(C) 2012 Louwtjie P. Snyman. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The Afrotropical Mantispidae genera have previously been neglected and are poorly known. The genera are revised and redescribed. A new genus Afromantispa Snyman and Ohl is described with Afromantispa tenella comb. n.as type species. Perlamantispa (Handschin, 1960) is synonymised with Sagittalata Handschin, 1959. The new combinations within the genus include Sagittalata austroafrica comb. n., Sagittalata bequaerti comb. n., Sagittalata dorsalis comb. n., Sagittalata girardi comb. n., Sagittalata nubila comb. n., Sagittalata perla comb. n., Sagittalata pusilla comb. n., Sagittalata similata comb. n., Sagittalata royi comb. n., Sagittalata tincta comb. n. and Sagittalata vassei comb. n. An illustrated key to the genera Afromantispa gen. n., Sagittalata Handschin, 1959, Mantispa Illiger, 1798, Cercomantispa Handschin, 1959, Rectinerva Handschin, 1959, Nampista Navás, 1914, and Pseudoclimaciella Handschin, 1960 is provided. The wing venation of Mantispidae is redescribed. Similarities between the genera are discussed. Subsequent studies will focus on revising the taxonomic status of species, which are not dealt with in this study.
Mantispidae, Neuroptera, Afrotropical, lacewings, key
The superorder Neuropterida is considered to comprise a diversity of clades, many of which are characterized by a large number of plesiomorphic characters. It includes the orders Raphidioptera, Megaloptera and Neuroptera (
The significance of the order Neuroptera is well documented in fields other than taxonomy. All larvae are obligate predators, while adults are predacious or pollen-feeders and consequently fulfil vital roles in the functioning of natural ecosystems. The order is therefore ideal for studies in biological fields other than taxonomy (
The Afrotropical Mantispidae are certainly one of the families that is in urgent need of revision (
The Mantispinae are the only subfamily known from the Afrotropical Region, and since the Afrotropical Mantispidae have received little attention, their potential impact on other fields of biology cannot be readily assessed. The positive impact of other families of Neuroptera has proven to be of great value in fields such as agriculture as biological control agents taxonomy (
This study forms part of two programmes, a southern African initiative and a global programme. The first will form part of the programme: ‘Monitoring lacewings (Insecta: Neuroptera) in southern Africa’ (
The ultimate long term aim of the study is to resolve the taxonomy of Afrotropical Mantispidae in order to facilitate further research on the group. Since the Afrotropical Mantispids has never received a comparative and large scale revision, this manuscript will form the basis of long term directional research. To achieve this, generic groups was redefined on the basis of clear autapomorphies to support their monophyly, and future molecular studies will be carried out to determine whether these morphologically-defined genera are supported by DNA evidence. Current as well as subsequent studies on the taxonomy and biology of the species can be carried out within the context of these generic concepts.
Biology of MantispinaeFemale Mantispinae lay large batches of stalked eggs ranging from several hundred to several thousand (
A campodeiform, triungulin larvae hatches from the stalked eggs and uses one of two strategies for locating a food source (
The larvae undergo a unique ontological pattern. It has been proposed that the developmental pattern can be termed hypermetamorphic ontogeny but some authors do not agree (
The mating behaviour of mantispids has received some attention (
The Afrotropical Mantispidae are taxonomically extremely complex. Interspecific morphology differs only slightly, while marked intraspecific variation adds to the complexity. Appropriate distinguishing characters must consequently be carefully sought. The literature on southern African taxa is limited. Most of the species descriptions are vague and not intelligible, often lacking sufficient illustrations. This is probably because several authors were not Neuropterists and described the specimens using inappropriate characters and often overlooked important distinguishing criteria. As an example,
Previous authors did little comparative studies and described new taxa without conclusively capering them with similar taxa (
The most recent publications on the Afrotropical taxa were by Poivre in the early 1980’s (
This Palaearctic genus was recently revised by
Mantispa Illiger, 1798 is treated as a Palaearctic genus and is not redescribed in this study. Only a few specimens were available for this revision. Material is currently being obtained for a full comparative study of Mantispa and Sagittalata Handschin, 1959.
