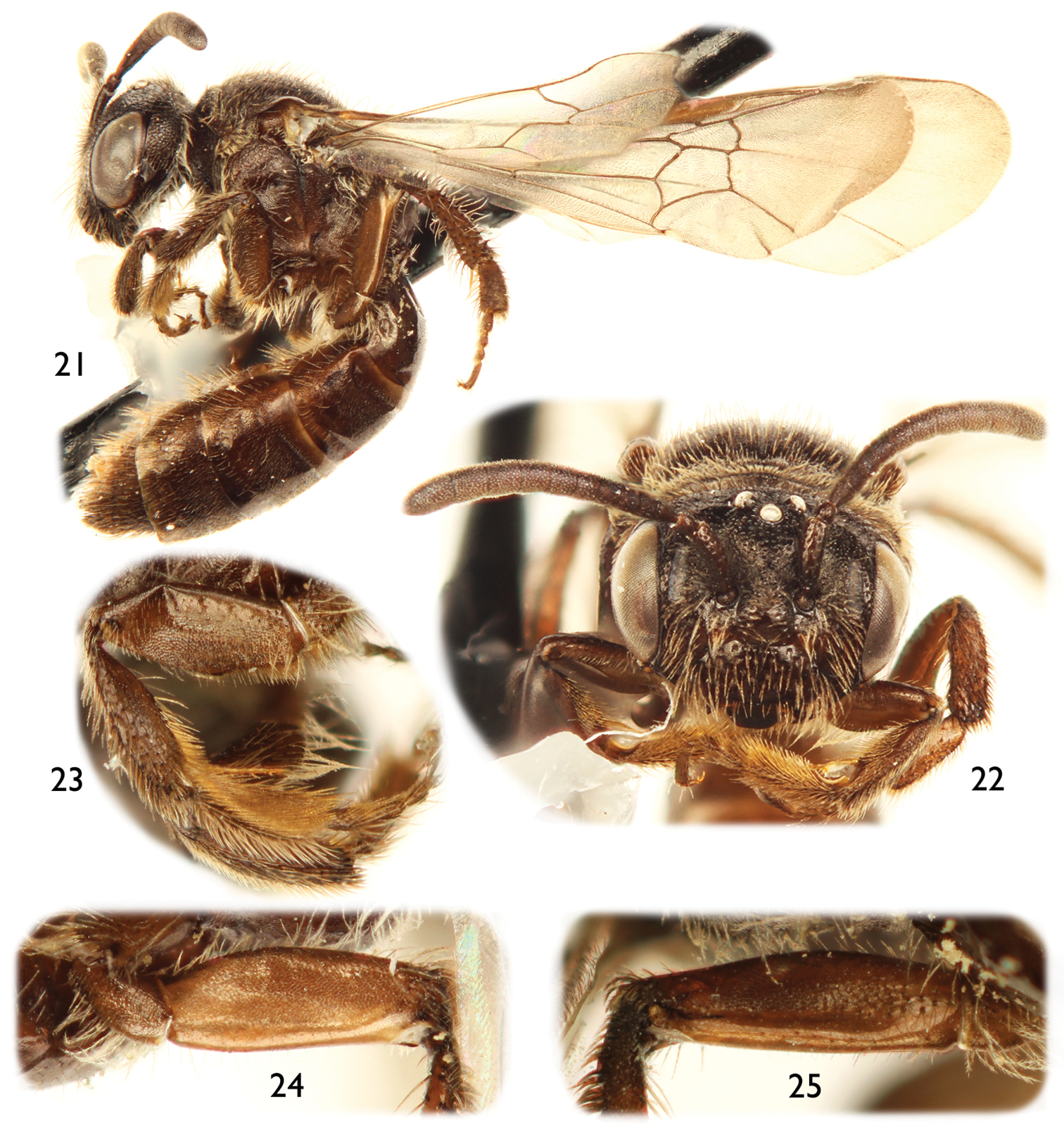
The male of this species can easily be recognized by the antennal flagellum weakly crenulate on the posterior surface (Fig. 2), the mandible distinctly broad apically (Figs 4, 5), and the posterior hypostomal carina strongly projecting into a tooth (Fig. 4).
