(C) 2012 Donald R. Davis. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The indigenous North American micropterigid genus Epimartyria Walsingham, 1898 is revised. Three species are recognized, including Epimartyria auricrinella Walsingham, 1898 which occurs widely over much of the northeastern United States and Canada, a new species, Epimartyria bimaculella Davis & Landry from northwestern United States and Canada, and Epimartyria pardella (Walsingham, 1880) from northern California to northern Oregon. The larva of Epimartyria auricrinella is described in detail, supplemented with illustrations of the external structure of the larval integument. The larval plastron is described and illustrated for Epimartyria, and this is compared with the plastrons of Neomicropteryx Issiki, 1931 and Micropterix Hübner, 1825. COI barcode sequences show that the three species are genetically distinct, congruent with morphological differences. Marked haplotype divergence within some Epimartyria auricrinella populations appears to be unrelated to morphology, geography or phenology.
Distribution, DNA barcodes, genital morphology, larval morphology, plastron
The archaic moth family Micropterigidae constitutes the only member of the suborder Zeugloptera and is one of three extant families whose adults are partially characterized as possessing articulated mandibles and in having never developed a coilable proboscis (
Micropterigidae typically occur in humid habitats where their larvae frequently feed on foliose liverworts or possibly on fungi within rotten logs or soil (
Major portions of the larval integument of Epimartyria auricrinella have been found to be densely covered with minute, irregularly shaped micropapillae (
Five monophyletic lineages have been determined within the Micropterigidae based on analysis using the 16S rRNA gene (
Specimens examined in this study are deposited in the following institutions:
BMNH The Natural History Museum (formerly the British Museum (Natural History), London, United Kingdom.
BIO Biodiversity Institute of Ontario, University of Guelph, Ontario, Canada.
CZC Collection of Christof Zeller-Lukashort, Thalgau, Austria.
CNC Canadian National Collection of Insects, Arachnids and Nematodes, Agriculture and Agri-Food Canada, Ottawa, Ontario, Canada.
ONPS Olympic National Park Service Collection, Port Angeles, Washington, USA.
UCB Essig Museum of Entomology, University of California, Berkeley, California, USA.
USNM Collections of the former United States National Museum, now deposited in the National Museum of Natural History, Smithsonian Institution, Washington, D.C., USA.
WSDAC Washington State Department of Agriculture Collection, Olympia, Washington, USA.
Methods Specimen preparationGenitalic dissections were cleared by heating in hot 10% KOH for ~ 30 minutes, and subsequently cleaned and stained with either 2% chlorazol black E or mercurochrome solutions. All genitalic illustrations were drawn from dissections temporarily stored in glycerine, which were later permanently embedded in Canada balsam or Euparal. Genitalic terminology follows
DNA barcodes were produced at the Canadian Centre for DNA barcoding at the Biodiversity Institute of Ontario, University of Guelph following standard protocols (
http://species-id.net/wiki/Epimartyria
Micropteryx pardella Walsingham, by original designation.
Epimartyria appears closely allied to the Asian genus Paramartyria as suggested by the similar elongate process arising from the inner base of the male valvae (Fig. 78) and by similar larval chaetotaxy (
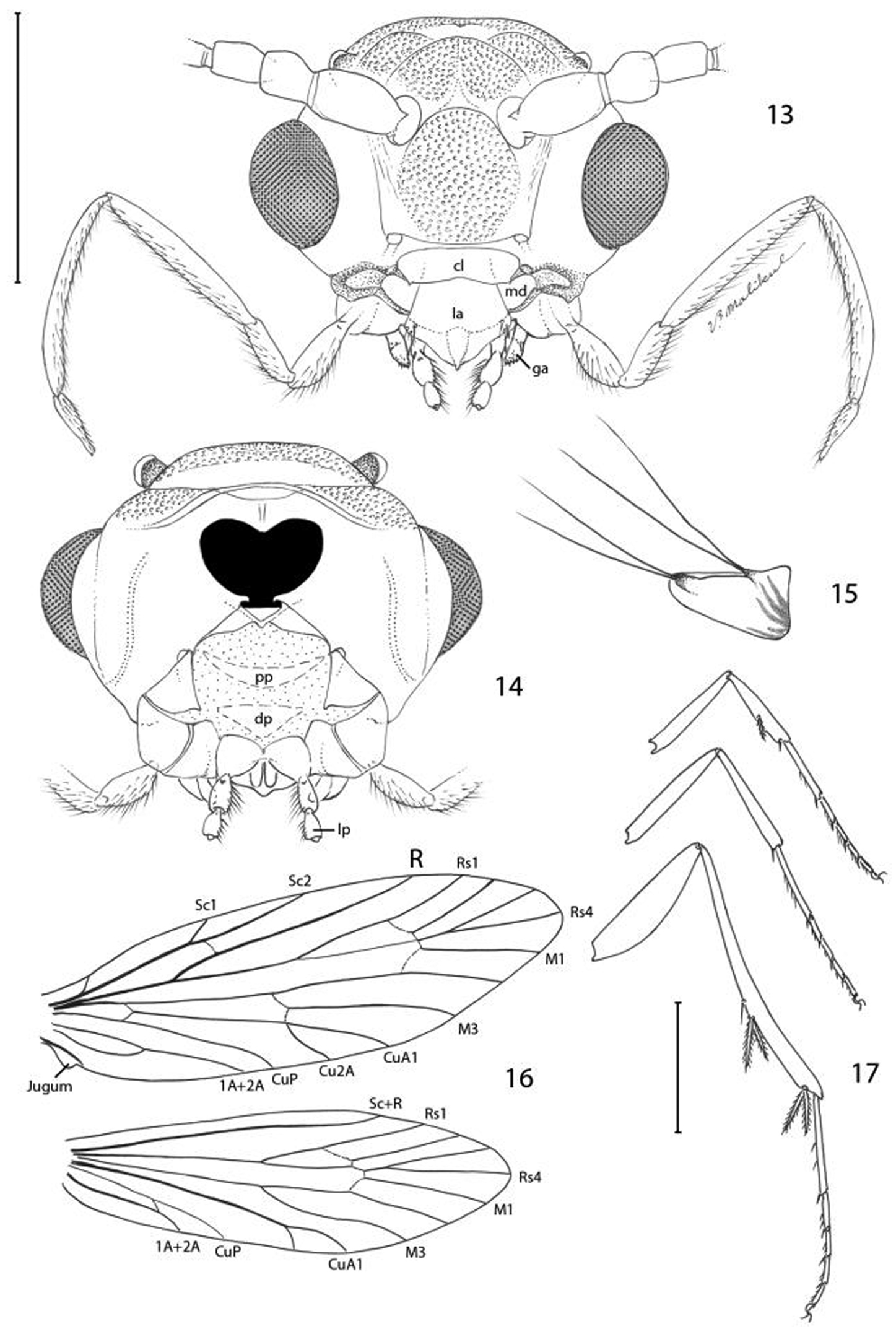
Head (Figs 13–15): Vestiture entirely hairy, scales erect and piliform with acute apices. Antenna (Figs 18–20) 0.75–0.9× length of forewing, slightly longer in male; pedicel enlarged, ~ 1.5× length of first flagellomere; flagellum moniliform, with 46–58 flagellomeres in male, 38–47 in female; flagellomeres mostly sparsely covered with long, piliform scales which exceed the length of their supporting flagellomere; basal 2–3 flagellomeres in male and 5–7 in female covered dorsally with moderately broad scales; a pair of large ascoid sensilla, opposite one another, with ~ 11–16 elongate, curved, sensory branches (Fig. 18) on each flagellomere; a single irregularly shaped and often bilobed multiporus sensillum placodeum (
Thorax: Scales of mesonotum broad, appressed. Metanotum mostly naked except for a few long, piliform scales. Tegulae rather sparsely covered with long piliform scales. Forewing length: 4.2–5.5 mm; forewing (Fig. 16) with humeral vein present; Sc deeply bifurcate; R simple; Sc-R crossvein present near fork of Sc; Rs with 4 veins; Rs3–4 fused to ~ basal 1/3; accessory cell present; M with 3 branches; 1A and 2A fused over distal half; 3A extending across base of moderately small jugal lobe. Wing scale morphology of the primitive, generally non-glossatan type (
Abdomen: Cuticle dark brown, sparsely covered with long, piliform scales. A pair of glands present, opening on sternum V in both sexes (
Male genitalia: Tergum X (uncus) ~ half the median ventral length of IX; apex deeply divided nearly half its length into two broad lobes. Sternum X (venter X) variously bilobed, with or without short lateral lobes. Segment IX a completely sclerotized ring, with dorsal median length ~ 1/6 of ventral length. Sternum IX (vinculum) a broad plate with subparallel lateral margins; anterior end as broad or broader than caudal end. Valva with a subacute to rounded apex; base of valva with a long digitate process from mesal surface. Medial plate (juxta) with a slender stalk-like base gradually expanding anteriorly to a small, flat, oval plate. Distal phallus divided into two slender branches ~ half the total length of phallus; shorter dorsal branch of phallus terminating in gonopore (phallotreme) with thickened radial folds; a pair of minute, acute spines present laterally near distal third of dorsal branch; apex of longer ventral branch densely covered with numerous minute flattened scutate processes with rounded apices directed basad; phallobase moderately inflated, as long as or slightly longer than divided branches.
Female genitalia: Abdominal segment IX a complete ring with mid dorsal length ~ 0.5–0.6× the mid ventral length. Segment X consisting of a pair of lateral, setose plates; cloaca ending terminally; X often telescoped into IX and VIII in repose. Apophyses absent. Genital chamber with thickened walls surrounding a variably shaped slerite; caudal end of sclerite furcate. Ductus spermatheca with a moderately enlarged, spindle-shaped reservoir (utriculus) located at varying distances along ductus. Corpus bursae gradually enlarging anteriorly, membranous, with four tridentaform signa equally spaced around middle of corpus bursae; enlarged bases of signa projecting externally beyond wall of corpus bursae, with spinose branches projecting internally.
For many years John Heath, formerly employed at the Experimental Research Station at Monks Wood in England, pursued research on the family Micropterigidae, resulting in about 20 papers on this group (
Because this is the first taxonomic revision of Epimartyria, there remain some gaps in our knowledge about their biology which cannot be answered with available material and evidence.
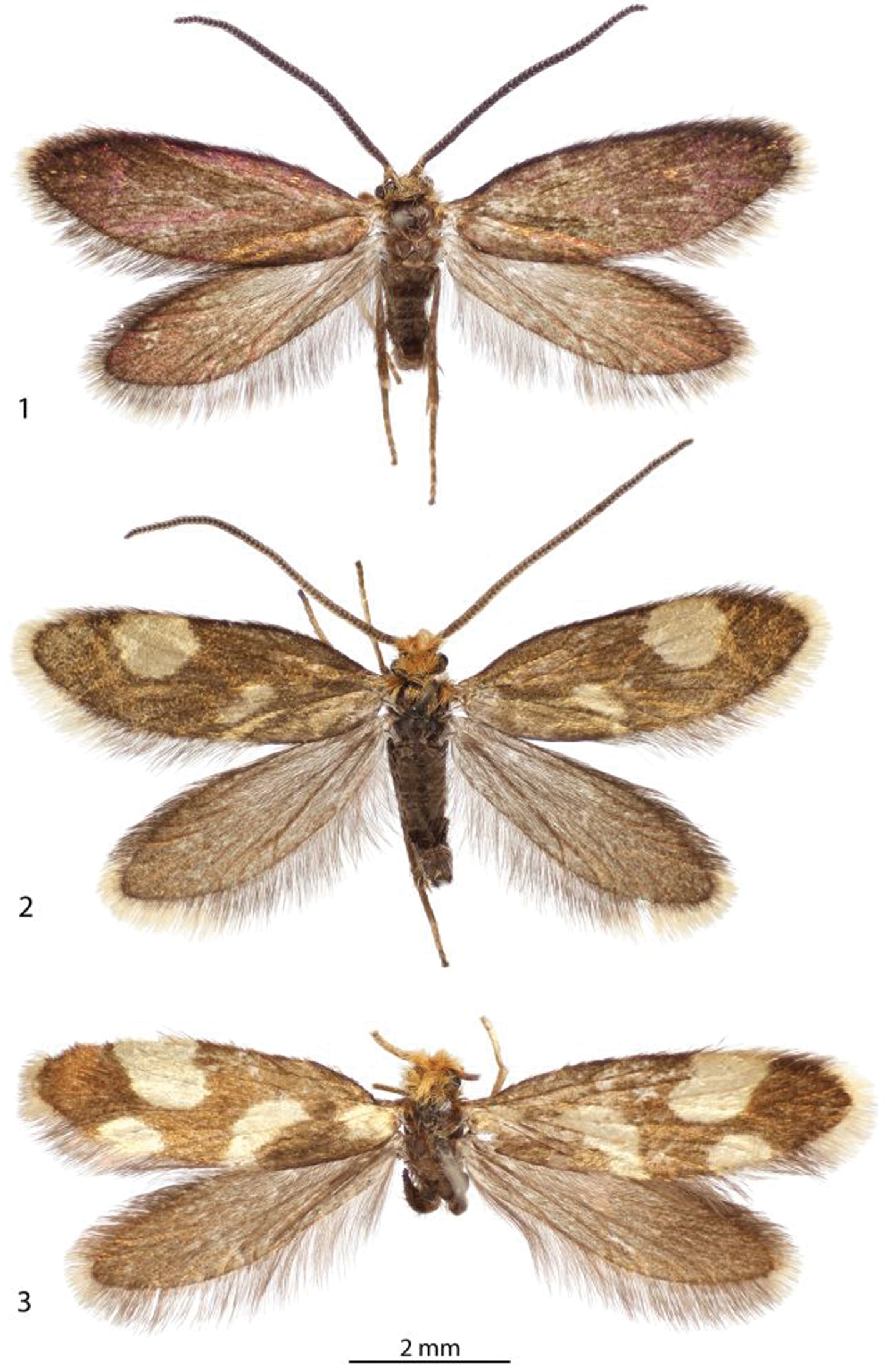
| 1 | Forewing without spots, uniformly dark fuscous with coppery to purplish luster (Fig. 1) | Epimartyria auricrinella |
| – | Forewingwith pale yellowish spots | 2 |
| 2 | Forewingwith 2 pale yellowish spots (Fig. 2); foretibia with epiphysis absent; caudal apex of male sternum X (gnathos) deeply divided, with apex of lobes acute (Fig. 82) | Epimartyria bimaculella |
| – | Forewingwith 4 pale yellowish spots (Fig. 3); foretibia with epiphysis present; caudal apex of male sternum X not deeply divided, with short, triangular, caudal lobes (Fig. 89) | Epimartyria pardella |
http://species-id.net/wiki/Epimartyria_auricrinella
Figs 1, 4–5, 10, 18–32, 33–59, 74–80Adult Epimartyria auricrinella are easily distinguished from those of the other members of Epimartyria in possessing uniformly dark fuscous forewings without the yellowish spots present in those species.
(Figs 1, 4–5). Head: Vestiture light orange brown. Antenna with vestiture of scape and pedicel concolorous with head; scales of flagellum dark brown to fuscous. Labial palpus cream.
Thorax: Dark fuscous with coppery to purplish luster. Tegula concolorous with head. Forewing dark fuscous with coppery or golden to purplish luster dorsally, less iridescent ventrally; fringe paler, more gray. Forewing length: 4.2–5.6 mm. Hindwing with scales over distal third nearly as broad, dark fuscous and iridescent as in forewing; scales gradually becoming more slender, more gray, and less iridescent over basal 2/3; fringe gray. Legs medium to dark brown dorsally, light brown ventrally and at apices of tarsomeres; epiphysis absent.
