(C) 2012 Jan Klimaszewski. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The aleocharine beetles of the Yukon Territory, Canada are reviewed based on material studied since the most recent survey of the territory in 2008. The present contribution recognizes a fauna of 125 species, of which 9 are new to science, 20 represent new territorial records and one represents a new Canadian record. Seventeen species are considered Holarctic, 6 introduced, and 2 species are of undetermined status (Holarctic or adventive). The Yukon fauna is classified in 32 genera and 8 tribes. The new species are: 1) Acrotona horwoodae Klimaszewski & Godin, sp. n.; 2) Atheta (Microdota) microelytrata Klimaszewski & Godin, sp. n.; 3) Atheta (Microdota) riparia Klimaszewski & Godin, sp. n.; 4) Atheta (Datomicra) whitehorsensis Klimaszewski & Godin, sp. n.; 5) Ocyusa yukonensis Klimaszewski & Godin, sp. n.; 6) Philhygra pseudolarsoniKlimaszewski & Godin, sp. n.; 7) Philhygra terrestris Klimaszewski & Godin, sp. n.; 8) Boreophilia davidgei Klimaszewski & Godin, sp. n.; and 9) Boreophilia herschelensis Klimaszewski & Godin, sp. n.
Canada, Coleoptera, Staphylinidae, Aleocharinae, taxonomy, Yukon
Aleocharinae is the largest subfamily of Staphylinidae and embraces a wide variety of morphologically and ecologically diverse species that are poorly documented in Canada. This subfamily is widely distributed in North America and occurs in almost all terrestrial habitats. Most species are found in forests where they occur in leaf litter, under bark, in fungi, in moss and within the nests of ants, mammals and birds. In forest litter, the aleocharine fauna is a dominant group and part of a complex ecological web that is responsible for nutrient cycling, which ultimately contributes to forest productivity and resilience (
Currently, over 400 species of Aleocharinae in 92 genera are recorded from Canada and Alaska (
The present paper provides an updated review of aleocharine beetles from the Yukon Territory and constitutes important baseline data for monitoring the impact of invasive species, pollution, natural resource extraction and climate change. Additionally, the information and illustrations contained herein will make it possible to incorporate this diverse subfamily into ongoing Canadian biodiversity inventories including those in the Canadian Arctic.
Materials and methodsOver 1, 226 adults of Aleocharinae from the Yukon Territory were studied and most specimens were dissected to examine genitalia. The genital structures were dehydrated in absolute alcohol, mounted in Canada balsam on celluloid microslides and pinned with the specimens from which they originated. Photographs of the entire body and the genital structures were taken using an image processing system (Nikon SMZ 1500 stereoscopic microscope; Nikon Digit-like Camera DXM 1200F) and Adobe Photoshop software.
Morphological terminology mainly follows that used by
Samples collected in this study include those from the Ecological Monitoring and Assessment Network (EMAN) plots. Two l ha plots, the Fireweed Drive (mixed pine and willow forest) and Cadet Camp (white spruce mature forest with feathermoss ground cover), have been reserved for long-term monitoring. All samples from these locations were collected from pitfall traps operating from late May to late September. Additional pitfall samples were collected by Donald Reid from early June to early August 2007, and early June to mid August 2008 at an alluvial fan on Hershel Island (dominated by Carex and grasses with some willows). All other sample collections were from organic litter sifting.
Depository/institutional abbreviations:CNC Canadian National Collection of Insects, Arachnids and Nematodes, Agriculture and Agri-Food Canada, Ottawa, Ontario, Canada.
ECW Environment Canada, Whitehorse, Yukon, Canada
LFC Natural Resources Canada, Canadian Forest Service, Laurentian Forestry Centre, René Martineau Insectarium, Québec City, Quebec, Canada.
ResultsIn this second recent survey of the Aleocharinae of the Yukon Territory, 125 species in 32 genera and 8 tribes are reported, including two tentative records. Nine species are newly described herein, 20 additional species constitute new territorial records and one species represents a new Canadian record. There are 6 adventive and 17 Holarctic species known from the territory and the status of two other species cannot yet be determined as belonging to either category. Adventive species constitute 4.8% of the total known aleocharine fauna of the Yukon.
DiscussionThe present survey increased the known Yukon aleocharine fauna from 95 to 125 species (
Intensive sampling of the aleocharine fauna of the Yukon is continuing by the second author and undoubtedly many more species will be discovered in the future. The study of the Yukon fauna is particularly significant for understanding the shift in some species distributions in response to climate warming and for establishing baseline biodiversity data for northern Canada. Additionally, the occurrence of a species in the Yukon Territory otherwise known only from the eastern part of the country provides some evidence for a natural Holarctic distribution. Therefore, a survey of the biodiversity of the Yukon also contributes to our knowledge of species suspected of being adventive.
Checklist of Aleocharinae species in the Yukon Territory(* adventive species, ** Holarctic species, NTR=new territorial record for the Yukon Territory, NCR=new Canadian record; taxa in phylogenetic order).
Order Coleoptera
Family Staphylinidae Latreille
Subfamily Aleocharinae Fleming
I. Tribe Gymnusini Heer
Gymnusa Gravenhorst
Brevicollis Group
1. Gymnusa atra Casey**
2. Gymnusa konopackii Klimaszewski
Variegata Group
3. Gymnusa pseudovariegata Klimaszewski
4. Gymnusa smetanai Klimaszewski**
5. Gymnusa campbelli Klimaszewski
II. Tribe Aleocharini Fleming
Aleochara Gravenhorst
Subgenus Aleochara s. str.
