(C) 2012 Roger Daniel Randrianiaina. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
This study provides detailed morphological descriptions of previously unknown tadpoles of the treefrog genus Boophis Tschudi and analyses of habitat preferences of several of these tadpoles in Ranomafana National Park. A total of twenty-two tadpoles determined via DNA barcoding are characterized morphologically herein, fourteen of them for the first time. Twelve of these tadpoles belong to taxonomically undescribed candidate species which in several cases are so far only known from their larval stages. Our data show that the larvae of some of these candidate species occur syntopically yet maintaining a clearly correlated genetic and morphological identity, suggesting that they indeed are true biological and evolutionary species. Tadpoles considered to belong to the “adherent” ecomorphological guild inhabit fast-running waters and their oral disc is commonly to continuously attached to the rocky substrate, supposedly to keep their position in the water current. Some of these species are characterized by the presence of a dorsal gap of papillae and the absence of an upper jaw sheath. This guild includes the tadpoles of the Boophis albipuncatus group (Boophis ankaratra, Boophis schuboeae, Boophis albipunctatus, Boophis sibilans, Boophis luciae), and of the Boophis mandraka group (Boophis sambirano and six candidate species related to this species and to Boophis mandraka). Tadpoles considered belonging to the “suctorial” guild inhabit fast-running waters where they use frequently their oral disc to attach to the substrate. They have an enlarged oral disc without any dorsal gap, including two nominal species (Boophis marojezensis, Boophis vittatus), and five candidate species related to Boophis marojezensis. An ecological analysis of the tadpoles of Boophis luciae, Boophis schuboeae and Boophis marojezensis [Ca51 JQ518198] from Ranomafana National Park did not provide evidence for a clear preference of these tadpoles to the fast flowing microhabitat sections of the stream, although the tadpoles discussed in this study are typically caught in this habitat.
Amphibia, Anura, Mantellidae, Boophis, larval morphology, oral disc, clasping, adherent, suctorial, candidate species, larval ecology, microhabitat preference
The genus Boophis Tschudi, 1938 is a species-rich group of treefrogs in the family Mantellidae which is endemic to Madagascar and to the Comoran island of Mayotte. Seventy-two nominal species and over 25 candidate species of Boophis are currently known (
Tadpoles have been described for 46 species of Boophis (e.g.,
The existence of strongly rheophilous tadpoles in species of Boophis has been known since the work of
In this study, morphological data on twenty-two strongly rheophilous tadpoles are provided, of which fourteen were previously unknown. Twelve of these larvae belong to candidate species which so far have not been scientifically named.
All these strongly rheophilous tadpoles are characterized by their “streamlined” (i.e., elongated, narrow and flat) body form, their wide oral disc containing many keratodont rows with all posterior rows uninterrupted, their completely keratinized jaw sheaths, of which the lower one is always “ribbed” and the upper one can be absent in some species, and rows of many small rounded marginal papillae with or without a dorsal gap. The absence of many of these characteristics in Boophis williamsi tadpoles (
In the context of grouping Malagasy tadpoles into different ecomorphological guilds, some Boophis tadpoles have been classified as “suctorial” and “adherent” by
The use of DNA barcoding to identify amphibian larvae from species-rich tropical communities to the species level has been tremendously successful within the last years (e.g.,
Tadpoles were collected by different kinds of nets having mesh sizes from 2 to 5 mm, depending on the size of the streams, the strength of the water current and the type of substrate. They were euthanised by immersion in chlorobutanol solution, and immediately sorted into homogeneous series based on morphological characters (body shape, relative tail length, eye position and direction, oral disc position, direction and configuration, general color pattern). From each series one specimen was selected and a tissue sample from its tail musculature or fin taken and preserved in 99% ethanol. This specimen is here called “DNA voucher”. All detailed morphological tadpole characterizations and drawings are based on this DNA voucher, whereas variation is sometimes described based on further specimens of the series. After tissue collection, all specimens were preserved in 5% formalin or 70% ethanol. Specimens were deposited in the Zoologische Staatssammlung München, Germany (ZSM). When referring to voucher specimens the original field numbers (FG/MV, FGZC, T, and ZCMV) are usually provided together with the final ZSM catalogue numbers. Tadpoles studied in this paper are summarized in Tables 1 and 2, including data concerning the site and its coordinates, the date of the capture and the collectors.
For detailed morphological examination, especially to determine developmental stages and assess characters of the oral disc, preserved tadpoles were stained slightly with methylene blue. Tadpoles were examined under water, and a few drops of methylene blue were applied to the oral disc, hind limb, spiracle, narial opening and vent tube to better discern their structures. Developmental stages were determined following
Morphological descriptions, measurements and drawings were done on digital pictures of the preserved tadpoles taken with a stereomicroscope Zeiss StereoDiscovery V12 connected to a computer, following landmarks, terminology and definitions of
In tadpoles of many frog species, pigmentless parts of the body wall become detached and apparently separated by a liquid-filled cavity from the underlying pigmented parts of the skin and the inner organs (among Malagasy frogs, for instance extremely expressed in the tadpoles of some Scaphiophryne; see
Summary of localities with geographic coordinates, and collection dates, of tadpole specimens studied herein.