Mantispa and PerlamantispaIt has been suggested that Perlamantispa Handschin, 1960 may be a synonym of Mantispa (
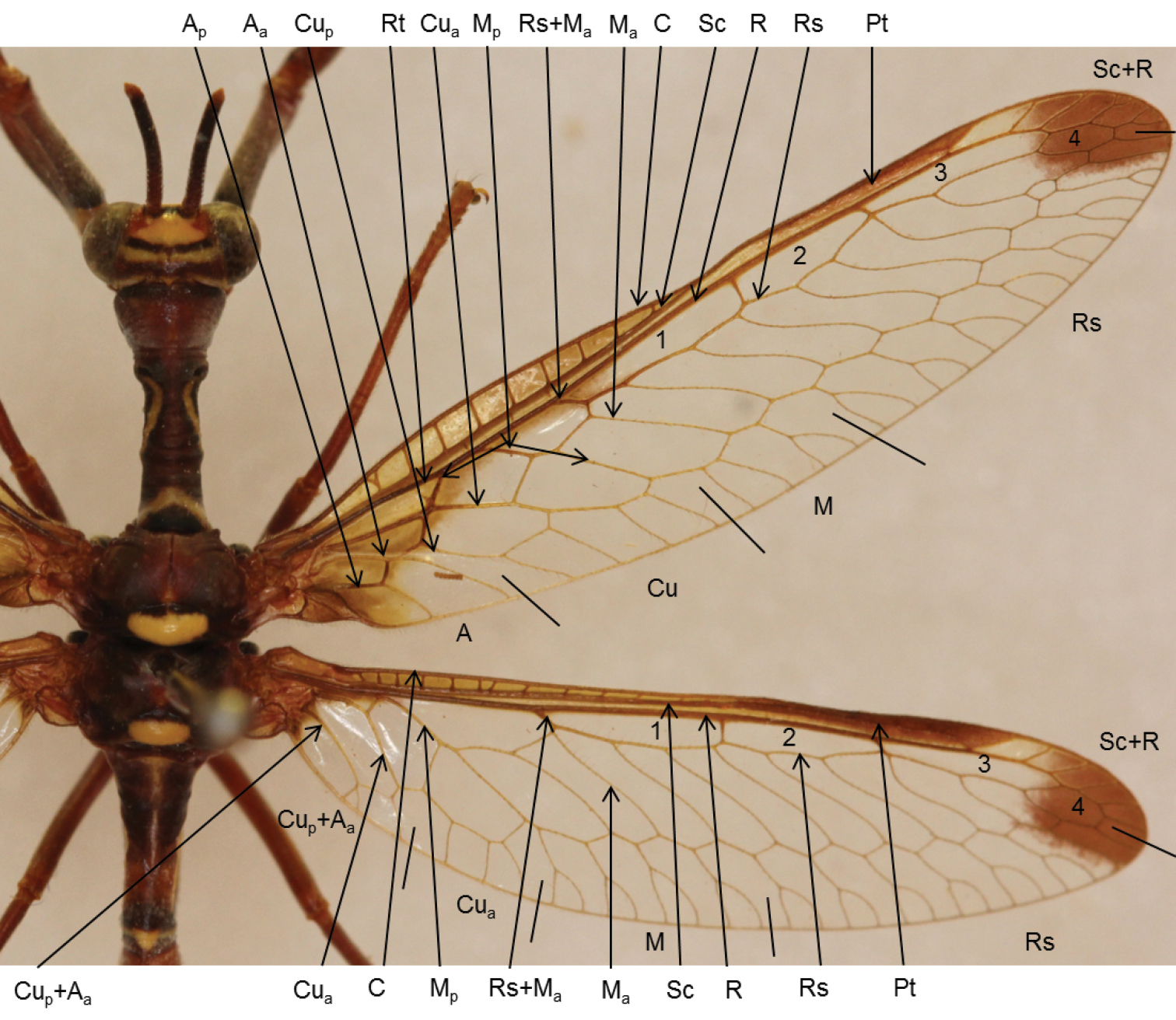
Wing terminology: This study followed the wing terminology of
Wing venation of Mantispidae. A Anal C Costa Cu Cubitus M Median Pt Pterostigma R Radius, Radial cells 1, 2, 3 and 4 Rt Radial triangle Rs Radial sector Sc Subcosta + indicates fused veins p posterior a anterior.
