(C) 2012 Anna Zhadan. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new species of the genus Laubieriopsis Petersen, 2000 is described based on 28 specimens collected in the north-east part of the North Sea. It is characterized by fixed number of chaetigers (22), paired genital papillae, bidentate neurochaeta of chaetigers 1–4, the absence of acicular chaetae on chaetigers 5–21 and, on the last chaetiger, one acicular and three capillary chaetae enlarged and directed backward. The present study brings the number of known species of Laubieriopsis to five and the number of Northeast Atlantic species of this genus to two.
Annelida, Polychaeta, Fauveliopsidae, Laubieriopsis norvegica, new species, taxonomy, Norway, Northeast Atlantic
The Fauveliopsidae are a small (about 20 species) family of detritus-feeding polychaetes. They are small benthic animals, mainly found on deep bottoms down to 6000 m (
The family Fauveliopsidae was erected by
The internal morphology and ultrastructure of fauveliopsids have been poorly studied.
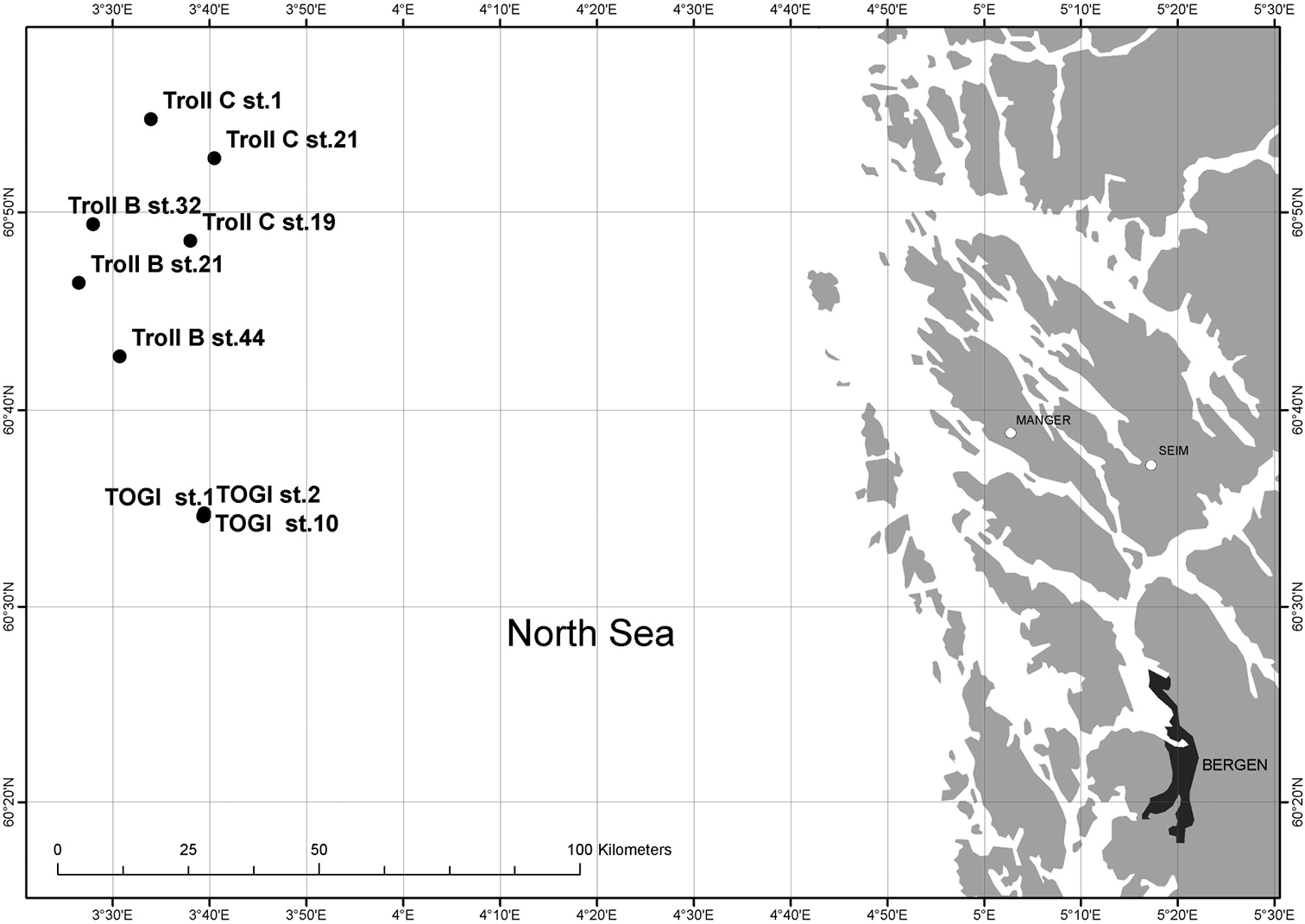
Specimens of Laubieriopsis sp. were collected in the North Sea (60°34'N, 03°41'E; 03°26'E, 60°54'N) (Fig. 1) by the company Akvaplan-niva in May 1998 (collector Andrey Sikorski). All specimens were fixed in a 4% formaldehyde seawater solution and later transferred to 70% ethanol. The material is stored in the Zoological Museum of M.V. Lomonosov Moscow State University (ZMMU).
Map of stations of Akvapla-niva, 1998, showing findings of Laubieriopsis norvegica sp.n.
Twenty-one specimens were examined under a stereomicroscope after dehydration in a glycerin series. Additional data were obtained using scanning electron microscopy: seven specimens were critical point-dried after dehydration in an ethanol series and acetone, and then coated with gold prior to examination with a Scanning Electron Microscope HITACHI S-405 A and Camscan-S2.
