(C) 2012 Gerrit Karssen. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Holo- and paratypes of the root-knot nematodes Meloidogyne mayaguensis Rammah & Hirschmann, 1988 and Meloidogyne enterolobii Yang & Eisenback, 1983 were morphometrically and morphologically compared. All observed female, male and second-stage juvenile morphometrical and morphological characters are identical for the two studied species. Additionally, contradictions between the original species descriptions were unravelled.
The present study of holo- and paratypes confirms the taxonomical status of Meloidogyne mayaguensis as a junior synonym for Meloidogyne enterolobii.
Junior synonym, Meloidogyne, Meloidogyne enterolobii, Meloidogyne mayaguensis, Nematoda, root-knot nematode, sy- nonymisation
In
It was
In their comprehensive studies on the characterisation of Meloidogyne species from China, with isozymes and mtDNA,
In 2005–2006 we compared the available holo- and paratypes of Meloidogyne enterolobii and Meloidogyne mayaguensis. Meanwhile our Chinese co-authors collected live Meloidogyne enterolobii material on Hainan Island at the type locality from the type host and we kindly received live Meloidogyne mayaguensis type material from Dr. V. Blok (originating from Dr. M. Fargette). The preliminary isozyme and morphological results were presented by the first author during a Pest Risk Analysis meeting on Meloidogyne enterolobii at EPPO in Paris (
Finally, as again at DNA level no differences were found, the two species were synonymised: “The species Meloidogyne enterolobii (syn. Meloidogyne mayaguensis)” and “…of Meloidogyne mayaguensis (junior synonym of Meloidogyne enterolobii)” (
Although taxonomical not strictly necessary, we present herein a morphological and morphometrical comparison between the holo- and paratype slides of Meloidogyne mayaguensis and Meloidogyne enterolobii. Additionally we discuss anomalies between the descriptions of Meloidogyne mayaguensis and Meloidogyne enterolobii.
Material and methodsHolo- and paratype slides (Table 1) originating from USDA Nematode Collection (USDANC), Beltsville, USA were kindly provided by Dr. Z. Handoo. The type slides are in good condition and includes female holotypes, male allotypes, perineal patterns and second-stage juvenile paratypes. These slides were observed by compound light microscopy (Olympus BH-2 and Zeiss Axio Imager), including Differential Interference Contrast and photographed by Leica DMC-50 digital camera. For the overall morphological and morphometrical comparison between the types we focussed on the most differential and supplementary Meloidogyne characters, as described by
Meloidogyne mayaguensis and Meloidogyne enterolobii holo-, allo- and paratype slides studied, including USDANC codes.
| Meloidogyne mayaguensis | Meloidogyne enterolobii | |||
|---|---|---|---|---|
| Holotype | 1 female | T-428t | 1 female | T-360t |
| Allotype* | 1 male | T-429t | 1 male | T-361t |
| Paratype | 10 perineal patterns | T-3849p | 8 perineal patterns | T-3147p |
| Paratype | 6 males | T-3843p | 10 males | T-3149p |
| Paratypes | 25 J2’s | T-3846/7p | 25 J2’s | T-3152p |
*According to the ICZN rules (4th edition) the allotype concept is no longer valid, and treated herein as a paratype.