Male: Body length 8.70 mm (8.50 mm); forewing length 6.50 mm (6.70 mm); head width 2.40 mm (2.48 mm). Head 1.4× wider than long; inner orbits of compound eyes subparallel (Fig. 3); intertorular distance 1.7× OD, 0.7× length of torulorbital distance; torulus diameter equal to OD; ocellocular distance 3.4× OD, 2.0× greater than ocelloccipital distance; interocellar distance 1.3× OD; compound eye 1.8× longer than wide; clypeus 2.6× broader than long, projecting about 0.4× compound eye width in lateral view; gena 1.2× broader than compound eye in profile; supraclypeal area, just below inferior torular tangent, distinctly protuberant; frontal line elevated just above antennal toruli to one-half distance between antennal toruli and median ocellus, ending at that point; inner subantennal sulcus about 0.7× length of outer subantennal sulcus; facial fovea 1.7× longer than broad, about one-half length of scape; scape 2.1× longer than broad, antennal flagellum slightly longer than head width, F1–F6 weakly crenulate on posterior surface, not forming deep concavity between flagellomeres (Fig. 2); pedicel about one-third length of F1, about as long as broad, F1 1.8× longer than broad, about 1.5× longer than F2 and F3 individually, remaining flagellomeres about as long as broad, except last flagellomere longer than broad; mandible distinctly broad apically (Figs 4, 5); posterior hypostomal carina strongly projecting into a tooth (Fig. 4). Forewing pterostigma 4.0× longer than broad; prestigma 3.1× longer than broad (prestigma width measured to its margin). Mesosoma slightly narrower than head width; mesoscutum 1.3× wider than long, 2.3× longer than mesoscutellum, 4.5× longer than metanotum; propodeum with basal part about three-fourths of mesoscutellum length in dorsal view; protibial spur with apical portion of rachis long, about three-fourths of malus length, with distinct row of 10 elongate branches (not including apical portion of rachis); mesotibial spur gently curved apically, with coarse branches, less than one-half of mesobasitarsus length; metatibia with posterior marginal carina weakly toothed on upper two-thirds; metatibial spurs slightly curved apically, inner spur slightly longer than outer; pretarsal claws cleft, inner ramus slightly shorter than the outer. Lateral fovea of TII ellipsoid, about 2.0× longer than broad; TVII with V-shaped median emargination on distal margin (Fig. 6); SV–SVIII, and genital capsule as in figures 7–12.
Color dark brown to black, except apex of mandible reddish brown and clypeus with yellow maculation as in figure 3. Wing membranes brownish, veins and pterostigma dark brown.
Head with sparse, long (2.5–3.0× OD), semierect, poorly-branched, black setae except brownish setae on condylar and outer grooves of mandible, gena posteriorly, and hypostomal area; scape with long setae, 2× as long as maximum scape diameter. Pronotum with short (0.5–1.0× OD), dense, brownish setae along dorsal margin and pronotal lobe; mesoscutum, mesoscutellum, and metanotum with two types of setae: sparse, long (2.5–3.0× OD), erect, poorly-branched, black setae, and dense, short (0.5× OD), brownish setae; mesepisternum and lateral and posterior areas of propodeum with mostly sparse, long (2.5–3.0× OD), erect, branched, brownish setae; legs with setae mostly brownish, longer and denser on coxae, trochanters, and profemur. Metasoma with terga mostly bare, with minute (≤ 0.3× OD), semierect, sparse ferruginous setae on discs, laterally with denser and longer setae; TVI with long (2× OD), semierect, dark brown setae on disc, setae denser on TVII; sterna with sparse, short (1× OD), semierect setae, denser and longer on sides of each sternum.