In the FW Rs+Ma splits into Rs and Ma at the first fork (Fig. 1). The Ma vein follows a path directly to the posterior margin. The Rs form the three radial cells and the anterior half of a hexagon-shaped cell (radial cell 4) before reaching the apical margin. In the FW
Male genitalia: No dissections were made because it proved to be unnecessary for the delimitation of monophyletic groups. Preliminary studies indicated that internal genitalic structures might not be a necessary character for the elucidation of the Afrotropical Mantispidae genera even though it is valuable for species delimitation. The only genus where the ectoprocts as well as the pseudopenis are autapomorphic for the Afrotropical genera is Cercomantispa Handschin, 1959 (Fig. 4f; also see discussion below Cercomantispa and Rectinerva Handschin, 1959).
Studied genera: Since the genera studied here are all distributed in the Afrotropics, the autapomorphies have not been compared to all Mantispidae genera. The genus Mantispa Illiger, 1798 is not assigned an autapomorphy because of the confusion between this genus and Sagittalata Handschin, 1959 (see Sagittalata below) that resulted in the lack of a character rather than the presence of one.
Madagascar: The Madagascar fauna was revised by
Name combinations and museums holding valuable collections were identified using LDL (
SANC South African National Collection of Insects, Pretoria, South Africa
MZB Museo de Zoologia, Barcelona, Spain
MRAC Musée Royal de l’Afrique Centrale, Tervuren, Belgium
ZMB Museum für Naturkunde an der Humboldt-Universität, Berlin, Germany
MNHN Museum National d’Histoire Naturelle, Paris, France
DMNH Ditsong Museum of Natural History (formerly Transvaal Museum of Natural History), Pretoria, South Africa
urn:lsid:zoobank.org:act:23AFF0D1-4D05-4E15-8B91-79F32AE4A9D0
http://species-id.net/wiki/Afromantispa
Both
Widespread throughout Africa. A few species have been collected in the Palaearctic Region sharing borders with Africa such as Spain and the Arabian Peninsula.
Prothorax granulated; granules dark (Fig. 3e). Antennae with distinct yellowish white band in the apical third (Fig. 4a). Even species with pale antennae have a few darker flagellomeres two-thirds apically from the base of the antennae and on the apex to form a yellowish-white band. The crossvein between Cua and Cup+Aa in hind wing attenuated or absent (Fig. 2b). These characteristics combined are unique to this genus and can be used to distinguish Afromantispa species from all other genera.
Wing variation in the Afrotropical Mantispinae. a Pseudoclimaciella apicipennis (Kolbe) b Afromantispa sp. c Sagittalata sp. d Cercomantispa sp. e Nampista ragazziana (Navás) f Rectinerva braconidiformis Handschin.
Head: Antennae moniliform; colour variable but all with conspicuous yellowish-white band in apical third; scape and pedicel yellow. Posterior vertex concave except for slight convex elevation directly posterior to and between antennal bases; median tubercle projection at posterior margin of vertex, vertex not visible in lateral view. Compound eyes large, each eye slightly broader medially at epistomal suture. Labrum circular. Mandible with dark apices; inner margins dark.
Thorax: Pronotum narrow and elongated; prothorax longer than pterothorax; granulated; granules dark; pronotum transversely slightly wrinkled or rugulose; setae present. Maculae slightly raised and inconspicuous; not pigmented in lighter coloured species, pigmented and shiny in darker species. Prozona slightly broader than base. Meso- and metathorax of similar size and distinctly separated by a deep cleft.
Wings (Fig. 2b): Wings always hyaline, lacking pigment except for the pterostigma. Pterostigma slightly concave in dorsal view; semi-circular and truncate appearance; pterostigma of most species with reddish appearance. Radial cell 1 and 2 of similar size with radial cell 3 smaller and narrower; a single crossvein from third radial cell to anterior margin (C). Hind wings: Crossvein between Cua and Cup+Aa attenuated or absent; Cua with sharp angle to and from attenuated crossvein to form inverted triangle.
Legs: Median line on the anterior surface of the forecoxae never continuous from thorax to femur (Fig. 4a). Mid- and hind legs differ considerably between species. Fore tarsal claw reduced to a single claw lacking arolium; Mid- and hind pretarsal claws pectinate (5 -6 teeth) with the middle tooth elongated giving the claw a sharp triangular appearance; arolium present on mid and hind tarsi.
Abdomen/Genitalia: Males with ectoprocts slightly enlarged (Fig. 4j). Pseudopenis visible in lateral and dorsal view. EEG present. No morphological significance regarding the female genitalia.