| Locality | Site | Species | Coordinates | Date | Collectors |
|---|---|---|---|---|---|
| Ankijagna Lalagna | Boophis sambirano[Ca49] | 14°14.055'S, 48°58.732'E 1187 m a.s.l. | 08.06.2010 | D.R. Vieites, F.M. Ratsoavina, A.S. Rasamison, A . Rakotoarisoa, M. Vences, R.D. Randrianiaina | |
| Ambohitsara | Boophis albipunctatus | 21°21.431'S, 47°48.941'E 294 m a.s.l. | 03.03.2007 | A. Strauß, J. Glos, E. Reeve, T. Rasolonjatovo-H., S. Ndriantsoa, M. Vences, R.D. Randrianiaina | |
| Ambinanitelo | Boophis marojezensis[Ca52] | 14°13.524'S, 48°57.808'E 1182 m a.s.l. | 09.06.2010 | D.R. Vieites, F.M. Ratsoavina, A.S. Rasamison, A . Rakotoarisoa, M. Vences, R.D. Randrianiaina | |
| Ambinanitelo | Boophis sambirano [Ca50] | 14°13.524'S, 48°57.808'E 1182 m a.s.l. | 09.06.2010 | D.R. Vieites, F.M. Ratsoavina, A.S. Rasamison, A . Rakotoarisoa, M. Vences, R.D. Randrianiaina | |
| An'Ala | Andohanisity | Boophis mandraka[Ca46] | 18°55.156'S, 48°29.278'E 889 m a.s.l. | 08.02.2006 | C. Patton, D.R. Vieites, J. Patton, L. Raharivololoniaina, M. Vences, R.D. Randrianiaina |
| Andasibe Special Reserve | Analamazaotra river | Boophis sibilans | 18°55.900'S, 48°25.733'E 900 m a.s.l. | 04.12.2001 | L. Raharivololoniaina, M Vences |
| Between Antsohihy and Bealanana | Anjingo river | Boophis sambirano[Ca47] | 14°44.929'S, 48° 29.491'E 925 m a.s.l. | 07.06.2010 | D.R. Vieites, F.M. Ratsoavina, A.S. Rasamison, A . Rakotoarisoa, M. Vences, R.D. Randrianiaina |
| Between Antsohihy and Bealanana | Anjingo river | Boophis sambirano[Ca48] | 14°44.929'S, 48°29.491'E 925 m a.s.l. | 07.06.2010 | D.R. Vieites, F.M. Ratsoavina, A.S. Rasamison, A . Rakotoarisoa, M. Vences, R.D. Randrianiaina |
| Manongarivo Special Reserve | Camp Norbert | Boophis sambirano | 13°56.053'S, 48°27.028’E 288 m a.s.l. | 31.01.2003 | F. Glaw, M. Vences, R.D. Randrianiaina |
| Marojejy National Park | Camp Mantella | Boophis vittatus | 14°26.972'S, 49°47.214'E 327 m a.s.l. | 14.02.2005 | F. Glaw, M. Vences, R.D. Randrianiaina |
| Marojejy National Park | Camp Marojejia | Boophis englaenderi | 14°26.070'S, 49°45.638'E 740 m a.s.l. | 18.02.2005 | F. Glaw, M. Vences, R.D. Randrianiaina |
| Marojejy National Park | Camp Mantella | Boophis englaenderi[Ca23] | 14°26.972'S, 49°47.214'E 327 m a.s.l. | 19.02.2005 | F. Glaw, M. Vences, R.D. Randrianiaina |
| Marojejy National Park | Camp Mantella | Boophis marojezensis[Ca25] | 14°26.972'S, 49°47.214'E 327 m a.s.l. | 19.02.2005 | F. Glaw, M. Vences, R.D. Randrianiaina |
| Marojejy National Park | Camp Mantella | Boophis marojezensis[Ca26] | 14°26.972'S, 49°47.214'E 327 m a.s.l. | 19.02.2005 | F. Glaw, M. Vences, R.D. Randrianiaina |
| Marojejy National Park | Camp Mantella | Boophis sibilans | 14°26.972'S, 49°47.214'E 327 m a.s.l. | 19.02.2005 | F. Glaw, M. Vences, R.D. Randrianiaina |
| Ranomafana National Park | Ambatolahyriver | Boophis andohahela | 21°14.897'S, 47°25.769'E 867 m a.s.l. | 27.07.2009 | R.D. Randrianiaina |
| Ranomafana National Park | Ambatolahyriver | Boophis marojezensis[Ca51] | 21°14.897'S, 47°25.769'E 867 m a.s.l. | 27.07.2009 | R.D. Randrianiaina |
| Ranomafana National Park | Ambatolahyriver | Boophis schuboeae | 21°14.897'S, 47°25.769'E 867 m a.s.l. | 27.07.2009 | R.D. Randrianiaina |
| Ranomafana National Park | Imaloka | Boophis marojezensis[Ca51] | 21°14.529'S, 47°27.938'E 957 m a.s.l. | 01.03.2007 | A. Strauß, J. Glos, E. Reeve, T. Rasolonjatovo-H., S. Ndriantsoa, M. Vences, R.D. Randrianiaina |
| Ranomafana National Park | In a poolbelow waterfall | Boophis schuboeae | 11.02.2003 | M. Teschke, M. Vences | |
| Ranomafana National Park | Marihy avaratra | Boophis luciae | 21°15.806'S, 47°25.548'E 1144 m a.s.l. | 20.02.2007 | A. Strauß, J. Glos, E. Reeve, T. Rasolonjatovo-H., S. Ndriantsoa, M. Vences, R.D. Randrianiaina |
| Ranomafana National Park | Marihy avaratra | Boophis mandraka[Ca38] | 21°15.806'S, 47°25.548'E 1144 m a.s.l. | 02.02.2007 | A. Strauß, J. Glos, E. Reeve, T. Rasolonjatovo-H., S. Ndriantsoa, M. Vences, R.D. Randrianiaina |
| Ranomafana National Park | Talatakely | Boophis luciae | 21°15.846'S, 47°25.161'E 966 m a.s.l. | 24.02.2006 | L. Raharivololoniaina, A.F. Ranjanaharisoa, T.J. Razafindrabe, D.R. Vieites, J. Patton, C. Patton, M. Vences, R.D. Randrianiaina |
| Ranomafana National Park | Talatakely | Boophis marojezensis[Ca51] | 21°15.846'S, 47°25.161'E 966 m a.s.l. | 24.02.2006 | L. Raharivololoniaina, A.F. Ranjanaharisoa, T.J. Razafindrabe, D.R. Vieites, J. Patton, C. Patton, M. Vences, R.D. Randrianiaina |
| Ranomafana National Park | Sahateza(Pond Donald) | Boophis ankaratra | 21°15.476'S, 47°21.583'E 1016 m a.s.l. | 03.03.2007 | A. Strauß, J. Glos, E. Reeve, T. Rasolonjatovo-H., S. Ndriantsoa, M. Vences, R.D. Randrianiaina |
| Ranomafana National Park | Vatoharana | Boophis andohahela | 21°17.338'S, 47°25.765'E 1016 m a.s.l. | 24.03.2007 | A. Strauß, J. Glos, E. Reeve, T. Rasolonjatovo-H., S. Ndriantsoa, M. Vences, R.D. Randrianiaina |
| Tsaratanana Strict Nature Reserve | Antevialambazaha | Boophis marojezensis[Ca53] | 14°10.455'S, 48°56.714'E 1699 m a.s.l. | 10.06.2010 | D.R. Vieites, F.M. Ratsoavina, A.S. Rasamison, A . Rakotoarisoa, M. Vences, R.D. Randrianiaina |
Collection numbers and Genbank accession numbers of the tadpoles studied. FG/MV, FGZC, LR, T, TAD, ZCMV (field numbers), ZSM (Zoologische Staatssammlung München). Missing accession numbers indicate that sequences were too short or of poor quality and were therefore not submitted to Genbank, or that they will be submitted to Genbank in the course of future studies.