Outer surface of mandible and basal area of labrum smooth and shiny, impunctate; clypeus with sparse (1–1.5× PW), faint punctures, integument between punctures imbricate; supraclypeal area with scattered punctures laterally, weakly imbricate, shinier than on clypeus medially; subantennal area and inferior paraocular area with punctures separated by a puncture width or less, integument strongly imbricate to nearly granular (as on remainder of face); remaining areas of face with coarse punctures, contiguous, smaller than on clypeus; gena strongly imbricate with faint punctures. Mesoscutum, mesoscutellum, and metanotum with small, dense punctures (≤ 1× PW), integument granular between punctures; mesepisternum strongly imbricate with scattered (1–2.0× PW), faint punctures, punctures coarser and denser dorsally; metepisternum transversely weakly striate near wing base, otherwise strongly imbricate. Propodeum strongly imbricate with fine and weak striae basally, lateral and posterior surfaces with faint, scattered punctures. Metasomal terga and sterna shiny, weakly imbricate with minute, scattered (2–3.0× PW) punctures on discs, punctures coarser and denser on TVII; distal margins of terga shiny, weakly imbricate, impunctate except on TVII.
Female: Unknown.
Male of Protandrena (Andinopanurgus) amyae Gonzalez and Engel, sp. n. 1 Lateral habitus of holotype. 2 Detail of paratype antenna showing crenulations. 3 Facial aspect of holotype. 4 Oblique ventral view of paratype head showing hypostomal tooth (arrow). 5 Lateral aspect of paratype head.
Male terminalia of Protandrena (Andinopanurgus) amyae Gonzalez and Engel, sp. n. 6 Apical view of tergum VII. 7 Sternum V. 8 Sternum VI 9 Sternum VII. 10 Sternum VIII. 11 Genital capsule (left half is dorsal aspect, right half is ventral aspect). 12 Lateral aspect of genital capsule.
The specific epithet is a matronym honoring Mrs. Amy Comfort de Gonzalez, loving and supporting wife of the senior author.
urn:lsid:zoobank.org:act:BB7E3450-892C-4DBA-8EBA-ADABB1135D4F
http://species-id.net/wiki/Protandrena_(Andinopanurgus)_femoralis
Figs 13–25♂, Peru: Pasco Dept. [Departamento] San Miguel Eneñas, NW Villa Rica-Puerto Bermudas Rd., 1780 m, 10°44'0"S, 75°11'54"W, 16 Oct 1999; R. Brooks, PERU1B99 037, ex: yellow composite / SM0149359, KUNHM-ENT [barcode label] (SEMC).
(n = 3♀♀, 12♂♂) 2♂♂ with same date as the holotype; 1♂, Pasco Dept. San Juan, Villa Rica-Puerto Bermudas Rd., Rio Cacazu, 1100 m, 10°39'12"S, 75°6'54"W, 16 Oct 1999; R. Brooks, PERU1B99 034A, ex: on flowering tree; 3♀♀, 9♂♂, Pasco Dept. Villa Rica Rd., 1475 m, 10°47'6"S, 75°18'54"W, 15 Oct 1999; R. Brooks, D. Brzoska, PERU1B99 030 (SEMC).