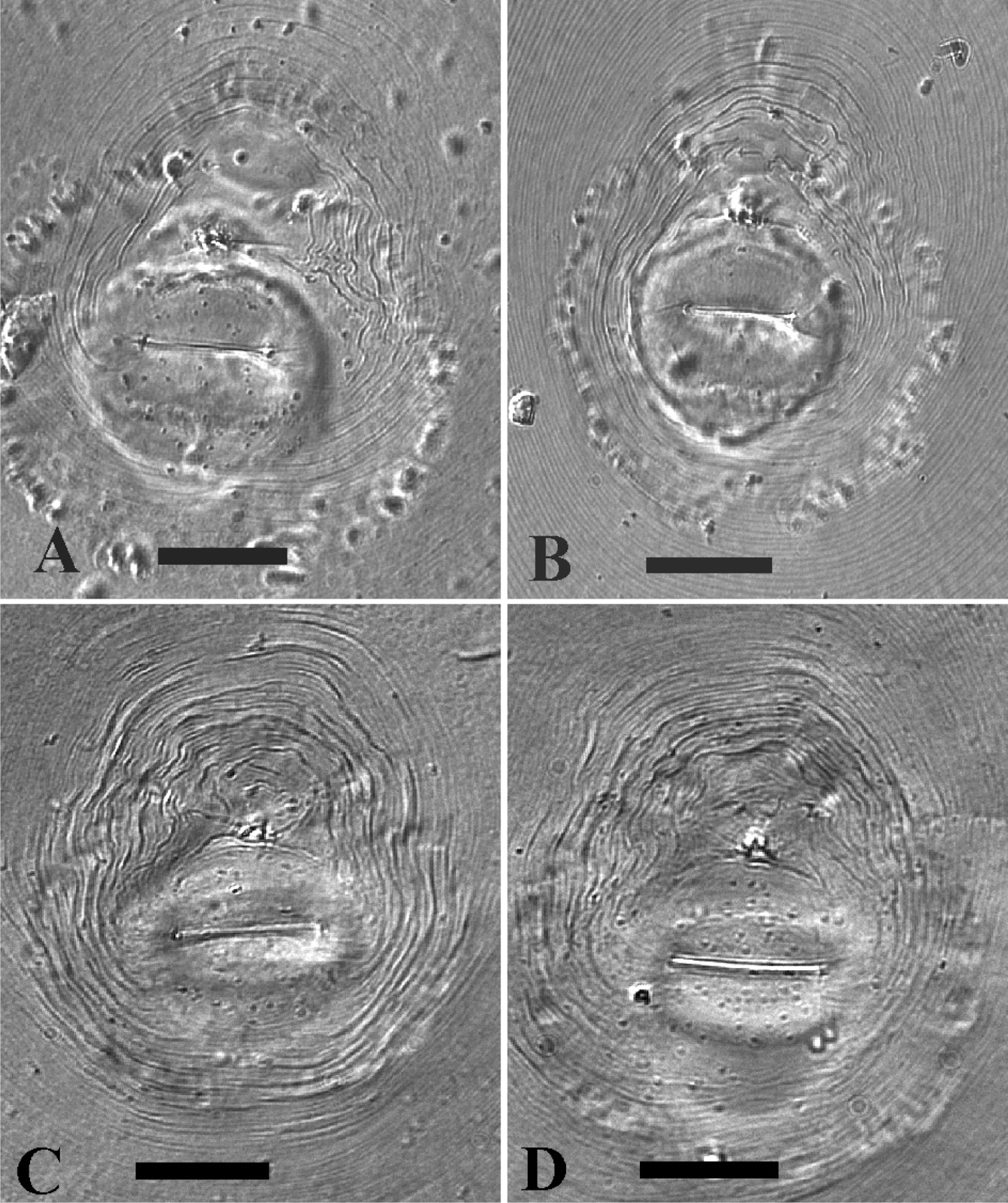
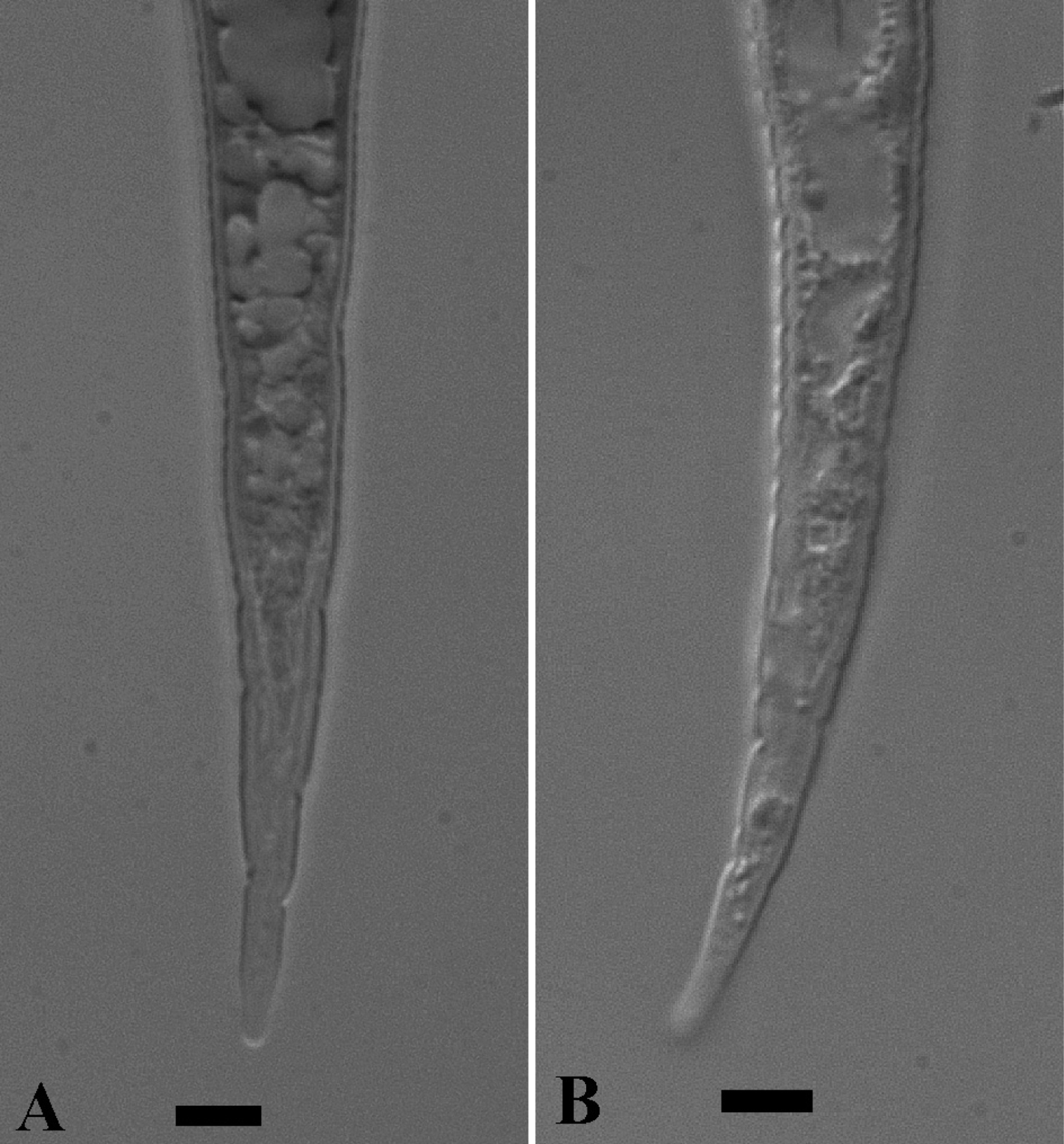
See Figure 1 and 2 for LM photographs of female and second-stage juvenile morphological characteristics.
LM photographs of perineal patterns of Meloidogyne mayaguensis (A, B) and Meloidogyne enterolobii (C, D). Bar = 25 µm.
LM photographs of second-stage juvenile tails of Meloidogyne mayaguensis (A) and Meloidogyne enterolobii (B). Bar = 5 µm.
See Table 2–5 for respectively female, male and second-stage juvenile morphological and morphometrical observations.