ResultsBody cylindrical, weakly divided into two regions by segment shape and type of chaetae. Fixed number of segments. Cuticle smooth, without papillae or papillae minute and inconspicuous. Chaetae include capillaries and acicular types, some might be bidentate. Interramal papillae small, mostly sessile. Last segment similar in size to preceding ones, often bilobed, pygidium retracted inside it.
urn:lsid:zoobank.org:act:5D46C0C3-6974-4683-81BF-C09964602392
http://species-id.net/wiki/Laubieriopsis_norvegica
Figs 2, 3, 4Norway, North Sea, 60°52.69'N, 03°40.48'E, 340 m, mud. Akvaplan-niva, Troll C stn. 21–4; 14.05.1998. coll. A. Sikorski.
Holotype female with 32 oocytes, 6.8 mm long; width in dorsal view on chaetiger 4–400 µm, stored in 70% alcohol. Original label: “Spec.: Polychaeta indet., stn.: 21–4, Date: 14.05. Troll C 1998. Akvaplan-niva A.Sikorski”. ZMMU № W141 HOLOTYPE, Laubieriopsis norvegica (printed label).
Paratypes: Akvaplan-niva Troll C stn. 21–4, 60°52.69'N, 03°40.48'E, 14.05.1998, 340 m, mud, one specimen without visible gametes, ZMMU № W142. Akvaplan-niva Troll C stn. 19–3, 60°48.54'N, 03°38.03'E, 14.05.1998, 334 m, three specimens, two without visible gametes, one female with 39 oocytes, ZMMU № W143. Akvaplan-niva Troll C stn 1–5, 60°54.65'N, 03°33.95'E, 15.05.1998, 341 m, two specimens without visible gametes, ZMMU № W144. Akvaplan-niva TOGI stn.10–4, 60°34.58'N, 03°39.34'E, 10.05.1998, 304 m, four specimens, three without visible gametes, one male with sperm, ZMMU № W144. Akvaplan-niva TOGI stn.1–4, 60°34.59'N, 03°39.45'E, 9.05.1998, 305 m, three specimens with small oocytes, ZMMU № W146. Akvaplan-niva TOGI stn. 2–4, 60°34.72'N, 03°39.46' E, 9.05.1998, 305 m, one specimen, male with sperm, ZMMU № W147. Akvaplan-niva Troll B, stn.32–3, 60°49.37'N, 03°27.98'E, 14.05.1998, 335 m, three specimens, two females with oocytes, one without visible gametes, ZMMU № W148. Akvaplan-niva Troll B, stn. 21–3, 60°46.40'N, 03°26.51'E, 14.05.1998, 322 m, two specimens without visible gametes, ZMMU № W149. Akvaplan-niva Troll B, stn.44–4, 60°42.68'N, 03°30.74'E, 14.05.1998, 319 m, one specimen without visible gametes, ZMMU № W150.