Abdomen: Piliform scales uniformly brown dorsally and ventrally. Paired glands of sternum 5 with muscle for opening glands originating on anterior edge of sternum 6 and inserted into each gland duct just inside aperature; gland reservoir slightly larger and more ovoid in female, but surrounding layer of secretory cells better developed and 2–3× thicker in female (
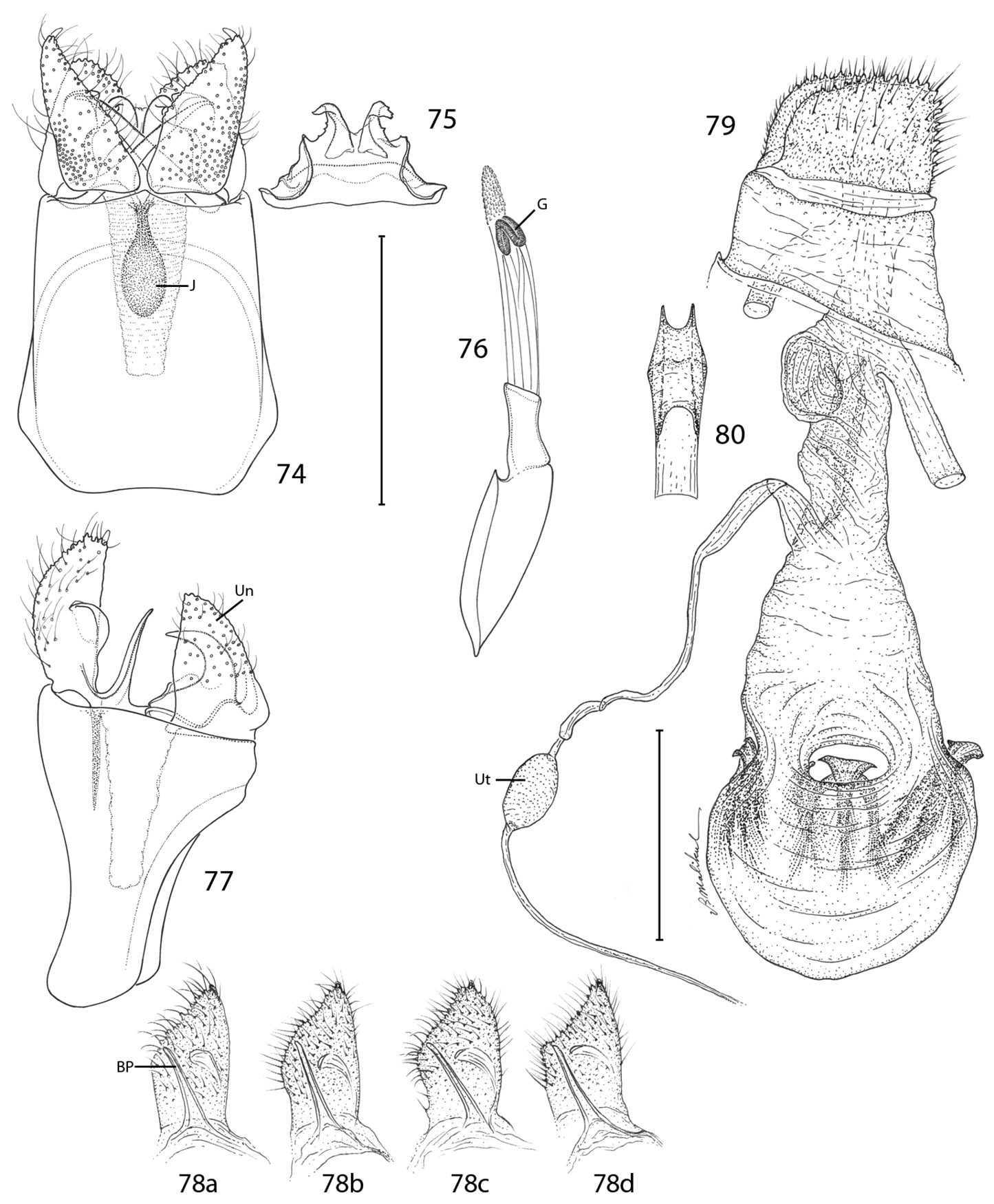
Male genitalia (Figs 74–78): Caudal lobes of tergum X broadly rounded. Caudal apex of sternum X deeply divided, with apex of lobes acute, recurved; a pair of short, lateral lobes present near base. Valvae moderately long, ventral length nearly half the maximum length of segment IX; apex subacute and bearing a short, slender, recurved spine; a short, triangular, rounded process arising midway from mesal surface; elongate basal process ~ 4/5 the length of valva; distal margin of valva variable within populations from slightly concave to convex (Figs 78a-d). Dorsal branch of phallus cylindrical and smooth.
Female genitalia (Figs 79–80): As described for genus. Caudal end of genital sclerite moderately furcate as in Epimartyria bimaculella; length of furcations ~ 0.2 that of relatively shorter, undivided base.
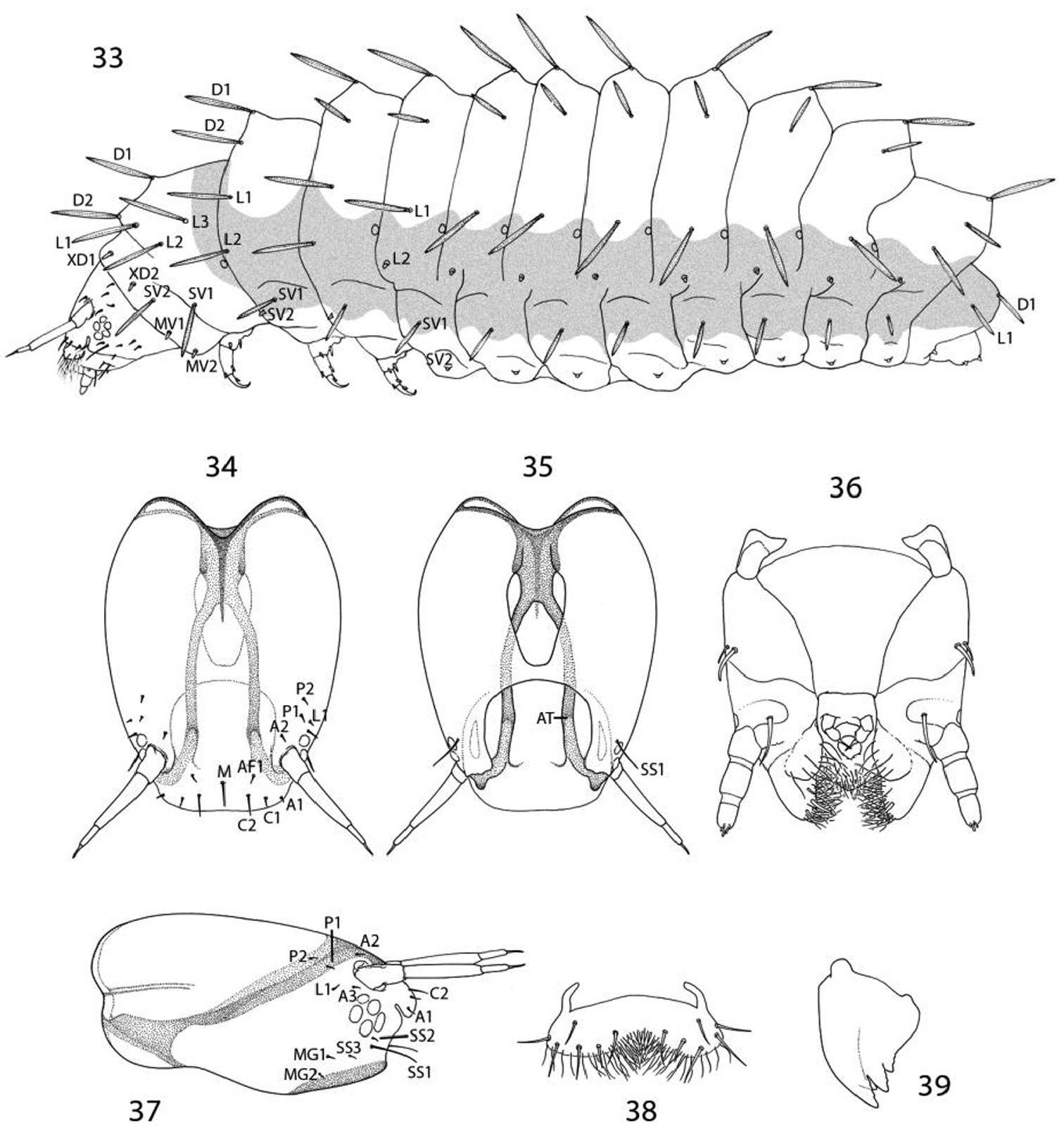
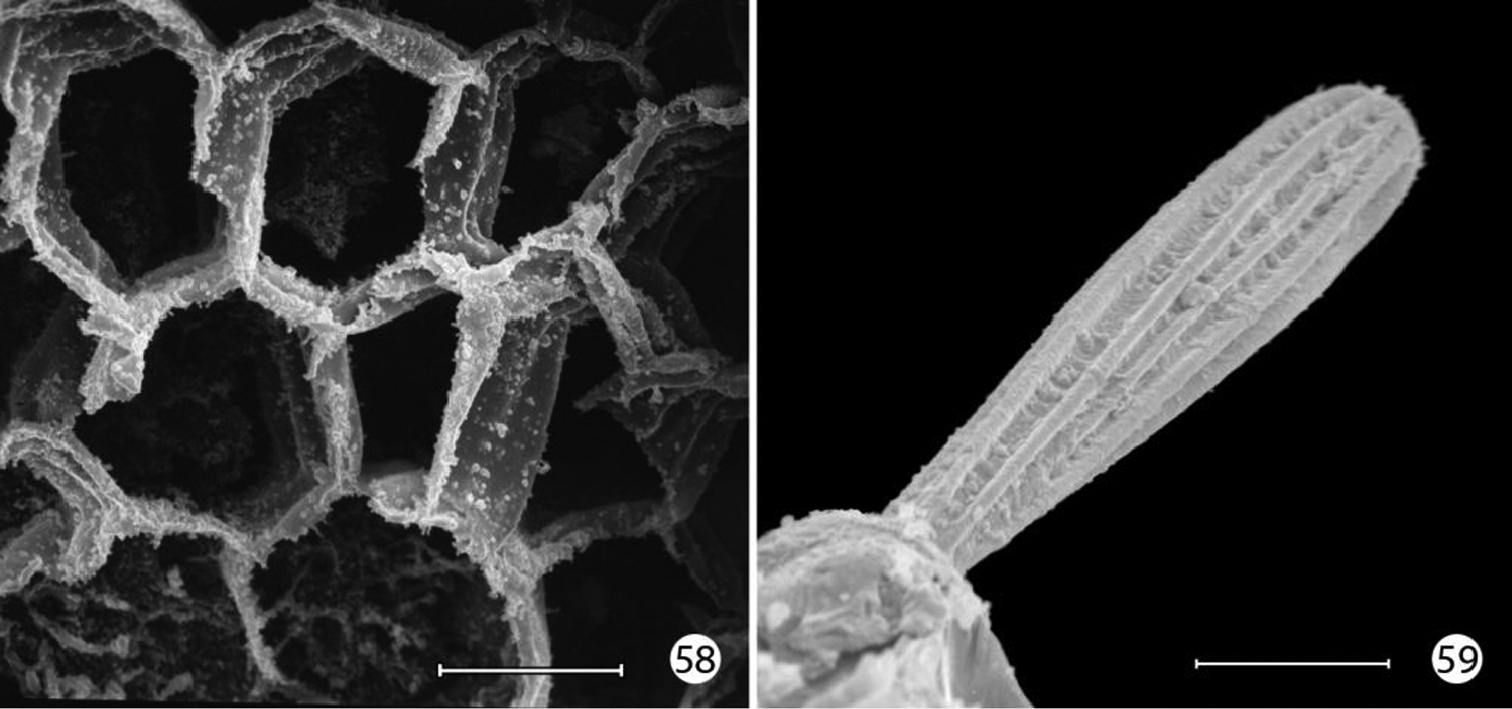
(33–59). Mature larva up to 5 mm in length. Body approximately hexagonal in cross section; color generally brown, lighter brown ventrally. Integument over dorsal half of body with a honeycomb-like surface of raised ridges (Figs 52, 58); integument of ventral half densely covered with micropapillae (Fig. 56) with an extensive plastron surface laterally (Fig. 52). Primary setae longitudinally ribbed, moderately slender, long, clavate.
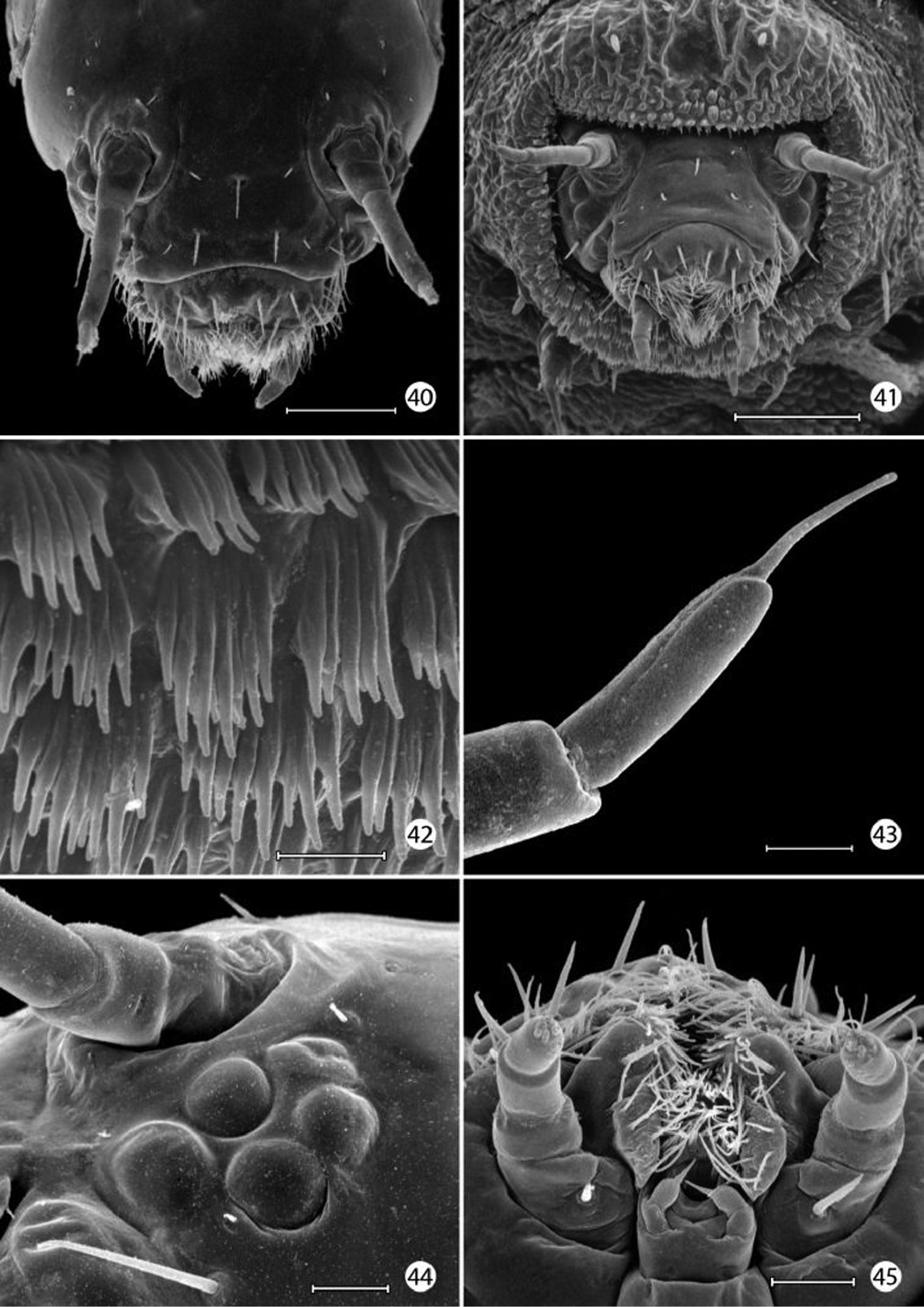
Head: Prognathous and capable of being retracted into prothorax. Antenna elongate, slender, 3- segmented, arising posterior of clypeal margin and dorsal to stemmata; second segment the longest, ~ 2× the length of basal segment; all antennal segments without sensory setae except for elongate terminal spinose seta. Five stemmata present, arranged in a tight circle. Adfrontal sutures vestigial, not extending to vertex; adfrontal ridges similarly undeveloped. Ecdysial lines externally indistinct. Tubular spinneret absent; external opening of labial salivary gland circular, relatively large, diameter ~ equal to length of second segment of labial palpus. Cranial setae reduced in length and number and concentrated over anterior third of head; stemmatal setae absent; a single medial (M) seta arising midway between antennae, without homology in other Lepidoptera but possibly homologous to campaniform sensillum in Trichoptera larva (
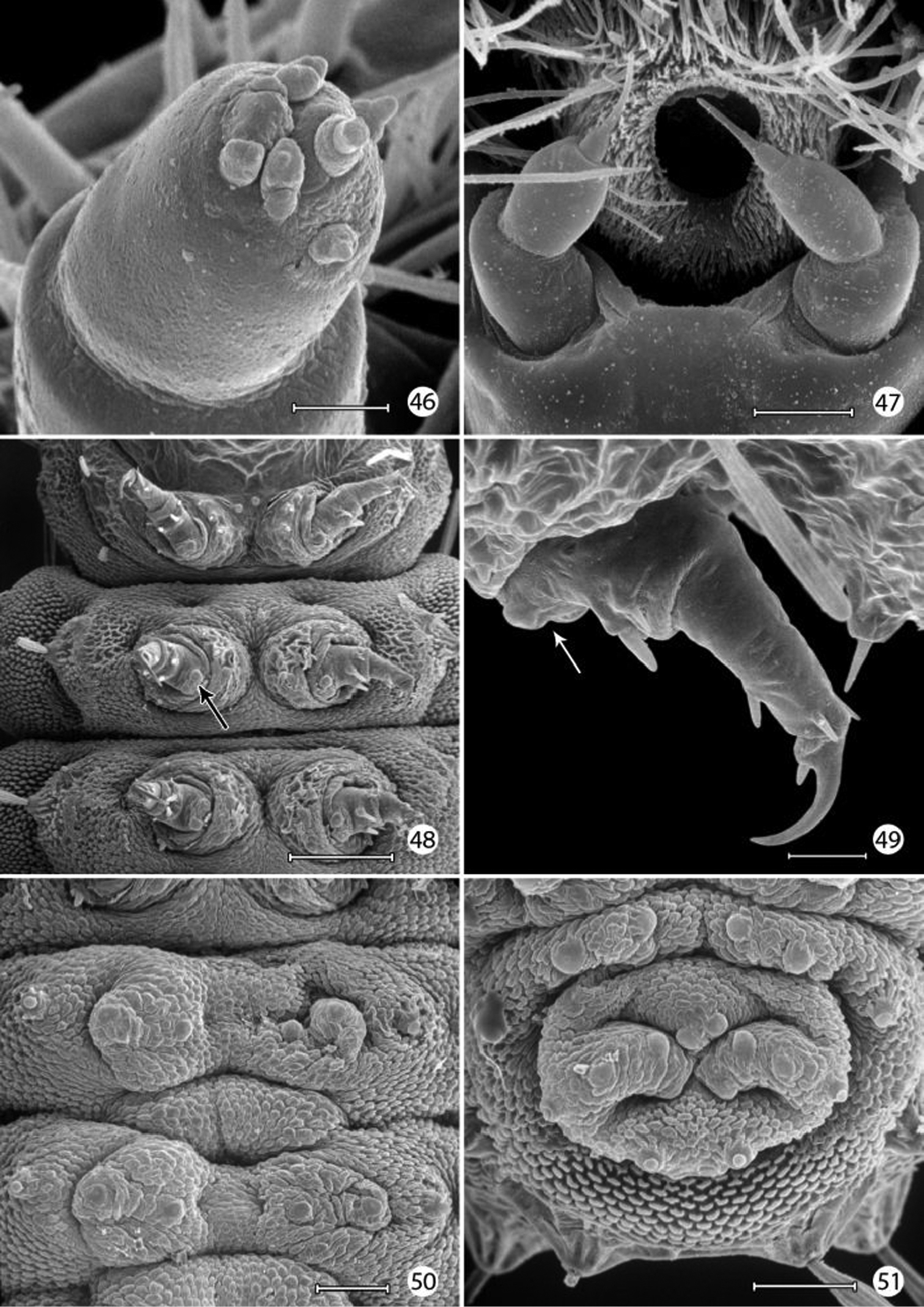
Thorax: Prothorax with 7 primary tactile setae and 4 peg-like microsetae, the latter located along anterior margin of prothorax near the head- prothoracic fold; XD1 and XD2 greatly reduced to peg-like microsetae along dorso-anterior margin of prothorax below D2; L1 posterior to XD1; L2 below L1 and anterior to spiracle. MV1 and MV2 short, peg-like, below SV2 and closer to anterior margin of prothorax; MV2 about 2 × length of MV1. Subdorsal setae absent on all body segments. Meso- and metathorax with 5 primary setae and one microseta (SV2); L1 and 2 well developed and equal in size. Legs with 3 well defined segments and large pretarsal segment; 4- segmented including reduced coxa; pretarsal claw curved, elongate, ~ 1/3 the length of remainder of leg; axial spine at base of claw well developed; femur and trochanter fused, as well as tibia and tarsus; coxa with a bilobed and possibly eversible tactile vesicle located posterior-mesally near base of femur (Figs 48–49);
Abdomen: Segments 1–8 with 4 primary setae and 2 peg-like (L2) to spherical microseta (SV2); segment 9 with only D1 and L1; segment 10 with 2 microsetae, possibly representing D1 and L1. Spiracles peripneustic, located anteriorly in intersegmental fold on segments 1–8; spiracle raised to form a small dome with walls subdivided into ~ 10–12 fimbriated bands. Abdominal segments 1- 8 with short, fleshy, nonmuscular prolegs with rounded apices (Fig. 50); crochets absent in all genera of Micropterigidae.
