(C) 2012 Michael M. Webber. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
A new scorpion species is described from the Inyo Mountains of California (USA). The presence of a strong subaculear spine, along with other characters, places the new species within Wernerius, an incredibly rare genus that until now consisted of only two species. Wernerius inyoensis sp. n. can be most easily distinguished from the other members of the genus by smaller adult size, femur and pedipalp dimensions, and differences in hemispermatophore morphology. Previous studies have suggested that the elusive nature of this genus may be attributed to low densities and sporadic surface activity. Herein, we provide another hypothesis, that Wernerius are primarily subterranean. Mitochondrial sequence data are provided for the holotype.
Vaejovis, taxonomy, 16S, COI, barcoding, Death Valley
The discovery that scorpions fluoresce under ultra-violet light (
Herein, we describe another new species of scorpion from southwestern North America that may never have been discovered without the use of ultra-violet light. The new species is represented by a single male collected from the Inyo Mountains of eastern California in 2009. Like the majority of recently discovered scorpions in North America, this species is particularly small (~16 mm) and was almost overlooked during our survey. The new species possesses a strong subaculear spine and other characters representative of Wernerius (
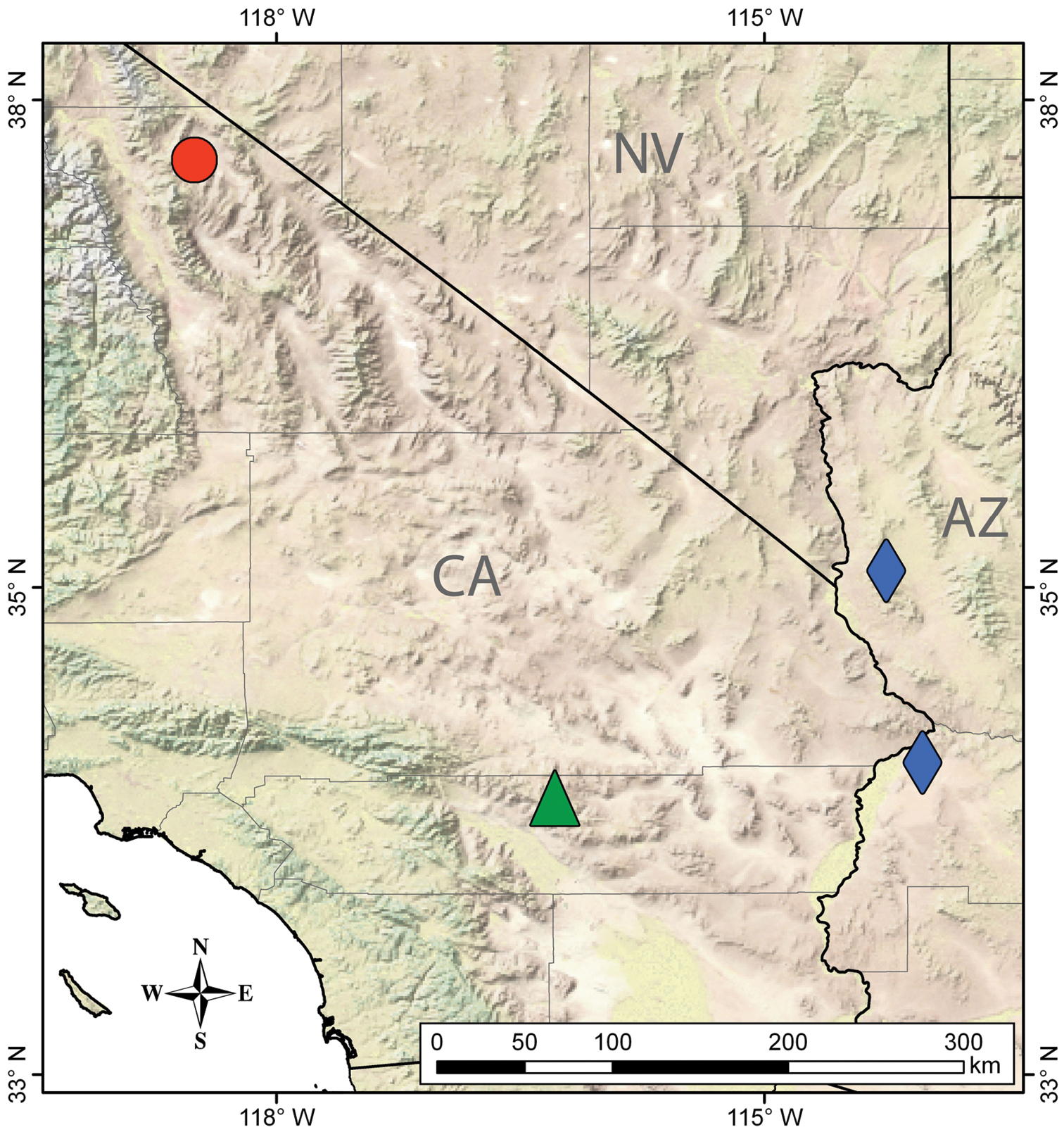
The discovery of Wernerius in the Inyo Mountains extends the range of the genus over 400 kilometers to the north, making its known distribution extremely disjunct (Fig. 1). We suspect that the three species of Wernerius must either occur at low densities, exhibit sporadic surface activity, or live subterraneously (or a combination of these) making these scorpions some of the most enigmatic and little-known in North America. For these reasons, and even though based on only a single specimen, the description of this new species is an important contribution to the growing knowledge of vaejovid scorpions. Since all three species of Wernerius are incredibly rare, we also sequenced portions of two mitochondrial genes to support DNA barcoding initiatives (
