(C) 2012 Adam J. Brunke. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
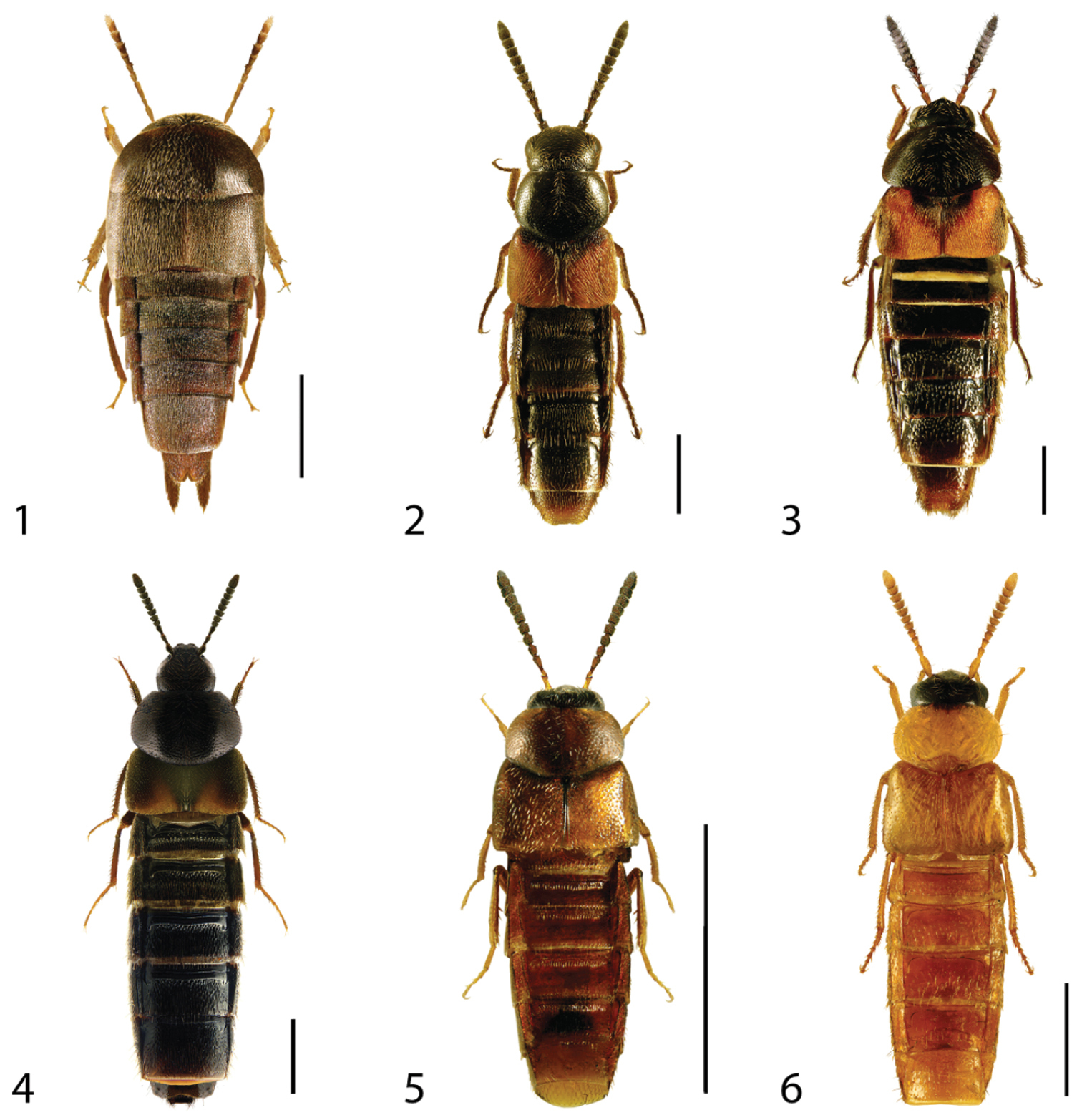
For reference, use of the paginated PDF or printed version of this article is recommended.
The Aleocharinae (Coleoptera: Staphylinidae) of Ontario were reviewed in the context of recently studied material, primarily from insect surveys conducted by the University of Guelph Insect Collection (Ontario, Canada). Aleochara daviesi Klimaszewski & Brunke sp. n., Agaricomorpha websteri Klimaszewski & Brunke sp. n., Atheta (Microdota) alesi Klimaszewski & Brunke sp. n., Dinaraea backusensis Klimaszewski & Brunke sp. n., and Strigota obscurata Klimaszewski & Brunke sp. n. are described as new to science. We also report 47 new Ontario records and 24 new Canadian records. Callicerus rigidicornis (Erichson) and Alevonota gracilenta (Erichson) are newly reported from North America as adventive species. A checklist, with Canadian distributions by province, of the 224 species of Aleocharinae known from Ontario is given. The following species are placed in subjective synonymy with Dexiogyia angustiventris (Casey): (Dexiogyia asperata (Casey) syn. n., Dexiogyia abscissa (Casey) syn. n., Dexiogyia tenuicauda (Casey) syn. n., Dexiogyia intenta (Casey) syn. n., Dexiogyia alticola (Casey) syn. n.). The following species are placed in subjective synonymy with Acrotona subpygmaea (Bernhauer): (Acrotona avia (Casey) syn. n., Acrotona puritana (Casey) syn. n.). Lectotypes are designated for Thiasophila angustiventris Casey, Thiasophila asperata Casey, Ischnoglossa intenta Casey, Oxypoda rubescans Casey, Chilopora americana Casey, Chilopora fuliginosa Casey, Coprothassa smithi Casey, Atheta subpygmaea Bernhauer, Colpodota puritana Casey, Strigota seducens Casey, Trichiusa compacta Casey, Trichiusa hirsuta Casey and Trichiusa robustula Casey.
Canada, Ontario, biodiversity, taxonomy, distributional records, Aleocharinae
Over the past ten years, the Aleocharinae (Coleoptera: Staphylinidae) have been one of the most active areas of beetle systematics research in Canada (see references in
The aleocharine fauna of Ontario was summarized by
Specimens were examined using Wild Heerbrugg M5A and Nikon SMZ 1000 stereomicroscopes, and nearly all were dissected to examine features of the genitalia. In many cases, tergite and sternite 8 were also examined. These structures were dehydrated in absolute alcohol and mounted in Canada balsam on celluloid microslides and pinned with the specimens from which they originated. Photographs were taken using an image processing system (Nikon SMZ 1500 stereoscopic microscope, Nikon Digital Camera DXM 1200F, and Adobe Photoshop software). Habitus photographs of all included species are provided, while genitalia are illustrated only for those species whose genitalia have not been shown in recent publications. Maps of each species’ distribution in Ontario, Canada were prepared using ARC MAP and Adobe Photoshop. In the species accounts, distributions are given by province or state (Canada, U.S.A.) or by country (elsewhere). These territories are abbreviated using Canada Post and United States Postal Service standards.
Morphological terminology mainly follows that used by
Material was examined from the following collections:
CNC Canadian National Collection of Insects, Ottawa, Ontario, Canada
DEBU University of Guelph Insect Collection, Guelph, Ontario, Canada
FMNH Field Museum of Natural History, Chicago, Illinois, USA
LFC Laurentian Forestry Centre, Quebec, Quebec, Canada
MZLU Museum of Lund University, Lund, Sweden
NMNH National Museum of Natural History, Smithsonian Institution, Washington D.C., USA
ZMB Museum für Naturkunde, Invalidenstrasse 43, 10115, Berlin, Germany
ZMUC Zoological Museum, University of Copenhagen, Copenhagen, Denmark
RWC Reginald Webster Collection, Charters Settlement, New Brunswick, Canada
Additionally, distributions of species included in this account were kindly checked by A. Davies (CNC) against his database of Canadian Staphylinidae to be published in the upcoming second edition of the ‘Checklist of Beetles of Canada and Alaska’ (Davies in Bousquet et al. in prep.). Distributions marked herein with an asterisk (*) represent records based entirely on these data. In the species accounts, the number of specimens for each collection event is given directly preceding the collection abbreviation in brackets. Where appropriate, short discussions pertaining to individual species are given in the species accounts under ‘comments’. We follow the higher taxonomic organization of Ashe in
As a result of the present study we recognize 224 species of Aleocharinae in Ontario. A checklist by tribe of all known Ontario Aleocharinae species and their distributions in Canada is given in Table 1. Aleochara daviesi Klimaszewski & Brunke sp. n., Agaricomorpha websteri Klimaszewski & Brunke sp. n., Atheta (Microdota) alesi Klimaszewski & Brunke sp. n., Dinaraea backusensis Klimaszewski & Brunke sp.n., and Strigota obscurata Klimaszewski & Brunke sp. n. are described as new to science. Twenty-four species are newly recorded from Canada, 47 species are newly recorded from Ontario, and the Palaearctic species Alevonota gracilenta (Erichson, 1839) and Callicerus rigidicornis (Erichson, 1839) are newly reported as introduced to North America. The genera Agaricomorpha Ashe, 1984, Alevonota Thomson, 1858, Callicerus Gravenhorst, 1802, Dexiogyia Thomson, 1858, Phanerota
Species of Aleocharinae recorded from Ontario and their provincial and territorial distribution within Canada. Provinces in bold denote new records given in the present publication. Additional records provided by A. Davies (see Methods) are marked by *.
| Tribe Gymnusini | |
| Gymnusa atra Casey | YT, NT, NU, BC, AB, MB, ON, QC, NB, NS, NL |
| Gymnusa campbelli Klimaszewski | YT, NT, MB*, ON, QC, NB, NL |
| Gymnusa grandiceps Casey | MB, ON, QC, NB, NS, NL |
| Gymnusa pseudovariegata Klimaszewski | YT, NT, BC, AB, MB, ON, QC, NB, NS, NL |
| Gymnusa smetanai Klimaszewski | YT, NT, MB, ON, NL |
| Tribe Deinopsini | |
| Deinopsis canadensis Klimaszewski | ON, NB, NL |
| Deinopsis harringtoni Casey | MB*, ON, QC, NB, NS, NL |
| Deinopsis illinoisensis Klimaszewski | ON |
| Deinopsis rhadina Klimaszewski | ON, QC*, NB |
| Tribe Aleocharini | |
| Aleochara assiniboin Klimaszewski | YT, BC, SK, MB, ON |
| Aleochara bilineata Gyllenhal † | BC, AB, SK*, MB, ON, QC, NB, NS, PE, NL |
| Aleochara bimaculata Gravenhorst | NT, BC, AB, SK, MB, ON, QC, NB, NS, NL |
| Aleochara castaneipennis Mannerheim | YT, NT*, BC, AB, ON, QC, NB, NS, NL |
| Aleochara curtula (Goeze) † | BC, ON, QC, NB, NS, PE, NL |
| Aleochara daviesi Klimaszewski & Brunke, sp.n. | ON |
| Aleochara fumata Gravenhorst † | YT, BC, AB, MB, ON, QC, NB, NS, PE, NL |
| Aleochara gracilicornis Bernhauer | NT, BC, SK, MB, ON, QC, NB, NS |
| Aleochara inexpectata Klimaszewski | ON, QC, NB, NS |
| Aleochara lacertina Sharp | BC, AB, SK, MB, ON, QC, NB, NS, NL |
| Aleochara lanuginosa Gravenhorst † | BC, AB, ON, QC, NB, NS, NL |
| Aleochara lata Gravenhorst† | YT, BC, SK, MB, ON, QC |
| Aleochara lustrica Say | ON |
| Aleochara ocularis Klimaszewski | MB, ON, QC |
| Aleochara rubricalis (Casey) | BC, ON (doubtful record) |
| Aleochara rubripennis (Casey) | MB, ON, QC, NB |
| Aleochara sculptiventris (Casey) | ON, QC, NB, NL |
| Aleochara sekanai Klimaszewski | YT, NT*, AB, MB, ON, NL |
| Aleochara speculicollis Bernhauer | AB, ON, QC |
| Aleochara tahoensis Casey | YT, NT, BC, AB, SK, MB, ON, NB, NS |
| Aleochara thoracica Casey | ON, QC, NB, NL |
| Aleochara tristis Gravenhorst† | ON, QC, NB, NL |
| Aleochara verna Say | YT, BC, AB, SK, MB, ON, QC, NB, NS, PE, NL |
| Tinotus caviceps Casey | ON, QC |
| Tinotus morion (Gravenhorst) † | BC, AB, ON, QC, NB, NS, NL |
| Tinotus trisectus Casey | ON |
| Tribe Hoplandriini | |
| Hoplandria klimaszewskii Génier | ON, QC |
| Hoplandria laevicollis (Notman) | ON, QC |
| Hoplandria laeviventris Casey | ON |
| Hoplandria lateralis (Melsheimer) | ON, QC, NB |
| Platandria carolinae Casey | ON |
| Tribe Oxypodini | |
| Amarochara brevios Assing | ON |
| Amarochara fenyesi Blatchley | ON |
| Calodera parviceps (Casey) | YT, ON, NB, NS |
| Crataraea suturalis (Mannerheim) † | BC, SK, ON, NB, NS, NL |
| Devia prospera (Erichson) | YT, NT, BC, AB, MB, ON, NB, NL |
| Dexiogyia angustiventris (Casey) | ON |
| Gennadota canadensis Casey | ON, QC, NB, NS |
| Hylota ochracea Casey | NT, ON, QC, NB, NS |
| Ilyobates bennetti Donisthorpe † | ON, QC, NB, NS |
| Meotica ‘pallens’ Redtenbacher † | BC, ON, NS |
| Ocyusa canadensis Lohse | YT, ON |
| Oxypoda amica Casey | YT, MB, ON, QC, NB, NS |
| Oxypoda brachyptera (Stephens) † | ON, QC, NB, NS, NL |
| Oxypoda canadensis Klimaszewski | YT, NT, AB, MB, ON, QC, NL |
| Oxypoda chantali Klimaszewski | ON, QC, NS |
| Oxypoda convergens Casey | AB, ON, QC, NB, NS, NL |
| Oxypoda demissa Casey | YT, ON, QC, NB, NS, NL |
| Oxypoda frigida Bernhauer | YT, NT, BC, AB, ON, QC, NB, NS, NL |
| Oxypoda gnara Casey | ON, QC, NB |
| Oxypoda grandipennis (Casey) | YT, BC, AB, ON, QC, NB, NS, NL |
| Oxypoda hiemalis Casey | YT, NT, ON, QC, NB, NS, NL |
| Oxypoda lacustris Casey | YT, NT, BC, AB, ON, QC, NB, NL |
| Oxypoda lucidula Casey | YT, NT, AB, MB, ON, QC, NB, NL |
| Oxypoda opaca (Gravenhorst) † | BC, ON, NB, NS, NL |
| Oxypoda operta Sjöberg | YT, AB, ON, QC, NS, NL |
| Oxypoda orbicollis Casey | YT, AB, ON, QC, NB, NS, NL |
| Oxypoda perexilis Casey | ON, QC, NS |
| Oxypoda pseudolacustris Klimaszewski | AB, ON, QC, NB, NS, NL |
| Oxypoda rubescans Casey | ON |
| Oxypoda vockerothi Klimaszewski | ON, NB |
| Parocyusa americana (Casey) | ON |
| Parocyusa fuliginosa (Casey) | ON, NL |
| Phloeopora arctica Lohse | YT, NT, ON |
| Tribe Tachyusini | |
| Brachyusa helenae (Casey) | YT, NT, ON, NB, NL |
| Gnypeta caerulea (Sahlberg) | YT, NT, BC*, AB, SK, MB, ON, QC, NB, NS, PE, NL |
| Gnypeta canadensis Klimaszewski | AB, ON |
| Gnypeta carbonaria (Mannerheim) | NT, AB, SK, MB, ON, QC, NB, NL |
| Gnypeta helenae Casey | BC, AB, ON |
| Gnypeta nigrella (LeConte) | ON, NB, NL |
| Meronera venustula (Erichson) | ON, QC, NB |
| Tachyusa americana Casey | ON, QC, NB |
| Tachyusa americanoides Paśnik | NT, BC, AB, MB, ON, QC, NB, NS, NL |
| Tribe Hypocyphtini | |
| Cypha inexpectata Klimaszewski & Godin | YT, ON |
| Tribe Myllaenini | |
| Myllaena arcana Casey | AB, ON, QC, NB, NS, NL |
| Myllaena audax Casey | NT, BC, ON, QC, NB, NL |
| Myllaena cuneata Notman | ON, NS |
| Myllaena insomnis Casey | YT, NT, BC, AB, SK, MB, ON, QC, NB, NS, NL |
| Myllaena lucidificans Casey | ON, QC, NB |
| Myllaena potawatomi Klimaszewski | ON |
| Myllaena vulpina Bernhauer | ON, NB, NS |
| Tribe Autaliini | |
| Autalia rivularis (Gravenhorst) | BC, AB, ON, QC, NB, NS, NL |
| Tribe Homalotini | |
| Agaricomorpha websteri Klimaszewski & Brunke, sp. n. | ON, QC, NB, NS |
| Eumicrota corruscula (Erichson) | ON, QC, NB |
| Eumicrota socia (Erichson) | ON, QC, NB, NS, PE |
| Euvira micmac Klimaszewski & Majka | ON, NB, NS |
| Gyrophaena affinis Mannerheim † | BC, MB, ON, QC, NB, NS, NL |
| Gyrophaena antennalis Casey | ON, NB, NS, NL |
| Gyrophaena brevicollis Seevers | ON |
| Gyrophaena caseyi Seevers | ON, QC |
| Gyrophaena criddlei Casey | YT? MB, ON, NB, NL |
| Gyrophaena dybasi Seevers | ON, NB |
| Gyrophaena egena Casey | ON, QC |
| Gyrophaena flavicornis Melsheimer | ON, QC, NB, NS |
| Gyrophaena fuscicollis Casey | ON, NB |
| Gyrophaena gaudens Casey | ON, QC, NB, PE |
| Gyrophaena gilvicollis Casey | ON, NB |
| Gyrophaena insolens Casey | MB*, ON, NB, NL |
| Gyrophaena keeni Casey | YT, BC, AB, ON, QC, NB, NL |
| Gyrophaena meduxnekeagensis Klimaszewski & Webster | ON, QC, NB |
| Gyrophaena modesta Casey | AB*, ON, NB, NS, NL |
| Gyrophaena nana (Paykull) | YT, BC, AB, MB, ON, NB, NL |
| Gyrophaena nanoides Seevers | ON, QC, NB, NL |
| Gyrophaena neonana Seevers | YT, ON, NB, NL |
| Gyrophaena stroheckeri Seevers | ON |
| Gyrophaena subnitens Casey | MB, ON, NB, NS |
| Gyrophaena uteana Casey | BC, AB*, ON, QC, NB |
| Gyrophaena vitrina Casey | ON, QC, NB, PE |
| Homalota plana (Gyllenhal) † | ON, NB, NS, NL |
| Leptusa brevicollis Casey | ON, QC, NB, NS, PE, NL |
| Leptusa canonica Casey | ON, QC, NB, NS, NL |
| Leptusa carolinensis Pace | ON, QC, NB, NS |
| Leptusa cribulata (Casey) | ON, QC |
| Leptusa elegans Blatchley | ON, QC |
| Leptusa gatineauensis Klimaszewski & Pelletier | BC, AB, ON, QC, NB, NS, NL |
| Leptusa jucunda Klimaszewski & Majka | ON, QC, NB, NS |
| Leptusa opaca Casey | ON, QC, NB, NS, PE, NL |
| Neotobia alberta Ashe | AB, MB, ON, QC, NB |
| Phanerota fasciata (Say) | ON |
| Phymatura blanchardi (Casey) | AB, ON, NB |
| Silusa alternans Sachse | ON, QC, NB, NS, PE |
| Silusa californica (Bernhauer) | YT, BC, AB, ON, QC, NB, NS, PE, NL |
| Silusida marginella (Casey) | ON, QC, NB, NS, PE, NL |
| Thecturota pusio (Casey) | ON |
| Tribe Placusini | |
| Placusa canadensis Klimaszewski | ON, QC, NS |
| Placusa despecta Erichson | ON, QC |
| Placusa incompleta Sjöberg | BC, ON, QC, NB, NS, NL |
| Placusa pseudosuecica Klimaszewski | BC, ON, QC |
| Placusa tachyporoides (Walt) | BC, ON, QC, NB, NS |
| Placusa tacomae Casey | YT, NT, BC, AB, ON, QC, NB, NS, NL |
| Placusa vaga Casey | YT, NT. BC, ON, QC, NB, NS |
| Tribe Athetini | |
| Acrotona smithi (Casey) | ON, NB |
| Acrotona subpygmaea (Bernhauer) | ON, NS |
| Alevonota gracilenta (Erichson) † | ON |
| Aloconota sulcifrons (Stephens) † | ON, QC, NB, NL |
| Atheta aemula (Erichson) | ON, QC, NB |
| Atheta alesi Klimaszewski and Brunke, sp. n. | ON |
| Atheta annexa Casey | ON, QC, NB, NS, NL |
| Atheta borealis Klimaszewski & Langor | ON, NL |
| Atheta brunswickensis Klimaszewski | YT, ON, NS |
| Atheta burwelli (Lohse) | YT, ON, QC, NB, NL |
| Atheta campbelli (Lohse) | YT, ON, NL |
| Atheta capsularis Klimaszewski | YT, ON, QC, NB, NL |
| Atheta circulicollis Lohse | ON, QC, NB, NL |
| Atheta crenuliventris Bernhauer | ON, QC, NB, NL |
| Atheta dadopora Thomson | YT, AB, ON, NB, NS, PE, NL |
| Atheta districta Casey | BC, AB, ON, NB, NS, NL |
| Atheta festinans (Erichson) | ON |
| Atheta frosti Bernhauer | BC, ON, QC, NB, NS, NL |
| Atheta graminicola (Gravenhorst) | YT, NT, BC, AB, MB, ON, QC, NB, NL |
| Atheta hampshirensis Bernhauer | BC, ON, QC, NB, NS, NL |
| Atheta klagesi Bernhauer | YT, BC, AB, ON, QC, NB, NS, PE, NL |
| Atheta modesta (Melsheimer) | AB, ON, QC, NB, NS |
| Atheta nescia (Casey) | BC, ON |
| Atheta particula (Casey) | ON, QC, NB, NS |
| Atheta pennsylvanica Bernhauer | ON, QC, NB, NS, NL |
| Atheta platonoffi Brundin | YT, BC, AB, ON, NB, NS, NL |
| Atheta prudhoensis (Lohse) | YT, ON, NB, NS, NL |
| Atheta pseudocrenuliventris Klimaszewski | YT, ON, NB, NS, NL |
| Atheta pseudomodesta Klimaszewski | ON, QC, NB, NS, NL |
| Atheta remulsa Casey | YT, BC, AB, ON, QC, NB, NS, NL |
| Atheta savardae Klimaszewski & Majka | ON, QC, NB, NS, NL |
| Atheta strigosula Casey | YT, BC, ON, QC, NB, NL |
| Atheta terranovae Klimaszewski & Langor | YT, ON, NB, NL |
| Atheta ventricosa Bernhauer | YT, BC, AB, ON, QC, NB, NS, NL |
| Callicerus obscurus Gravenhorst † | ON |
| Callicerus rigidicornis (Erichson) † | ON |
| Clusiota impressicollis (Bernhauer) | BC, ON, NB, NL |
| Dalotia coriaria (Kraatz)† | BC, AB, ON, NB, NS |
| Dinaraea angustula (Gyllenhal) † | YT, AB, ON, QC, NB, NS, PE, NL |
| Dinaraea backusensis Klimaszewki & Brunke, sp.n. | ON |
| Earota dentata (Bernhauer) | YT, BC, AB, MB, ON, QC, NB, NS, NL |
| Hydrosmecta pseudodiosica Lohse | YT, ON, NB |
| Liogluta aloconotoides Lohse | YT, ON, QC, NB, NS, NL |
| Lypoglossa franclemonti Hoebeke | YT, NT, AB, MB, ON, QC, NB, NS, NL |
| Mocyta breviuscula (Mäklin) | YT, BC, AB, ON, QC, NB, NS, NL |
| Mocyta fungi (Gravenhorst) † | YT, BC, AB, ON, QC, NB, NS, PE, NL |
| Philhygra botanicarum (Muona) | YT, BC, ON, NB, NS, NL |
| Philhygra clemens (Casey) | YT, BC, ON, QC, NB, NS |
| Philhygra jarmilae Klimaszewski & Langor | YT, ON, NB, NL |
| Philhygra laevicollis (Mäklin) | BC, ON, NB, NS |
| Philhygra luridipennis (Mannerheim) | ON, NB, NL |
| Philhygra proterminalis (Bernhauer) | ON |
| Nehemitropia lividipennis (Mannerheim) † | ON, QC, NB, NS, PE, NL |
| Schistoglossa blatchleyi (Bernhauer & Scheerpeltz) | YT, NT, MB, ON, QC, NB |
| Schistoglossa brunswickensis Klimaszewski & Webster | ON, QC, NB |
| Seeversiella globicollis (Bernhauer) | BC, AB, ON, QC, NS, NL |
| Stethusa klimschi (Bernhauer) | ON |
| Stethusa spuriella (Casey) | ON |
| Strigota ambigua (Erichson) | YT, ON, NS, PE, NL |
| Strigota obscurata Klimaszewski & Brunke, sp. n. | ON |
| Strophogastra penicillata Fenyes | AB, MB, ON, QC, NB, NS |
| Thamiaraea brittoni (Casey) | ON, QC, NB |
| Trichiusa compacta Casey | ON |
| Trichiusa hirsuta Casey | ON |
| Trichiusa postica Casey | ON, NS |
| Trichiusa robustula Casey | ON |
| Tribe Falagriini | |
| Aleodorus bilobatus (Say) | ON |
| Aleodorus scutellaris (LeConte) | ON |
| Cordalia obscura (Gravenhorst) † | ON, QC, NB, NS |
| Falagria dissecta Erichson | BC, AB, SK, MB, ON, QC, NB, NS |
| Falagria sulcata Paykull† | AB, ON, QC, NB |
| Myrmecocephalus cingulatus (LeConte) | ON, NS |
| Tribe Lomechusini | |
| Drusilla canaliculata (Fabricius) † | ON, QC, NB, NS, PE, NL |
| Myrmecoecia lauta (Casey) | ON |
| Myrmedonota aidani Maruyama & Klimaszewski | ON |
| Pella carolinae (Casey) | ON |
| Pella gesneri Klimaszewski | AB, MB, ON, NB |
| Pella loricata (Casey) | ON, NS |
| Pella schmitti (Hamilton) | ON, QC |
| Platyusa sonomae Casey | ON |
| Xenodusa cava (LeConte) | ON |
| Xenodusa reflexa (Walker) | BC, AB, SK, MB, ON, QC, NB, NS |
| Zyras obliquus (Casey) | BC, AB, MB, ON, QC, NB, NS, NL |
| Zyras planifer (Casey) | ON |
† Considered adventive in North America.