The new genus name is a combination of Afro- and Mantispa, which emphasises the African distribution of this Mantispa-like taxon.
Besides the type species, 18 confirmed and 7 unconfirmed species names will be added in the future. These numbers are, however, certain to change. Synonyms need to be identified and new species described. A subsequent full revision of Afromantispa is currently in progress. The genus for the time being will therefore be based on the type species only.
http://species-id.net/wiki/Mantispa
The genus was described by
Palaearctic genus with some species records from the Afrotropical Region. These countries include Morocco and countries bordering the Arabian Peninsula.
Ectoprocts sligtly swollen/enlarged. Pterostigma elongated and dark red. Prothorax with setae and slightly transversely rugulose (Fig. 3g). Fore coxae lack continuous median line on anterior surface (Fig. 4b).
Pronotum variation in the Afrotropical Mantispinae a Pseudoclimaciella apicipennis (Kolbe) b Sagittalata hilaris (Navás) c Cercomantispa sp. d Rectinerva braconidiformis Handschin e Afromantispa sp. f Nampista ragazziana (Navás)g Mantispa mandarina Navás.
Apomorphic characters of the Afrotropical Mantispinae a Afromantispa sp., antennal band and discontinuous fore-coxal line b Sagittalata sp., continuous line on fore-coxae c Rectinerva braconidiformis Handschin, Elongated antennae and light pleura d Nampista ragazziana (Navás), lamellate flagella e Pseudoclimaciella apicipennis (Kolbe), black maculae and pronotal markings f Male Cercomantispa perparva (Esben-Peterson), elongated ectoprocts and pseudopenis gMale Sagittalata sp., slightly enlarged/swollen ectoprocts h Cercomantispa sp., anterior scape and pedicel always yellow i Male Cercomantispa perparva (Esben-Peterson), inner femoral surface.
http://species-id.net/wiki/Sagittalata
austroafrica (Poivre)
Perlamantispa austroafrica Poivre, 1984b: 642. syn. n.
bequaerti (Navás)
Mantispilla bequaerti Navás, 1932: 279. Synonymized with Perlamantispa bequaerti (Navás) by Handschin, 1960a: 197.
Mantispilla bequaerti var. decolor Navás, 1932: 280. Synonymized with Perlamantispa bequaerti (Navás) by Handschin, 1960a: 197.
Mantispilla kibumbana Navás, 1936c: 355.. Synonymized with Perlamantispa bequaerti (Navás) by Handschin, 1960a: 197.
Perlamantispa bequaerti (Navás). As a new combination by
dorsalis (Erichson)
Mantispa dorsalis Erichson, 1839: 168.
Mantispilla hemichroa Navás, 1931: 129.
Mantispilla hypophoea Navás, 1932: 279.
Perlamantispa dorsalis (Erichson). As a new combination by
girardi Poivre
Perlamantispa girardi Poivre, 1982a: 194.syn. n.
nubila (Stitz)
Mantispilla nubila Stitz, 1913: 15.
Mantispa nubila (Stitz, 1913) syn. n.
perla (Pallas)
Mantis perla Pallas, 1772: 14.
Mantispa christiana Charpentier, 1825: 93. Synonymized with Mantispa perla by
Mantispa flaveola Erichson, 1839: 168.
Mantispa victorii Guérin-Méneville, 1844: 391. Synonymized with Mantispa perla by
Mantispa perla var. brunnea Navás, 1906: 102.
Perlamantispa perla (Pallas, 1772). As a new combination by
pusilla (Pallas)
Mantis pusilla Pallas, 1772: 15
Mantis brevicornis De Geer, 1778: 620, pl. 46, fig. 9–10. Synonymized with Mantispa pusilla by
Perlamantispa pusilla (Pallas, 1772) As a new combination by
similata (Navás)
Mantispilla similata Navás, 1922: 396.
Perlamantispa similata (Navás, 1922). Listed as valid combination in
royi Poivre
Perlamantispa royi Poivre, 1982a: 191. syn. n.
tincta (Navás)
Mantispilla tincta Navás, 1929: 107
Perlamantispa tincta (Navás, 1929). As a new combination by
vassei (Navás)
Mantispa vassei Navás, 1909: 474.
Mantispa (Mantispilla) lineatifrons Enderlein, 1910: 346. Synonymized with Perlamantispa vassei by Handschin, 1960a: 193.
Mantispilla sankitana Navás, 1922: 395. Synonymized with Perlamantispa vassei by Handschin, 1960a: 193.