Adult specimens with 22 chaetigers. First four chaetigers with sigmoidal acicular chaetae, neurochaetae often bidentate. Chaetigers 5–21 without acicular chaetae, with one thin capillary chaeta and one very small and thin additional chaeta in each ramus. Chaetiger 22 with one thick acicular notochaetae and three capillary neurochaetae. Two weltlike genital papillae present at posterior edge of segment 8.
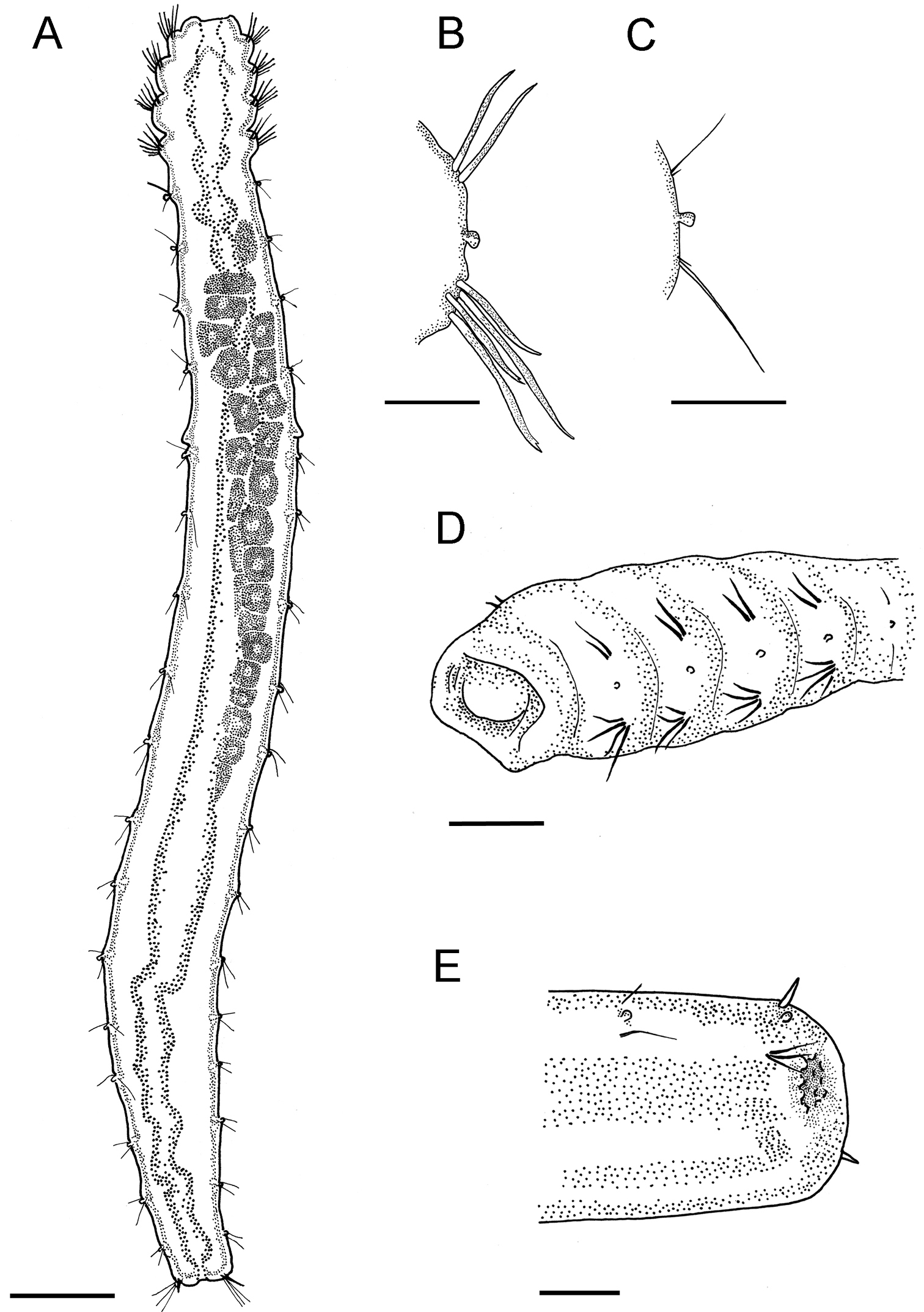
Adult specimens 6–8 mm long and 0.35–0.4 mm wide with 22 chaetigers. Only one specimen has 23 chaetigers. Body slender, often c–shaped or s-shaped by fixation, colorless in preserved material. Four anteriormost chaetigers swollen, following segments cylindrical, without clear borders (Fig. 2A, 3A, B).
Laubieriopsis norvegica sp.n. A dorsal view of female with oocytes B parapodia of chaetiger 3 C parapodia of chaetiger 14 D anterolateral view of anterior end (prostomium visible) E dorsolateral view of posterior end. Scale (μm): A – 400, B, D, E – 100, C – 30.