Morphological observations of primary female, male and second-stage juvenile characters of Meloidogyne mayaguensis and Meloidogyne enterolobii holo- and paratypes compared to described data.
| Species | Meloidogyne mayaguensis | Meloidogyne enterolobii | |
| Character | described | observed | |
| Female | |||
| Stylet knobs | knobs reniform or transversely elongated, distinctly indented, merging gradually with shaft | knobs set off from shaft, and divided longitudinally by groove so that each knob appears as two | oval, anteriorly often indented, slightly sloping backward to set off |
| Perineal pattern | round to dorso-ventrally ovoid, dorsal arch rounded, striae fine, single lateral line may occur | oval shaped, with coarse and smooth striae, dorsal arch moderately high to high, often rounded, nearly square in some, lateral lines not distinct | oval shaped, striae mostly fine, dorsal arch rounded to square, weak lateral line(s) sometimes present |
| Male | |||
| Head shape | head not set off, shallowly rounded to truncate, head region high without annulations | head cap high and rounded, head region only slightly set off from body | head cap high and rounded, head region slightly set off, not annulated |
| Stylet knobs | knobs large, set off from shaft, rounded, sloping backward, dorsal knob base concave | knobs large, rounded, distinctly set off, in some specimens each knob divided longitudinally | knobs large, ovoid to rounded, slightly sloping backwards |
| Second-stage juvenile | |||
| Stylet knobs | knobs small, rounded, set off from shaft, distinctly sloping backward | knobs large, rounded, set off from shaft | knobs ovoid to rounded, slightly sloping backwards |
| Tail shape | slender, gradually tapering to bluntly rounded tip | very thin, tip broad, bluntly rounded | slender, posterior part nearly straight and parallel, tapering to rounded tip |
| Hyaline tail part | distinctly set off, often containing small fat droplet at tip | clearly defined, a few fat droplets may occur in terminus | anterior part not clearly delimitated |
The important morphological characters, like female stylet knob and perineal pattern shape do not differ between the species, as can already be observed by comparing the original illustrations between Meloidogyne mayaguensis and Meloidogyne enterolobii (see original descriptions respectively Fig. 2 A–D & Fig. 3 A–D). This perineal pattern type is not species specific within the genus Meloidogyne and can best be marked as typical for many species within the Meloidogyne incognita-group, including the observed variation within the dorsal part. Additionally we observed a relatively large tail remnant area, free of any striae, just above the covered anus (Fig. 1 A–D). Also the observed stylet knob position variation, slightly sloping backward to set off from the shaft, is a common Meloidogyne feature. Strangely this variation is also clearly visible in the SEM photographs of excised female stylets of Meloidogyne mayaguensis (see original description, Fig. 3 A-C), but not described. With the light microscope one can observe a weak longitudinal indention, for both species, in the female stylet knobs at the anterior side. The reported differences “not divided so conspicuously as those of Meloidogyne enterolobii” as mentioned in the Meloidogyne mayaguensis description (see diagnosis original description), was not confirmed by our observations. Also the described position of one of the Meloidogyne mayaguensis stylet knobs “the dorsal knob is slightly sloping posteriad in lateral view” was not observed by us.
MalesThe male head shape for Meloidogyne mayaguensis is described as “not set off”, while a slightly set off head region was observed as described for Meloidogyne enterolobii. Comparing the original SEM pictures of the head for Meloidogyne mayaguensis and Meloidogyne enterolobii (see original descriptions respectively Fig. 6 A–D & 5 A, B) shows clearly not any differences in head morphology. Also the male stylet knobs have been SEM studied for the original descriptions (Fig. 3 E, F & Fig. 6 B) of both species. Large oval to rounded shaped knobs, slightly sloping backwards are clearly visible. This was also observed by LM for both species, however described as “rounded and set off” for Meloidogyne enterolobii and “set off from the shaft, rounded, sloping backward” for Meloidogyne mayaguensis. The later description of the knobs is rather odd, i.e. set off and sloping backward at the same time!The same results were described and observed for the second-stage juvenile knobs for both species.
Second-stage juvenilesThe second-stage juvenile stylet knob size is described as small for Meloidogyne mayaguensis and large for Meloidogyne enterolobii. We indeed observed a larger size variation for Meloidogyne enterolobii stylet knob width (2.5 – 4.0 µm) compared to Meloidogyne mayaguensis (2.2 – 2.9 µm). However when observing live second-stage juveniles, the same large stylet knob width variation was observed for both species.