6. Aleochara (s. str.) assiniboin Klimaszewski
7. Aleochara (s. str.) lata Gravenhorst*
8. Aleochara (s. str.) sekanai Klimaszewski
9. Aleochara (s. str.) tahoensis Casey
Subgenus Coprochara
10. Aleochara (Coprochara) verna Say
Subgenus Xenochara
11. Aleochara (Xenochara) castaneipennis Mannerheim
12. Aleochara (Xenochara) fumata Gravenhorst*
III. Tribe Oxypodini Thomson
Calodera Mannerheim
13. Calodera parviceps (Casey) (NTR)
Devia Blackwelder
14. Devia prospera (Erichson)**
Gnathusa Fenyes
15. Gnathusa caribou Lohse
16. Gnathusa evaFenyes (NTR)
17. Gnathusa tenuicornis Fenyes (NTR)
Parocalea Bernhauer
18. Parocalea nearctica Lohse
19. Parocalea pseudobaicalica Lohse
Neothetalia Klimaszewski
20. Neothetalia canadiana Klimaszewski
Ocyusa Kraatz
21. Ocyusa yukonensis Klimaszewski & Godin, sp. n.
22. Ocyusa canadensis Lohse
Oxypoda Mannerheim
Convergens Group
23. Oxypoda pseudoconvergens Klimaszewski & Godin
24. Oxypoda canadensis Klimaszewski (NTR)
Lacustris Group
25. Oxypoda lacustris Casey
26. Oxypoda hiemalis Casey
Lucidula Group
27. Oxypoda lucidula Casey
28. Oxypoda demissa Casey
Operta Group
29. Oxypoda operta Sjöberg* (NTR)
Irrasa Group
30. Oxypoda irrasa Mäklin
Inimica Group
31. Oxypoda yukonensis Klimaszewski & Godin
Orbicollis Group
32. Oxypoda orbicollis Casey
33. Oxypoda frigida Bernhauer
Grandipennis Group
34. Oxypoda grandipennis (Casey)
Amica Group
35. Oxypoda amica Casey (NTR)
Phloeopora Erichson
36. Phloeopora arctica Lohse
Brachyusa Mulsant and Rey
37. Brachyusa helenae (Casey) (NTR)
Gnypeta Thomson
Selmani Group
38. Gnypeta ashei Klimaszewski
39. Gnypeta brincki Palm
40. Gnypeta sellmani Brundin**
Caerulea Group
41. Gnypeta caerulea** (C.R. Sahlberg)
IV. Tribe Hypocyphtini
Cypha Leach
42. Cypha inexpectata Klimaszewski & Godin
V. Tribe Myllaenini Ganglbauer
Myllaena Erichson
Insomnis Group
43. Myllaena insomnis Casey
VI. Tribe Homalotini Heer
Gyrophaena Mannerheim
Nana Group
44. Gyrophaena nana (Paykull)**
45. Gyrophaena neonana Seevers
Keeni Group
46. Gyrophaena keeni Casey
Pulchella Group
47. Gyrophaena criddlei Casey (NTR) [tentative]
Silusa Erichson
48. Silusa californica (Bernhauer)
VII. Tribe Placusini Mulsant and Rey
Placusa Erichson
49. Placusa tacomae Casey
50. Placusa vaga Casey
VIII. Tribe Athetini Casey
Acrotona Thomson
51. Acrotona onthophila Lohse
52. Acrotona horwoodae Klimaszewski & Godin, sp. n.
Mocyta Mulsant and Rey
53. Mocyta breviuscula (Mäklin)
54. Mocyta fungi (Gravenhorst)*
Strigota Casey
55. Strigota ambigua (Erichson) (NTR)
Amischa Thomson
56. Amischa praelonga (Casey) (NCR, NTR)
57. Amischa tersa Casey [tentative]
Atheta Thomson
Subgenus Atheta Thomson
58. Atheta (s. str.) graminicola (Gravenhorst)**
59. Atheta (s. str.) martini Lohse
Subgenus Pseudota Casey
Klagesi Group
60. Atheta (Pseudota) klagesi Bernhauer
Subgenus Oreostiba Ganglbauer
61. Atheta (Oreostiba) sparreschneideri Munster**
Subgenus Alaobia Thomson
62. Atheta (Alaobia) ventricosa Bernhauer
Subgenus Bessobia Thomson
63. Atheta (Bessobia) cryptica (Lohse)
Subgenus Dimetrota Mulsant & Rey
Altaica Group
64. Atheta (Dimetrota) altaica Bernhauer **
65. Atheta (Dimetrota) nearctica (Lohse)
Prudhoensis Group
66. Atheta (Dimetrota) prudhoensis (Lohse)
67. Atheta (Dimetrota) burwelli (Lohse)
68. Atheta (Dimetrota) terranovae Klimaszewski & Langor (NTR)
69. Atheta (Dimetrota) caribou (Lohse)
70. Atheta (Dimetrota) strigosula Casey
71. Atheta (Dimetrota) pseudometlakatlana Klimaszewski & Godin
Modesta Group
72. Atheta (Dimetrota) pseudocrenuliventris Klimaszewski
Campbelli Group
73. Atheta (Dimetrota) smetanai (Lohse)
74. Atheta (Dimetrota) campbelli (Lohse)
Fanatica Group
75. Atheta (Dimetrota) fanatica Casey(NTR)
76. Atheta (Dimetrota) munsteri Bernhauer**
Cadeti Group
77. Atheta (Dimetrota) cadeti Klimaszewski and Godin
Subgenus Rhagocneme Munster
78. Atheta (Rhagocneme) subsinuata (Erichson)*
Subgenus Datomicra Mulsant and Rey
79. Atheta (Datomicra) dadopora Thomson* or **
80. Atheta (Datomicra) whitehorsensis Klimaszewski & Godin, sp. n.
Subgenus Microdota Mulsant and Rey
81. Atheta (Microdota) platonoffi Brundin** (NTR)
82. Atheta (Microdota) pratensis (Mäklin) (NTR)
83. Atheta (Microdota) microelytrata Klimaszewski & Godin, sp. n.
84. Atheta (Microdota) riparia Klimaszewski & Godin, sp. n.
SUBGENUS UNCERTAIN
85. Atheta brunswickensis Klimaszewski
86. Atheta capsularis Klimaszewski
87. Atheta remulsa Casey
Dinaraea Thomson
88. Dinaraea angustula (Gyllenhal)* (NTR)
89. Dinaraea planaris (Mäklin)
Dochmonota Thomson
90. Dochmonota rudiventris (Eppelsheim)* or **
Hydrosmecta Thomson
91. Hydrosmecta pseudodiosica Lohse
Earota Mulsant and Rey
92. Earota dentata (Bernhauer)
Emmelostiba Pace
93. Emmelostiba microptera (Lohse)
Liogluta Thomson
94. Liogluta aloconotoides Lohse
95. Liogluta granulosa Lohse
96. Liogluta trapezicollis Lohse
97. Liogluta nigropolita (Bernhauer)
Lypoglossa Fenyes
98. Lypoglossa angularis (Mäklin)
99. Lypoglossa franclemonti Hoebeke (NTR)
Philhygra Mulsant and Rey
100. Philhygra pseudopolaris Klimaszewski and Langor [listed as Philhygra polaris (Bernhauer) by
101. Philhygra botanicarum (Muona)**
102. Philhygra pseudolarsoniKlimaszewski & Godin, sp. n.
103. Philhygra sinuipennis Klimaszewski & Langor (NTR)
104. Philhygra malleoides Lohse
105. Philhygra leechi Lohse (NTR)
106. Philhygra ripicoloides Lohse
107. Philhygra pseudoboreostiba Lohse
108. Philhygra juni Lohse
109. Philhygra clemens (Casey) (NTR)
110. Philhygra terrestris Klimaszewski & Godin, sp. n.
111. Philhygra jarmilae Klimaszewski & Langor (NTR)
Boreophilia Benick
112. Boreophilia islandica (Kraatz)**
113. Boreophilia nearctica Lohse
114. Boreophilia blatchleyi (Bernhauer & Scheerpeltz)
115. Boreophilia venti (Lohse)
116. Boreophilia nomensis (Casey) [
117. Boreophilia caseyi Lohse
118. Boreophilia insecuta (Eppelsheim)**
119. Boreophilia gelida (J. Sahlberg)**
120. Boreophilia herschelensis Klimaszewski & Godin, sp. n.
121. Boreophilia davidgei Klimaszewski & Godin, sp. n.
Boreostiba Lohse
122. Boreostiba frigida (J. Sahlberg)** [= sibirica sensu Lohse in
123. Boreostiba sibirica (Mäklin)**
124. Boreostiba parvipennis (Bernhauer)
125. Boreostiba lagunae Lohse
Systematic account of new records and new species of Aleocharinae from the Yukon territoryI. Tribe Oxypodini Thomson
| Origin | Nearctic |
|---|---|
| Nearctic distribution | Canada: NS, NB, ON, YT; USA: RI |
| YT distribution | YUKON (NTR): Whitehorse, Paddy’s Pond, 60.7067, -135.0917, 6.V.2007, 649 m, litter sifting, mixed aspen and white spruce forest, B. Godin (ECW, LFC) 2 females |
| References |
|
| Origin | Nearctic |
| Nearctic distribution | Canada (NTR): BC, YT; USA: CA |
| YT distribution | YUKON: Whitehorse, Granger subdivision, coniferous woodchip pile, 60.7097, -135.0996, 2.IX.2007, 661 m, pitfall trap, B. Godin (LFC) 1 male; same data except: 3.V.2008 (LFC, ECW) 4 males, 2 females |
| References |
|
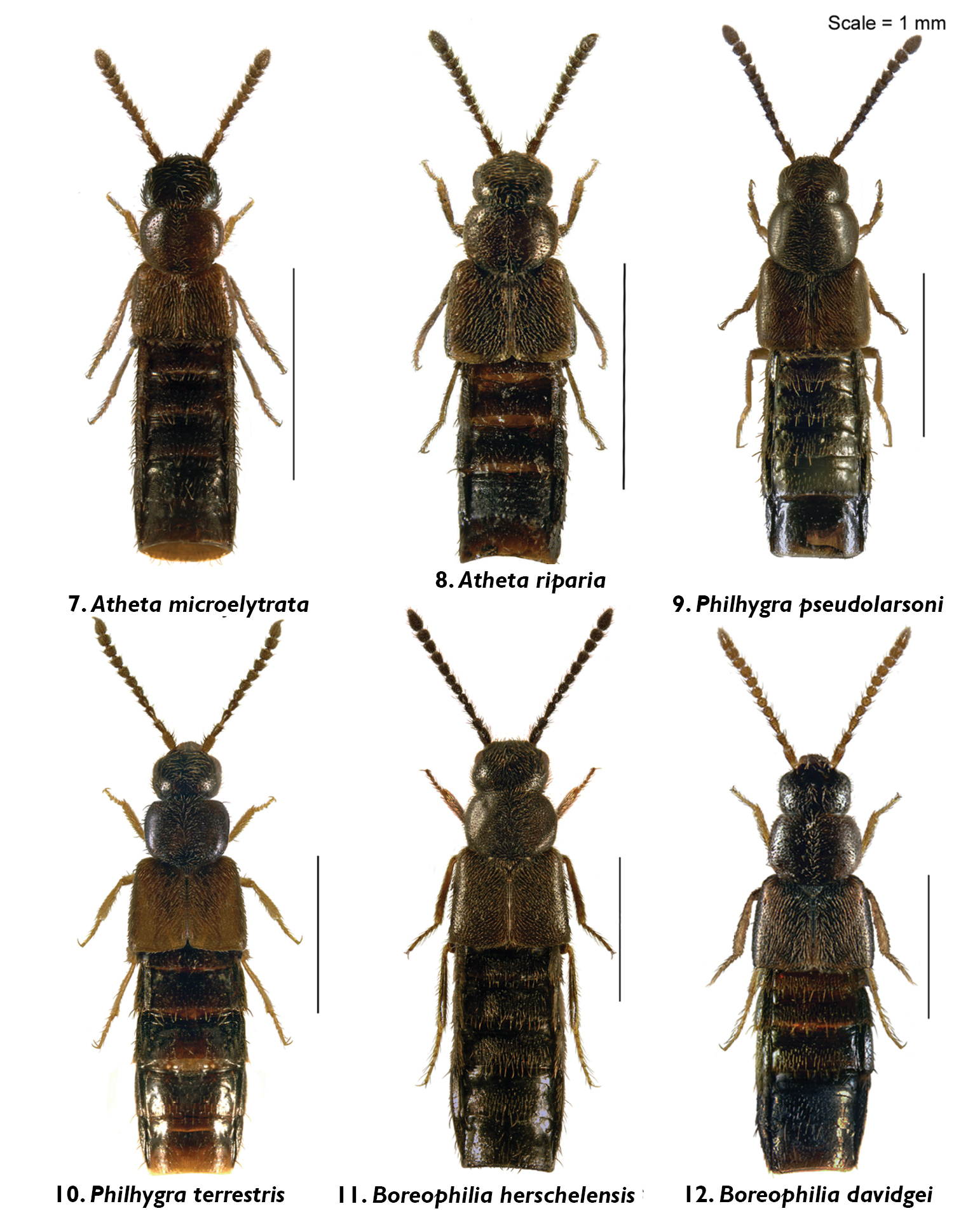
Body images in dorsal view: 1 Gnathusa eva Fenyes 2 Gnathusa tenuicornis Fenyes 3 Ocyusa yukonensis Klimaszewski & Godin, sp. n. 4 Acrotona horwoodae Klimaszewski & Godin, sp. n. 5 Amischa praelonga (Casey) 6 Atheta (Datomicra) whitehorsensis Klimaszewski & Godin, sp. n.
