(C) 2011 C. H. Dietrich. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new cicadellid tribe, Tungurahualini, is recognized to include Tungurahuala Kramer, and a related new genus, Ilyapa gen. n., based on six new species. The tribe is included in subfamily Mileewinae, the concept of which is further expanded to include tribes Makilingiini Baker, and Tinteromini Godoy and Webb, taxa previously treated as separate subfamilies. Keys to tribes of Mileewinae (sensu lato) and genera of Tungurahualini are provided. A new species of Tungurahuala, Tungurahuala acuminata sp. n., is also described and keys to species of Tungurahuala and Ilyapa are provided. The new tribe is presently recorded only from cloud forests in the northern Andes Mountains of South America.
Homoptera, Auchenorrhyncha, morphology, identification, distribution
The leafhopper subfamily Mileewinae
comprises small to medium-sized, slender, usually darkly pigmented
species that inhabit wet tropical forests worldwide. Most species appear
to inhabit montane cloud forests where they occur on herbaceous
vegetation in the understory. The group was established by
Phylogenetic analyses of both morphological and molecular data (
In a recent taxonomic review and morphology-based phylogenetic analysis of Evacanthinae sensu lato (including tribes Balbillini, Evacanthini, Nirvanini, and Pagaroniini),
Morphological terminology follows
Specimens examined are deposited in the following institutions: Humboldt Institute, Villa Leyva, Colombia (HIC); Illinois Natural History Survey (INHS); North Carolina State University, Raleigh (NCSU); and Universidad de San Marcos, Lima, Peru (USML).
Resultshtp://species-id.net/wiki/Mileewinae
Head with lorum extended little or no farther
dorsad than clypeal suture; gena partially or entirely concealing
triangular proepisternum; anteclypeus strongly convex, tapered
distally; frontoclypeus without median longitudinal carina; ocelli on
crown anteromesad of eyes, well separated from anterior margin; crown
glabrous or punctate, without oblique lateral submarginal carinae;
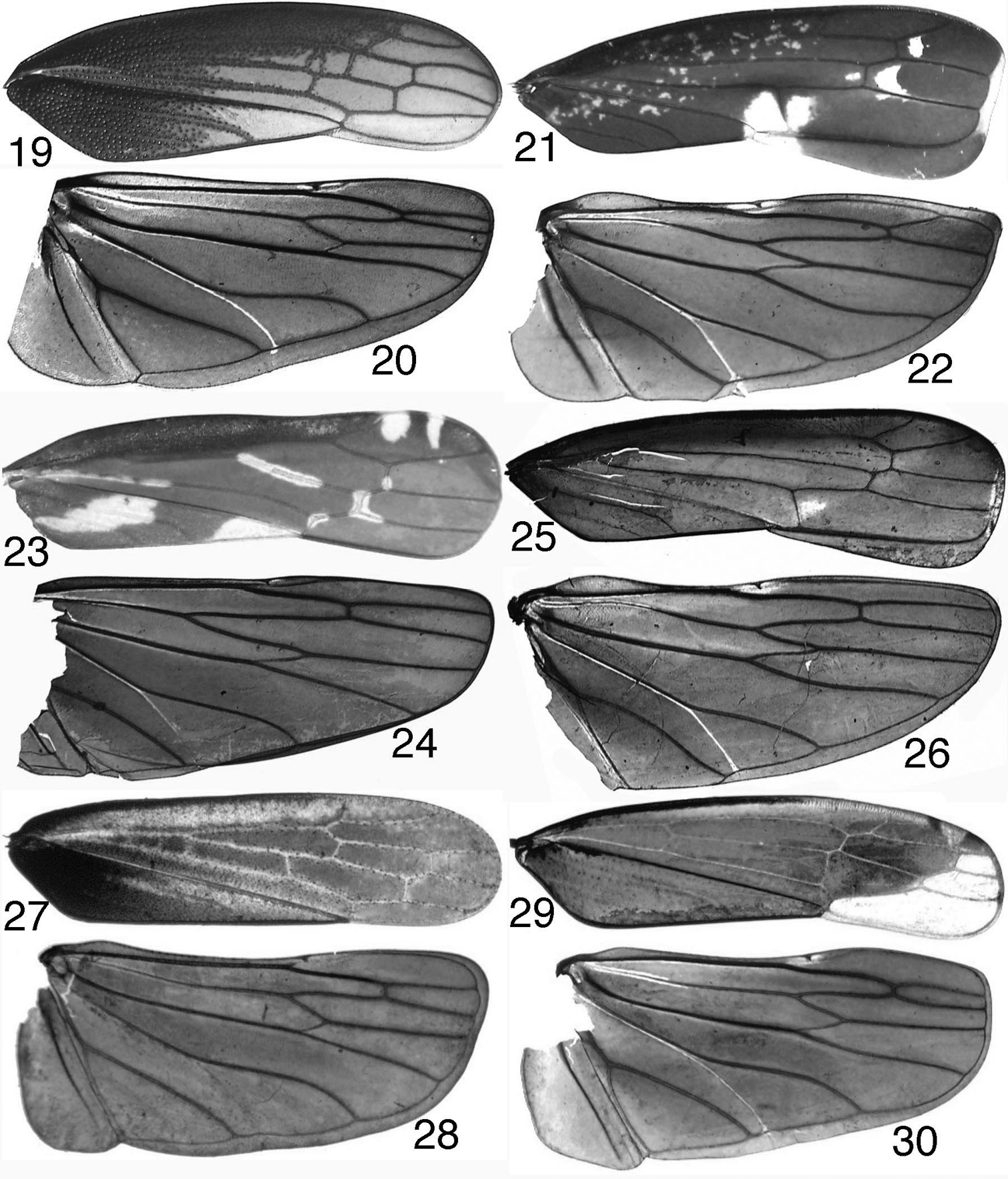
antennal base near anterodorsal corner of eye. Forewing (Figs 19, 21, 23, 25, 27, 29) with crossvein s (= “r" of
Mileewinae, as here redefined, are most readily distinguished from other Cicadellidae
by the following combination of features: head with ocelli on crown
distant from eyes and margin, frontoclypeus without median longitudinal
carina; forewing with only two anteapical cells; hind wing submarginal
vein very close to margin at wing apex; female second valvulae with
paired distal blades occupying 50% or more of their length. They key to
couplet 46 in
The hind wing venation is very similar among the tribes here included in Mileewinae, although the extant Old World genera currently included in Mileewini (Mileewa Distant, Ujna Distant, and Processina Yang, Deitz & Li ) share a unique pattern in which vein R2+3 is complete but does not extend to the wing apex (Fig. 22);
thus in these three genera there appear to be only three closed apical
cells reaching the wing apex instead of the usual four (Fig. 26). Interestingly, this pattern does not occur in the New World mileewine genus Amahuaka Melichar, or in the two genera of Mileewini described from Eocene Baltic amber (
.