Hepaticophyta: Lepidoziaceae: Bazzania trilobata (L.) S. Gray.
Unknown.
(Figs 9–10). The species occurs in shaded locations, in wet swampy woods, boggy ditches, or creek sides where leafy (moss-like) liverworts, the probable larval host, grow. Such habitats can be periodically or seasonally flooded. Larvae possess a plastron which indicates the capacity to live for short periods in a subaquatic environment or, at least in a habitat that is water-saturated. Adults are diurnal and are best obtained by gently sweeping the understory or clumps of liverworts (
♂, USA: North Carolina, 1884, H. K. Morrison, Type No. 35325, slide BM 8947 (BMNH).
CANADA: NOVA SCOTIA: Baddeck: 1 ♂, 23 Jun 1936; 1 ♂, 30 Jun 1936, T.N. Freeman, specimens # CNCLEP00077282–00077283, slide MIC1825 (CNC); Parrsboro: 1 (abdomen missing), 12 Jul 1944, J. McDunnough, (CNC). ONTARIO: Ottawa: 4 ♂, 19 Jun 1906, C.H. Young, slide USNM 16615 (USNM, CNC); 5 ♂, 20 Jun 1906, C.H. Young, slide USNM 34372, specimens # CNCLEP00077266–00077268, CNC slide MIC1822 (CNC, USNM); 1 ♂, 27 Jun 1906, slide USNM 98008 (USNM); 1 ♂, 12 Jun 1946, G.S. Walley, specimen # CNCLEP00077269 (CNC). Black Lake, N of Burgess Township: 1 ♂, 22 Jun 1974; 2 ♂, 1 ♀, 14 Jun 1975, J.A. Downes, specimens # CNCLEP00077277–00077280 (CNC). Moosonee: 1 ♀, 18 Jul 1934, G.S. Walley, specimen # CNCLEP00077288 (CNC). Orillia: 3 ♂, 26 Jun 1926; 1 ♂, 3 ♀, 2 Jul 1926, C.H. Curran, specimens # CNCLEP00077276–00077270–00077276, CNC slide MIC1823 (CNC). Thunder Bay: 1 ♀, Jul 1945, H. S. Parish. QUEBEC: Havre-Saint-Pierre: 4 ex., 3–17 Jul 2010, malaise trap, C. Bélanger. Gaspé Peninsula: Mont Albert: 2 ♂, 1 ♀, 18 July 1940, A. E. Brower, slides USNM 16621, 17501, SEM slide 18394 (USNM); 11 ♂, 1 ♀, 19 Jul 1940, 2♂, 22 Jul 1940; side Mt. Albert: 2 ♂, 2 ♀, wing slide USNM 16157, slides USNM 18396, 18408, 98007, SEM slide 18430, (USNM, CNC). Mansonville: 1 ♂, 18 Jun 1928, W.J. Brown, specimen # CNCLEP00077286, CNC slide MIC1824 (CNC). Ste-Agathe-des-Monts, Lac Brûlé, 46.0903°N, 74.26°W, 370 m: 11 ♂, 6 ♀, 26 Jun 1991, afternoon sweeping in swampy ditch with liverworts and mosses at edge of spruce forest, J.-F. Landry, specimens # CNCLEP00076615–00076631 (CNC); Lac Brûlé, 46.0909°N, 74.2756°W, 370 m: 9 ♂, 3 ♀, 8 Jul 1992, 2 ♂, 16 Jul 1992, day sweeping on shaded liverworts near boggy marsh, J.-F. Landry; specimens # CNCLEP00068799–00068800 (CNC, USNM); Lac Brûlé, 46.0903°N, 74.26°W, 370 m: 3 ♂, 2 ♀, 1 Jul 1993, afternoon sweeping liverworts and mosses, J.-F. Landry, specimens # CNCLEP00067565–00067569 (CNC); Lac Brûlé, 46.0885°N, 74.2789°W, 370 m: 1 ♂, 4 Jul 1993, day sweep in mixed forest, J.-F. Landry, specimen # CNCLEP00076570 (CNC); Lac Brûlé, 46.0909°N, 74.2756°W, 370 m: 4 ♂, 2 ♀, 7 Jul 1993, day sweeping in shaded spruce-birch forest swamp, J.-F. Landry, specimens # CNCLEP00076571–00076576 (CNC); Lac Brûlé, 46.0885°N, 74.2789°W, 370 m: 1 ♂, 9 Jul 1993, at mercury light in mixed forest, J.-F. Landry, specimens # CNCLEP00076577 (CNC); Lac Brûlé, 46.0885°N, 74.2789°W, 370 m: 1 ♂, 1 Jul 1996, sweeping ferns at forest edge in afternoon, J.-F. Landry, specimens # CNCLEP00076577 (CNC); Lac Brûlé, 46.0885°N, 74.2789°W, 370 m: 1 ♂, 30 Jun 1997; 1 ♂, 1 Jul 1997, day sweep in mixed forest, J.-F. Landry, specimen # CNCLEP00076578 (CNC); Lac Brûlé, 46.0812°N, 74.2833°W, 370 m: 2 ♀, 1 Jul 1997, sweep in forest trail ca 18:00H, sunny, J.-F. Landry, specimens # CNCLEP00076580–00076581 (CNC); Lac Brûlé, 46.0919°N, 74.2756°W, 370 m: 13 ♂, 5 ♀, 2 Jul 1997, in swampy wood perched on vegetation 11:30H–12:30H overcast just before thunderstorm, J.-F. Landry, specimens # CNCLEP00076582–00076599 (CNC); Lac Brûlé, 46.0921°N, 74.2756°W, 370 m: 1 ♂, 1 ♀, 2 Jul 2000, afternoon sweeping liverworts and low vegetation in forest swamp, specimens # CNCLEP00067787–00067788, CNC slide MIC5756, DNA barcoded (CNC); 14 ♂, 5 ♀, 8 Jul 2002, day sweeping shaded liverworts near boggy marsh, specimens # CNCLEP00007712–00007720, 00068787–00068788, CNC slides MIC5753, MIC5755, MIC5757, MIC5758, MIC5760, MIC5762, MIC5763, 9 DNA barcoded (CNC); 6 ♂, 29 Jun 2003, in mixed forest swamp day-sweeping herbaceous and shrub vegetation, J.-F. Landry, specimens # CNCLEP00002816–00002821, CNC slide MIC5761, DNA barcoded (CNC); Lac Brûlé, 46.0881°N, 74.2788°W, 370 m: 1 ♂, 1 ♀, 4 Jul 2004, day sweep in forest swamp with liverwort, J.-F. Landry, specimens # CNCLEP00006682–00006683, CNC slide MIC5754, 1 DNA barcoded (CNC). Gatineau Park, Ramsey Lake, Hopkin’s Hole, 45.6025°N, 76.1079°W, 245 m: 12 ♂, 3 ♀, 11 Jun 1991, afternoon sweeping in forest swamp, J.-F. Landry, specimens # CNCLEP00076600–00076614 (CNC). Gatineau, Masham Township, 45.68°N, 76.05°W: 1 ♂, 26 Jun 1974; 1 ♀, 30 Jun 1974, D.M. Wood, specimens # CNCLEP00077284–00077285 (CNC). UNITED STATES: GEORGIA: Rabun Co: Chattahochee National Forest, Tate Br. Campground: 1 ♀, 16–17 May 1970, O. S. Flint, Jr. (USNM). KENTUCKY: Powell Co: 1 ♂, 23 Nov 1909, 1 ♂, 25 May 1924 (USNM). MAINE: Aroostook Co: Round Mountain: 1 ♂, 20 Jul 1956. Piscataquis Co: Greenville: 1 ♂, 9 Jul (USNM). Franklin Co: West Farmington: 1 ♂, 29 Jun 1966, A. E. Brower (USNM). Hancock Co: Acadia National Park, Mt. Desert Island: 1 ♂, 30 Jun 1933, (USNM). Penobscot Co: Passadumkeag: 1 ♀, 25 Jun 1938 (USNM). Piscataquis Co: Baxter State Park, Mt. Katahdin, Hunt Trail, 2400 feet: 1 ♂, 17 Jul 1948, bushes by brook, A. E. Brower, slides 16388, wing USNM 29861 (USNM). Sagadahoc Co: Woolwich: 1 ♀, 29 Jun 1965, A. E. Brower, slide USNM 33917 (USNM). MARYLAND: Montgomery Co: Cabin John: 1 ♂, 30 Apr 1921, A. Busck (USNM). MICHIGAN: Keweenaw Co: Isle Royale National Park: 2 ♂, 10 Jul 1957, R. W. Hodges (USNM). Emmet Co: Wilderness State Park, 45.7119°N, 84.9402°W, 180 m: 6 ♂, 30 Jun 1992, 17:00–18:00 hrs sweeping liverworts on banks of shaded stream in oak-pine forest with thuja, J.-F. and B. Landry, specimens # CNCLEP00068781–00068786, CNC slides MIC5752, MIC5764, DNA barcoded (CNC). NEW HAMPSHIRE: Rockingham Co: Hampton: 1 ♂, 6–11–1904, S.A. Shaw (USNM). NEW JERSEY: Essex Co: Essex Co. Park: 1 ♂, 3 Jun 1900, W. D. Kearfott (USNM). Essex Co: 1 ♂, 3 Jun; 7 ♂, 3 ♀, 8 Jun 1907, W. D. Kearfott, slides USNM 18409, 91794, 91795 (USNM). NEW YORK: Essex Co: [Keene]: Table Top Mountain, 3500 feet: 2 ♂, 1 ♀, 21 Jul 1940 (USNM). NORTH CAROLINA: Swain Co: Great Smoky Mountains National Park: Oconaluftee River at Towstring Road: 1 ♂, 11 May 1970, SEM slide USNM 17565 (USNM). Smokemont Campground and nearby: 2 ♀, 11–14 May 1970, slide USNM 33920, head slide 16614 (USNM). Whitewater River at rt. 171: 1 ♂, 18 May 1970, O. S. Flint, Jr. (USNM). PENNSYLVANIA: Dauphin Co: Inglenook: 1 ♀, 30 May 1911 (USNM). SOUTH CAROLINA: Pickens Co: Clemson, Wildcat Creek: 1 ♂, 25 Apr 1968, P. Carlson, J. Morse (USNM). TENNESSEE: Sevier Co: University of Tennessee Field Station, 35.739°N, 83.4235°W, 503 m: 1 ♀, 22 May 2005, afternoon sweeping vegetation along forest creek, J.-F. Landry, specimen # CNCLEP00016403, CNC slide MIC5759, DNA barcoded (CNC). VIRGINIA: Falls Church: 1 ?, 1908, A. Busck, wing slide USNM 91787 (USNM). WEST VIRGINIA: Pendleton Co., Smoke Hole State Park, Briggs Run: 3 ♂, 2 ♀, 28 May 1977, [sweeping low vegetation 8–11 AM], D. & M. Davis, slide USNM 20690 (USNM).
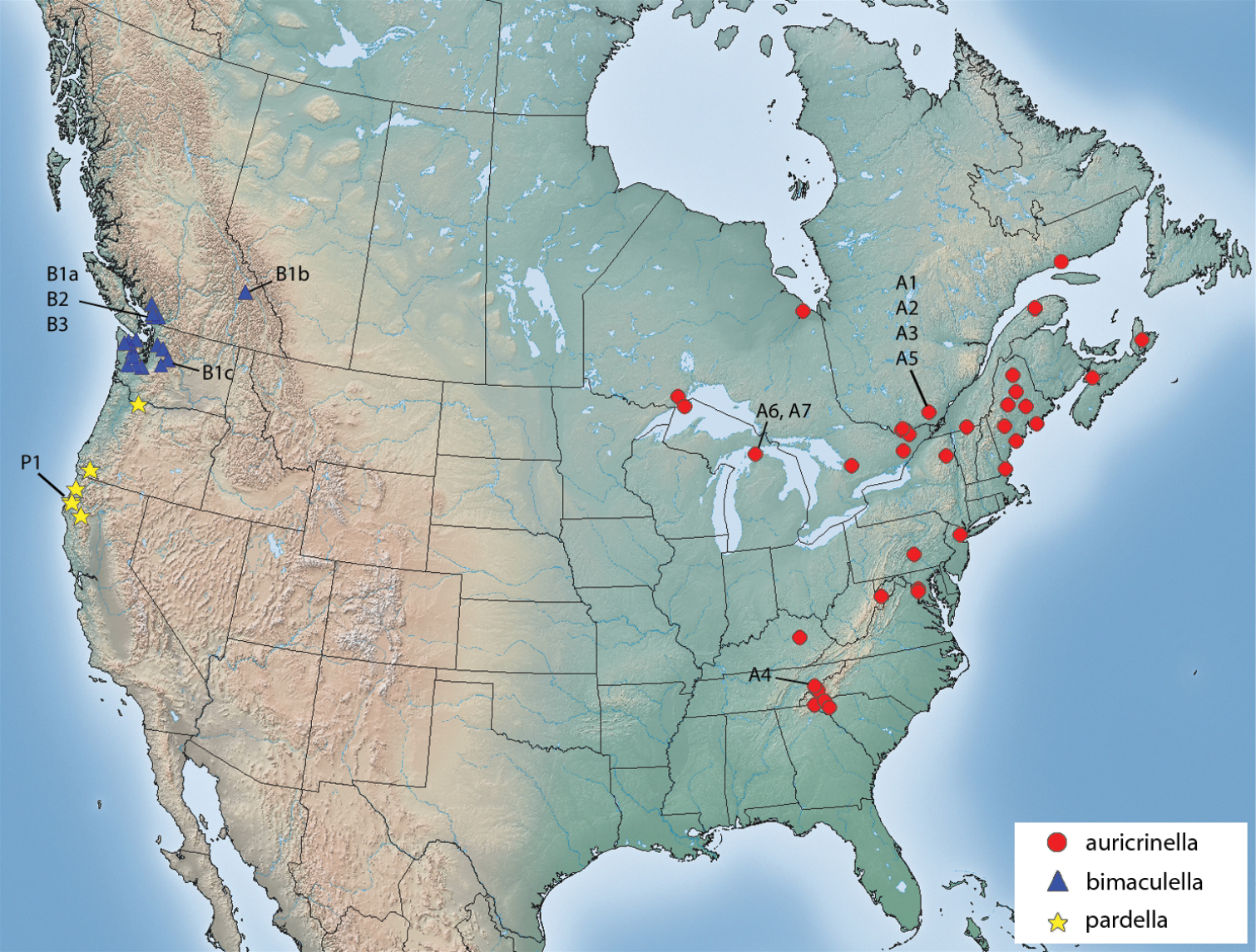
(Fig. 32).Epimartyria auricrinella occurs widely over eastern North America, in Canada from Nova Scotia to Ontario, and in the U.S. from Maine to Michigan and south to Tennessee and Georgia.