http://species-id.net/wiki/Deinopsis_illinoisensis
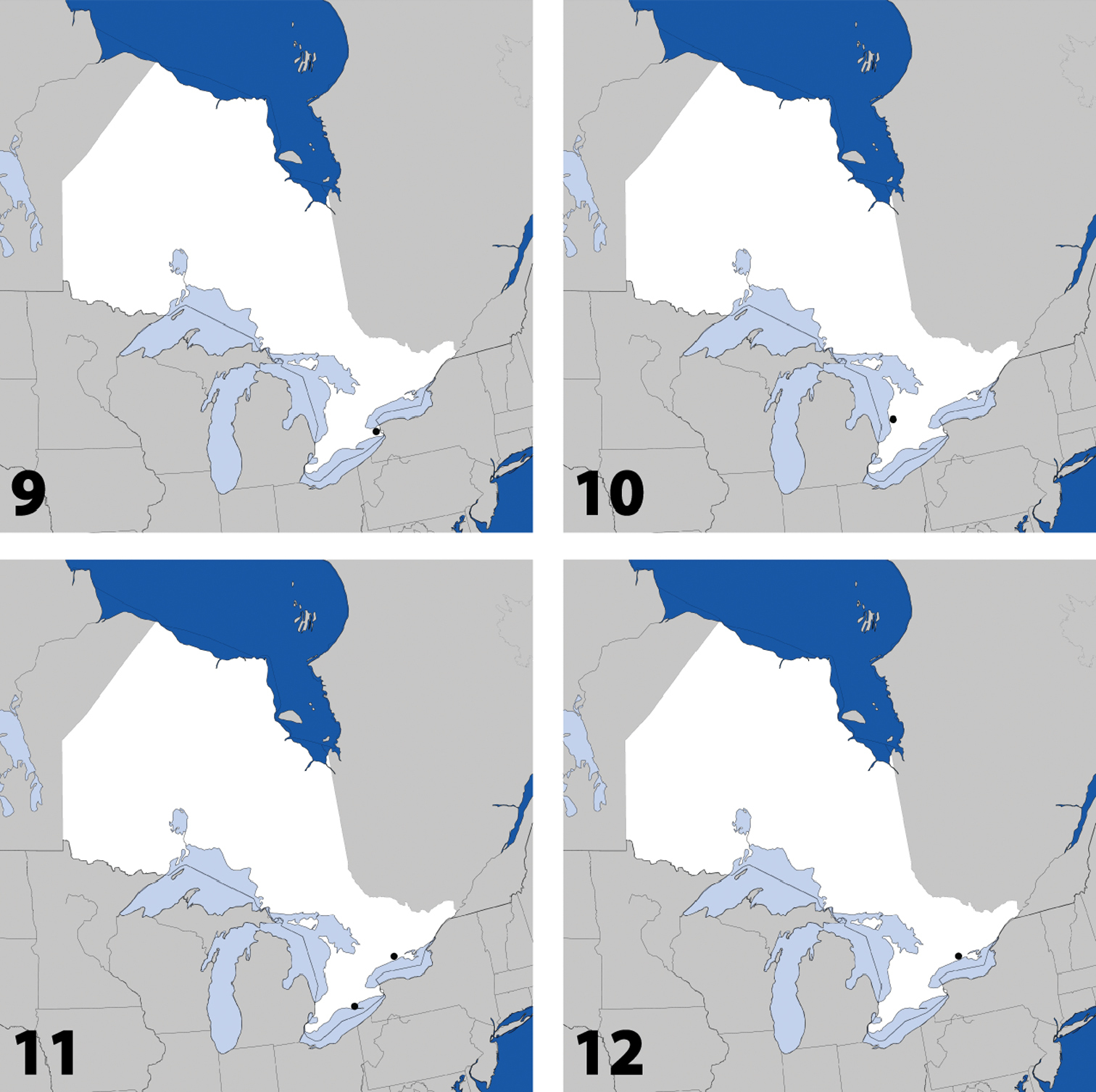
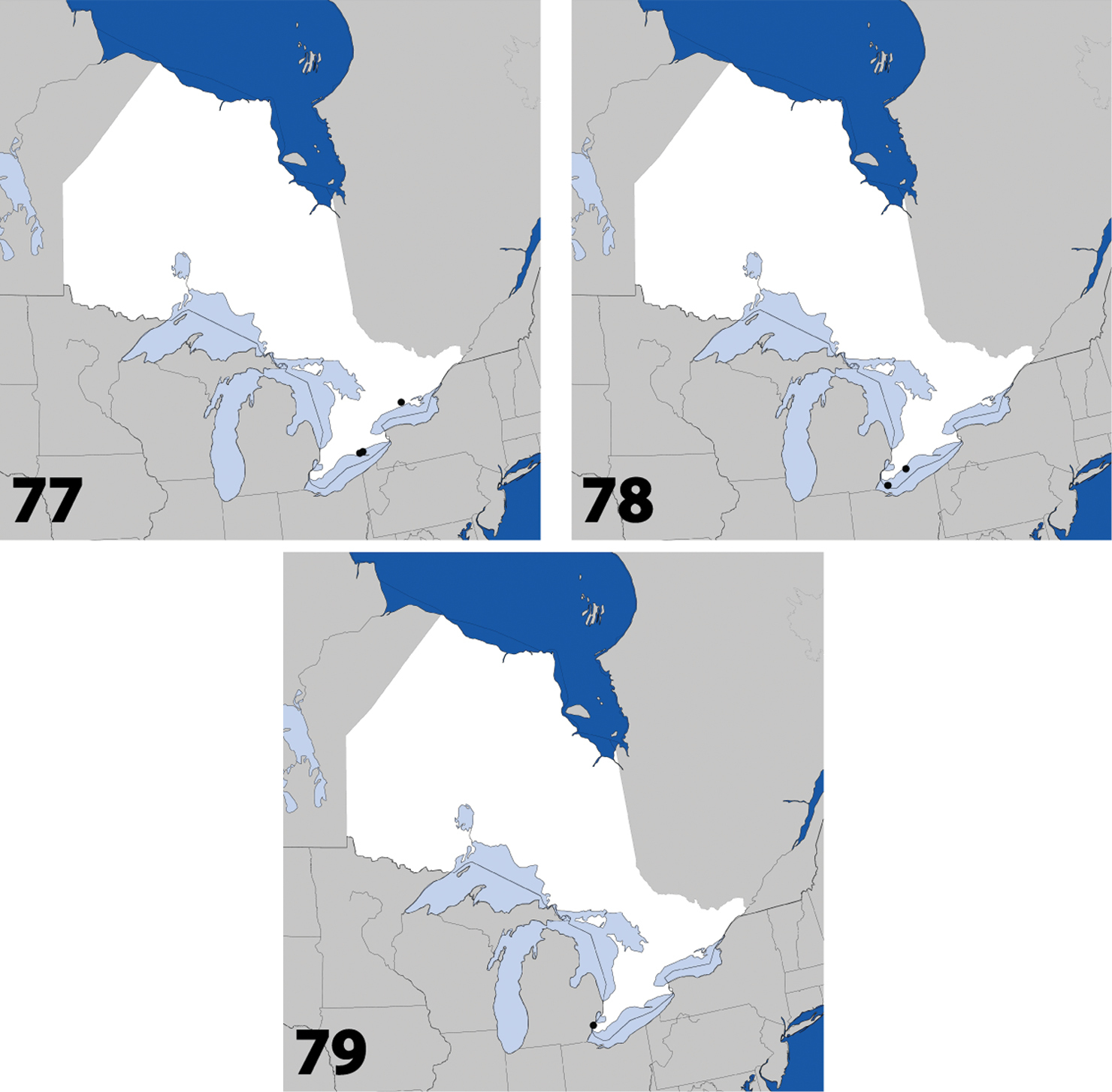
Fig. 1, Map 1, genitalia inNew Canadian Record
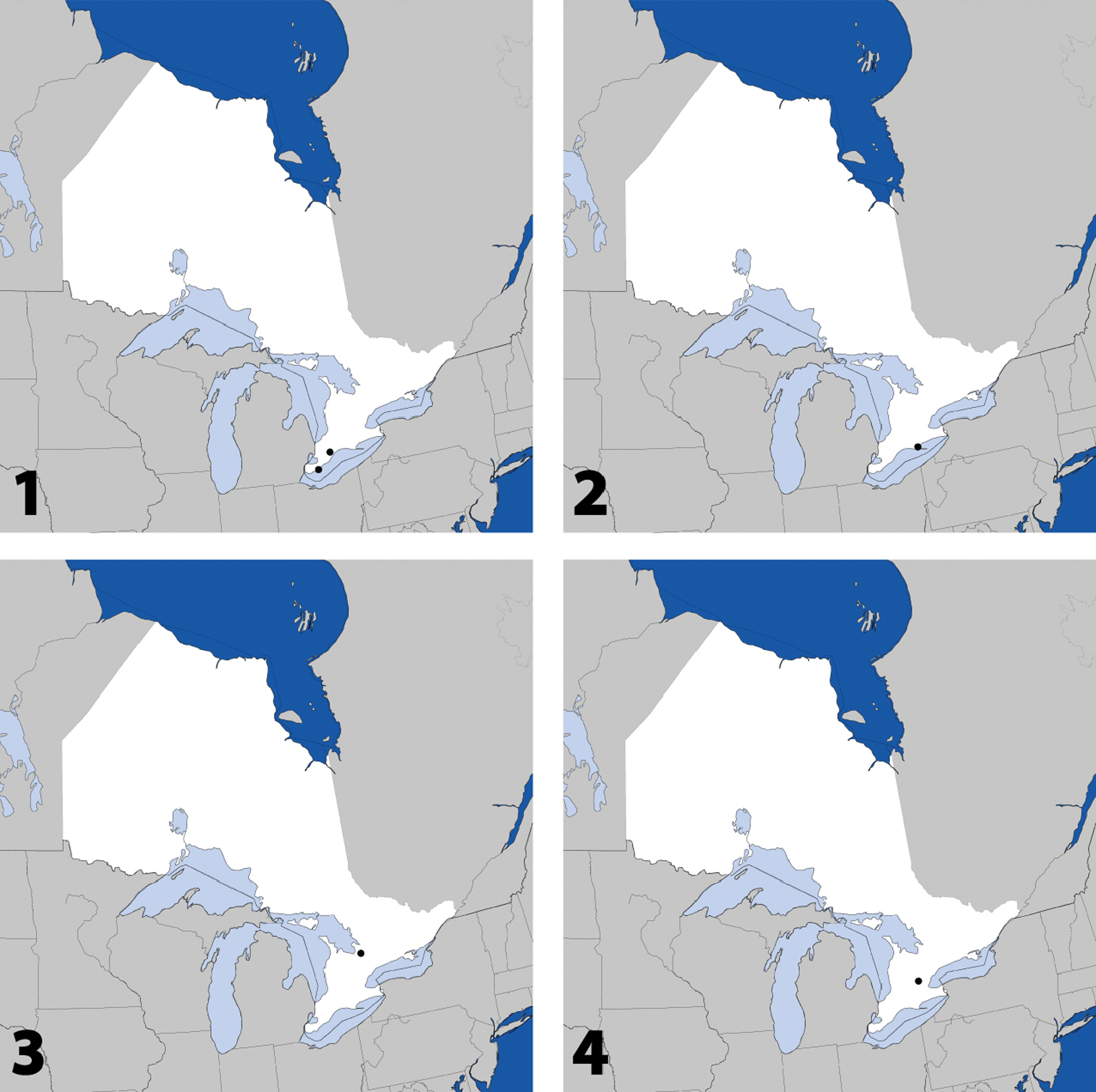
CANADA: ON: Chatham-Kent Co., Wheatley Provincial Park, treading at waters edge, 23.vii.2011, S.M. Paiero, 4 (DEBU); Elgin Co., Newport Forest, ~3km SW of Wardsville, 42°37'52"N, 81°46'43"W, 30.vii.2009, A. Brunke, 1 (DEBU).
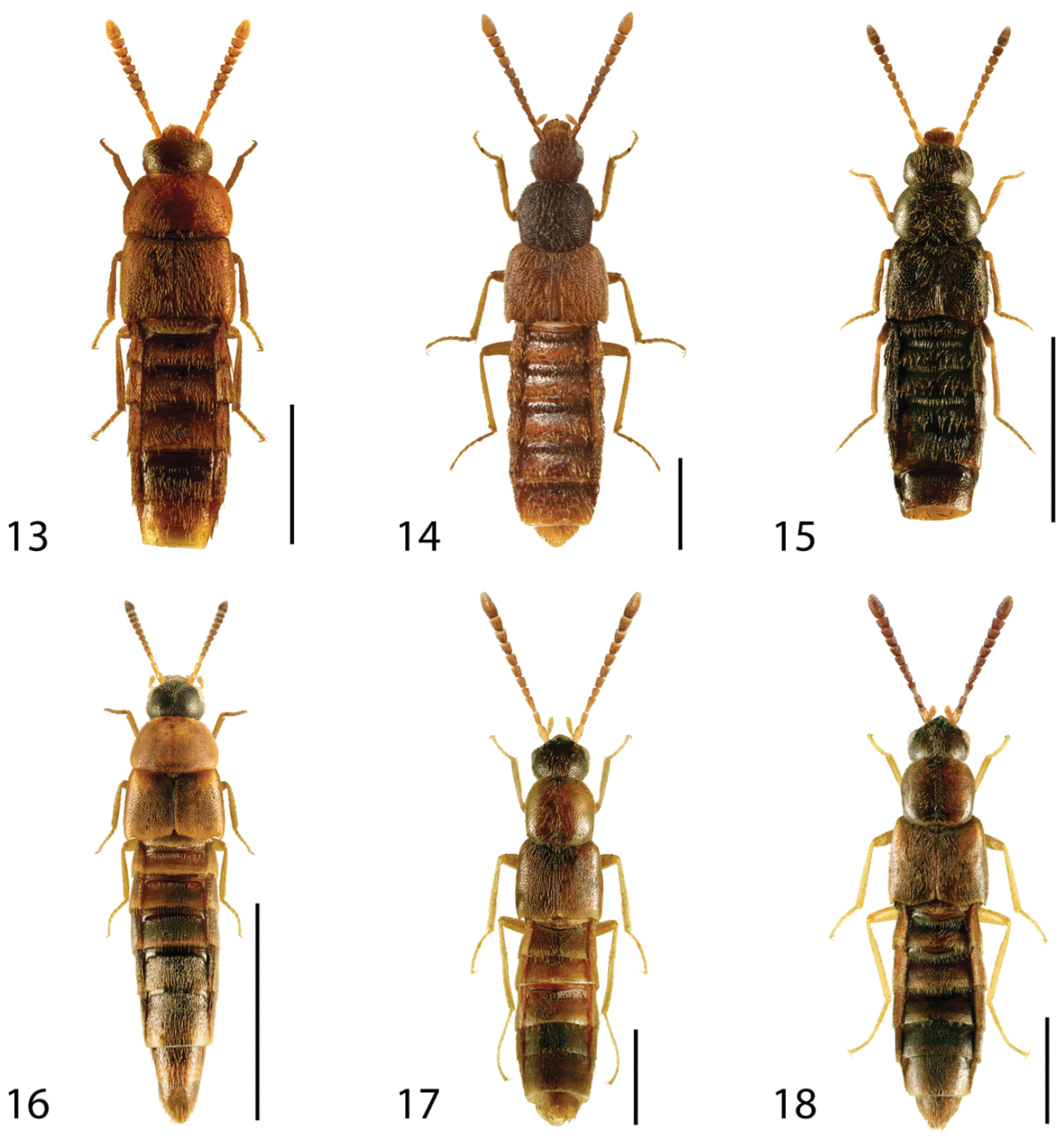
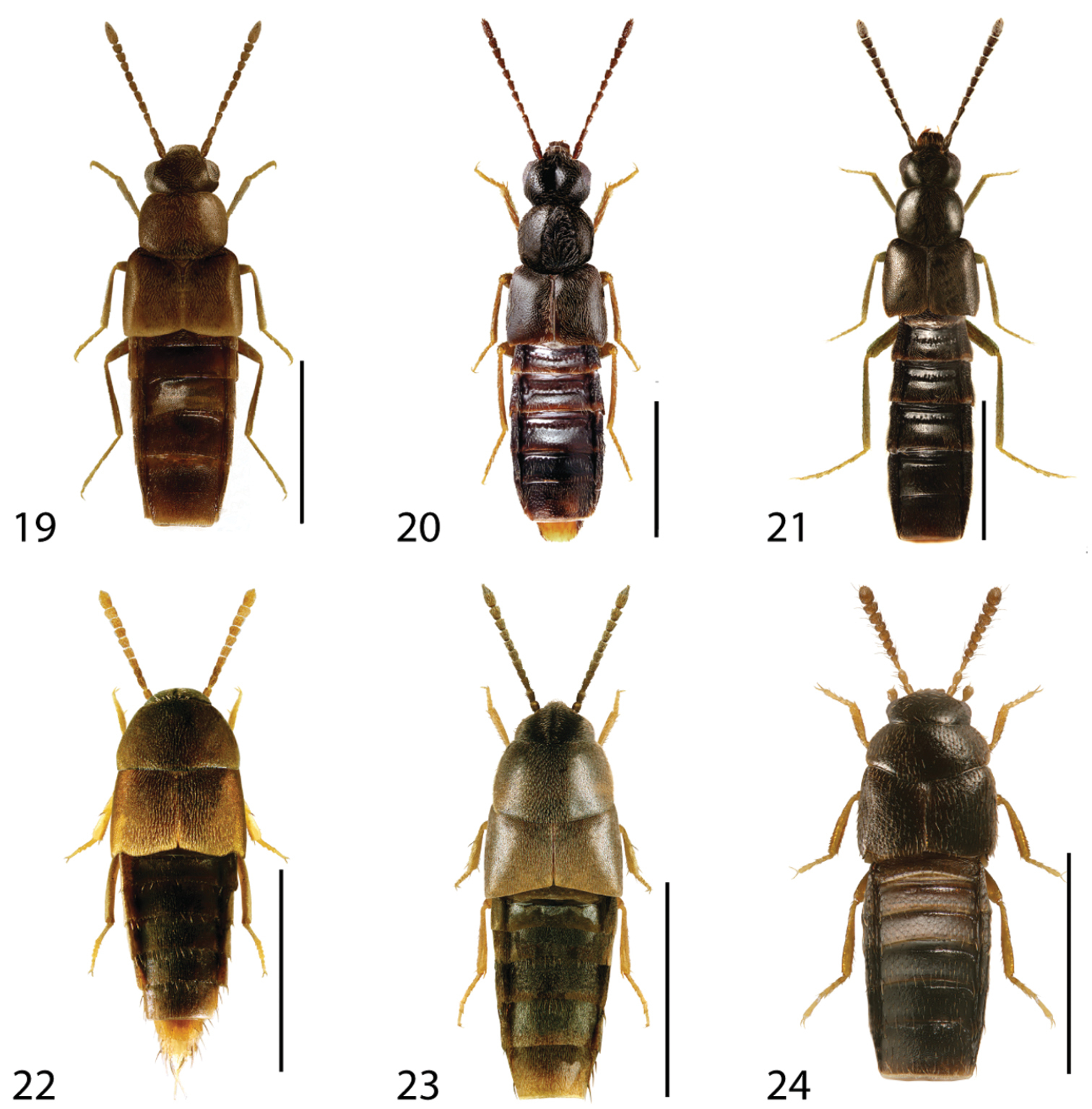
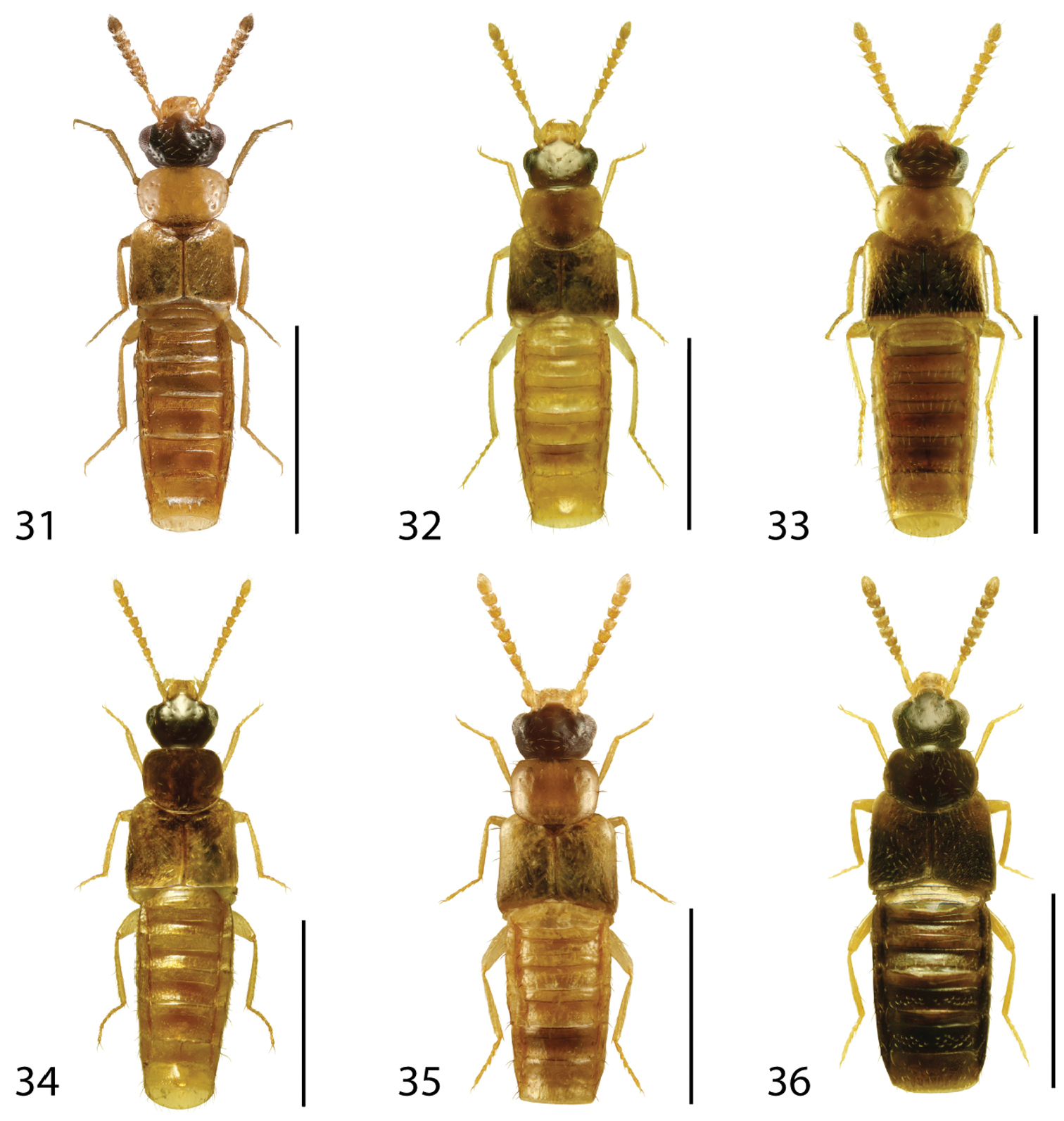
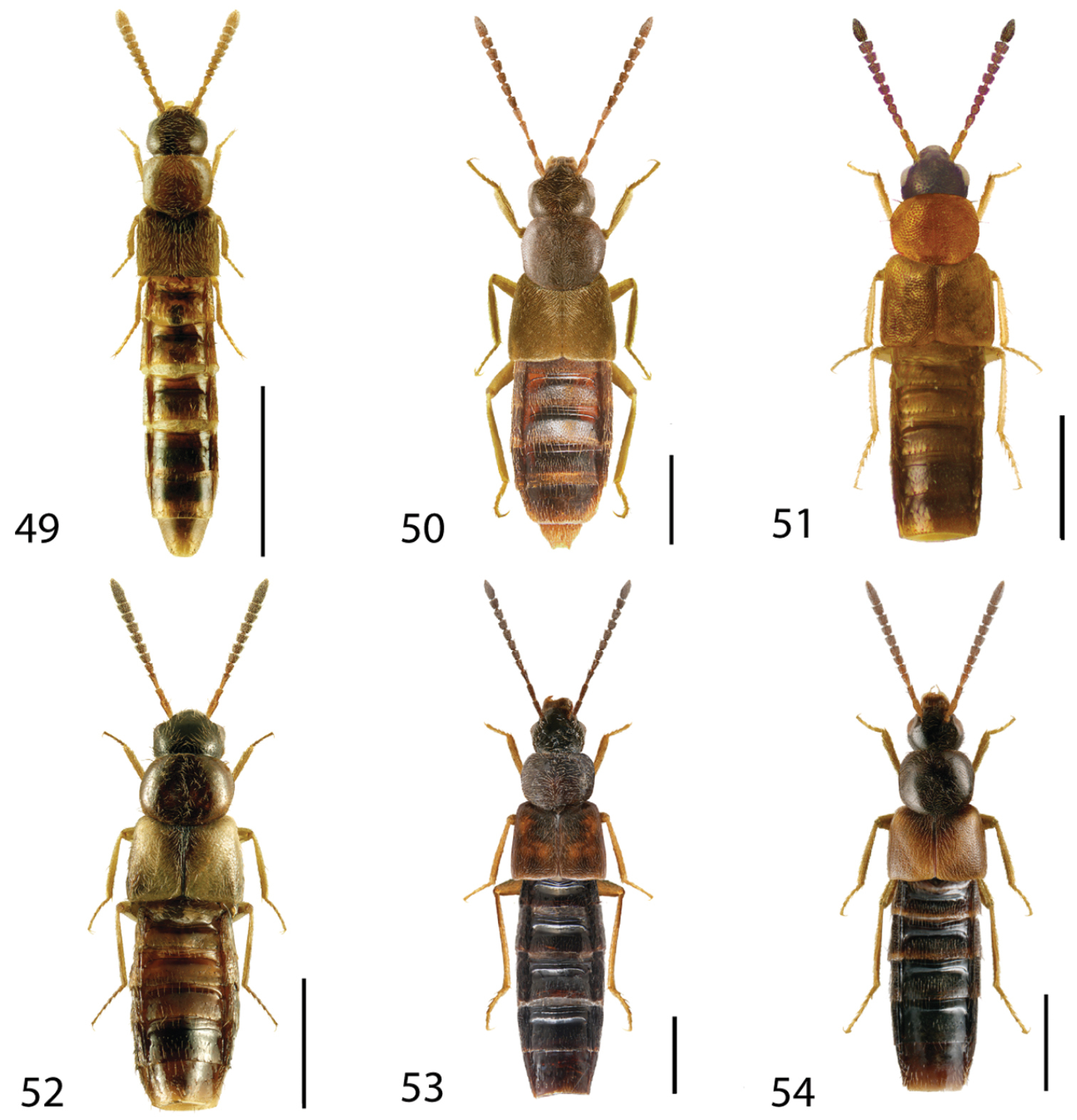
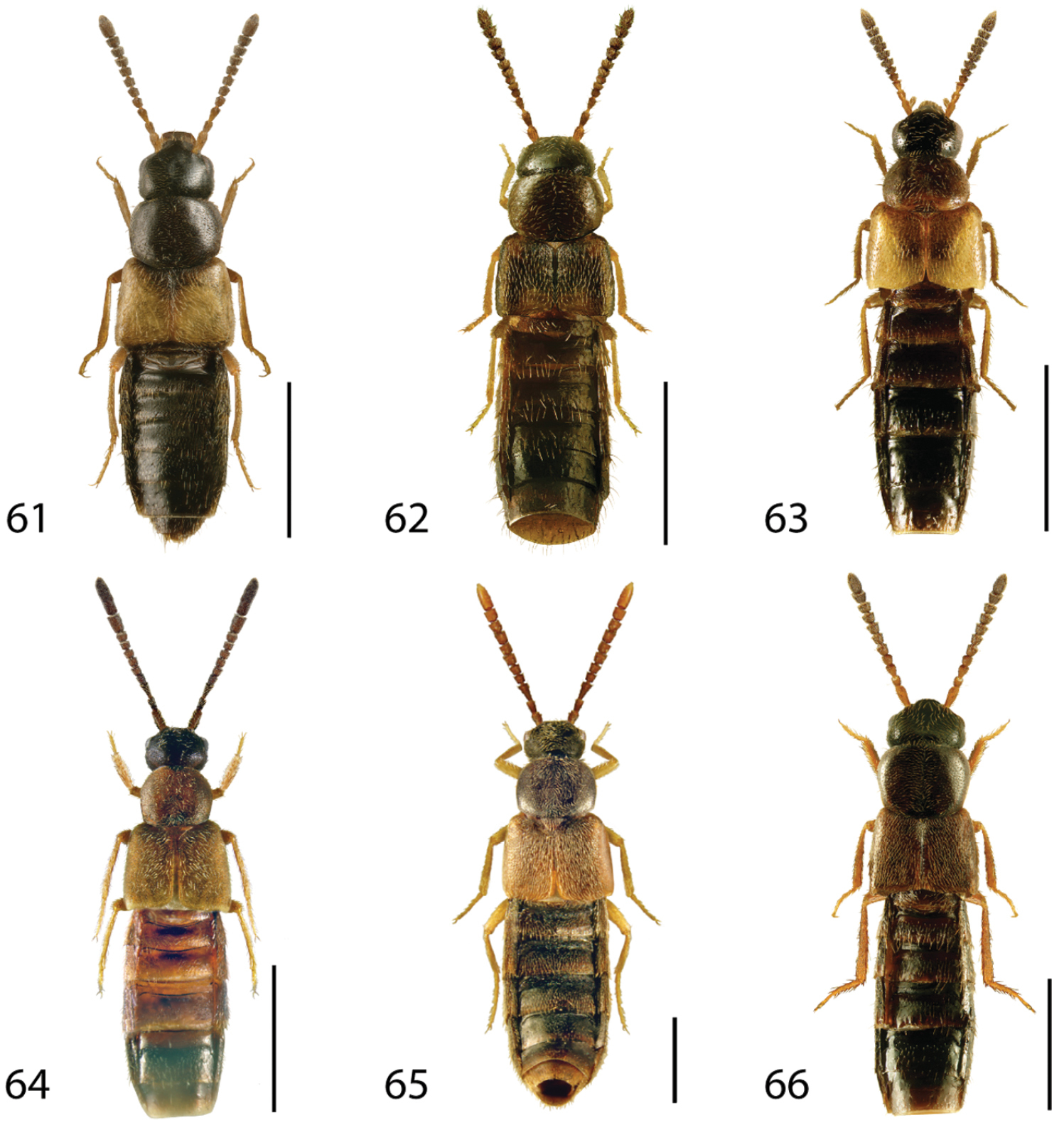
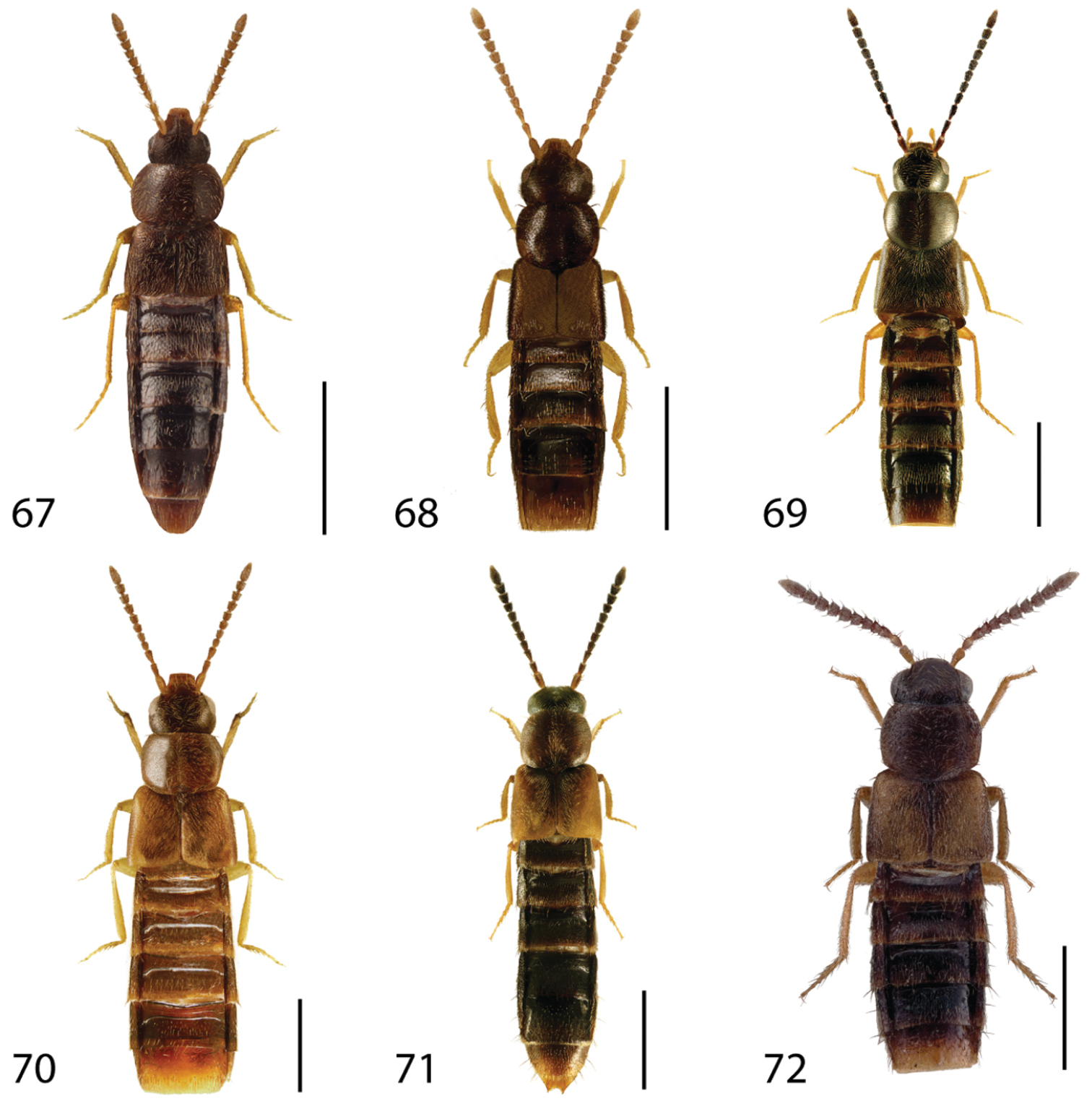
Dorsal habitus of: 1 Deinopsis illinoisensis Klimaszewski 2 Aleochara daviesi Klimaszewski & Brunke sp. n. 3 Aleochara lustrica Say 4 Aleochara tristis Gravenhorst 5 Tinotus trisectus Casey 6 Hoplandria klimaszewskii Génier. Scale 1mm.