| Origin | Nearctic |
| Nearctic distribution | Canada: YT, BC; USA: AK, CA |
| YT distribution | YUKON (NTR): Whitehorse, Paddy’s Pond, 60.7067, -135.0917, 6.V.2007, 649 m, litter sifting, mixed aspen and white spruce forest, B. Godin (ECW) 1 male |
| References |
|
Body images in dorsal view: 7 Atheta (Microdota) microelytrata Klimaszewski & Godin, sp. n. 8 Atheta (Microdota) riparia Klimaszewski & Godin, sp. n. 9 Philhygra pseudolarsoni Klimaszewski & Godin, sp. n. 10 Philhygra terrestris Klimaszewski & Godin, sp. n. 11 Boreophilia herschelensis Klimaszewski and Godin, sp. n. 12 Boreophilia davidgei Klimaszewski & Godin, sp. n.
urn:lsid:zoobank.org:act:CAF7FE71-43FD-4C09-9B9C-FE58D3D72F29
http://species-id.net/wiki/Ocyusa_yukonensis
Figs 3, 16, 32, 33(male). Canada, Yukon, EMAN Plot (Ecological Monitoring and Assessment Network), mature white spruce and feathermoss forest, 60.5963, -134.9522, 8.VII.2003, 738 m, yellow pitfall trap (LMKM31Y), (LFC).
Yukon, EMAN Plot, 60.5963, -134.9522, 24.VII.2003, 738 m, black pitfall trap (LMKM31B), (ECW) 1 male.
Yukonensis - a Latin adjective derived from the Yukon Territory, Canada.
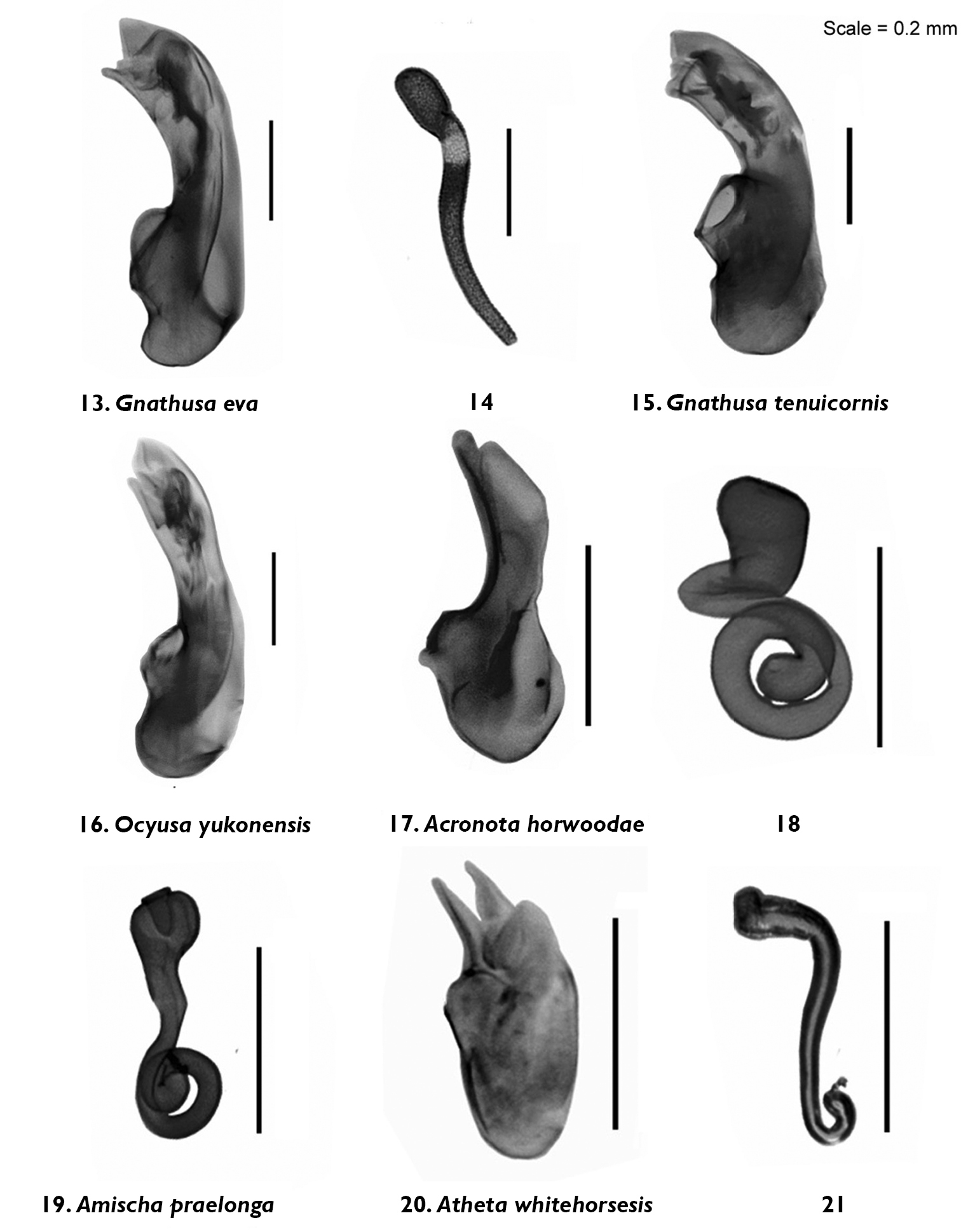
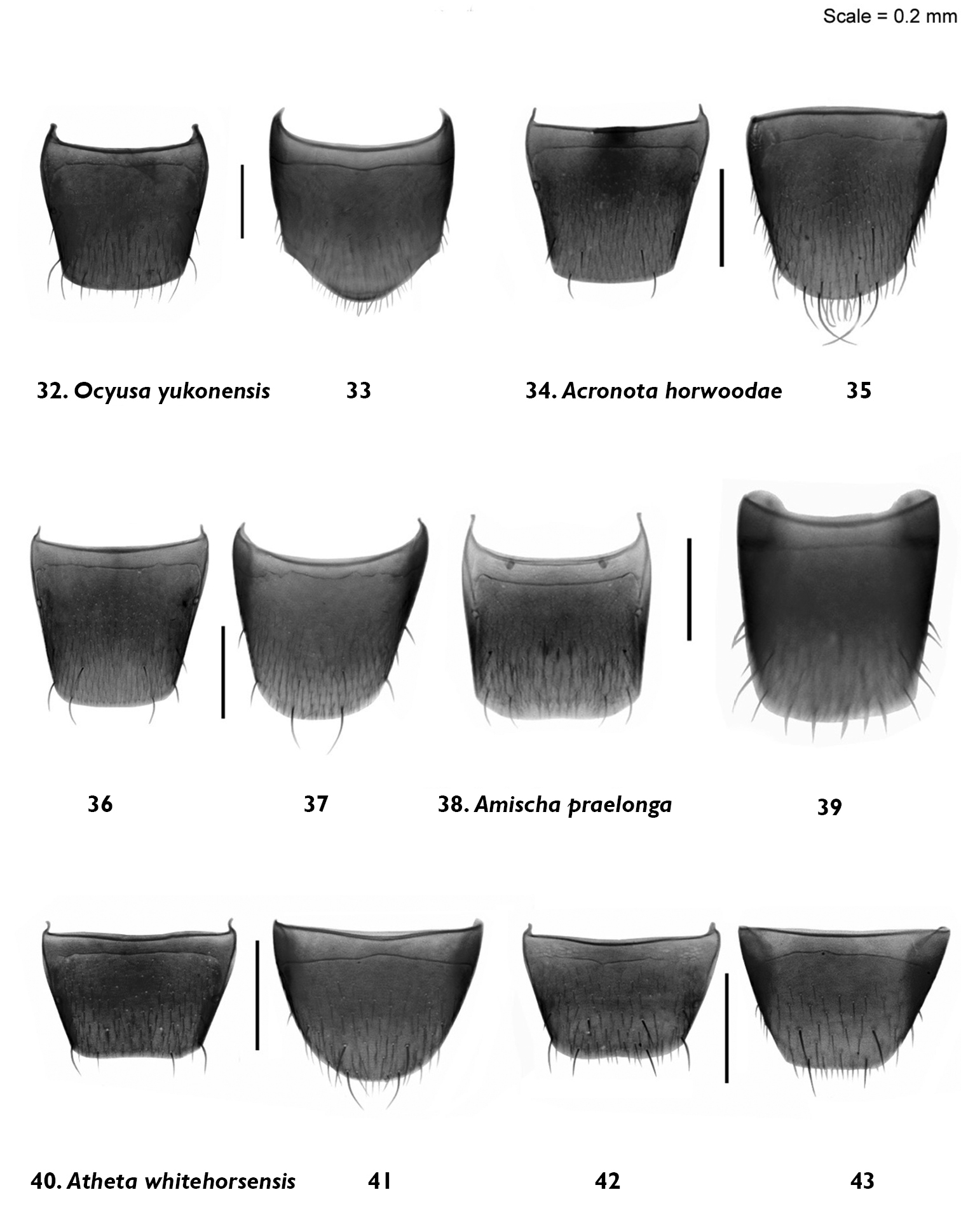
Body small, subparallel, robust, uniformly dark brown, almost black; length 2.8–3.0 mm; head round in outline and almost as wide as pronotum; antennae with article 4 subquadrate, 5–10 moderately transverse, increasingly wider apicad; pronotum transverse, angular posteriad and slightly narrower than maximum width of elytra; abdomen subparallel, at base as wide as elytra (Fig. 3). MALE: male tergite 8 widely truncate apically (Fig. 32); sternite 8 slightly produced at apex (Fig. 33); median lobe of aedeagus as illustrated (Fig. 16). FEMALE: unknown.