Distribution of Wernerius inyoensis sp. n. (red circle, Inyo Mountains, California) and closely related species: Wernerius spicatus (green triangle, San Bernardino Mountains, California) and Wernerius mumai (blue diamond, Parker and Black Mountain, Arizona).
Measurements are as described by
Acronyms of depositories.— DEVA, Death Valley National Park, USA; SDNHM, San Diego Natural History Museum, San Diego, California, USA.
Molecular analysisTotal genomic DNA was extracted from leg and pedipalp tissue from the left side of the holotype using a DNeasy Extraction Kit (Qiagen Inc.), leaving the right side of the voucher intact. A portion of the cytochrome oxidase subunit I (COI) gene was amplified with forward primer COI_modF (5’- ATCATAAGGATATTGGGACTATGT - 3’,
Subfamily Syntropinae Kraepelin, 1905
Tribe Stahnkeini Soleglad & Fet, 2006
Genus Wernerius Soleglad & Fet, 2008
urn:lsid:zoobank.org:act:A50D0CA5-CBE7-4C5F-9BC6-EFB155F7EFA2
http://species-id.net/wiki/Wernerius_inyoensis
Figs 1 – 14United States: California: male holotype, Loretta Mine Road, Inyo Mountains, Death Valley National Park, 37.2299°N, 117.9568°W, 1706 m, 9 September 2009, M.R. Graham and G.M. Graham Jr. (DEVA 54174).
The specific epithet refers to the type locality in the Inyo Mountains, California.
Small in size, with the only known adult male less than 17 mm in total length. Yellow-orange base color with darker carinae on the pedipalps, and segments of the metasoma. Genital operculum divided below posterior one fifth, carapace very slightly emarginate; pectine count 11–11; 7 inner (ID) denticles on the pedipalp movable finger and 6 on the fixed finger.
Although specimens of Wernerius spicatus and Wernerius mumai were not available for study (Sissom pers. comm.), based on the original descriptions of these species (
Wernerius inyoensis sp. n. in vivo.
Wernerius inyoensis sp. n.can be distinguished from Wernerius mumai by the following combination of characters: smaller adult size (of the holotype, < 17 mm), more robust femur (L/W ratio 3.54) and a shorter, thinner pedipalp, 5 OD denticles on the pedipalp movable finger in addition to 5 on the fixed finger, and ventral metasomal setae counts. Inframedian carinae are crenulate and complete on metasomal segment I, and cover the posterior half of metasomal segments II and III. A comparison of characters is provided in Table 1.