As for the males, the published SEM second-stage juvenile head shape is absolute identical for Meloidogyne mayaguensis and Meloidogyne enterolobii (see original descriptions respectively Fig. 7 A–D & Fig. 8 A, B). The tail is distinctly tapering and in the posterior tail (roughly the hyaline tail part) nearly straight and running parallel for both second-stage juvenile paratypes. Also, for both species the hyaline tail part is described as “distinctly set off” or “clearly defined”. We observed for both species however not a clearly anterior delimitated hyaline tail part, in fact the body content runs deep into the hyaline tail part (Fig 2 A, B), as comparable to Meloidogyne hapla (Karssen, 2002). The second-stage juvenile drawings for both species descriptions (Fig. 4 E, F & Fig. 7 E–F) show a clearly delimitated anterior hyaline tail part, while the original photographs (Fig. 5 F, G & Fig. 9 B) do not show this at all. The fact that both descriptions did not include the hyaline tail measurements (a standard procedure), suggest strongly that the hyaline tail part is not clearly defined. Also in live second-stage juveniles we did not observe a clearly defined hyaline tail part (Table 2).
MorphometricsThe morphometrical characters between the types of Meloidogyne mayaguensis and Meloidogyne enterolobii (Table 3–5), are comparable for the described and observed data, i.e. all mean data are the same or at least within the calculated range. Body length and body width data are generally slightly smaller when comparing observed to described data, this is a well known effect due to a slight shrinking of the nematode body within permanent slides. For Meloidogyne enterolobii males we noticed however an unusual difference in greatest body width between the described 42.3 µm (37–48 µm) and observed 32.0 µm (24–39) µm data. The differences can not only be explained due to a shrinking effect, particularly as the observed greatest body width data agrees with the observed data for Meloidogyne mayaguensis. Also for the Meloidogyne enterolobii female holotype unexplainable differences were noticed between described and observed data for the DGO (3.7 µm versus 4.8 µm) and stylet length (13.4 µm versus 14.7 µm).
Morphometrical (in µm) observations (mean, SD & range) of female Meloidogyne mayaguensis and Meloidogyne enterolobii holo- (single female) and paratypes (perineal patterns) compared to described data.
| Species | Meloidogyne mayaguensis | Meloidogyne enterolobii | ||
|---|---|---|---|---|
| Character | description | observed | description | observed |
| Holotype (N) | 1 | 1 | 1 | 1 |
| Body length | 720 | 674 | 667 | 693 |
| Body width | 570 | 576 | 415 | 462 |
| Neck length | 190 | 168 | 265 | 262 |
| Neck width | 160 | 169 | -- | -- |
| DGO | 6.2 | 6.4 | 3.7 | 4.8 |
| Excretory pore tohead end | 46.4 | 45.8 | 44.8 | 64.0 |
| Stylet length | 15.1 | 15.7 | 13.4 | 14.7 |
| Stylet knob height | 2.2 | 2.0 | 2.7 | 2.3 |
| Stylet knob width | 4.4 | 4.5 | 4.3 | 4.5 |
| Paratypes (N) | 35 | 10 | 20 | 8 |
| Interphasmidial dist. | 23.2 ± 2.5 (18.1–29.6) | 28.8 ± 3.7 (24.3–33.3) | 30.7 ± 4.8 (22.2–42.0) | 33.5 ± 7.6 (22.4–41.9) |
| Vulval slit length | 26.1 ± 1.9 (20.9–30.4) | 27.0 ± 1.4 (25.0–29.4) | 28.7 ± 2.0 (25.3–32.4) | 28.0 ± 1.0 (25.9–29.1) |
| Vulva-anus distance | 18.4 ± 1.5 (12.7–21.1) | 21.4 ± 3.1 (17.0–27.1) | 22.2 ± 1.8 (19.7–26.6) | 23.4 ± 1.6 (21.1–26.2) |
| DGO | 4.8 ± 0.8 (3.5–6.7) | – | 4.9 ± 0.8 (3.7–6.2) | – |
| Excretory pore to head end | 48.2 ± 13.6 (25.9–86.6) | – | 62.9 ± 10.5 (42.3–80.6) | – |
| Stylet length | 15.8 ± 0.8 (13.8–16.8) | – | 15.1 ± 1.4 (13.2–18.0) | – |
Morphometrical (in µm) observations (mean, SD & range) of male Meloidogyne mayaguensis and Meloidogyne enterolobii paratypes compared to described data.
