(C) 2012 Robin E. Thomson. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Eight new species of Hydroptilidae (Trichoptera) from Venezuela are described: Acostatrichia digitata sp. n., Hydroptila cressae sp. n., Metrichia botrychion sp. n., Ochrotrichia spira sp. n., Oxyethira bettyae sp. n., Oxyethira quiramae sp. n., Oxyethira redunca sp. n., and Rhyacopsyche shorti sp. n.New country records for Venezuela of 2 additional species, Neotrichia feolai Santos & Nessimian, 2009 and Oxyethira picita Harris & Davenport, 1999, are also provided. Illustrations of male genitalia are provided with each description.
Trichoptera, caddisflies, Hydroptilidae, Anchitrichia, Hydroptila, Metrichia, Neotrichia, Ochrotrichia, Oxyethira, Rhyacopsyche, new species, Neotropical
Caddisflies, or Trichoptera, are a diverse order of insects with ~15, 000 described species and 100s of new species awaiting description (
In this paper, we describe 8 new hydroptilid species in 6 genera from Venezuela.We also provide new country records for Venezuela for 2 species, Neotrichia feolai Santos & Nessimian, 2009 and Oxyethira picita Harris & Davenport, 1999. The material was collected as part of a project under the direction of Dr. Andrew Short, University of Kansas, USA, to inventory the aquatic Coleoptera and other aquatic insect orders of Venezuela. In June, 2010, a team of 4 American and 4 Venezuelan entomologists collected aquatic insects in the southern half of Venezuela, including the llanos of Guárico state, the southern tributaries of the upper to middle Orinoco River basin, and the Gran Sabana of Bolívar state, and, in northern Venezuela, the Turimiquire Mountains of Monagas state. About 90 species of Trichoptera were collected, including about 25 new species of which the new Hydroptilidae are described here.
Materials and methodsMorphological terminology used for male genitalia of specimens in the genus Oxyethira follows that of
urn:lsid:zoobank.org:act:85981ECC-3397-4968-ACBC-B6EE2098DA5B
http://species-id.net/wiki/Acostatrichia_digitata
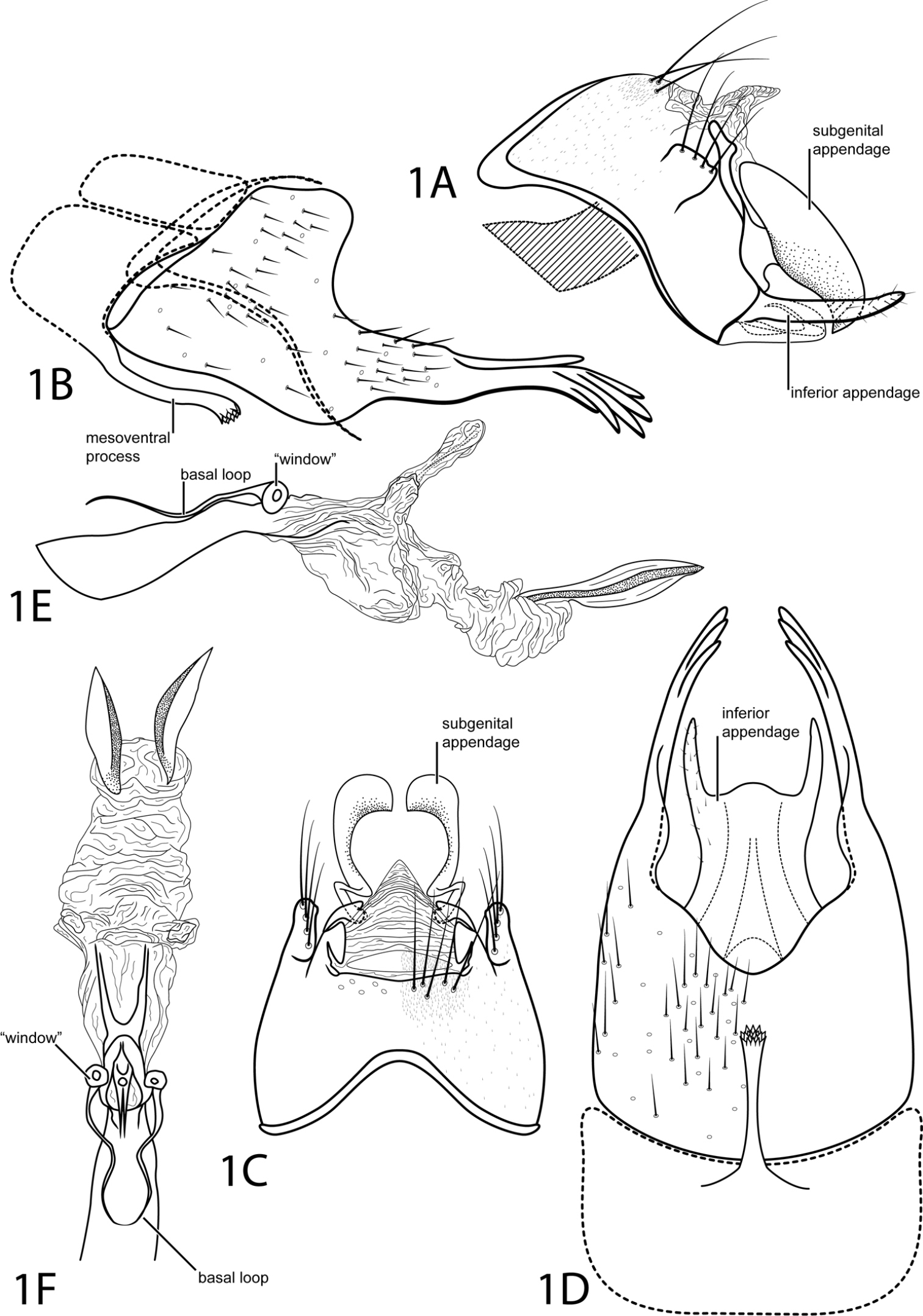
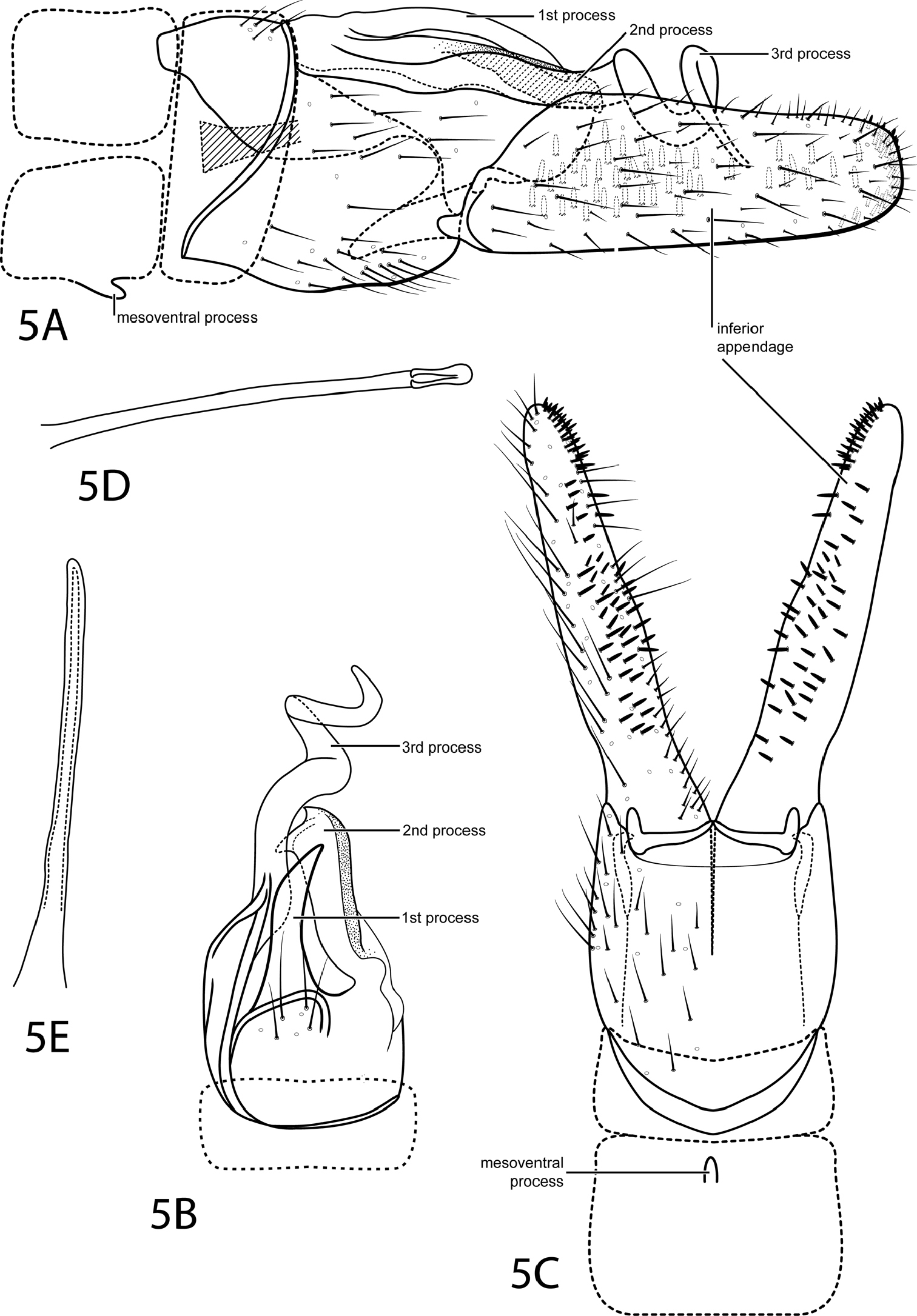
Fig. 1This species is most similar to Acostatrichia fimbriata Flint, 1974 but can be distinguished by a mesoventral process on abdominal segment VII with an apex that is truncate and rugose, not pointed. The posterolateral process of abdominal segment VIII bears digitate projections apically, unlike the spines on Acostatrichia fimbriata. Additionally, the subgenital appendage of Acostatrichia digitatus is pointed apically instead of rounded.