| Species | Locality | ZSM- and Field number | Accession number |
|---|---|---|---|
| Boophis englaenderi | MaroJeJy National Park | FGZC 2244- ZSM 623/2008 | HM769921 |
| Boophis englaenderi [Ca23] | MaroJeJy National Park | FGZC 2957- ZSM 1632/2007 | JQ518193 |
| FGZC 2241- ZSM 1499/2007 | --- | ||
| FGZC 2243- ZSM 527/2008 | FJ559144 | ||
| FGZC 2248- ZSM 1508/2007 | --- | ||
| FGZC 2250- ZSM 1502/2007 | --- | ||
| FGZC 2252- ZSM 1503/2007 | --- | ||
| FGZC 2257- ZSM 529/2008 | --- | ||
| FGZC 2260- ZSM 530/2008 | --- | ||
| FGZC 2273- ZSM 1514/2007 | --- | ||
| FGZC 2275- ZSM 1516/2007 | --- | ||
| Boophis andohahela | Ranomafana National Park | T 60- ZSM 912/2007 | GU974437 |
| T 107- ZSM 1319/2007 | GU974422 | ||
| T 125- ZSM 1321/2007 | GU974423 | ||
| T 127- ZSM 1162/2007 | GU974424 | ||
| T 131- ZSM 1351/2007 | GU974425 | ||
| T 150- ZSM 910/2007 | GU974427 | ||
| T 222- ZSM 566/2007 | GU974435 | ||
| T 428- ZSM 998/2007 | GU974449 | ||
| T 09/273- ZSM 282/2009 | --- | ||
| Boophis ankaratra | Ranomafana National Park | FGMV 2003.1698- ZSM 816/2004 | --- |
| ZCMV 3803- ZSM 168/2008 | --- | ||
| ZCMV 4917- ZSM 876/2007 | GU974476 | ||
| Boophis schuboeae | Ranomafana National Park | FGMV 2002.1800- ZSM 978/2004 | DQ068394 |
| Tad 2004-780- ZSM 1339/2004 | --- | ||
| Tad 2004-797- ZSM 1356-2004 | --- | ||
| T 09/980- ZSM 743/2008 | --- | ||
| T 09/968- ZSM 739/2008 | --- | ||
| T 09/971- ZSM 740/2008 | --- | ||
| T 09/998- ZSM 749/2008 | --- | ||
| Boophis albipunctatus | Ambohitsara | ZCMV 4942- ZSM 78/2008 | GU974373 |
| ZCMV 4946- ZSM 82/2008 | GU974374 | ||
| Boophis sibilans | An'Ala | ZCMV 3450- ZSM 1754/2007 | --- |
| Boophis sibilans | Andasibe | LR 269- ZSM 557/2004 | DQ792492 |
| Boophis sibilans | MaroJeJy National Park | FGZC 2956- ZSM 1631/2007 | JQ518194 |
| Boophis luciae | Ranomafana National Park | T 176- ZSM 792/2007 | --- |
| T 177- ZSM 593/2007 | GU975090 | ||
| T 178- ZSM 541/2007 | GU975094 | ||
| T 179- ZSM 976/2007 | --- | ||
| T 224- ZSM 264/2007 | --- | ||
| T 430- ZSM 274/2007 | GU975096 | ||
| ZCMV 3619- ZSM 1587/2006 | HM769939 | ||
| ZCMV 3631- ZSM 1588/2006 | HM769940 | ||
| ZCMV 3686- ZSM 634/2008 | HM769938 | ||
| ZCMV 4024- ZSM 688/2007 | --- | ||
| ZCMV 5146- ZSM 730/2007 | --- | ||
| Boophis sambirano | Manongarivo Special Reserve | FGMV 2002.1904- ZSM 678/2004 | EU717863 |
| FGMV 2002.1902- ZSM 672/2004 | EU717861 | ||
| Boophis mandraka [Ca38] | Ranomafana National Park | ZCMV 4261- ZSM 456/2007 | FJ559153 |
| Boophis mandraka [Ca46] | An'Ala | ZCMV 3479- ZSM 1784/2007 | JQ518195 |
| Boophis sambirano [Ca47] | Between Antsohihy and Bealanana | ZCMV 13105- ZSM 482/2010 | JQ518203 |
| ZCMV 13110- ZSM 486/2010 | JQ518204 | ||
| Boophis sambirano [Ca48] | Between Antsohihy and Bealanana | ZCMV 13107- ZSM 484/2010 | JQ518206 |
| ZCMV 13108- ZSM 485/2010 | JQ518207 | ||
| ZCMV 13109- ZSM 485/2010 | JQ518205 | ||
| Boophis sambirano [Ca49] | AnkiJagna Lalagna | ZCMV 13150- ZSM 523/2010 | JQ518209 |
| ZCMV 13155- ZSM 528/2010 | JQ518208 | ||
| ZCMV 13156- ZSM 529/2010 | JQ518210 | ||
| Boophis sambirano [Ca50] | Ambinanitelo | ZCMV 13171- ZSM 544/2010 | JQ518212 |
| ZCMV 13172- ZSM 545/2010 | JQ518211 | ||
| ZCMV 13173- ZSM 546/2010 | JQ518213 | ||
| ZCMV 13174- ZSM 547/2010 | JQ518214 | ||
| Boophis marojezensis | MaroJeJy National Park | FGZC 2277- ZSM 1528/2007 | JQ518196 |
| FGZC 2953- ZSM 1628/2007 | JQ518199 | ||
| Boophis marojezensis [Ca25] | MaroJeJy National Park | FGZC 2929- ZSM 1611/2007 | FJ559146 |
| Boophis marojezensis [Ca26] | MaroJeJy National Park | FGZC 2930- ZSM 1612/2007 | JQ518197 |