Key to tribes of Mileewinae
| 1 | Head with margin of crown sharply carinate and encroaching onto eye laterally (Figs 13, 16); pronotum distinctly punctate; front femur with setae AM1 and AV1 small or absent (Phillippines, Thailand) | Makilingiini |
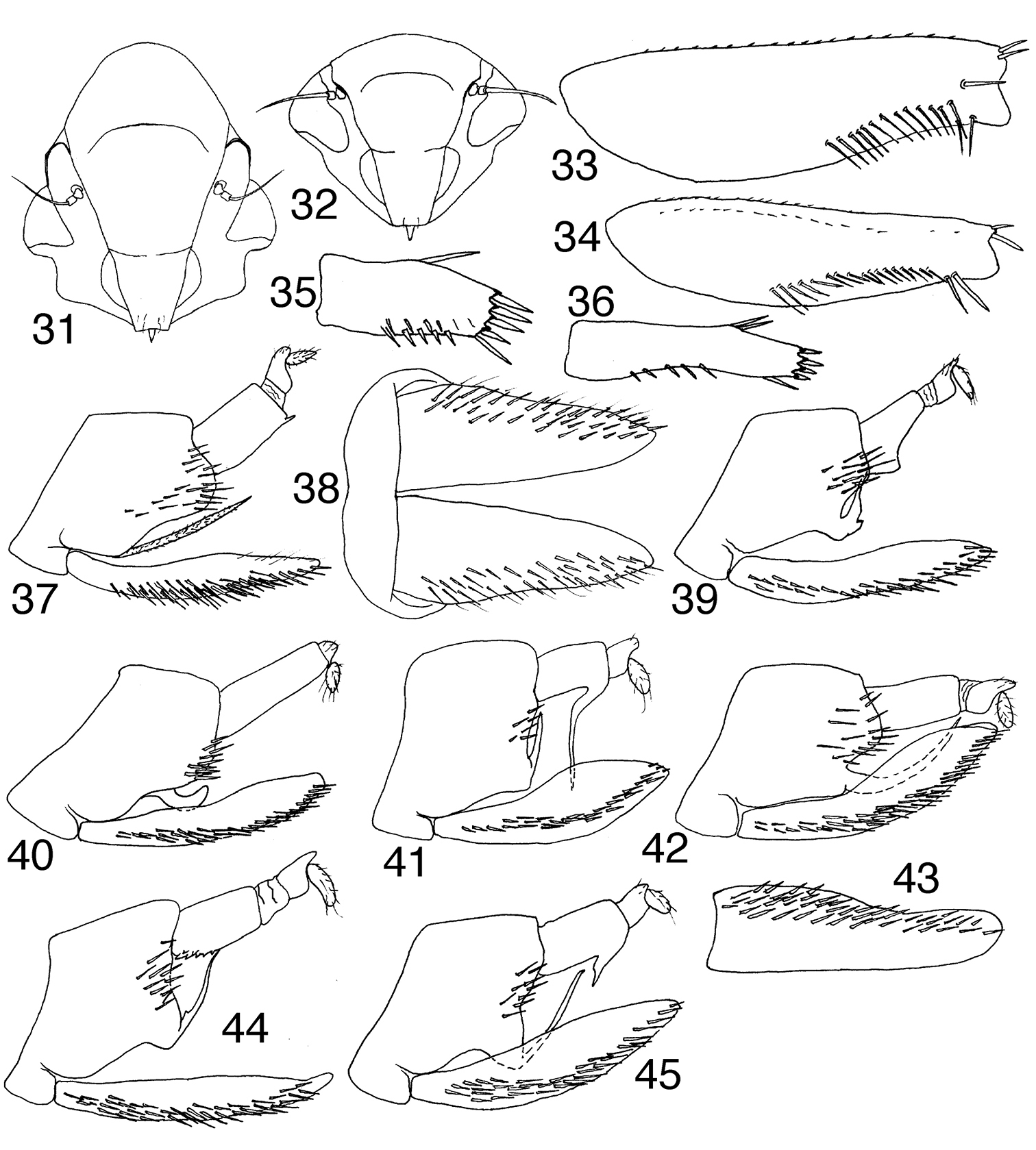
| – | Head with margin of crown at most weakly carinate, not encroaching onto eye laterally (Figs 12, 17, 18); pronotum without distinct punctations or, if punctures present, then pronotum also strongly rugose (Figs 1–3); front femur with one or more enlarged ventral setae at or near apex (Figs 33–34) | 2 |
| 2 | Body strongly depressed (Figs 11–12), rostrum not exceeding front trochanters, frontoclypeus with distinct transverse ridge visible as shelf in lateral view (Figs 11–12); forewing crossvein r-m1 present (Figs 27, 29); hind tarsus much less than half length of tibia, tarsomere I without platellae (pale, baloonlike setae), apical pecten bearing spiniform macrosetae (Figs 35–36); female second valvulae (Figs 71, 75) with dorsal margin bearing numerous large teeth with smaller serrations between teeth (Neotropical region) | Tungurahualini |
| – | Body not depressed (Figs 16–18), rostrum extended well beyond front trochanters; frontoclypeus evenly convex or flat in profile without transverse ridge; forewing with or without crossvein r-m1 (Figs 21, 23); hind tarsus approximately half length of tibia or longer (Fig. 18), tarsomere I with apical pecten bearing one or more platellae; second valvulae with dorsal margin smooth or minutely serrate, without large teeth | 3 |
| 3 | Antennae longer than body; forewing crossvein r-m1 present, appendix absent (Fig. 23) | Tinteromini |
| – | Antennae shorter than body; forewing crossvein r-m1 absent, appendix well developed (Figs 21, 25) | Mileewini |
Mileewinae, scale bars = 1 mm 1–10 Tungurahualini, dorsal habitus 1 Tungurahuala basilisca, male from Colombia 2 Tungurahuala acuminata, male 3 same, female 4 Ilyapa bifida, male 5 Ilyapa loca, male 6 Ilyapa longispina, male 7 Ilyapa ochrescens, male 8 Ilyapa recurvata, male 9 Ilyapa viridis, male 10 same, 5th instar nymph 11–12 Tungurahualini, lateral habitus 11 Tungurahuala acuminata, male 12 Ilyapa viridis, male 13–18 Other Mileewinae 13 Makilingia sp., male from Thailand, dorsal habitus 14 same, Mileewa margheritae, male 15 same, Tinteromus sp., male from Colombia (full length of antenna not shown) 16 Makilingia sp., lateral habitus 17 same, Mileewa margheritae 18 same, Tinteromus sp.