Mantispilla burgeoni Navás, 1923: 77., Probable synonym of Perlamantispa vassei according to Handschin, 1960a: 193.
Perlamantispa vassei (Navás, 1909) As a new combination by Handschin, 1960a: 193. syn. n.
Handschin seemed to confuse the female Cercomantispa specimens and the genus he described as Sagittalata. In his revision (1960a) he mentioned that types of Sagittalata tristis and Sagittalata tristella are both female and that he is certain they are Sagittalata species. He mentions that the wing venation and prothorax corresponds with Sagittalata. However, the complete fusion between the Cua and Cup+Aa veins in the hind wing to form a rectangle (Fig. 2d) occurs in Sagittalata tristis (= Cercomantispa tristis) and Sagittalata tristella (= Cercomantispa tristella) corresponds with Cercomantispa and not with the type species Sagittalata hilaris or any of the other species (Sagittalata lugubris
Widespread in the Afrotropical Region. Also occur in the Palaearctic and Oriental Regions
An Afrotropical genus with four species currently known from the Palaearctic Region. Ectoprocts of males sligtly swollen (Fig. 4g), pseudopenis visible in dorsal view. Pterostigma elongated and dark red or black. Prothorax transversly rugulose; lacks setae (Fig. 3b). Fore coxae with continuous median line on anterior surface (Fig. 4b).
Head: Antennae moniliform. Flagellum dark, may end in two or three yellow flagellomeres. Anterior scape and pedicel either yellow or black; vertex flat, not visible in lateral view; frons and mouthparts vary in colour; eye margin yellow in dark species and black/dark brown in light species.
Thorax: Maculae inconspicuous, never pigmented in a different colour from the surrounding pronotum; pronotum lacks setae, transversely rugulose; prothorax longer than pterothorax.
Wings (Fig. 2c): Wings usually hyaline, may be partly or completely pigmented, pterostigma elongated and robust, always reddish or black; crossvein between radial cells 1 and 2 perpendicular to R; a single crossvein from third radial cell to anterior margin (C); Hind wings: Crossvein between Cua and Cup+Aa attenuated, rarely absent; Cua with sharp angle to and from attenuated crossvein to form inverted triangle shape.
Legs: Raptorial legs differ in colour, coxal sulcus conspicuous, surrounding patterns never visible on sulcus; continuous line on anterior surface of fore coxae; fore tarsal claw reduced to a single claw lacking an arolium. Mid- and hind pretarsal claws pectinate (5–6 teeth); median tooth longer than surrounding teeth; pointed appearance; arolium present on mid and hind tarsi.
Abdomen/Genitalia: EEG present. Ectoprocts of male slightly swollen; slightly smaller than ectoprocts of members of Afromantispa and Mantispa; pseudopenis visible in dorsal view.
Afromantispa, Mantispa and Sagittalata seem to form a group with several similar aspects regarding their morphology. All three genera seem to have similar genitalic structures. In addition to the genitalia, the general wing venation is extremely similar with only the pterostigma of Afromantispa slightly different with a reddish, roundish and truncate appearance. In the hind wing, the inverted “V” shape made by the Cua when descending towards the attenuated or absent crossvein extending to Cup+Aa and again ascending after the crossvein is prominent and easily identified in this group (Figs 2b, c). The median coxal line is not a strong autapomorphy since some of the Mantispa specimens studied had a discontinuous line on the anterior coxa, but the geographic distribution of the genera does support separate genera. A decision to keep the genera separate has consequently been made, thereby ensuring that relevant morphological information is not lost before a conclusive result is achieved. Of significant importance is the presence of the EEG that manifests in this group only.
http://species-id.net/wiki/Cercomantispa
Cercomantispa is probably the most complex of all the Afrotropical genera. This is not only because of the sexual dimorphism and the general small size, but because of the confusion in the literature and physical state of the type specimen. Males are easily recognised by their elongated ectoprocts, but females do not have conspicuous genitalia and differ morphologically from the males in terms of colour and patterns. Females were therefore described as different species from the males and placed in several other genera. In addition to the confusion between the female Cercomantispa and Sagittalata there is a lack of clarity regarding the generic boundaries of Cercomantispa, Necyla and Orientispa. Necyla and Cercomantispa could be synonyms (
Widespread throughout the Afrotropical Region
The flagella of the antennae are very dark with the anterior surface of the scape and pedicel always yellow, even in the very dark species (Figs 3c, 4h). Pronotum smooth, lacks setae (Fig. 3c). The rectangular cell formed by the fusion of A2 and Cup in the hind wing is very diagnostic and no other mantispid genus has such a structure (Fig. 2d). All wing cells lacks pigment except for the pterostigma. The mid- and hind legs yellowish-brown to yellow covered in black setae. The males have elongated ectoprocts as well as an elongated pseudopenis, both longer than the 8th tergite, and bent ventrally (Fig. 4f).