Measurements (in millimeters) of all known adult specimens in genus Wernerius.
| Wernerius inyoensis sp. n. | Wernerius spicatus | Wernerius spicatus | Wernerius spicatus | Wernerius mumai | |
|---|---|---|---|---|---|
| Sex | Male | Male | Female (Holo) | Female (Para) | Female (Holo) |
| Total Length | 16.4 | 15.9 | 17.3 | 16.1 | 24.5 |
| Carapace Length | 2.38 | 2.2 | 2.35 | 2.25 | 3.5 |
| Mesosoma Length | 4.89 | 4.95 | 6.1 | 5.65 | 8.5 |
| Metasoma Length | 7.48 | 6.65 | 6.6 | 6.05 | 9.15 |
| Met I Length | 1.05 | 0.95 | 0.9 | 0.8 | 1.35 |
| Met I Width | 1.29 | 1.1 | 1.15 | 1.1 | 1.8 |
| Met I L/W | 0.81 | 0.86 | 0.78 | 0.73 | 0.75 |
| Met I Diameter | 1.12 | N/A | N/A | N/A | N/A |
| Met II Length | 1.19 | 1.05 | 1.05 | 0.95 | 1.5 |
| Met II Width | 1.31 | 1.1 | 1.15 | 1.1 | 1.8 |
| Met II Diameter | 1.10 | N/A | N/A | N/A | N/A |
| Met III Length | 1.24 | 1.1 | 1.15 | 1.05 | 1.6 |
| Met III Width | 1.40 | 1.15 | 1.15 | 1.1 | 1.85 |
| Met III L/W | 0.89 | 0.96 | 1.0 | 0.95 | 0.86 |
| Met III Diameter | 1.05 | N/A | N/A | N/A | N/A |
| Met IV Length | 1.71 | 1.5 | 1.35 | 1.25 | 2.2 |
| Met IV Width | 1.50 | N/A | 1.25 | 1.2 | 2.1 |
| Met IV Diameter | 1.07 | N/A | N/A | N/A | N/A |
| Met V Length | 2.29 | 2.05 | 2.15 | 2 | 2.6 |
| Met V Width | 1.45 | 1.4 | 1.3 | 1.2 | 2.05 |
| Met V L/W | 1.58 | 1.46 | 1.65 | 1.67 | 1.27 |
| Met V Diameter | 1.07 | N/A | N/A | N/A | N/A |
| Telson Length | 2.27 | 2.1 | 2.25 | 2.15 | 3.35 |
| Vesicle Length | 1.67 | 1.5 | 1.65 | 1.6 | 2.55 |
| Vesicle Width | 1.10 | 1.05 | 1.05 | 1.15 | 1.85 |
| Vesicle L/W | 1.52 | 1.43 | 1.57 | 1.39 | 1.38 |
| Vesicle Diameter | 0.76 | 0.75 | 0.8 | 0.8 | 1.3 |
| Aculeus Length | 0.60 | 0.55 | 0.6 | 0.55 | 0.8 |
| Pedipalp Length | 7.95 | 6.95 | N/A | N/A | 11.6 |
| Femur Length | 2.02 | 1.95 | 2 | 1.9 | 3 |
| Femur Width | 0.57 | 0.55 | 0.6 | 0.55 | 0.95 |
| Femur L/W | 3.54 | 3.55 | 3.33 | 3.45 | 3.16 |
| Patella Length | 2.36 | 2.15 | 2.3 | 2.15 | 3.25 |
| Patella Width | 0.64 | 0.6 | 0.7 | 0.65 | 1.1 |
| Patella L/W | 3.69 | 3.58 | 3.29 | 3.31 | 2.95 |
| Chela Length | 3.57 | 2.85 | 3.65 | 3.5 | 5.35 |
| Chela L/W | 3.84 | 3.35 | 3.65 | 3.50 | 3.45 |
| Palm Length | 1.98 | N/A | 1.85 | 1.8 | N/A |
| Palm Width | 0.93 | 0.85 | 1 | 1 | 1.55 |
| Palm Diameter | 1.12 | 0.95 | N/A | N/A | 1.65 |
| Movable Finger L | 2.24 | 2.05 | 2.2 | 2.1 | 3.15 |
| Fixed Finger L | 1.79 | 1.6 | 1.8 | 1.7 | 2.45 |
| Pectine count | 11, 11 | 12, 12 | 11, 11 | 10, 11 | N/A |
| Middle lamellae count | 5, 6 | N/A | 6, 6 | 6, 6 | N/A |
| Fixed Finger L/Carapace L | 0.75 | 0.73 | 0.77 | 0.76 | 0.70 |
Color: Carapace, tergites, femur, patella, and metasoma have a yellow-orange base color with dark brown to black markings on the chela and along carinae of the metasoma. Legs are yellow and slightly lighter in color than the rest of the body. Pedipalp chela is yellow-orange in color with darker reddish-brown coloration at the anterior portion of the palm where the fixed finger and movable finger meet. Chelicerae are yellow with mottling on distal half. Telson is dark-yellow to orange bordered by dark brown carinae. Pectines and genital operculum are light yellow.