urn:lsid:zoobank.org:act:E466750A-38A1-4798-A4E4-4E169D37F6E8
Tungurahuala Kramer
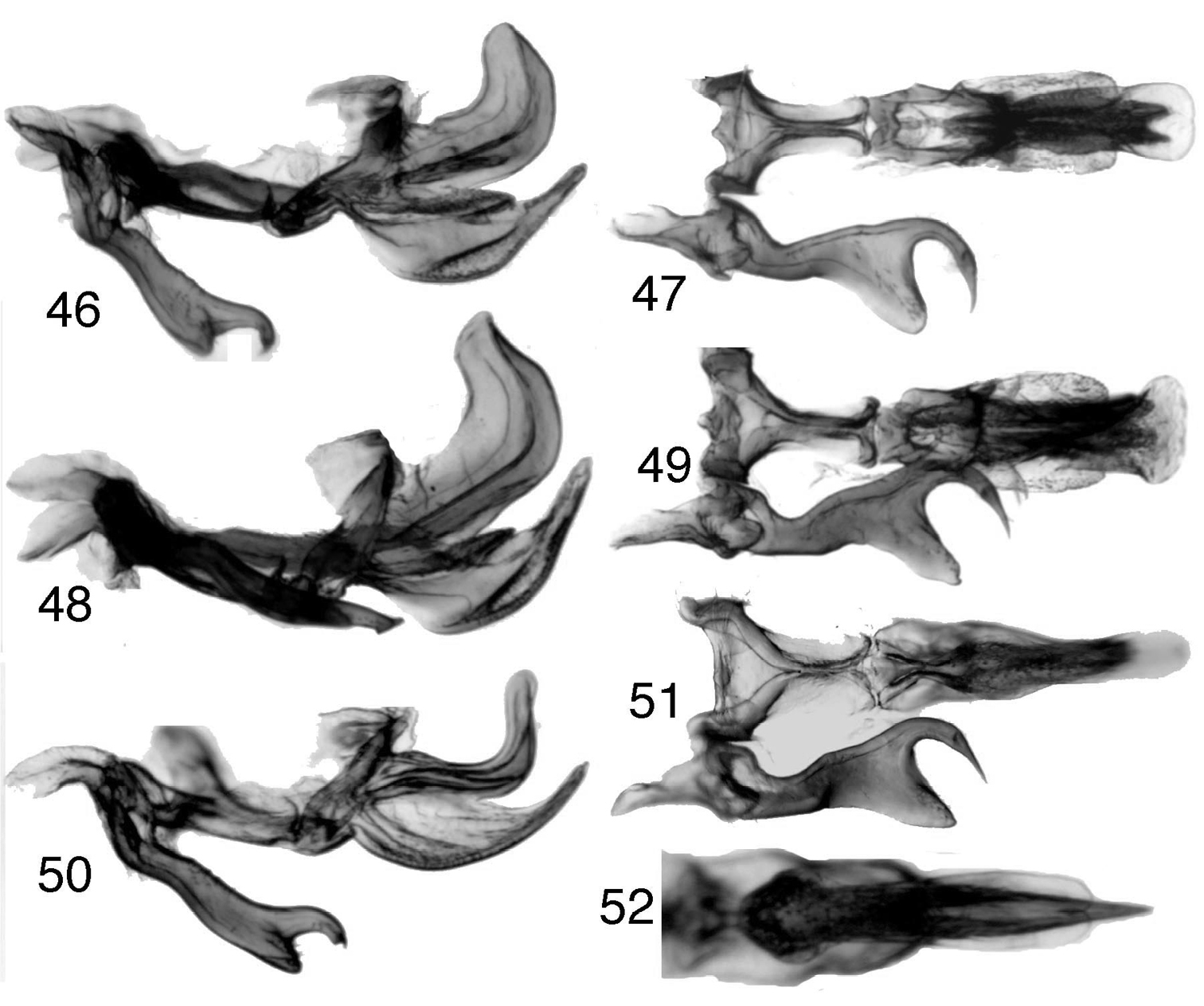
Medium-sized leafhoppers (~6–8 mm), body depressed, head produced, face horizontal in profile, antenna shorter than width of head, frontoclypeus with transverse carina forming distinct shelf in lateral view (Figs 11–12, 31–32); anteclypeus extended to or slightly beyond lower margin of gena; lorum with lateral margin not extended to lateral margin of gena; rostrum short, not surpassing front trochanters. Front femur (Figs 33–34) with AM1 and AV1 enlarged, row AV with 0–1 preapical setae; intercalary row with 12 or more slender setae; hind tarsomere I without platellae, pecten with four tapered macrosetae. Forewing (Figs 27, 29) vein R with three branches; two r-m crossveins present; appendix absent or very narrow; hind wing vein R2+3 complete, extended to wing apex. Male pygofer (Fig. 37) with well developed ventral appendage, dorsal appendage absent, several macrosetae present distally; anal tube usually with paired ventrolateral processes; valve (Fig. 38) short, transverse, broadly fused to pygofer; subgenital plate broadest near base in ventral view, expanded medially in lateral view, with numerous scattered stout submarginal setae; connective stem as long as or longer than arms; style (Figs 47–51) cheliform with preapical lobe greatly enlarged and preapical tooth distinct. Female first valvulae with dorsal sculpturing strigate (Fig. 70) or concatenate (Fig. 73); second valvulae (Figs 71, 74) with small serrations between larger teeth.
Tungurahualini resemble other Mileewinae in having the ocelli on the crown distant from the margin, the frontoclypeus and clypellus strongly convex, the forewing with the inner apical cell elongate and parallel-sided, the hind femur with macrosetal formula 2+1+1, and the second valvulae with the toothed distal blades longer than the basal fused section. They differ from other Mileewinae in having the head depressed with the face horizontal, the frontoclypeus with a transverse carina (Figs 31–32) forming a distinct shelf in profile, the first hind tarsomere pecten with spiniform setae (platellae absent), and the subgenital plate with numerous scattered macrosetae (see also Key).
A previous cladistic analysis (
Tungurahualini resemble Nirvanini (Evacanthinae, sensu
Species of Tungurahualini are presently known only from cloud forests in the northern and central Andean regions of the New World tropics.
.