Chaetotaxy:Because the larvae of Micropterigidaelacksome thoracic and abdominal setae present in higher Lepidoptera, determining the homology of those setae present is subject to uncertainty. Various assumptions have been made as to which setae are present, based in part on their position to longitudinal muscle groups or to various body ridges (
Prolegs: The larval prolegs of Micropterigidae, which occur on abdominal segments 1–8 and 10, differ in their morphology from those of all other Lepidoptera where crochet- bearing prolegs are typically present only on segments 3–6 and 10.
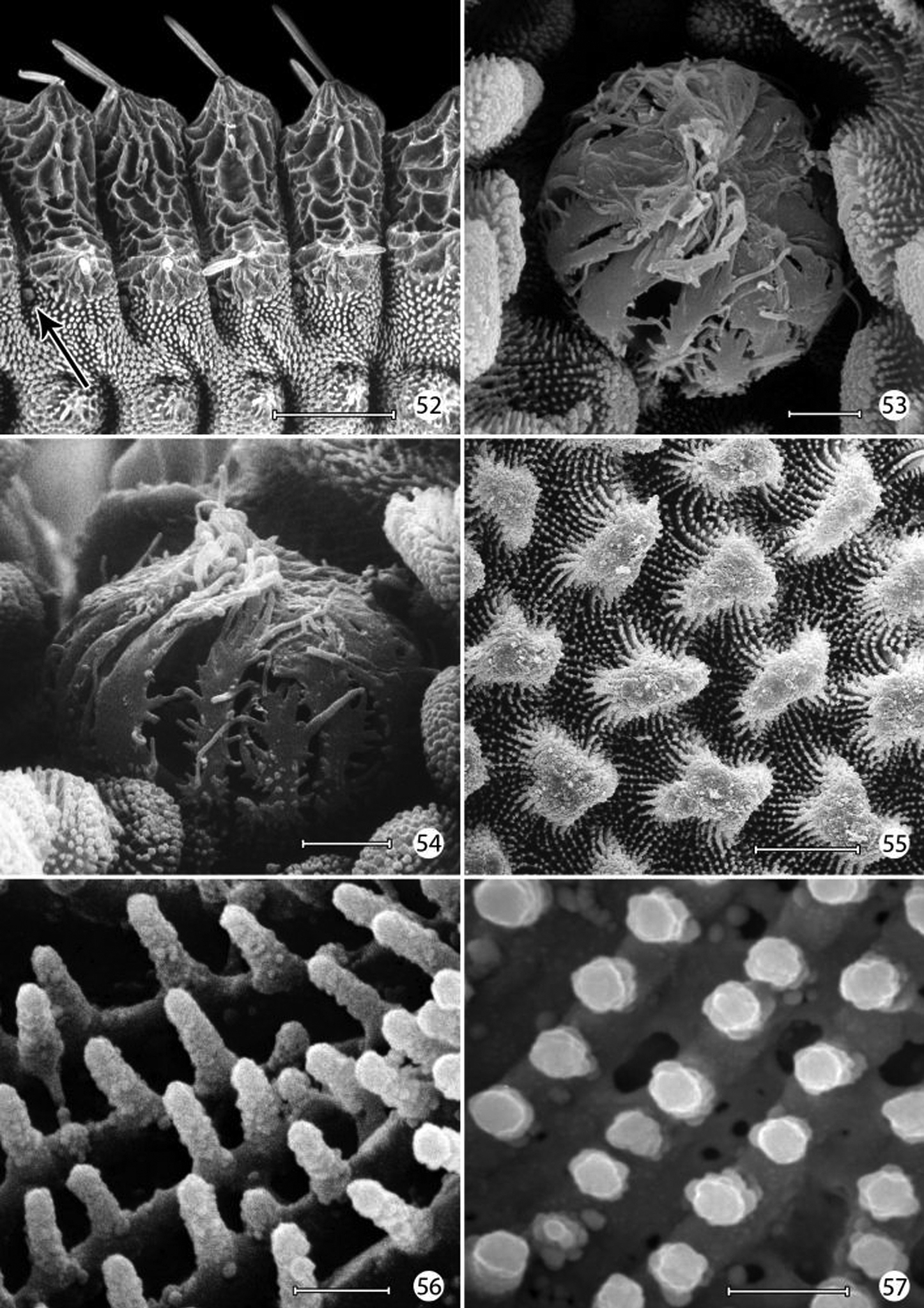
Integumental specializations: Larvae of Micropterigidae often occur close to the ground in habitats more likely to be subjected to periodic flooding and drying. As an adaptation to such conditions, the larvae have developed an unusual cuticular morphology in the form of a physical gill, or plastron (
An extensive plastron area has been observed in Epimartyria (
The multidentate, scale-like cuticular outgrowths (Figs 41–42) covering the intersegmental membrane between the head and prothorax of Epimartyria auricrinella may further assist in a respiratory function. These structures superficially resemble the plastron scales present in certain Coleoptera (
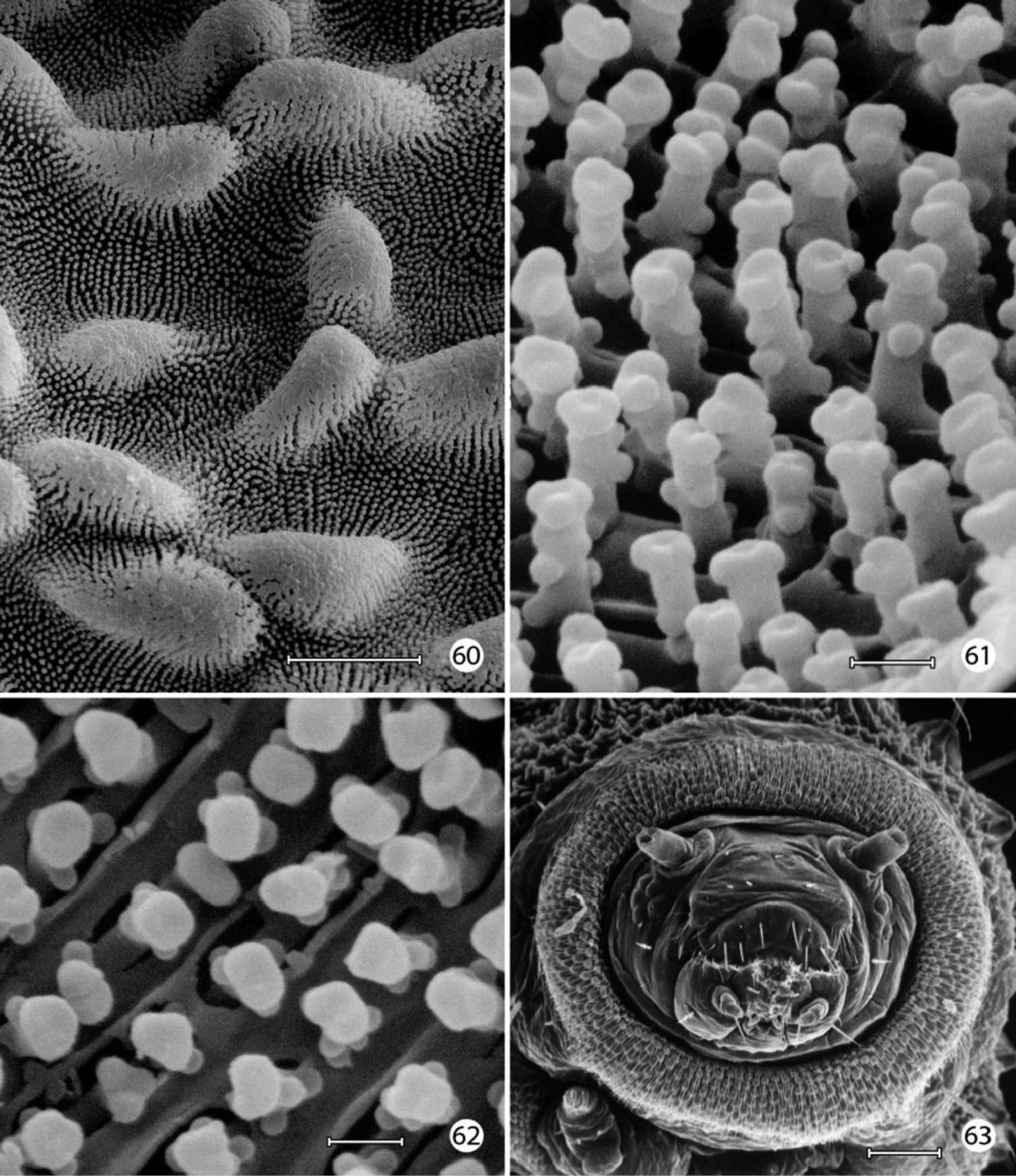
The larval plastron of Neomicropteryx nipponensis Issiki is similar to that of Epimartyria auricrinella in possessing a dense zone of minute, irregularly shaped micropapillae interconnected by dense radiations of smaller ridges bearing rows of knobby microtubercules (Figs 60–62). Scutate outgrowths also arise from the intersegmental head-prothoracic membrane (Fig. 63) as in Epimartyria. It is likely that larvae of all members of the northern hemisphere group of micropterigid genera proposed by
Adults. 1 ♂, Epimartyria auricrinella, (4.9 mm) Canada: Quebec 2 ♂, Epimartyria bimaculella (5.5 mm) Holotype, Canada: British Columbia 3 ♀, Epimartyria pardella (5.5 mm) USA: California. (Forewing length in parentheses).
Adults and habitat. 4–5 Epimartyria auricrinella, at Lac Brûlé, Québec, 30 Jun 1997, ca 0700 hrs. on dewy Solidago leaf 6–7 Epimartyria bimaculella 6 at Washington, Olympic National Park, Hoh Rainforest Road, 22 Jun 2010 (photo by Zeller-Lukashort) 7 at British Columbia, Vancouver area, Belcarra, 24 May 2009, ca 1000 hrs (photo by Holden) 8 Epimartyria pardella, California, Redwood National Park, Gold Bluffs State Beach, Fern Canyon 9 Habitat, clump of the liverwort Bazzania trilobata at Lac Brûlé, Québec in which larvae of Epimartyria auricrinella were found.
Habitats of Epimartyria 10 Swampy forest at Lac Brûlé, Quebec where larvae and numerous adults of Epimartyria auricrinella were collected 11 Douglas fir forest where adults of Epimartyria bimaculella were observed swarming around the ferns (photo by Zeller-Lukashort).
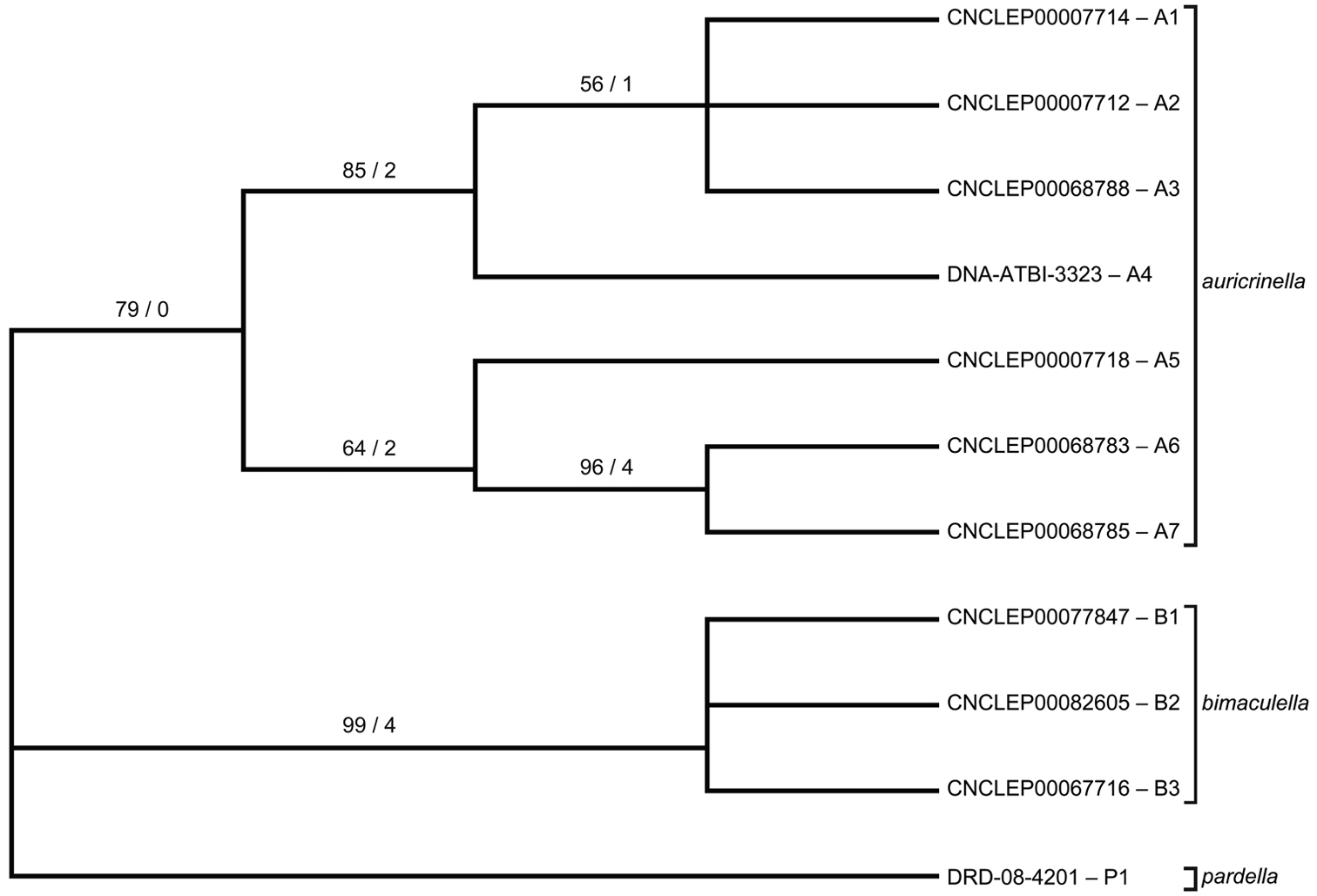
Neighbour-joining tree of genetic distances (K2P model) for cytochrome c oxidase I (COI) in species of Epimartyria (total = 44 specimens). End-branch labels are specimen Sample IDs followed by the geographic area in parentheses: BC = British Columbia; CA = California; MI = Michigan; QC = Quebec; TN = Tennessee; WA = Washington. Sequence lengths are 658bp unless otherwise indicated (xn in square brackets indicates the number of ambiguous positions). Distinct haplotypes are designated by a capital letter and digit.