Carapace: anterior margin very slightly emarginate, with three lateral eyes on each side; moderately convex dorsolaterally; finely granular with scattered small granules, with larger granules symmetrically flanking the median furrow; median furrow is slight and traverses length of carapace, excluding the median eyes; ratio of median eyes location (from anterior edge)/carapace length = 0.32; carapace length/width at median eyes = 1.44. Tergites: slightly granular with weak median carinae from distal half of tergite I, and terminating at the middle of segment VII; strong granular dorsolateral and lateral supramedian carina on posterior 4/5s of VII; pretergites very finely granular. Sternites: I–V smooth to very finely granular and without carinae; V with granular ventral lateral carinae on posterior 1/5 to posterior 3/5. Spiracles: ovoid with median side parallel to posterior sternite margin. Genital Operculum: sclerites separated on posterior 1/5 with genital papillae protruding slightly beyond posterior of operculum plates. Pectines:tooth count 11/11; middle lamellae 5/6. Metasoma: ratio of segment I length/width 0.81; segment II length/width 0.91; segment III length/width 0.89; segment IV length/width 1.14; segment V length/width 1.58. Segments I–IV: dorsolateral carinae are strong and serrate, with distal denticle of I–IV enlarged and spinoid; denticle size is largest on segments III and IV and smaller on segments I and II; possesses intermediary carinae on segments I, II, and III; inframedian carinae are crenulate, and traverse the entire length of segment I, and ½ of the posterior portion of segments II and III, lateral supramedian carinae I–III possesses serrated granules and enlarged spinoid distal denticle; carinae of segment IV are less pronounced, crenulate to serrate, and flared on distal terminus; a space exists between the dorsolateral and supramedian carinae of segments I–III, and 1/3 of segment III; intermediary carinae are less distinct and are more granular than the ventrolateral carinae; ventral carinae are weakly serrate, but less distinct than dorsal carinae; ventrolateral carinae I strong, crenulate to serrulate; on II–III serrulate to serrate; on IV crenulate to serrate; ventral submedian setae 3/3:3/3:3/3:3/3:3/3. Segment V: dorsolateral carinae moderate, granular; lateromedian carinae moderate and granular on anterior 4/5, obsolete on distal 1/5; ventrolateral and ventromedian carinae crenulate to weakly serrate; intercarinal spaces are finely granular; ventrolateral setae 2/2:2/2:2/2:2/2:3/3.