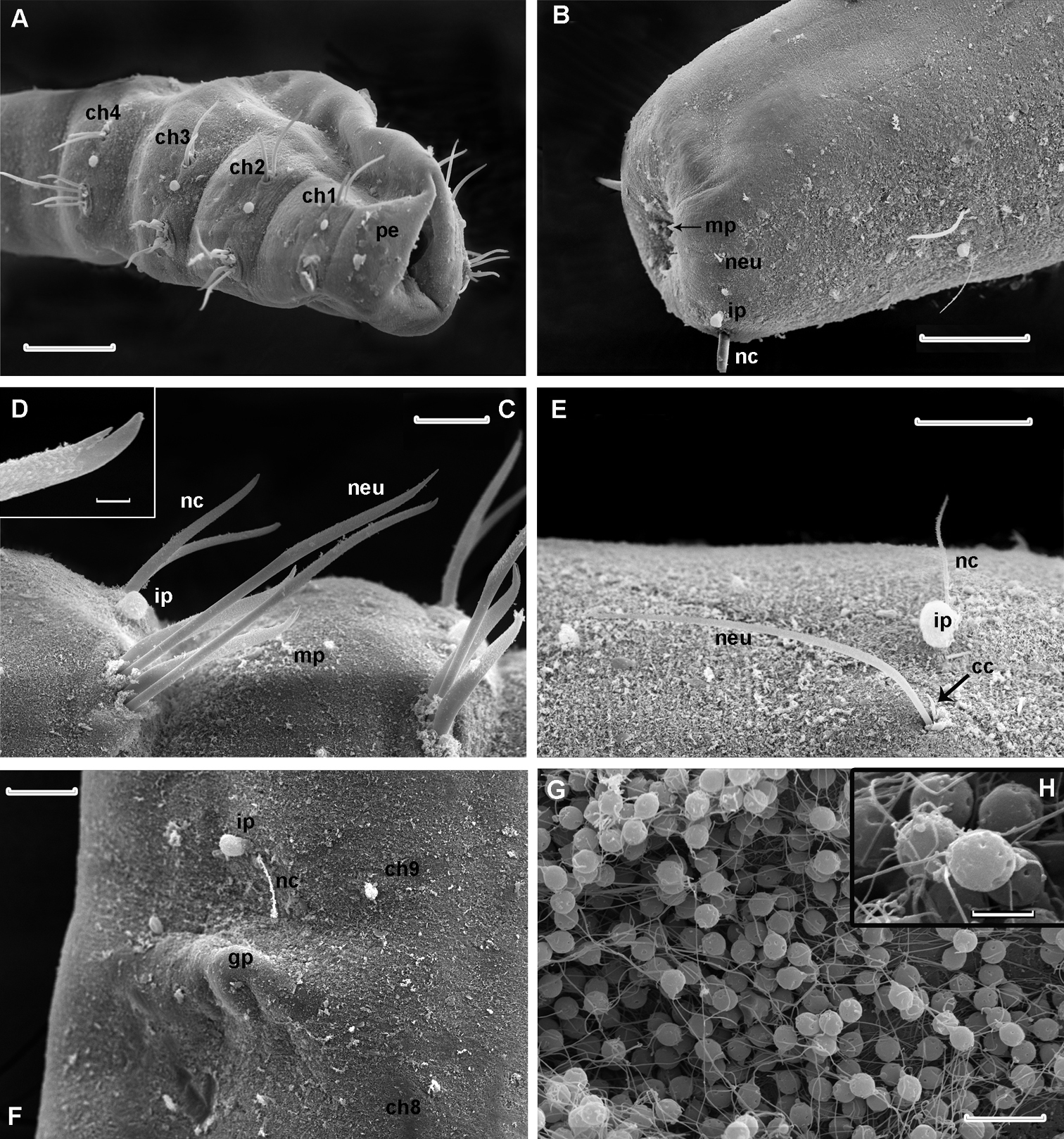
Laubieriopsis norvegica sp.n., A entire view from the dorsal side B anterior end, ventral view C posterior end, ventral view D micropapillae E area of chaetigers 7–9, genital papillae. Scale (μm): A – 500; B– 200; C, E – 100; D– 50.
Laubieriopsis norvegica sp.n. A anterior end, lateral view B posterior end, ventro-lateral view C parapodia of chaetigers 2–3 D tip of neurochaeta enlarged E parapodia of chaetiger 12 F genital papilla and parapodia of chaetiger 9; neurochaetae are broken G, H sperm cell. Scale (μm): A, B – 100, C–30, D – 3, E, F – 30, G – 10, H – 2. Abbreviation: cc accessory chaeta, ch1–ch4 chaetigers 1–4, ch8 chaetiger 8, ch9 chaetiger 9, gp genital papilla, ip interramal papilla, mp micropapilla, neu neurochaeta, nc notochaeta, oc oocyte, pe peristomium, pr prostomium
Cuticle thick (about 8 μm), smooth, with very thin ring wrinkles, bearing scattered minute inconspicuous micropapillae (Fig. 3D, 4C), visible under higher magnification and in SEM. Relatively larger micropapillae surrounding inverted parts – prostomium and pygidium (Fig. 4B).
Ventral nerve cord visible by transparency. Ganglia longitudinally elongated and indistinctly separated from each other.
Prostomium small, from round to triangular, lacking appendages, with ciliary nuchal organs, without eyes (Fig. 2D, 3B). In most specimens prostomium completely retracted into peristomium (Fig. 4A). Peristomium forms complete ring; it bears micropapillae.
Parapodia biramous, best developed on chaetigers 1–4 and hardly distinct on posteriormost segments except last one (22). Interramal papillae short–stalked, pyriform, situated midway between noto- and neuropodia (Fig. 3C, 4A, B, C, E, F). Two epidermal glands visible in each parapodium.