Head: Antennae long, moniliform; flagellomeres black; the apical three flagellomeres might be lighter in colour; anterior scape and pedicel always yellow, even in very dark species; vertex medially convex, clearly visible in lateral view; vertex bordered by conspicuous yellow eye margin; frons with longitudinal dark median line, not visible in very dark species (e.g. C. tristis); mandibles usually yellow or lighter than coloration of frons; black tipped with black inner margin
Thorax: Pronotum smooth, lacking setae; maculae conspicuous, not always pigmented; similar in length or slightly longer than pterothorax; in most species a dark median line forms two circular dorso-lateral yellow markings on prozona; prozona much wider than metazona, metazona narrow;
Wings (Fig. 2d): Wing venation comparatively simple; always lacks pigmented cells; pterostigma elongated, narrow; dark brown; a single crossvein from third radial cell to anterior margin (C); a single radial sector vein extending posteriorly from each radial cell 1, 2 and 3 respectively; four or five crossveins reaching posterior wing margin from Mp in hind wing; a rectangle shaped cell formed by the fusion of Aa+Cup and Cua.
Legs: Raptorial forelegs yellow; fore tarsal claw reduced to a single claw lacking an arolium; inner femoral surface dark in females; often only distal half dark in males (Fig. 4i), outer femur of both sexes with a narrow, brown latero-dorsal line; middle and hind legs yellow-brown to yellow covered in setae; most species with a narrow dark longitudinal line along femur and tibia; pretarsal claws pectinate; middle tooth projecting beyond the others giving the claw a sharp appearance.
Abdomen/Genitalia: Male: Ectoprocts elongated, longer than tergite 8; slightly swollen apically; apices bent downwards; pseudopenis elongated and bent ventrally; visible between ectoprocts in ventral and dorsal view; EEG absent
http://species-id.net/wiki/Rectinerva
Rectinerva is a monotypic genus. Only two female specimens have been collected, one being the holotype collected in 1933 (MRAC) the other collected in 1976 (MNHN) and described by
Katanga (Democratic Republic of Congo) and Cameroon
Light red-brown. The antennal flagellae long slender and black, proximal half covered in prominent thick black setae (Fig. 4c). The anepimeron, anepisternum, katepimeron as well as katepisternum much lighter than the rest of the body, almost white (Fig. 4c). Three radial sector veins extending posteriad from radial cells 1–3. The wing colouration is unique among mantispids from the region (Fig. 2f).
Head: Head capsule light reddish-brown except for black tipped mandibles, vertex and pedicels. Scape light reddish-brown and pedicel black; flagellum long slender, black, proximal half covered in prominent thick black setae. Vertex medially raised in convex shape, visible in lateral view; raised vertex from antennal bases to posterior margin; black. Inner mandible margins lack black pigment. Eyes small; black to dark grey.
Thorax: Pronotum light reddish-brown; smooth; covered in light inconspicuous setae. Maculae inconspicuous; same colour as pronotum. Pterothorax uniform light red-brown; sutures inconspicuous and smooth; lacks deep clefts. Anepimeron, anepisternum, katepimeron as well as katepisternum lighter, almost white, conspicuous against the uniform light red-brown of the pteronota.
Wings (Fig. 2f): Both wings pigmented in banded formation with colours ranging from dark-brown to light red-brown. Pterostigma black and slightly concave in dorsal view. A single vein from radial cell 3 to the anterior wing margin (C). Radial cells broad, Radial cell 1 being the largest, radial cell 2 somewhat smaller and rectangular in shape with the radial cell 3 being the smallest. Lacks the hexagonal radial cell 4 found in other Afrotropical Mantispidae genera. Three radial sector veins extending in posterior direction from radial cells 1–3. Hind wing: Cua parallel with A2+Cup. Cua - A2+Cup crossvein not attenuated and close to posterior margin.