Male. Length of forewing 2.7 mm (n=1). Head unmodified, with 3 ocelli; antennae unmodified. Tibial spur count 1, 3, 4. Dorsum of head dark brown with pale yellow setae; thorax dark brown with pale yellow setae dorsally, light brown ventrally; leg segments with light brown setae. Forewings covered with fine yellow setae and small scattered patches of dark brown setae. Genitalia. Abdominal sternum VII with long mesoventral process, apex truncate, rugose. Segment VIII anterolateral margin straight, posterolateral margin greatly elongate into narrow structure bearing digitate apical projections; ventrally posterior margin concave, mildly crenulated. Segment IX anterolateral margin acute, posterolateral margin broadly convex; with mesolateral quadrate structure bearing prominent setae (see Fig. 1A); dorsally with posterior margin straight. Subgenital appendage paired, broadly rounded with apicoventral point, dorsally with rounded emargination on inner edge. Inferior appendage setose, narrow, rod-like, fused latero-ventrally with subgenital appendage, in ventral view with semiquadrate apical emargination (see Fig. 1D). Tergum X membranous, triangular in dorsal view. Phallus tubular basally with median complex bearing basal loop and pair of circular “windows”, apex with pair of elliptic plates, strongly sclerotized mesolaterally.
Acostatrichia digitata sp. n. Male genitalia: A segments IX–X, lateral (base of phallus crosshatched) B segments VII–VIII and segment IX anterolateral margin, lateral C segments IX–X, dorsal D segments VII–IX, ventral E phallus, lateral F phallus, dorsal.
Holotype male: VENEZUELA: Bolívar: E Tumeremo, W Bochinche, Río Botonamo, 07°25.462'N, 61°14.318'W, 150 m, 13.vii.2010, UV light, Holzenthal, Thomson, Cressa (UMSP000095201) (UMSP).
The Latin word digitatus meaning “having fingers”, referring to the digitate projections on the posterolateral process of the VIIIth segment.
urn:lsid:zoobank.org:act:22765A69-4EE1-4DA6-BB78-E100D0C6EF33
http://species-id.net/wiki/Hydroptila_cressae
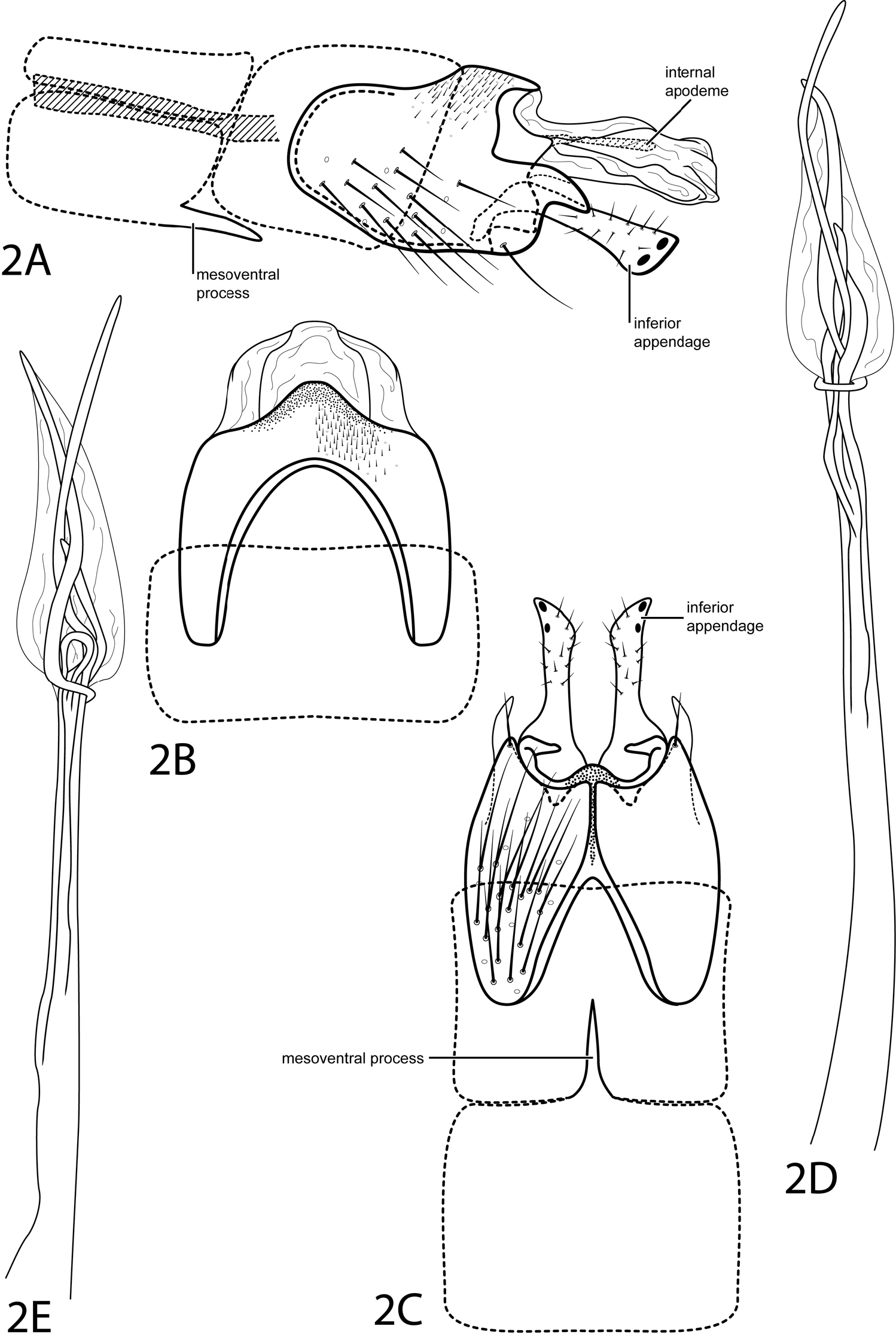
Fig. 2This species is most similar to Hydroptila denza Ross, 1948, but differs in the shape of the projection on the posterolateral margin of abdominal segment IX. This projection is more pointed and is curved downward, or decurved, in Hydroptila cressae, while it is straight and more blunt in Hydroptila denza. The triangular subgenital process seen in Hydroptila denza is not apparent in Hydroptila cressae.Additionally, tergum X of Hydroptila cressae contains an internal apodeme that is not apparent in Hydroptila denza.