This native Nearctic species is known only from the type locality in the Yukon.
Two adults were collected in July.
Median lobe of aedeagus and spermatheca in lateral view of Gnathusa eva Fenyes 13, 14 Gnathusa tenuicornis Fenyes 15 Ocyusa yukonensis Klimaszewski & Godin, sp. n. 16 Acrotona horwoodae Klimaszewski & Godin, sp. n. 17, 18 Amischa praelonga (Casey) 19 Atheta (Datomicra) whitehorsensis Klimaszewski & Godin, sp. n. 20, 21.
http://species-id.net/wiki/Oxypoda_canadensis
Figs 5, 41, 80–82, 171, 203, 204, 209, 210, in| Origin | Nearctic |
| Nearctic distribution | Canada: NL, QC, ON, MB, AB, YT, NT; USA: AK, NH |
| YT distribution | YUKON (NTR): Whitehorse, Paddy’s Pond, 60.7067, -135.0917, 6.V.2007, 649 m, litter sifting, mixed aspen and white spruce forest, B. Godin (ECW) 1 male, 1 female; Watson Lake - Watson Creek, 60.1272, -128.805, 7.VII.2008, 697 m, deciduous debris soil sifting, B. Godin (ECW) 1 male, 2 females; Contact Creek, 65 km E Watson Lake; 59.9995, -127.7241, 8.VI.2008, 621 m, litter sifting, creek bank, B. Godin (ECW) 1 male; Upper Liard, Albert Creek, 60.0522, -128.928, 8.VII.2008, 619 m, deciduous forest soil sifting, B. Godin (ECW, LFC) 3 males, 4 females |
| References |
|
http://species-id.net/wiki/Oxypoda_operta
Figs 16, 52, 104, 105, 181, 245, 246, 249, 250, in| Origin | Holarctic or Palaearctic |
| Nearctic distribution | Canada: NL, NS, QC, ON, AB, YT; USA: NH |
| YT distribution | YUKON (NTR): Watson Lake - Watson Creek, 60.1272, -128.805, 4.VI.2008, 697 m, deciduous debris, soil sifting, B. Godin (ECW) 1 male, 1 female |
| References |
|
http://species-id.net/wiki/Brachyusa_helenae
Figs 48, 49, 222a-c, in| Origin | Nearctic |
| Distribution | Canada: NL, YT, NT; USA: AK, MT |
| YT distribution | YUKON (NTR): Nisutlin Wildlife Area, 60.2317, -132.5632, 17.IX.2007, 679 m, pitfall – Willow stand #2 (ECW, LFC) 2 females |
| References |
|
II. Tribe Homalotini Heer
http://species-id.net/wiki/Gyrophaena_criddlei
Figs 16, 107–110, in| Origin | Nearctic |
| Distribution | Canada: NL, NB, MB, YT |
| YT distribution | YUKON (NTR): Watson Lake – Watson Creek, 60.12723, -128.8053, 16. VIII.2007, 697 m, mushrooms, B. Godin (LFC) 1 female; Granger, 60.7078, 135.0971, 25.VIII.2007, 657 m, B. Godin (LFC) 1 female. |
| References |
|
The two females are tentatively identified as Gyrophaena criddlei but a male is needed for positive confirmation of this species in the Yukon Territory.
III. Tribe Athetini Casey
urn:lsid:zoobank.org:act:D5CA8598-36E8-40B4-AEAD-20D013A6964E
http://species-id.net/wiki/Acrotona_horwoodae
Figs 4, 17, 18, 34–37(male). Canada, Yukon, Whitehorse, Paddy’s Pond, 60.7067, -135.0917, 27.V.2008, 649 m, litter sifting, mixed aspen and white spruce forest, B. Godin (LFC).
(female). Same data as the holotype (ECW).
This species name is dedicated to Denise Horwood, wife of the second author, who assisted him in numerous aleocharine sample collections.
Body narrowly oval, moderately convex, uniformly black, punctation on forebody fine, dense and not asperate, microsculpture fine but not pronounced; length 2.4 mm; head narrower than pronotum, ratio of maximum width of head to maximum width of pronotum 0.7; antennal articles 7–10 slightly transverse; pronotum moderately transverse, ratio of maximum width to length 1.4, about as wide as elytra; elytra at suture about as long as pronotum; abdomen slightly narrowed posteriad (Fig. 4). MALE: tergite 8 moderately elongate and truncate apically (Fig. 34); sternite 8 widely arcuate apically (Fig. 35); median lobe of aedeagus as illustrated (Fig. 17). FEMALE: tergite 8 moderately elongate and truncate apically, base not sinuate (Fig. 36); sternite 8 widely arcuate apically, base not sinuate (Fig. 37); spermatheca with capsule tulip-shaped and stem coiled posteriorly (Fig. 18).
Bionomics. The specimens were found by sifting forest litter in May.
Comments. The shape of the median lobe of the aedeagus and the spermatheca of Acrotona horwoodae are different from all recorded species of Nearctic Acrotona, and they are generally similar to those of the Palaearctic species Acrotona aterrima Gravenhorst, which is brown and has a much broader body.
http://species-id.net/wiki/Strigota_ambigua
Figs 88, 261a-c, in| Origin | Nearctic |
| Distribution | Canada: NL, NS, PE, YT; USA: CA, CO, CT, IA, KS, MA, MO, NC, NJ, NV, NY, TX |
| YT distribution | YUKON (NTR): Whitehorse, 60.7328, -135.0986 18.VI.2007, 717 m, hand collected, parking lot asphalt, (ECW) 1 female |
| References |
|
| Origin | Nearctic |
| Distribution | Canada (NTR): YT; USA: WY |
| YT distribution | YUKON (NTR): Whitehorse, McIntyre Creek, 60.7398, -135.1462, 25.IV.2007, 744 m, litter sifting, willow stand by creek bank, B. Godin (ECW, LFC) 2 females; EP Impact, south, 60.7336, -135.0946, 19.VII.2001, 695 m, pitfall trap, disturbed land, grasses, B. Godin (ECW, LFC) 3 females |
| References |
|
Two additional Amischa morphotypes were recognized in the Yukon material on the basis of external body characters and the shape of the spermatheca. They are not included in this account because they are difficult to associate with any of the recorded species. The first morphospecies is represented by three narrowly elongate bicoloured specimens with the head and 4–5 basal abdominal tergites almost black, with the pronotum brown and the appendages and posterior of the elytra light brown, and with the spermathecal capsule moderately elongate with a moderately long apical invagination. The second morphospecies is represented by three specimens, which are broader, with the body uniformly dark brown to almost black, and the spermathecal capsule broader and shorter apically and with a longer apical invagination. Both groups have the apex of tergite 8 deeply notched. We need more specimens and representatives of both sexes to establish the status of these morphotypes.