| Boophis marojezensis [Ca51] | Ranomafana National Park | T 394- ZSM 1008/2007 | GU974657 |
| T 432- ZSM 117/2007 | GU974658 | ||
| T 09/1088- ZSM 779/2008 | --- | ||
| T 09/1091- ZSM 780/2008 | --- | ||
| T 09/1094- ZSM 781/2008 | --- | ||
| ZCMV 3691- ZSM 267/2008 | --- | ||
| ZCMV 3629- ZSM 318/2008 | --- | ||
| ZCMV 3635- ZSM 232/2008 | --- | ||
| ZCMV 3690- ZSM 266/2008 | --- | ||
| ZCMV 3742- ZSM 481/2008 | --- | ||
| ZCMV 4203- ZSM 401/2007 | --- | ||
| ZCMV 4264- ZSM 457/2007 | GU974654 | ||
| ZCMV 4376- ZSM 1453/2007 | GU974647 | ||
| ZCMV 4531- ZSM 532/2007 | GU974648 | ||
| ZCMV 4541- ZSM 504/2007 | GU974650 | ||
| ZCMV 4547- ZSM 1390/2007 | GU974651 | ||
| ZCMV 4550- ZSM 509/2007 | GU974652 | ||
| ZCMV 4931- ZSM 838/2007 | GU974656 | ||
| ZCMV 5098- ZSM 913/2007 | GU974646 | ||
| ZCMV 5986- ZSM 1212/2007 | GU974655 | ||
| ZCMV 1395- ZSM 0025/2007 | GU974653 | ||
| T 09/1085- ZSM 778/2008 | --- | ||
| Boophis marojezensis [Ca52] | Ambinanitelo | ZCMV 13168- ZSM 541/2010 | JQ518215 |
| ZCMV 13169- ZSM 542/2010 | --- | ||
| Boophis marojezensis [Ca53] | Tsaratanana Strict Nature Reserve | ZCMV 13200- ZSM 573/2010 | JQ518216 |
| ZCMV 13201- ZSM 574/2010 | --- | ||
| ZCMV 13202- ZSM 575/2010 | --- | ||
| ZCMV 13203- ZSM 576/2010 | --- | ||
| ZCMV 13204- ZSM 577/2010 | --- | ||
| ZCMV 13205- ZSM 578/2010 | JQ518217 | ||
| Boophis vittatus | MaroJeJy National Park | FGZC 2237- ZSM 5219/2005 | --- |
| FGZC 2238- ZSM 1906/2007 | JQ518200 | ||
| FGZC 2251- ZSM 1907/2007 | JQ518201 | ||
| FGZC 2914- ZSM 1601/2007 | JQ518202 |
DNA barcoding was based on a fragment of the mitochondrial 16S rRNA gene, which is known to be sufficiently variable among species of Malagasy frogs (
Candidate species nomenclature followed the scheme developed by
During a study on stream tadpole communities in Ranomafana National Park (RNP) in the south eastern escarpment of Madagascar, we exhaustively sampled 33 stream sections for tadpoles (
We used data from this study for an exemplary analysis of breeding site choice and microhabitat use of syntopic species of strongly rheophilous tadpoles. To identify the habitat variables of the stream and the surrounding forest that may be important for breeding site choice, we performed a principal component analysis (PCA) and plotted species according to their incidence as supplementary variables in the PCA biplot. For PCA, we used all ten habitat variables of all 33 streams sampled during the tadpole community study. PCA was run on the correlation matrix in order to standardize for the influence of unequal variance. To evaluate data outliers and linear interdependence of variables, box-plots and pair-plots (
To analyze the use of microhabitats within streams, we first constructed graphs of raw data to display the species specific distribution between microhabitats. In order to quantify true preferences for microhabitats, Ivlev's electivity index (E,
Statistical analysis were performed in R 2.9.2 (
We here provide a summary of the most important morphological characteristics of one representative species per species group, and brief accounts for all other species and candidate species in which we mainly emphasize their difference to the species described more completely, or to other species belonging to the same group. Standardized, detailed descriptions and assessments of variation for all species and candidate species are found in the electronic supplement. Original measurements and ratios are given in Tables 3–5 which are equally included as electronic supplement.