Key to genera of Tungurahualini
| 1 | Head and pronotum uniformly black dorsally; crown margin pentagonal in dorsal view | Tungurahuala Kramer |
| – | Head and pronotum pale orange, white or green with distinct reddish orange markings; crown margin parabolic in dorsal view | Ilyapa gen. n. |
htp://species-id.net/wiki/Tungurahuala
Elongate, strongly depressed, leafhoppers (Figs 1–3, 11). Coloration dark brown to black; face with dull yellow band extended from lora across base of clypellus and apex of clypeus. Crown unevenly convex, coarsely granulose and densely clothed with minute setae, pentagonal in dorsal view; marginal carina present apically, becoming obsolete posterolaterally; median longitudinal carina weakly delimited; ocelli on crown anterad of eyes, slightly closer to lateral margin than to midline; antennal ledge broad, depressed, coincident with lateral margin of crown; flagellum slightly shorter than crown width; mesal margin of eye entire; lateral frontal suture absent dorsad of antennal ledge; frontoclypeus (Fig. 31) rugulose medially with well developed muscle scars laterally, oblique anteroventral section separated from nearly horizontal posteroventral section by transverse ridge; transclypeal suture indistinct; anteclypeus tapered, weakly convex, apex wider than lorum; lorum well separated from ventral genal margin; gena angulately produced, largely concealing proepisternum. Pronotum depressed, rugulose and minutely setose, narrower than head, lateral margins strongly carinate, carina even with eye, margins subparallel in dorsal view; exposed part of mesonotum and scutellum together wider than long. Forewing (Fig. 27) opaque basally, gradually becoming translucent distally; veins raised and well delimited, with marginal setae; costal flange well developed basally; R three-branched (rarely with 1–2 supranumerary branches), branches not reflexed; crossvein s absent (outer anteapical cell open distally); two r-m and three m-cu crossveins present; inner apical cell narrow; appendix absent. Hind wing (Fig. 28) with cell distad of r-m crossvein broadened distally. Prothoracic femur (Fig. 33) stout, AM1 and AV1 well developed, intercalary row with ~13 close-set preapical setae; tibia short, weakly expanded distally, dorsal rows with few indistinct, widely spaced setae, AV well developed, PV with few scattered setae. Mesothoracic femur longer and wider than prothoracic femur, compressed, AV and PV each with several irregular setae, tibial rows with numerous poorly differentiated setae. Metathoracic tibia row AV with setae evenly spaced from base to apex; tarsomere I with several scattered plantar setae. Male sternum III apodemes well developed; pygofer (Fig. 37) short with scattered macrosetae dorsolaterally, with long, slender process densely clothed with minute spicules arising from ventrolateral margin and extending mesad into genital capsule, then dorsad; anal tube well sclerotized, broader than long in dorsal view, venter flat; valve (Fig. 38) short, straplike, narrowly fused to pygofer; plates triangular, depressed, extended well beyond posterior margin of pygofer, with lateral band and irregular submedial row of macrosetae, dorsolateral margin weakly sinuate in lateral view, base weakly constricted in ventral view; aedeagus (Figs 46–52) in lateral view with shaft split into dorsal gonopore-bearing section and tapered ventral process; connective (Figs 47–51) trilobed basally; style with large preapical lobe and attenuated, hooked apex. Female sternite VII (Fig. 65) subtruncate, concealing base of ovipositor; first valvulae (Fig. 69) slender, with dorsal and ventral preapical sculpturing irregularly strigate; second valvulae (Fig. 71) with basal fused area short, distal blades large, dorsal margin ascending in straight line, then gradually descending toward apex, declivous portion with ~7 widely spaced conical teeth and intervening serrations, apical fourth serrate, without teeth; third valvulae without macrosetae. Nymph unknown.
.
Key to species of Tungurahuala (males)
| 1 | Anal tube without distinct apical ventrolateral spines; aedeagal shaft (Figs 46, 48) broad in lateral view, ventral process with dorsolateral lobes | Tungurahuala basilisca Kramer |
| – | Anal tube with pair of distinct apical ventrolateral spines (Fig. 37); aedeagal shaft (Fig. 50) narrow in lateral view, ventral process without dorsolateral lobes | Tungurahuala acuminata, sp. n. |
htp://species-id.net/wiki/Tungurahuala_basilisca
Figs 1, 46–49Length male 7.6–7.9. Head (male) approximately ¼ length of forewing. Forewing dark brown to black. Male anal tube with apical ventrolateral spines very weakly developed or absent. Aedeagus with gonopore-bearing shaft in lateral view broad, apex obliquely truncate, posteroapical margin concave; ventral process with pair of microtrichiate dorsolateral lobes, in ventral view abruptly expanded preapically, apex broadly bilobed, in lateral view with apex convergent toward shaft and with dorsal margin entire.
Holotype male: ECUADOR: Mt. Tungurahua, Baños, 2500m, August 20, 1937 (W. Clarke-Macintyre) [USNM]. Other material: 1 male, COLOMBIA, Cundinamarca, PNN Chingaza Charrascales, 4°31'N, 73°45'W, 2990m, Malaise, 4–18 October 2001 (L. Cifuentes), M.2551 [HIC]; 1 male, Cundinamarca, PNN Chingaza Alto de la Bandera, 4°31'N, 73°45'W, 3660m, Malaise, 30 March -12 April 2001 (L. Cifuentes), M.1585 [HIC]; 1 male, same data except 27 December 2001–11 January 2002 (E. Raigoso), M.3023 [INHS].
The specimens examined from Colombia are here considered conspecific with the holotype from Ecuador, although there is slight variation among specimens in size, coloration, and the shape of the aedeagus. Given the small number of specimens available, it seems prudent to consider these minor variations to be intra-specific, despite the considerable geographic disjunction among the known populations.
Although
Mileewinae, wings 19–20 Makilingia sp. (Thailand), fore- and hind wing 21–22 same, Mileewa margheritae 23–24 same, Tinteromus sp. (Colombia) 25–26 same, Amahuaka sp. (Mexico) 27–28 Tungurahuala acuminata 29–30 same, Ilyapa viridis.