http://species-id.net/wiki/Atheta_terranovae
Figs 107, 280a–c, 407a–d, in| Origin | Nearctic |
| Distribution | Canada: NL, YT |
| YT distribution | YUKON (NTR): Whitehorse, Granger, 60.7078, -135.0971, 1.VIII.2007, 657 m, mushrooms, B. Godin (ECW) 2 females; same data except: 60.7366, 135.097, 15.VIII.2008, 743 m, pitfall trap, ski trail, birch stand, B.Godin (ECW) 1 male; EMAN Plot, Fireweed Dr., 60.6014, -134.9387, 8.VIII.2006, 772 m, pitfall trap, mixed pine and willow forest (ECW) 1 male; same data except: 23.VII.2006 (ECW) 1 female; EMAN Plot, Cadet Camp, 60.5951, -134.9499, 23.VIII.2006, 760 m, pitfall trap, mature white spruce and feathermoss forest, (ECW) 1 female |
| References |
|
http://species-id.net/wiki/Atheta_fanatica
Figs 134, 307a–c, in| Origin | Nearctic |
| Distribution | Canada: NL, NS, NB, QC, YT, BC; USA: AK, NV |
| YT distribution | YUKON (NTR): Whitehorse, Paddy’s Pond, 60.7067, -135.0917, 20.V.2007, 649 m, litter sifting, B. Godin (ECW) 1 male; Whitehorse, Granger, 60.7078, -135.0971, 5.VIII.2007, 657 m, soil sifting, B. Godin (ECW) 1 male; same data except: 27.IX.2008, compost (LFC) 1 male, 1 female |
| References |
|
urn:lsid:zoobank.org:act:9ACD0F86-341A-4855-925A-51104BB8C8F4
http://species-id.net/wiki/Atheta_whitehorsensis
Figs 6, 20, 21, 40–43Canada, Yukon, Whitehorse, Granger, 60.7078, -135.0971, 25.VIII.2007, 657 m, soil sifting, black spruce stand, AWT, B. Godin (LFC).
Canada, Yukon, Whitehorse, Granger, 60.7078, -135.0971, 5.VIII.2007, 657 m, soil sifting, black spruce stand, AWT, B. Godin (ECW) 1 female.
The specific name derives from the name of the type locality, which is Whitehorse, Yukon.
Body narrowly oval, dark brown to black, with bases of antennae and legs rust-brown, surface matte, with asperate dense punctation on forebody and strong meshed microsculpture (Fig. 6); length 1.9–2.0 mm; head narrower than pronotum and elytra, with short postocular area, eyes large and slightly protruding; antennae slender, slightly incrassate apically, article 4 subquadrate, 5 slightly elongate and 6–10 slightly to strongly transverse; pronotum strongly transverse and broadest in the middle; elytra transverse, longer than pronotum; abdomen broadly arcuate laterally (Fig. 6). MALE: tergite 8 transverse and truncate apically (Fig. 40); sternite 8 widely rounded apically (Fig. 41); median lobe of aedeagus with venter of tubus straight and short, and apex sharply produced (Fig. 20). FEMALE: tergite and sternite 8 truncate apically (Figs 42, 43); spermatheca with pipe-shaped capsule and long stem hooked posteriorly (Fig. 21).
This species is similar externally to Atheta (Dimetrota) hampshirensis Bernhauer and Atheta (Datomicra) dadopora Thomson but differs in the shape of the spermatheca and median lobe of the aedeagus, and has a broader body than the latter species.
This native Nearctic species is known only from the type locality in the Yukon Territory.
Adults were captured by sifting soil in a black spruce stand.
http://species-id.net/wiki/Atheta_platonoffi
Figs 127, 300a-c, 423, in| Origin | Holarctic |
| Distribution | Canada: NL, NS, NB, ON, AB, BC, YT; USA: AK |
| YT distribution | YUKON (NTR): Whitehorse, Granger, 60.7078, -135.0971, 25.VIII.2007, 657 m, soil sifting, black spruce stand, B. Godin (ECW, LFC) 3 males, 2 females; same data except: 1.VIII.2008, mushrooms (ECW, LFC) 3 males; 16.VIII.2007, mushrooms (ECW) 1 female; Upper Liard, Albert Creek, 60.0522, -128.928, 8.VII.2007, 699 m, deciduous debris, soil sifting, B. Godin (ECW) 1 female |
| References |
|
http://species-id.net/wiki/Atheta_pratensis
Figs 128, 301a–c, 428, in| Origin | Nearctic |
| Distribution | Canada: NL, YT; USA: AK |
| YT distribution | YUKON (NTR): Tagish, Tagish Lake; 60.2658, -134.2873, 20.VIII.2007, 654 m, mushroom, B. Godin (ECW) 1 male |
| References |
|
urn:lsid:zoobank.org:act:A75DCD78-E696-4AE7-8E8C-ACAF8F3B3F7E
http://species-id.net/wiki/Atheta_microelytrata
Figs 7, 22, 23, 44–47(male). Canada, Yukon, Whitehorse, Takhini, hotsprings, 60.8769, -135.3596, 30.IV.2009, 716 m, aspen litter – soil sifting, B. Godin (LFC).
. Canada, Yukon, Whitehorse, Takhini, hotsprings, 60.8769, -135.3596, 19.IX.2009, 716 m, alder/willow litter, soil sifting, B. Godin (ECW) 2 males; same data except: 3.V.2009 (ECW, LFC) 2 females.
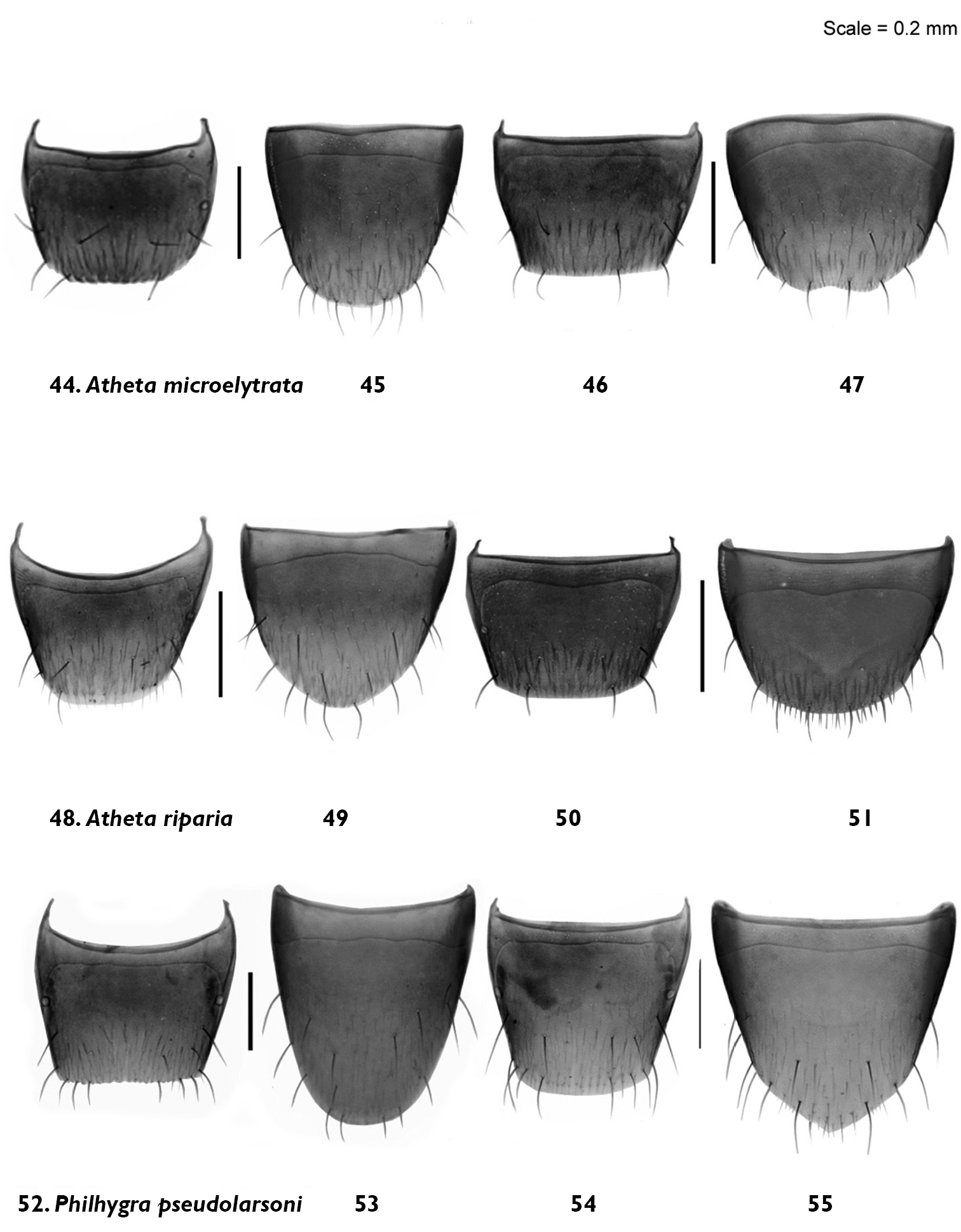
The specific name derives from the word micro, meaning small, and elytra, in allusion to the small and short elytra of this species.