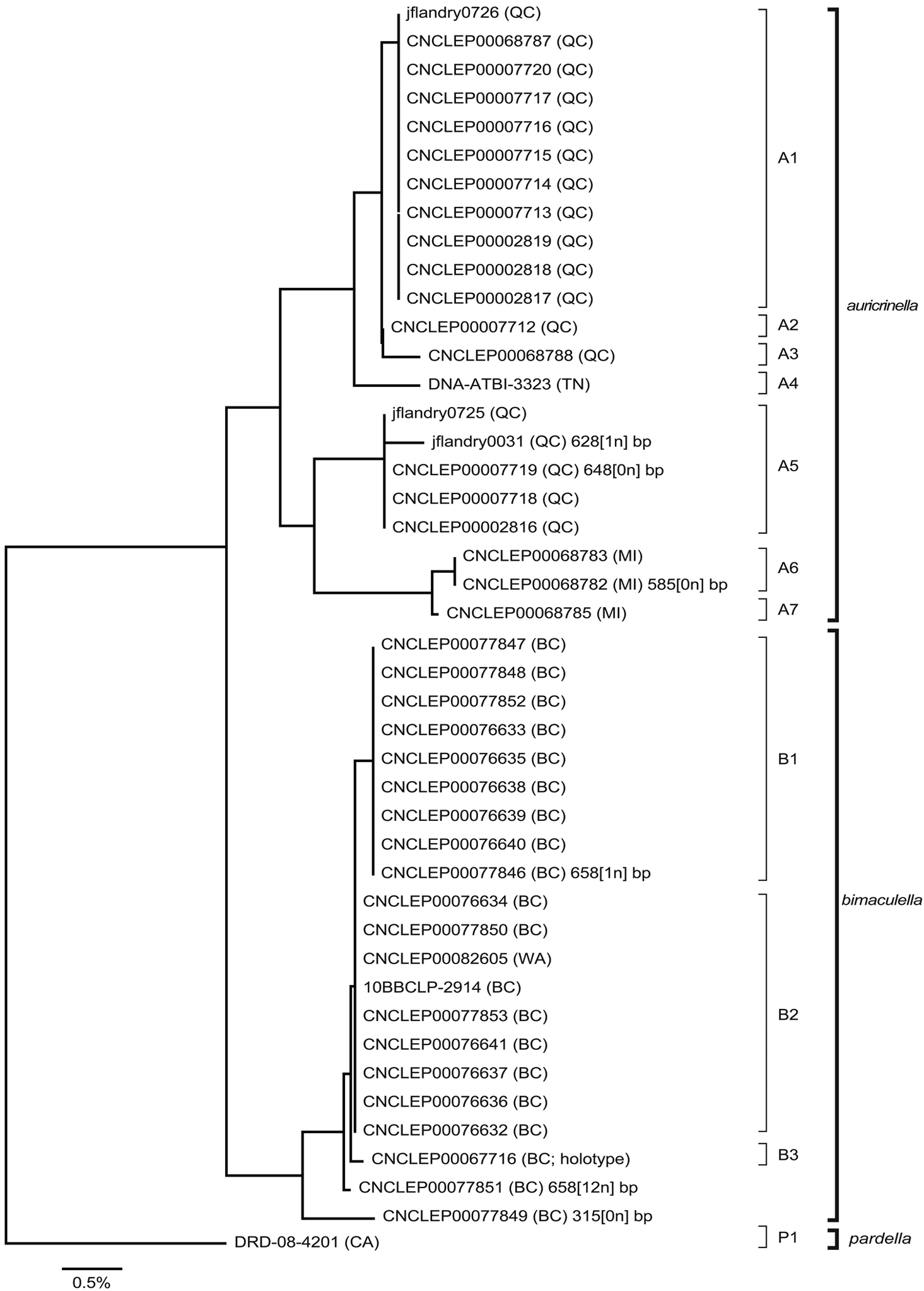
Strict consensus tree of three most parsimonious trees (length = 65, CI = 0.877, RI = 0.857) based on 11 unique DNA barcode haplotypes in species of Epimartyria. End-branch alphanumeric labels are specimen SampleIDs with haplotype designations (A1, A2, etc.). Numbers above branches are bootstrap values (1000 replicates) / Bremer support values.
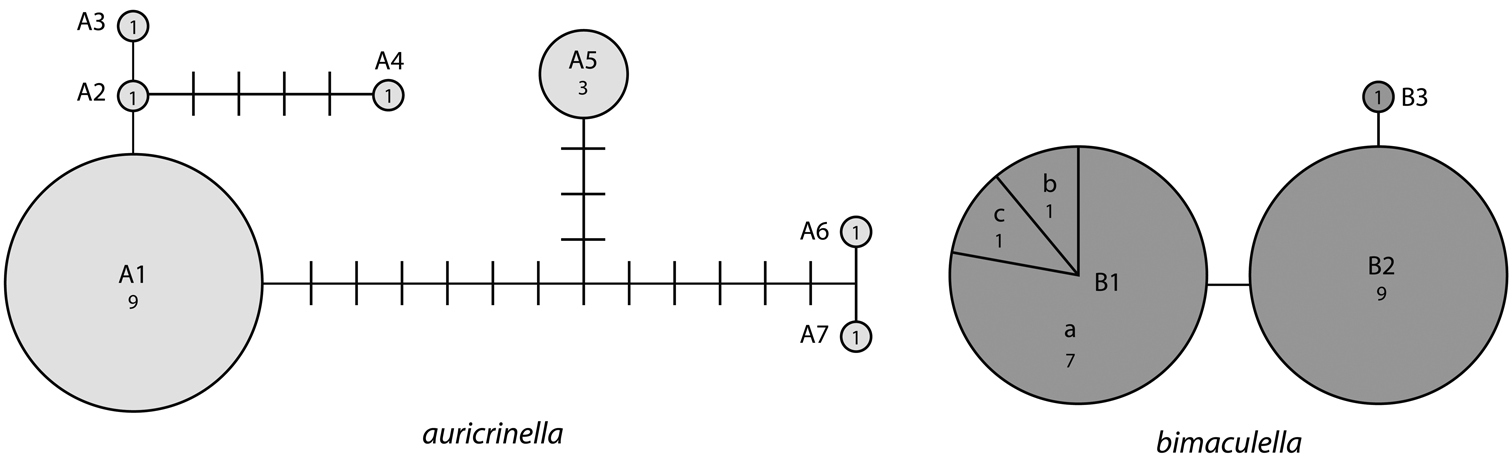
Haplotype network for 10 distinct haplotypes detected in two species of Epimartyria (7 for Epimartyria auricrinella, 4 for Epimartyria bimaculella). Circles are labelled with the haplotype name (capital letter), and the number of specimens per haplotype; lower case letters refer to localities indicated on the distribution map (Fig. 32). The single sequence of Epimartyria pardella, which separated out, is not shown.
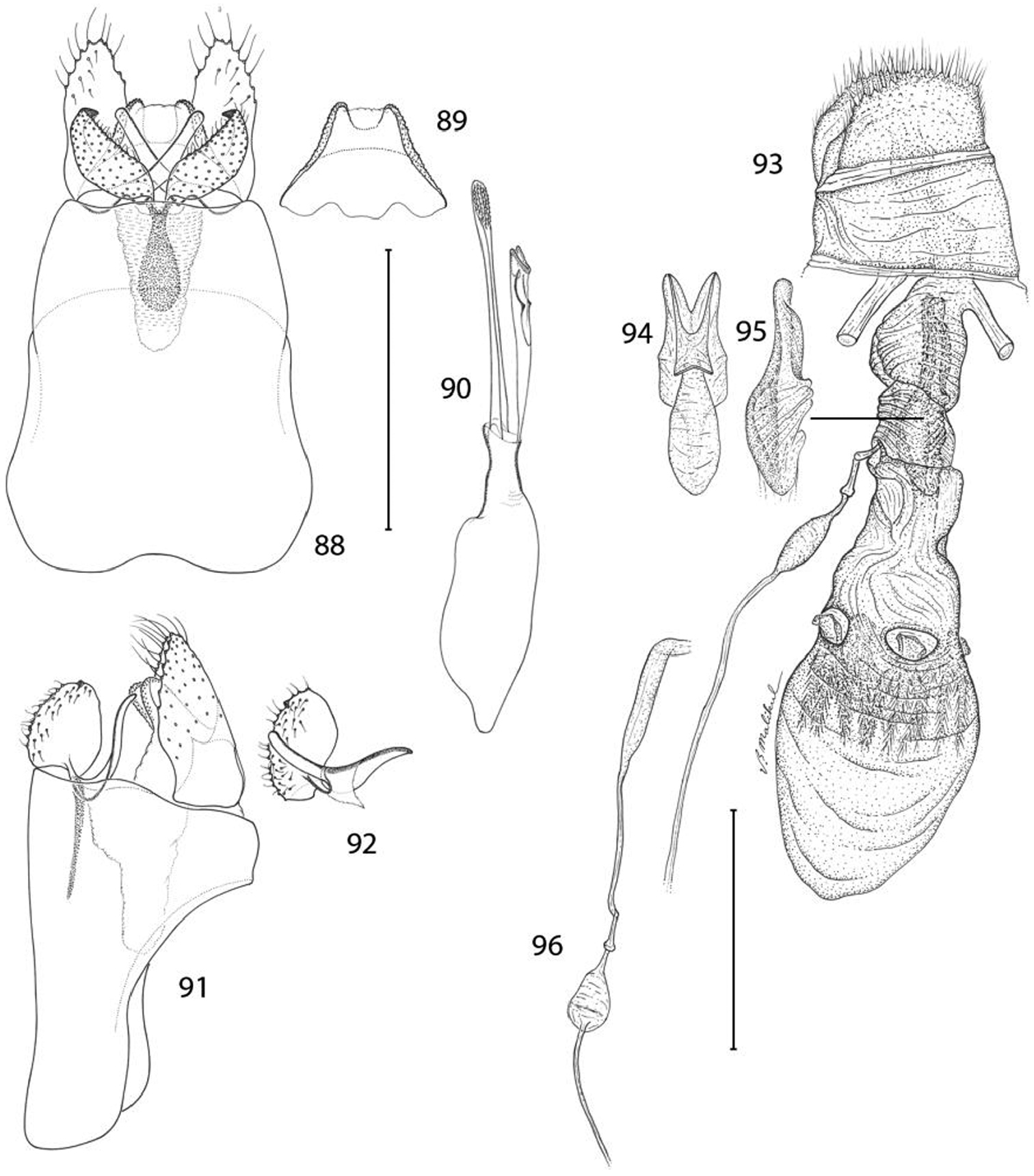
Epimartyria pardella, Adult morphology 13 Head (cl: clypeus; ga: galea; la: labrum; md: mandible) (0.5 mm) 14 Head, ventral view ( pp: proximal prelabium; dp: distal prelabium; lp: labial palpus) 15 right mandible 16 Wing venation, USNM slide 16613. 17 Legs (1.0 mm). (Scale lengths in parentheses).
urn:lsid:zoobank.org:act:6A40EFD1-BC6D-4DF3-AB1C-D4031870E61D
http://species-id.net/wiki/Epimartyria_bimaculella
Figs 2, 6–7, 11, 32, 81–87Adultsof Epimartyria bimaculella most resemble those of Epimartyria pardella in possessing dark fuscous forewings marked by pale golden spots. A total of two yellowish spots occur in bimaculella, with only a single large costal spot present beyond the middle of the forewing. Four spots are present on the forewing of pardella, with two of these located across the distal third of the wing on the costal and dorsal margins respectively.
(Figs 2, 6–7). Head: Vestiture similar to Epimartyria auricrinella, light orange brown. Antenna with vestiture of scape and pedicel concolorous with head; scales of flagellum mostly pale golden brown, becoming darker, more fuscous over distal third. Labial palpus cream.
Thorax: Dark fuscous with coppery to purplish luster. Tegula concolorous with head. Forewing mostly dark fuscous with coppery to purplish luster dorsally, marked with two pale yellowish spots; the largest, irregularly oval to rectangular spot extends from the costa approximately halfway across the distal third of wing; a second smaller, more slender spot extends diagonally from about midway along dorsal margin to midway on discal cell; a slight suffusion of pale yellowish scales may be sometimes evident at the base of the forewing, but only seldom does this occur; forewing less iridescent ventrally; fringe pale yellow along termen, fuscous along dorsal margin. Forewing length: 4.6–5.3 mm. Hindwing mostly gray, becoming darker and slightly iridescent toward apex; fringe gray. Legs medium to dark brown dorsally with a slight purplish luster, light brown to cream ventrally; epiphysis absent.
Abdomen: Piliform scales dark brown dorsally, paler brown ventrally.
Male genitalia (Figs 81–85): Tergum X similar to Epimartyria auricrinella, broadly bilobed. Caudal apex of sternum X deeply divided, with apex of lobes acute, only slightly curved; a pair of short, lateral lobes present near base. Valva moderately long, ventral length ~ half the maximum length of segment IX; apex subacute and bearing a short, slender, recurved spine similar to Epimartyria auricrinella; a short but broader and more triangular, rounded process arising midway from mesal surface; elongate basal process ~ 4/5 the length of valva; distal margin of valva variable within populations from slightly convex to ~ straight. Dorsal branch of phallus cylindrical and smooth.
Female genitalia (Figs 86–87): As described for genus. Caudal end of genital sclerite moderately furcate as in Epimartyria auricrinella; length of furcations ~ 0.3 that of moderately long, undivided base.
Unknown.
(Figs 6–7, 11). At the type locality, specimens were captured by sweeping low lying vegetation or during diurnal flight along a shaded seepage in a Douglas Fir–Western Red Cedar forest where leafy liverworts grew. Adults were also observed perching on lower parts of plants such as Salmonberry (Rubus spectabilis Pursh) no more than approximately 25 metres from the liverwort habitat (D.G. Holden, pers. comm.). In different parts of the range, specimens were collected from late April to mid August, with most records in June. Late records (July and August) are from higher elevations.
♂, CANADA: BRITISH COLUMBIA: Belcarra, 49°17'59.11"N, 122°55'30.88"W, Alt. 25 m., 8 Jun 2008, visual sweep, Dave G. Holden, specimen # CNCLEP00067716, CNC slide MIC5768, Barcode of Life Project, leg removed, DNA extracted, digital image captured, (CNC).
CANADA: BRITISH COLUMBIA: Belcarra Park, 49.3107°N, 122.9263°W, alt. 13 m: 2 ♂, 24 May 2009, day sweep, Dave G. Holden, specimen # CNCLEP00076632–00076633 [both DNA barcoded] (CNC); 13 ♂, 1 ♀, 1 Jun 2009, day sweep, Dave G. Holden, specimens # CNCLEP00076634–00076639, 00077846–00077853, CNC slides MIC5765, MIC5767, MIC5766, MIC5570, MIC5571 [all DNA barcoded] (CNC); 2 ♂, 2 Jun 2009, day sweep, Dave G. Holden, specimens # CNCLEP00076640–00076641 [both DNA barcoded] (CNC). Maple Ridge, Univ. of British Columbia Research Forest, 49.277679°N, 122.553870°W, 259 m: 1 ♂, 1 Jun 2011, visual sweep, Dave G.Holden (CNC). Fraser Mills: 6 ♂, 7 ♀, 11 June 1921, L. E. Marmont, SEM slide USNM 18431, slides USNM 33919, 98001, 98002, 98004; 27 ♂, 7 ♀, 15 Jun 1922, E. H. Blackmore collector, slides USNM 17503, 18410–18411, 34282, 91785, 97991, 98000, 98003, 98005, 98009–98017 (BMNH, CNC, USNM). Squamish, Diamond Head Trail: 1 ♂, 12 Aug 1963; 2 ♂, 14 Aug 1963, W.R.M. Mason, specimens # CNCLEP00077292–00077294, CNC slide MIC1826 (CNC). Mt Seymour, 49.337368°N, 122.957695°W, 292 m: 1 ♂, 21 Jun 2011, visual sweep, Dave G.Holden (CNC). Glacier National Park, Loop Trail, 1140 m, 51.254°N, 117.538°W, 1 ♀, 16 Jul 2010, malaise trap, specimen #10BBCLP-2914 [DNA barcoded], CNC slide MIC5769 (BIO). UNITED STATES: Washington: Clallam Co: Olympic National Park, sweeping on Soleduck Trail to Deer Lake, 1000 m: 3 ♂, 15 Jul 1998, D. R. Davis, slide USNM 34302 (USNM). Olympic Peninsula, Port Angeles, 245m, 48, 07924°N, 123, 42990°W ± 50m: 3 ♂ , 1 ♀, 20 Jun 2010; 1 ♂, 20.6.2010, 15:45h, Hausenblas and Zeller-Lukashort (ONPS). Olympic Peninsula, Sol Duc Hot Springs Rd, 390m, 48.06385°N, 123.99565°W ± 50m: 1 ♂, 21 Jun 2010, 13:00h, Hausenblas and Zeller-Lukashort, slide AP-Nr 42/2010 Christof Zeller (ONPS). Olympic Peninsula, Kalaloch, 10m 47.61131°N, 124.37588°W ± 50m: 1 ♂, 23 Jun 2010, 17:00h, Hausenblas and Zeller-Lukashort (ONPS). Olympic Peninsula, Hoh Rainforest Rd, 130m 47.81641°N, 124.05161°W ± 50m: 21 ♂ , 3 ♀, 22 Jun 2010, 16:15h, Hausenblas and Zeller-Lukashort (CZC). Grays Harbor Co: Elma: 46.9738°N, 123.2945°W, yel st trp, 1 ♀, 27 Jun 2011, G. Kohler, WSDA 978–1008A (WSCAD). King Co: Asahel Curtis picnic area: 47.3951°N, 121.4677°W, 1 ♂, 27 Jul 2011, hand col, C. Looney, WSDA W666–1129A, B (WSCAD, USNM). Stevens Pass, Hwy 2, 14.5 km E Skykomish, 645 m., 47.7143°N, 121.1722°W: 1 ♂, 8 Jul 2010, afternoon sweep, J.-F. Landry and D.G. Holden, specimen #CNCLEP00082605, CNC slide MIC5739 [DNA barcoded] (CNC). Mason Co: Skokomish River Rd: 47.3019°N, 123.1858°W, 4 ♂ , 2 ♀, 17 Jun 2011, hand col, C. Looney, WSDA W666–1131A-E (WSCAD, USNM). Pierce Co: Fort Lewis: 1 ♂, 29 May 1951, R. Schuster, Essig Museum slide 0152, (UCB). Snohomish Co: East Arlington Co. Park: 1 ♂, 29 Apr 1979, L. Massell, e. Lepidozia liverwort, slide USNM 98006 (USNM). 6 mi. E of Verlot: 1 ♂, collected 26 Mar 1979, emerged 2 May 1979, reared from liverwort, “Jungermannia obovata” L. Russell (USNM). Thurston Co: Evergreen State College, 47.0791°N, 122.9750°W, 2 ♂, 25 Jun 2011, hand col, C. Looney & E. Lagasa, WSDA 666–1130A, B (WSCAD, USNM).