Coloration in life of strongly rheophilous tadpoles of Boophis (dorsal, lateral and ventral views): A Boophis andohahela (T 09/273-ZSM 282/2009) B Boophis ankaratra (ZCMV 4917-ZSM 876/2007) C Boophis schuboeae (T 09/980-743/2008) D Boophis sibilans (ZCMV 11548 - to be catalogued in ZSM) E Boophis luciae (ZCMV 11548-to be catalogued in ZSM) F Boophis albipunctatus (ZCMV 4946-ZSM 82/2008) G Boophis mandraka [Ca38](ZCMV 4261-ZSM 456/2007) H Boophis sambirano [Ca47](ZCMV 13105-ZSM 482/2010) I Boophis sambirano [Ca48](ZCMV 13109-ZSM 486/2010) J Boophis sambirano [Ca49](ZCMV 13155-ZSM 528/2010) K Boophis sambirano [Ca50](ZCMV 13172-ZSM 545/2010) L Boophis marojezensis [Ca51] (ZCMV 13550-ZSM 721/2010) M Boophis marojezensis [Ca52] (ZCMV 13168-ZSM 541/2010) N Boophis marojezensis [Ca53] (ZCMV 13200-ZSM 573/2010).
Pictures of the oral discs of the voucher specimens (stained with methylene blue for better visibility of morphological structures). Scale bars represent 1 mm.