Legs: Raptorial legs uniformly light reddish-brown; coxal sulcus same colour and inconspicuous; tibia-tarsal joint and fist tarsal segment black; fore tarsal claw reduced to a single claw lacking an arolium. Mid- and hind pretarsal claws pectinate (5–6 teeth); median tooth longer than surrounding teeth; pointed; arolium present on mid and hind tarsi. The rest of the mid leg light red-brown. Femur of hind leg light red-brown as well as the proximal third of the tibia, distal two-thirds and tarsal segments black; pre-tarsus light red-brown with some dark brown at pretarsal-claw bases.
Genitalia: At the time of this study the macerated female genitalia (prepared by Ragner Hall in 1983) were missing.
Cercomantispa and Rectinerva form a group because of synapomorphies. The male of Rectinerva is not yet known, so the genitalia cannot be used as a morphological character and sexual dimorphism cannot be excluded. However, the antennae of both genera are quite long compared to other Afrotropical taxa, and the flagellomeres are black with the anterior surface of the scape and pedicel yellow (Fig. 4h). Furthermore, the pronota of members of both genera are very similar in structure, smooth and narrow posterior to the maculae (Figs 3c, d). The rectangular shape of the cell formed by the fusion of Aa+Cup and Cua is present in only Cercomantispa. However, the second cell between the Cup+Aa and the posterior wing margin of Rectinerva is quite similar in shape but lacks the fusion between Aa+Cup and Cua. In addition to these, the comparatively simple wing venation and reduced number of radial sector cross veins in both genera seems to confirm the close relationship (Figs 2d, f).
http://species-id.net/wiki/Nampista
Predominantly a Palaearctic genus In the Afrotropics the genus is found only in the countries bordering the Arabian Peninsula where it is represented by three species (
The only Afrotropical genus close to Nampista is Pseudoclimaciella. It can easily be distinguished from Pseudoclimaciella by the following characteristics: Flagellomeres asymmetrically lamellate (Fig. 4d); deeply incised ventrally. Prothorax shorter than pterothorax (Fig. 3f). The basal half of the forewings always pigmented; the majority of the basal half of the hind wings clear (Fig. 2e).
Revised by
http://species-id.net/wiki/Pseudoclimaciella
Pseudoclimaciella can easily be recognised and confusion with other genera is unlikely. Tuberonotha
The genus is probably confined to woodland and forests in the Eastern Tropical Corridor. All the locality data indicate a C-shaped distribution extending from the tropical areas in western Africa such as Sierra Leone through central Africa extending down into South Africa east of the plateau, and into Madagascar.
All members of Pseudoclimaciella are rusty reddish to brown. Basal cells of forewings always pigmented; basal cells of the hind wing always clear (Fig. 2a). Two or three veins originate from radial cell 3 and terminate at anterior wing margin (Fig. 2a); radial cells 1–3 elongated and narrow (Fig. 2a). Two yellow bands extend from pronotal maculae to ventral basal margin forming an inverted “V” shape on the dorsal side (Figs 3a, 4e). Hind tibia rusty reddish at joints and yellow in middle.
Head: Antennae moniliform; most flagellomeres dark in colour; twice as broad as long, one to three bright yellow flagellomeres apically; scape and pedicel rusty reddish. Vertex convex, not visible in lateral view; slightly raised posteriorly; vertex always yellow or rusty reddish; epistomal suture black except in Pseudoclimaciella elisabethae (
Thorax: Pronotal maculae conspicuous, from the pronotal maculae two yellow bands with black margins extend to ventral basal margin forming an inverted “V” shape on the dorsal side; prescutum with yellow margin forming another inverted “V” shape; prothorax longer than pterothorax, prozona relatively smooth, anterior margin of prothorax black, might be discontinuous medially; metazona with transversely rugulose, lacks setae. Postnota 2 and 3 often yellow as well as posterior abdominal margins providing a vespid wasp-like appearance.
Wings (Fig. 2a): Wing venation complex. Pterostigma elongated, narrow, rusty-reddish. Radial cells 1–3 elongated and narrow; of similar length; at least 8 radial sector veins extending in posterior direction from radial cells 1–3. Two veins from radial cell 3 extending towards anterior margin (C), (very few specimens with three such veins but only in one wing so some individual variation present). All species except for above mentioned 4 with apical pigmentation in both wings. Hind wings: Crossvein between Cua and Cup+Aa prominent; Cua almost parallel with basal half of Cup+Aa; inverted triangle formed by Cua shallow.