Additional specimen examined, excluded from type material: CANADA: BRITISH COLUMBIA: Glacier National Park, Loop Trail, 1140 m, 51.254°N, 117.538°W, 1 ♀, 16 Jul 2010, malaise trap, specimen #10BBCLP-2914 [DNA barcoded] (BIO).
(Fig. 32). Epimartyria bimaculella is known from northwestern Washington and southern British Columbia. Most British Columbia records are from the southwesternmost corner in the periphery of the Vancouver area, reflecting a more intense collecting effort in that region. One record from the Rocky Mountains of Glacier National Park, BC suggests a significantly broader distribution.
The species nameis derived from the Latin bi; (two, double) and maculella (little spot) in reference to the two, small, pale yellowish spots present on the forewings.
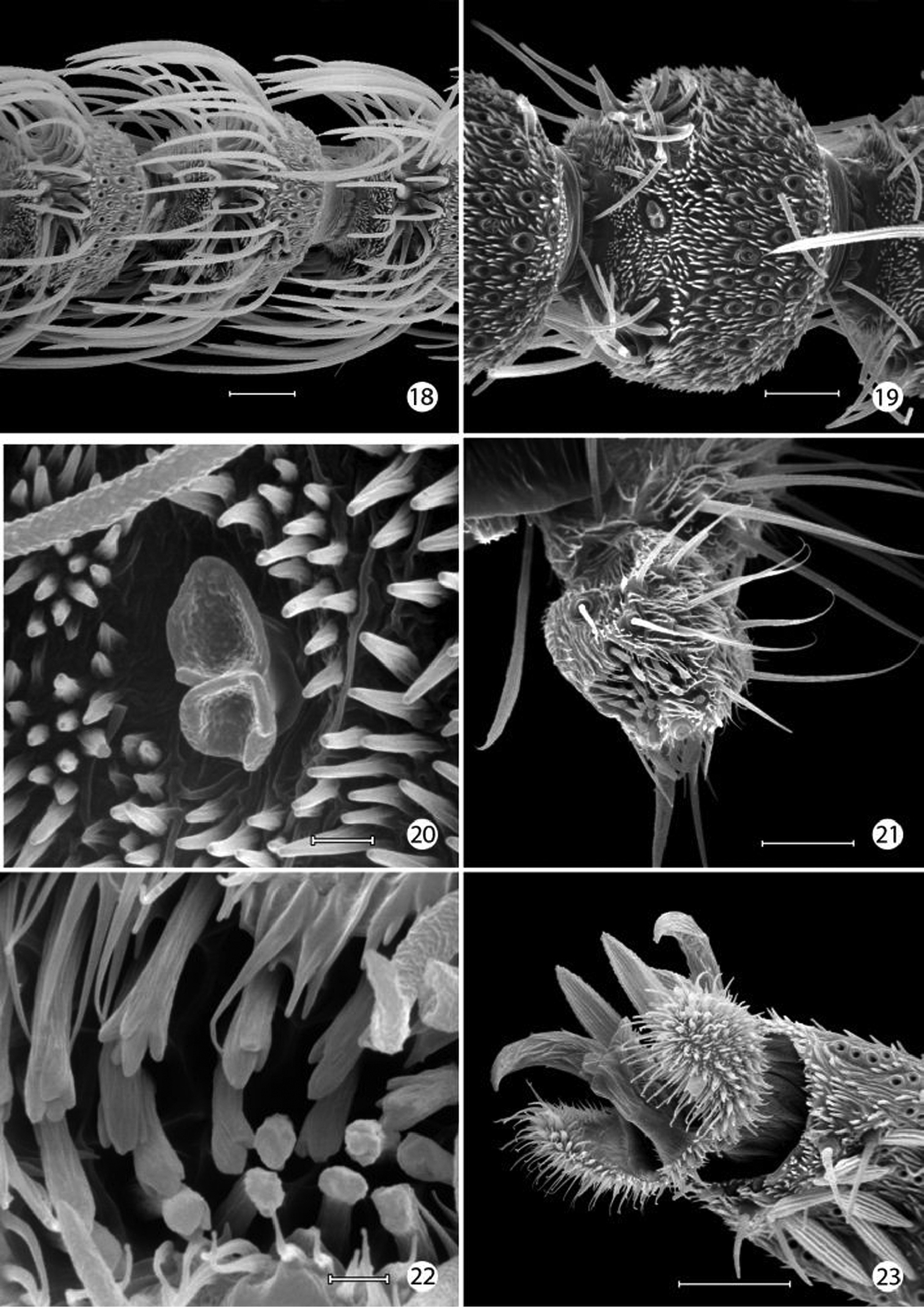
Epimartyria auricrinella, Adultmorphology 18 Flagellomeres with ascoid sensilla (20 µm) 19 Flagellomere with multiporus sensillum placodeum (20 µm) 20 Detail of multiporus sensillum placodeum in Fig. 19 (2 µm) 21 Apical segment of labial palpus with distal organ vom Rath (20 µm). 22 Sensilla of organ vom Rath (2 µm) 23 Mesothorcic pretarsus (20 µm). (Scale lengths in parentheses).
Epimartyria auricrinella, Adultmorphology 24 Mesothorcic pretarsus (20 µm) 25 Detail of pseudempodium of pretarsus (2 µm) 26 Arolium (5 µm) 27 Detail of surface of arolium (1 µm). (Scale lengths in parentheses).
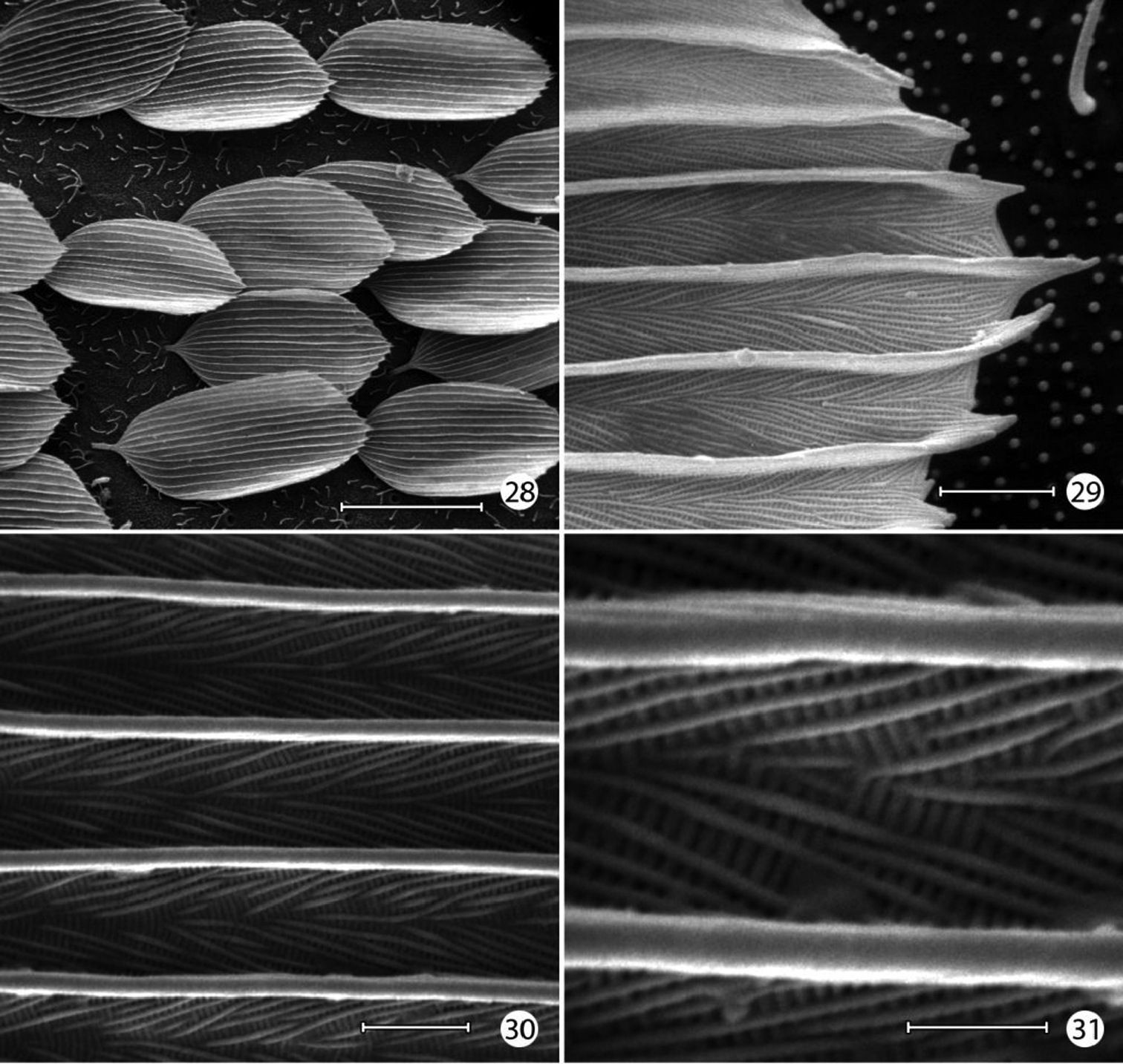
Epimartyria auricrinella, Forewing scale structure 28 Dorsal forewing scales from discal cell (40 µm) 29 Apical margin of scale in Fig. 28 (2 µm) 30 detail of Fig. 29 (2 µm) 31 Detail of Fig. 30 (1 µm). (Scale lengths in parentheses).
Distribution of Epimartyria species. Alphanumeric designations refer to haplotypes shown in the haplotype network of Fig. 12c.
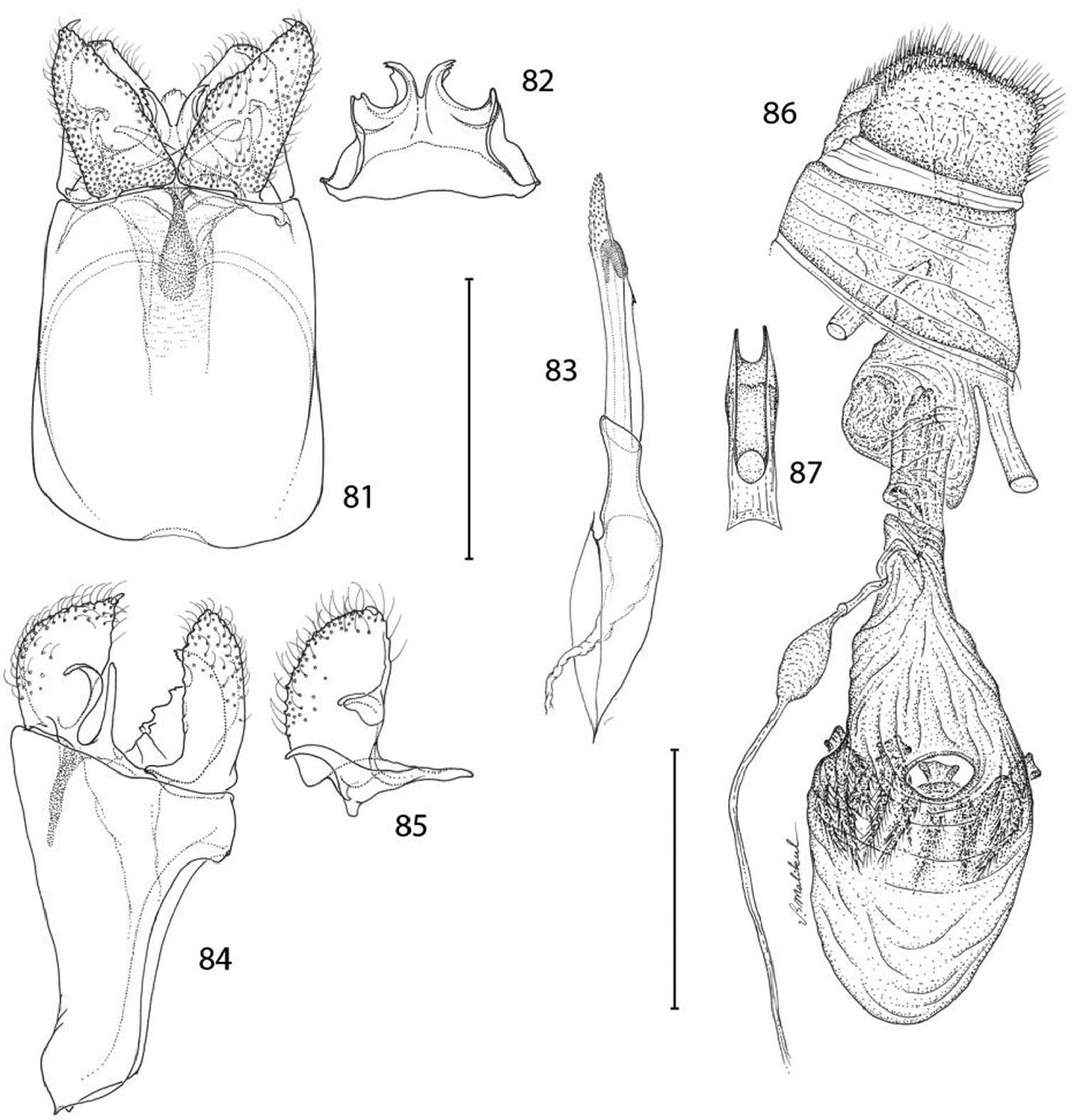
Epimartyria auricrinella, larval morphology 33 Chaetotaxy; shaded area indicates extent of epidermal plastron34 Head, dorsal view(M: medial seta) 35 Head, ventral view (AT: anterior arm of tentorium) 36 Ventralview of maxilla and labrum 37 Head, lateral view 38 Labrum, dorsal view 39 Mandible.
Epimartyria auricrinella, larval morphology 40 Head, dorsal view (100 µm) 41 head, anterior view (100 µm) 42 Scutate cuticular outgrowths from head-prothoracic fold (of Fig. 41) (10 µm) 43 Apex of antenna (10 µm). 44 Stemmata, 5 total (25 µm) 45 Ventral view of maxilla and labium (20 µm). (Scale lengths in parentheses).
Epimartyria auricrinella, larval morphology 46 Apical sensilla of maxillary palpus (5 µm) 47 Labial palpi and opening of labial salivary gland (100 µm) 48 Thoracic legs (arrow: tactile vesicle of coxa) (200 µm) 49 Mesothoracic leg (arrow: tactile vesicle of coxa) (25 µm) 50 Abdominal prolegs, segments 1–2 (100 µm) 51 Anal prolegs (100 µm). (Scale lengths in parentheses).
http://species-id.net/wiki/Epimartyria_pardella
Figs 3, 8, 13–17, 32, 88–96.Adults of Epimartyria pardella most resemble those of Epimartyria bimaculella in possessing dark fuscous forewings marked by pale golden spots. Four spots are present on the forewing of pardella with two of these located across the distal third of the wing. In contrast, a total of two yellowish spots occur in bimaculella, with only a single large costal spot present beyond the middle of the forewing. In the male genitalia, the caudal lobes of sternum X (uncus) are more simple than those of the other members of Epimartyria in consisting of more shallow, rounded lobes compared to being curved and more slender in the males of auricrinella and bimaculella.
(Figs 3, 8). Head: Vestiture light orange brown. Antenna with vestiture of scape and pedicel concolorous with head; scales of flagellum pale golden yellow. Labial palpus pale brown to cream.