This group is characterized by tadpoles having a generalized oral disc without lateral emargination and ventral gap of papillae, but the dorsal gap is wide to very wide. The anterior margin of the oral disc is a continuation of the snout. Usually A1 is uninterrupted and P1 is interrupted, except the three species described herein which are the ones in the group having the strongest expression of adaptations that we interpret as rheophilous. The jaw sheaths are very strong with smooth surface and completely or partially keratinized in some species. The upper sheath is always made up by a medial convexity. Dorsolateral glands which exist in some other Boophis tadpoles are absent.
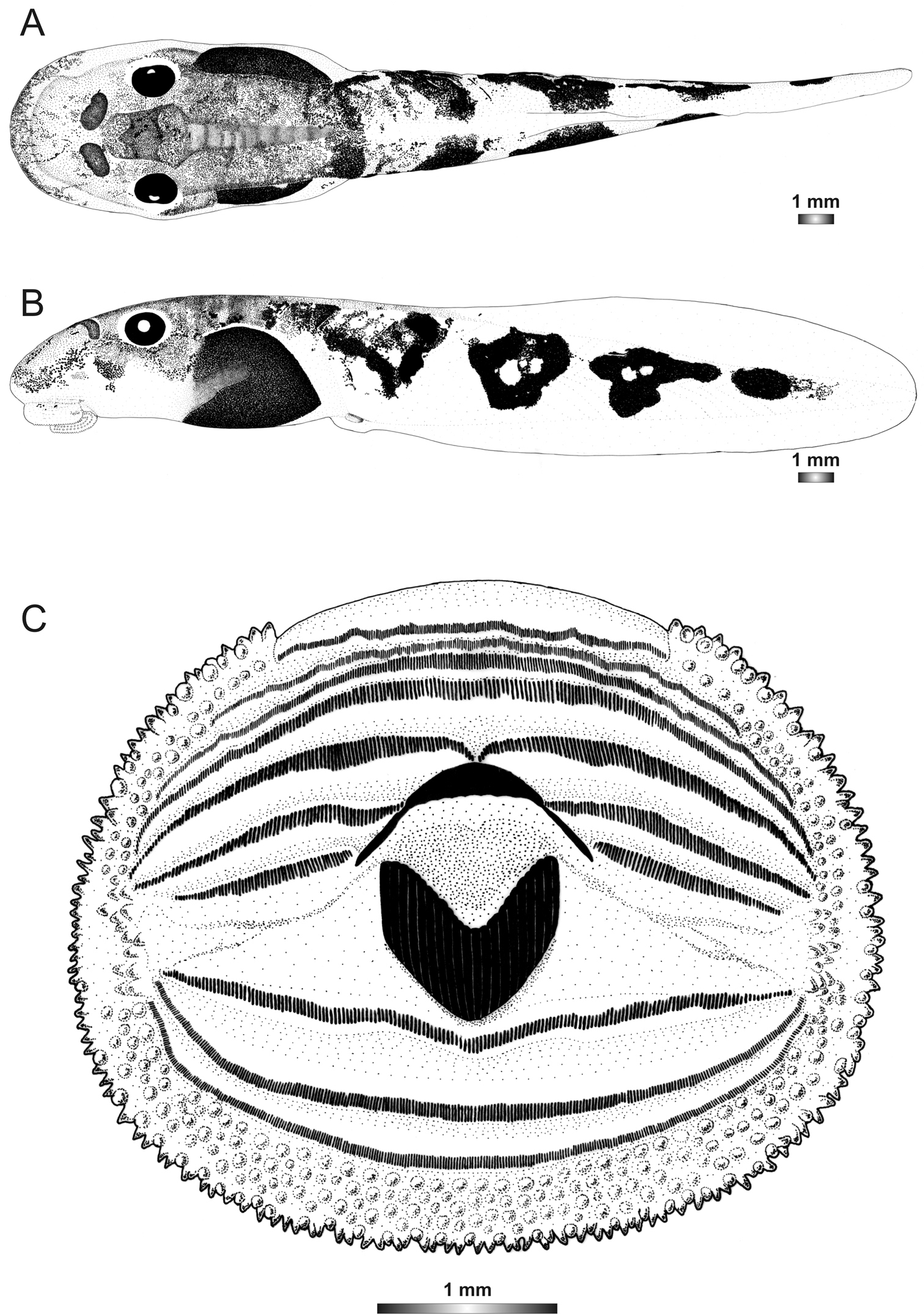
Boophis englaenderi Glaw & Vences, 1994
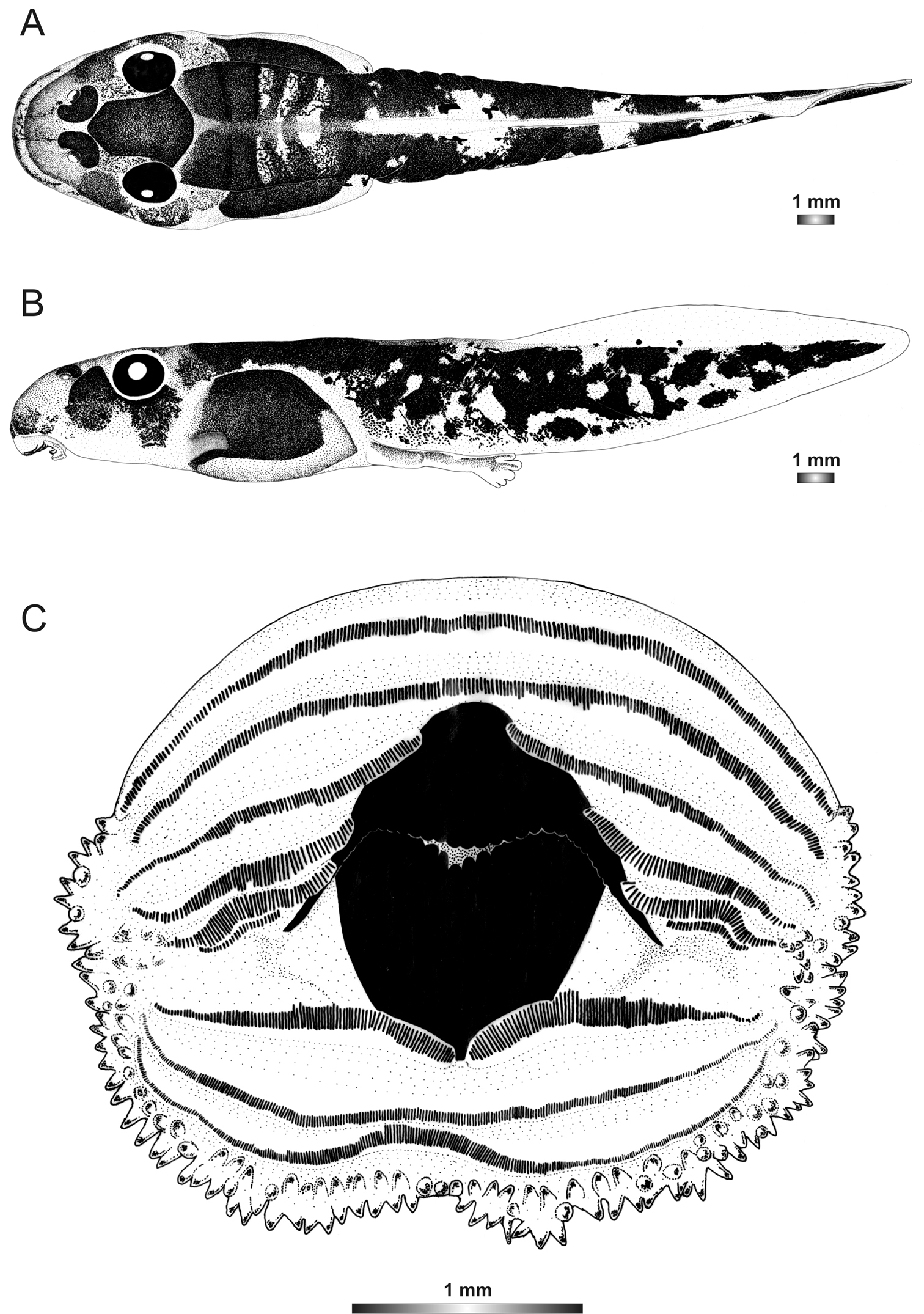
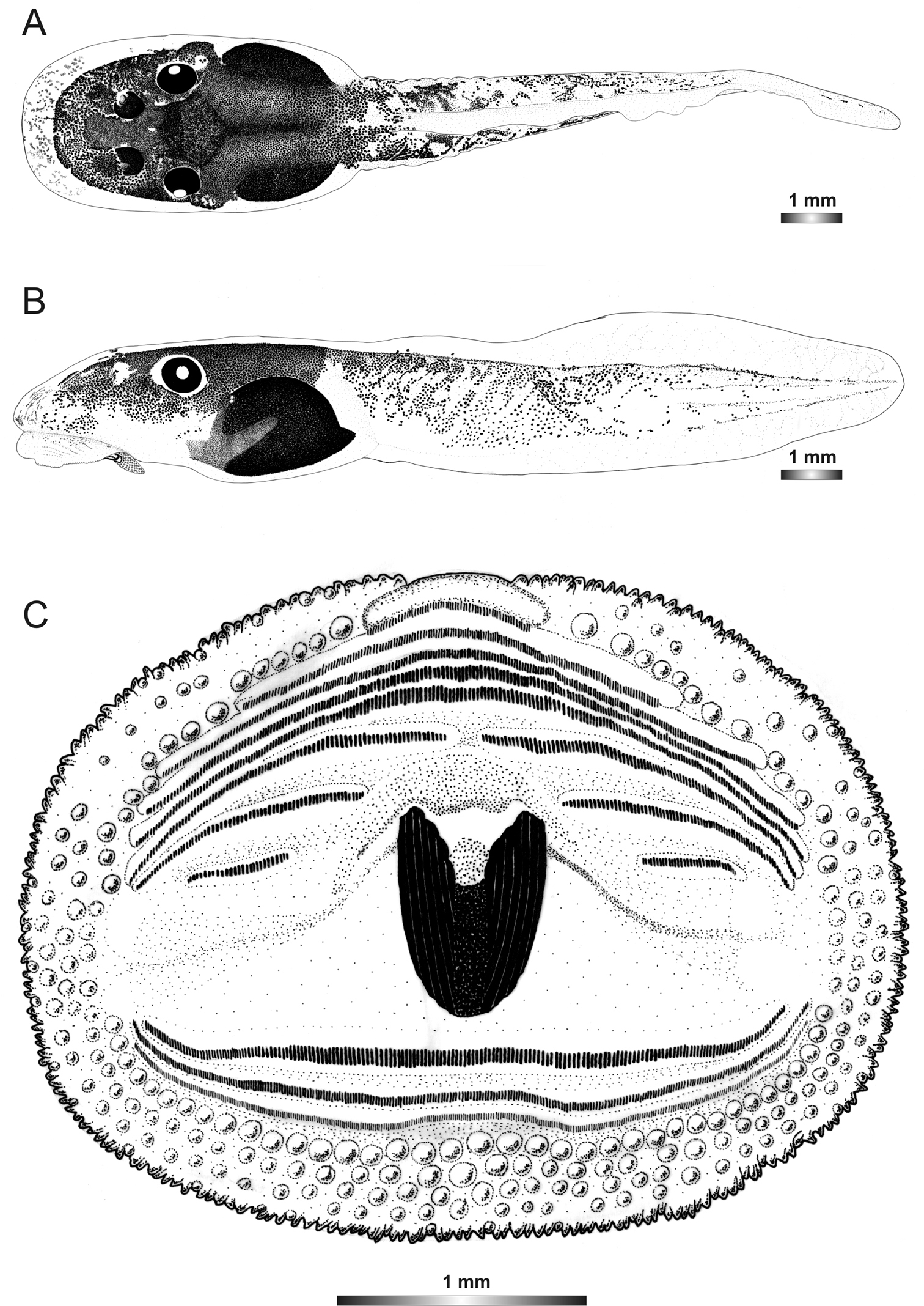
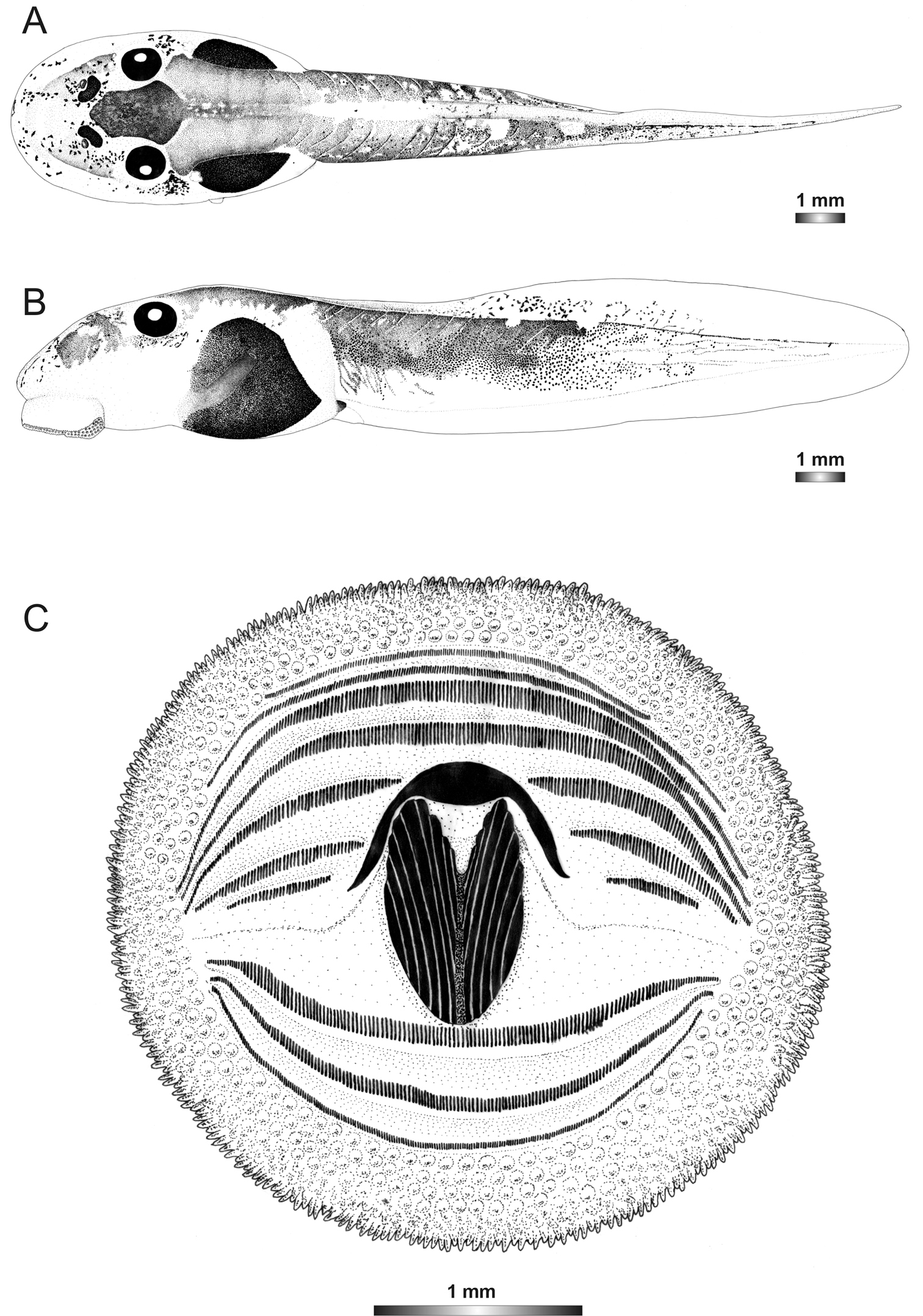