Both sexes of Protandrena femoralis are most similar to Protandrena guarnensis
from northwestern Colombia in their small body size (4.5–6.0 mm in body
length), F1 about as long as F2, absence of maculations on the male
clypeus, male TVII with a straight distal margin, male SV without
spines on the midapical margin, and general shape of the genitalia and
hidden sterna. The male can easily be separated by the structure of
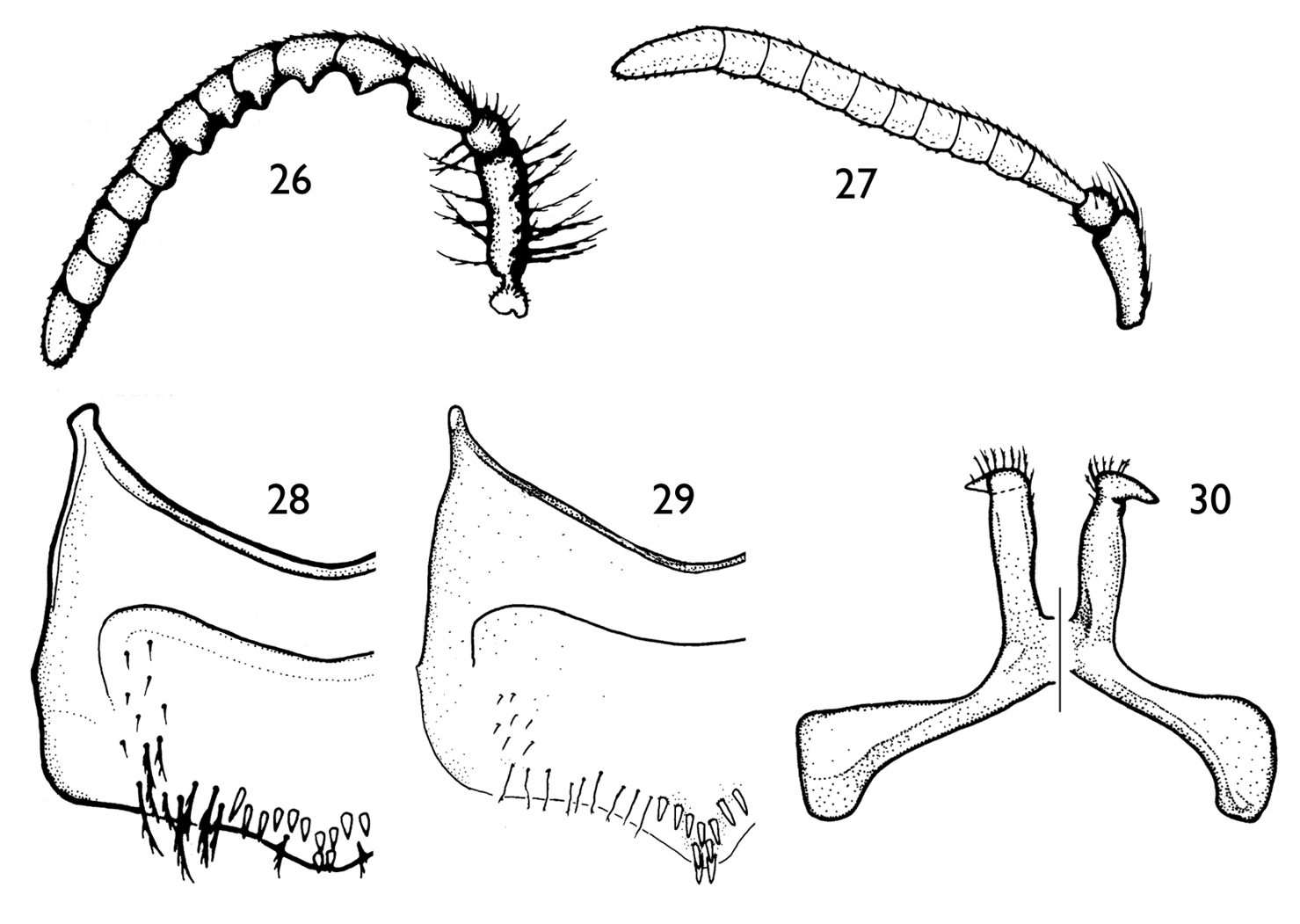
SVII, which has broader apical lobes (cf. Figs 17 and 30), and the gonostylus, which is more robust in profile than that of Protandrena guarnensis, about the same width across its length, and basally not protuberant on the medial margin in dorsal view (Figs 19, 20).
The female can be recognized by the posterior surface of the mesofemur
and anterior and posterior surfaces of the metafemur distinctly
depressed (Figs 23–25). In Protandrena guarnensis the apical lobes of SVII are narrow, parallel-sided, with the retrorse section of the apex comma-shaped (Fig. 30);
the gonostylus is slender in profile, slightly tapering towards the
apex, and strongly protuberant basally on the medial margin in dorsal
view (cf.