Male. Length of forewing 2.0 mm (n=1). Head unmodified, without ocelli; antennae unmodified. Tibial spur count 0, 2, 4. Dorsum of head brown with pale yellow setae; thorax brown with light brown setae dorsally, light brown ventrally; leg segments with light brown setae. Forewings covered with fine light brown setae with small dark brown patch of setae at apex. Genitalia. Abdominal sternum VII with simple, slender, pointed mesoventral process. Segment VIII unmodified. Segment IX anterolateral margin convex, posterolateral margin with pointed projection, curving slightly ventrad; dorsally with posterior margin convex. Inferior appendage setose, with narrow base, apex truncate with pair of dark points on internal face. Tergum X membranous, extending past inferior appendage, containing internal sclerotized apodeme (see Fig. 2A). Phallus narrow, elongate; apex membranous, ovate, with elongate, slender spines extending past membranous region.
Hydroptila cressae sp. n. Male genitalia: A segments VII–X, lateral (base of phallus crosshatched) B segments VIII-X, dorsal C segments VII–IX, ventral D phallus, lateral E phallus, dorsal.
Holotypemale: VENEZUELA: Bolívar: Gran Sabana, E. Pauji, “Río Curvita”, 04°31.237'N, 61°31.591'W, 869 m, 15–16.vii.2010, UV light, Holzenthal, Thomson, Cressa (UMSP000095196) (UMSP).
Named in honor of Dr. Claudia Cressa, an aquatic ecologist at the Universidad Central de Venezuela and friend and colleague of the authors.
urn:lsid:zoobank.org:act:13F7C864-63DB-4360-92FA-D3CB3331DBA3
http://species-id.net/wiki/Metrichia_bostrychion
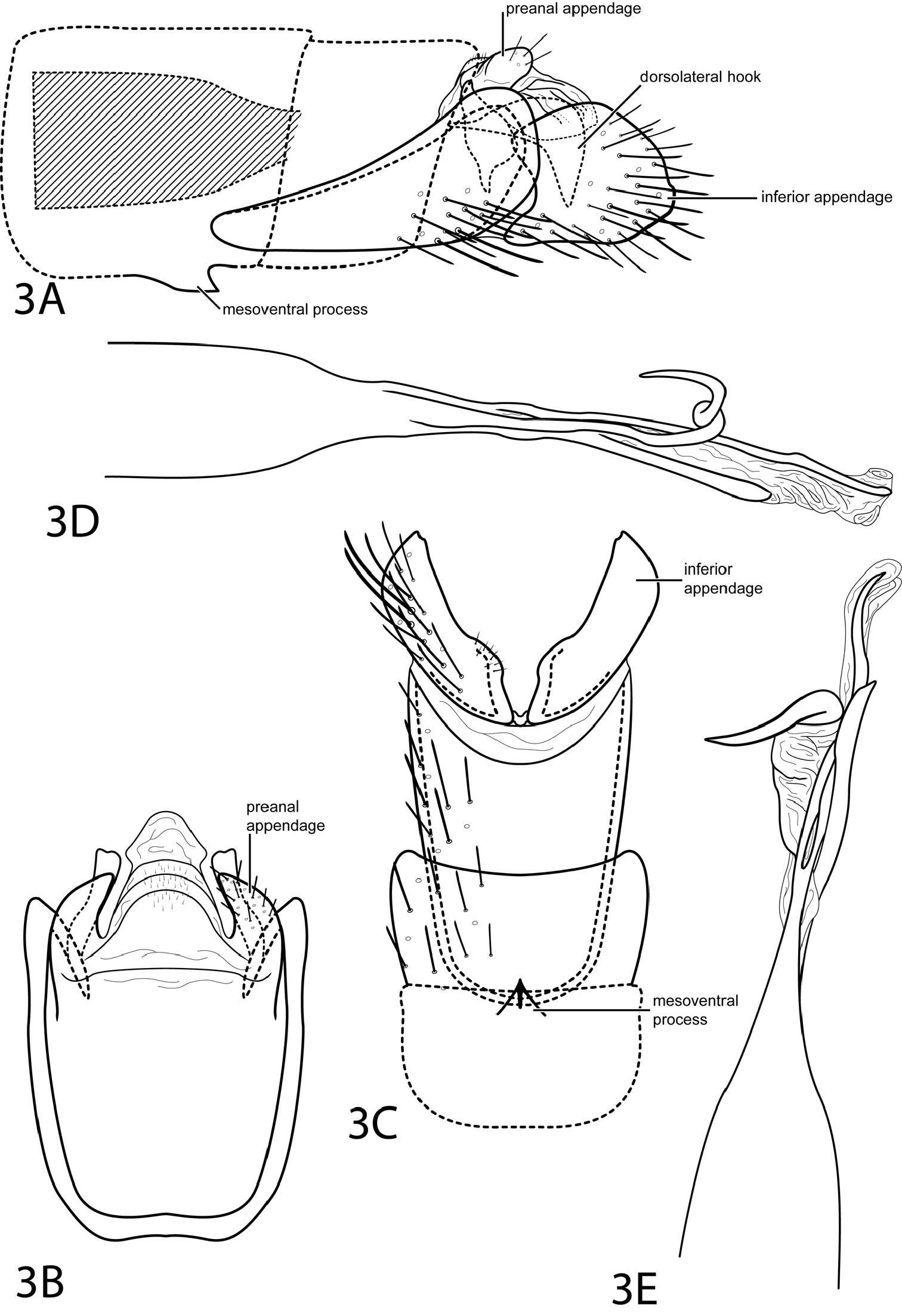
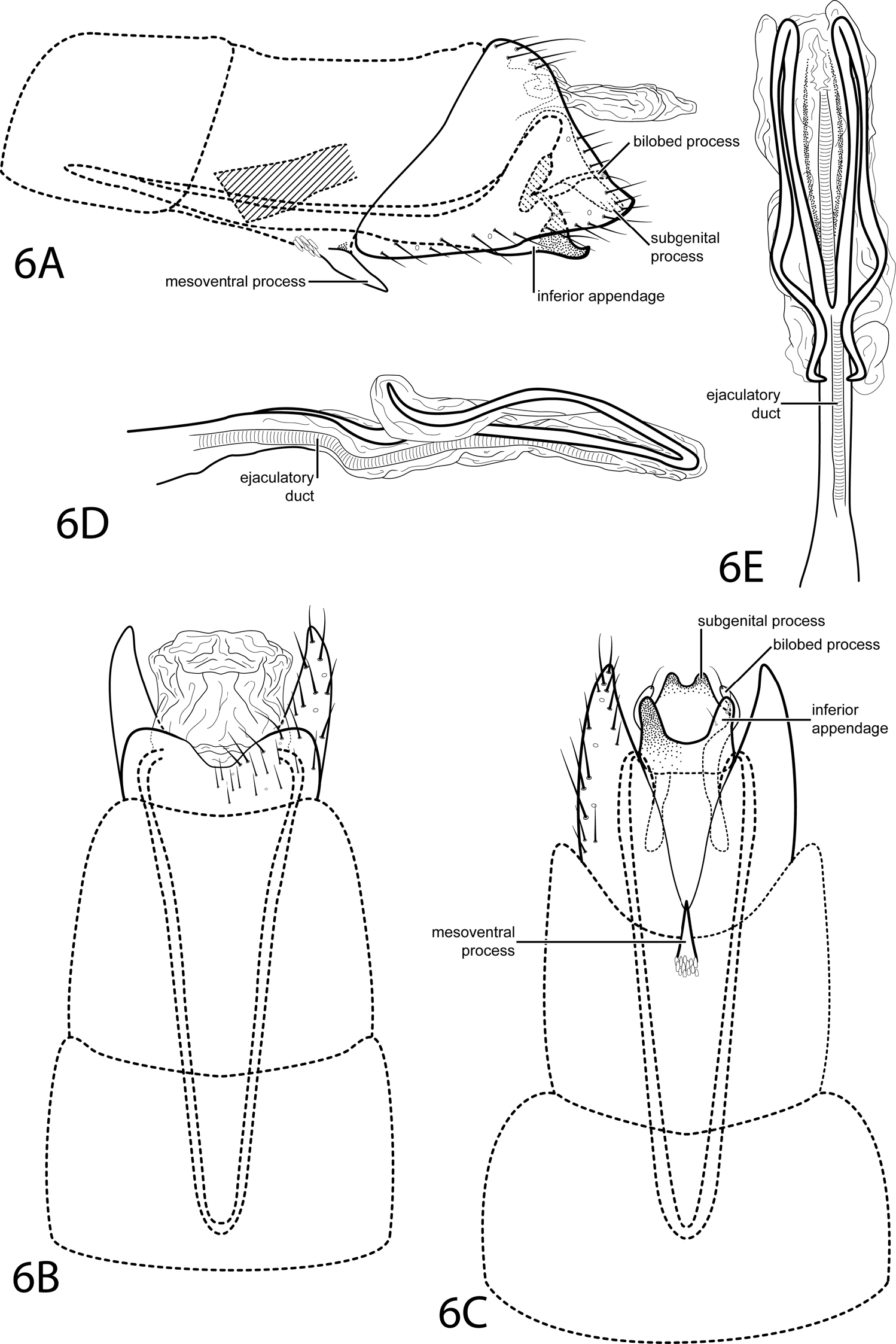
Fig. 3This species is most similar to Metrichia anisoscola (Flint, 1991), but differs in the shape of the inferior appendage, which is less elongate in Metrichia bostrychion and more suborbicular. The dorsolateral hook in Metrichia bostrychion is also stouter than that of Metrichia anisoscola. Metrichia bostrychion can also be distinguished by the 3rd spine on the phallus which spirals dorsally.