Canada: ON; USA: CT, FL, IL, KY, LA, MA, MI, MS, OK, PA, TX (
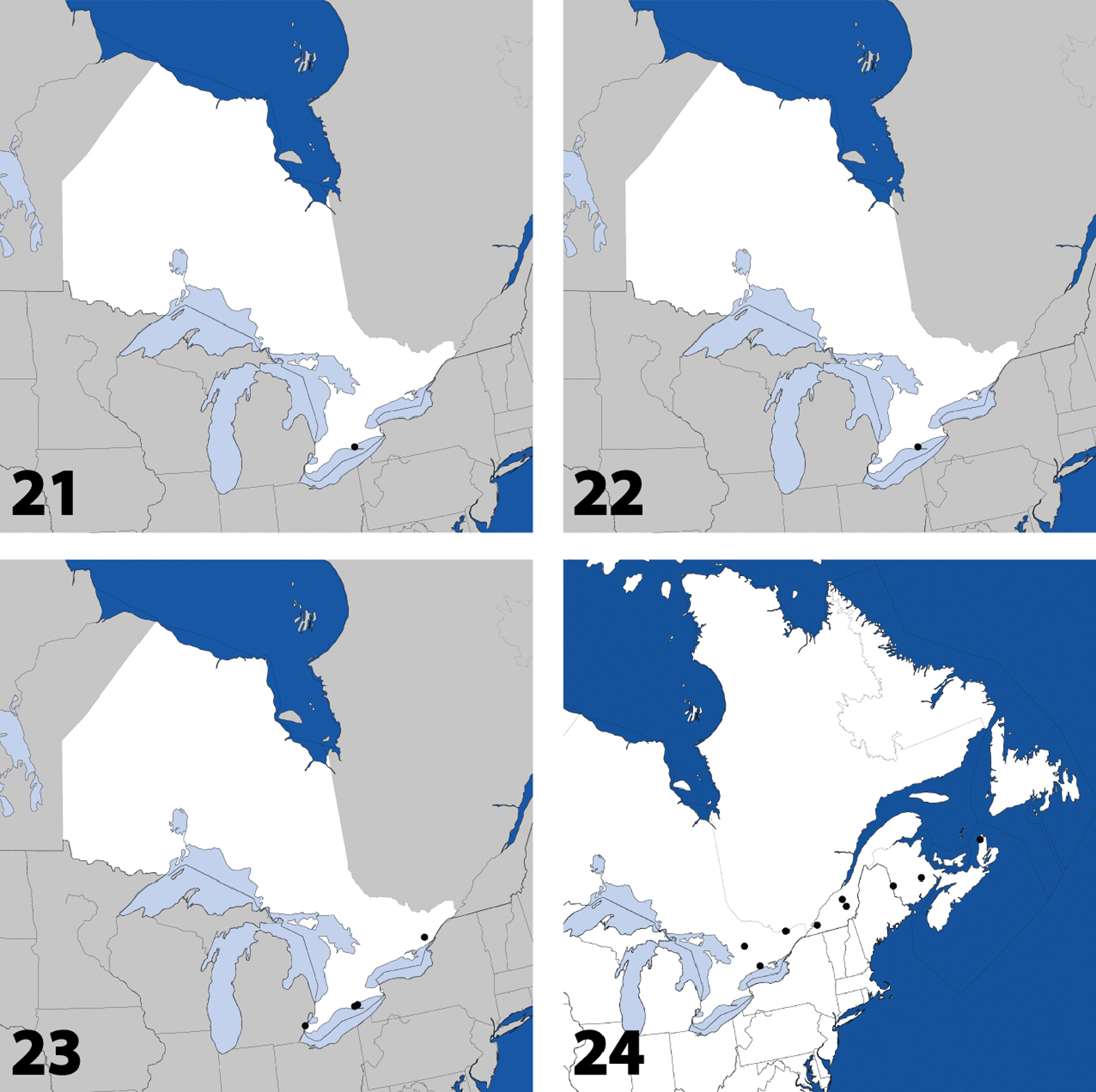
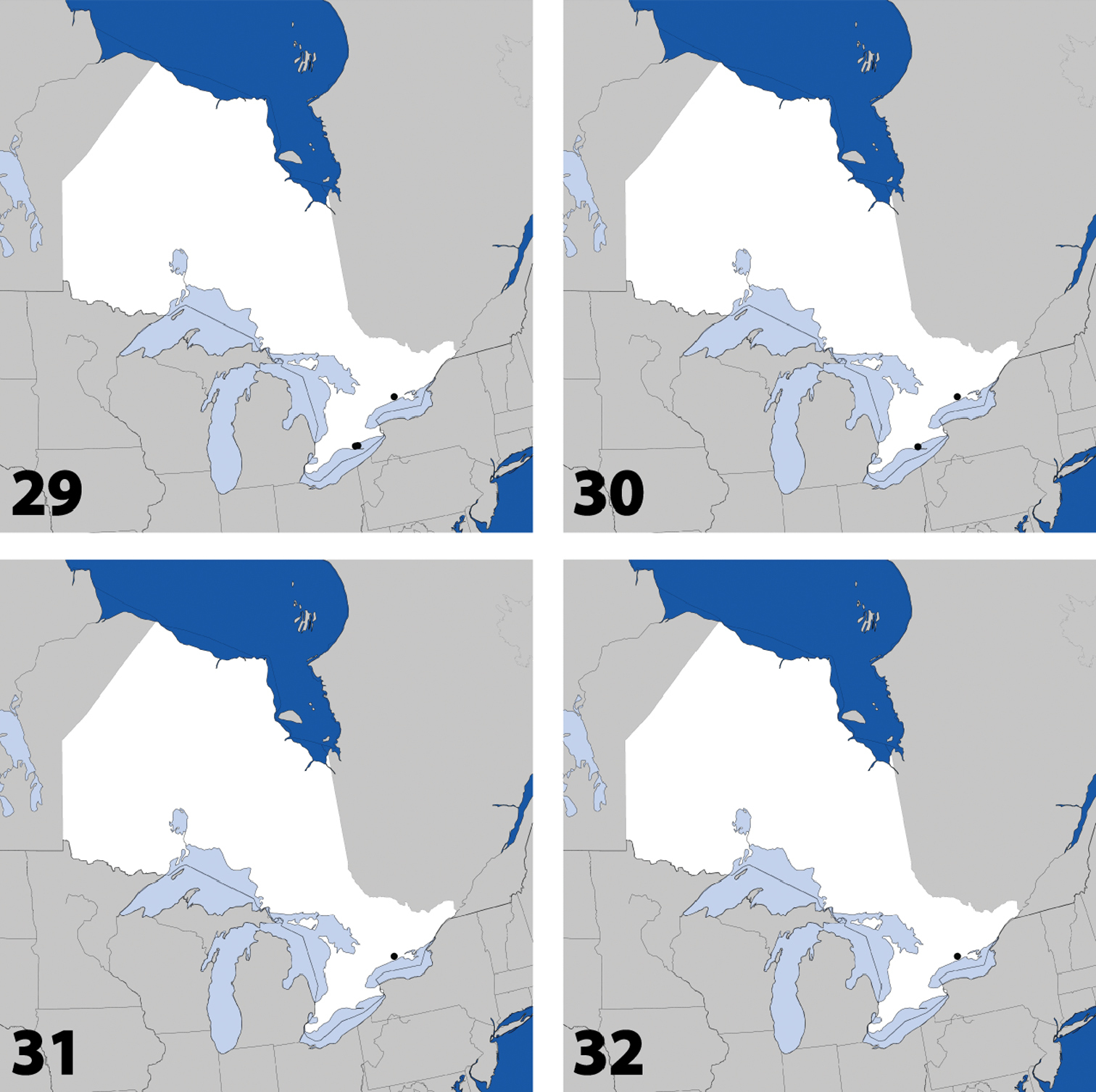
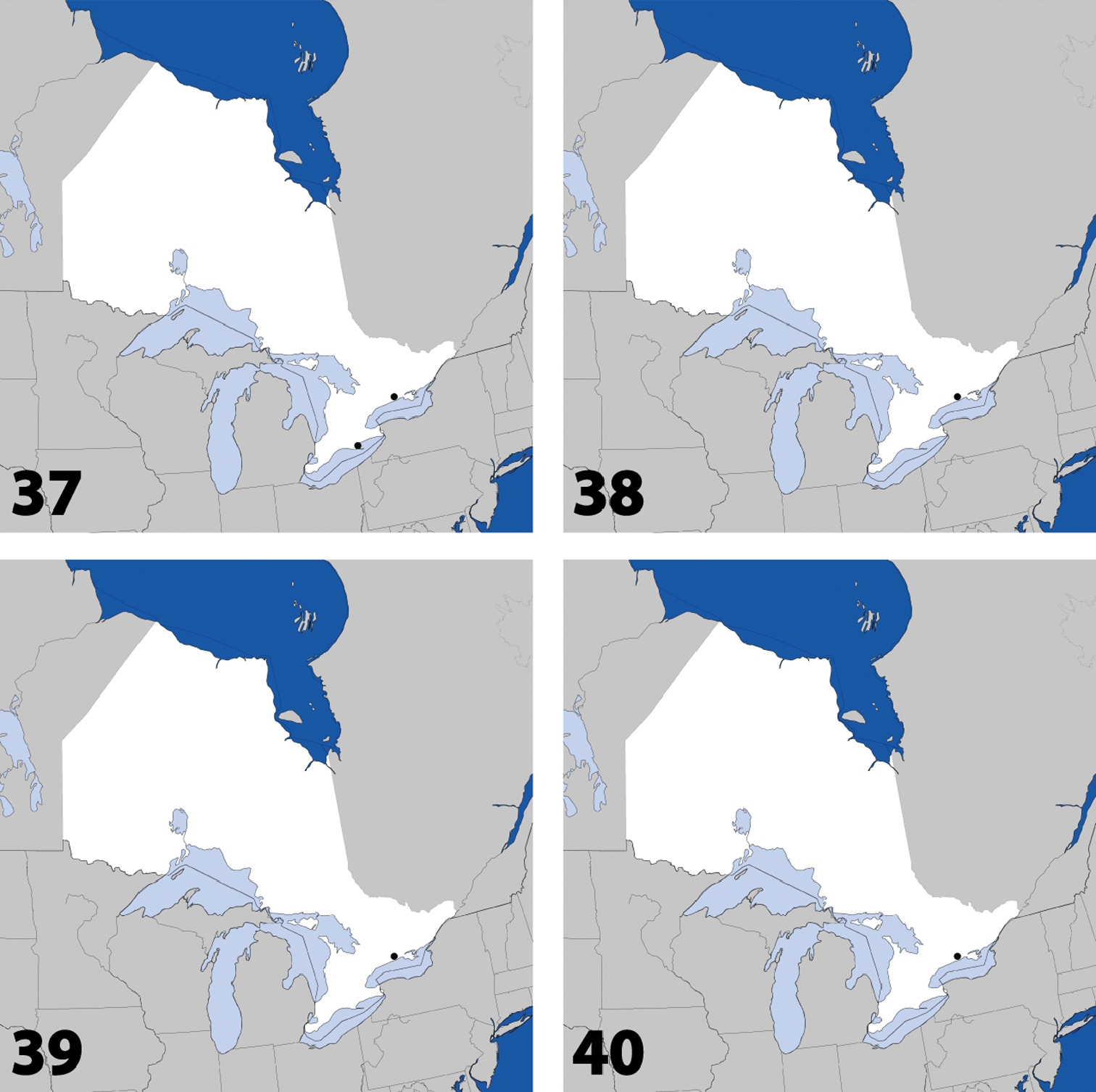
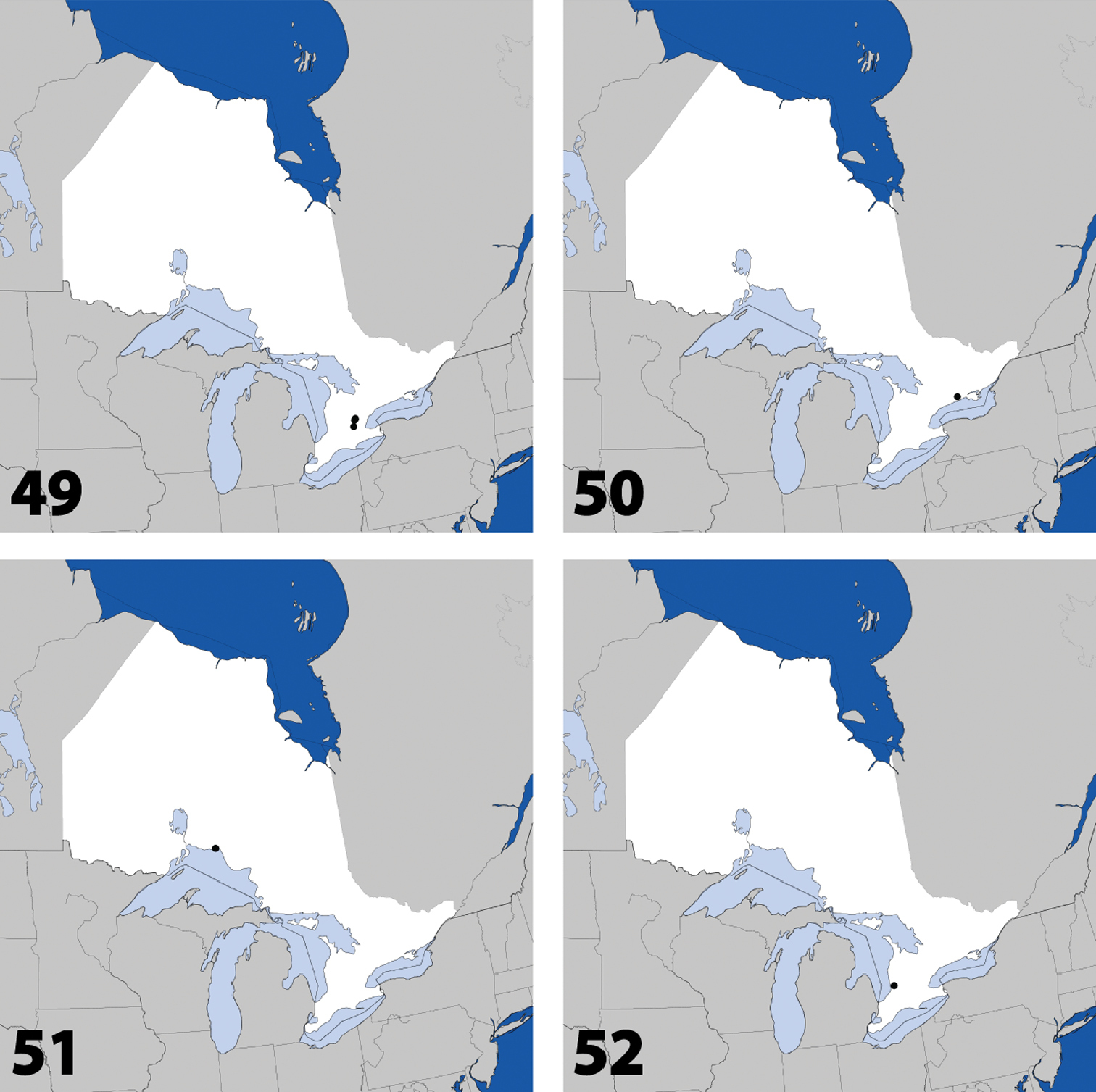
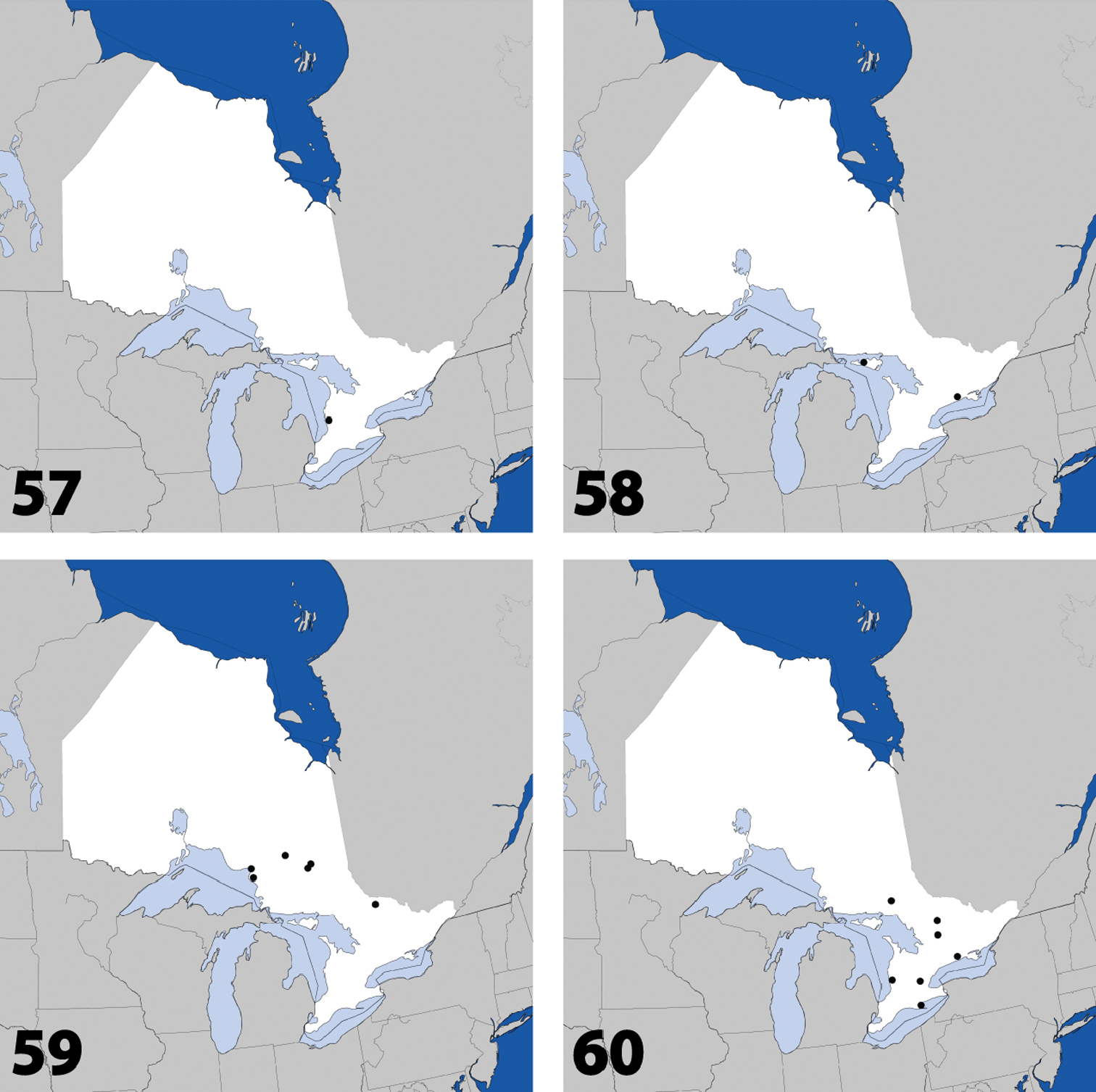
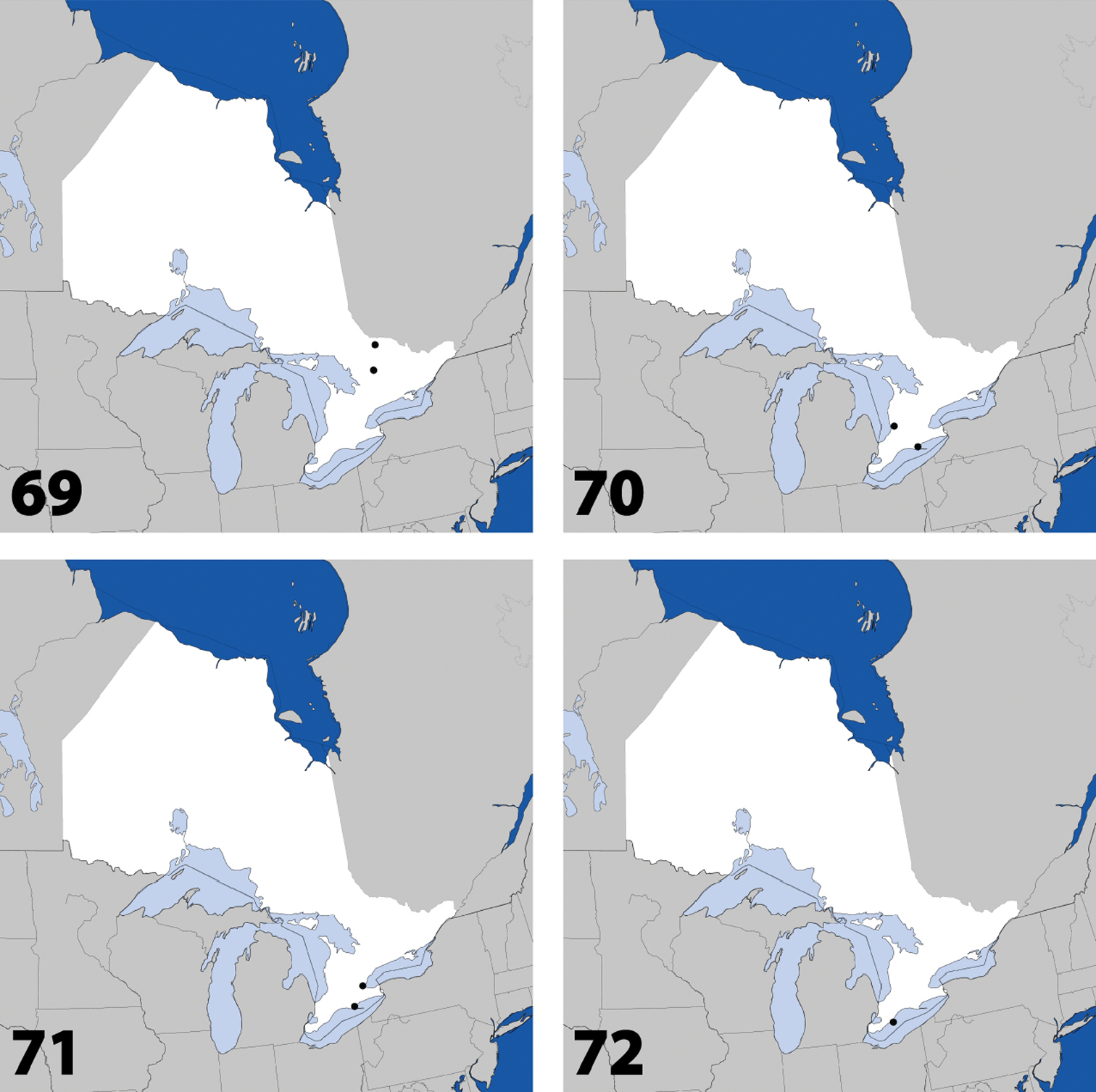
Distribution in Ontario of: 1 Deinopsis illinoisensis Klimaszewski 2 Aleochara daviesi Klimaszewski & Brunke sp. n. 3 Aleochara lustrica Say 4 Aleochara tristis Gravenhorst.
urn:lsid:zoobank.org:act:F542DCE5-7DB7-4F47-B16A-65C6189899D4
http://species-id.net/wiki/Aleochara_daviesi
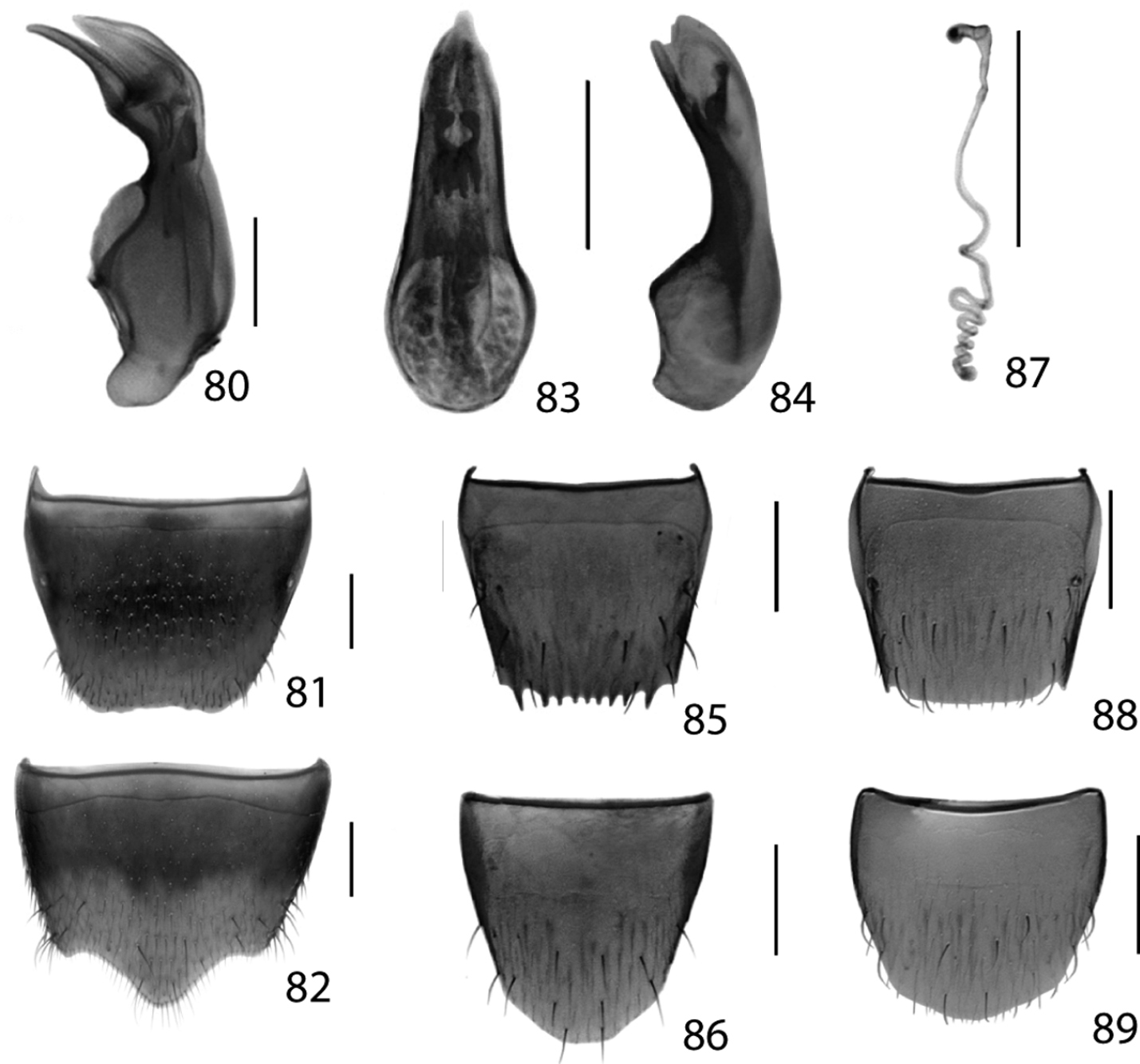
Figs 2, 80–82; Map 2Canada, Ontario, Haldimand-Norfolk Reg., 6 km W of Saint Williams, Backus Woods, slough forest, 42°40'7"N, 80°29'34"W.
Holotype (male): CANADA, ON:Hald.-Norfolk Reg., Backus Woods, North Block, 42°40'7"N, 80°29'34"W, 23.iv.2011, Brunke & Marshall, debu00340040 (DEBU).
Distinguished from other Aleochara by the following combination of characters: antennomere 4 subquadrate and 5–10 slightly transverse (Fig. 2); eyes extremely large, protruding laterally and close to frontal margin, postocular area of head about as large as eye in lateral view, postocular carina strong and complete; pronotum slightly transverse, with basal margin arcuate; elytra slightly longer than pronotum; abdomen subparallel for most of its length; basal metatarsomere slightly longer than the following tarsomere, tarsal claws exceptionally large, narrowly elongate; median lobe of aedeagus with large and narrowly elongate crista apicalis, tubus in lateral view swollen ventrally and sharply produced apically (Fig. 80). Aleochara daviesi is very similar externally to the western North American Aleochara lobata Klimaszewski from which it may be readily distinguished by the shape of the median lobe.
. Body length 4.9 mm; black with legs, elytra (except narrowly at base) and abdominal tergites VII and VIII, rust brown; punctation of forebody coarse, dense and flattened, interspaces between punctures with fine meshed microsculpture (Fig. 2); head broadest apically with very short frons and with strong and complete postocular carina, pubescence of dorsal surface directed toward midline of disc, eyes extremely large, protruding laterally, and close to frontal margin of head, postocular area about as long as eye; antennae with antennomeres 1–3 elongate, antennomere 4 subquadrate and 5–10 slightly transverse; pronotum slightly transverse, shorter than elytra, pubescence directed obliquely posteriad from midline of disc, punctation flattened and forming transversely impressed line at base of disc; elytra with posterior margin nearly straight with slight lateral emargination, pubescence directed lateroposteriad from suture; abdomen subparallel for most of its length, tergites II-IV with deep and V with shallow impression, impressions with dense punctures separated from each other by a distance equal to or less than diameter of a puncture, punctures often touching; basal metatarsomere slightly longer than the following segment; tarsal claws exceptionally large, elongate and with surface smooth.
Male. Tergite 8 bicolored, dark brown/black basally and yellowish apically, truncate apically and with margin slightly crenulate (Fig. 81); sternite eight produced apically (Fig. 82); median lobe of aedeagus in lateral view with large and elongate bulbus produced ventrally at base, crista apicalis narrowly elongate and large, tubus swollen ventrally and sharply produced apically (Fig. 80).
Female: Unknown.
Presently known only from Backus Woods, an old growth deciduous forest in southern Ontario. Aleochara daviesi almost certainly occurs in the eastern United States and elsewhere in southern Canada.
The holotype was collected by submerging forest litter near the margins of forest pools (some permanent). Other members of the subgenus Echochara are inhabitants of mammal burrows or caves (
This species is dedicated to our colleague Anthony Davies (CNC, Ottawa, Ontario, Canada) in recognition of his contribution to the knowledge of Canadian Staphylinidae and in appreciation of his assistance over the years in specimen loans, distributional records and curatorial matters, especially those relevant for this project.
http://species-id.net/wiki/Aleochara_lustrica
Fig. 3, Map 3, genitalia inCANADA: ON:Simcoe Co., Midhurst, 28.ix.2008, carrion, forest nr. Neretva St., A. Brunke, 1 (DEBU).
Canada: ON; USA: AL, AZ, AR, DC, FL, GA, IL, IN, KS, KY, LA, MD, MA, MI, MO, MS, NC, NH, NJ, NY, OH, OK, PA, SC, TN, TX, VA, WV, WI.Also known from Mexico and South America (Trinidad and Tobago) (
http://species-id.net/wiki/Aleochara_tristis
Fig. 4, Map 4, genitalia inNew Ontario Record
CANADA: ON:Wellington Co., Guelph, field, 20.ix.1984, Brian Wisenden, 1 (DEBU).
Canada: ON, QC, NB, NL; USA: CA, MN, NE, PA, VT. Widespread in Palaearctic, Oriental and Afrotropical Regions (
Aleochara tristis was intentionally released in the United States in 1965 to control populations of Face Fly (Musca autumnalis DeGeer), a nuisance pest of and disease vector for livestock, which breeds in their dung (
http://species-id.net/wiki/Tinotus_trisectus
Fig. 5, Map 5, genitalia inCANADA: ON:Bruce Co., Port Elgin, 15.vii.1980, P.F. Karrow, 1 (DEBU); Chatham-Kent Co., Glencoe, carrot field, pitfall, 17.v.2007, A. Brunke, 1 (DEBU); Hald.-Norfolk Reg., Turkey Point Prov. Park, site 2, 42°42'28"N, 80°20'29"W, savannah, at lights, 5.vii.2011, Brunke & Paiero, 1 (DEBU); Wellington Co., Guelph, Victoria Rd. & Conservation Line, soybean field, pitfall, 4.viii.2009, A. Brunke, 2 (DEBU), Guelph, woodland edge, 9.x.1991, C.S. Blanev, 1 (DEBU).
Canada: ON; USA: AZ, CA, ID, NY, OR, PA, TN (
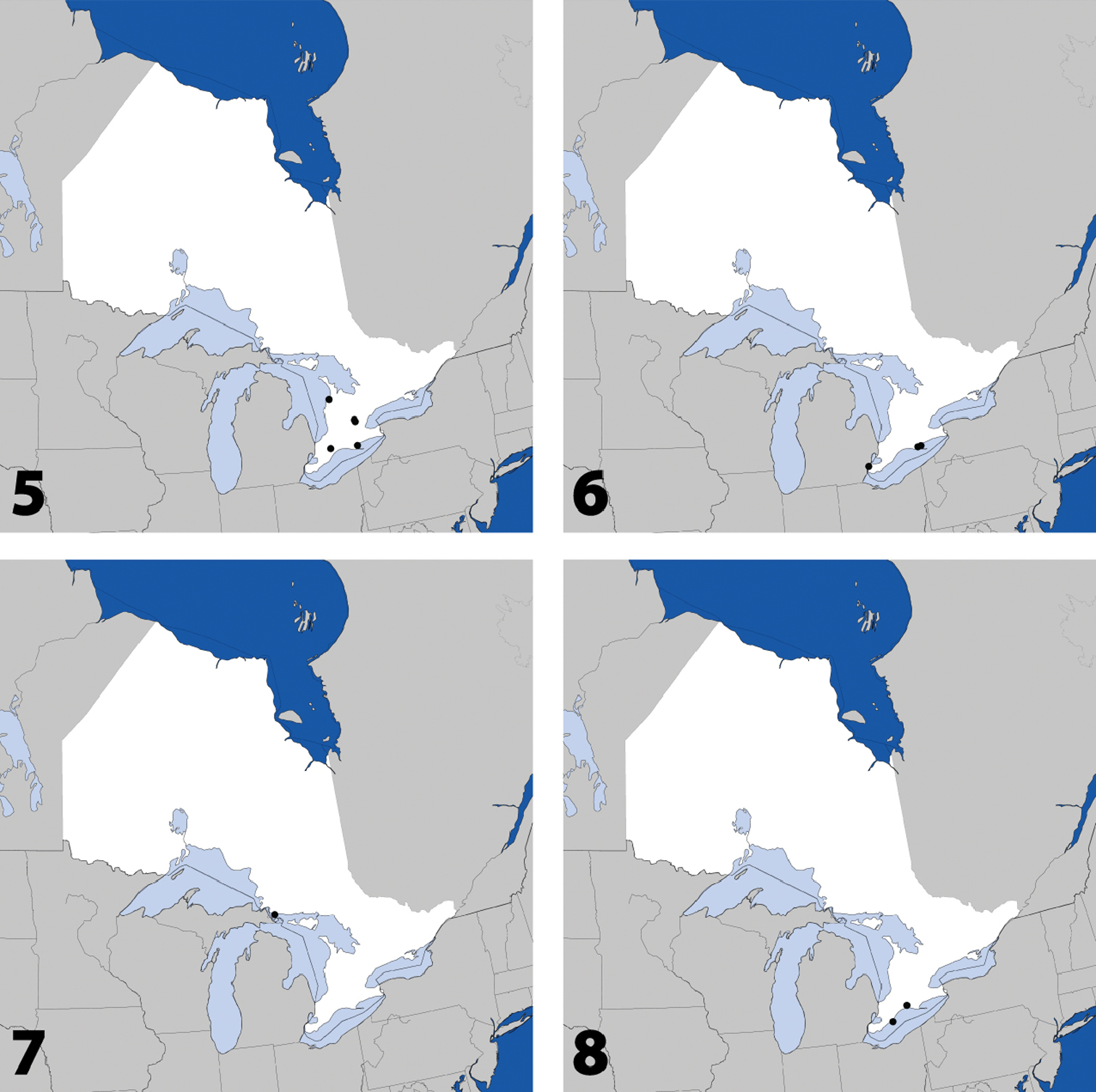
Distribution in Ontario of: 5 Tinotus trisectus Casey 6 Hoplandria klimaszewskii Génier 7 Hoplandria laevicollis (Notman) 8. Hoplandria laeviventris Casey.