Telson: smooth to slightly granular with very pronounced subaculear tubercule; 3/1 LAS denticles (
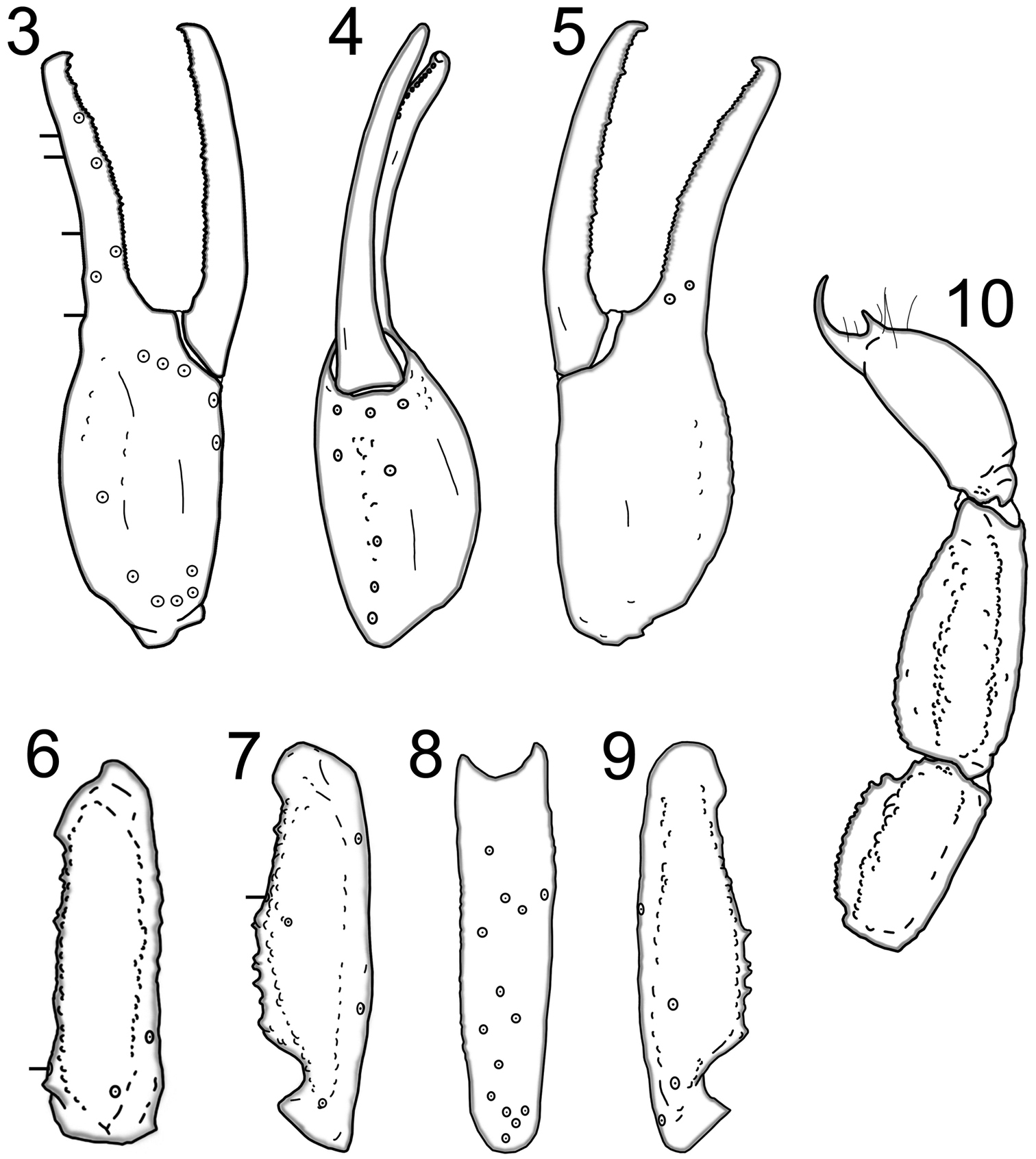
Trichobothrial patterns of Wernerius inyoensis sp. n. based on male holotype 3 Right pedipalp chela, external 4 Right pedipalp chela, ventral 5 Right pedipalp chela, internal 6 Right pedipalp femur, dorsal 7 Right pedipalp patella, dorsal 8 Right pedipalp patella, internal 9 Right pedipalp patella, ventral 10 Lateral aspect of metasomal segments IV and V, and telson.