Male. Length of forewing 1.8 mm (n=1). Head unmodified, with 3 ocelli; antennae unmodified. Tibial spur count 1, 3, 4. Dorsum of head dark brown with white setae; thorax dark brown with white and dark brown setae dorsally, brown ventrally; leg segments with brown setae. Forewings covered with fine dark brown setae with small patch of light brown setae at apex. Abdomen with internal sacs between segments IV–V. Dorsolateral setal brushes on segments IV and V. Genitalia. Abdominal sternum VII with short, pointed mesoventral process. Segment VIII unmodified. Segment IX anterolateral margin very elongate, narrowing, withdrawn into segments VII–VIII, posterolateral margin convex; dorsally with posterior margin membranous, flat. Preanal appendage (cercus) short, rounded. Dorsolateral hook stout, strongly decurved (see Fig. 3A). Inferior appendage suborbicular with shallow posterolateral emargination, extends as high as segment IX. Tergum X membranous, apex subdeltoid in dorsal view. Phallus widest at base, narrowing to median constriction, membranous apex with 3 spines, 1st and 3rd slender, elongate, 2nd spiraling dorsad.
Metrichia bostrychion sp. n. Male genitalia: A segments VII–X, lateral (base of phallus crosshatched) B segments IX–X, dorsal C segments VII–IX, ventral D phallus, lateral E phallus, dorsal.
Holotype male: VENEZUELA: Monagas: Guachero Cave National Park at La Paila waterfall, 10°10.322'N, 63°33.315'W, 1110 m, 20–21.vii.2010, sweep net, Holzenthal, Thomson (UMSP000095197) (UMSP).
same data as holotype, 1 female (UMSP).
The diminutive of the Greek word bostrychos meaning “curl”, referring to the small spiral in the second apical spine on the phallus.