This species may be distinguished from all eastern Tinotus but Tinotus caviceps based on the combination of reddish body and elytra with short, bristle-like setae that are directed obliquely laterad (
Tinotus trisectus appears to prefer open habitats including woodland edges, agricultural fields and oak savannah. Previously, nothing was known about its habitat associations. This species is probably broadly distributed across North America, reaching its northern limit in southern Canada.
http://species-id.net/wiki/Hoplandria_klimaszewskii
Fig. 6, Map 6, genitalia inCANADA: ON:Essex Co., Windsor, Ojibway Prairie, unburnt forest, yellow pans, 19 to 22.vi.2001, S.M. Paiero, 1 (DEBU); Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'21"N, 80°29'26"W, forest, at lights, 20.vii.2011, Brunke & Paiero, 1 (DEBU), Turkey Point Prov. Park, site 1, 42°41'48"N, 80°19'48"W, forest, malaise pans, 15.vi to 5.vii.2011, Brunke & Paiero, 1 (DEBU).
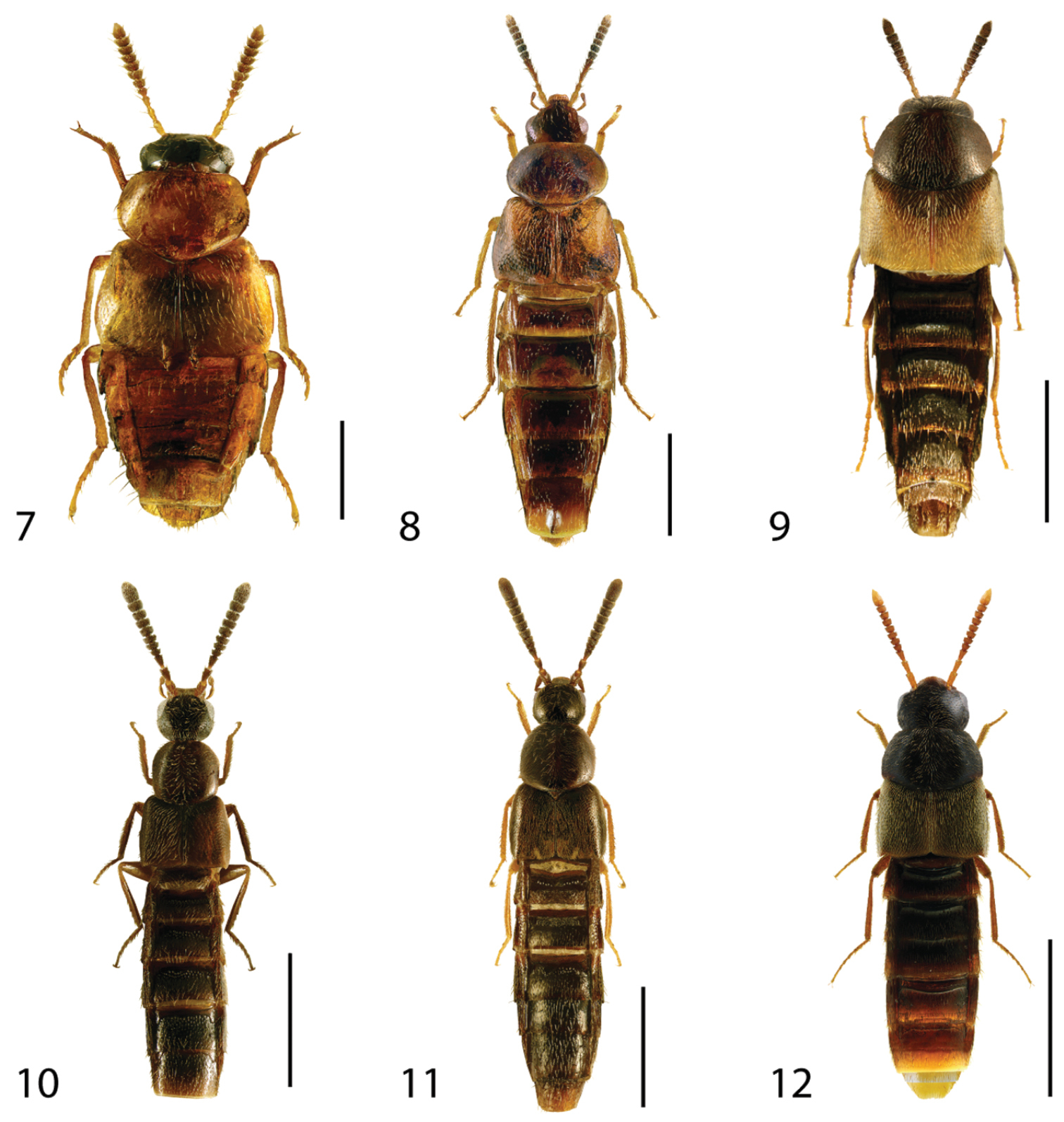
Canada: ON, QC; USA: AR, DC, FL, IL, MD, NJ, NY, NC, VA, WV (
http://species-id.net/wiki/Hoplandria_laevicollis
Fig. 7, Map 7, genitalia inCANADA: ON:Algoma Distr., Hilton Beach, hardwood forest and field, malaise, 14 to 17.vii.1987, F.W. & J.H. Swann, 1 (DEBU).
Dorsal habitus of: 7 Hoplandria laevicollis (Notman) 8 Hoplandria laeviventris Casey 9 Platandria carolinae Gyllenhal 10 Amarochara brevios Assing 11 Amarochara fenyesi Blatchley 12 Crataraea suturalis (Mannerheim). Scale 1mm.
Canada: ON, QC; USA: DC, FL, GA, LA, NC, NJ, NY, VA (
http://species-id.net/wiki/Hoplandria_laeviventris
Fig. 8, Map 8, genitalia inCANADA: ON: Chatham-Kent Co., Rondeau Prov. Park, int. tr. 4 (=intercept trap 4), in a white pine stand, 2.vi to 6.vi.1985, L. LeSage & A. Smetana, 1 (CNC); Elgin Co., Orwell, 15.vi.1978, J.M. Cumming, 1 (DEBU).
Canada: ON; USA: AL, AR, CT, DC, GA, IL, IN, KY, LA, MA, MD, NJ, NY, NC, OH, PA, TN, TX, VA, WV (
http://species-id.net/wiki/Platandria_carolinae
Fig. 9, Map 9, genitalia inCANADA: ON:Lincoln Co., Short Hills, Wildlife Pres., 1 mi E of N. Pelham, flowers of ‘Cornis florida’ L., 5.vi.1973, H. Frania, 2 (CNC).
Canada: ON; USA: DC, GA, IL, IN, IA, KA, LA, NE, NJ, NC, PA, TN, VA (
Distribution in Ontario of: 9. Platandria carolinae Gyllenhal 10. Amarochara brevios Assing 11 Amarochara fenyesi Blatchley 12 Crataraea suturalis (Mannerheim).
This is the only eastern species of Platandria (
http://species-id.net/wiki/Amarochara_brevios
Fig. 10, Map 10, genitalia inCANADA: ON:Huron Co., Auburn, hedgerow, pitfall trap, 26.v.2010, A. Brunke, 1 (DEBU), Auburn, soybean field, 23.vi.2010, 1 (DEBU), same data except: 7.vii.2010, 1 (DEBU), 4.viii.2010, 3 (DEBU).
Canada: ON; USA: KS (
This species is distinguished from other Nearctic Amarochara based on the extremely dense punctation of the abdominal tergites, weak microsculpture of the forebody and shape of the median lobe of the aedeagus in lateral view.
Amarochara brevios was previously known only from the holotype collected in Kansas via flight intercept trap. Here, we report this species from Ontario, Canada based on numerous specimens collected using pitfall and raised pan traps in soybean fields (only 6 specimens kept as vouchers). While Amarochara inquilina (Casey) and Amarochara formicina Assing are associated with mound-building Formica ants, other species of the genus appear to be general inhabitants of decaying litter and only occasionally inhabit ant nests (
http://species-id.net/wiki/Amarochara_fenyesi
Fig. 11, Map 11, genitalia inCANADA: ON:Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'18"N, 80°29'24"W, low forest, malaise pans, 5 to 17.viii.2011, Brunke & Paiero, 1 (DEBU), same data except: 42°40'20"N, 80°29'29"W, ridge forest, malaise pans, 17.viii to 1.ix.2011, 1 (DEBU), 42°40'20"N, 80°29'29"W, ridge forest, malaise pans, 1.ix to 20.ix.2011, Brunke & Paiero, 1 (DEBU; Northumberland Co., Peter's Woods Prov. Nat. Res., 44°7'26"N, 78°2'31"W, forest, malaise pans, backwoods, 19.v to 1.vi.2011, Brunke & Paiero, 1 (DEBU), same data except: front woods, 16 to 27.vi.2011, 1 (DEBU), 12 to 26.vii.2011, 1 (DEBU), 12 to 26.viii.2011, 2 (DEBU).
Canada: ON; USA: GA, IN, KS (
This species can by distinguished from other Nearctic Amarochara by the following combination of characters: head and pronotum with weak microsculpture; first segment of metatarsus about as long as second to fourth segments combined; punctation of abdominal tergites sparse (
All specimens of this species with collection data were collected in forested reserves using flight intercept traps (
http://species-id.net/wiki/Crataraea_suturalis
Fig. 12, Map 12, genitalia inCANADA: ON:Northumberland Co., Barr property, 7 km NE Centreton, site 1, 44°7'40"N, 77°58'57"W, savannah, malaise pans, 16 to 27.vi.2011, Brunke & Paiero, 1 (DEBU).
Canada: BC, SK, ON, NB, NS, NL; USA: CA, IA, IL, IN, MA, MO, PA, SC, VA, VT; widespread in Palaearctic (
http://species-id.net/wiki/Dexiogyia_angustiventris
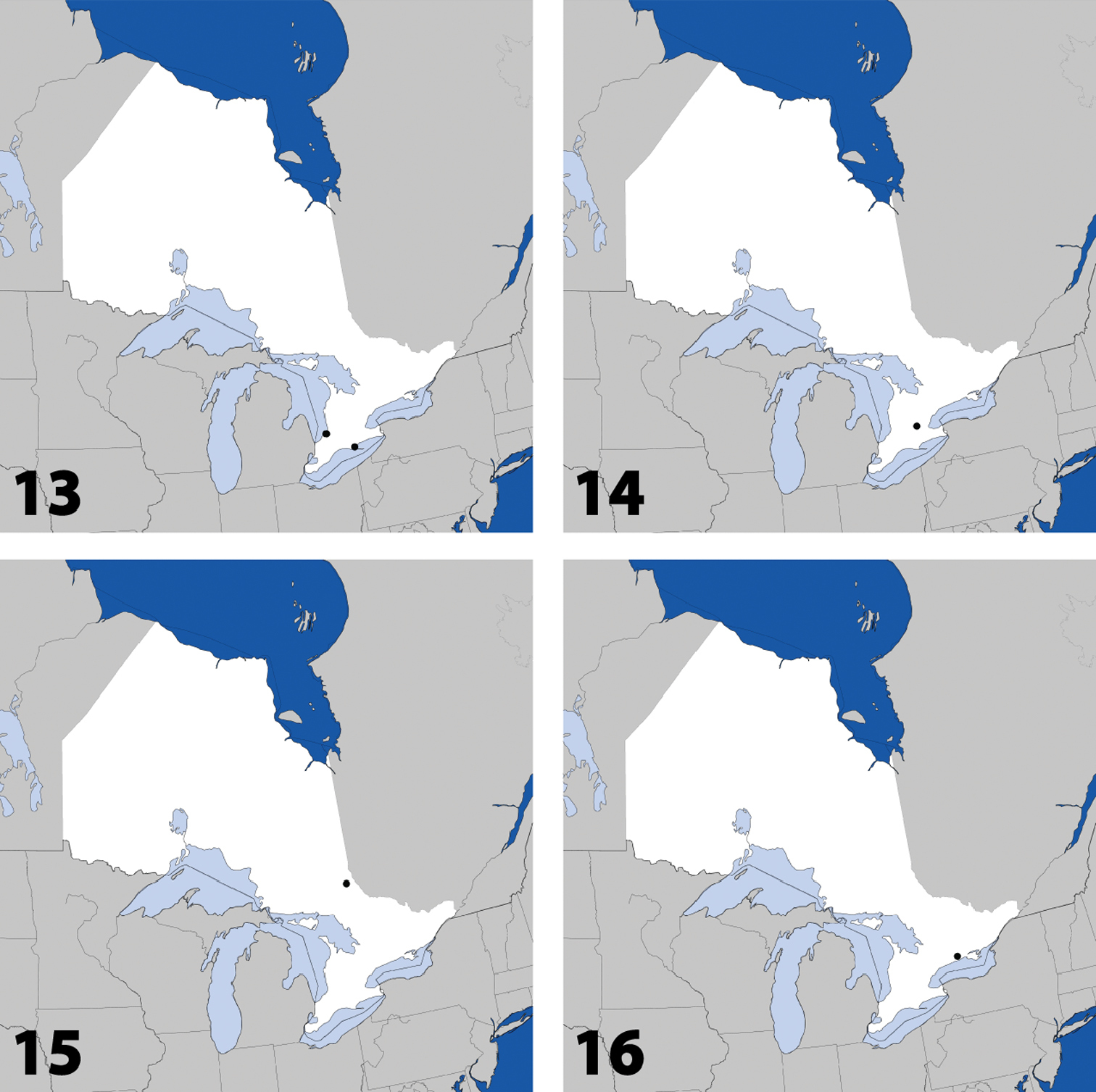
Figs 13, 83–89; Map 13(Type material – see above). CANADA: ON:Hald.-Norfolk Reg., Cronmiller prop., 6 km W St Williams, site 2, 42°40'18"N, 80°29'24"W, forest, malaise pans, 17.v to 31.v.2011, A. Brunke & S.M. Paiero, 1 (DEBU) same data except: 31.v. to 15.vi; Cronmiller prop., 6 km W St Williams, 42°40'21"N, 80°29'26"W, forest, 5.vii.2011, A. Brunke, 1 (DEBU); Lambton Co., Pinery Prov. Pk., under white pine bark, 17.iv.2010, A. Brunke, 2 (DEBU).
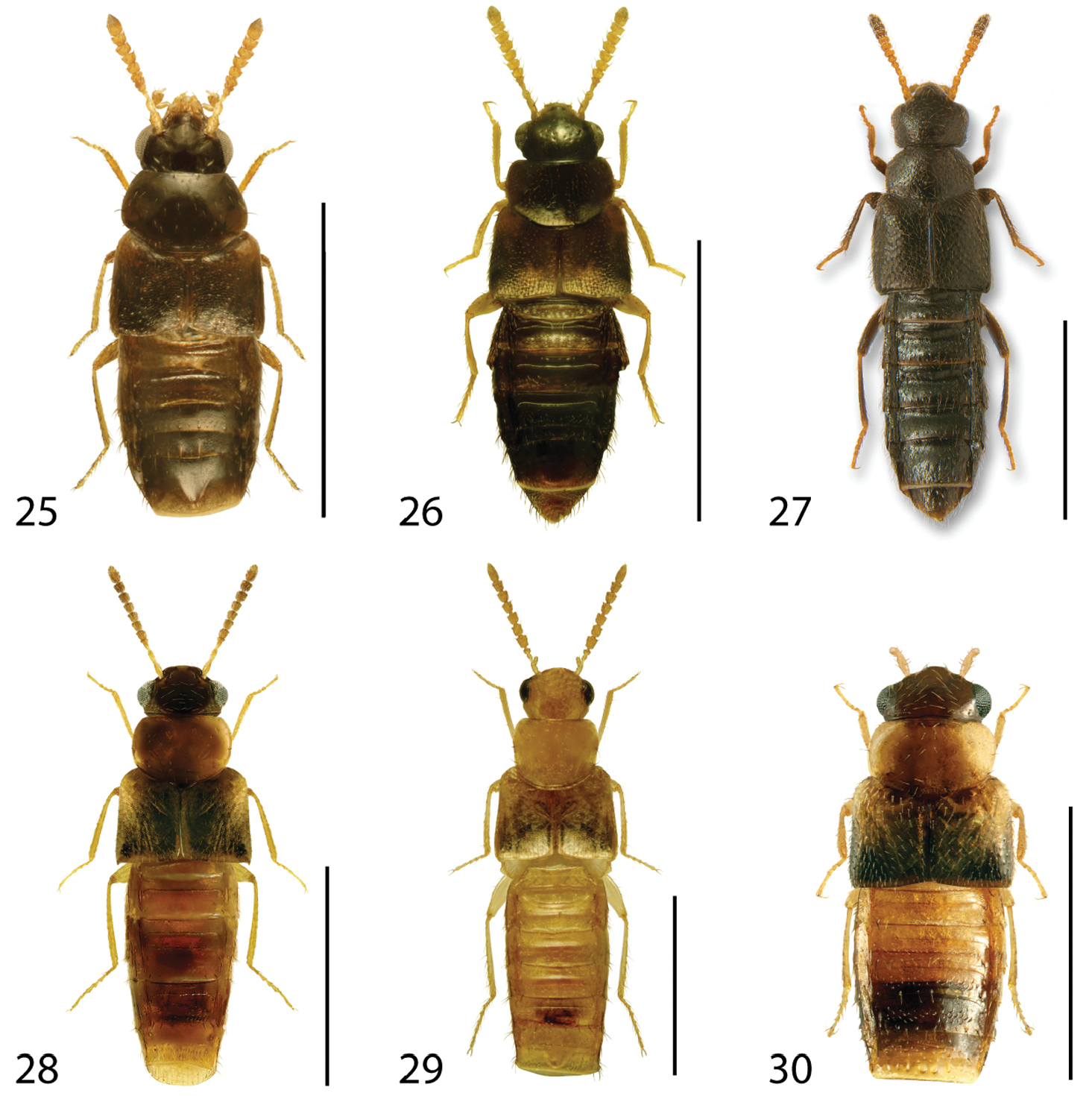
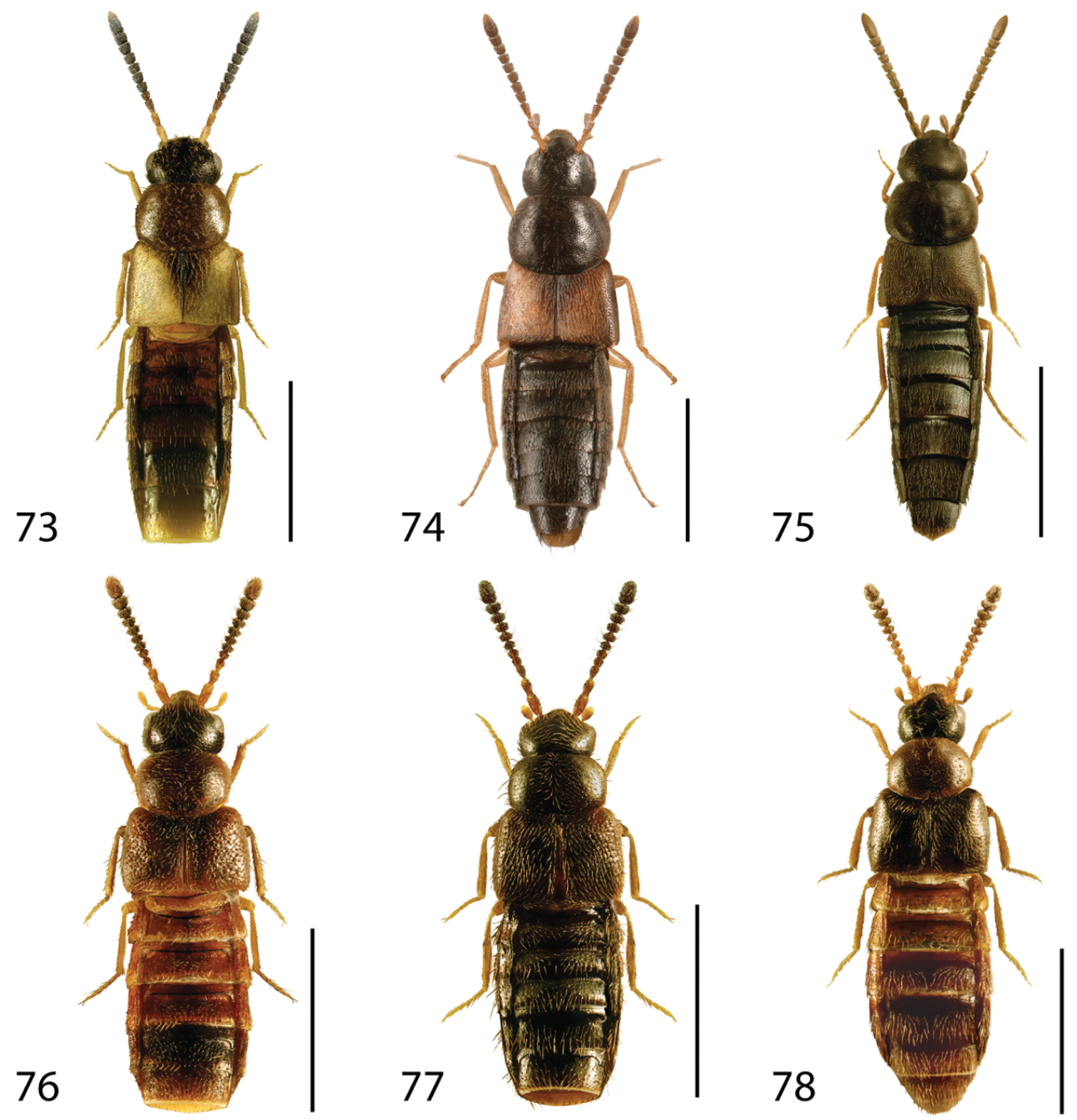
Dorsal habitus of: 13 Dexiogyia angustiventris (Casey) 14 Ilyobates bennetti Donisthorpe 15 Ocyusa canadensis Lohse 16 Oxypoda rubescans Casey 17 Parocyusa americana (Casey) 18 Parocyusa fuliginosa (Casey). Scale 1mm.
Canada: ON; USA: CA, FL, IA, RI. Native.
All North American species of Dexiogyia were described by
Dexiogyia has been associated with subcortical microhabitats, especially those of pine and in the ‘burrows of wood-boring beetles’ (
http://species-id.net/wiki/Ilyobates_bennetti
Fig. 14, Map 14, genitalia inNew Ontario Record
CANADA: ON:Waterloo Reg., Blair, hedgerow, pitfall trap, 5.v.2009, A. Brunke, 5 (DEBU), same data except: 19.v.2009, 1 (DEBU).
Canada: ON, QC, NB, NS; widespread in Palaearctic (
http://species-id.net/wiki/Ocyusa_canadensis
Fig. 15, Map 15, genitalia inNew Ontario Record
CANADA: ON: Timiskaming Distr., 52 mi S of Armstrong, 27.vi.1973, R. Parry & J.M. Campbell, 7 (CNC).
Canada: YT, ON; USA: AK (
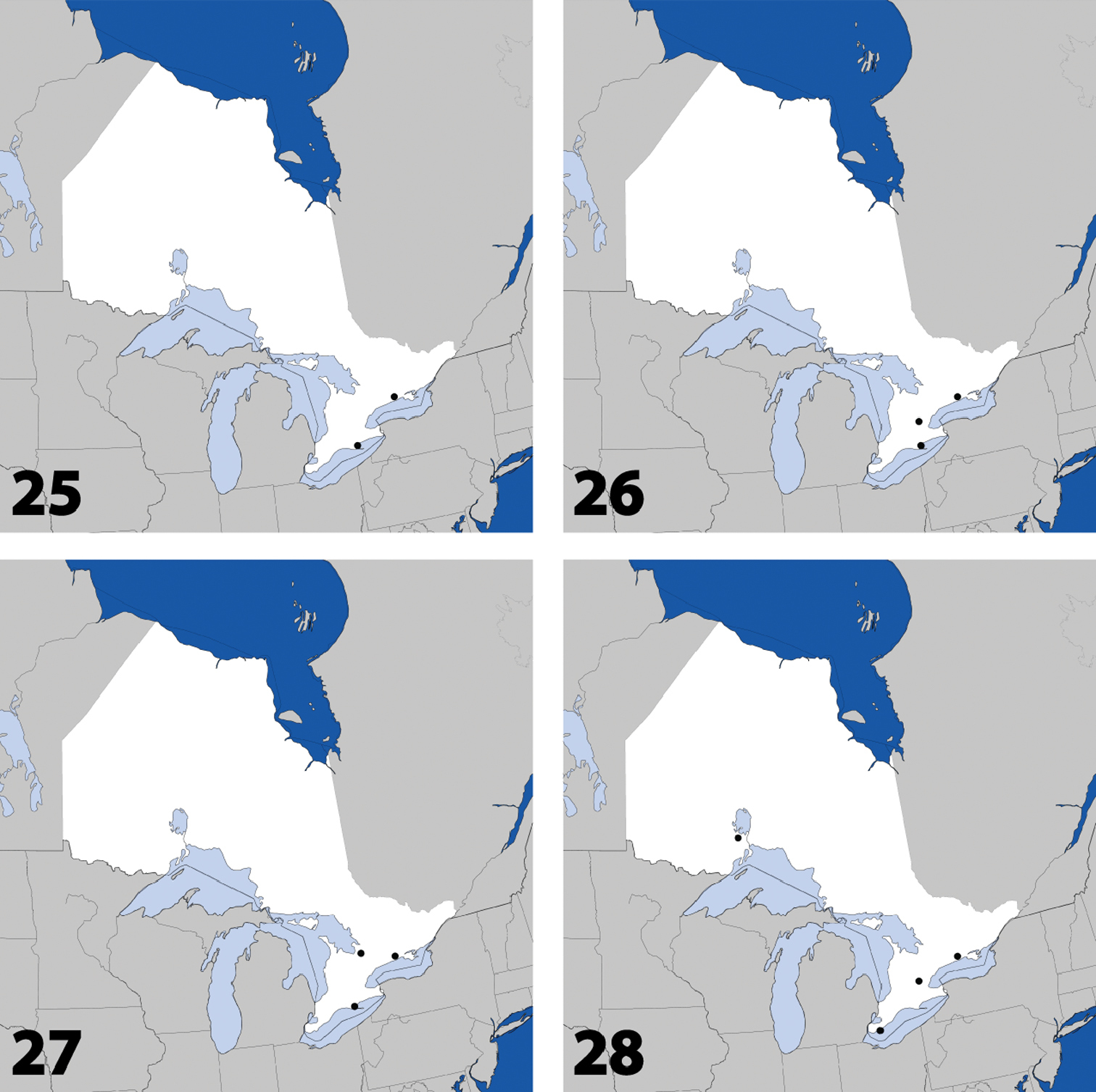
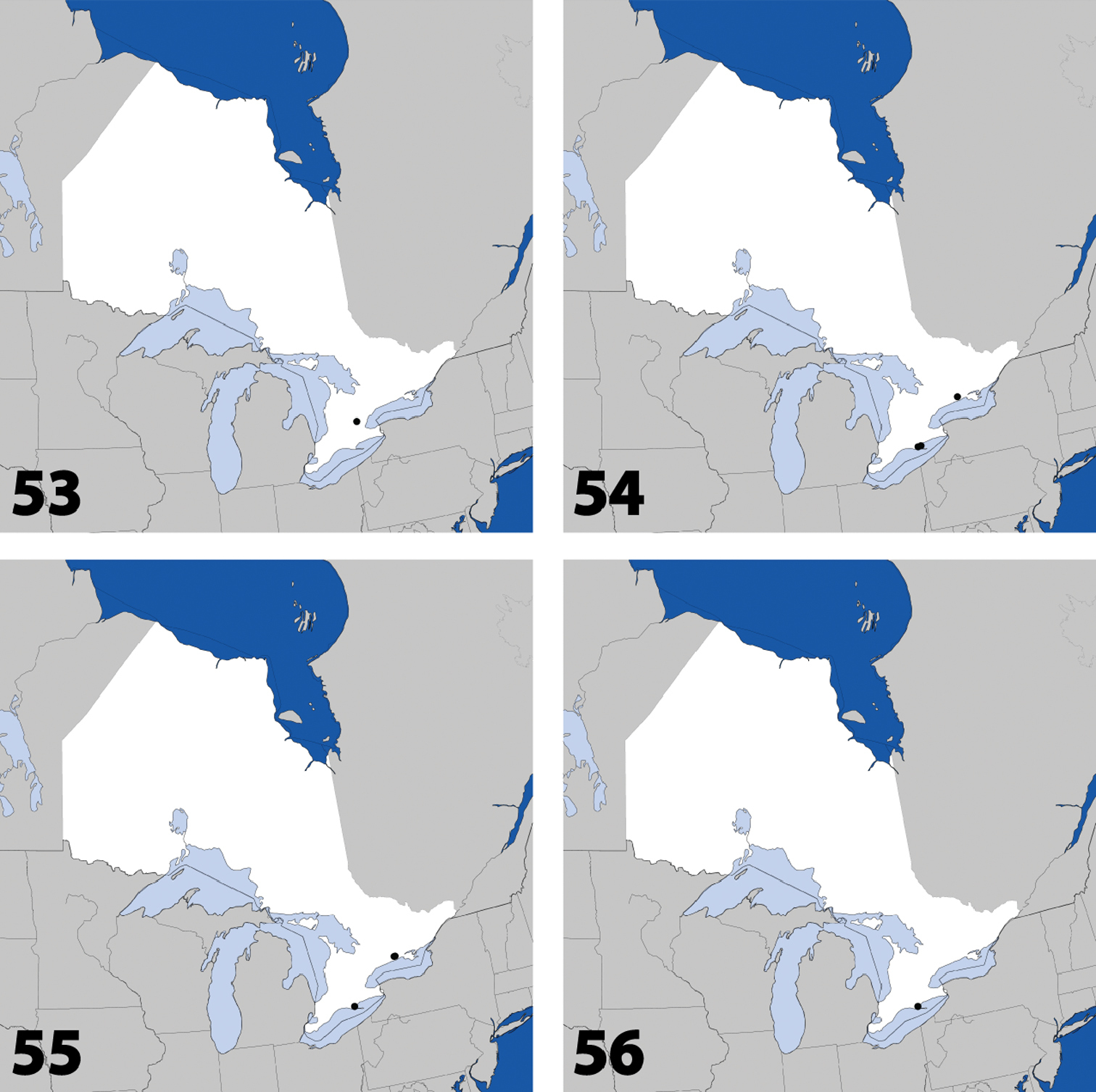
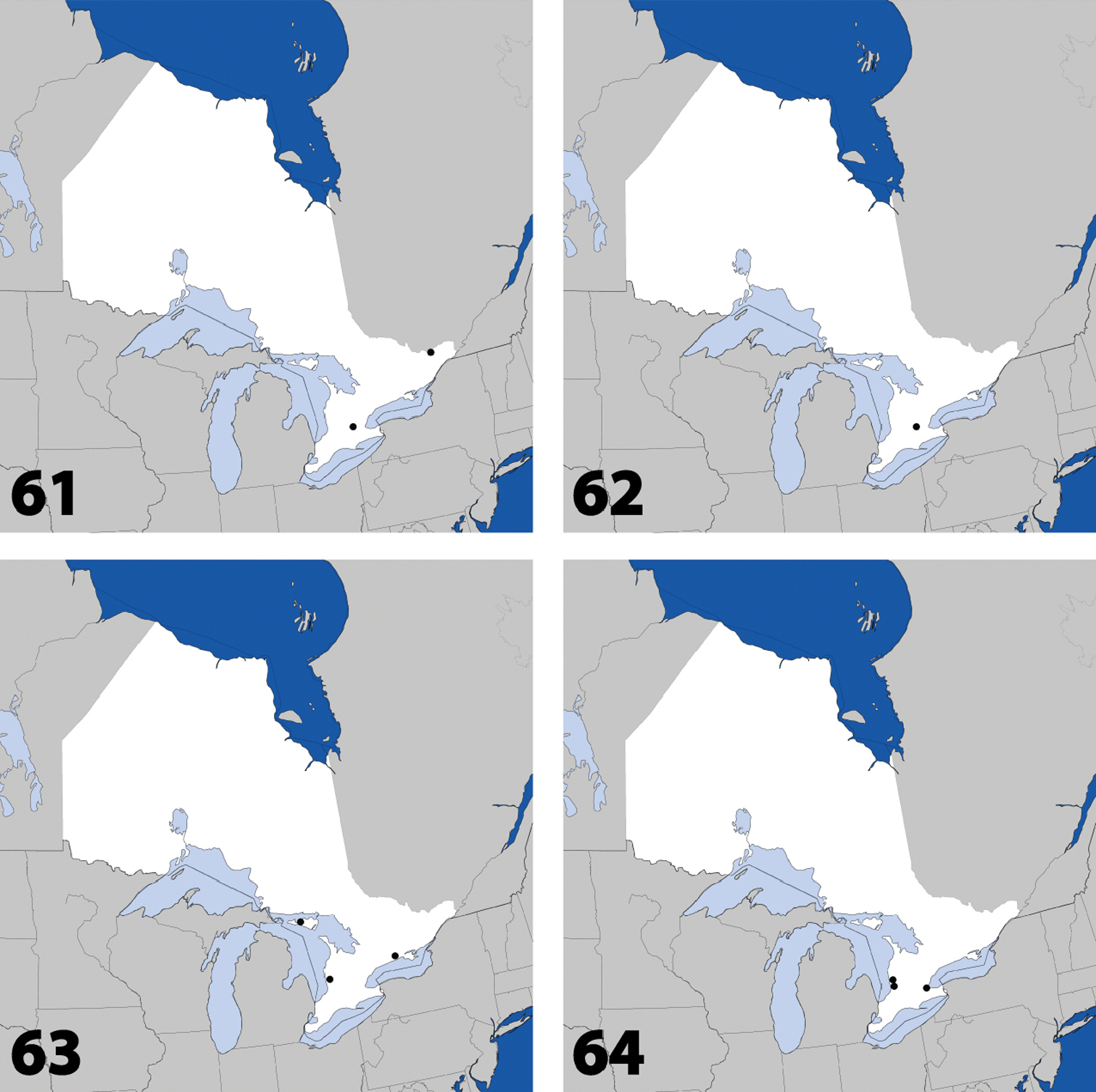
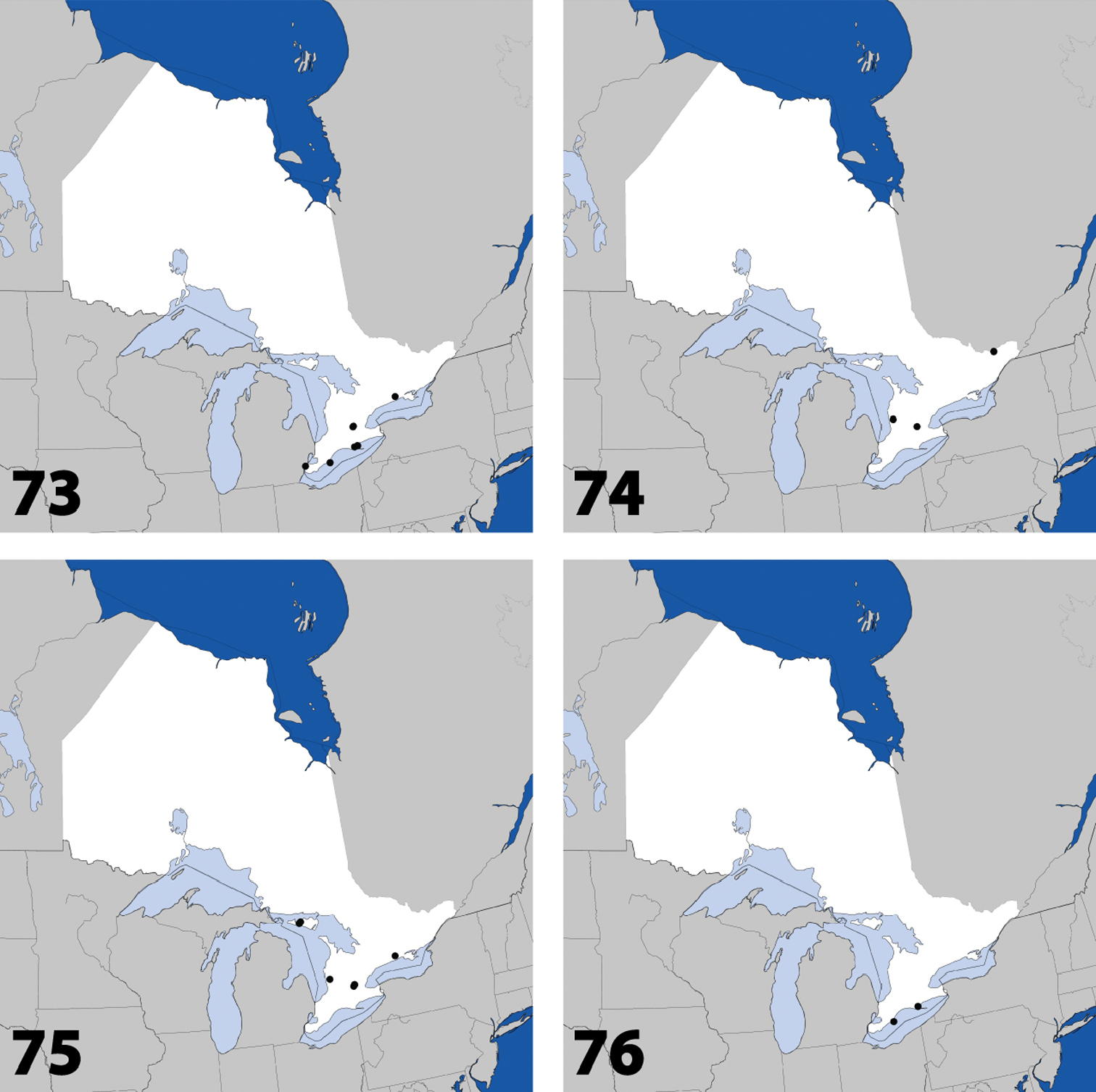
Distribution in Ontario of: 13 Dexiogyia angustiventris (Casey) 14 Ilyobates bennetti Donisthorpe 15 Ocyusa canadensis Lohse 16 Oxypoda rubescens Casey.