Tungurahualini 31–32 head, anteroventral view 31 Tungurahuala acuminata 32 Ilyapa viridis 33–34 prothoracic femur, anterior view 33 Tungurahuala acuminata 34 Ilyapa viridis 35–36 hind tarsomere I, ventral view 35 Tungurahuala acuminata 36 Ilyapa viridis 37 Tungurahuala acuminata, genital capsule, lateral view 38 same, valve and subgenital plates, ventral view 39 Ilyapa bifida, genital capsule, lateral view 40 same, Ilyapa loca 41 same, Ilyapa longispina 42 same, Ilyapa ochrescens 43 Ilyapa ochrescens, left subgenital plate, ventral view 44 Ilyapa recurvata, genital capsule, lateral view 45 same, Ilyapa viridis.
urn:lsid:zoobank.org:act:FD180D56-E0FC-4CCC-9C28-4F4C8FB090FE
htp://species-id.net/wiki/Tungurahuala_acuminata
Figs 2, 3, 11, 27, 28, 31, 33, 35, 37, 38, 50–52, 65, 69–71Length male 7.5–7.6 mm, female 7.6 mm. Head (male) approximately 1/3 as long as forewing (female with head proportionately longer compared to forewing). Forewing dark brown with tan pigment along costal area, mostly tan in female. Male anal tube with apical ventrolateral spine well developed. Aedeagus with gonopore-bearing shaft slender, apex rounded; ventral process acuminate, without dorsolateral lobes, apex in ventral view narrower than shaft.
Holotype male: COLOMBIA, Boyacá, SFF Iguaque Lagunillas, 5°25'N, 73°27'W, 3380m, Malaise 2–18 May 2001 (P. Reina), M.1756. Paratypes: 1 male, same data except 28 June–19 July 2001, M.1966 [HIC]; 1 male, Boyacá, SFF Iguaque Qda. Carrizal, 5°25'N, 73°27'W, 3350m, Malaise 13 – 30 July 2000 (P. Reina), M.379; 1 male, same data except 21 January–9 February 2001; 1 male and 1 female, Boyacá, SFF Iguaque Cabaña Mamarramos, 5°25'N, 73°27'W, 2855m, Malaise 7–21 January 2001 (P. Reina), M.1252; 1 male, same data except 21 December 2000–7 January 2001, M.1072 [HIC, INHS].
The name refers to the acuminate ventral process of the aedeagus.
This species resembles Tungurahuala basilisca in overall structure, but the head is proportionately longer and the coloration of the forewing is lighter overall, although variable among specimens examined.
Ilyapa longispina, sp. n.
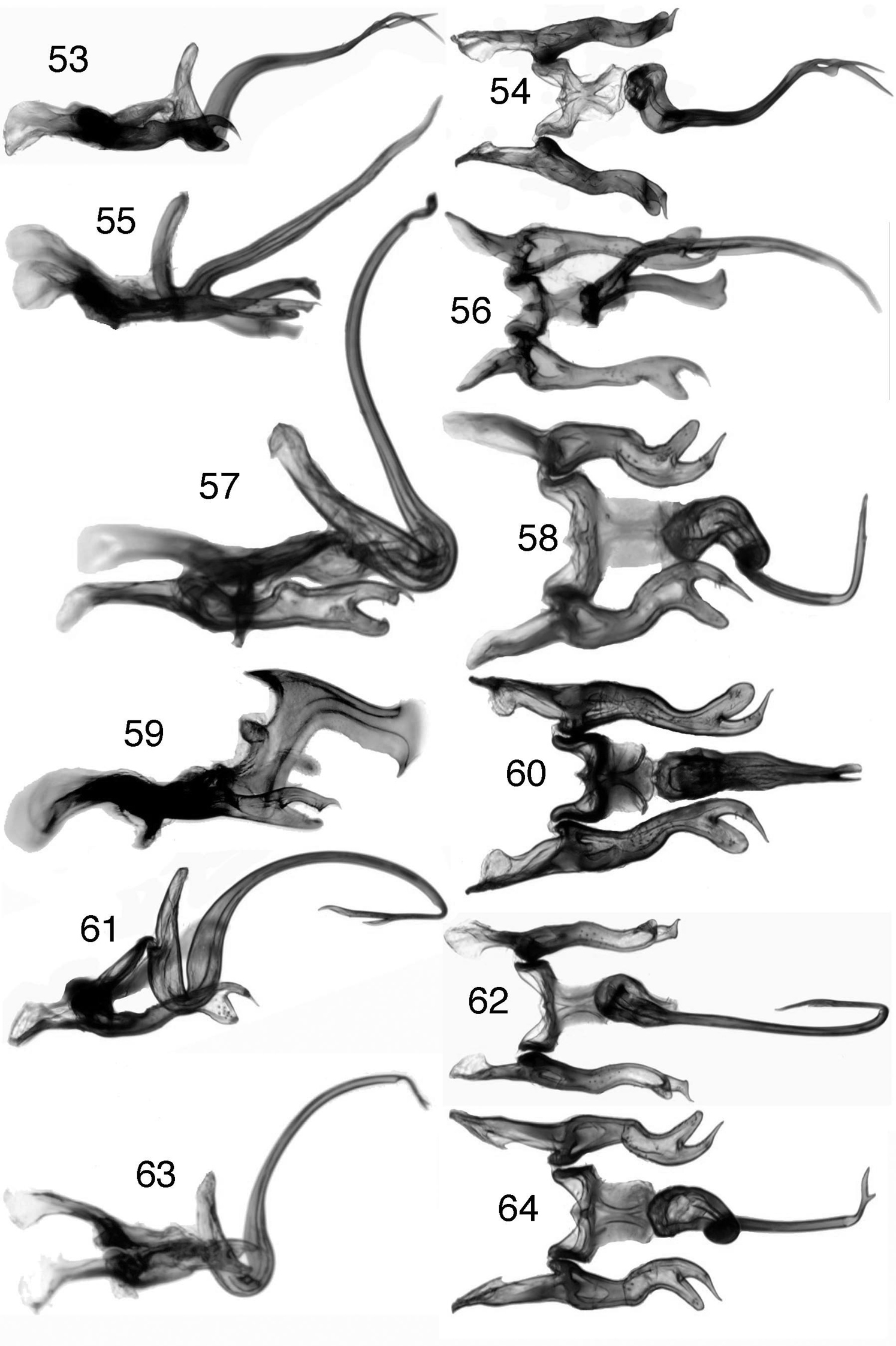
Medium sized, depressed leafhoppers (Figs 4–10, 12). Coloration pale orange or green with red/orange markings dorsally; crown with pair of oblique red maculae mesad of ocelli, pronotum with semicircular orange macula anteriorly, face and thoracic sternites black, legs dark basally and pale distally, abdomen heavily marked with dark brown dorsally and with varying amounts of dark brown pigmentation ventrally. Crown depressed, finely granulose, without setae, weakly pentagonal in dorsal view, marginal and medial carinae absent; ocelli on crown anterad of eyes slightly closer to lateral margin than to midline; antennal ledge broadly depressed, coincident with crown margin; flagellum slightly shorter than crown width; mesal margin of eye emarginate; frontoclypeus granulose with oblique anterodorsal section separated from nearly horizontal posteroventral section by distinct transverse ridge; muscle scars distinct laterally; clypeal suture obsolete medially; anteclypeus convex, tapered, apex narrower than lorum; lorum well separated from ventral genal margin; gena weakly produced laterally, partially concealing proepisternum. Pronotum weakly convex with irregular transverse striations, lateral margins divergent posterad, slightly wider than head, strongly carinate, carina even with eye. Exposed part of mesonotum and scutellum wider than long. Forewing (Fig. 29) opaquely sclerotized except apical cells, veins distinct but without marginal setae; R three branched, crossvein s absent; two r-m and 3–4 m-cu crossveins present; apical cells 2 and 3 very short; inner apical cell relatively broad; appendix very narrow. Hind wing (Fig. 30) with cell distad of r-m crossvein parallel-sided or narrowed distally. Prothoracic femur (Fig. 34) slender, AM1 large, located on ventral margin, AV1 well developed, row AV without preapical setae; intercalary row with ~17 slender, close-set setae; tibia 1+1, PV absent. Mesothoracic femur equal in length but wider than prothoracic femur; AV and PV with few widely separated setae, tibial row PD with apical seta, other rows with few irregularly spaced setae. Metathoracic femur macrosetal formula 2+1+1, rarely 2+1+1+1, tibia and tarsus as in Tungurahuala. Male with apodemes of sternite III well developed; pygofer (Figs 39–45) short with scattered macrosetae dorsolaterally, with ventral process glabrous, slender, arising posteroventrally and curved posterodorsad; anal tube in dorsal view as long as broad, flat ventrally, with or without pair of ventrolateral processes distally; valve short, rectangular, narrowly fused to pygofer; plates depressed basally, expanded and slightly compressed distally with band of macrosetae extended from lateral margin of base posteriorly across middle of