– GCTTCTATGGTAGGGACAGCTTTGAGAT TAATAATTCGTATTGAGATTGGAAGTCCTGGGTCTTTTATTGGAGA TGATCAAATTTATAATGTTGTTGTTACTGCTCATGCTTTTGTAAT GATTTTTTTTATGGTAATACCAATTATAATTGGAGGTTTTGGAAATTG GTTAGTCCCTTTAATGTTGGGGGCTCCTGATATGGCTTTCCCTCGTT TAAATAATATAAGTTTTTGGTTATTACCTCCTGCATTTTTTTTATTATT AGGGTCAGCTTCATTGGAAAGAGGCGCAGGGACAGGCTGAACTGT GTACCCGCCTCTTTCCTCATATATGTTCCATTCTGGTGGTTCTGTT GATATGACTATTTTTTCTTTACATTTAGCTGGAGTTTCTTCAATTT TAGGAGCTATTAATTTTATTACTACTATTTTAAATATACGTATAAGTG GAATATTATTGGAGCGTATTCCTTTGTTTGTATGATCTGTAAGGAT TACTGCTATTTTATTACTTCTTTCTCTTCCCGTTCTTGCAGGGGC TATTACTATACTATTAACTGATCGAAATTTTAATACTTCTTTTTTT GATCCTGCAGGAGGGGGAGATCCCATTTTATATCAGCATT TATTTTGATTTTTTGGACATCCTGAAGTTTATATTTTAATTCTTC CTGGGTTTGGAATGGTTTCTCATATTATTAGTCATCATACTG GAAAGAGGGAGCCTTTTGGAGCTTTGGGAATGATTTATGCAATG GTTGCTATTGGGTTTTTAGGATTTGTTGTTTGGGCTCATCATAT GTTTACTGTTGGAATAGATGTTGATACTCGAGCTTATTTTACT GCTGCTACTATGGTTATTGCTGTTCCTACTGGGATCAAAATTTT TAGATGATTAGCTACTTTACATGGTTCTTATTTTGTCTTTACGC CCCCTCTTTTGTGGGCTTTGGGATTTGTTTTTCTATTTACTG TAGGAGGTTTAACTGGTGTAATTTTAGCTAATTCTTCTTTGGA TATTGTTCTTCATGATACTTATTATGTTGTAGCTCATTTTCAT TATGTTTTGTCTATAGGAGCAGTTTTTGCCATTATTGCTGGAATT GTTGAATGGTTTCCTCTATTTTTAGGTTGTCAGATGAGTGAGCG TATATTAAAAATTCATTTTTTTGTGATGCTTTTGGGGGTAAAT
Male holotype: total length 16.4; carapace length 2.38; mesosoma length 4.89; metasoma length 7.48 (excluding telson); Metasoma: segment I length/width 1.05/1.29; segment II length/width 1.19/1.31; segment III length/width 1.24/1.40; segment IV length/width 1.71/1.50; segment V length/width 2.29/1.45. Telson: length 2.27; vesicle length/width/depth 1.67/1.10/0.76; aculeus length 0.60. Pedipalps: total length 7.95; femur length/width 2.02/0.57; patella length/width 2.36/0.64; chela length 3.57; palm length/width/depth 1.98/0.93/1.12; movable finger length 2.24; fixed finger length 1.79.
Known only from the type locality in the Inyo Mountains of California where it was collected at an elevation of 1706 m.