The specimens from boreal Ontario represent the first record of this species in eastern North America and suggest a transboreal
http://species-id.net/wiki/Oxypoda_rubescans
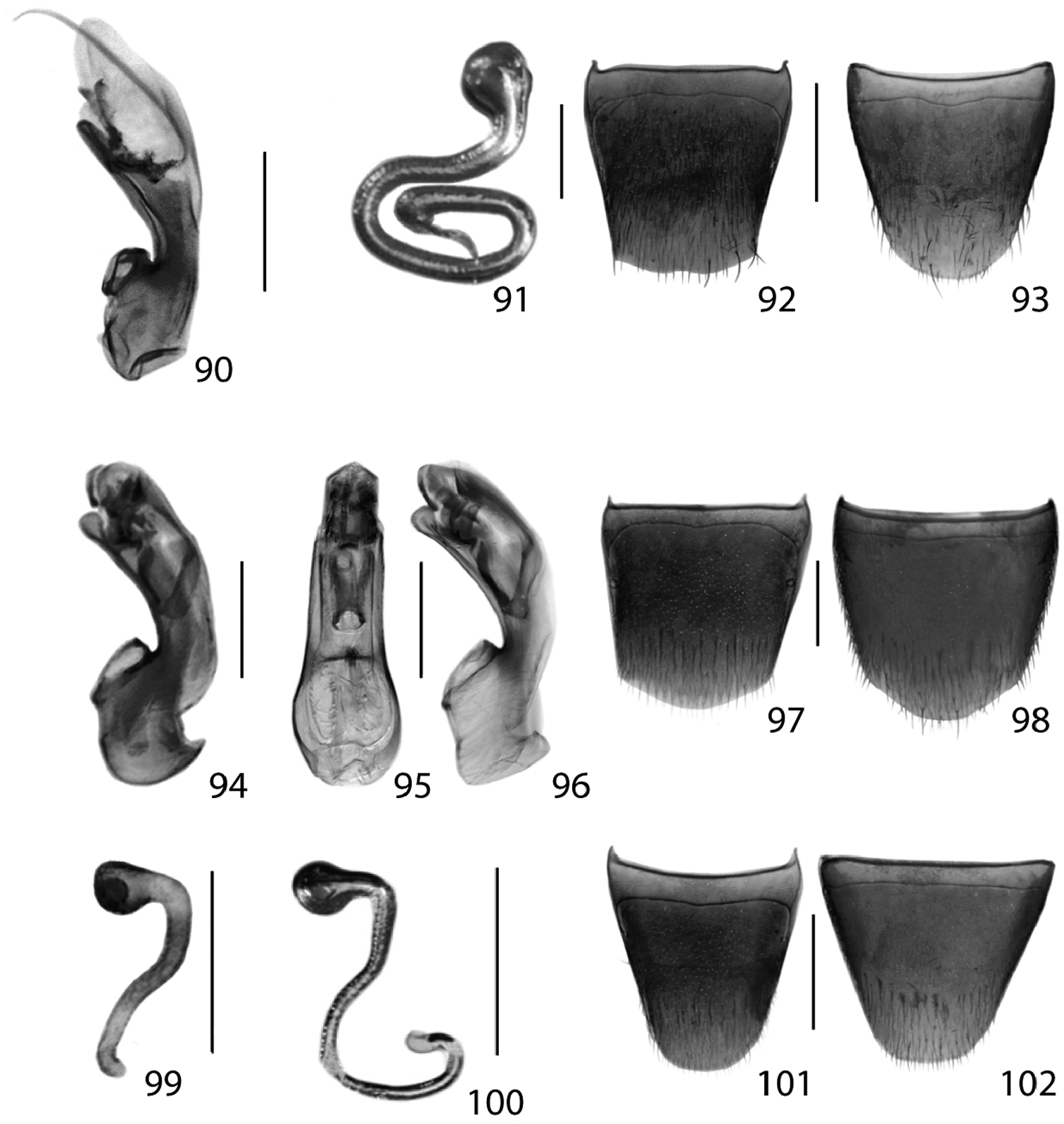
Figs 16, 90; Map 16New Canadian Record
(Type material – see above). CANADA: ON:Northumberland Co., Barr prop., 7 km NE Centreton, site 2, 44°7'48"N, 77°59'3"W, field, malaise pans, 19.v to 1.vi.2011, Brunke & Paiero, 1 (DEBU).
Canada: ON; USA: NY. Native.
This is the first collection of Oxypoda rubescans since its description based on a male specimen collected in the Catskill Mountains of New York (
http://species-id.net/wiki/Parocyusa_americana
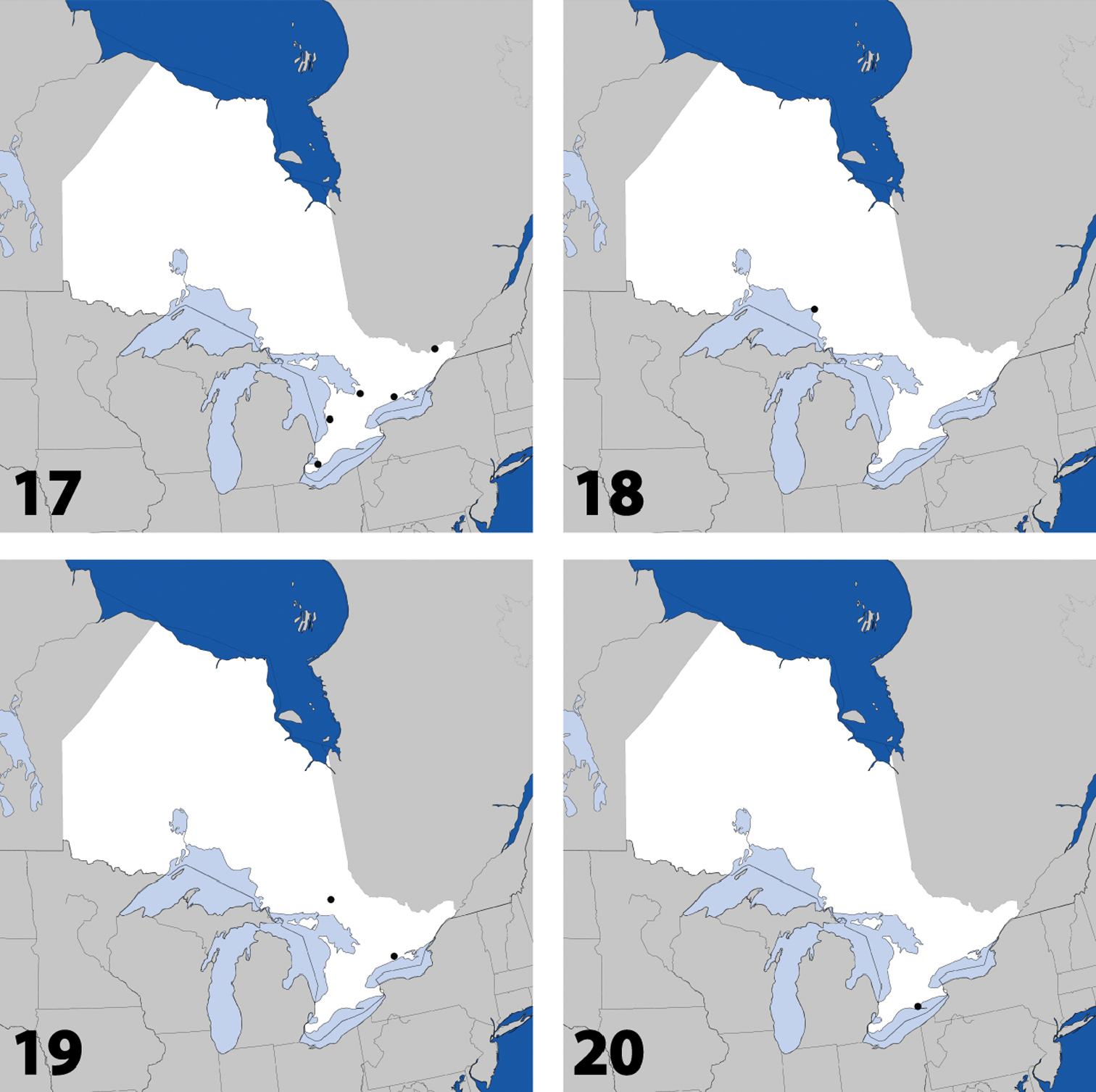
Figs 17, 91–93; Map 17New Canadian Record
(Type material – see above). CANADA: ON:Chatham-Kent Co., Tilbury, pitfall trap, 23.vi.1994, T. Savinski, 1 (DEBU); Huron Co., Auburn, hedgerow, pitfall, 27.x.2010, A. Brunke, 1 (DEBU); Northumberland Co., Peter's Woods PNR, 44°7'27"N, 78°2'21"W, dry streambed, under rock, 12.ix.2011, Brunke & Paiero, 1 (DEBU); Ottawa Division, Mer Bleue, 20.ix.1980, leg. R. Baranowski, 1 (MZLU); Simcoe Co., Midhurst, Finlay Mills Rd., Willow Creek, 44°26'24"N, 79°43'48"W, splashing sandy bank, 13.vi.2010, A. & K. Brunke, 1 (DEBU).
Canada: ON; USA: NY. Native
Distribution in Ontario of: 17 Parocyusa americana (Casey) 18 Parocyusa fuliginosa (Casey) 19 Brachyusa helenae (Casey) 20 Gnypeta helenae Casey.
This is the first record of Parocyusa americana since its description based on a female specimen collected from Peekskill, New York (
Specimens of Parocyusa americana were found on a stream bank and in a dry streambed under a rock. We expect Parocyusa americana to occur broadly over northeastern North America in habitats near running water.
http://species-id.net/wiki/Parocyusa_fuliginosa
Figs 18, 94–101; Map 18, genitalia inNew Ontario Record
CANADA: ON: Algoma Distr., Michipicoten River, south of Wawa, 5.ix.1980, leg. R. Baranowski, 1 (MZLU).
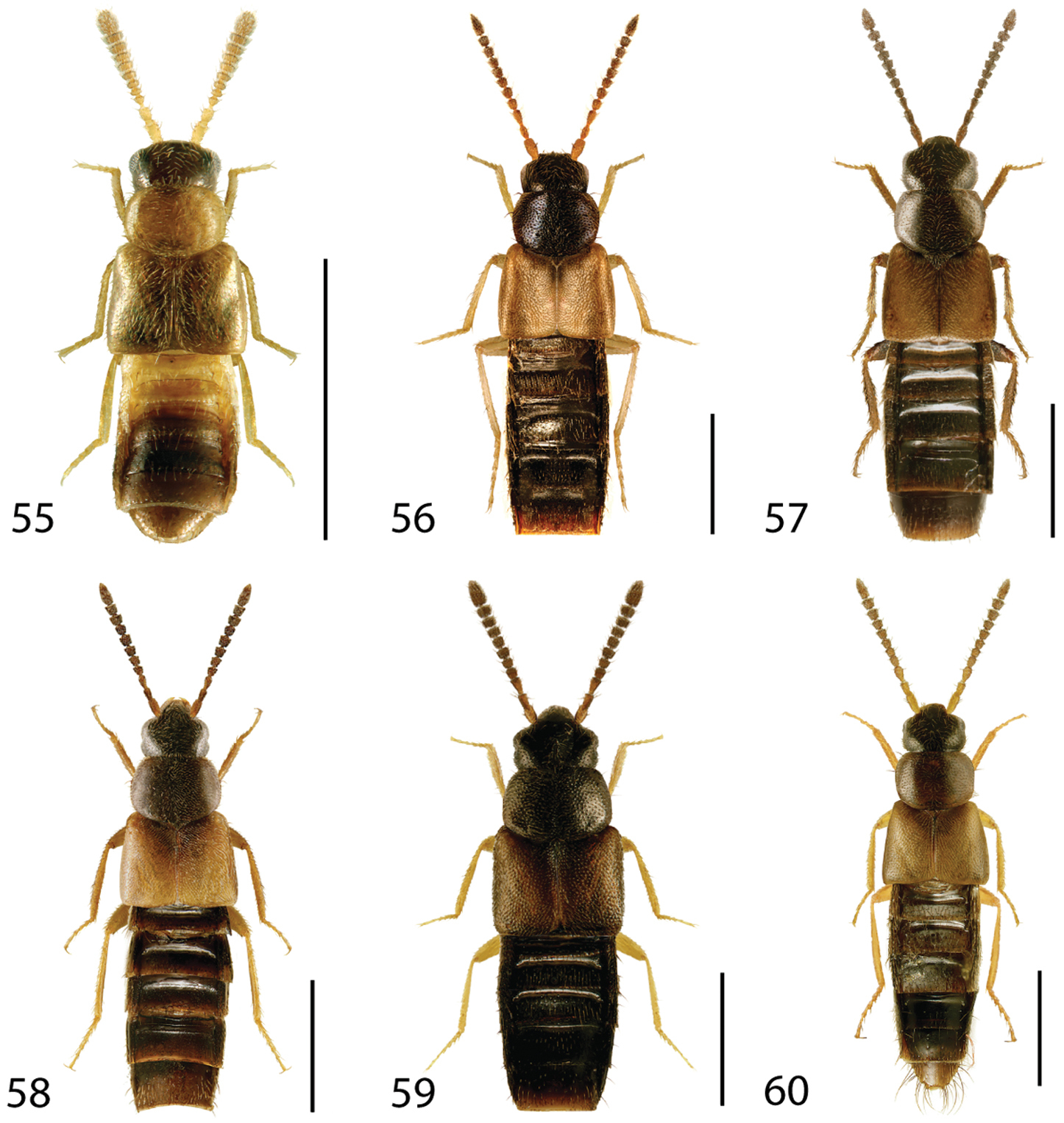
Dorsal habitus of: 19 Brachyusa helenae (Casey) 20 Gnypeta helenae Casey 21 Gnypeta nigrella (LeConte) 22 Myllaena cuneata Notman 23 Myllaena potawatomi Klimaszewski 24 Agaricomorpha websteri Klimaszewski & Brunke sp. n.Scale 1mm.
Canada: ON, NL; USA: MA, NC, PA (
This species was recorded from Canada for the first time by
http://species-id.net/wiki/Brachyusa_helenae
Fig. 19, Map 19, genitalia inNew Ontario Record
CANADA: ON:Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, 12.vii.2011, A. Brunke, 1 (DEBU); Greater Sudbury Div., Wahnapitae, 22.viii.1980, leg. R. Baronowski, 1 (MZLU).
Canada: YT, NT, ON, NL; USA: AK, MT (
http://species-id.net/wiki/Gnypeta_helenae
Fig. 20, Map 20, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Cronmiller prop., ~6km W St. Williams, 42°40'20"N, 80°29'29"W, eutrophic pond edge, 17.viii.2011, A. Brunke, 1 (DEBU), same data except S.M. Paiero, 1 (DEBU).
Canada: BC, AB, ON; USA: AZ, MT, NM, OR (
This is the first record of this species from eastern North America. Gnypeta helenae is indistinguishable externally from Gnypeta canadensis Klimaszewski, which was described based on characters of the male and female genitalia (
http://species-id.net/wiki/Gnypeta_nigrella
Fig. 21, Map 21, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'20"N, 80°29'29"W, eutrophic pond, treading edge, 4.viii.2011, Brunke & Paiero, 1 (DEBU), same data except: 17.viii.2011, A. Brunke, 1 (DEBU).
Canada: ON, NB, NL;USA: MA, MD, PA, VT (
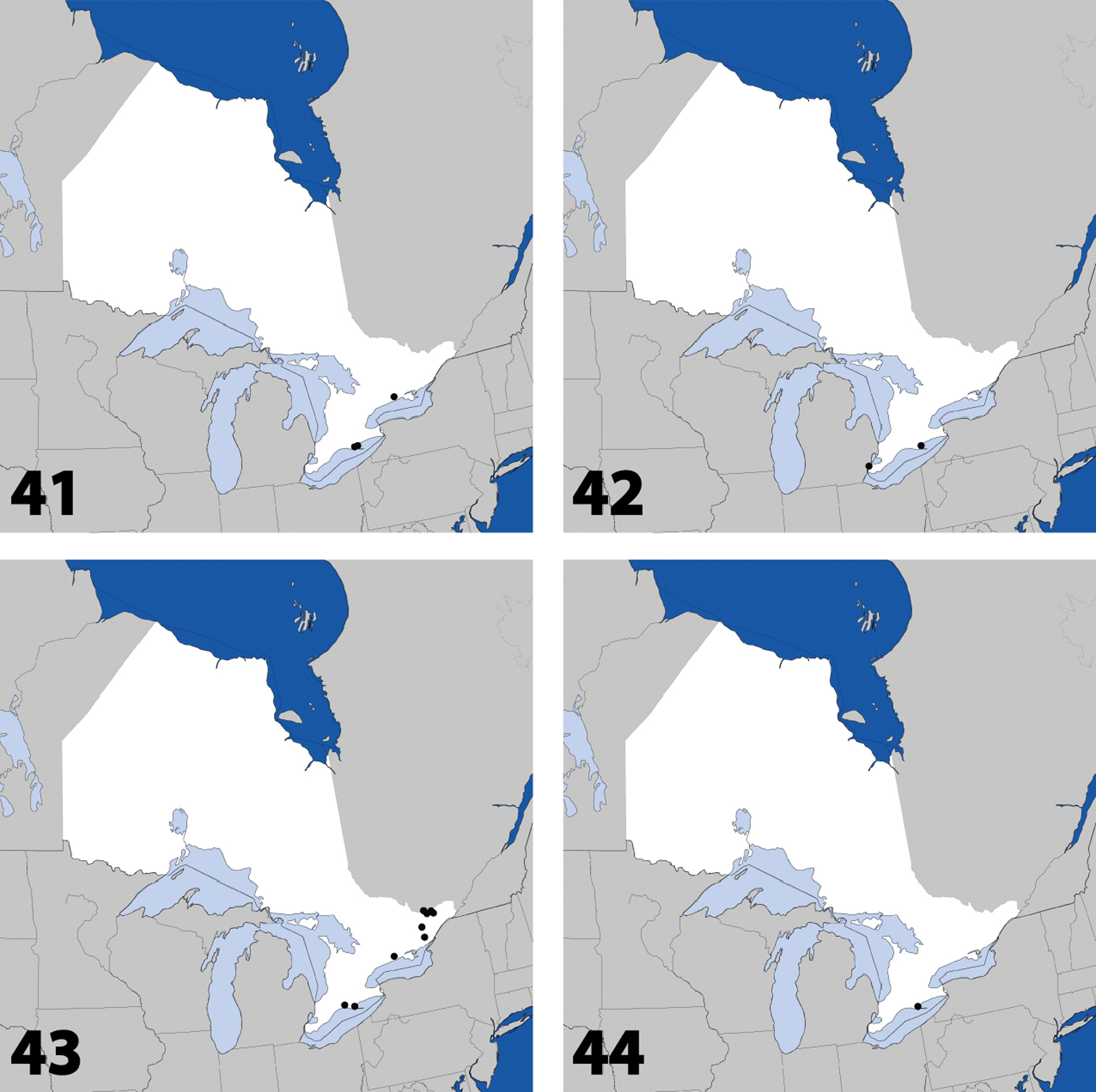
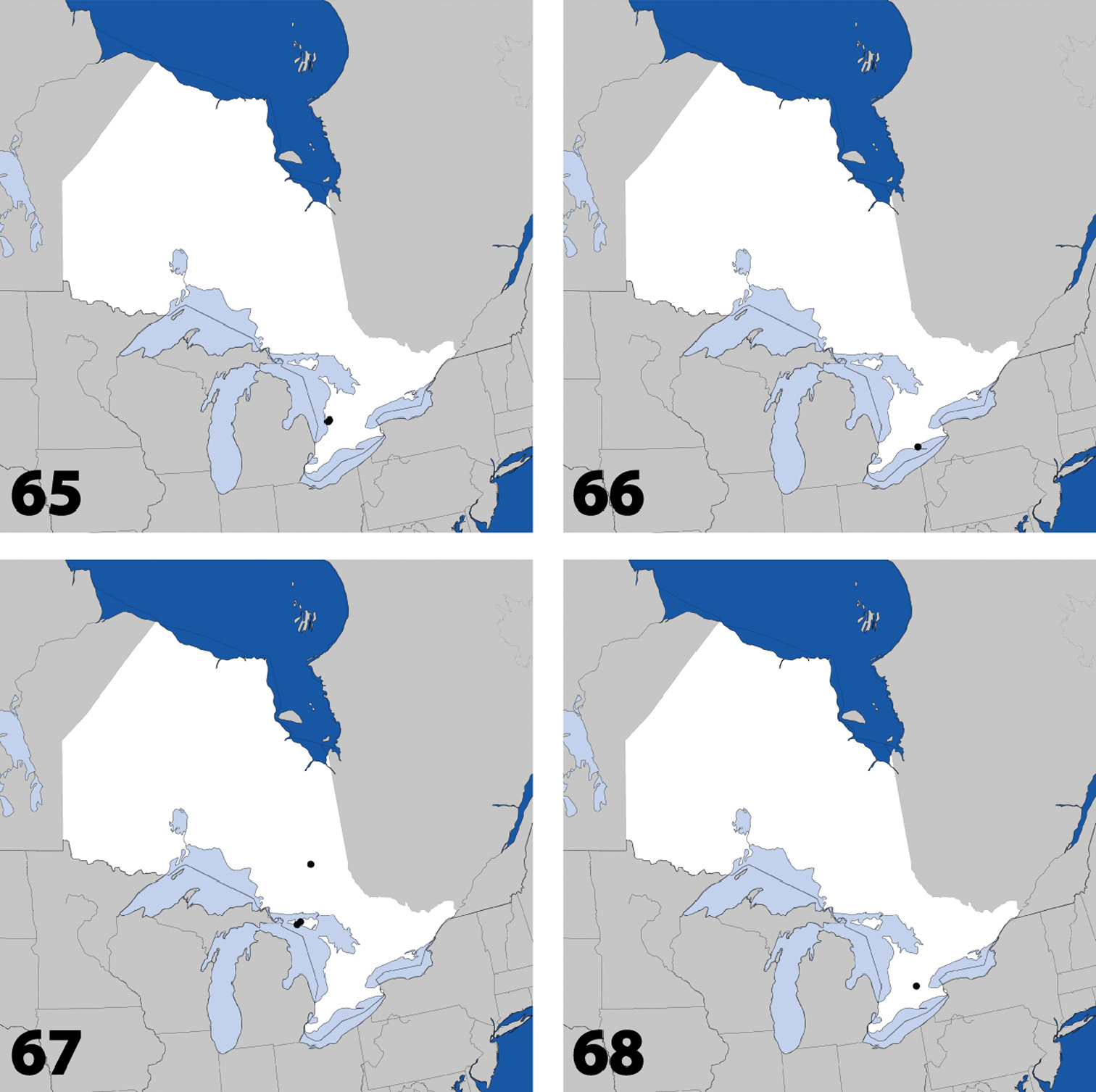
Distribution in Ontario of: 21 Gnypeta nigrella (LeConte) 22 Myllaena cuneata Notman 23 Myllaena potawatomi Klimaszewski. World distribution of: 24 Agaricomorpha websteri Klimaszewski & Brunke sp. n.
http://species-id.net/wiki/Myllaena_cuneata
Fig. 22, Map 22, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'21"N, 80°29'26"W, forest, at lights, 20.vii.2011, Brunke & Paiero, 1 (DEBU), Cronmiller Prop., ~6km W St. Williams, 42°40'21"N, 80°29'26"W, forest, Berlese leaf and log litter, 20.ix.2011, A. Brunke, 1 (DEBU).
Canada: ON, NS; USA: AR, FL, GA, IL, LA, MA, MD, NH, OK, TN, VA (
http://species-id.net/wiki/Myllaena_potawatomi
Fig. 23, Map 23, genitalia inNew Canadian Record
CANADA: ON:Essex Co., Ojibway Prairie Prov. Nat. Reserve, pond edge, 23.vii.2011, S.M. Paiero, 1 (DEBU); Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'21"N, 80°29'26"W, treading edge, eutrophic pond, 4.viii.2011, Brunke & Paiero, 2 (DEBU) same data except: 12.viii.2011, S.M. Paiero, 1 (DEBU), Turkey Point Prov. Park, marsh nr. fish hatchery, treading vegetation, 20.vii.2011, A. Brunke, 3 (DEBU); Leeds and Grenville Co., Chaffey’s Locks, Queens Univ. Biol. Station, 44.56–76.32, in decaying veg. on lake shore, 16 to 17.viii.2010, A. Brunke, 1 (DEBU).
Canada: ON; USA: AZ, AL, CA, FL, GA, IL, IN, MA, OK, TX, VA, WI;Mexico, Haiti, Jamaica (
urn:lsid:zoobank.org:act:684EAFCD-F04D-4237-A3EA-140839A5C588
http://species-id.net/wiki/Agaricomorpha_websteri
Figs 24, 103–105; Map 24Canada, New Brunswick, Queens Co., Cranberry Lake P.N.A., red oak forest, 46.1125°N, 65.6075°W.
Holotype(male): CANADA: NB:Queens Co., Cranberry Lake P.N.A., 46.1125°N, 65.6075°W, 25.vi-1.vii.2009, Red oak forest, Lindgren funnel trap, R. Webster & M-A. Giguère (LFC).