http://species-id.net/wiki/Neotrichia_feolai
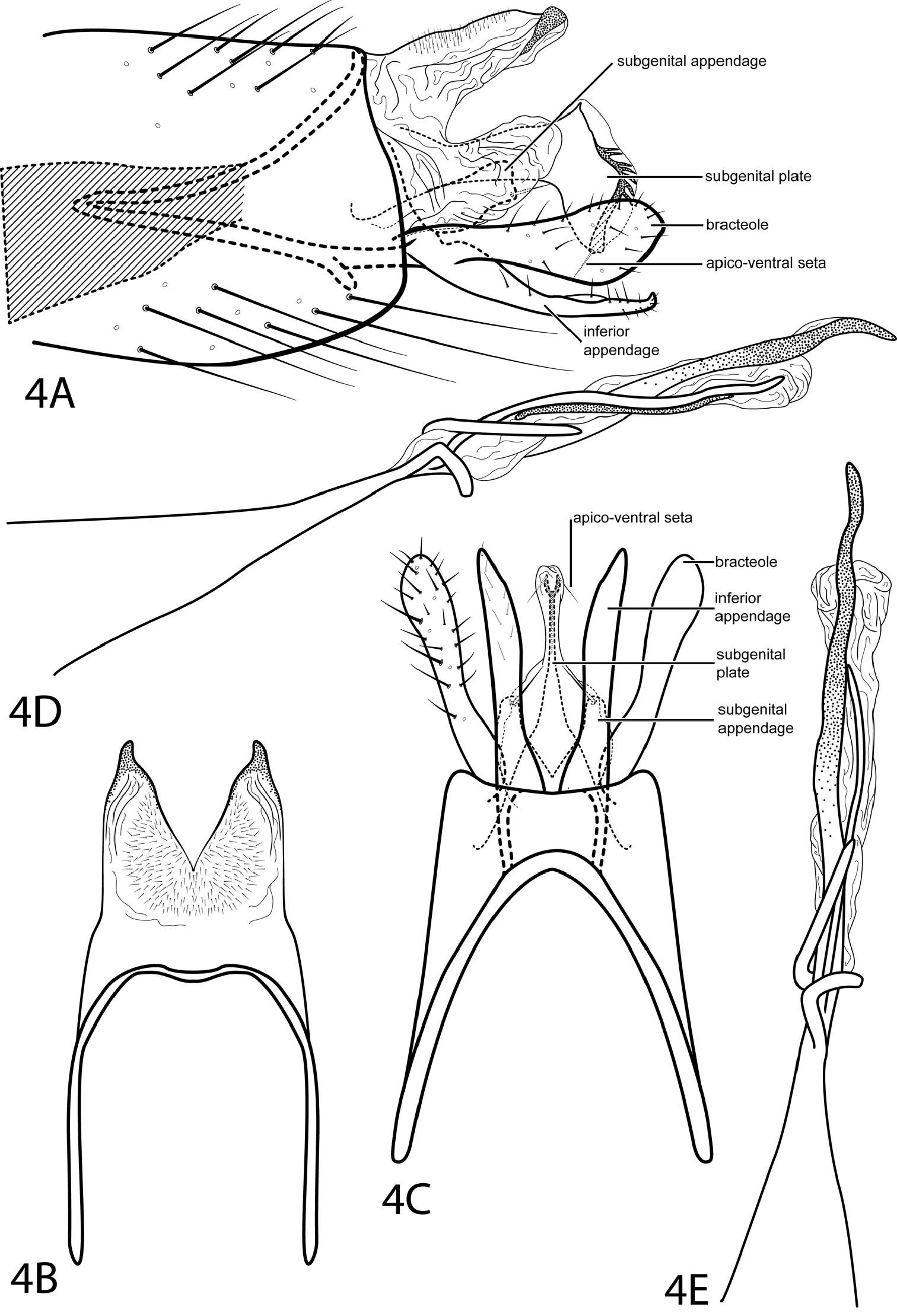
Fig. 4Neotrichia feolai was previously only known from the male holotype collected from Brazil, Amazonas. Eight males were collected for the first time from Venezuela, representing a new record in this study for the country. Original illustrations did not include the distinctive subgenital appendage, but specimens from Venezuela match all other characteristics of the Brazilian species perfectly. Some of our specimens are dry, while the holotype was collected in alcohol, allowing us to to describe coloration. We have also described and illustrated the subgenital plate not seen in the original illustration.
According to the original authors, this species is most similar to Neotrichia biuncifera Flint, 1974. The shapes and lengths of the bracteole and inferior appendage are similar, but Neotrichia feolai can be distinguished by having only a single spine at the apex of the phallus.
Male. Length of forewing 1.6–1.9 mm (n=8). Head unmodified, with 3 ocelli; antennae unmodified. Tibial spur count 0, 2, 3. Dorsum of head brown with light brown setae; thorax brown with light brown setae dorsally, light brown ventrally; leg segments with light brown setae. Forewings covered with fine light brown setae with small patches of dark brown setae. Genitalia. Abdominal sternum VII without mesoventral process. Segment VIII unmodified. Segment IX anterolateral margin strongly narrowing, withdrawn into segment VIII, posterolateral margin fused dorsally with tergum X (see Fig. 4B). Subgenital plate fused, diamond-shaped with pair of apico-ventral setae, posterior margin bearing paired row of sclerotized spines within membranous layer (see Figs. 4A, 4C). Bracteole spatulate, extended evenly with inferior appendage. Inferior appendage setose, laterally narrow and rod-like, fused latero-ventrally with subgenital appendages, ventrally with semiquadrate apical emargination. Tergum X membranous, bearing minute dorsal setae, with deep emargination both laterally and dorsally, dorsal lobe with sclerotized apex. Phallus with wide tubular base narrowing to median constriction, membranous apex with spiral process and slender apical spines.
Neotrichia feolai Santos & Nessimian, 2009. Male genitalia: A segments VIII–X, lateral (base of phallus crosshatched) B segments IX–X, dorsal C segment IX, ventral D phallus, lateral E phallus, dorsal.
VENEZUELA: Guárico: Santa Rita, Morichal de los Becerros, 08°09.044’N, 62°35.149’W, 66 m, 6.vii.2010, UV light, Holzenthal, Thomson, 8 males (5 in alcohol) (UMSP, NMNH, MIZA).
urn:lsid:zoobank.org:act:83832CD2-268B-4AA3-A5EC-F6FAB463D9F6
http://species-id.net/wiki/Ochrotrichia_spira
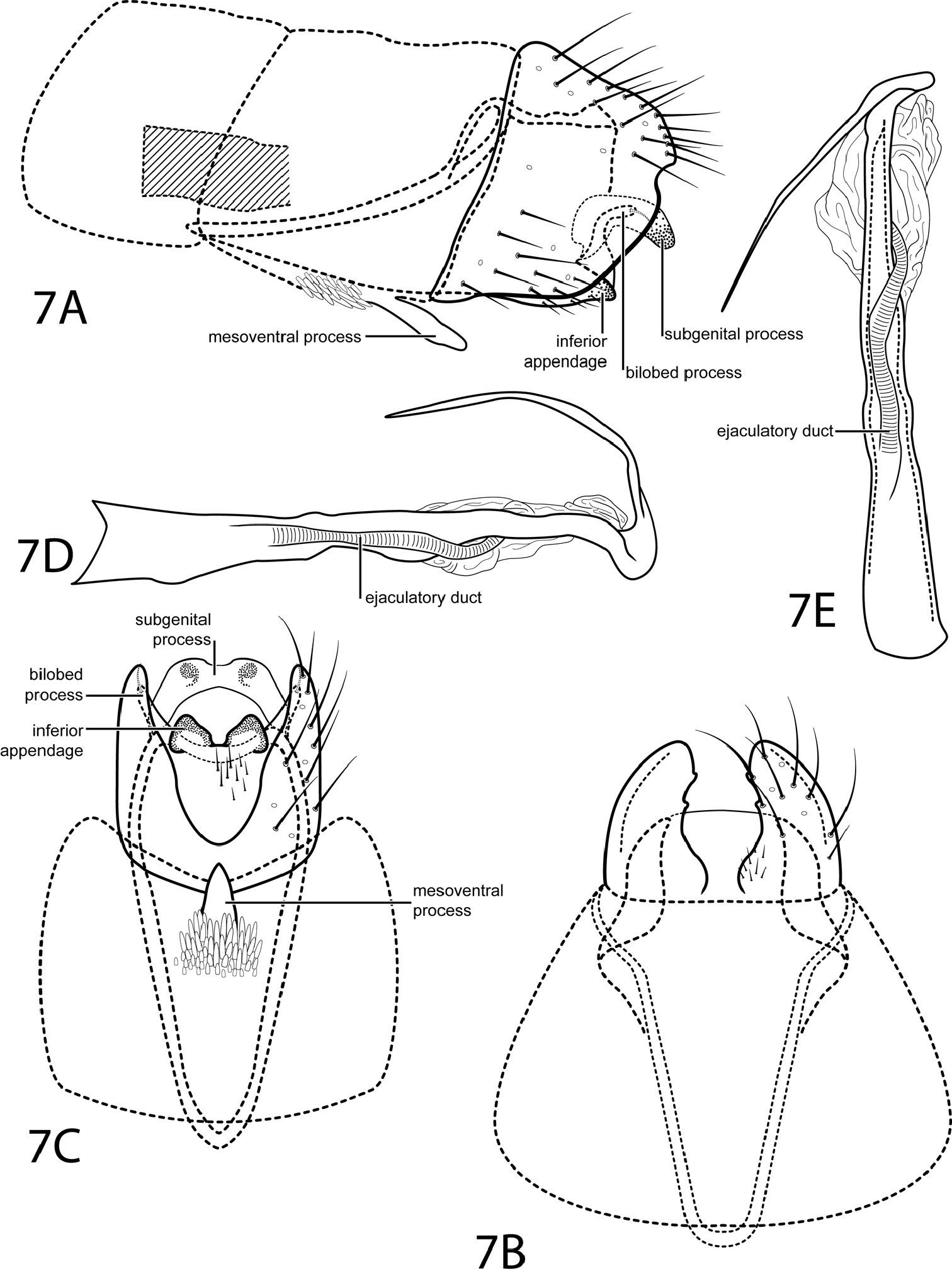
Fig. 5This species is most similar to Ochrotrichia raposa Bueno-Soria & Santiago-Fragoso, 1992. Both have a simple, threadlike phallus and the large inferior appendage bears patches of black pegs on its internal face. The inferior appendage of Ochrotrichia spiralis, however, is parallel-sided with a truncate apex. Also, the sclerotized processes extending from tergum X of Ochrotrichia spiralis are easily distinguishable from those of Ochrotrichia raposa, in particular the large, spiral process.