Thorax: Dark fuscous with coppery to purplish luster. Tegula concolorous with head. Forewing dark fuscous with coppery to purplish luster dorsally, marked with four, pale yellowish spots; the largest, irregularly rectangular and slightly oblique spot extends from costa approximately halfway across the distal third of wing; a smaller, more oval spot opposite costal spot on dorsal margin; an oblique basal spot arising midway along dorsal margin and extending halfway across wing to base of radial vein; a fourth, smallest spot at base of wing; forewing less iridescent ventrally; fringe pale yellow along termen, more gray along dorsal margin. Forewing length: 5.0–5.5 mm. Hindwing mostly gray, becoming darker and slightly iridescent toward apex; fringe gray; fringe light to dark gray. Legs medium to dark brown dorsally, paler brown ventrally and over tarsomeres; epiphysis present, ~ 1/3 the length of foretibia and arising slightly beyond its midlength.
Abdomen: Piliform scales light to dark brown.
Male genitalia (Figs 88–92): Tergum X with more slender caudal lobes. Caudal apex of sternum X (gnathos) not deeply divided, with short, triangular caudal lobes and without accessory lateral lobes. Valva short, ventral length less than 1/3 the maximum midventral length of segment IX; apex rounded and bearing a short, stout subapical spine; mesal surface smooth, without median process; elongate basal process nearly equal to length of valva. Dorsal branch of phallus more depressed, with subapical margins bearing short, paired spines, gonopore with less thickened radial folds than in previous two species.
Female genitalia: (Figs 93–96): As described for genus. Caudal end of genital sclerite deeply furcated; length of furcations exceeding length of short, undivided base.
Egg. White; dimensions 0.44 × 0.44 mm.
Larva. Not examined. The following description has been summarized from
Head. Length 0.5 mm, diameter 0.27 mm. Brown. Antennae prominent, 3-segmented and situated on small tubercles located on dorsal lateral portion of head. Stemmata with 5 facets and located near base of antenna. Labrum simple with a pair of 3-segmented palpi. Mandibles simple and dark brown. Head diameter of first and second instar larvae 0.11 and 0.22 mm, respectively.
Thorax: Prothorax distinctly narrower than mesothorax. Prothoracic shield with 10 peg-like setae, 8 on the anterior and lateral border and 2 dorsally. Mesothorax with 8 setae, 6 on dorsal and lateral anterior portion of gray brown pigmented area, and 2 just ventral to this pigmented area. Setae of metathoracic segment similar to those of mesothorax except subdorsal (D2) seta is greatly reduced in size. All thoracic segments have additional small micro-seta just dorsal to each true leg. Thoracic legs brown, with 3 segments (excluding coxa) and simple claw.
Abdomen: Abdominal segments Al to A8 (and T2 and T3) with serrated knobs which form a dorsal and lateral ridge; areas between ridges concave. The mid-dorsal area concave with a small dark depression present on posterior of segments T2 to A8. Segments Al to A8 each with one dorsal seta (length 0.18 mm) atop dorsal ridge. Segments Al to A8 with reduced, almost microscopic subdorsal (D2) seta (length 0.04 mm) and prominent lateral seta (length 0.12 mm) on lateral ridge. Dorsal. subdorsal and lateral setae occur in brownish pigmented area which has rough and wrinkled appearance. Dorsal and lateral intersegmental area constricted and may contain series of 8 to 20 microscopic dots. Cuticle ventral to lateral ridge smooth and light brown. Series of brown dots form pattern around protuberance that usually support a small seta. Conical ventral prolegs occur on segments Al to A8, with a small, sclerotized protuberance present on ventral surface of each. Segments A9 and Al0 fused and with enlarged simple sucker. Spiracles posterior and ventral to lateral setae.
Hepaticophyta: Conocephalaceae: Conocephalum conicum (L.) Dumort.; Pelliaceae: Pellia species, with the latter host preferred from rearings (
Not examined. Exarate, decticous; white to light brown.
Not examined. Brown, oval in general form, measuring 5.5 × 4.5 mm; primarily of silk with small fragments of vegetation attached.
(Fig. 8).
Adults begin to emerge in late May with the flight season ranging from late May to mid- July and peak flight activity in June at the Prairie Creek State Redwood Park locality. Tuskes and Smith reported the adults to be relatively inactive, remaining motionless for hours and seldom travelling more than 30 cm. They are known to be diurnal and most active between 0900 and 1930 h. Adult feeding by Epimartyria pardella has not been reported, but the adults were observed drinking water by lowering their heads to the moisture. Moths can die in less than two days if deprived of moisture but may survive in captivity from 9 to 18 days when provided with water.
Tuskes and Smith concluded that Epimartyria pardella possessed a two year life cycle similar to that proposed for Epimartyria auricrinella (
♂ (present designation), “OREGON: Klamath Co: nr. Redwood Creek, Coast: 26 June 1872, Wlsm. 90591; B.M. Genitalia Slide No. 25352; Epimartyria (= Micropteryx Wlsm.) pardella Wlsm. PARATYPE; Lectotype ♂, Epimartyria pardella Wlsm. (BMNH).” The lectotype has been selected from a series of five syntypes collected “on the borders of the forest of “redwood” (Taxodium sempervirens) near the coast, in southern Oregon, at the beginning of June 1872” (
UNITED STATES: CALIFORNIA: Humbolt Co: Arcata: 1 ♂, 11 Jun 1969, slide USNM 16613, wing slide USNM 18441 (USNM). Fern Canyon: 1 ♀, 18 Jun 1977, N. J. Smith, slide DRD 4529 (UCB). Fern Canyon, Prairie Creek State Park: 3 ♂, 19 Jun 1981, Ann & Paul Tuskes; 1 ♂, 20 Jun 1981, P. Tuskes, 3 ♂, Ann & Paul Tuskes (USNM). Kneeland: 69 Prairie Lane: 1 ♂, 17 Jun 2001, 2 ♂, 18 Jun 2001, 1 ♂, 29 Jun 2001, 1 ♂, 30 Jun 2001, R. S. Wielgus, diurnal flight in wet meadow below house, slide USNM 34278 (USNM); 1 ♀, 22 Jun 2003, R. S. Wielgus, slides DRD 4528, 4529 (UCB). Trinity Co: Forest Glen: 2 ♀, 25 May 1973, J. Doyen (UCB). OREGON: Klamath Co: nr. Redwood Creek, Coast: 1 ♂ (lectotype), 26 June 1872, Wlsm. 90591; B.M. Genitalia Slide No. 25352, (BMNH). Multnomah Co: Benson State Park, Multnomah Falls: 2 ♂, C. V. Piper, 1 ♂, wing slide USNM 91788 (USNM).
(Fig. 32). Epimartyria pardella is known from northwestern California and northern Oregon. California localities and the type locality in Oregon are near the coast in redwood forests. The most northern Oregon locality occurs in the Columbia River valley.
Information included in this report on the immature stages and life history of Epimartyria pardella has been quoted or summarized from the thorough study of this species by
Epimartyria auricrinella, larval morphology 52 Abdominal segments 1–4, lateral view, showing sculptured epicuticle of dorsal half and plastron region (shaded area) of lower half (arrow indicates spiracle) (200 µm) 53 Spiracle, apical view (5 µm) 54 Spiracle, lateral view (5 µm) 55 Plastron of lateral surface of abdomen with numerous, irregular micropapillae (10 µm) 56 Detail of fig. 55 showing parallel rows of microtubercules extending between micropapillae (1 µm) 57 View looking down on microtubules in fig. 56 showing cuticular openings between rows (1 µm). (Scale lengths in parentheses).
Epimartyria auricrinella, larval morphology 58 Honeycombed chambers of abdominal exocuticle with pellicle removed, in dorsal half (dorsal to spiracle) of abdominal segment 4 (10 µm) 59 Abdominal seta D1 showing longitudinal ridges (20 µm). (Scale lengths in parentheses).
Neomicropteryx nipponensis, larval morphology 60 Plastron from lateral surface of abdomen with numerous, irregular micropapillae (10 µm) 61 Detail of Fig. 55 showing parallel rows of microtubercules extending between micropapillae (0.5 µm) 62 View looking down on microtubules in fig. 61 showing cuticular openings between rows (0.5 µm) 63 Head, anterior view (100 µm). (Scale lengths in parentheses).
Micropterix species (England), larval morphology 64 Prothoracic leg (arrow: tactile vesicle of coxa) (50 µm) 65 Detail of tactile vesicle in fig. 64 (10 µm) 66 Abdominal segments 5–10 showing prolegs 5–8 and sucker-like anal proleg (100 µm) 67 Abdominal proleg (20 µm) 68 Detail of apex of abdominal proleg (10 µm) 69 Dorsal abdominal setae (50 µm). (Scale lengths in parentheses).
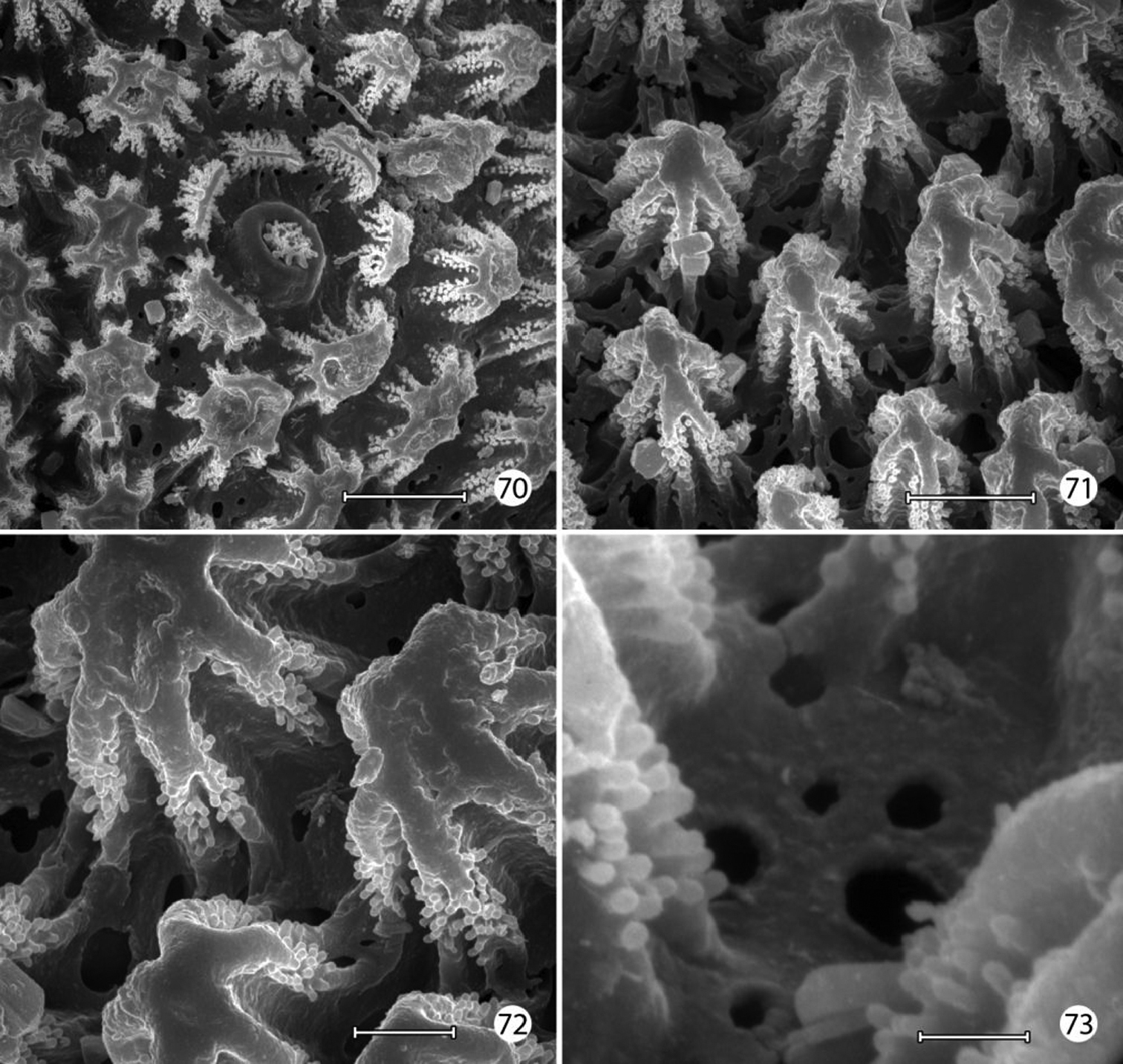
Micropterix species (England), larval morphology 70 Lateral plastron surface of abdomen showing micropapillae around broken scale base (20 µm) 71 Detail of plastron Fig. 70 showing cuticular openings between micropapillae (10 µm) 72 Detail of fig. 71 (5 µm) 73 Detail ofcuticular openings in Fig. 71 (2 µm). (Scale lengths in parentheses).
Epimartyria auricrinella, Genitalic morphology 74–78 Male, USNM slides 16615, 34372 74 Genital capsule, ventral view (0.5 mm); J: juxta (medial plate) 75 Sternum X (gnathos) 76 Aedeagus (G: gonopore (phallotreme) 77 Genital capsule, lateral view (Un: uncus, (tergum X) 78a Valva, lateral view, inner side (BP: basal process), slide USNM, 34372, Ottawa, Ontario 78b slide MIC5762, Lac Brûlé, Quebec 78c slide MIC5764, Wilderness State Park, Michigan 78d slide MIC5761, Lac Brûlé, Quebec 79–80 Female, USNM slide 17501, Mt. Albert, Quebec 79 Oviscape, lateral view (Ut: utriculus) (0.5 mm) 80 Genital sclerite, ventral view. (Scale lengths in parentheses).
Epimartyria bimaculella, Genitalic morphology 81–85 Male, USNM slide 18410, Fraser Mill, British Columbia 81 Genital capsule, ventral view (0.5 mm) 82 Sternum X (gnathos) 83 Aedeagus 84 Genital capsule, lateral view 85 Valva 86–87 Female, USNM slide 33919, Fraser Mill, British Columbia 86 Oviscape, lateral view (0.5 mm) 87 Genital sclerite, ventral view. (Scale lengths in parentheses).
Epimartyria pardella, Genitalic morphology 88–92 Male, USNM slide 16613, Arcata, California 88 Genital capsule, ventral view (0.5 mm) 89 Sternum X (gnathos) 90 Aedeagus 91 Genital capsule, lateral view 92 Valva 93–96 Female, DRD slide 4528, Kneeland, California 93 Oviscape, lateral view (0.5 mm) 94 Genital sclerite, ventral view 95 Genital sclerite, lateral view 96 Ductus spermathecae, showing variation of vesicle position. (Scale lengths in parentheses).
A total of 44 specimens yielded barcode sequences, of which 40 were full-length at 658 bp (Appendix 1). Geographic representation of barcoded specimens was primarily dictated by the availability of recently collected material (<20 years), and consequently restricted to a few localities which do not represent the entire range of the species (Fig. 32). Three barcode sequences of Epimartyria were available in GenBank, two for auricrinella and one for pardella (from
Neighbour-joining analysis resulted in three distinct clusters that corresponded to the three species as defined here on the basis of morphology (Fig. 12a). Epimartyria pardella was the most distant species with mean distances of 4.52% and 5.09% from Epimartyria bimaculella and Epimartyria auricrinella, respectively. Morphologically it is the most distinct of the three species in genitalia. Epimartyria auricrinella and Epimartyria bimaculella seemed to be genetically closer to each other, with a mean distance of 2.57%. Morphologically, their genitalia are also more similar. Intraspecific sequence variation was small in Epimartyria bimaculella at 0.2% ± 0.1 and a minimum of three haplotypes could be distinguished. Two specimens with either short sequences or ambiguous sites were not assigned as haplotypes. In contrast, Epimartyria auricrinella showed a high amount of sequence divergence resulting in 7 subclusters representing different haplotypes. Pairwise divergence ranged from 0.16–2.69% (1.63% ± 0.832), with 9 out of 21 comparisons showing over 2% divergence (Table 1).
To assess whether the high amount of intraspecific divergence may be correlated with morphological variation, geography, or both, representatives of each haplotype were further subjected to a parsimony analysis. Maximum-parsimony analysis performed on unique haplotypes (7 for auricrinella, 3 for bimaculella, 1 for pardella) resulted in three most parsimonious trees, of which the strict consensus is illustrated (Fig. 12b). Of the 658 base pairs of the full barcode dataset, 607 were constant and 51 were variable, of which only 25 were parsimony-informative. The MP cladogram was similar to the NJ tree in that the three morphospecies were retained as separate, well-supported clades. Despite high sequence variation within the auricrinella clade, support for internal nodes was generally weak.