apex; aedeagal shaft (Figs 53–62) arcuate posteriorly, often asymmetrical, gonopore apical; connective trilobed anteriorly, stem broad and depressed; style sinuate with large, sparsely setose preapical lobe, apophysis acuminate with ventral preapical tooth. Female seventh sternite (Figs 65–68) longer than sixth and concealing basal half of ovipositor in repose, posterior margin produced; first valvulae (Fig. 72–73) slender, dorsal sculpturing concatenate, second valvulae (Fig. 74) similar to those of Tungurahuala but with dorsal teeth more numerous, prominent and closely spaced, and dorsum at base of blade evenly rounded rather than angulate. Fifth instar nymph (Fig. 10) with overall form and chaetotaxy similar to that of adult except coloration pale greenish yellow with median dorsal longitudinal white stripe; crown with acrometope well delimited, longer than wide; metope well delimited; ocellar precursors well delimited and positioned as in adult on coryphe anteromesad of eyes, well separated from margin; face with distinct transverse shelf corresponding to epistomal suture (as in adult), cibarial muscle scars distinct; dorsum glabrous with scattered sparse, minute setae; enlarged setae absent; hind tarsomere I with three apical platellae.
The name Ilyapa is based on that of the Inca god of thunder, lightning, and rain, but the gender is here considered feminine due to its ending.
This genus is closely related to Tungurahuala, as indicated by the similarities in cephalic structure (ocelli distant from margin, frontoclypeus with transverse carina), forewing venation (crossvein s lacking), leg chaetotaxy (hind tarsomere I pecten with tapered macrosetae), and male genitalia (pygofer with recurved posteroventral process, style with strong preapical lobe). It differs from Tungurahuala in the characters noted in the key.
The genus is described based on six species from the Andean region of South America. The species inhabit cloud forests and have been collected by sweeping grasses and other herbaceous vegetation in the understory. They are readily distinguished by differences in coloration, head proportions, and the structure of the male genitalia.
.
Key to species of Ilyapa (males)
| 1 | Aedeagal shaft (Figs 59–60) bilaterally symmetrical, compressed and broad in lateral view | Ilyapa ochrescens, sp. n. |
| – | Aedeagal shaft strongly asymmetrical, elongate, slender and tubular | 2 |
| 2 | Aedeagus (Figs 55–56) with depressed, apically truncate ventral process arising near base; gonopore-bearing shaft elongate, spinelike, evenly tapered distally, without process | Ilyapa loca, sp. n. |
| – | Aedeagus without basal process, shaft with one or more distal processes | 3 |
| 3 | Aedeagus (Figs 58, 64) with distal process extended laterad at approximately right angle in ventral view | 4 |
| – | Aedeagus with distal process not extended laterad at right angle, either strongly recurved ventrad or more or less continuing in line with shaft | 5 |
| 4 | Distal process of aedeagus bifid (Fig. 64) | Ilyapa viridis, sp. n. |
| – | Distal process of aedeagus unbranched (Fig. 58) | Ilyapa longispina, sp. n. |
| 5 | Aedeagus (Fig. 61) with single distal process strongly recurved ventrad, apex branched; anal tube with several small, irregular ventrolateral teeth (Fig. 44) | Ilyapa recurvata, sp. n. |
| – | Aedeagus (Figs 53–54) with two distal processes more or less aligned with shaft, asymmetrically curved, one process with angulate projection near base; anal tube with pair of short triangular projections near base (Fig. 39) | Ilyapa bifida, sp. n. |
urn:lsid:zoobank.org:act:26BD4DCA-8225-43E5-AD09-1D484CC59C40
htp://species-id.net/wiki/Ilyapa_bifida
Figs 4, 12, 39, 53, 54Length male 6.6–6.9 mm, female 7.0 mm. Crown pale orange-yellow, orange-red maculae broad, overlapping ocelli, anterior margin forming acute angle; pronotum and opaque areas of forewing bright green (mottled with yellow in specimens removed from ethanol), pronotum with semicircular macula distinct. Male pygofer processes extended mesad and curved dorsad but not or only slightly crossing midline; anal tube process short, triangular. Aedeagus asymmetrical, shaft tubular, in lateral view V-shaped basally, arched and sinuate distally, terminating in two slender, bladelike processes continuing in line with shaft but asymmetrically curved, one process with angulate projection near base. Female seventh sternite with posterior margin slightly produced, rounded medially. Fifth instar nymph pale olive green dorsally with broad white median longitidinal stripe extended entire length of body; venter white.
Holotype male: PERU, Pasco, Yanachaga-Chemillén N.P., Refugio El Cedro, 2420 m, 10°33'07"S, 75°21'27"W, 10 October 2002 (D. M. Takiya) PE07 [USML]. Paratypes: 1 male, same data except 12 October 2002 (R. Rakitov) [INHS].
The species name refers to the bifid apex of the aedeagus.