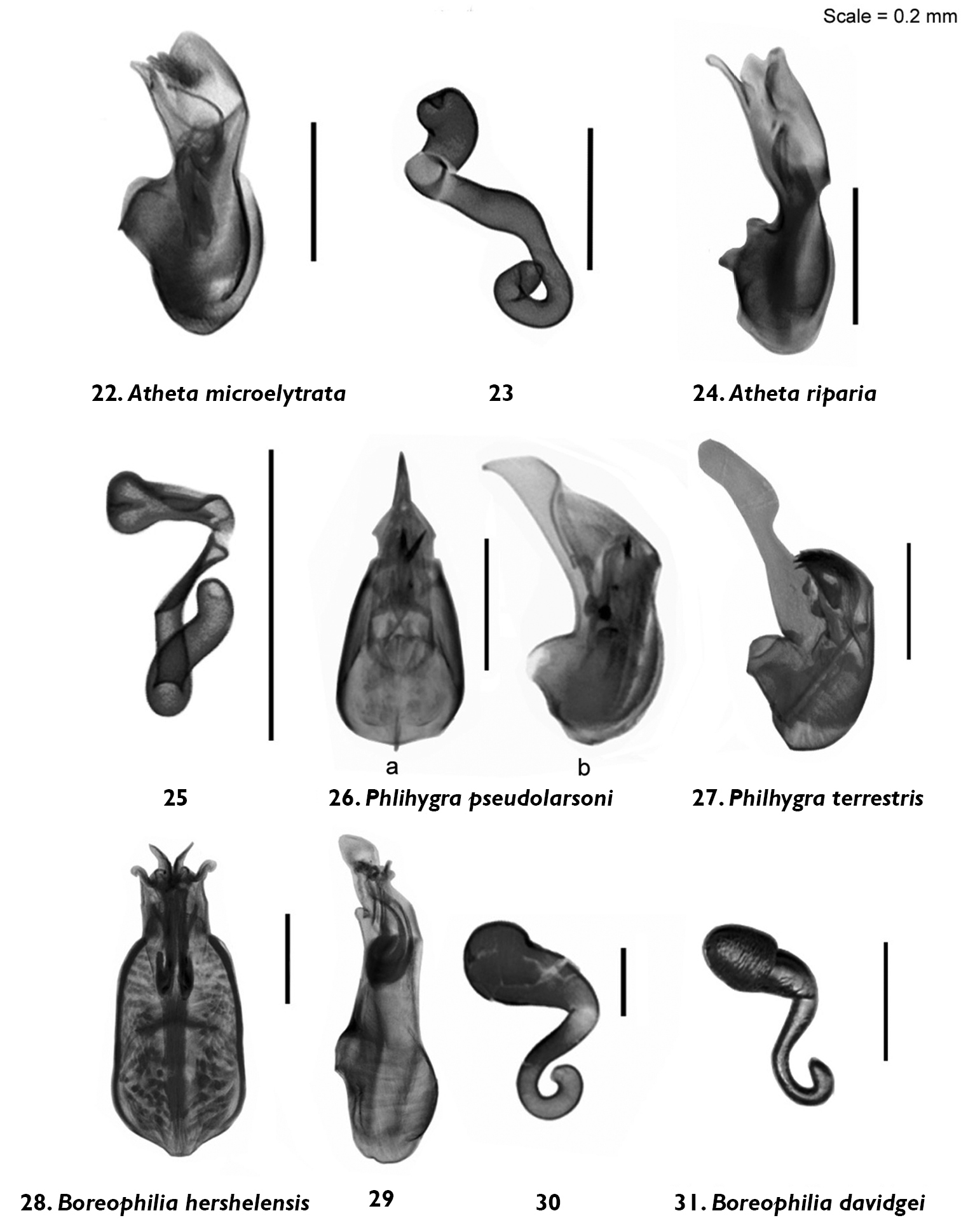
Body narrowly subparallel; dark brown, with bases of antennae and legs rust-brown; strongly glossy, with fine and moderately dense punctation on forebody and strong, meshed microsculpture (Fig. 7); head as wide as pronotum and elytra, with long postocular area, eyes moderately small and slightly protruding; antennae slender, slightly incrassate apicad, articles 4–5 subquadrate and 6–10 slightly to strongly transverse; pronotum narrower at base and broadening apicad; elytra transverse, shorter than pronotum; abdomen widest subapically; length 1.9–2.0 mm (Fig. 7). MALE: tergite 8 truncate apically and with crenulation scarcely visible (Fig. 44); sternite 8 widely rounded apically (Fig. 45); median lobe of aedeagus with apex narrow and ventrally produced, athetine bridge well developed (Fig. 22). FEMALE: tergite 8 truncate apically (Fig. 46); sternite 8 truncate and slightly emarginate medially (Fig. 47); spermatheca with pipe-shaped capsule and long, posteriorly-coiled stem (Fig. 23).
This species bears some superficial external similarity to Geostiba and Emmelostiba but has typical Atheta-like genitalia.
This native Nearctic species is known only from the type locality in the Yukon Territory.
Adults were found in aspen, alder and willow litter in March, May and September.
urn:lsid:zoobank.org:act:BC82DFB4-F60B-4758-9860-BC23B2F3D6DC
http://species-id.net/wiki/Atheta_riparia
Figs 8, 24, 25, 48–51(male). Canada, Yukon, Whitehorse, Paddy’s Pond, 60.7067, -135.0917, 16.IX.2007, 649 m, litter sifting, mixed aspen and white spruce forest, B. Godin (LFC).
Same data as the holotype (ECW) 1 male.
Canada, Yukon, Watson Lake, Watson Creek, 60.12723, -128.8053, 16.VIII.2007, 697 m, mushrooms, B. Godin (LFC) 1 female.
The name of this species derives from the Latin adjective riparius, -a, -um, in allusion to the wet litter where the types were found.
Body small and narrow, subparallel; black, with tarsi reddish-brown; moderately glossy, with fine, dense punctation and meshed microsculpture on forebody (Fig. 8); head approximately as wide as pronotum, depressed medially, eyes slightly protruding; antennae slender, slightly incrassate apicad, articles 4–10 slightly to strongly transverse; pronotum emarginate laterally; elytra broader and longer at suture than pronotum; head, pronotum and base of abdomen of the same width; sides of abdomen subparallel; length 1.9–2.0 mm (Fig. 8). MALE: tergite 8 truncate apically and with smooth margin (Fig. 48); sternite 8 widely rounded apically (Fig. 49); median lobe of aedeagus with apex narrow and ventrally produced (Fig. 24). FEMALE (non-paratype): tergite 8 truncate apically (Fig. 50); sternite 8 broadly rounded apically (Fig. 51); spermatheca slightly distorted but with club-shaped capsule and posteriorly-twisted stem (Fig. 25).
This species differs from other Nearctic Microdota by the combination of body shape, strongly punctate surface and the shape of the median lobe of the aedeagus and spermatheca.
Distribution. This native Nearctic species is known only from the Yukon Territory but it is probably more widely distributed in northern Canada.
Bionomics. The two males were captured in September in wet, organic litter and the female was found in mushrooms in mid-August.
http://species-id.net/wiki/Dinaraea_angustula
Figs 141, 314a–c, 442, in| Origin | Palaearctic |
| Distribution | Canada: NL, NS, NB, PE, QC, ON, AB, YT; USA: CA, NY |
| YT distribution | YUKON (NTR): EMAN plot, Cadet Camp, 60.5951, -134.9499, 26.V.2006, 760 m, pitfall trap, mature white spruce and feathermoss forest, B. Godin (LFC) 1 male |
| References |
|
http://species-id.net/wiki/Lypoglossa_franclemonti
Figs 154, 328a–c, 455, in| Origin | Nearctic |
| Distribution | Canada: NL, NB, NS, QC, ON, MB, AB, YT, NT; USA: ME, NH, NY, VT |
| YT distribution | YUKON (NTR): Upper Liard, Albert Creek, 60.0522, -128.9279, 3.VI.2007, 699 m, deciduous litter sifting, B. Godin (ECW, LFC) 4 males, 2 females; same data except: 4.VI.2007 (ECW, LFC) 1 male, 2 females, 7.VII.2008 (ECW, LFC) 2 males; Watson Lake, Watson Creek, 60.12723, -128.8053, 16.VIII.2007, 697 m (ECW) 1 male |
| References |
|
urn:lsid:zoobank.org:act:64A996FC-47AE-453A-A112-B57D0C0D950F
http://species-id.net/wiki/Philhygra_pseudolarsoni
Figs 9, 26, 52–55Canada, Yukon, Whitehorse, Paddy’s Pond, 60.7067, -135.0917, 26.V.2007, 649 m, litter sifting, mixed aspen and white spruce forest, B. Godin (LFC).
same label data as the holotype (ECW) 1 male; Watson Lake, Watson Creek, 60.1272, -128.8053, 4.VI.2007, 697 m, deciduous forest soil sifting, B. Godin (ECW) 1 male, 1 female.