Male: Body length 5.0 mm (4.73–5.33 mm); forewing length 4.47 mm (4.47–4.60 mm); head width 1.50 mm (1.50–1.60 mm). Head 1.4× wider than long; inner orbits of compound eyes converging below (Figs 14); intertorular distance 2.3× OD, 0.9× length of torulorbital distance; torulus diameter equal to OD; ocellocular distance 3.4× OD, 2.8× greater than ocelloccipital distance; interocellar distance 1.3× OD; compound eye 1.8× longer than wide; clypeus 2.4× broader than long, projecting about 0.3× compound eye width in lateral view; gena 0.8× width of compound eye in profile; supraclypeal area, just below inferior torular tangent, distinctly protuberant; frontal line weakly elevated just above antennal toruli to one-half distance between antennal toruli and median ocellus, ending at that point; inner subantennal sulcus about 0.7× length of outer subantennal sulcus; facial fovea about 2.0× longer than broad, 0.4× length of scape; scape 2.1× longer than broad, antennal flagellum unmodified, slightly longer than head width (Figs 13–14); pedicel slightly shorter than F1, about as long as broad, F1 about as long as broad, subequal to F2 and F3 individually, remaining flagellomeres about as long as broad, except last flagellomere longer than broad; mandible pointed. Forewing prestigma 3.2× longer than broad (prestigma width measured to its margin); pterostigma 3.6× longer than broad. Mesosoma narrower than head width; mesoscutum 1.3× wider than long, 2.7× longer than mesoscutellum, 5.7× longer than metanotum; propodeum with basal part about three-fourths of mesoscutellum length in dorsal view; protibial spur with apical portion of rachis long, about one-half length of malus, with distinct row of about 10 elongate branches (not including apical portion of rachis); mesotibial spur straight or nearly so, with coarse branches, slightly more than one-half mesobasitarsus length; metatibia with posterior marginal carina weakly toothed on upper third; metatibial spurs of similar length, slightly curved apically; pretarsal claws with rami of similar length. Lateral fovea of TII elongate, about 4.0× longer than broad; TVII with distal margin straight or nearly so; SV–SVIII, and genital capsule as in figures 15–20.
Color dark reddish brown to black, without yellow maculations. Wing membranes subhyaline, slightly brownish, veins and pterostigma dark brown.
Head with sparse, long (2.5–3.0× OD), semierect, poorly-branched, black setae except brownish setae on condylar and outer grooves of mandible, gena posteriorly, and hypostomal area; scape with long setae, 2× as long as maximum scape diameter. Pronotum with short (0.5–1.0× OD), dense, brownish setae along dorsal margin and pronotal lobe; mesoscutum and mesoscutellum with two types of dark brown setae: sparse, long (1.5–2.0× OD), erect, poorly-branched setae, and short (0.5–1.0× OD), slightly denser setae; metanotum with short setae as on mesoscutellum; mesepisternum and lateral and posterior areas of propodeum with very sparse, long (1.5–2.0× OD), erect, branched, brownish setae; legs with setae mostly brownish, longer and denser on coxae, trochanters, and profemur. Metasomal terga with minute (≤ 0.3× OD), semierect, dense ferruginous setae on discs, laterally with denser and longer setae; TVI with long (1.5–2× OD), semierect, dark brown setae on disc, setae denser on TVII; sterna with sparse, short, semierect setae (1.5× OD) on discs, denser and longer laterally; preapical margin of SIV with few, semierect thick setae, each seta consisting of short rachis with three or four long branches, resembling scales or bundles of several setae at low magnifications.