This species may be distinguished by its relatively long crown and the bifid apex of the aedeagus.
urn:lsid:zoobank.org:act:8E950B3B-7F16-47E6-96F5-C5729EA156C0
htp://species-id.net/wiki/Ilyapa_loca
Figs 5, 40, 55–56Length male 6.1 mm. Coloration as described for Ilyapa bifida except crown margin white, orange-red maculae broader, and pronotum almost entirely orange; apical margin of crown forming obtuse angle. Male pygofer processes short, broad, and digitiform; anal tube without processes. Aedeagus highly asymmetrical; gonopore-bearing shaft acuminate, extended to left posterodorsad and gradually curved mesad, apex without process; ventral process depressed, apex expanded, truncate, and even with style apices. Female unknown.
Holotype male, PERU, Chanchamayo, 25 July 1960 (Young and Ramirez) [NCSU].
The species name means “crazy" and refers to the bizarre, asymmetrical aedeagus.
This species may be distinguished by its relatively short crown and by the presence of a long, unpaired ventral process arising from the base of the aedeagal shaft.
urn:lsid:zoobank.org:act:00015CA5-8E24-4357-A657-7B8D3B5F6638
htp://species-id.net/wiki/Ilyapa_longispina
Figs 6, 41, 57–58, 67, 72–74Length male 5.9–6.2 mm; female 7.1–7.5 mm. Coloration as described for Ilyapa bifida except crown margin mostly white; apical margin of crown forming approximately right angle. Male pygofer processes slender, crossing posteromedially; anal tube with pair of retrorse posterolateral spines; plate apex angulate mesally. Anal tube processes long with apices curved ventrad; pygofer processes shorter, not meeting medially; apical process of aedeagus more elongate, with minute preapical spine posteriorly. Female seventh sternite with posterior margin trilobed with median lobe acute and larger than lateral lobes.
Holotype male: PERU, Chanchamayo, 25 July 1960 (Salazar and Ramirez) [NCSU]. Paratypes: 1 male, same locality, 22 July 1960 (C. Ramirez) [INHS]; 3 females, same locality except 21 and 25 July 1960 [NCSU, INHS]. Other material: 3 males, PERU, Ucayali, 8 km E Abra La Divisoria, 1250 m, 9°9'57"S, 75°48'11"W, 25 October 2002 (R. A. Rakitov) sweeping, 02-39-2; 1 male, 1 female, PERU, Pasco, Yanachaga-Chemillén N.P., Puesto de Control Huampal, 1050 m, 10°1'09"S, 75°34'27"W, 8 October 2002 (R. A. Rakitov) on grass; 1 male, same data except Refugio El Cedro, 2420 m, 10°33'07"S, 75°21'27"W, 10 October 2002, D.M. Takiya, PE07; 1 female PERU, Junin, 1 km S Minapichita, 2100 m 11°6'1"S, 75°25'30"W, 19 October 2002 (C. H. Dietrich) sweeping, 02-20-1 [INHS].
The species name refers to the long distal spine of the aedeagus.
This species may be distinguished by its moderately long crown and by the elongate, laterally directed distal spine of the aedeagus.
One specimen from Yanachaga-Chemillén National Park, Peru, has the distal spine of the aedeagus extended to the right, mirroring the condition found in other examined specimens of this species. Specimens examined from Yanachaga-Chemillén National Park have the anal tube processes considerably shorter than those in the type series from Chanchamayo, but such variation is here considered to be intraspecific. Based on the material available for study, this is the most widespread and common species of the genus.
urn:lsid:zoobank.org:act:1D932E94-61AA-400E-B8D3-40F45CEF0D3D
htp://species-id.net/wiki/Ilyapa_ochrescens
Figs 7, 42, 59–60Length male 6.5–6.8 mm. Nearly uniform pale orange dorsally, orange-red maculae slender; apical margin of crown forming acute angle, pronotal macula indistinct. Male pygofer processes robust, not crossing posteromedially; anal tube without ventrolateral processes; aedeagal shaft symmetrical, pillarlike, compressed, in lateral view extended dorsad and bent posterad at right angle, with pair of acute anterodorsal processes extended anterad, posterodorsal extension with ventral margin irregular, apex expanded with ventral spine. Female unknown.
Holotype male: COLOMBIA, Cundinamarca, PNN Chingaza Valle Del Fraylejon, 4°31'N, 73°45'W, 3170m, Malaise 31 August–13 September 2000 (A. Pérez), M.732. Paratypes: 1 male, same coordinates, Chingaza Bosque Palacio, 2930m, Malaise 8–22 December 2000 (A. Cifuentes), M.1027; 1 male, same data except 3–16 March 2001(C. Vinchira and A. Cifuentes), M.1492 [HIC].
The species name refers to the mostly orange coloration of the dorsum.
This species may be distinguished by its relatively long crown, predominantly orange coloration, and broad, strongly compressed aedeagal shaft.
urn:lsid:zoobank.org:act:987F8A00-CC54-494D-AE56-7FDAD4F92AF3
htp://species-id.net/wiki/Ilyapa_recurvata
Figs 8, 44, 61–62Length male 5.9 mm. External morphology and male terminalia similar to those of Ilyapa longispina, except as follows: anal tube without pair of ventrolateral processes but with several irregular teeth; aedeagus with distal process strongly recurved ventrad and anterad, branched near midlength with one branch about half length of other. Female unknown.
Holotype male: PERU, Huánuco, Carpish Pass, 2600 m, 9°43'3"S, 76°5'38"W, 27 October 2002 (C. H. Dietrich) sweeping, 02-45-1 [USML].
The species name refers to the strongly recurved apex of the aedeagus.
This species may be distinguished by its relatively short crown and strongly recurved aedeagal apex.
urn:lsid:zoobank.org:act:D530E660-0B0C-4159-9EDF-4FD394B048C4
htp://species-id.net/wiki/Ilyapa_viridis
Figs 9, 29–30, 32, 34, 45, 63–64, 68Length male 5.8–6.0, female 7.0 mm. Crown pale yellow medially, white laterally, orange/red maculae broad, overlapping ocelli; anterior margin forming approximately right angle; pronotum and opaque areas of forewing dark green (mottled with yellow in specimens removed from ethanol), pronotum with semicircular macula distinct. Male pygofer processes slender, crossing posteromedially; anal tube with pair of retrorse posterolateral spines. Aedeagus asymmetrical, shaft narrow, tubular and gradually tapered distally, in lateral view narrowly U-shaped with distal part attenuated and arcuate; apex with slender bifurcate process extended to left at right angle to shaft. Female seventh sternite posterior margin with small acute median tooth.