Male. Length of forewing 2.6–2.7 mm (n=3). Head unmodified, with 3 ocelli; antennae unmodified. Tibial spur count 0, 3, 4. Dorsum of head dark brown with pale yellow setae; thorax light brown with light brown setae dorsally, light brown ventrally; leg segments with light brown setae and patches of dark brown. Forewings covered with fine brown setae with small patches of dark brown setae near apex. Genitalia. Abdominal sternum VII with short, rounded mesoventral process. Segment VIII unmodified. Segment IX anterolateral margin concave, posterolateral margin fused dorsally with tergum X. Inferior appendage setose, 3 times longer than wide, parallel-sided, apex truncate, inner surface bearing many short, stout spines distributed as in Figs. 5A, 5C. Tergum X sclerotized, highly developed with 3 processes: 1st simple, slender pointed; 2nd with heavily sclerotized edge, large subapical point, small apical point; 3rd strongly spiraled, extended past other processes. Phallus tubular, elongate, threadlike.
Ochrotrichia spira sp. n. Male genitalia: A segments VII-X, lateral (base of phallus crosshatched) B segments VIII–X, dorsal C segments VII–IX, ventral D phallus, lateral E phallus, dorsal.
Holotypemale: VENEZUELA: Monagas: Guachero Cave National Park, 10°10.322'N, 63°33.315'W, 1110 m, 20–21.vii.2010, UV light, Holzenthal, Thomson, Cressa (UMSP000095193) (UMSP).
same data as holotype, 2 males (UMSP, NMNH).
The Latin word spiralis meaing “spiral”, referring to the strongly spiraled process extending from tergum X.
urn:lsid:zoobank.org:act:9B85F4D2-070A-4192-BD8D-E4DF432D19AA
http://species-id.net/wiki/Oxyethira_bettyae
Fig. 6This species is placed in the subgenus Tanytrichia according to the characters given by Kelley (1985): segment VIII venter excised to anterior margin, segment IX elongate and extending into segment VI, the absence of segment IX dorsum, and a phallus bearing two long lateral processes originating at the midlength. This species is most similar to Oxyethira longissima Flint, 1974. The phallus is very similar, bearing long paired processes sharply bent back anteriorly. However, the subgenital process of Oxyethira longissima is more strongly arched and much more slender in lateral view than that of Oxyethira bettyae. Also, when viewed ventrally, the bilobed process of Oxyethira bettyae is wider basally than Oxyethira longissima.
Male. Length of forewing 2.0–2.2 mm (n=6). Head unmodified, with 3 ocelli; antennae unmodified. Tibial spur count 0, 3, 4. Dorsum of head dark brown with pale yellow setae; thorax brown with light brown setae dorsally; leg segments with light brown setae. Forewings covered with fine light brown setae and small scattered patches of dark brown setae and golden brown setae. Genitalia. Abdominal sternum VII with simple, pointed mesoventral process with small patch of stout pegs basally. Segment VIII anterolateral margin straight, posterolateral margin pointed; dorsally posterior margin with rounded emargination; ventrally posterior margin with deeply divided. Segment IX anterolateral margin very narrow and very elongate, withdrawn into segments VI–VIII, posterolateral margin straight, not extended posteriorly past segment VIII. Subgenital process fused, apex with small rounded emargination (see Fig. 6C). Bilobed process slender, extending posteriad. Inferior appendage fused with deep apical emargination, sparsely setose, heavily sclerotized, apex acute, upturned in lateral view. Tergum X membranous, quadrate dorsally, oblong ventrally. Phallus with tubular basal half, apical half membranous; 2 long, lateral processes beginning at midlength, very sharply curved backward, or recurved.
Oxyethira bettyae sp. n. Male genitalia: A segments VI–X, lateral (base of phallus crosshatched) B segments VI–X, dorsal C segments VI–IX, ventral D phallus, lateral E phallus, dorsal.
Holotypemale: VENEZUELA: Guárico: UCV San Nicolasito Field Station, 08°8.296'N, 66°24.459'W, 62 m, 5.vii.2010, UV light, Holzenthal, Thomson (UMSP000095178) (UMSP).
same data as holotype, 1 male (UMSP); Venezuela: Bolívar: Los Pijiguaos at rock outcrop, 6°35.617'N, 66°49.238'W, 80 m, 7–8.vii.2010, UV light trap, Holzenthal, Thomson, 5 males (in alcohol) (UMSP, NMNH, MIZA).
Named in honor of the first author’s grandmother, Betty Welter, who passed away while this work was in progress.
http://species-id.net/wiki/Oxyethira_picita
Oxyethira picita was previously known only from the male holotype collected from Peru, Loreto. A single male was collected at a later date from Brazil, Amazonas (
Oxyethira picita was placed in the subgenus Tanytrichia by Harris & Davenport (1999), although it was suggested that it also displayed some similarity to the subgenus Loxotrichia. The original description and illustration of Oxyethira picita are detailed and well done; further description or illustration was not thought necessary.
VENEZUELA: Bolívar: Campamento Río Aro, 07°37.443'N, 64°08.324'W, 90 m, 10–11.vii.2010, UV light, Holzenthal, Thomson, 4 males (in alcohol) (UMSP, MIZA). VENEZUELA: Bolívar: 30 km S Upata, roadside marsh, 07°22.239'N, 61°44.233'W, 163 m, 12.viii.2010, Short, Tellez, Camacho, 1 male (in alcohol) (NMNH).