The haplotype network (Fig. 12c) resulted in a similar topology, with the three species separated from each other. In some cases, several haplotypes were present among specimens from the same locality (Fig. 32). For bimaculella, there were three haplotypes with 1–2 base pair differences from the type locality of Belcarra, BC, which were collected microsympatrically, two of which on the same date (Appendix 2). Similarly, for auricrinella, four haplotypes were present at Lac Brûlé, QC, of which three were 1–2 base pair apart but one (A5) was more than 10 bp divergent. At that locality haplotypes A1, A2 and A5 were present among specimens collected microsympatrically on the same date on two consecutive years (8 Jul 2002, 29 Jun 2003). Haplotype A4 from Tennessee, was closer to haplotypes A1–A3 from Lac Brûlé than to A5 from the same locality. The two localities are over 1300 km apart. Haplotypes A6 and A7 from Michigan were the most divergent, despite being geographically closer to Lac Brûlé than to Tennessee. The majority of barcode sequences came from a single locality for each of auricrinella and bimaculella. Thus it appears that higher haplotype diversity is associated with denser barcode sampling at single localities.
Genitalia were examined in several specimens of auricrinella representing the different haplotypes (Appendix 2). This showed slight variation in the shape of the male valva, in which the inner margin varied from evenly rounded to medially angulate (Fig. 78b–d). Several specimens showed various intermediate states of this condition from having a barely suggested median angle to a sharp one. The angulation differed slightly between the two valvae on some specimens. Likewise slight variation was observed in the depth of the apical notch of the uncus, which was a little deeper or a little more sharply V-shaped in some whereas it was proportionally shallower and more obtusely V-shaped in others. The lateral dentation and recurved distal lobes of the gnathos also displayed minor variations. The variation observed in male genitalia was present across haplotypes from the same locality and seemed unrelated to geography. Males were predominant in all series examined, thus fewer females were compared. No detectable variation was observed in the latter.
Thus we consider the variation in both haplotypes and morphology to be intraspecific. Although a 2% minimal divergence threshold is commonly observed to separate species, and in particular Lepidoptera (
Percent mitochondrial cytochrome c oxidase I (COI) sequence divergence among 11 unique haplotypes of three Epimartyria species. Cells below diagonal = distances in %; cells above diagonal = standard error estimates.
| A1 | A2 | A3 | A4 | A5 | A6 | A7 | B1 | B2 | B3 | P1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Epimartyria auricrinella A1 | 0.15 | 0.26 | 0.36 | 0.49 | 0.57 | 0.52 | 0.62 | 0.60 | 0.60 | 0.78 | |
| Epimartyria auricrinella A2 | 0.16 | 0.21 | 0.33 | 0.51 | 0.59 | 0.54 | 0.60 | 0.58 | 0.58 | 0.78 | |
| Epimartyria auricrinella A3 | 0.47 | 0.31 | 0.40 | 0.57 | 0.64 | 0.59 | 0.65 | 0.64 | 0.63 | 0.82 | |
| Epimartyria auricrinella A4 | 0.94 | 0.78 | 1.10 | 0.57 | 0.59 | 0.59 | 0.63 | 0.62 | 0.62 | 0.81 | |
| Epimartyria auricrinella A5 | 1.73 | 1.89 | 2.21 | 2.37 | 0.49 | 0.50 | 0.59 | 0.57 | 0.59 | 0.84 | |
| Epimartyria auricrinella A6 | 2.20 | 2.37 | 2.69 | 2.53 | 1.73 | 0.21 | 0.69 | 0.67 | 0.67 | 0.89 | |
| Epimartyria auricrinella A7 | 1.88 | 2.04 | 2.37 | 2.53 | 1.73 | 0.31 | 0.65 | 0.63 | 0.63 | 0.87 | |
| Epimartyria bimaculella B1 | 2.53 | 2.37 | 2.69 | 2.69 | 2.37 | 3.18 | 2.85 | 0.14 | 0.21 | 0.79 | |
| Epimartyria bimaculella B2 | 2.37 | 2.20 | 2.53 | 2.53 | 2.21 | 3.01 | 2.69 | 0.16 | 0.15 | 0.78 | |
| Epimartyria bimaculella B3 | 2.36 | 2.20 | 2.52 | 2.52 | 2.37 | 3.01 | 2.69 | 0.31 | 0.15 | 0.78 | |
| Epimartyria pardella P1 | 4.80 | 4.80 | 5.14 | 5.14 | 5.14 | 5.47 | 5.13 | 4.63 | 4.47 | 4.47 |
We wish to thank Vichai Malikul and Young Sohn of the Department of Entomology, Smithsonian Institution for the line illustrations. Donald Harvey, Patricia Gentili-Poole, and Karolyn Darrow of the Department of Entomology, Smithsonian Institution, assisted with graphics and final preparation of plates. SEM images were done by DRD, assisted by Susann Braden, formerly of the Scanning Electron Microscope Laboratory, National Museum of Natural History. Mignon Davis of the Department of Entomology, Smithsonian Institution recorded specimen data and assisted with specimen curation. Vazrick Nazari, Agriculture & Agri-Food Canada, Ottawa, assisted JFL with barcoding, databasing, analysis, and specimen curation. We also wish to thank several individuals who have assisted us during the course of this study by providing critical information, photographs, loans of specimens under their care, or other special assistance: Steen Dupont, Natural History Museum of Denmark, Copenhagen, Denmark; George Gibbs, Victoria University of Wellington, Wellington, New Zealand; Eric LaGasa, Washington State Department of Agriculture, Olympia, WA; Niels Kristensen, Universitetes Zoologisk Museum, Copenhagen, Denmark; Chris Looney, Washington State Department of Agriculture, Olympia WA; Kim Mitter, Lepidoptera Tree of Life, University of Maryland, College Park, MD; Jerry Powell, University of California, Berkeley, CA; Kevin Tuck, Department of Entomology, The Natural History Museum, London, United Kingdom; Christof Zeller-Lukashort, Thalgau, Austria; David Holden, Port Coquitlam, BC; Paul Hebert, Biodiversity Institute of Ontario, Guelph, Ontario; Irwin and Fenya Brodo, Canadian Museum of Nature, Ottawa, Ontario; and Carle Bélanger, Havre-Saint-Pierre, Québec. François Dumas and David Walker gave permission to collect at the private sites of Lac Brûlé, Québec. We thank David Lees, Natural History Museum, London, UK, for a copy of John Heath’s unpublished manuscript on Epimartyria and Lees and George Gibbs, Wellington, New Zealand, for their thoughtful comments and suggestions to improve the manuscript. Support for DNA barcoding was provided by The National Science and Engineering Council of Canada (Canadian Barcode of Life Network sub-grant 047888 to JFL), Genome Canada through the Ontario Genomics Institute to P.D.N. Hebert, and other sponsors listed at www.BOLDNET.ca
Sample information for the Epimartyria specimens included in the DNA barcode analysis. Sample IDs are specimen identifiers; Barcode IDs (or Process IDs in BOLD) are sequence identifiers. Details of collecting data, images, sequences, and trace files for the 44 specimens listed are available in the Barcode of Life Database (BOLD) (www.barcodinglife.org) in the project codes indicated.
| Species of Epimartyria | Locality | Sample ID | Barcode ID | GenBank Accession | Sequence Length | BOLD Project Code |
|---|---|---|---|---|---|---|
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00002816 | MNAC969-11 | JN306512 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00002817 | MNAC970-11 | JN306513 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00002818 | MNAC971-11 | JN306514 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00002819 | MNAC972-11 | JN306515 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00007712 | MNAC974-11 | JN306516 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00007713 | MNAC975-11 | JN306517 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00007714 | MNAC976-11 | JN306518 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00007715 | MNAC977-11 | JN306519 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00007716 | MNAC978-11 | JN306520 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00007717 | MNAC979-11 | JN306521 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00007718 | MNAC980-11 | JN306522 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00007719 | MNAC981-11 | JN306523 | 648[0n] | ZEUNA |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00007720 | MNAC982-11 | JN306524 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | USA: MI: Wilderness SP | CNCLEP00068782 | MNAC984-11 | JN306525 | 585[0n] | ZEUNA |
| Epimartyria auricrinella | USA: MI: Wilderness SP | CNCLEP00068783 | MNAC985-11 | JN306526 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | USA: MI: Wilderness SP | CNCLEP00068785 | MNAC987-11 | JN306527 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00068787 | MNAC989-11 | JN306528 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | CNCLEP00068788 | MNAC990-11 | JN306529 | 658[0n] | ZEUNA |
| Epimartyria auricrinella | USA: TN: UoTennessee Field Stn | DNA-ATBI-3323 | LGSMD549-05 | GU088371 | 658[0n] | LGSMD |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | jflandry0031 | MEC031-04 | GU095823 | 628[1n] | MEC |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | jflandry0725 | MEC725-04 | GU095821 | 658[0n] | MEC |
| Epimartyria auricrinella | Canada: QC: lac Brûlé | jflandry0726 | MEC726-04 | GU095822 | 658[0n] | MEC |
| Epimartyria bimaculella | Canada: BC: Glacier NP | 10BBCLP-2914 | BBLPD916-10 | JN801469 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00067716 | MNAJ565-09 | GU693563 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00076632 | MNAC991-11 | JN306530 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00076633 | MNAC992-11 | JN306531 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00076634 | MNAC993-11 | JN306532 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00076635 | MNAC994-11 | JN306533 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00076636 | MNAC995-11 | JN306534 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00076637 | MNAC996-11 | JN306535 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00076638 | MNAC997-11 | JN306536 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00076639 | MNAC998-11 | JN306537 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00076640 | MNAC999-11 | JN306538 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00076641 | MNAC1000-11 | JN306509 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00077846 | MNAL648-10 | HQ965294 | 658[1n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00077847 | MNAL649-10 | HQ965295 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00077848 | MNAL650-10 | HQ965296 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00077849 | MNAL651-10 | JN801470 | 315[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00077850 | MNAL652-10 | HQ965297 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00077851 | MNAL653-10 | JN801471 | 658[12n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00077852 | MNAL654-10 | HQ965298 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | Canada: BC: Belcarra Pk | CNCLEP00077853 | MNAL655-10 | HQ965299 | 658[0n] | ZEUNA |
| Epimartyria bimaculella | USA: WA: 14.5 km E Skykomish | CNCLEP00082605 | MNAD991-11 | JN801472 | 658[0n] | ZEUNA |
| Epimartyria pardella | USA: CA: Kneeland | DRD-08-4201 | NAMUM303-08 | JN801473 | 658[0n] | ZEUNA |
Specimen data for haplotype analysis of Epimartyria. Haplotypes marked with an asterisk were selected for the parsimony analysis.
| Species | Hap | SampleID | Length | Dissection | Sex | Locality | Collecting Date |
|---|---|---|---|---|---|---|---|
| Epimartyria auricrinella | A1 | CNCLEP00002817 | 658 | M | QC: lac Brûlé | 29-Jun-2003 | |
| Epimartyria auricrinella | A1 | CNCLEP00002818 | 658 | M | QC: lac Brûlé | 29-Jun-2003 | |
| Epimartyria auricrinella | A1 | CNCLEP00002819 | 658 | M | QC: lac Brûlé | 29-Jun-2003 | |
| Epimartyria auricrinella | A1 | CNCLEP00007713 | 658 | MIC 5758 | F | QC: lac Brûlé | 08-Jul-2002 |
| Epimartyria auricrinella | A1* | CNCLEP00007714 | 658 | MIC 5762 | M | QC: lac Brûlé | 08-Jul-2002 |
| Epimartyria auricrinella | A1 | CNCLEP00007715 | 658 | M | QC: lac Brûlé | 08-Jul-2002 | |
| Epimartyria auricrinella | A1 | CNCLEP00007716 | 658 | F | QC: lac Brûlé | 08-Jul-2002 | |
| Epimartyria auricrinella | A1 | CNCLEP00007717 | 658 | MIC 5763 | F | QC: lac Brûlé | 08-Jul-2002 |
| Epimartyria auricrinella | A1 | CNCLEP00007720 | 658 | MIC 5757 | M | QC: lac Brûlé | 08-Jul-2002 |
| Epimartyria auricrinella | A1 | CNCLEP00068787 | 658 | F | QC: lac Brûlé | 02-Jul-2000 | |
| Epimartyria auricrinella | A1 | jflandry0726 | 658 | USNM 34322 | M | QC: lac Brûlé | 04-Jul-2004 |
| Epimartyria auricrinella | A2* | CNCLEP00007712 | 658 | MIC 5755 | M | QC: lac Brûlé | 08-Jul-2002 |
| Epimartyria auricrinella | A3* | CNCLEP00068788 | 658 | MIC 5756 | M | QC: lac Brûlé | 02-Jul-2000 |
| Epimartyria auricrinella | A4* | DNA-ATBI-3323 | 658 | MIC 5759 | F | TN: UoTN stn | 22-May-2005 |
| Epimartyria auricrinella | A5 | CNCLEP00002816 | 658 | M | QC: lac Brûlé | 29-Jun-2003 | |
| Epimartyria auricrinella | A5* | CNCLEP00007718 | 658 | MIC 5753 | M | QC: lac Brûlé | 08-Jul-2002 |
| Epimartyria auricrinella | A5 | jflandry0725 | 658 | MIC 5754 | F | QC: lac Brûlé | 04-Jul-2004 |
| Epimartyria auricrinella | A5? | CNCLEP00007719 | 648 | MIC 5760 | M | QC: lac Brûlé | 08-Jul-2002 |
| Epimartyria auricrinella | A5? | jflandry0031 | 628 [1n] | MIC 5761 | M | QC: lac Brûlé | 29-Jun-2003 |
| Epimartyria auricrinella | A6* | CNCLEP00068783 | 658 | MIC 5752 | M | MI: Wilderness SP | 30-Jun-1992 |
| Epimartyria auricrinella | A6? | CNCLEP00068782 | 585 | M | MI: Wilderness SP | 30-Jun-1992 | |
| Epimartyria auricrinella | A7* | CNCLEP00068785 | 658 | MIC 5764 | M | MI: Wilderness SP | 30-Jun-1992 |
| Epimartyria bimaculella | B1 | CNCLEP00076633 | 658 | M | BC: Belcarra | 24-May-2009 | |
| Epimartyria bimaculella | B1 | CNCLEP00076635 | 658 | MIC 5771 | F | BC: Belcarra | 01-Jun-2009 |
| Epimartyria bimaculella | B1 | CNCLEP00076638 | 658 | M | BC: Belcarra | 01-Jun-2009 | |
| Epimartyria bimaculella | B1 | CNCLEP00076639 | 658 | MIC 5767 | M | BC: Belcarra | 01-Jun-2009 |
| Epimartyria bimaculella | B1 | CNCLEP00076640 | 658 | M | BC: Belcarra | 02-Jun-2009 | |
| Epimartyria bimaculella | B1 | CNCLEP00077846 | 658 | M | BC: Belcarra | 01-Jun-2009 | |
| Epimartyria bimaculella | B1* | CNCLEP00077847 | 658 | MIC 5765 | M | BC: Belcarra | 01-Jun-2009 |
| Epimartyria bimaculella | B1 | CNCLEP00077848 | 658 | M | BC: Belcarra | 01-Jun-2009 | |
| Epimartyria bimaculella | B1 | CNCLEP00077852 | 658 | M | BC: Belcarra | 01-Jun-2009 | |
| Epimartyria bimaculella | B2 | 10BBCLP-2914 | 658 | MIC 5769 | F | BC: Glacier NP | 16-Jul-2010 |
| Epimartyria bimaculella | B2 | CNCLEP00076632 | 658 | M | BC: Belcarra | 24-May-2009 | |
| Epimartyria bimaculella | B2 | CNCLEP00076634 | 658 | MIC 5770 | M | BC: Belcarra | 01-Jun-2009 |
| Epimartyria bimaculella | B2 | CNCLEP00076636 | 658 | M | BC: Belcarra | 01-Jun-2009 | |
| Epimartyria bimaculella | B2 | CNCLEP00076637 | 658 | M | BC: Belcarra | 01-Jun-2009 | |
| Epimartyria bimaculella | B2 | CNCLEP00076641 | 658 | M | BC: Belcarra | 02-Jun-2009 | |
| Epimartyria bimaculella | B2 | CNCLEP00077850 | 658 | M | BC: Belcarra | 01-Jun-2009 | |
| Epimartyria bimaculella | B2 | CNCLEP00077853 | 658 | M | BC: Belcarra | 01-Jun-2009 | |
| Epimartyria bimaculella | B2* | CNCLEP00082605 | 658 | MIC 5739 | M | WA: Skykomish | 08-Jul-2010 |
| Epimartyria bimaculella | B3* | CNCLEP00067716 | 658 | MIC 5768 | M | BC: Belcarra | 08-Jun-2008 |
| Epimartyria bimaculella | B? | CNCLEP00077849 | 315 | M | BC: Belcarra | 01-Jun-2009 | |
| Epimartyria bimaculella | B? | CNCLEP00077851 | 658[12n] | MIC 5766 | M | BC: Belcarra | 01-Jun-2009 |
| Epimartyria pardella | P1* | DRD-08-4201 | 658 | ? | CA: Kneeland | 21-Jun-2008 |