The southwestern United States is one of most well-studied areas in the world in terms of scorpions, so it is puzzling that a genus as widespread as Wernerius is so infrequently encountered. Previous authors have attributed their rarity to low densities or sporadic surface activity (
Recent studies of invertebrates inhabiting the deep soil strata (euedaphon) in Bulgaria have revealed a rich spider fauna (
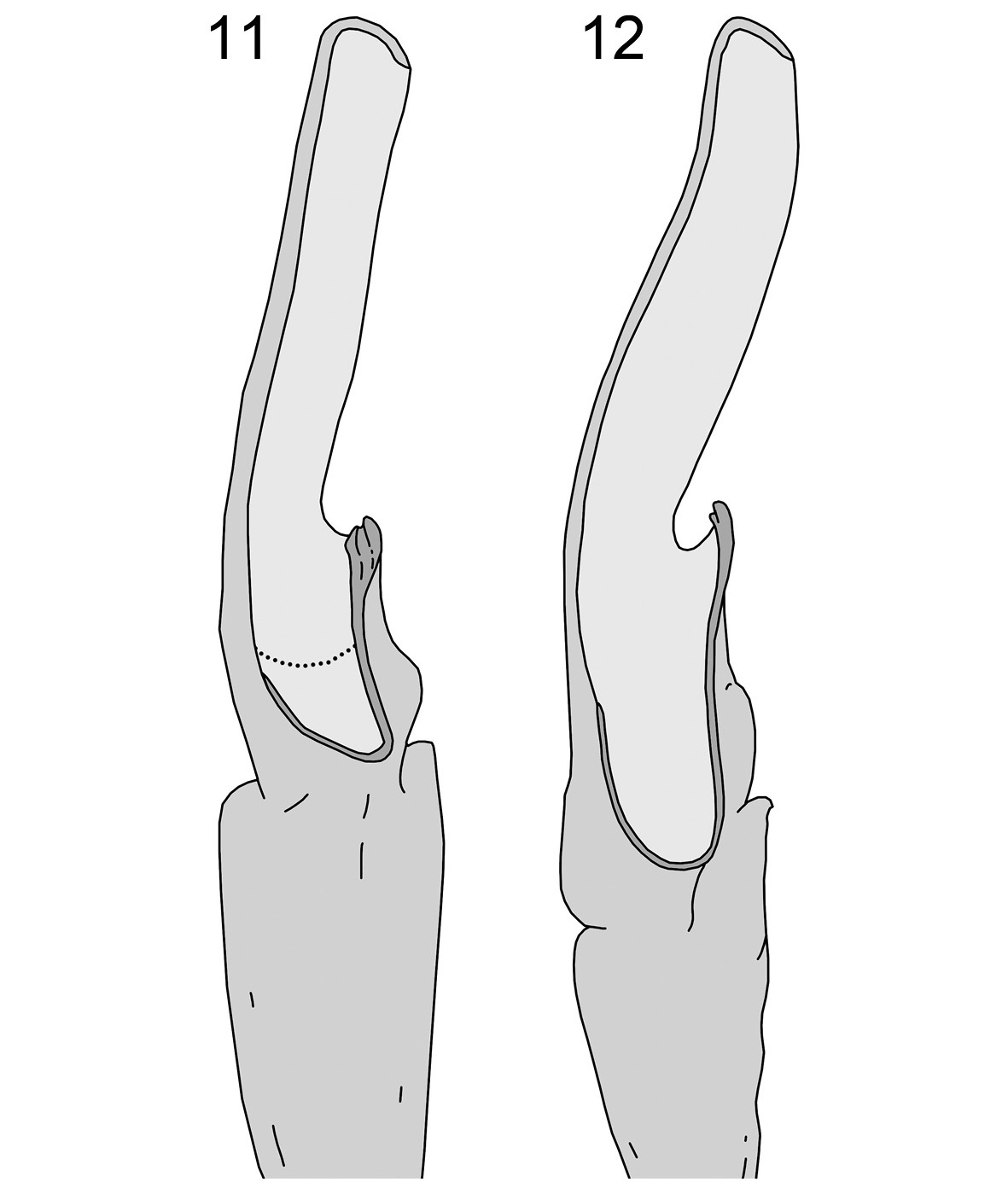
Dorsal aspect of right hemispermatophore: 11 Wernerius inyoensis sp. n. (dotted line indicates the ventral trough) 12 Wernerius spicatus (redrawn from
Type locality of Wernerius inyoensis sp. n. 13 Desert wash where the species was first discovered. 14 Talus slope that might provide a subterranean habitat for Wernerius inyoensis sp. n.
We are greatly indebted to George Graham Jr., Penny Graham, George Webber Jr., and Margaret Irick for assistance in the field. We thank David Ek for help initiating this project and Angela Evenden for her support. This research was conducted under a task agreement with the National Park Service (J8R07080009) administered through the Great Basin Cooperative Ecosystem Studies Unit.