Legs: Raptorial femur, tibia and tarsi uniformly red; lacks patterns on inner femoral surface; suture in fore coxa prominent and paler; single fore tarsal claw claw lacking an arolium. Mid- and hind pretarsal claws pectinate (5–6 teeth); teeth of similar size; spoon-shaped appearance; arolium present on mid- and hind-tarsi. Proximal joint of hind tibia dark rusty-red, distal joint lighter rusty red; proximad third of tibia same dark rusty red as joint, two distal thirds of tibia yellow.
Abdomen/Genitalia: Males with ectoprocts inconspicuous to slightly enlarged. Pseudopenis visible in lateral and dorsal view; continuous variation in both ectoproct size and pseudopenis size. EEG absent. No morphological significance regarding the female genitalia.
Pseudoclimaciella and Nampista are quite similar in many aspects. Species of both genera are generally quite large, reddish in appearance with wings often pigmented with a similar colour. From an Afrotropical perspective, they may form a group, but with other genera such as Tuberonotha from the Oriental Region that is in most aspects identical to Pseudoclimaciella, it is likely that Nampista is not the most closely related taxon to any of the Afrotropical genera.
| 1a | Flagellomeres asymmetrical and lamellate (Fig. 4d) | Nampista |
| – | Flagellomeres symmetrical and moniliform (Fig. 4a, b, c, e) | 2 |
| 2a | Crossvein between A2 and Cup in hind wing attenuated or absent (Fig. 2b, c, f) | 3 |
| – | A2 and Cup in hind wing fused (Fig. 2d) or crossvein between A2 and Cup in hind wing prominent (Fig. 2a) | 4 |
| 3a | Prothorax granulated (Fig. 3e), white band present in distal third of antennae (Fig. 4a) | Afromantispa |
| – | Prothorax either smooth (Fig. 3c, d), rugulose (Fig. 3a, f) or covered in setae but lack granules (Fig. 3g) and white band in distal third of the antenna absent | 7 |
| 4a | Prothorax smooth | 5 |
| – | Prothorax rugulose | Pseudoclimaciella |
| 5a | Prothorax smooth and rectangle cell formed by fusion of Aa+Cup and Cua in hind wing (Fig. 2d) | Cercomantispa |
| – | Prothorax smooth (Fig. 3d, 4c) but no fusion between A2 and Cup in hind wing (Fig. 2f) | Rectinerva (in part) |
| 7a | In the hind wing Cup forms a sharp angle when bending towards A2 (Fig. 2c) | 8 |
| – | In the hind wing Cup do not form a sharp angle towards A2 (Fig. 2f) | Rectinerva (in part) |
| 8a | Median line on anterior surface of fore coxae present (Fig. 4b), prothorax lacks setae (Fig. 3b), Afrotropical distribution | Sagittalata |
| – | Median line absent on anterior surface of fore coxae, setae present on prothorax (Fig. 3g), Palaearctic distribution | Mantispa |
The taxonomic ultimate aim is to comprehensively revise the family. The present study will serve as a basis for future taxonomic research on the lower taxa of Afrotropical Mantispidae. The complexity of the Mantispidae has consequently been arranged into smaller monophyletic groups each with at least one autapomorphy. Future studies should therefore focus on revising each genus thereby avoiding an unnecessary increase in the already confusing list of invalid names in the region.
Genitalic structures may prove to be important to elucidate the species and should be thoroughly investigated. Given the diversity and complexity of the Afrotropical Mantispidae, molecular and behavioural studies may delimit what traditional morphological tools cannot resolve.
Fundamental knowledge regarding the basic biology of Mantispidae is sparse and requires attention. Studies that aim to investigate the oviposition preference of females, larval cues used for locating food sources and host specificity should be priority. This might allow for easier collection of specimens as well as raising other important questions regarding their evolution, behaviour and ecology.
We would like to extend our gratitude to the University of Pretoria and the Scarab Research Group (University of Pretoria) for funding the project. Thanks to the various museum curators for their assistance. Personally, I would also like to thank Christian Pirk, Catherine Sole, Cornel du Toit and Werner Strümpher for their support and patience as well as the staff of the ZMB for providing me with all the images which without the study could not have been possible. Lastly I thank the reviewers for their helpful comments.