Paratypes (5 males, 2 females, 6 sex unknown): CANADA: NB: Carleton Co., near Belleville, 1.3 km E ict. Rt. 540 & Plymouth Rd., 46.1867°N, 67.6817°W, 7-v-2008, R. Webster coll., 1 male (RWC); NS: Cape Breton H.N.P., Lone Shieling, vii.1983, Malaise trap, R. Vockeroth, PG729861, 2 sex? (CNC); ON: Haliburton Co., 10km SE Dorset, 45.16–78.84, vernal pool litter, previously wet, 25-ix-2009, S. Kullik, 1 male (DEBU); Northumberland Co., Peter's Woods PNR, back woods, 44°7'28"N, 78°2'14"W, forest, malaise pans, 19-v to 1-vi-2011, Brunke & Paiero, debu01146638, 1 female (DEBU); QC: Communaute-Urbainé-de-l'Outaouais, Gatineau Pk., near Hull, 28.iv.1974, A. Davies, 1 sex? (CNC); L'Aminate, Ste-Praxède, 6–13.vii.1999, Lindgren trap # 3, 99–3-0461, 2 sex? (LFC), Saint-Jacques-de-Leeds, 46°16'N, 71°23'W, 7.vii-9.vii.1993, Plan Vert ‘93, Lindgren trap # 1, Dispositif B, Ėrabliėre [=sugar bush], ‘1993–3-0381', Hébert & Jobin, 1 female (LFC); Rousillon, Ste-Catherine, Port., 29.vi.1961, 5.viii, 9.viii, 26.viii.1961, J-C. Aubé, 3 males, 1 sex? (CNC).
Body small, compact and oval in outline;length 1.6–1.8 mm; body dark brown with legs, maxillary palpi and 2–3 basal antennomeres yellowish-brown, or body dark brown with pronotum and elytra slightly paler, and appendages and basal part of abdomen yellowish-brown (Fig. 24); forebody with strong meshed microsculpture, punctation coarse, sparse and flatly impressed, pubescence sparse and approximately evenly distributed on forebody; head transverse and produced anteriad, eyes large and longer than postocular area, pubescence directed posteriad and obliquely mediad; ligula narrowly elongate and divided almost to base; antennae slightly incrassate, basal 3 antennomeres elongate, 4 subquadrate, 5–10 increasingly broadening apically, 11 oval and elongate; maxillary palpi with 4 articles, penultimate article expanded apically, and apical article acicular; pronotum strongly transverse, base strongly sinuate, converging apicad, disc with pubescence directed posteriad except for some setae at base directed laterad; elytra at suture distinctly longer than pronotum, pubescence directed straight posteriad; abdomen gradually but weakly tapering apicad, tergites II and III impressed basally, and with elevated punctures.
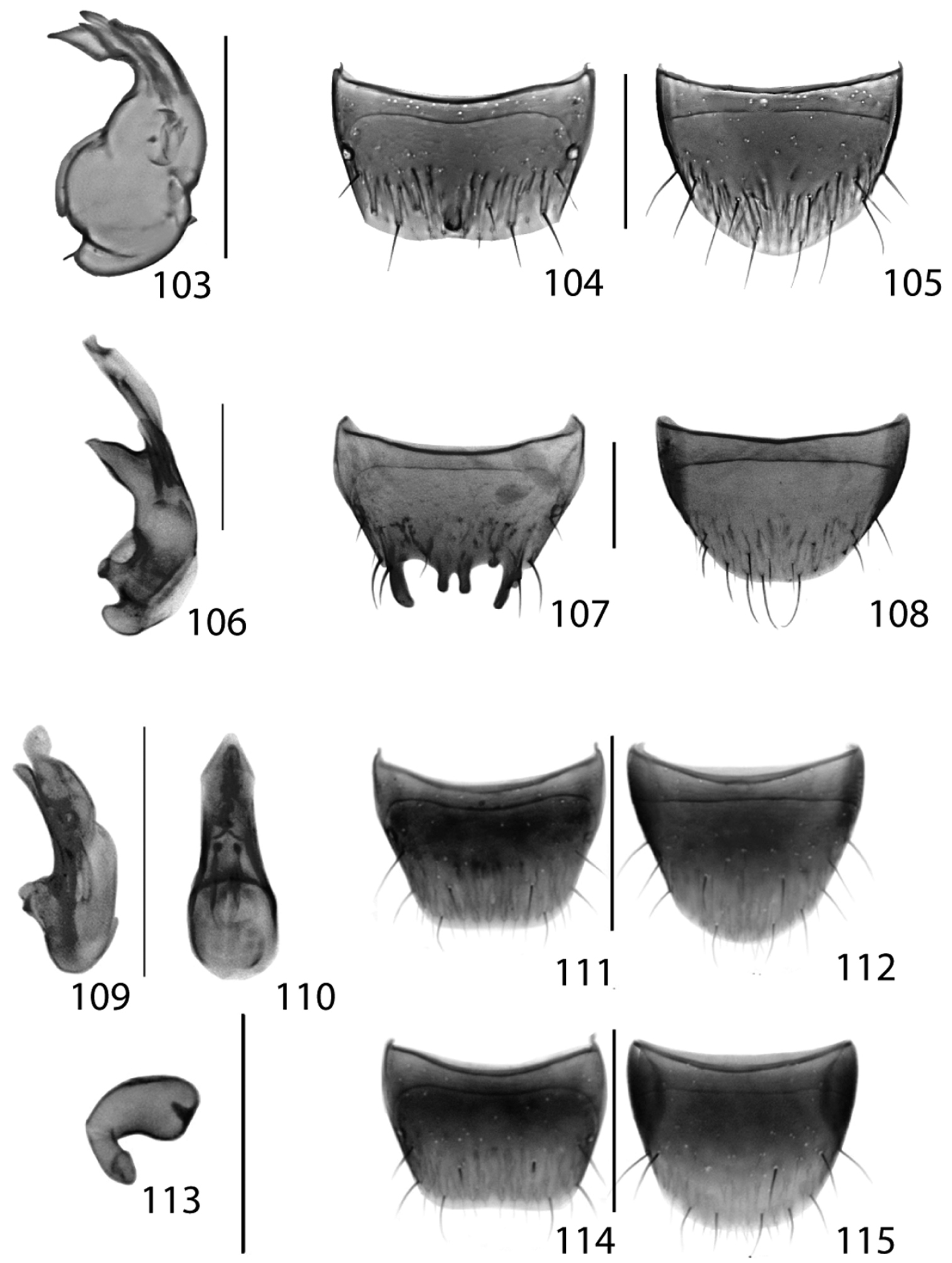
Male. Tergite VIII transverse, shallowly emarginate medially at the apical margin and with short medio-apical carinate protuberance (Fig. 104); sternite VIII broadly rounded apically (Fig. 105); median lobe of aedeagus in lateral view with large bulbus and U-shaped, narrow tubus with broad and angular swelling subapically; flagellum long, thin, everted and about 3 times as long as tubus (difficult to see in Fig. 103).
Female. Tergite VIII strongly transverse and similar to that of male but lacking median carina; sternite VIII transverse and arcuate apically; spermatheca with spherical capsule and inconspicuous short stem, in general similar to those of Gyrophaena and Eumicrota.
Known from Ontario, Quebec, New Brunswick and Nova Scotia. Agaricomorpha websteri is probably broadly distributed in northeastern North America, south of the boreal forest zone.
Little is known about the natural history of this species but all specimens were collected in deciduous forests, mostly by passive, above-ground traps indicating high flight capability. Other species of the genus are found on woody and leathery polypore fungi (Ashe in Newton et al. 2000), which commonly grow on dead or dying standing trees. Interestingly, several individuals were captured by Lindgren funnel traps, which typically attract species associated with this type of coarse woody debris.
This species is dedicated to our colleague Reginald P. Webster of Charters Settlement, New Brunswick, who collected the holotype and whose material has contributed much to the knowledge of Canadian biodiversity.
Agaricomorpha websteri is the only known species of the genus in eastern North America. This genus was erected by
http://species-id.net/wiki/Eumicrota_corruscula
Fig. 25, Map 25, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Turkey Point Prov. Park, site 2, 42°42'28"N, 80°20'29"W, savannah, at lights, 5.viii.2011, Brunke & Paiero, 3 (DEBU); Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, on fungi, 12.viii.2011, A. Brunke, 1 (DEBU).
Dorsal habitus of: 25 Eumicrota corruscula (Erichson) 26 Eumicrota socia (Erichson) 27 Euvira micmac Klimaszewski & Majka 28 Gyrophaena affinis Mannerheim 29 Gyrophaena antennalis Casey 30 Gyrophaena brevicollis Seevers. Scale 1mm.
Canada: ON, QC, NB; USA: AL, CT*, DC, FL, GA, IL, IN, IA, KS, KY, LA, MA, MI, MO, NJ, NY, OH, PA, SC, TN, TX, VA, WI, WV (
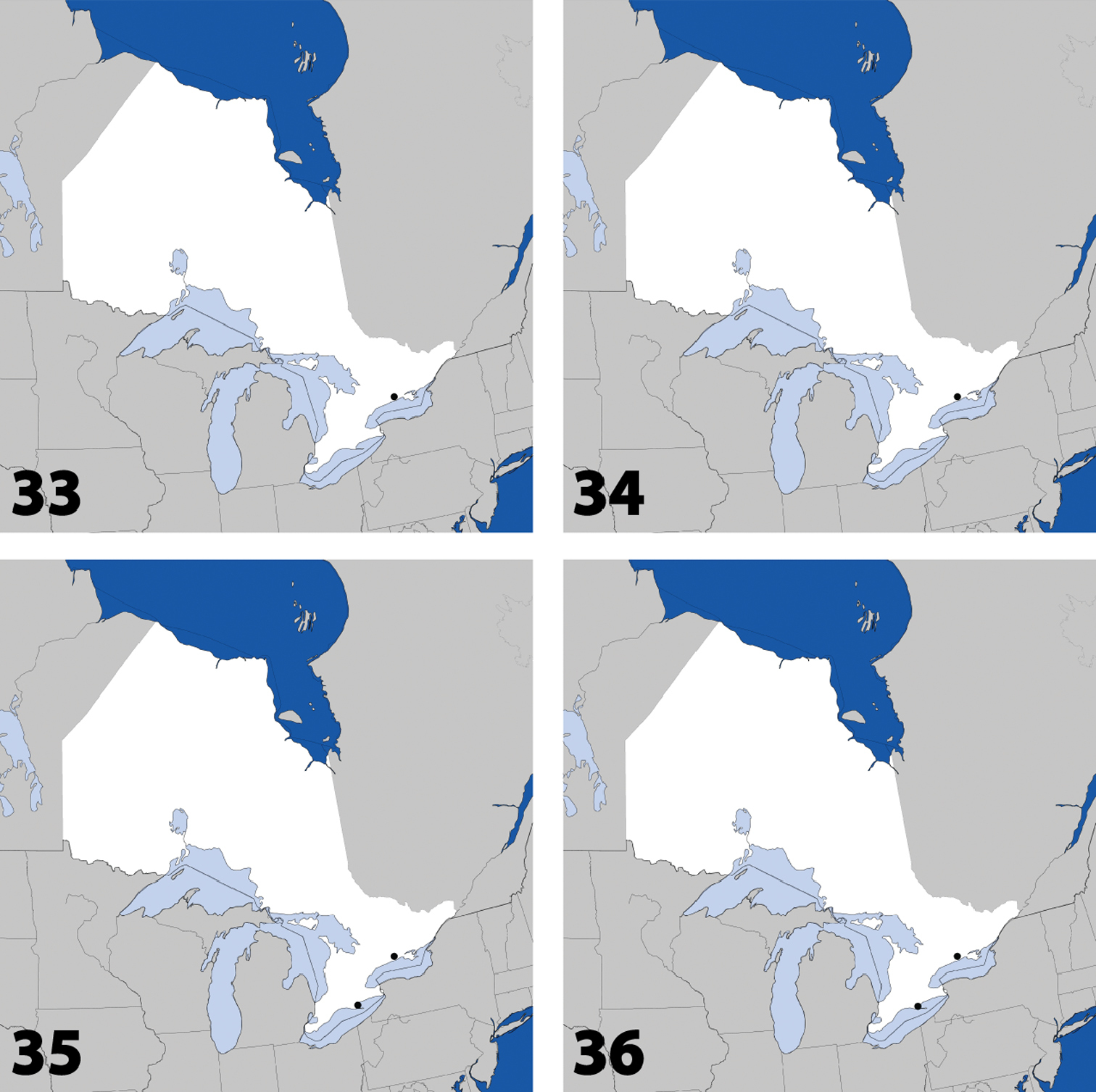
Distribution in Ontario of: 25 Eumicrota corruscula (Erichson) 26 Eumicrota socia (Erichson) 27 Euvira micmac Klimaszewski & Majka 28 Gyrophaena affinis Mannerheim.
http://species-id.net/wiki/Eumicrota_socia
Fig. 26, Map 26, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Turkey Point Prov. Park, site 1, 42°41'48"N, 80°19'48"W, forest, on fungus, 17.viii.2011, A. Brunke, 1 (DEBU), Turkey Point Prov. Park, site 2, 42°42'28"N, 80°20'29"W, savannah, Berlese leaf and log litter w. fungus, 17.v.2011, A. Brunke, 1 (DEBU); Northumberland Co., Peter’s Woods Prov. Nat. Res., 44°7'27"N, 78°2'21"W, forest, Berlese leaf & log litter, 1.vi.2011, Brunke & Paiero, 1 (DEBU), Peter’s Woods Prov. Nat. Res., 44°7'26"N, 78°2'31"W, forest, malaise pans, 19.v to 1.vi.2011, Brunke & Paiero, 1 (DEBU), same data except: 16 to 27.vi.2011, 2 (DEBU); Wellington Co., Guelph, Arboretum, 11.ix.2007, A. Brunke, 1 (DEBU).
Canada: ON, QC, NB, NS, PE; USA: AR, DC, FL, IL, IN, KS, KY, LA, ME, MD, MI, MO, NY, NC, OH, PA, SC, TN, TX, VA, WI, WV (
http://species-id.net/wiki/Euvira_micmac
Fig. 27, Map 27, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'20"N, 80°29'29"W, ridge forest, malaise pans, 20.ix to 12.x.2011, Brunke & Paiero, 1 (DEBU);Northumberland Co., Barr Property, ~ 7km NE Centreton, 44°7'44"N, 77°59'0"W, forest, sappy Populus wood, 12.vii.2011, A. Brunke, 1 (DEBU), Barr Property, ~ 7km NE Centreton, 44°7'48"N, 77°59'3"W, old field, malaise pans, 1 to 16.vi.2011, Brunke & Paiero, 1 (DEBU), Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, Berlese streamside litter, 19.v.2011, A. Brunke, 1 (DEBU); Simcoe Co., Midhurst, forest nr. Neretva St., 44°26'22"N, 79°42'40"W, leaf litter, 10.x.2010, A. Brunke, 2 (DEBU).
Canada: ON, NB, NS; USA: OH, MI (
This species has previously been associated with Red Oak (Quercus rubra L.) and some specimens have been collected inside spherical Red Oak galls (
http://species-id.net/wiki/Gyrophaena_affinis
Fig. 28, Map 28, genitalia inNew Ontario Record
CANADA: ON:Essex Co., Point Pelee, 24.vi.1925, G.S. Walley, 1 (CNC); Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, on fungus, 12.viii.2011, S.M. Paiero, 1 (DEBU); Thunder Bay Distr., Black Sturgeon Lake, 1 to 5.viii.1956, Lindberg, 7 (CNC); Wellington Co., Guelph, reared from fungus, 23.viii.1990, H. Dewer, 1 (DEBU).
Canada: BC, MB, ON, QC, NB, NS, NL; USA: AZ*, DC, IL, IN, IA, KY, ME, MA, MI, MN, MO, NC, NH, NJ, NM, NY, OH*, PA, TN, WA, WI, WV (
This adventive species was accidentally listed as occurring in Ontario in
http://species-id.net/wiki/Gyrophaena_antennalis
Fig. 29, Map 29, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Manester Tract, 6km NNW St. Williams, 17.ix.2008, A. Brunke, 2 (DEBU), Turkey Point Prov. Park, site 1, 42°41'48"N, 80°19'48"W, forest, on fungi, 20.ix.2011, S.M. Paiero, 1 (DEBU); Northumberland Co., Peter’s Woods PNR, 44°7'26"N, 78°2'31"W, forest, gilled mushrooms, 12.ix.2011, A. Brunke, 1 (DEBU).
Canada: ON, NB, NS, NL; USA: MA, NC, NY, TN* (
Distribution in Ontario of: 29 Gyrophaena antennalis Casey 30 Gyrophaena brevicollis Seevers 31 Gyrophaena caseyi Seevers 32 Gyrophaena criddlei Casey.
This species was newly recorded from Nova Scotia by
http://species-id.net/wiki/Gyrophaena_brevicollis
Fig. 30, Map 30, genitalia inNew Canadian Record
CANADA: ON:Hald.-Norfolk Reg., Cronmiller prop., 6km W St. Williams, 42°40'21"N, 80°29'26"W, forest, 17.viii.2011, A. Brunke, 1 (DEBU), same data except: 42°40'20"N, 80°29'29"W, forest, site 1, malaise pans, 20.ix to 12.x.2011, Brunke & Paiero, 1 (DEBU); Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, gilled mushrooms, 12.ix.2011, A. Brunke, 1 (DEBU).
Canada: ON; USA: IN, IL, MS, NC (
http://species-id.net/wiki/Gyrophaena_caseyi
Figs 31, 106–108; Map 31New Ontario Record
CANADA: ON:Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, gilled mushrooms, 12.ix.2011, A. Brunke, 1 (DEBU).
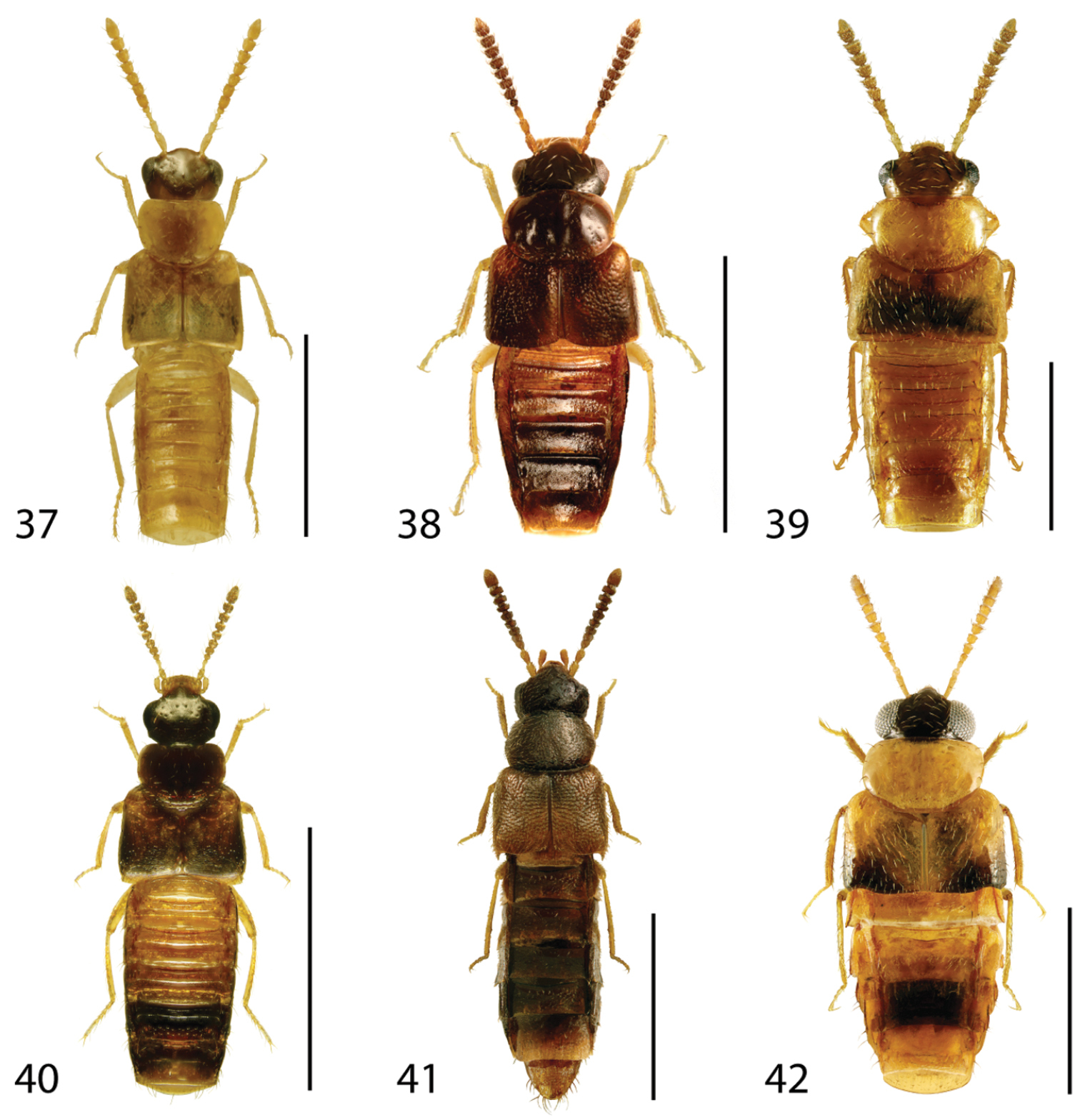
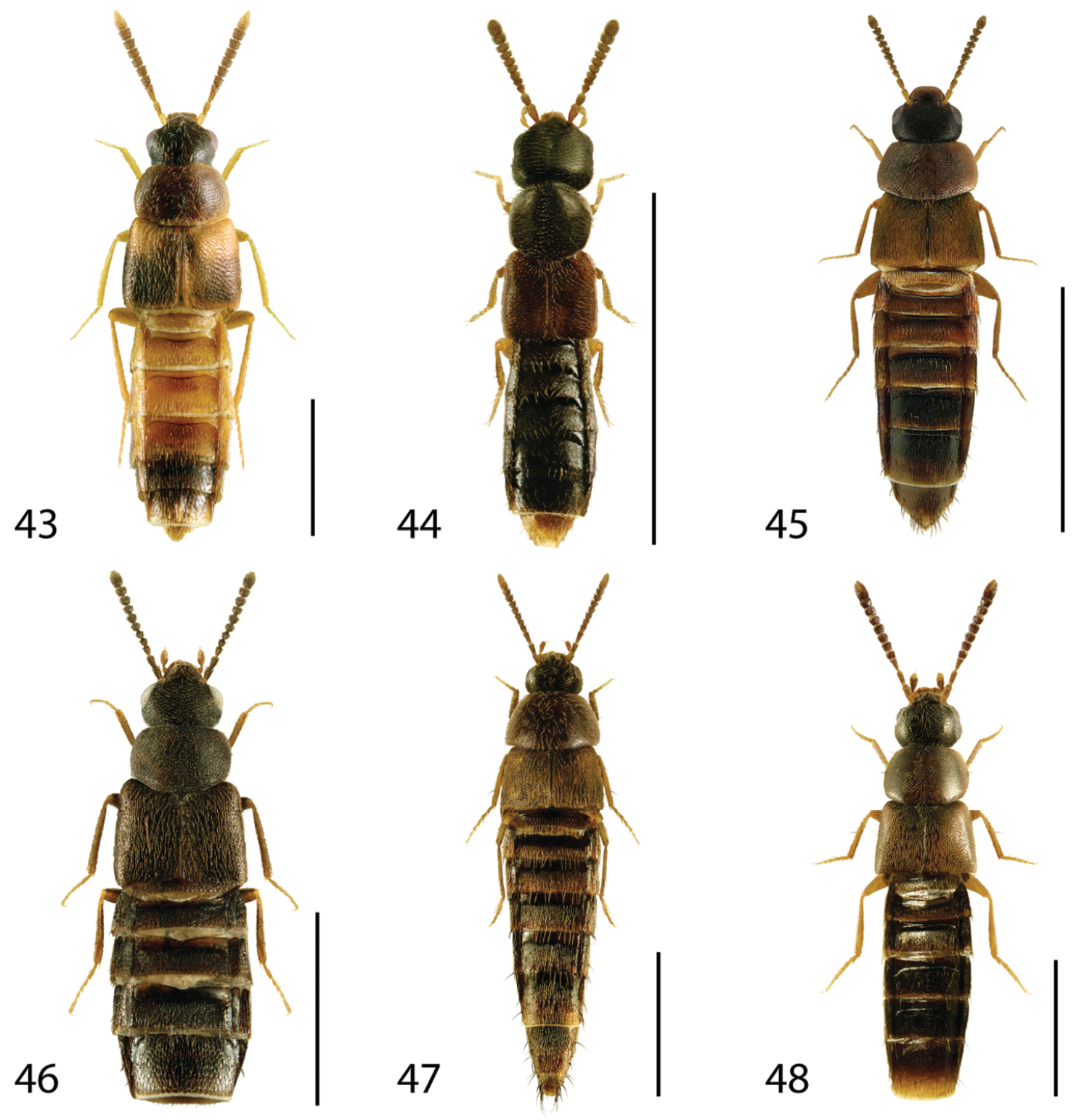
Dorsal habitus of: 31 Gyrophaena caseyi Seevers 32 Gyrophaena criddlei Casey 33 Gyrophaena dybasi Seevers 34 Gyrophaena fuscicollis Casey 35 Gyrophaena gilvicollis Casey 36 Gyrophaena meduxnekeagensis Klimaszewski and Webster. Scale 1mm.
Canada: ON, QC, NB; USA: MI, NC, NY, PA (
This species was erroneously reported from New Brunswick by
http://species-id.net/wiki/Gyrophaena_criddlei
Fig. 32, Map 32, genitalia inNew Ontario Record
CANADA: ON:Northumberland Co., Peter’s Woods PNR, 44°7'26"N, 78°2'31"W, forest, gilled mushrooms, 12.ix.2011, A. Brunke, 2 (DEBU).
Canada: YT (tentative), MB, ON, NB, NL (
http://species-id.net/wiki/Gyrophaena_dybasi
Fig. 33, Map 33, genitalia inNew Ontario Record
CANADA: ON:Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, on fungus, 12.viii.2011, A. Brunke, 1 (DEBU).
http://species-id.net/wiki/Gyrophaena_fuscicollis
Fig. 34, Map 34, genitalia inNew Ontario Record
CANADA: ON:Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, on fungus, 12.viii.2011, S.M. Paiero, 1 (DEBU).
Canada: ON, NB; USA: DC, IL, NY, PA, WI (
http://species-id.net/wiki/Gyrophaena_gilvicollis
Fig. 35, Map 35, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Turkey Point Prov. Park, site 1, 42°41'48"N, 80°19'48"W, forest, on fungus, 20.ix.2011, S.M. Paiero, 1 (DEBU);Northumberland Co., Peter’s Woods PNR, 44°7'26"N, 78°2'31"W, forest, gilled mushrooms, 12.ix.2011, A. Brunke, 2 (DEBU).
Canada: ON, NB; USA: DC, IL, IN, MI, NY, PA, VA, WV (
This species was listed as questionably occurring in Ontario by
http://species-id.net/wiki/Gyrophaena_meduxnekeagensis
Fig. 36, Map 36, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'18"N, 80°29'24"W, forest, malaise pans, 17 to 31.v.2011, Brunke & Paiero, 1 (DEBU); Northumberland Co., Peter’s Woods PNR, 44°7'26"N, 78°2'31"W, forest, malaise pans, 27.vi to 12.vii.2011, Brunke & Paiero, 1 (DEBU).
Canada: ON, QC, NB (
http://species-id.net/wiki/Gyrophaena_modesta
Fig. 37, Map 37, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Turkey Point Prov. Park, site 1, 42°41'48"N, 80°19'48"W, forest, on fungus, 20.ix.2011, A. Brunke, 1 (DEBU);Northumberland Co., Peter’s Woods PNR, 44°7'26"N, 78°2'31"W, forest, gilled mushrooms, 12.ix.2011, A. Brunke, 2 (DEBU).
Dorsal habitus of: 37 Gyrophaena modesta Casey 38 Gyrophaena neonana Seevers 39 Gyrophaena stroheckeri Seevers 40 Gyrophaena uteana Casey 41 Leptusa carolinensis Pace 42 Phanerota fasciata (Say). Scale 1mm.
Canada: AB*, ON, NB, NS, NL; USA: IL, IN, MI, MN, NH, NY (
Distribution in Ontario of: 37 Gyrophaena modesta Casey 38 Gyrophaena neonana Seevers 39 Gyrophaena stroheckeri Seevers 40 Gyrophaena uteana Casey.
http://species-id.net/wiki/Gyrophaena_neonana
Fig. 38, Map 38, genitalia inNew Ontario Record
CANADA: ON:Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, fungus on log, 27.vii.2011, S.M. Paiero, 3 (DEBU).
Canada: YT, ON, NB, NL; USA: IN, NC, PA, WI (
http://species-id.net/wiki/Gyrophaena_stroheckeri
Fig. 39, Map 39, genitalia inNew Canadian Record
CANADA: ON:Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, fungus, 12.viii.2011, S.M. Paiero, 1 (DEBU).
Canada: ON; USA: IN, NC, WI (
http://species-id.net/wiki/Gyrophaena_uteana
Fig. 40, Map 40, genitalia inCANADA: ON:Northumberland Co., Barr Property, ~ 7km NE Centreton, 44°7'44"N, 77°59'0"W, field, malaise pans, 16 to 27.vi.2011, Brunke & Paiero, 1 (DEBU), Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, maple-beech forest, Berlese leaf and log litter, 19.v.2011, A. Brunke, 1 (DEBU), same as previous except: 1.vi.2011, 1 (DEBU), Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, malaise pans, 16 to 27.vi.2011, Brunke & Paiero, 2 (DEBU).
Canada: BC, AB*, ON, QC, NB; USA: CA, CO, UT (
http://species-id.net/wiki/Leptusa_carolinensis
Fig. 41, Map 41, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'20"N, 80°29'29"W, sand ridge forest, malaise pans, 17 to 31.v.2011, Brunke & Paiero, 3 (DEBU), Turkey Point Prov. Park, wilderness area, forest, under bark, 17.v.2011, A. Brunke, 1 (DEBU);Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, under bark, large sugar maple, 6.x.2011, A. Brunke, 1 (DEBU).
http://species-id.net/wiki/Phanerota_fasciata
Fig. 42, Map 42, genitalia inNew Canadian Record
CANADA: ON:Essex Co., La Salle, Brunet Park, 29.vii.2005, S.M. Paiero, 2 (DEBU); Hald.-Norfolk Reg., Turkey Point Prov. Park, 42°41'48"N, 80°19'48"W, forest, gilled mushrooms, 12.x.2011, A. Brunke, 2 (DEBU).
Canada: ON; USA: AR, DC, FL, GA, IA, IL, IN, KY, KS, LA, MD, MI, MO, MS, NC, NJ, NY, OH, PA, TN, TX, VA, WI (
. The genus Phanerota is newly recorded in Canada based on specimens collected on mushrooms in extreme southern Ontario. This genus may reach its northern distributional limit in southern Ontario, as it was not reported in a recent review of New Brunswick Gyrophaenina (
http://species-id.net/wiki/Phymatura_blanchardi
Fig. 43, Map 43, genitalia inNew Ontario Record
CANADA: ON:Elgin Co., Aylmer West, malaise trap, 7 to 15.ix.1972, 1 (CNC); Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'21"N, 80°29'26"W, forest, fungi, 12.viii.2011, S.M. Paiero, 1 (DEBU), same data except: 20.ix.2011, S.M. Paiero, 1 (DEBU);Lanark Co., Bell’s Corners, 14.x.1967, A. Smetana, 3 (CNC); Leeds and Grenville United Co., Chaffey’s Locks Biol. Stn., 16.x.1986, A. Smetana, 1 (CNC); Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, 6.x.2011, A. Brunke, 1 (DEBU);Ottawa Div., Constance Bay, x.1970, S. Peck, 1 (CNC), Leitrim, ex. Ganoderma applanatum, 5.x.1985, R.S. Skidmore, 1 (CNC), Ottawa, Beaulieu, 29.viii.1912, 5 (CNC), South March, 11.x.1967, J.M. Campbell & A. Smetana, 1 (CNC).
Dorsal habitus of: 43 Phymatura blanchardi (Casey) 44 Thecturota pusio (Casey) 45 Placusa incompleta Sjöberg 46 Placusa vaga Casey 47 Acrotona smithi (Casey) 48 Acrotona subpygmaea (Bernhauer). Scale 1mm.
Canada: AB, ON, NB; USA: IA, IN, MO, NY (
http://species-id.net/wiki/Thecturota_pusio
Figs 44, 109–115; Map 44New Canadian Record
CANADA: ON: Hald.-Norfolk Reg., Turkey Point Prov. Pk., site 2, 42°42'28"N, 80°20'29"W, savannah, Berlese leaf, log and grass litter, 12.x.2011, A. Brunke, 11 (DEBU).