| Species | Meloidogyne mayaguensis | Meloidogyne enterolobii | ||
|---|---|---|---|---|
| Character | description | observed | description | observed |
| N | 30 | 7 | 20 | 11 |
| Body length | 1503 ± 142 (1175–1742) | 1431 ± 63 (1337–1496) | 1600 ± 160 (1349–1913) | 1230 ± 316 (865–1667) |
| Greatest body width | 37.8 ± 3.1 (32.2–44.4) | 34.5 ± 1.9 (32.0–37.4) | 42.3 ± 3.6 (37.0–48.3) | 32.0 ± 6.0 (23.7–39.2) |
| Stylet length | 22.9 ± 0.8 (20.7–24.6) | 22.1 ± 0.7 (20.8–23.0) | 23.4 ± 1.0 (21.2–25.5) | 21.5 ± 1.7 (19.2–23.4) |
| Stylet knob height | 3.0 ± 0.3 (2.4–3.7) | 3.2 ± 0.3 (2.6–3.4) | 3.3 ± 0.3 (2.6–3.9) | 2.5 ± 0.3 (2.1–3.2) |
| Stylet knob width | 5.0 ± 0.3 (4.3–5.6) | 5.3 ± 0.5 (4.5–5.8) | 5.4 ± 0.3 (4.5–5.8) | 4.5 ± 0.6 (3.5–5.0) |
| DGO | 4.1 ± 0.4 (3.3–5.0) | 4.1 ± 0.7 (3.2–5.1) | 4.7 ± 0.4 (3.7–5.3) | 4.7 ± 0.6 (3.7–5.8) |
| Excretory pore to head end | 166.4 ± 8.8 (147.2–180.8) | 158.6 ± 14.9 (132.5–177.9) | 178.2 ± 11.2 (159.7–206.2) | 155.8 ± 22.3 (129.9–199.7) |
| Spicule length | 28.3 ± 1.5 (24.4–31.3) | 29.0 ± 2.4 (25.6–32.3) | 30.4 ± 1.2 (27.3–32.1) | 28.0 ± 1.1 (26.2–29.4) |
| Gubernaculum length | 7.1 ± 0.6 (6.1–9.3) | 7.5 ± 1.0 (6.4–9.0) | 6.2 ± 1.0 (4.8–8.0) | 6.5 ± 0.8 (6.1–8.0) |
| Tail length | 14.3 ± 1.1 (11.3–16.3) | 13.0 ± 1.1 (10.9–14.7) | 12.5 ± 2.2 (8.6–20.2) | 11.9 ± 1.2 (10.2–13.4) |
| A | 39.9 ± 3.9 (31.1–49.6) | 41.6 ± 2, 9 (37.2–44.7) | 37.9 ± 3.2 (34.1–45.5) | 38.1 ± 4.0 (30.0–43.4) |
| C | 105.7 ± 10.0 (85.8–124.3) | 110.5 ± 10.8 (98.5–133.7) | 131.6 ± 24.2 (72.0–173.4) | 103.2 ± 23.7 (71.4–135.9) |
Morphometrical (in µm) observations (mean, SD & range) of second-stage juvenile Meloidogyne mayaguensis and Meloidogyne enterolobii paratypes compared to described data.