Outer surface of mandible and basal area of labrum smooth and shiny, impunctate; clypeus with sparse (1–1.5× PW), faint punctures, integument between punctures weakly imbricate basally, becoming nearly smooth and shiny toward apex; supraclypeal area with scattered punctures laterally, weakly imbricate, medially shiny as on clypeus; remaining areas of face with coarse punctures separated by a puncture width or less, integument strongly imbricate to nearly granular, punctures becoming weaker and sparser on vertex; gena strongly imbricate with faint punctures. Mesoscutum, mesoscutellum, and metanotum with small, sparse punctures, integument granular between punctures; mesepisternum strongly imbricate with scattered (1–2.0× PW), faint punctures, punctures coarser and denser dorsally; metepisternum transversely weakly striate near wing base, otherwise strongly imbricate. Propodeum strongly imbricate with few fine, weak striae basally (barely visible), lateral and posterior surfaces with faint, scattered punctures. Metasomal terga and sterna shiny, lineolate-imbricate with minute punctures separated by about two puncture widths on discs, punctures coarser and denser on TVII, sparser on sterna; distal margins of terga shiny, weakly imbricate, impunctate except on TVII.
Female: As in male except shorter body pubescence, lighter and shinier integument (Fig. 21), and the following: Body length 5.53–5.73 mm; forewing length 4.87–5.0 mm; head width 1.60–1.67 mm. Inner orbits of compound eyes subparallel (Fig. 22); intertorular distance 2.6× OD, about as long as torulorbital distance; torulus diameter subequal to OD; ocellocular distance 3.5× OD, 2.5× greater than ocelloccipital distance; interocellar distance 1.6× OD; compound eye 2.1× longer than wide; clypeus 2.9× broader than long, projecting about 0.4× compound eye width in lateral view; supraclypeal area gently convex, not distinctly protuberant medially; facial fovea 3.3× times longer than broad, 0.7× length of scape; scape 2.7× longer than broad, antennal flagellum about as long as head width. Forewing prestigma 5.0× longer than broad (prestigma width measured to its margin); pterostigma 4.5× longer than broad. Mesoscutum about 5.0× longer than metanotum; protibial spur with apical portion of rachis about three-fourths length of malus, with about five branches (not including apical portion of rachis); mesotibial spur about 0.7× mesobasitarsus length; mesofemur with posterior surface and metafemur with anterior and posterior surfaces distinctly depressed (Figs 23–25); inner metatibial spur slightly shorter than outer; pretarsal claws with inner ramus shorter than the outer. Lateral fovea of TII about 3.0× longer than broad.
Male of Protandrena (Andinopanurgus) femoralis Gonzalez and Engel, sp. n. 13 Lateral aspect. 14 Facial aspect.
Male terminalia of Protandrena (Andinopanurgus) femoralis Gonzalez and Engel, sp. n. 15 Sternum V. 16 Sternum VI. 17 Sternum VII. 18 Sternum VIII. 19 Genital capsule (left half is dorsal aspect, right half is ventral aspect). 20 Lateral aspect of genital capsule.
Female of Protandrena (Andinopanurgus) femoralis Gonzalez and Engel, sp. n. 21 Lateral habitus. 22 Facial aspect. 23 Posterior surface of mesofemur. 24 Anterior surface of metafemur. 25 Posterior surface of metafemur.
The specific epithet refers to the distinctly depressed meso- and metafemora of the female of the species.
http://species-id.net/wiki/Protandrena_(Andinopanurgus)_wayruronga
1♂, Ecuador, Pich. [Pichincha], Quito (48 KmS), 6 May 1975, Ashley Gurney (USNM).
This species was previously known from the male holotype collected in Papallacta, Napo (Ecuador).