Canada: ON; USA: IN. Native.
This is the first collection of Thecturota pusio since Casey’s description (1894) based on the female holotype from ‘Indiana’ and the first Canadian record of the genus. We have dissected the female holotype for comparison with the Ontario specimens and illustrate the male and female sexual characters for the first time (Figs 109–115). Live specimens of Thecturota pusio were extremely slow-moving and the use of a Berlese funnel likely facilitated the capture of this minute (<2mm) species.
http://species-id.net/wiki/Placusa_incompleta
Fig. 45, Map 45, genitalia inNew Ontario Record
CANADA: ON:Northumberland Co., Peter’s Woods PNR, 44°7'26"N, 78°2'31"W, front woods, forest, malaise pans, 19.v to 1.vi.2011, Brunke & Paiero, 1 (DEBU), Barr Property, ~ 7km NE Centreton, 44°7'44"N, 77°59'0"W, forest, sappy Populus wood, 12.vii.2011, A. Brunke, 1 (DEBU), Barr Property, ~ 7km NE Centreton, 44°7'44"N, 77°59'0"W, 12.viii.2011, A. Brunke, 1 (DEBU).
Canada: BC, ON, QC, NB, NS, NL; USA: WA; western Palaearctic (
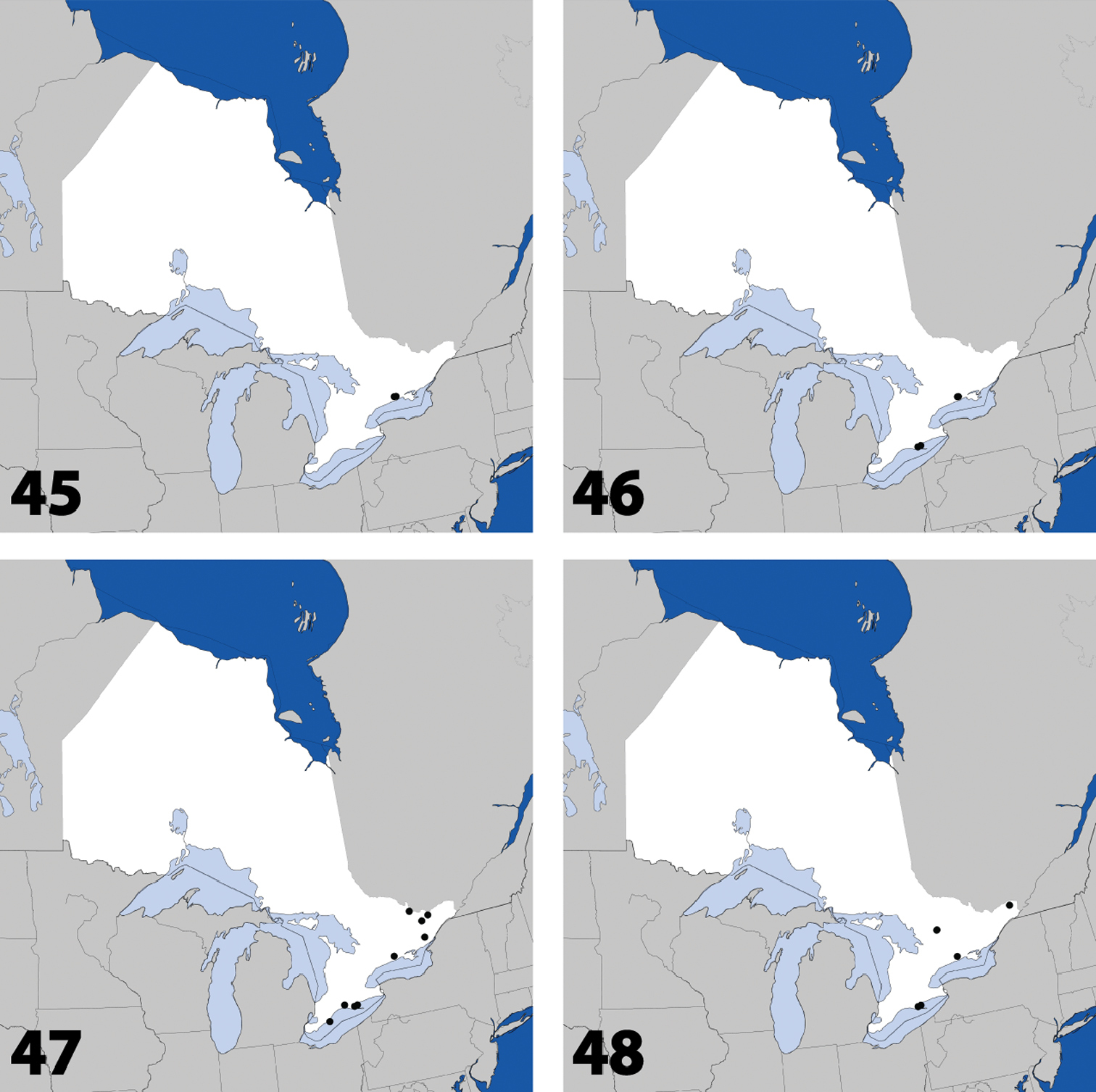
Distribution in Ontario of: 45 Placusa incompleta Sjöberg 46 Placusa vaga Casey 47 Acrotona smithi (Casey) 48 Acrotona subpygmaea (Bernhauer).
http://species-id.net/wiki/Placusa_vaga
Fig. 46, Map 46, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'20"N, 80°29'29"W, forest, sand ridge, malaise, 20.vii to 5.viii.2011, Brunke & Paiero, 1 (DEBU), Turkey Point Prov. Park, 42°42'28"N, 80°20'29"W, oak savannah, malaise, 20.ix to 12.x.2011, Brunke & Paiero, 1 (DEBU); Northumberland Co., Barr Property, ~ 7km NE Centreton, 44°7'44"N, 77°59'0"W, forest, sappy Populus wood, 12.vii.2011, A. Brunke, 2 (DEBU), Barr Property, ~ 7km NE Centreton, 44°7'44"N, 77°59'0"W, 12.viii.2011, S.M. Paiero, 1 (DEBU), Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, 27.vii.2011, S.M. Paiero, 1 (DEBU).
Canada: YT, NT, BC, ON, QC, NB, NS; USA: CA (
http://species-id.net/wiki/Acrotona_smithi
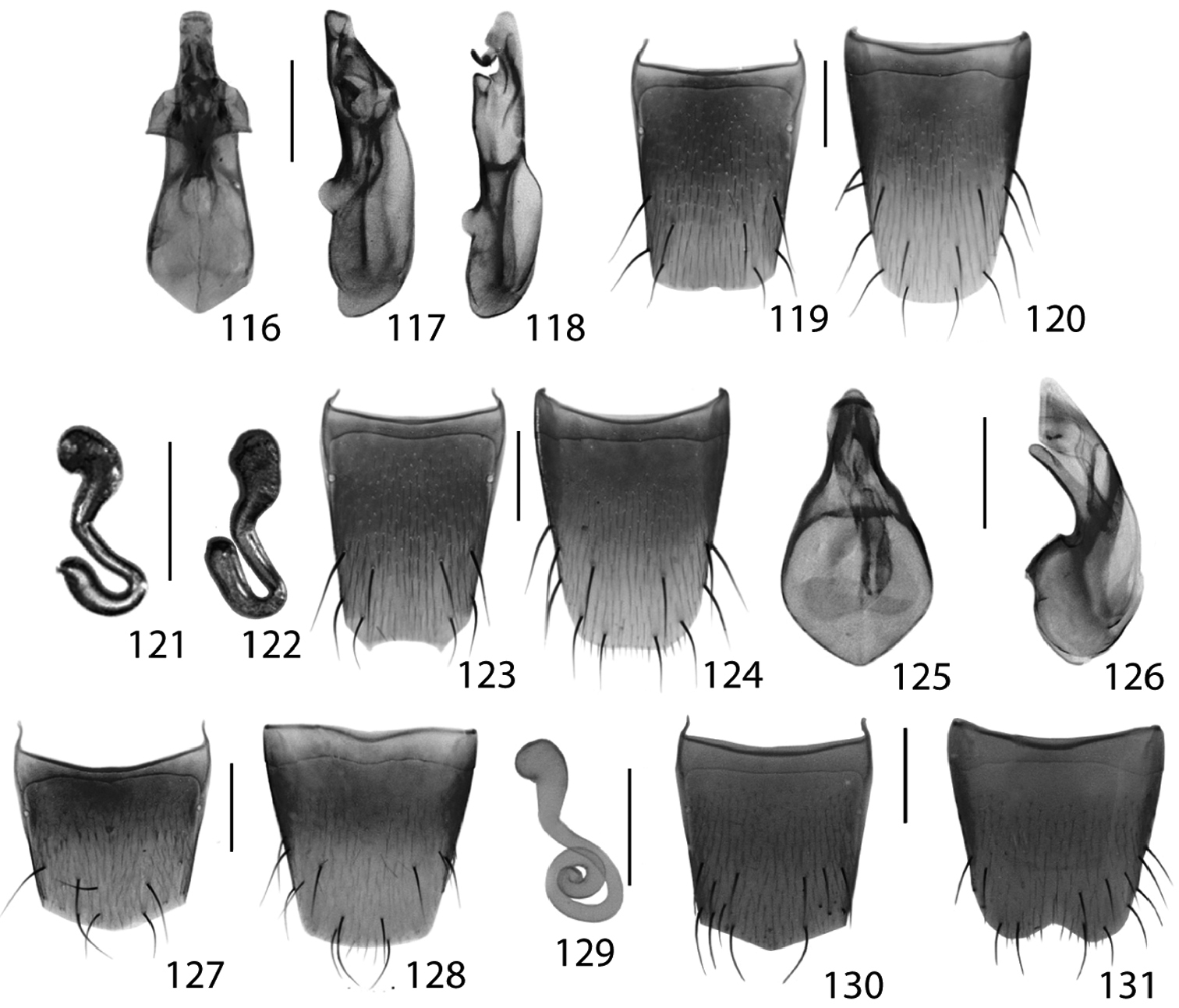
Fig. 47, 116–124; Map 47New Canadian Record
(Type material – see above). CANADA: ON:Chatham-Kent Co., Rondeau Prov. Park, end Lakeshore Rd., 1.vi.1985, A. Davies & J.M. Campbell, 1 (CNC), same data except: 5.vi.1985, sifted grass pile & leaves, 3 (CNC), Rondeau Prov. Park, deciduous forest, 19.v to 6.vii.1976, Dondale & Redner, 2 (CNC), Rondeau Prov. Park, intercept trap, on sand beach, edge oak forest, 22 to 31.vii.1985, L. LeSage & A. Woodliffe, 2 (CNC), same data except: 1 to 9.viii.1985, 1 (CNC), 9 to 17.viii.1985, 1 (CNC), Rondeau Prov. Park, intercept trap, maple-beech forest, 13 to 22.vii.1985, L. LeSage & A. Woodliffe, 1 (CNC), Rondeau Prov. Park, intercept trap, white pine stand, 1 to 9.viii.1985, L. LeSage & A. Woodliffe (2), Rondeau Prov. Park, Lakeshore Rd., 30.v.1985, A. Smetana, 2 (CNC); Elgin Co., Aylmer West, Malaise trap, 24 to 31.viii.1972, 1 (CNC), same data except: 15 to 22.ix.1972, 1 (CNC); Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'20"N, 80°29'29"W, sand ridge, forest, malaise pans, 15.vi to 5.vii.2011, Brunke & Paiero, 1 (DEBU), same data except: 5.vii to 20.vii.2011, 1 (DEBU), 31.v to 15.vi.2011, 1 (DEBU), Cronmiller Prop., ~6km W St. Williams, 42°40'20"N, 80°29'29"W, low forest, malaise pans, 5.vii to 20.vii.2011, Brunke & Paiero, 2 (DEBU), Turkey Point Prov. Park, site 2, 42°42'28"N, 80°20'29"W, oak savannah, Lindgren funnel, 15.vi to 5.vii.2011, Brunke & Paiero, 1 (DEBU); Lanark Co., 7 mi W of Carleton Place, 15.v.1980, A. Smetana, 3 (CNC); Leeds and Grenville Co., ‘Chaffey’s Locks’ Biol. Stn., 16.x.1986, A. Smetana, 2 (CNC); Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, 12.viii.2011, A. Brunke, 1 (DEBU); Ottawa Div., Kanata, 25.v.1979, A. & Z. Smetana, 2 (CNC); Renfrew Co., Haley Sta., 15km NW Renfrew, mixed forest malaise, 2 to 30.ix.1979, S. Peck, 1 (CNC).
Canada: ON, NB; USA: NY (
Acrotona smithi is newly recorded in Canada based on numerous collections across Ontario. Ontario material was compared with the type series of Acrotona smithi from New York. This species is easily recognized amongst other northeastern Acrotona by the large and fusiform body (Oxypoda-like habitus), the distinctive shape of the aedeagus in lateral view (Figs 117–118), the broadly and shallowly emarginate female tergite VIII (Fig. 123), and, despite some variation, the general shape of the spermatheca (Figs 121–122). Acrotona smithi appears to be a common species inhabiting deciduous to mixed forests and semi-open habitat (e.g. oak savannah) and is probably broadly distributed across northeastern North America.
http://species-id.net/wiki/Acrotona_subpygmaea
Figs 48, 125–131; Map 48New Ontario Record
(Type material – see above). CANADA: ON: Hald.-Norfolk Reg., Backus Woods, Wetland trail, 42°39'54"N, 80°29'34"W, sugar maple dom. mesic forest, sift litter, 2.iv.2010, A. Brunke, 3 (DEBU), Backus Woods, 4.x.2010, 1 (DEBU), Backus Woods, north block, 42°40'7"N, 80°29'34"W, ex. sifted litter, berlese, 23.iv.2011, Brunke and Marshall, 1 (DEBU), Cronmiller Prop., ~6km W St. Williams, 42°40'20"N, 80°29'29"W, forest, sand ridge, malaise pans, 17 to 31.v.2011, Brunke & Paiero, 1 (DEBU), Cronmiller Prop., ~6km W St. Williams, 42°40'21"N, 80°29'26"W, forest, berlese vernal pool litter, 17.v.2011, A. Brunke, 1 (DEBU), Turkey Point Prov. Pk., site 1, 42°41'48"N, 80°19'48"W, forest, sift tree hole litter, 12.x.2011, A. Brunke, 1 (DEBU); Haliburton Co., 10 km SE Dorset, 45.16, -78.84, vernal pool litter (previously wet), 19.vi.2011, S. Kullik, 1 (DEBU), same data except: 45.17 -78.82, 17.x.2009, 1 (DEBU); Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, ex. cold wet moss on rocks and edge of spring, 15.ix.2011, A. Brunke, 1 (DEBU); Prescott and Russell United Co., Alfred Bog, berlese litter, forest trail, 17.vii.1982, L. LeSage, 2 (CNC).
Canada: ON, NS; USA: IN, MA, RI (
In an online catalog of North American Athetini (
There are some Canadian specimens currently identified as Acrotona subpygmaea that possess very short elytra and slightly different sexual characters (R. Webster and J. Klimaszewski unpublished data) including one Ontario female [Backus Woods, Wetland trail, 42°39'54"N, 80°29'34"W, sugar maple dom. mesic forest, sift litter, 2.iv.2010]. Therefore, we recommend that identifications of Acrotona subpygmaea be based on the distinctive sexual characteristics of either sex (Figs 125–131) until the Nearctic diversity of this genus is more adequately known. Acrotona subpygmaea is a common species occurring in a variety of forest litter microhabitats and has been collected in both spring and fall. We expect this species to occur broadly across northeastern North America.
http://species-id.net/wiki/Alevonota_gracilenta
Figs 49, 132–134; Map 49, spermatheca inNew North American Record
CANADA: ON:Waterloo Reg., Blair, Whistle Bare Rd. and Township Rd.1, 43.372 -80.362, soybean field, pitfall trap, 29.vi.2010, A. Brunke, 2 (DEBU); Wellington Co., Eramosa, hedgerow, pitfall, 4.v.2010, A. Brunke, 1 (DEBU), same data except: 13.vii.2010, 1 (DEBU), Guelph, hedgerow, pitfall, 19.v.2009, 1 (DEBU), same data except: 1.ix.2009, 1 (DEBU).
Dorsal habitus of: 49 Alevonota gracilenta (Erichson) 50 Aloconota sulcifrons (Stephens) 51 Atheta capsularis Klimaszewski 52 Atheta aemula (Erichson) 53 Atheta borealis Klimaszewski & Langor 54 Atheta circulicollis Lohse. Scale 1mm.
Canada: ON; widespread in western Palaearctic (
Distribution in Ontario of: 49 Alevonota gracilenta (Erichson) 50 Aloconota sulcifrons (Stephens) 51 Atheta capsularis Klimaszewski 52 Atheta aemula (Erichson).
Alevonota gracilenta is recorded here for the first time in North America as an adventive species. It is rather easily recognized in North America by the narrow, linear habitus, small eyes and distinctive aedeagus with a long flagellum (Fig. 132).
Alevonota gracilenta apparently prefers a wide range of unforested habitats in its native range but is usually only collected in small numbers and using passive traps (
http://species-id.net/wiki/Aloconota_sulcifrons
Fig. 50, Map 50, genitalia inNew Ontario Record
CANADA: ON:Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, 12.viii.2011, A. Brunke, 1 (DEBU).
Canada: ON, QC, NB, NL; USA: AL, IL, IN, KY, MO, NH, NY, TN, VA, WA, WV; widespread in Palaearctic region, possibly cosmopolitan (
http://species-id.net/wiki/Atheta_capsularis
Fig. 51, Map 51, genitalia inNew Ontario Record
CANADA: ON:Thunder Bay Distr., Neys Provincial Park, campground area 2, 48°47'17"N, 86°37'32"W, forest, dung pans, 16 to 19.vii.2002, M. Buck, 1 (DEBU).
Canada: YT, ON, QC, NB, NL (
http://species-id.net/wiki/Atheta_aemula
Fig. 52, Map 52, genitalia inNew Ontario Record
CANADA: ON:Huron Co., Brucefield, hedgerow, pitfall, 11.v.2009 (1), 8.vi.2009 (1), A. Brunke (DEBU).
Canada: ON, QC, NB; USA: CA, IA, KS, MA, MS, NC, NH, NJ, NY, PA, TX (
http://species-id.net/wiki/Atheta_borealis
Fig. 53, Map 53, genitalia inNew Ontario Record
CANADA: ON:Wellington Co., Arkell, wet sedge meadow, sweep, 7.x.1993, C.S. Blaney, 1 (DEBU).
http://species-id.net/wiki/Atheta_circulicollis
Fig. 54, Map 54, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'18"N, 80°29'24"W, forest, site 2, malaise pans, 17 to 31.v.2011, Brunke & Paiero, 1 (DEBU), Turkey Point Prov. Park, 42°41'48"N, 80°19'48"W, forest, malaise pans, 31.v to 15.vi.2011, Brunke & Paiero, 1 (DEBU); Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, forest, malaise, 19.v to 1.vi.2011, Brunke & Paiero, 1 (DEBU), same data except: back woods, forest, malaise pans, 1 to 16.vi.2011, 1 (DEBU).
Canada: ON, QC, NB, NL (
This species was previously known only from relatively northern, forested localities in Canada, including near the tree line in Quebec (
http://species-id.net/wiki/Atheta_particula
Fig. 55, Map 55, genitalia in Klimaszewski et al. (2005) New Ontario RecordCANADA: ON:Hald.-Norfolk Reg., Cronmiller Prop., ~6 km W St. Williams, 42°40'18"N, 80°29'24"W, forest, nr. vernal pools, malaise pans, 31.v to 15.vi.2011, Brunke & Paiero, 2 (DEBU), Cronmiller Prop., ~6 km W St. Williams, 42°40'18"N, 80°29'24"W, forest, 17.viii.2011, A. Brunke, 1 (DEBU); Northumberland Co., Barr Property, ~ 7km NE Centreton, 44°7'48"N, 77°59'3"W, old field, malaise pans, 1 to 16.vi.2011, Brunke & Paiero, 1 (DEBU), Peter’s Woods PNR, 44°7'26"N, 78°2'31"W, forest, on fungus, 12.viii.2011, S.M. Paiero, 1 (DEBU), Peter’s Woods PNR, 44°7'26"N, 78°2'31"W, forest, 12.viii.2011, A. Brunke, 1 (DEBU).
Dorsal habitus of: 55 Atheta particula (Casey) 56 Atheta burwelli (Lohse) 57 Atheta campbelli Lohse 58 Atheta pseudocrenuliventris Klimaszewski 59 Atheta terranovae Klimaszewski & Langor 60 Atheta savardae Klimaszewski & Majka. Scale 1mm.
Canada: ON, QC, NB, NS; USA: NY, RI (
http://species-id.net/wiki/Atheta_burwelli
Fig. 56, Map 56, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'20"N, 80°29'29"W, site 2, forest, malaise pans, 17 to 31.v.2011, Brunke & Paiero, 2 (DEBU).
Canada: YT, ON, QC, NB, NL (
http://species-id.net/wiki/Atheta_campbelli
Fig. 57, Map 57, genitalia inNew Ontario Record
CANADA: ON:Huron Co., Auburn, hedgerow, pitfall, 26.v.2010, A. Brunke, 1 (DEBU).
http://species-id.net/wiki/Atheta_pseudocrenuliventris
Fig. 58, Map 58, genitalia inNew Ontario Record
CANADA: ON:Manitoulin Distr., Manitoulin Is., Kip Fleming Tract, ~8km SW Gore Bay, 45°52'13"N, 82°32'31"W, oak savannah/alvar, RET over burrow, 15.vi to 16.vii.2010, Marshall et al., 1 (DEBU); Northumberland Co., Peter’s Woods PNR, 44°7'27"N, 78°2'21"W, front woods, forest, malaise pans, 16 to 27.vi.2011, Brunke & Paiero, 1 (DEBU).
Canada: YT, ON, NB, NS, NL (
http://species-id.net/wiki/Atheta_terranovae
Fig. 59, Map 59, genitalia inNew Ontario Record
CANADA: ON:Algoma Distr., Lake Superior Prov. Pk., 2.ix.1980, leg. R. Baranowski, 6 (MZLU), same data except: 3.ix.1980, 1 (MZLU), 6.ix.1980, 4 (MZLU), Michipicoten River (south of Wawa), 5.ix.1980, leg. R. Baranowski, 7 (MZLU), same data except: 8.ix.1980, leg. R. Baranowski, 2 (MZLU); Nipissing Distr., Algonquin Prov. Park nr. Brent, 20.viii.1980, leg. R. Baranowski, 5 (MZLU), same data except: 21.viii.1980, 1 (MZLU); Sudbury Distr., 30 km W of Foleyet, 30.viii.1980, leg. R. Baranowski, 2 (MZLU), Gogama, Mattagami River, 24.viii.1980, leg. R. Baranowski, 1 (MZLU), same data except: 27.viii.1980, 4 (MZLU), Mattagami, 25.viii.1980, leg. R. Baranowski, 1 (MZLU), same data except: 27.viii.1980, 4 (MZLU).
Canada: YT, ON, NB, NL (
The above Ontario collections of this recently described species suggest a transcontinental distribution in Canada.
http://species-id.net/wiki/Atheta_savardae
Fig. 60, Map 60, genitalia inNew Ontario Record
CANADA: ON:Greater Sudbury Div., Sudbury, Laurentian Univ. Campus, 46°27'38"N, 80°57'33"W, forest, pitfall trap, 1.ix.2010 (2), 24.ix.2010 (4), 27.ix.2010 (4), 29.ix.2010 (4), 4.x.2010 (4), 6.x.2010 (1), 8.x.2010 (1), J.S. Jackson (DEBU); Hald.-Norfolk Reg., Turkey Point Prov. Park, 42°41'48"N, 80°19'48"W, forest, on fungi, 12.x.2011, S.M. Paiero, 1 (DEBU); Haliburton Co., Dorset, 18 km S of Frost Centre, fungus, 19.ix.2008, S. Kullik, 1 (DEBU); Huron Co., Auburn, hedgerow, pitfall, 10.ix.2010, A. Brunke, 1 (DEBU); Nipissing Distr., Algonquin Prov. Park, Swan Lake station, Scott Lk., 45°29'15" 78°43'20"W, shore site, pan traps, 4.vii.1995, S. A. Marshall, 1 (DEBU); Northumberland Co., Peter’s Woods PNR, 44°7'26"N, 78°2'31"W, forest, 6.x.2011, A. Brunke, 1 (DEBU); Wellington Co., Arkell, field vegetation, 1.x.1993, C. Krupke, 1 (DEBU).
Canada: ON, QC, NB, NS, NL (
This species appears to be associated with decaying fungi in forested habitats as all known specimens with microhabitat data were collected this way.
urn:lsid:zoobank.org:act:76CCEC54-23E7-4DB2-B34F-B4D14BFDE7BC
http://species-id.net/wiki/Atheta_alesi
Figs 61, 135–141; Map 61Canada, Ontario, Ottawa Div., Ottawa, Central Experimental Farm, Marmota burrow.
Holotype (male): CANADA: ON: Ottawa, Centr. Exp. Farm, Marmota burrows, 20.iv.2009, A. Smetana leg. (LFC).
Paratypes (6 males, 8 females): 13 with same data as holotype: (2 male, 5 female, CNC; 4 male, 2 female, LFC); Waterloo Reg., Blair, 43.37 -80.39, hedgerow, canopy trap, 19.v.2009, A. Brunke, 1 female (DEBU).
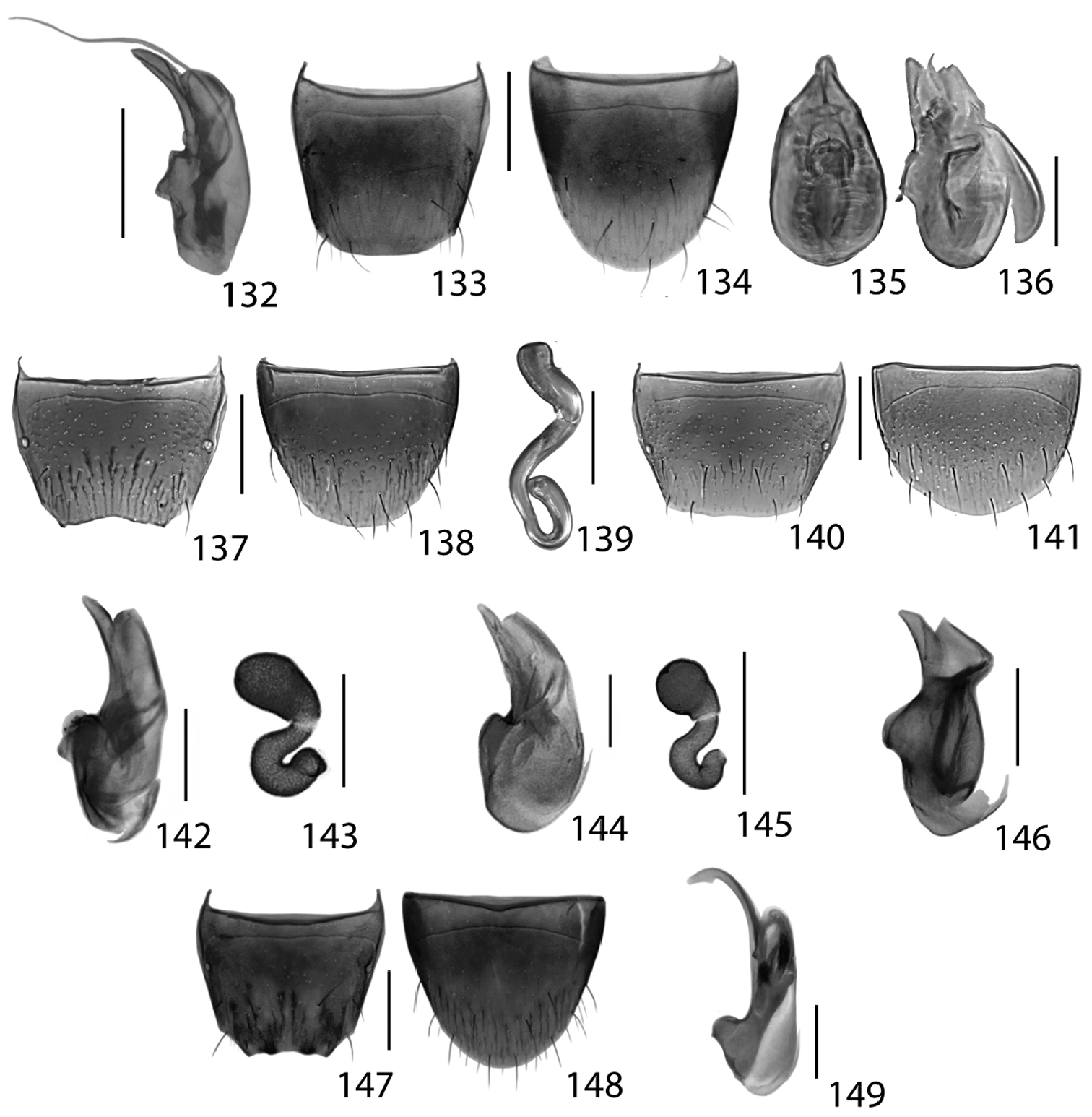
Dorsal habitus of: 61 Atheta alesi Klimaszewski & Brunke sp. n. 62 Atheta festinans (Erichson) 63 Atheta nescia (Casey) 64 Callicerus obscurus Gravenhorst 65 Callicerus rigidicornis (Erichson) 66 Dinaraea backusensis Klimaszewski & Brunke sp. n.Scale 1mm.
This species may be distinguished from all other Atheta (Microdota) species by the following combination of characters: body dark brown with legs, 2–3 basal antennomeres and elytra yellowish; forebody strongly glossy and with microsculpture; distal antennomeres only moderately transverse; male tergite VIII with distinctive shallow and wide emargination (Fig. 137), median lobe of aedeagus in lateral view with large bulbus and straight tubus (Fig. 136), internal sac in lateral view with distinctive, large, curved sclerite that is bifurcate basally (Fig. 136); and spermatheca S-shaped, with elongate, tubular capsule that bears a moderately long and broad apical invagination, stem sinuate and apically looped (Fig. 139).
Body small, length 2.4–2.6 mm, narrowly subparallel, forebody with strong meshed microsculpture and strongly glossy, abdomen strongly glossy and with moderately sparse pubescence; head, pronotum and abdomen dark brown, elytra, legs and antennomeres 2–3 yellowish; head subquadrate, flattened and slightly impressed medially, with postocular area at least as long as diameter of eye, eyes large and slightly protruding, pubescence directed inwards in central part of disc; antennae slender, antennomeres 1–3 strongly elongate, 4–5 subquadrate, 6–10 moderately transverse, apical antennomere strongly elongate, longer than 9–10 combined; pronotum moderately transverse, margined laterally and posteriorly, pubescence radiating laterad and obliquely posteriad from the midline of disc, with 4 macrosetae close to lateral margin; elytra slightly elongate, at suture longer than pronotum, pubescence directed obliquely latero-posteriad; abdomen subparallel, tergites III to V with basal impression; legs moderately elongate.
Male. Tergite VIII truncate apically and with shallow, wide emargination (Fig. 137); sternite VIII rounded apically or sometimes slightly pointed medially (Fig. 138); median lobe of aedeagus with large, broad bulbus and short triangular tubus in parameral view; in lateral view, tubus straight ventrally and narrowly rounded at apex (Fig. 136); internal sac in abparameral view with distinct structures as illustrated in Fig. 135, internal sac in lateral view with distinctive curved sclerite that is bifurcate basally (Fig. 136).
Female. Tergite VIII truncate apically (Fig. 140); sternite VIII rounded and slightly pointed medially (Fig. 141); spermatheca S-shaped, with elongate, tubular capsule that bears a moderately long and broad apical invagination, stem sinuate and apically looped (Fig. 139).
Atheta alesi is currently only known from Ontario but is expected to occur broadly across eastern North America.
Distribution in Ontario of: 61 Atheta alesi Klimaszewski & Brunke sp. n. 62 Atheta festinans (Erichson) 63 Atheta nescia (Casey) 64 Callicerus obscurus Gravenhorst.