urn:lsid:zoobank.org:act:6C447B87-EA39-4D06-A28A-0702345D2930
http://species-id.net/wiki/Oxyethira_quiramae
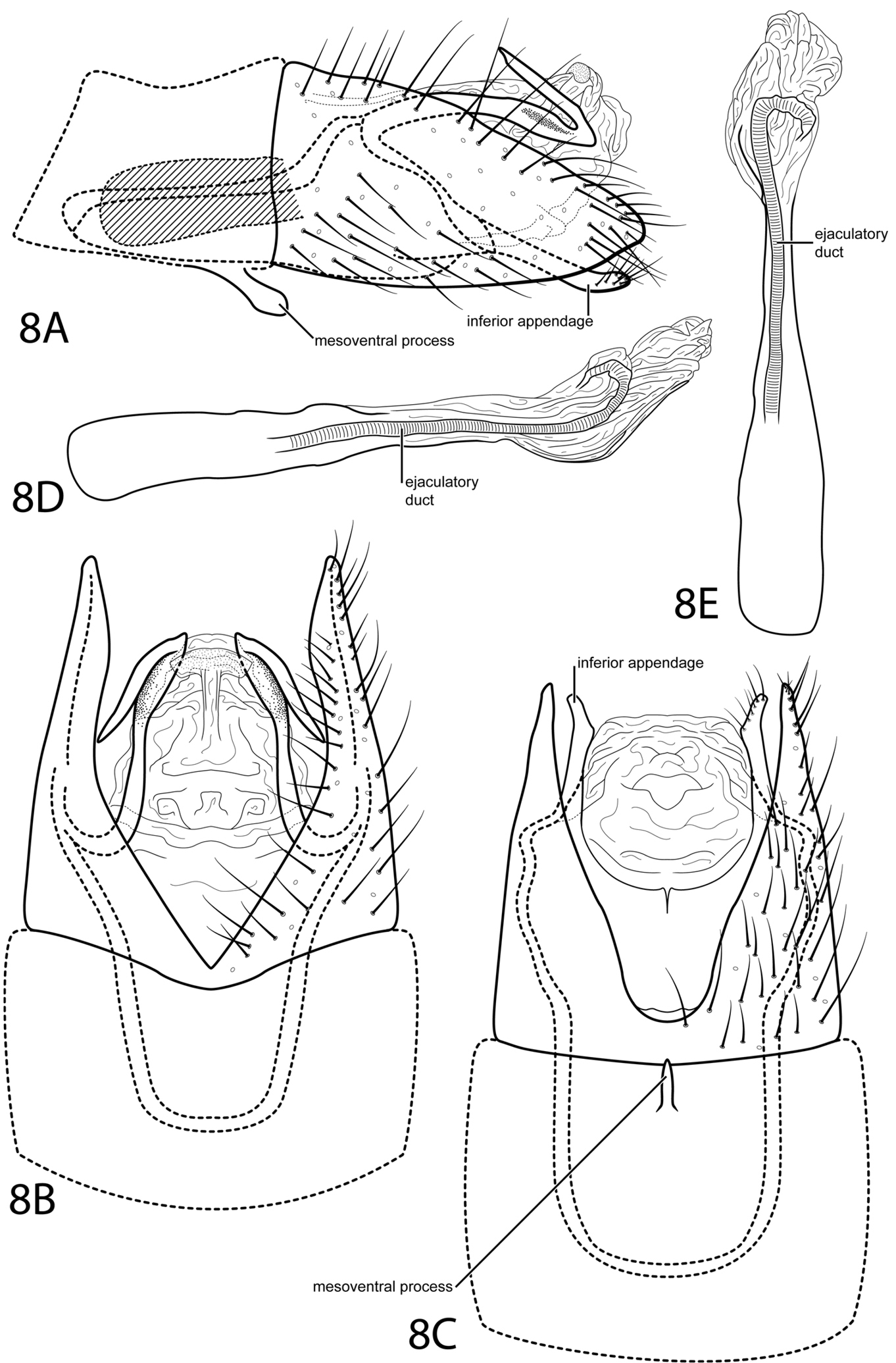
Fig. 7This species is placed in the subgenus Dactylotrichia according to the characters given by Kelley (1985): segment VIII venter excised nearly to the anterior margin and segment IX venter extending anteriorly through segments VIII–VI. This species is most similar to Oxyethira hozosa Harris & Davenport, 1999. Both species have short, blunt, ventrally triangular inferior appendages and a phallus with a distal curved process and an ejaculatory duct enclosed within the membranous apex. Oxyethira quiramae can be distinguished by a subgenital plate that is not as strongly decurved and lacks an acute apex in lateral view. Also, in Oxyethira quiramae, segment IX extends anteriorly past the posterior margin of abdominal segment VI.
Male. Length of forewing 1.8–1.9 mm (n=3). Head unmodified, with 3 ocelli; antennae unmodified. Tibial spur count 0, 3, 4. Dorsum of head brown with light brown setae; thorax brown with light brown setae dorsally, light brown ventrally; leg segments with light brown setae. Forewings covered with fine brown setae with small scattered patches of light brown setae and small patches of dark brown setae near margins and apex. Genitalia. Abdominal sternum VII with simple, digitate mesoventral process with large patch of stout pegs basally. Segment VIII anterolateral margin straight, posterolateral margin convex with small mesal emargination; dorsally posterior margin with rounded emargination; ventrally deeply excised. Segment IX anterolateral margin narrow and elongate, withdrawn into segments VI–VIII, posterolateral margin straight, not extended posteriorly past segment VIII. Subgenital process fused distomesally, apex with shallow emargination, curving ventrad (see Fig. 7C). Bilobed process slender, curved, not extending posteriorly past segment VIII. Inferior appendage reduced, triangular, heavily sclerotized (see Fig. 7C). Tergum X not apparent. Phallus with tubular basal half, apical half membranous; apex elongate, slender, pointed, curving dorsad and sharply recurved.
Oxyethira quiramae sp. n. Male genitalia: A segments VI–IX, lateral (base of phallus crosshatched) B segments VII–IX, dorsal C segments VII–IX, ventral D phallus, lateral E phallus, dorsal.
Holotype male: VENEZUELA: Guárico: UCV San Nicolasito Field Station, 08°8.296'N, 66°24.459'W, 62 m, 5.vii.2010, UV light, Holzenthal, Thomson (UMSP000095179) (UMSP).
same data as holotype, 2 males (NMNH, MIZA).
Named in honor of Gina Quiram, a friend and colleague of the first author, for all her help in the field.
urn:lsid:zoobank.org:act:C8A428D1-6558-479C-8AF8-8857D6E2A5FB
http://species-id.net/wiki/Oxyethira_redunca
Fig. 8We have been unable to assign this species to a subgenus. The deep ventral excision of abdominal segment VIII and the extension of segment IX anteriorly into segment VII make it somewhat similar to Loxotrichia. However, the absence of a subgenital process precludes it from being placed with certainty in any of the current subgenera and distinguishes it from all other species.
Male. Length of forewing 2.4 mm (n=1). Head unmodified, with 3 ocelli; antennae unmodified. Tibial spur count 0, 3, 4. Dorsum of head brown with pale yellow setae; thorax brown with pale yellow setae dorsally, pale yellow ventrally; leg segments with brown setae. Forewings covered with fine brown setae and elongate patches of light brown setae and small patches of dark brown setae near margins and apex. Genitalia. Abdominal sternum VII with spatulate mesoventral process. Segment VIII anterolateral margin straight, posterolateral margin acutely convex; dorsally posterior margin with deep acute emargination; ventrally posterior margin with deep rounded emargination. Segment IX anterolateral margin very elongate, narrowing, withdrawn into segments VII–VIII, posterolateral margin acute, not extending past segment VIII; dorsally bearing paired, elongate, slender processes, basal half extending posteriorly, apical half strongly bent anteriad. Subgenital process not apparent. Bilobed process not apparent. Inferior appendage setose, laterally narrow and rod-like, fused latero-ventrally with subgenital appendages, ventrally with semiquadrate apical emargination. Tergum X membranous, large, bearing elliptic patch of minute setae dorsally (see Fig. 8B). Phallus with tubular basal half, apical half membranous, convex ventrally, apex curving dorsad.