Males
| 1 | Forewing with three submarginal cells (occasional individuals have only two) (North and Central America) | Protandrena (Protandrena s. str.) |
| – | Forewing with two submarginal cells | 2 |
| 2(1) | Propodeal triangle basally pubescent (metasoma often red or largely so) (Nearctic) | Protandrena (Metapsaenythia) |
| – | Propodeal triangle basally glabrous | 3 |
| 3(2) | Metatibial spurs strongly curved at apices; first submarginal cell on posterior margin shorter than second (Chile) | Protandrena (Austropanurgus) |
| – | Metatibial spurs or at least one of them slightly curved or almost straight; first submarginal cell on posterior margin about as long as or longer than second | 4 |
| 4(3) | Gonostylus less than one-third as long as gonocoxite; SVI scarcely notched apically (face black) (South America) | Protandrena (Parasarus) |
| – | Gonostylus over one-half as long as gonocoxite; SVI with deep midapical notch or slit | 5 |
| 5(4) | Mesoscutum with punctures well marked, many of them separated by spaces larger than their diameters; SVI with midapical emargination narrow, deep (North and Central America) | Protandrena (Pterosarus) |
| – | Mesoscutum with punctures very small, homogeneous, commonly dense; SVI midapical emargination U- or V-shaped | 6 |
| 6(5) | TVII gently or strongly projected medially on distal margin; gonostylus with long branched setae apically, partially fused to gonocoxite, at least ventrally | Protandrena (Heterosarus) |
| – | TVII not projecting medially on distal margin, straight or with V-shaped median emargination (Fig. 6); gonostylus without long, branched setae on apex, fully fused to gonocoxite (Figs 11, 12, 19, 20) | Protandrena (Andinopanurgus) |
Females
| 1 | Forewing with three submarginal cells (only two in occasional individuals) (North and Central America) | Protandrena (Protandrena s. str.) |
| – | Forewing with two submarginal cells | 2 |
| 2(1) | Tibial scopa of rather long, abundant setae with clearly visible branches (North and Central America) | Protandrena (Pterosarus) |
| – | Tibial scopa of sparser setae that lack branches, or some of them with few, minute branches | 3 |
| 3(2) | Propodeal triangle basally pubescent (metasoma often largely red) (Nearctic) | Protandrena (Metapsaenythia) |
| – | Propodeal triangle basally glabrous | 4 |
| 4(3) | Metatibial spurs strongly curved at apices; anterior tentorial pit at intersection between outer subantennal and epistomal sulci | 5 |
| – | Metatibial spurs not strongly curved at apices; anterior tentorial pit not at intersection between outer subantennal and epistomal sulci, just below or above intersection | 6 |
| 5(4) | First submarginal cell on posterior margin shorter than second; face with yellow areas (Chile) | Protandrena (Austropanurgus) |
| – | First submarginal cell on posterior margin longer than second; face black (South America) | Protandrena (Parasarus) |
| 6(4) | Anterior tentorial pit in epistomal sulcus slightly to distinctly below intersection between outer subantennal and epistomal sulci; propodeum usually with dorsal surface at most as long as metanotum | Protandrena (Heterosarus) |
| – | Anterior tentorial pit at outer subantennal sulcus, just above intersection between outer subantennal and epistomal sulci; propodeum with dorsal surface longer than metanotum | Protandrena (Andinopanurgus) |
Males
Note that the male of Protandrena maximina is unknown.