| Species | Meloidogyne mayaguensis | Meloidogyne enterolobii | ||
|---|---|---|---|---|
| Character | description | observed | description | observed |
| N | 35 | 25 | 30 | 25 |
| Body length | 454 ± 28 (390–528) | 420 ± 21 (386–456) | 437 ± 17 (405–473) | 408 ± 18 (380–442) |
| Greatest body width | 14.7 ± 0.5 (13.8–15.8) | 13.9 ± 0.7 (13.1–15.4) | 15.3 ± 0.9 (13.9–17.8) | 14.8 ± 2.1 (11.0–18.0) |
| Body width at anus | 10.9 ± 0.5 (10.2–12.2) | 9.8 ± 0.6 (9.0–11.2) | – | 9.8 ± 0.9 (8.0–11.0) |
| Stylet length | 11.6 ± 0.3 (11.1–12.2) | 11.5 ± 0.4 (10.9–12.1) | 11.7 ± 0.5 (10.8–13.0) | 11.3 ± 0.7 (10.5–13.0) |
| Stylet base to head end | 15.2 ± 0.3 (14.8–15.8) | 15.4 ± 0.3 (14.7–16.0) | – | 15.0 ± 0.7 (14.0–16.0) |
| Stylet knob height | – | 1.5 ± 0.1 (1.2–1.7) | 1.6 ± 0.1 (1.3–1.8) | 1.8 ± 0.3 (1.5–2.0) |
| Stylet knob width | – | 2.5 ± 0.2 (2.2–2.9) | 2.9 ± 0.3 (2.4–3.4) | 3.0 ± 0.4 (2.5–4.0) |
| DGO | 3.9 ± 0.2 (3.3–4.3) | 3.7 ± 0.4 (3.2–4.2) | 3.4 ± 0.3 (2.8–4.3) | 3.8 ± 0.3 (3.0–4.5) |
| Excretory pore to head end | 87.6 ± 3.3 (79.9–97.9) | 88.3 ± 3.0 (83.5–95.3) | 91.7 ± 3.3 (84.0–98.6) | 80.8 ± 4.4 (70.0–88.0) |
| Tail length | 54.4 ± 3.6 (49.2–62.9) | 54.2 ± 2.7 (48.7–58.5) | 56.4 ± 4.5 (41.5–63.4) | 52.1 ± 3.4 (45.0–57.0) |
| a | 30.9 ± 1.9 (26.4–34.7) | 30.1 ± 1.6 (26.9–32.8) | 28.6 ± 1.9 (24.0–32.5) | 28.0 ± 3.7 (23.3–34.6) |
| c | 8.3 ± 0.4 (7.0–9.2) | 7.8 ± 0.3 (7.1–8.4) | 7.8 ± 0.7 (6.8–10.1) | 7.9 ± 0.6 (7.0–9.0) |
| Excretory pore (%) | 19.4 ± 1.0 (17.8–22.3) | 21.1 ± 0.9 (19.2–22.7) | – | 19.8 ± 1.1 (17.6–21.9) |
The described and discussed Meloidogyne mayaguensis differences (see diagnosis original description)within the female perineal pattern for the interphasmidial distance, vulval slit length and vulva-anus distance is not confirmed by our observations. All these measurements are within the observed range. Perineal pattern measurements are generally highly variable and a logical reason for
The two species descriptions report also on the mode of reproduction and number of chromosomes, both reproduce by mitotic parthenogenesis (= apomixes) and have a somatic chromosome number of 2n = 44–45 for Meloidogyne mayaguensis and 2n = 44–46 for Meloidogyne enterolobii. In conclusion, both species have the same mode of reproduction and somatic chromosome number.
Host plantsAdditionally, both species descriptions report in their introduction part some hosts, i.e. they both previously applied the North Carolina differential host test (
The observed esterase (VS1-S1 type) and malate dehydrogenase (N1a type) isozyme patters are identical for both species and agrees with previous results (
In conclusion, the holo- and paratype material of Meloidogyne mayaguensis and Meloidogyne enterolobii is morphological and morphometrical identical and it confirms the taxonomical status of Meloidogyne mayaguensis as a junior synonym for Meloidogyne enterolobii.
This work was supported by the special fund for agro-scientific research in the public interest of China (grant no. 201103018).