This species name derives from the specific name larsoni (Philhygra larsoni Klimaszewski and Langor), and the prefix pseudo (false) in relation to the similarity of the two species in external and, to a lesser degree, genitalic morphology.
Body narrowly subparallel, uniformly black or black with legs and sutural part of elytra reddish-brown (Fig. 9); moderately glossy, with fine, dense punctation and meshed microsculpture on forebody; head round, distinctly narrower than pronotum, with eyes as long as postocular region of head; antennae slender with articles 4–5 elongate, 6–10 subquadrate to slightly transverse; pronotum slightly transverse and almost as wide as elytra; elytra at suture as long as or slightly longer than pronotum; length 2.9–3.0 mm (Fig. 9). MALE: tergite 8 widely arcuate apically (Fig. 52); sternite 8 elongate and rounded apically (Fig. 53); median lobe of aedeagus with apex triangularly produced in lateral view (Fig. 26).
Female. tergite 8 truncate apically (Fig. 54); sternite 8 produced medially (Fig. 55); pygidium with ventral structure weakly sclerotized.
This species is known only from Whitehorse and Watson Lake in the Yukon Territory.
. This species was collected in May and June from ground litter.
Philhygra pseudolarsoni is similar in both external morphology and genitalia to Philhygra larsoni Klimaszewski and Langor. However, it may be distinguished from Philhygra larsoni by the smaller and darker body, quadrate or transverse antennal articles 4–10 and by the median lobe of the aedeagus with a more elongate apical part of the tubus in lateral view.
http://species-id.net/wiki/Philhygra_sinuipennis
Figs 161, 335a, b, 462a, b, in| Origin | Nearctic |
| Distribution | Canada: NL, YT |
| YT distribution | YUKON (NTR): Watson Lake, Watson Creek, 60.1272, -128.8053, 4.VI.2007, 697 m, deciduous litter sifting, B. Godin (ECW, LFC) 2 males |
| References |
http://species-id.net/wiki/Philhygra_leechi
Figs 118, 119, in| Origin | Nearctic |
| Distribution | Canada: MB, YT, NT |
| YT distribution | YUKON (NTR): Nisutlin Wildlife Area, 60.2317, -132.5632, 21.VIII.2007, 679 m, pitfall – Willow stand # 2, B. Godin (LFC) 1 male. |
| References |
|
urn:lsid:zoobank.org:act:246EBFF8-C0AE-43D6-98D9-C99289EE7B47
http://species-id.net/wiki/Philhygra_terrestris
Figs 10, 27, 56, 57(male). Canada, Yukon, Whitehorse, Paddy’s Pond, 60.7067, -135.0917, 26.V.2007, 649 m, litter sifting, mixed forest (aspen and white spruce), B. Godin (LFC).
Etymology. This species name is an adjective that derives from the Latin word terra (ground, earth, soil).
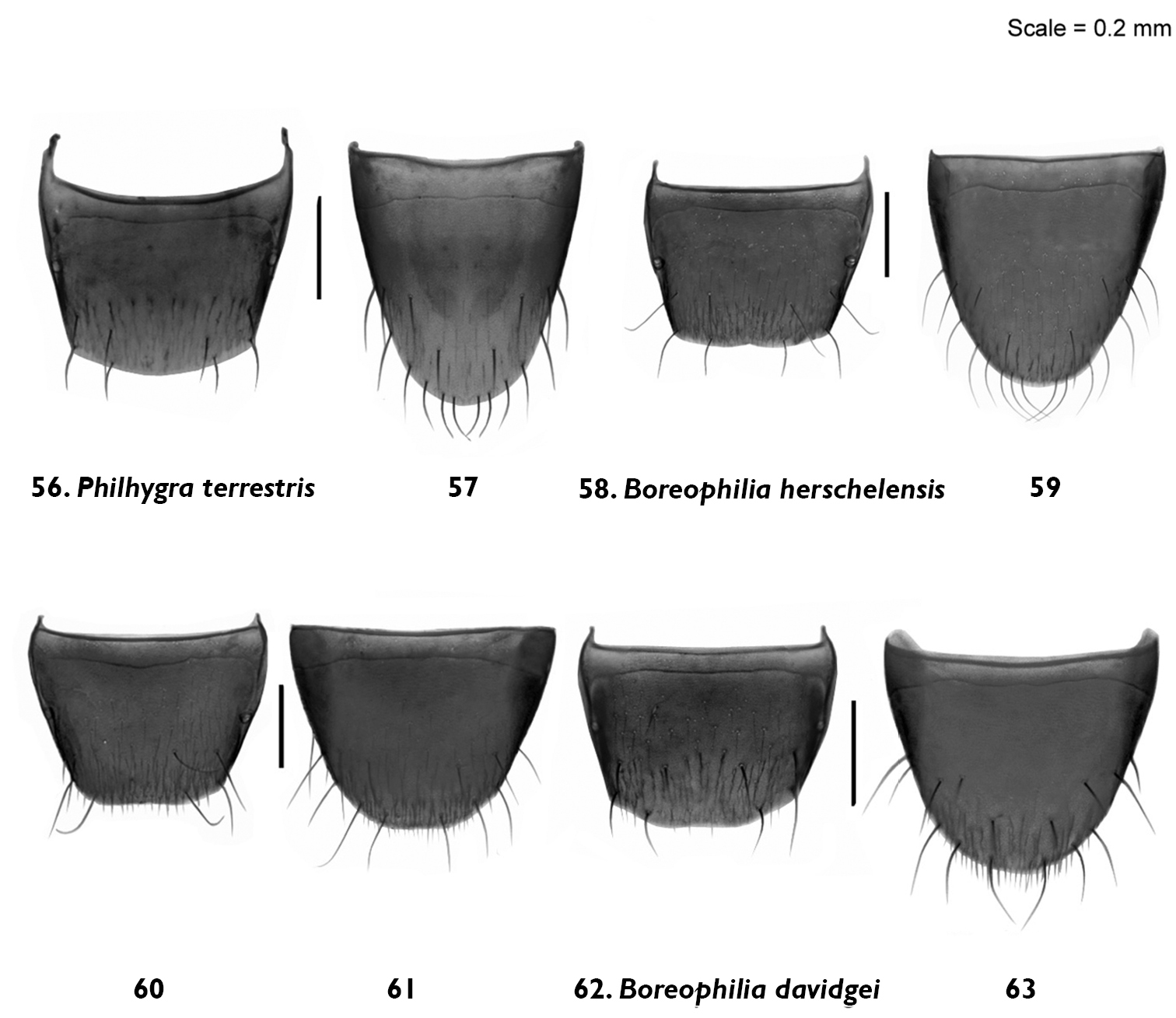
Diagnosis. Body narrowly subparallel, head and abdomen black, pronotum and elytra brown, basal article of antenna and legs yellowish (Fig. 10); strongly glossy, with fine, dense punctation and meshed microsculpture on forebody; head round, distinctly narrower than pronotum with eyes as long as postocular region of head; antennae slender with articles 4–5 elongate, 6–10 subquadrate; pronotum slightly transverse and almost as wide as elytra; elytra at suture slightly longer than pronotum; length 2.9–3.0 mm (Fig. 10). MALE: tergite 8 widely arcuate apically (Fig. 56); sternite 8 elongate and rounded apically (Fig. 57); aedeagus with apex of median lobe broadly produced and with tubus constricted basally in lateral view (Fig. 27).
Female. unknown.
Distribution. This species is known only from Whitehorse in the Yukon but it may be more widely distributed in the boreal zone of Canada and Alaska.
Bionomics. This species was collected in May from ground litter.