| 1 | Clypeus without cream or yellow maculations; F1 short, about as long as F2; face and disc of mesoscutum weakly shiny; SV without spines on midapical margin, with fringe of normal, minutely-branched setae; TVII with distal margin straight, not medially emarginate | 2 |
| – | Clypeus with cream or yellow maculations; F1 distinctly longer than F2; face and disc of mesoscutum dull; SV with distinctly stout, short spines on midapical margin (Figs 7, 28, 29); TVII with V-shaped median emargination on distal margin | 3 |
| 2(1) | SVII with apical lobes narrow, parallel-sided, retrorse section of apex comma-shaped (Fig. 30); gonostylus slender in profile, slightly tapering towards apex, basally strongly protuberant on medial margin in dorsal view (Colombia: Antioquia) | Protandrena guarnensis Gonzalez & Ruz |
| – | SVII with apical lobes not parallel-sided, much broader apically (apex about twice as broad as base), retrorse section of apex not comma-shaped (Fig. 17); gonostylus more robust in profile, about same width across its length, basally not protuberant on medial margin in dorsal view (Peru) | Protandrena femoralis sp. n. |
| 3(1) | Antennal flagellum weakly (Fig. 2) or strongly (Fig. 26) crenulate on posterior surface; SV with more than four spines on midapical margin; larger bees (body length 7.9–11.8 mm) | 4 |
| – | Antennal flagellum unmodified, not crenulate on posterior surface (Fig. 27); SV with a row of four spines on midapical margin; small bees (body length 5.7–6.1 mm) (Colombia: Boyacá, Cundinamarca) | Protandrena rangeliGonzalez & Ruz |
| 4(3) | Antennal flagellum strongly crenulate on posterior surface, with deep concavity between flagellomeres (Fig. 26); mandible not distinctly broad apically; posterior hypostomal carina unmodified, without a tooth; protibial spur with apex of rachis very short (less than one-third of malus length), with less than five elongate branches (not including apical portion of rachis); SIII–SV with distal margins distinctly convex; SV with midapical row of spines medially projecting (Figs 28, 29) | 5 |
| – | Antennal flagellum weakly crenulate on posterior surface, without deep concavity between flagellomeres (Fig. 2); mandible distinctly broad apically (Figs 4, 5); posterior hypostomal carina with strong tooth (Fig. 4); protibial spur with apex long, about three-fourths of malus length, with a distinct row of 10 elongate branches (not including apical portion of rachis); SIII–SIV with distal margins gently convex; SV with midapical row of spines straight, not medially projecting (Fig. 7) (Ecuador: Napo) | Protandrena amyae sp. n. |
| 5(4) | F8 and F9 crenulate; SV midapical row of spines of unequal sizes, distal two spines distinctly longer (Fig. 29) (Ecuador: Quito, Napo) | Protandrena wayruronga Gonzalez & Ruz |
| – | F8 and F9 unmodified, not crenulate (Fig. 26); SV with midapical row of spines of about same size, without two distinctly long spines distally (Fig. 28) (Colombia: Boyacá, Cundinamarca) | Protandrena bachue Gonzalez & Ruz |
Females
Note that the females of Protandrena amyae and Protandrena wayruronga are unknown. However, given that the male of these species have crenulate antennal flagella they likely should run to Protandrena bachue in the key.
| 1 | Antennal flagellum unmodified, not crenulate | 2 |
| – | Antennal flagellum modified, weakly crenulate on posterior surface of F1–F5 (Colombia: Boyacá, Cundinamarca) | Protandrena bachue Gonzalez & Ruz |
| 2(1) | F1 about as long as F2; discs of mesoscutum and mesoscutellum shiny, weakly imbricate between punctures; metatibia with brownish to whitish scopal setae | 3 |
| – | F1 distinctly longer than F2; discs of mesoscutum and mesoscutellum dull, strongly imbricate between punctures; metatibia with dark brown to black scopal setae | 4 |
| 3(2) | Mesofemur with posterior surface and metafemur with anterior and posterior surfaces distinctly depressed (Figs 23–25) (Peru) | Protandrena femoralis sp. n. |
| – | Meso- and metafemora unmodified, not distinctly depressed (Colombia: Antioquia) | Protandrena guarnensis Gonzalez & Ruz |
| 4(2) | Small bees (head width 1.6–1.7 mm; body length 5.6–6.3 mm) (Colombia: Boyacá, Cundinamarca) | Protandrena rangeli Gonzalez & Ruz |
| – | Larger bees (head width 1.9–2.1 mm; body length 7.8 mm) (Venezuela) | Protandrena maximina Gonzalez & Ruz |
Representative features of Andinopanurgus species (from
We are grateful to S. Brady and B. Harris for bringing this material to our attention and permitting its study, to I.A. Hinojosa-Díaz for assistance with microphotography (Figs 1–5, 13–15, 21–25), and to two anonymous reviewers for their constructive comments. Partial support was provided by US National Science Foundation grant DBI-1057366 (to MSE). This is a contribution of the Division of Entomology, University of Kansas Natural History Museum.