Chaetigers 1–4 bear thick sigmoidal hirsute chaetae. Two chaetae in notopodia and four (2 long and 2 shorter ones) in neuropodia (Fig. 2B, 4C). Some specimens possess five instead of four neurochaetae in anterior segments. Neurochaetae mostly bidentate (Fig. 4D), notochaetae usually unidentate, sometimes slightly bidentate. Some specimens possess 3–4 bidentate neurochaetae whereas others have only 1–2 bidentate neurochaetae. Both short and long neurochaetae can be bidentate or unidentate. Two specimens have transitional fifth segment with chaetae more similar to acicular chaetae of anterior four segments than to thin capillary chaetae of rest of the body.
Chaetigers 5–21 bear one thin capillary chaeta and one accessory very short, thin and barely distinct capillary chaeta per ramus (Fig. 2C, 4E, F). Notopodial chaetae thinner and shorter than neuropodial ones; accessory chaetae in notopodia often absent.
Chaetiger 22 bears one thick acicular chaeta in notopodia and three capillary chaetae in neuropodia (Fig. 2E, 3C, 4B).
Paired genital papillae (Fig. 3E, 4F) situated at posterior edge of chaetiger 8 in all studied individuals with gametes as well as in specimens without recognizable gametes, except for one specimen with unpaired genital papilla.
Pygidium retracted within last chaetiger, anus terminal. Boundary between distal part of last segment and pygidium indistinct. Inverted part (distal to chaetae) with a number of conical papillae, which are larger than in rest of body (Fig. 2E, 3C, 4B).
Oocytes up to 90 μm in diameter, arranged in two ovisacs, extend through chaetigers 6–15, each contains 20–40 oocytes (Fig. 2A, 3A, E).
Sperm cells hardly visible through body wall, but observed in SEM in body cavity of dissected specimen. They have rounded heads about 3 μm in diameter, an acrosome with elongated distal part and free flagellum at least 10 μm long (Fig. 4G, H).
The species name refers to its type locality.
Northeast part of the North Sea.
Inhabits muddy sediments with small admixture (0.8 – 7.7 %) of fine and medium sand, in depth of 300–350 m.
Laubieriopsis norvegica sp. n. differs from all previously described species of the family Fauveliopsidae by the highly reduced chaetal bunds in median and posterior parapodia. Whereas all fauveliopsid species have one, rarely two acicular spines in each parapodial ramus, noto- and neuropodia of chaetigers 5–21 in Laubieriopsis norvegica sp. n bear one fine, slender capillary and one even smaller chaeta which may be absent.
Species of the genus Laubieriopsis are characterized by a fixed number of chaetigers; previously described species have 16, 21 and 25 chaetigers (
Our study confirms Petersen’s (2000) statement on characters useful for distinguishing species in the genus Laubieriopsis: number of chaetigers, number of anterior chaetigers with acicular chaetae, presence of bidentate anterior neurochaetae, enlarged and directed backward chaetae of the last chaetiger, and paired/unpaired genital papilla. Interestingly, one of 28 specimens studied here has 23 instead 22 thoracic chaetigers and another one has an unpaired genital papilla. As intraspecific variability was noted for all characters (present work,
There was only one species of the genus Laubieriopsis previously known from the Northeast Atlantic, Laubieriopsis cabiochi. The present study increases the number of known species of Laubieriopsis to five and the number of Northeast Atlantic species of this genus to two.
We are grateful to Andrey Sikorski (Akvaplan-niva, Norway) who kindly provided the material. We thank Anton Makarov (Moscow State University, Russia) for making the map. We are very thankful to Alexander Tzetlin (Moscow State University, Russia) for his permanent patience and great support. The present study was supported by the Russian Foundation for Basic Research, projects № 11-04-01695, 10-04-01547, and federal target program of the Ministry of Education and Science of Russian Federation.