Nearly all specimens were collected from debris in groundhog (Marmota monax (L.)) burrows. Atheta alesi may be another member of the rich insect assemblage associated with groundhog burrows but further collections in this microhabitat are needed to confirm this. Although one specimen was collected in a raised pan trap placed in an agricultural hedgerow, other groundhog-associated staphylinids were collected in this series including Aleochara ocularis Klimaszewski and Bisnius pugetensis (Hatch).
This species is named in honor of Dr. Aleš Smetana, Ottawa, Ontario, Canada, in recognition of his excellent collections from groundhog (Marmota monax (L.)) burrows, which have revealed many interesting species that may be restricted to this microhabitat (e.g. species in
This species is tentatively assigned to the subgenus Microdota based on the following combination of characters present in other Canadian species: small body size, antennomeres 6–10 subquadrate to moderately transverse, Y-shaped ligula, simply formed median lobe of the aedeagus and overall shape of the spermatheca. Atheta (Microdota) alesi is most similar externally and in sexual characters to the type species of Microdota, Atheta (Microdota) amicula (Stephens), which has become introduced into North America. The new species can be separated from Atheta amicula by the longer apical antennomeres (strongly transverse in Atheta amicula), the straight ventral surface of the tubus in lateral view, the differently shaped sclerites of the internal sac in lateral view and the narrower capsule of the spermatheca. The sexual characters of Atheta amicula are illustrated in
http://species-id.net/wiki/Atheta_festinans
Fig. 62, Map 62, genitalia inCANADA: ON:Waterloo Reg., Blair, 43.374 -80.397, hedgerow, pitfall trap, 16.vi.2009, A. Brunke, 1 (DEBU).
Canada: ON; USA: AZ, CT, IA, IN, KS, NY, PA, RI (
This species was previously reported from Ontario by
http://species-id.net/wiki/Atheta_nescia
Fig. 63, Map 63, genitalia inNew Ontario Record
CANADA: ON:Huron Co., Auburn, hedgerow, 26.v.2010, A. Brunke, 1 (DEBU); Manitoulin Distr., Manitoulin I., Kip Fleming Tract, 8 km SW Gore Bay, 45°52'13"N, 82°32'31"W, oak savannah/alvar, pans nr. log, 23.viii to 30.viii.2010, Marshall et al., 2 (DEBU);same data except:malaise pans, 12.vi to 16.vi.2010, 1 (DEBU); Northumberland Co., Barr prop., 7 km NE Centreton, site 1, 44°7'40"N, 77°58'57"W, savannah, malaise pans, 16.vi to 27.vi.2011, Brunke and Paiero, 1 (DEBU).
Canada: BC, ON (
The specimens form Ontario agree in most characteristics with British Columbian specimens of Atheta nescia except for the less robust antennae, particularly in males, and the median lobe in lateral view, with a slightly narrower tubus and more rounded apex (for illustrations of the genitalia of Atheta nescia see Figs 51–53 in
The Ontario specimens were captured in sparsely treed, open habitats including savannahs and an agricultural hedgerow. Similarly, the specimens of Atheta nescia collected in British Columbia were primarily collected in clear-cut forests (
http://species-id.net/wiki/Callicerus_obscurus
Figs 64, 142–143; Map 64New Canadian Record
CANADA: ON:Hamilton Div., Hamilton, 15.v.1985, M. Sanborne, 1 (CNC); Huron Co., Brucefield, hedgerow, pitfall, 11.v.2009, A. Brunke, 1 (DEBU), same data except: 22.vi.2009, 1 (DEBU), Auburn, hedgerow, pitfall, 11.v.2010, A. Brunke, 1 (DEBU).
Canada: ON; western Palaearctic (
Callicerus obscurus is recorded from Canada for the first time based on Ontario specimens mostly collected in agricultural hedgerows.
Callicerus obscurus inhabits open and forested habitats in its native range and was suggested to be largely subterranean by
http://species-id.net/wiki/Callicerus_rigidicornis
Figs 65, 144–145; Map 65New North American Record
CANADA: ON: Huron Co., Auburn, hedgerow, pitfall, 11.v.2010, A. Brunke, 3 (DEBU), Benmiller, hedgerow, pitfall, 22.vi.2009, A. Brunke, 1 (DEBU).
Canada: ON; western Palaearctic (
Distribution in Ontario of: 65 Callicerus rigidicornis (Erichson) 66 Dinaraea backusensis Klimaszewski & Brunke sp. n. 67 Mocyta breviuscula (Mäklin) 68 Philhygra jarmilae Klimaszewski & Langor.
Callicerus rigidicornis is recorded from North America as an adventive species for the first time based on Ontario specimens collected in agricultural hedgerows. Males of this species do not have their antennomere 10 conspicuously elongate as in Callicerus obscurus. Callicerus rigidicornis is separated from Callicerus obscurus by the more transverse pronotum (Fig. 65). In both its native range and in Canada, this species is collected from the same habitats as Callicerus obscurus though the true microhabitat may be subterranean (
urn:lsid:zoobank.org:act:81B9BEBD-F6ED-4893-A5C1-2094599C88CC
http://species-id.net/wiki/Dinaraea_backusensis
Figs 66, 146–149; Map 66Canada, Ontario, Haldimand-Norfolk Reg., 6 km W of Saint Williams, Backus Woods, Wetland trail, sugar maple-dominated mesic forest, 42°39'54"N, 80°29'34"W.
Holotype (male): CANADA, ON:Hald.-Norfolk Reg., Backus Woods, Wetland trail, 42°39'54"N, 80°29'34"W, sugar maple-dominated mesic forest, sifted litter, 2.iv.2010, A. Brunke, debu00331025 (DEBU).
This species may be distinguished from all other Nearctic Dinaraea by the following combination of characters: postocular area slightly longer than eye; pronotum trapezoidal in form and slightly (not distinctly) transverse; antennomeres 1–3 elongate, 4–7 subquadrate, 8–10 slightly transverse; elytra flat, transverse, at suture about as long as pronotum; male tergite eight with median and lateral teeth (Fig. 147); and median lobe of aedeagus of distinctive shape in lateral view (Figs 146).
Body narrowly subparallel, flattened, length 3.1 mm, dark brown, with legs, maxillary palpi, and basal 1–3 antennomeres yellowish brown, forebody moderately glossy with strong meshed microsculpture, abdomen strongly glossy with weaker microsculpture, pubescence moderately dense, denser on pronotum and elytra than on abdomen (Fig. 66); head transverse, impressed medially, rounded laterally, postocular area slightly longer than eye, pubescence sparse and directed mediad; antennae with antennomeres 1–3 elongate, 4–7 subquadrate, 8–10 slightly transverse; maxillary palpi with penultimate article broad and last article acicular; pronotum slightly transverse, trapezoidal, basal margin arcuate, with obtuse hind angles, broadest in apical third, flattened medially, margined, pubescence sparser than that on elytra and directed laterad on disc and forming arcuate lines, pubescence at midline directed anteriad in apical portion and posteriad in basal portion, pronotum with 4 lateral macrosetae on each side; elytra flat, transverse, subequal in length to pronotum at midline, pubescence directed straight or obliquely posteriad, punctation granulose; abdomen with tergites II-IV strongly impressed and sparsely pubescent.
Male. Tergite VIII truncate apically with two lateral teeth and two median protuberances (Fig. 147); sternite VIII with apex arcuate but slightly pointed medially (Fig. 148); median lobe of aedeagus in lateral view with moderately large bulbus and short tubus with angulate apex, ventral side of tubus weakly arcuate; internal sac in lateral view with a narrow, elongate and recurved sclerite (Fig. 146).
Female. Unknown.
At present, Dinaraea backusensis is known only from southern Ontario but should occur across eastern North America, at least as far north as southern Canada.
The holotype was collected in a sugar maple dominated forest with a rich diversity of other deciduous trees by sifting deep pockets of leaf litter beside large, old logs. Other native species of Dinaraea have been associated with subcortical habitats (
This species is named after Backus Woods, a 704-acre, old growth, Carolinian forest in Ontario, Canada where the holotypewas collected. We would like to recognize the conservation efforts of the Nature Conservancy of Canada in this region and their recent work in acquiring this property for permanent protection.
Using previous literature, Dinaraea backusensis can be distinguished from all known Nearctic species of the genus except Dinaraea borealis Lohse and Dinaraea planaris (Mäklin) by the distinctive shape of the median lobe in lateral view (see figures in
http://species-id.net/wiki/Mocyta_breviuscula
Fig. 67, Map 67, genitalia inNew Ontario Record
CANADA: ON:Manitoulin Distr., Manitoulin Island, Kip Fleming Tract, ~8km SW Gore Bay, 45°52'13"N, 82°32'31"W, oak savannah/alvar, sifted litter, 29.ix.2010, S.M. Paiero, 1 (DEBU), Misery Bay Prov. Nat. Res., 45°47'28"N, 82°44'58"W, alvar, 29.ix.2010, S.M. Paiero, 1 (DEBU); Sudbury Co., Mattagami, 24.viii.1980, leg. R. Baranowski, 1 (MZLU).
Dorsal habitus of: 67 Mocyta breviuscula (Mäklin) 68 Philhygra jarmilae Klimaszewski & Langor 69 Philhygra laevicollis (Mäklin) 70. Philhygra luridipennis (Mannerheim) 71 Philhygra proterminalis (Bernhauer) 72 Stethusa klimschi (Bernhauer). Scale 1mm.
Canada: YT, BC, AB, ON, QC, NB, NS, NL; USA: AK, CA, NV (
http://species-id.net/wiki/Philhygra_jarmilae
Fig. 68, Map 68, genitalia inNew Ontario Record
CANADA: ON:Waterloo Reg., Blair, hedgerow, pitfall, 5.x.2010, A. Brunke, 1 (DEBU).
Canada: YT, ON, NB, NL (
The specimen from southern Ontario suggests a broad, transcontinental distribution in Canada for this recently described species. It likely occurs broadly in eastern United States as well.
http://species-id.net/wiki/Philhygra_laevicollis
Fig. 69, Map 69, genitalia inNew Ontario Record
CANADA: ON:Haliburton Co., 9 km SE of Dorset, vernal pool litter (previously wet), 45.17–78.84, 17.vii.2009, S. Kullik, 1 (DEBU), same data except: 45.18–78.83, 17.viii.2009, 1 (DEBU); Nipissing Distr., Algonquin Prov. Park, nr. Brent, 19.viii.1980, R. Baranowski, 1 (MZLU), same data except: 21.viii.1980, 1 (MZLU).
Canada: BC, ON, NB, NS; USA: AK, WA (
Distribution in Ontario of: 69 Philhygra laevicollis (Mäklin) 70 Philhygra luridipennis (Mannerheim) 71. Philhygra proterminalis (Bernhauer) 72 Stethusa klimschi (Bernhauer).
http://species-id.net/wiki/Philhygra_luridipennis
Fig. 70, Map 70, genitalia inNew Ontario Record
CANADA: ON:Hald.-Norfolk Reg., Cronmiller prop., ~6km W St. Williams, 42°40'21"N, 80°29'26"W, forest, at lights, 20.vii.2011, Brunke & Paiero, 1 (DEBU); Huron Co., Brucefield, hedgerow, pitfall trap, 28.ix.2009, A. Brunke, 1 (DEBU).
Canada: ON, NB, NL; Palaearctic: Europe and North Africa (
http://species-id.net/wiki/Philhygra_proterminalis
Figs 71, 149; Map 71New Canadian Record
CANADA: ON:Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'21"N, 80°29'26"W, forest, 31.v.2011, A. Brunke, 1 (DEBU), same data except: Lindgren funnel, 20.vii to 5.viii.2011, Brunke & Paiero, 1 (DEBU); Hamilton Div., Waterdown, madicolous spring, 5.viii.1985, B. Sinclair, 1 (DEBU).
Canada: ON; USA: CO, PA. Native.
http://species-id.net/wiki/Stethusa_klimschi
Fig. 72, Map 72, genitalia inNew Canadian Record
CANADA: ON:Chatham-Kent Co., Rondeau Prov. Pk., south point trail, nr. east parking lot, 42°15'42"N, 81°50'49"W, savannah, malaise, 14.viii to 7.ix.2003, Buck and Marshall, 1 (DEBU).
Canada: ON; USA: IN, LA, MS (
This species is newly recorded from Canada, extending its known distribution considerably northward. Stethusa klimschi appears to be less common in Ontario than Stethusa spuriella (see below) as only one female specimen was found.
http://species-id.net/wiki/Stethusa_spuriella
Fig. 73, Map 73, genitalia inNew Canadian Record
CANADA: ON:Chatham-Kent Co., Rondeau Prov. Park, south point east pkng lot, 42 15 42N, 81 50 49W, oak savannah, white pans, 29.v.2003, Buck & Paiero, 1 (DEBU); Essex Co., Windsor, Ojibway Prairie, burnt prairie, yellow pans, 15 to 18.v.2001, S. M. Paiero, 1 (DEBU); Hald.-Norfolk Reg., Cronmiller Prop., ~6km W St. Williams, 42°40'18"N, 80°29'24"W, low forest, malaise pans, 20.vii to 5.viii.2011, Brunke & Paiero, 1 (DEBU), Cronmiller Prop., ~6km W St. Williams, 42°40'20"N, 80°29'29"W, ridge forest, malaise pans, 5.vii to 20.vii.2011, Brunke & Paiero, 1 (DEBU), Turkey Point Prov. Park, site 1, 42°41'48"N, 80°19'48"W, forest, malaise pans, 5.viii to 17.viii.2011, Brunke & Paiero, 1 (DEBU), same data except: on fungus, 12.x.2011, A. Brunke, 1 (DEBU), Turkey Point Prov. Park, site 2, 42°42'28"N, 80°20'29"W, oak savannah, Berlese leaf, log and grass litter, 12.x.2011, A. Brunke, 1 (DEBU); Northumberland Co., Barr Property, ~ 7km NE Centreton, 44°7'44"N, 77°59'0"W, savannah, malaise pans, 26.vii to 12.viii.2011, Brunke & Paiero, 1 (DEBU); Waterloo Reg., Blair, soybean field, pitfall, 23.vi.2009, A. Brunke, 1 (DEBU), Cambridge, soybean field, pitfall, 23.vi.2009, A. Brunke, 1 (DEBU).
Dorsal habitus of: 73 Stethusa spuriella (Casey) 74 Strigota ambigua (Erichson) 75 Strigota obscurata Klimaszewski & Brunke sp. n. 76 Trichiusa compacta Casey 77 Trichiusa hirsuta Casey 78 Trichiusa robustula (Casey). Scale 1mm.
Canada: ON; USA: DE, FL, GA, IN, MO, NJ, NY, OH, PA, (
Distribution in Ontario of: 73 Stethusa spuriella (Casey) 74 Strigota ambigua (Erichson) 75 Strigota obscurata Klimaszewski & Brunke sp. n. 76 Trichiusa compacta Casey.
Stethusa spuriella appears to be a common species in both forested and open habitats in Ontario. No Canadian specimens of the third eastern species, Stethusa dichroa (Gravenhorst), were discovered in our material despite its widespread occurrence in the eastern United States; it is expected to occur in southern Ontario.
http://species-id.net/wiki/Strigota_ambigua
Fig. 74, Map 74, genitalia inNew Ontario Record
CANADA: ON:Huron Co., Auburn, soybean field, pitfall, 23.vi.2010, A. Brunke, 2 (DEBU); Ottawa Div., Ottawa, Centr. Exp. Farm, Marmota burrows, 20.iv.2009, A. Smetana, 5 (CNC); Waterloo Reg., Blair, soybean field, pitfall, 23.vi.2009, A. Brunke, 1 (DEBU).
Canada: YT, ON, NS, PE, NL; USA: CA, CO, CT, IA, KS, MA, MO, NC, NJ, NM, NY, NV, TX (
This widespread species apparently prefers open habitats with well-drained soil including dunes, beaches, limestone barrens, soybean fields, old fields, open gaps in spruce forest, riverbanks and on pavement (see references under ‘distribution’). The specimens from groundhog burrows were probably overwintering there.
urn:lsid:zoobank.org:act:9AD7A325-4D27-411A-9748-93BF8829BD63
http://species-id.net/wiki/Strigota_obscurata
Figs 75, 150–154; Map 75Canada, Ontario, Wellington Co., Eramosa, Wellington Rd. 124 and 29, hedgerow nr. soybean field, 43.61 -80.21.
Holotype (male): CANADA, ON: Wellington Co., Eramosa, Wellington Rd. 124 and 29, hedgerow, pitfall, 15.vi.2010, A. Brunke (DEBU).
Paratypes(2 males, 5 females, 7 sex unknown):labeled as the holotype, 6 sex? (DEBU); Huron Co., Auburn, soybean field, pitfall, 23.vi.2010, A. Brunke, 1 female, 1 male (DEBU); Manitoulin Distr., Manitoulin Is., Misery Bay Prov. Nat. Res., 45°47'28"N, 82°44'58"W, alvar, malaise trap, 15.vi to 2.vii.2010, Pivar et al., debu00325236, 1 female (DEBU), Manitoulin Is., Kip Fleming Tract, 8km SW Gore Bay, 45°52'13"N, 82°32'31"W, oak savannah/alvar, under stones, 27–29.v.2010, A. Brunke, debu00323337, 1 female (DEBU); Northumberland Co., Barr property, 7 km NE Centreton, site 2, 44°7'48"N, 77°59'3"W, field, malaise pans, 16–27.vi.2011, Brunke & Paiero, debu01147152, 1 female (LFC), same data except: malaise, 26.vii to 12.viii.2011, debu01149211, 1 female (DEBU); Wellington Co., Guelph, hedgerow, 5.v.2009, A. Brunke, 1 male (LFC).
Strigota obscurata is readily separated from the other Strigota species by the combination of: median lobe constricted basally in parameral view (Fig. 150), male and female tergite VIII with apical margin sharply produced (Fig. 152), the dark coloration, including the legs, the body size (2.2–2.5mm) and elytra at suture distinctly shorter than the pronotum at midline (Fig. 75).
Body narrowly elongate, dark brown to black, with legs and/or tarsi brown, central disc of elytra sometimes with traces of reddish tinge, length 2.2–2.5 mm, moderately glossy, with dense, meshed microsculpture, pubescence short, dense and appearing somewhat silky; head convex, rounded posteriorly, postocular area at least slightly longer than the length of eye, pubescence directed towards midline of disc; antennae stout, antennomeres 1–3 strongly elongate, 4–5 subquadrate and 6–10 moderately transverse; pronotum slightly transverse, widest in basal third, pubescence directed obliquely posteriad, posteriad at midline; elytra transverse, at suture shorter than pronotum at midline, pubescence directed straight posteriad; abdomen subparallel with tergites II–IV deeply impressed basally; metatarsus with basal article as long as two following articles combined.
Male. Tergite VIII with bisinuate base and acutely produced apex, (Fig. 152); sternite VIII elongate with broad distance between base and antecostal suture, apex truncate (Fig. 153); median lobe of aedeagus in lateral view with moderately sized bulbus, tubus of median lobe slightly produced ventrad, internal sac in lateral view with several short, inconspicuous sclerites (Fig. 151); median lobe of aedeagus in ventral (parameral) view with tubus constricted basally (Fig. 150).
Female. Tergite and sternite VIII similar to those of male; spermatheca with club-shaped capsule bearing a small invagination, stem sinuate and coiled apically (Fig. 154). The spermatheca of this species is nearly identical to that of Strigota ambigua except for the capsule, which is more sharply deflexed and of a different shape (Fig. 154, compare with illustrations in
Presently, Strigota obscurata is known only from Ontario but it is expected to occur widely in northeastern North America.
Strigota obscurata occurs in many of the same habitats as Strigota ambigua and was the most commonly collected rove beetle in southern Ontario soybean fields, frequently co-occurring with the latter species (Brunke et al. in prep.).
The specific name is the Latin word for ‘darkened’. This is in reference to the distinct, overall darker body colorationcompared to Strigota ambigua, the only other eastern species of the genus.
Prior to this publication there were five valid species of Strigota in North America: Strigota ambigua (Erichson) with numerous synonyms (see
The type series of Strigota seducens contains 6 specimens with the following data: Cal; ‘seducens-6’, Paratype USNM 39047; Casey bequest 1925; Gusarov lect. des. 2003 [unpublished designation]; our lectotype label, present designation, [1 male, dissected, with genitalia scarcely visible] (NMNH). Same data except: ‘seducens-2’; Gusarov paralect. des. 2003 [unpublished designation]; our paralectotype label, present designation, [1 female, dissected, spermatheca not located] (NMNH). Same data as first paralectotype except: ‘seducens-3’; [1 male, dissected, aedeagus not located] (NMNH). Same except: ‘seducens-4’; [1 female, dissected, with spermatheca] (NMNH). Same except: ‘seducens-5’; [1 sex?, not dissected]. Same except: ‘seducens-Type 39047’; seducens; [1 sex?, damaged, abdomen missing].
For the purpose of nomenclatural stability, we here designate the first mentioned specimen as a lectotype and the other 5 as paralectotypes. The spermatheca of one of the paralectotypes was compared to our specimens of Strigota obscurata and no important differences could be found; the only available aedeagus of Strigota seducens was barely visible in the permanent mount and could not be compared in detail. However, Strigota obscurata may be differentiated from Strigota seducens by the combination of characters in the diagnosis and the uniformly colored elytra (light brown in centre of the disc in Strigota seducens). The only other eastern species of the genus, Strigota ambigua, is easily separated from Strigota obscurata by the larger size (2.4–3.0mm), less produced tergite 8 in both sexes, differently shaped aedeagus and spermatheca (Figs 150–151, 154 vs. illustrations in
http://species-id.net/wiki/Trichiusa_compacta
Figs 76, 155–157; Map 76New Canadian Record
CANADA: ON: Chatham-Kent Co., Rondeau Prov. Pk., 1 to 9.viii.1985, Int. trap at edge of oak forest, L. LeSage & A. Woodliffe, 1 (CNC), Rondeau Prov. Pk., South Point Trail, slough forest, leaf litter, 27.ix.2009, Brunke & Cheung, 1 (DEBU); Hald.-Norfolk Reg., Cronmiller prop., 6 km W St. Williams, 42°40'21"N, 80°29'26"W, forest, Berlese vernal pool litter, 17.v.2011, A. Brunke, 1 (DEBU), Cronmiller prop., 6 km W St. Williams, 42°40'20"N, 80°29'29"W, forest, sand ridge, malaise pans, 17 to 31.v.2011, Brunke & Paiero, 1 (DEBU), Cronmiller prop., 6 km W St. Williams, 42°40'21"N, 80°29'26"W, low forest, malaise, 17 to 31.v.2011, Brunke & Paiero, 1 (DEBU).
Canada: ON; USA: DC, OH. Native.
The species of Trichiusa are currently under revision by V. Gusarov and so Trichiusa compacta is currently best recognized by the combination of habitus and the following sexual characters: median lobe of aedeagus in lateral view with tubus narrow, evenly subparallel and narrowly rounded apically (not sharp) (Fig. 156); spermatheca with moderately large, spherical and basally narrowed capsule bearing a deep apical invagination, stem C-shaped, looped and twisted posteriad (Fig. 157).
Trichiusa compacta appears to be forest inhabiting and was collected from a variety of passive traps and by sifting litter near vernal and semi-permanent forest pools.
http://species-id.net/wiki/Trichiusa_hirsuta
Figs 77, 158–160; Map 77New Canadian Record
CANADA: ON:Hald.-Norfolk Reg., Cronmiller prop., 6 km W St Williams, 42°40'20"N, 80°29'29"W, forest, sand ridge, malaise pan, 17.v to 31.v.2011, Brunke & Paiero, 1 (DEBU), Turkey Point Prov. Pk., site 1, 42°41'48"N, 80°19'48"W, forest, malaise pans, 17 to 31.v.2011, Brunke & Paiero, 1 (DEBU); Northumberland Co., Barr prop., 7 km NE Centreton, site 1, 44°7'40"N, 77°58'57W, savannah, malaise pans, 16 to 27.vi.2011, Brunke & Paiero, 1 (DEBU).
Canada: ON; USA: VA. Native.
This species is currently recognizable only by the combination of habitus (Fig. 77) and the following sexual characters: median lobe of aedeagus in lateral view with tubus narrowed toward apex and sharply pointed apically (not rounded) (Figs 158–159); spermatheca with large, spherical and basally narrowed capsule bearing a small apical invagination, stem relatively straight, looped and twisted posteriad (Fig. 160).
Unlike Trichiusa robustula, Trichiusa hirsuta was collected from upland forested or semi-forested habitats on sandy soil. More collections will help elucidate the habitat requirements of this species.
http://species-id.net/wiki/Trichiusa_robustula
Figs 78, 161–164; Map 78New Canadian Record
CANADA: ON: Chatham-Kent Co., Rondeau Provincial Park, beach near entrance, 3.vi.1985, in debris on beach at high water line, A. Davies & J.M. Campbell, 2 (CNC), Rondeau Provincial Pk., South Beach, 5.vi.1985, in debris on beach at high water line, A. Davies & J.M. Campbell, 1 (CNC), Rondeau Provincial Park, Lakeshore Road, 6.vi.1985, sifted grass pile and leaves, A. Davies & J.M. Campbell, 3 (CNC);Essex Co., East Sister I. Prov. Nat. Res., 41°49’N 82°51’W, 30.vii.2003, shore, yellow pans, S.A. Marshall, 1 (DEBU).
Canada: ON; USA: IA. Native.
Distribution in Ontario of: 77 Trichiusa hirsuta Casey 78 Trichiusa robustula (Casey) 79 Zyras planifer (Casey).
This species is currently recognizable only by the combination of habitus (Fig. 78) and the following sexual characteristics: median lobe of aedeagus in lateral view with tubus relatively broad, evenly subparallel and rounded apically (not sharp at apex) (Fig. 162); spermatheca with tubular capsule bearing large and deep apical invagination, stem sinuate, looped and twisted posteriad (Figs 163–164).
This species has been collected from the shoreline of the Great Lakes or from debris nearby. Further collecting is needed to determine whether or not Trichiusa robustula is typical of lakeshore habitat.
http://species-id.net/wiki/Zyras_planifer
Fig. 79, Map 79, genitalia inNew Canadian Record
CANADA: ON:Essex Co., Windsor, Ojibway Prairie, unburnt forest, yellow pans, 8 to 12.vi.2001, S.M. Paiero, 1 (DEBU).
Dorsal habitus of Zyras planifer (Casey). Scale 1mm.
Canada: ON; USA: DC, IN, NC (
Aleochara daviesi Klimaszewski & Brunke sp. n.: 80 aedeagus in lateral view 81 male tergite 8 82 male sternite 8. Dexiogyia angustiventris (Casey) 83 aedeagus in abparameral view 84 aedeagus in lateral view 85 male tergite 8 86 male sternite 8 87 spermatheca 88 female tergite 8 89 female sternite 8. Scale 0.2 mm.
Oxypoda rubescans Casey 90 aedeagus lateral view. Parocyusa americana (Casey) 91 spermatheca 92 female tergite 8 93 female sternite 8. Parocyusa fuliginosa (Casey) 94 aedeagus in lateral view [specimen from type series North Carolina] 95 aedeagus in abparameral view [Newfoundland] 96 aedeagus in lateral view [Newfoundland] 97 male tergite 8 98 male sternite 8 99 spermatheca [specimen from type series North Carolina] 100 spermatheca [Newfoundland] 101 female tergite 8 102 female sternite 8. Scale 0.2 mm.
Agaricomorpha websteri Klimaszewski & Brunke sp. n.: 103 aedeagus lateral view 104 male tergite 8 105 male sternite 8. Gyrophaena caseyi Seevers: 106 aedeagus lateral view 107 male tergite 8 108 male sternite 8. Thecturota pusio (Casey) 109 aedeagus lateral view 110 aedeagus abparameral view 111 male tergite 8 112 male sternite 8 113 spermatheca 114 female tergite 8 115 female sternite 8. Scale 0.2 mm.
Acrotona smithi (Casey) 116 aedeagus abparameral view 117 aedeagus lateral view internal sac retracted 118 aedeagus lateral view internal sac everted 119 male tergite 8 120 male sternite 8 121–122 spermathecae 123 female tergite 8 124 female sternite 8. Acrotona subpygmaea (Bernhauer): 125 aedeagus abparameral view 126 aedeagus lateral view 127 male tergite 8 128 male sternite 8 129 spermatheca 130 female tergite 8 131 female sternite 8. Scale 0.2 mm.
Alevonota gracilenta (Erichson) 132 aedeagus lateral view 133 male tergite 8 134 male sternite 8. Atheta (Microdota) alesi Klimaszewski & Brunke sp. n.: 135 aedeagus abparameral view 136 aedeagus lateral view 137 male tergite 8 138 male sternite 8 139 spermatheca 140 female tergite 8 141 female sternite 8. Callicerus obscurus Gravenhorst: 142 aedeagus lateral view 143 spermatheca. Callicerus rigidicornis (Erichson) 144 aedeagus lateral view 145 spermatheca. Dinaraea backusensis Klimaszewski and Brunke sp. n.: 146 aedeagus lateral view 147 male tergite 8 148 male sternite 8. Philhygra proterminalis (Bernhauer): 149 aedeagus lateral view. Scale 0.2 mm.
Strigota obscurata Klimaszewski & Brunke sp. n.: 150 aedeagus parameral view 151 aedeagus lateral view 152 male tergite 8 153 male sternite 8 154 spermatheca. Trichiusa compacta Casey 155 aedeagus abparameral view 156 aedeagus lateral view 157 spermatheca. Trichiusa hirsuta Casey 158 aedeagus lateral view internal sac retracted 159 aedeagus lateral view internal sac everted 160 spermatheca. Trichiusa robustula Casey 161 aedeagus abparameral view 162 aedeagus lateral view 163–164 spermathecae. Scale 0.2 mm.
This project is part of a recent effort to document the Canadian biodiversity of the large staphylinid subfamily Aleocharinae. Prior investigations have involved the faunas of Vancouver Island (BC) (
Although the specimens studied over the course of this project were from a variety of localities and survey projects, 62% of new distributional records were derived wholly or in part form material generated from a one-year Ontario arthropod survey, a partnership between the University of Guelph Insect Collection, Nature Conservancy of Canada and Ontario Ministry of Natural Resources. We feel that the results of the present study demonstrate that species-level inventories can contribute data that significantly enrich our knowledge of Canadian biodiversity, both adventive and native. In the United States, all-taxa biological inventory projects such as the Great Smokey Mountains ATBI and the Boston Harbor Islands ATBI are making similar contributions to our knowledge of arthropod biodiversity (examples for Coleoptera: Park, Carlton et al. 2010;
Considering the high return of discoveries made during the present study from a relatively small amount of material, it is clear that the checklist of Ontario Aleocharinae provided here represents only a preliminary but important baseline. Undoubtedly, more new species await description and several adventive species known elsewhere in eastern Canada have not yet been recorded from Ontario. We hope that this new baseline will act as a useful intermediate step towards the documentation of Canada’s arthropod biodiversity.
Funding for the Ontario Arthropod Surveys (2010, 20111) was provided by the Nature Conservancy of Canada (NCC) and the Ontario Ministry of Natural Resources (OMNR). JK thanks Natural Resources Canada for their support of this project. The samples from Ontario soybean fields were collected over the course of the Masters thesis of AJB and supported by funding from the Grain Farmers of Ontario, an OMAFRA-University of Guelph Sustainable Production Systems research grant awarded to Rebecca Hallett (University of Guelph, Guelph, Ontario, Canada) and an NSERC PGS-M and rare charitable research scholarship awarded to AJB. We thank the following for assistance with and permission to conduct sampling on their properties: NCC, OMNR, Ontario Parks, rare charitable research reserve, University of Guelph Arboretum, and A.K. Dewdney (Newport Forest, Wardsville, Ontario, Canada). We thank everyone who provided field assistance for the survey projects especially: Cody Anderson, Lauren DesMarteaux, David Makynen, Adam Jewiss-Gaines and Robert Pivar. We are grateful to the following people who provided access to specimens under their care: A. Davies (CNC), A. Newton and M. Thayer (FMNH), M. Sörensson (MZLU), D. Furth and F. Shockley (NMNH), R. Webster (cRW) and J. Frisch (ZMB). We thank one anonymous reviewer and A. Davies for corrections and correspondence that greatly improved the manuscript. Additionally, A. Davies graciously provided published and unpublished distribution data from his database. We are grateful to M. Thayer for producing the habitus image of Stethusa klimschi. V. Gusarov (University of Oslo, Norway) kindly provided information regarding the online North American record of Callicerus obscurus and other helpful correspondence.