Comments. This species is unique in the shape of the median lobe of the aedeagus in lateral view.
http://species-id.net/wiki/Philhygra_jarmilae
Figs 159, 333a, b, 460a-d, in| Origin | Nearctic |
| Distribution | Canada: YT, NL |
| YT distribution | YUKON (NTR): Albert Creek, 60.0522, -128.9279, 3.VI.2007, soil sifting, willow stand, B. Godin (LFC) 1 male. |
| References |
|
urn:lsid:zoobank.org:act:DD1259D2-69BE-4A73-B26F-DEA59F7F47D0
http://species-id.net/wiki/Boreophilia_herschelensis
Figs 11, 28–30, 58–61(female). Canada, Yukon, Herschel Island, 69.5706, -138.902, 13.VI.2007, 5 m, pitfall trap, site dominated by Carex and grasses with presence of willows (ATOR) – alluvial fan, D.G. Reid (LFC).
Labeled as the holotype except: 1–3.VI.2007 (ECW) 1 male; 7.VI.2007 (ECW) 2 males; 10.VI.2007 (CNC) 1 male; 15.VI.2007 (ECW) 1 female; 17.VI.2007 (ECW) 1 male, 1 female; 19.VI.2007 (ECW) 1 female; 16.VII.2007 (LFC) 1 male, 1 female; 21.VII.2007 (ECW) 2 females; 31.VII.2007 (LFC) 1 male; 7.VI.2008 (ECW) 2 females; 7.VII.2008 (ECW) 2 females; 15.VII.2008 (ECW) 1 female; 11.VIII.2008 (ECW) 1 female.
Named for the type locality, Herschel Island.
Body narrow, subparallel, head and pronotum about the same width, elytra and abdomen slightly wider, uniformly black (Fig. 11); surface matte except for slightly glossy abdomen; pubescence fine, punctation weak and moderately dense, meshed microsculpture pronounced on forebody; head round, slightly flattened medially and with eyes about as long as postocular region of head; antennae slender, articles 4–5 slightly elongate, 6–10 subquadrate, last article elongate; pronotum transverse, narrower at base and widest at middle; elytra at suture slightly longer than or as long as pronotum; abdomen subparallel for most of its length; length 2.8–3.0 mm (Fig. 11). MALE: tergite 8 transverse and truncate apically (Fig. 58); sternite 8 slightly elongate and rounded apically (Fig. 59); median lobe of aedeagus with straight tubus in lateral view and with apex short and narrow (Fig. 29), dorsal aspect as illustrated (Fig. 28). FEMALE: tergite 8 transverse and truncate apically (Fig. 60); sternite 8 slightly elongate and rounded apically (Fig. 61); spermatheca S-shaped, capsule consisting of a globular apical part with a small invagination, stem sinuate (Fig. 30).
The following combination of characters distinguishes this species from other congeners: narrow, subparallel and uniformly black body, integument of forebody matte and with dense microsculpture, median lobe of aedeagus narrow apically and spermatheca S-shaped.
This Nearctic species is known only from the type locality on Herschel Island, Yukon.
Adults were collected in June and July on an alluvial fan.
This species is superficially similar to Boreophilia nomensis Casey (=Boreophilia caseyiana Lohse) but differs by its uniformly black body and aedeagus with evenly narrow apical part of median lobe in lateral view.
urn:lsid:zoobank.org:act:6561B1F8-3DFD-4745-B5F3-7A3131152979
http://species-id.net/wiki/Boreophilia_davidgei
Figs 12, 31, 62, 63(female). Canada, Yukon, EMAN Plot, Cadet Camp, 60.5951, -134.9499, 20.IX.2006, 760 m, pitfall trap, mature white spruce and feathermoss forest, coll. EP Yukon, AJK (LFC).
Canada, Yukon, EMAN Plot, Cadet Camp, 60.5951, -134.9499, 29.V.2006, 760 m, pitfall trap, mature white spruce and feathermoss forest, EP Yukon, AHW (ECW) 1 female; same data except: 15.V.2002, JF (ECW) 1 female; 12.VI.2002, EV (ECW) 1 female; 18.X.2002, FD (CNC) 2 females; 8.VII.2003, LMK31Y. LJ (ECW) 1 female; Fireweed Dr., 60.6014, -134.9387, 23.IX.2000, 772 m, pitfall trap, mixed pine and willow forest, EP Yukon (ECW) 1 female; Whitehorse, Granger, 60.7078, -135.0971, 5.VIII.2007, 657 m, soil sifting, black spruce stand, B. Godin (ECW, LFC) 2 females; same data except: 25.VIII.2007 (LFC) 1 female; Whitehorse, Paddy’s Pond, 60.7067, -135.0917, 16.IX.2007, 649 m, litter sifting, mixed aspen and white spruce forest, B. Godin (ECW) 1 female; Upper Liard, Albert Creek, 60.0522, -128.928, 8.VII.2000, 699 m, deciduous litter sifting, B. Godin (ECW, LFC) 2 females.
Named for Douglas Davidge, biological technician (ECW), who supported the second author in his work for 20 years.
Body narrow, subparallel, head narrower than pronotum, elytra and abdomen slightly wider, uniformly brown with appendages yellowish-brown and antennae yellow, or with head and abdomen dark brown and rest of body light brown (Fig. 12); surface moderately glossy; pubescence fine, punctation weak and moderately dense, meshed microsculpture pronounced on forebody; head round, slightly flattened medially and with eyes about as long as postocular region of head; antennae slender, articles 4–5 slightly elongate, 6–10 subquadrate to slightly transverse, last article elongate; pronotum transverse, widest in basal half; elytra at suture slightly longer than pronotum; abdomen broadly arcuate laterally; length 2.8–2.9 mm (Fig. 12). MALE: unknown. FEMALE: tergite 8 transverse and truncate apically (Fig. 62); sternite 8 slightly elongate and rounded apically (Fig. 63); spermatheca S-shaped, capsule elongate, stem short and sinuate (Fig. 31).
The following combination of characters distinguishes this species from other congeners: body narrow, subparallel and brown, with pronotum, elytra and legs lighter, antennae yellowish, surface of forebody moderately glossy and with dense microsculpture, and spermatheca short and S-shaped.
This Nearctic species is known only from the type localities in the Yukon Territory.
Adults were collected from May to September from soil and organic litter.
This species may be easily distinguished by the unique shape of the spermatheca.
Median lobe of aedeagus and spermatheca (view as specified) of Atheta (Microdota) microelytrata Klimaszewski and Godin, sp. n. 22 lateral 23 lateral; Atheta (Microdota) riparia Klimaszewski & Godin, sp. n. 24 lateral 25 lateral; Philhygra pseudolarsoni Klimaszewski & Godin, sp. n. 26 lateral; Philhygra terrestris Klimaszewski & Godin, sp. n. 27 lateral; Boreophilia herschelensis Klimaszewski & Godin, sp. n. 28 dorsal 29 lateral 30 lateral; Boreophilia davidgei Klimaszewski & Godin, sp. n. 31 lateral.
Male and female tergite and sternite 8: Ocyusa yukonensis Klimaszewski & Godin, sp. n. 32, 33 male; Acrotona horwoodae Klimaszewski & Godin, sp. n. 34, 35, male 36, 37, female Amischa praelonga (Casey) 38, 39 female; Atheta (Datomicra) whitehorsensis Klimaszewski & Godin, sp. n. 40, 41 male 42, 43 female.
Male and female terite and sternite 8: Atheta (Microdota) microelytrata Klimaszewski & Godin, sp. n. 44, 45 male 46, 47 female; Atheta (Microdota) riparia Klimaszewski & Godin, sp. n. 48, 49 male 50, 51 female; Philhygra pseudolarsoni Klimaszewski & Godin, sp. n. 52, 53, male 54, 55 female.
Male and female tergite and sternite 8: Philhygra terrestris Klimaszewski & Godin, sp. n. 56, 57 male; Boreophilia herschelensis Klimaszewski & Godin, p. n. 58, 59 male60, 61 female; Boreophilia davidgei Klimaszewski & Godin, sp. n. 62, 63 female.
Pamela Cheers, English Editor (LFC) edited the first draft of the manuscript. Adam Brunke (University of Guelph, Ontario) corrected the first draft of this manuscript and provided many useful comments. Thesecond author thanks the following individuals for supporting this project: Douglas Davidge (ECW) for coordinating the collection of samples in the Nisutlin Wildlfe Area in conjunction with Debie van de Wetering; his wife Denise Horwood who assisted with the collectionof most of his samples; Donald G. Reid (Witehorse) for the collection of specimens from Herschel Island, Elise Bolduc (Université du Québec à Rimouski) for sorting Staphylinidae from the Herschel Island samples. The collection of samples from Herschel Island was supported by grants from the Government of Canada’s International Polar Year program (Indian and Northern Affairs), the Natural Sciences and Engineering Research Council of Canada, Environment Canada, and the Polar Continental Shelf Program (Natural Resources Canada), the Arctic Wildlife Observatories Linking Vulnerable EcoSystems International Polar Year poject (project #11, ArcticWOLVES) of the Centre d’études nordiques, Université Laval, Québec. We are most obliged to A. Smetana (CNC) for his comments on and signficant improvements of the oriinal manuscript.