Oxyethira redunca sp. n. Male genitalia: A segments VII–X, lateral (base of phallus crosshatched) B segments VII–X, dorsal C segments VII–X, ventral D phallus, lateral E phallus, dorsal.
Holotype male: VENEZUELA: Bolívar: Gran Sabana, E. Pauji, “Río Curvita”, 4°31.237'N, 61°31.591'W, 869 m, 15–16.vii.2010, UV light, Holzenthal, Thomson, Cressa (UMSP000095176) (UMSP).
The Latin word reduncus meaning “bent backward”, referring to the sharply bent dorsal processes of segment IX.
urn:lsid:zoobank.org:act:56EB1974-52AA-4EE1-9F95-357CD2AC3A4A
http://species-id.net/wiki/Rhyacopsyche_shorti
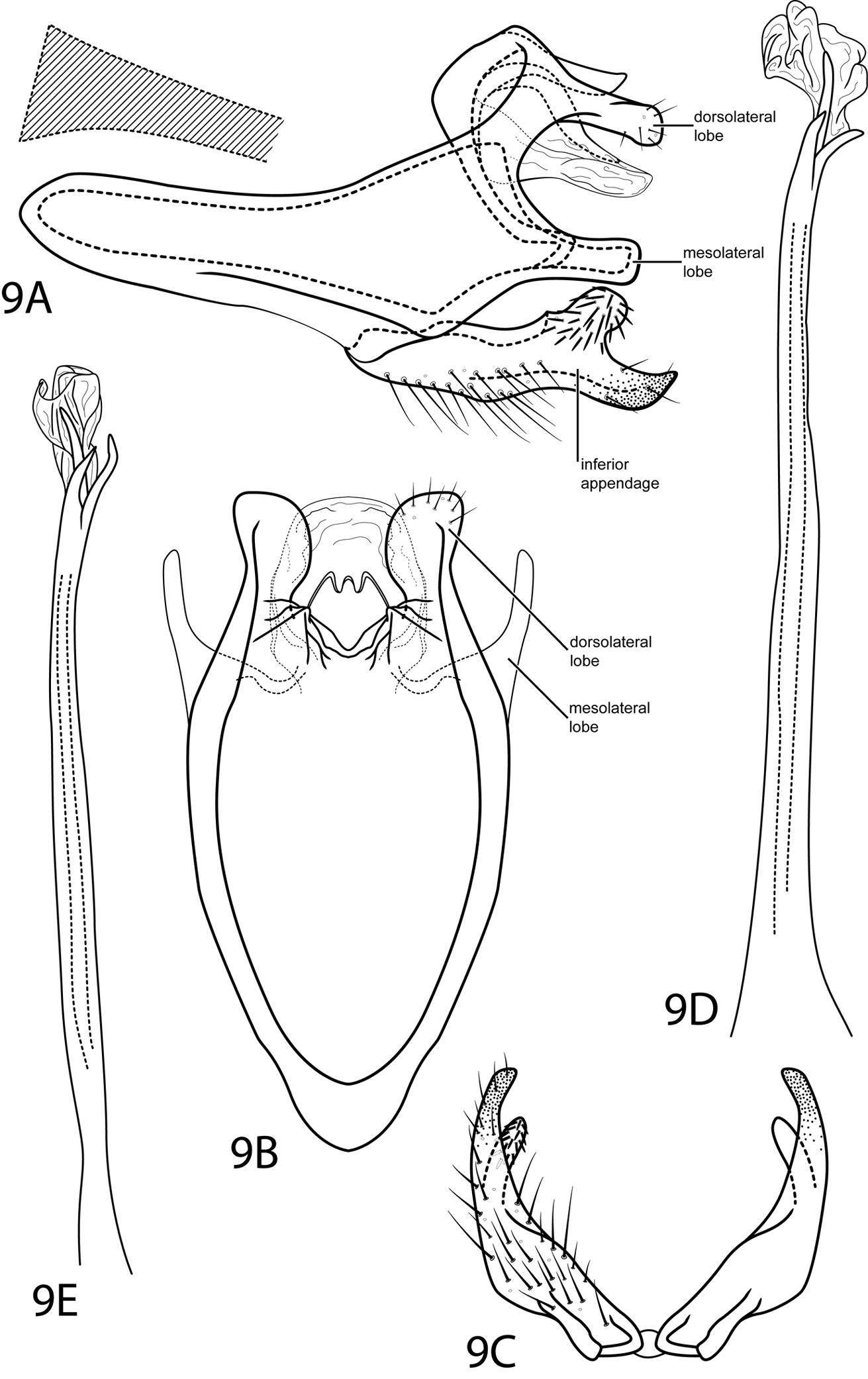
Fig. 9This species is most similar to Rhyacopsyche otarosa Wasmund & Holzenthal, 2007. Both species display an inferior appendage with a bifid apex bearing a large rounded dorsal lobe. However, the ventral lobe is broadly pointed in Rhyacopsyche shorti and truncate in Rhyacopsyche otarosa. Additionally, when seen dorsally, the dorsolateral lobes of segment IX are rounded in Rhyacopsyche shorti, not acicular as in Rhyacopsyche otarosa.
Male. Length of forewing 2.6–2.7 mm (n=2). Head unmodified, with 3 ocelli; antennae unmodified. Tibial spur count 1, 3, 4. Dorsum of head brown with dark brown setae and light brown patch between antennae; thorax brown with light brown setae dorsally, light brown ventrally; leg segments with dark brown setae. Forewings covered with golden brown setae with small patches of dark brown setae at margins and apex. Genitalia. Abdominal sternum VII without mesoventral process. Segment VIII unmodified. Segment IX anterolateral margin very elongate, narrowing, withdrawn into segments VII–VIII, posterolateral margin with rounded setae-bearing dorsolateral lobe and truncate mesolateral lobe. Inferior appendage with rounded mesodorsal projection bearing setae, setae directed anteriad; apex heavily sclerotized, curving dorsad, acute. Tergum X membranous, round in dorsal view, contracted inside dorsolateral lobes of segment IX. Phallus basally tubular, elongate, narrow, apex membranous and with thickened spines.
Rhyacopsyche shorti sp. n. Male genitalia: A segments IX–X, lateral (base of phallus crosshatched) B segments IX–X, dorsal C inferior appendages, ventral D phallus, lateral E phallus, dorsal.
Holotype male: VENEZUELA: Bolívar: Gran Sabana, E. Pauji, “Río Curvita”, 04°31.237'N, 61°31.591'W, 869 m, 15–16.vii.2010, UV light, Holzenthal, Thomson, Cressa (UMSP000095199) (UMSP).
same data as holotype, 1 male (UMSP).
Named in honor of Dr. Andrew Short, an entomologist at the University of Kansas and friend and colleague of the authors.
We are grateful to Dr. Andrew Short, University of Kansas, for giving us the opportunity to collect fresh material in Venezuela and for his expertise as expedition leader. For assistance in the field, we would like to thank Dr. Claudia Cressa, Jesús Camacho, Monica Tellez, Sergio Pacheco, and Quintin Arias. Special thanks are extended to Dr. José Clavijo, MIZA, for administrative and logistic support and for his hospitality. Dr. OS Flint, Jr., Smithsonian Institution, provided invaluable comments on the identity and placement of these new species. This material is based on work supported by the National Science Foundation under Grant Nos. DEB 0816904 to AEZ Short and KB Miller and 0816865 to KM Kjer and RW Holzenthal. RE Thomson was supported also by a CIC Smithsonian Institution Fellowship. This support is gratefully acknowledged.