Holotype male: PERU, Pasco, Yanachaga-Chemillén N.P., 10°32'39.7"S, 75°22'00.1"W, 2300m, 10–13 October 2002 (D. Takiya, C. Peña, R. Rakitov) Malaise trap across Rio San Alberto [USML]. Paratypes: 1 male and 1 female, same data; 2 males, Yanachaga-Chemillén N.P., 10°32'S, 75°21'W, S. Alberto Valley ca. Refugio El Cedro, 2270–2420m, 12 October 2002 (R. Rakitov); 2 males, 1 female, same data except 10 October 2002 (D. M. Takiya); 8 females, same data except 12 October 2002 (D. M. Takiya) [INHS].
The species name refers to the mostly pale green coloration of the dorsum.
This species closely resembles Ilyapa longispina in external morphology and in the male genitalia, but may be distinguished by the shorter, distinctly branched distal aedeagal process and by the distinctly smaller median lobe of female abdominal sternite VII.
In one examined male specimen, the configuration of the aedeagus is the mirror image of that of the other specimens.
Tungurahuala, male genitalia 46 Tungurahuala basilisca (specimen from PNN Chingaza Alto de la Bandera, Cundinamarca, Colombia), genitalia, lateral view 47 same, ventral view (only right style shown) 48 Tungurahuala basilisca (specimen from PNN Chingaza Churrascales, Cundinamarca, Colombia), genitalia, lateral view 49 same, ventral view 50 Tungurahuala acuminata, genitalia, lateral view 51 same, ventral view 52 same, aedeagus, posteroventral view.
Ilyapa, male genitalia, lateral and ventral views 53–54 Ilyapa bifida 55–56 Ilyapa loca 57–58 Ilyapa longispina 59–60 Ilyapa ochrescens 61–62 Ilyapa recurvata 63–64 Ilyapa viridis.
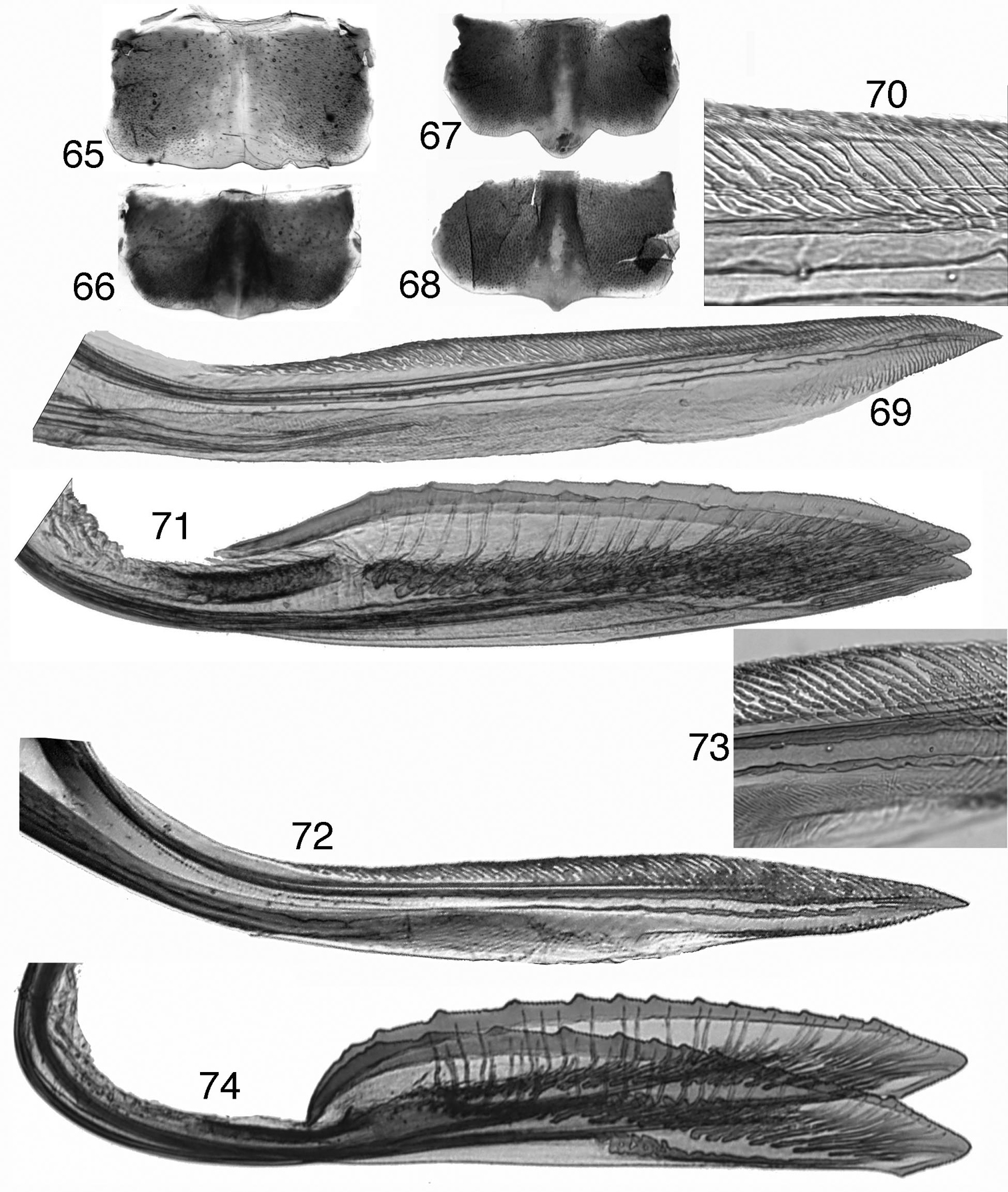
Tungurahualini, female terminalia 65–68 sternite VII 65 Tungurahuala acuminata 66 Ilyapa bifida 67 Ilyapa longispina 68 Ilyapa viridis 69–71 Tungurahuala acuminata 69 first valvula 70 same, detail of dorsal sculptured area 71 second valvulae 72–74 Ilyapa longispina 72 first valvula 73 same, detail of dorsal sculptured area 74 second valvulae
I am indebted to Paul Freytag and Michael Sharkey (University of Kentucky) for access to their Malaise trap samples from Colombia, to Robert Blinn (NCSU) for the loan of additional specimens, to Carlos Peña and Pedro Lozada for help with field work in Peru, to Daniela Takiya and Dmitry Dmitriev for helpful discussions, and to Mick Webb and Chandra Viraktamath for constructive criticism of the manuscript. This work was supported in part by grants from the U.S. National Science Foundation.