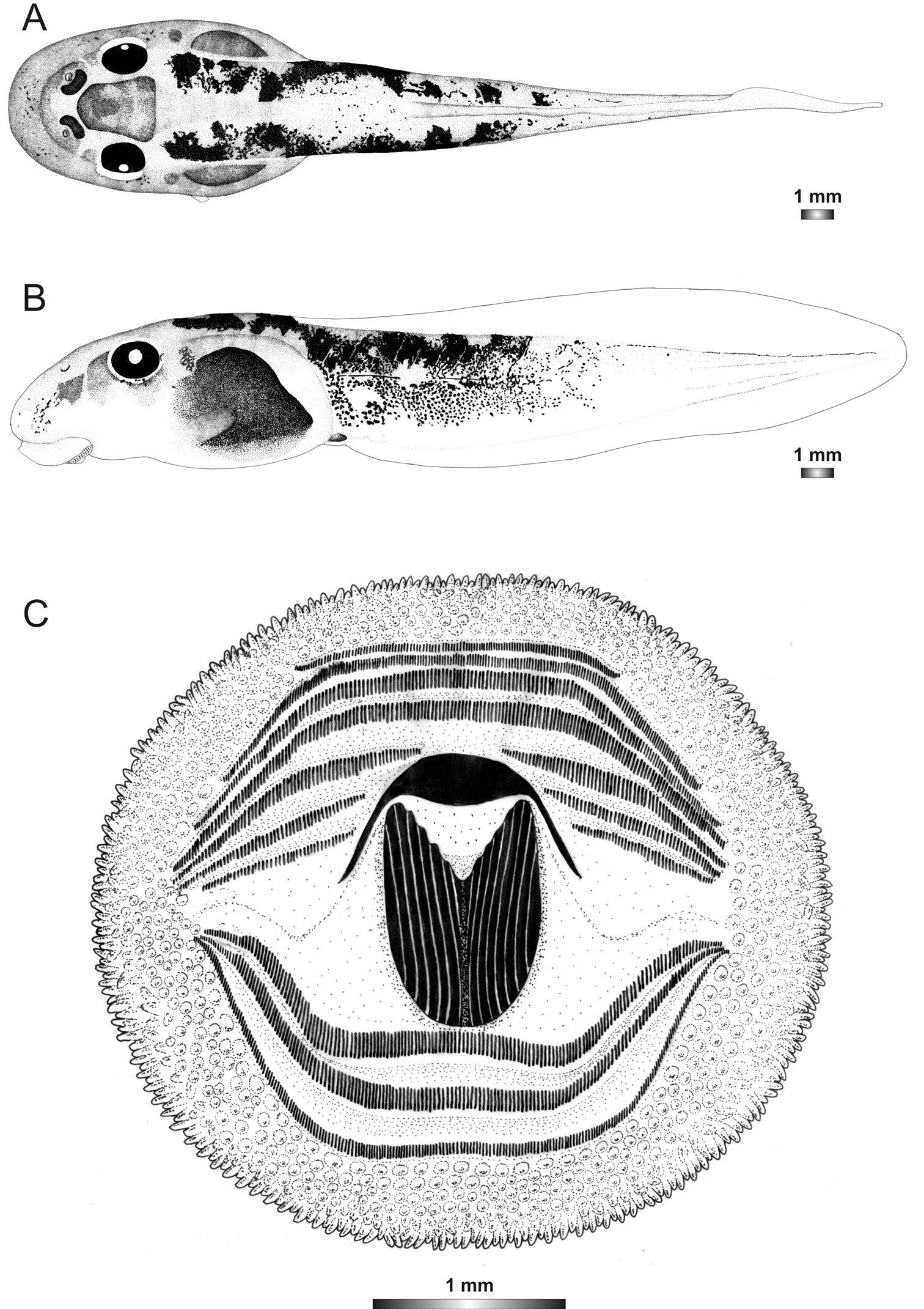
Morphological data were assessed in one tadpole (Figures 2 and 3) in developmental stage 36 (field number FGZC 2244; ZSM 623/2008, BL 11.8 mm, TL 25.4 mm, accession number HM769921) from Marojejy National Park (previously described by
The tadpoles of this species have an elliptical body, a flatly rounded snout in dorsal view and a short tail. The distance between eyes is wide and nares are very large, round, positioned very high dorsally, and situated nearer to snout than to eye and at eye level. LTRF is 6(3–6)/3(1). The upper jaw sheath is totally keratinized with rounded serrations, moderately wide with a very short widely rounded medial convexity. The lower sheath is V-shaped, completely keratinized and partially hidden by the upper one. Both jaw sheaths have a smooth surface.
In preservative, the tadpole is generally dark brown. Dark brown spots condensed to form a hexagonal mark above the neurocranium; a dark semicircular patch situated posterior to each narial opening and dark patches between the vertebral area and the abdominal region are present. The snout is spotted. The transversal lines between the vertebral area and the abdominal region are perceivable which make the domino-like structure on this noticeable. The dorsal part of the tail muscle has five dark brown and four light alternating bands. The prominent dark brown band is the extension of the patches between the vertebral area and the abdominal region. The myosepta are visible on the dorsal part of the tail. Laterally, the jugal area is covered by dense dark brown patches and the dorsolateral part of the flank is identical to the dorsal pattern; the ventrolateral part is pale and the abdominal region is very dark leaving an opaque discernible spiracle. Ventrally, oral disc, gular and branchial regions are pale; the venter is more or less transparent and the intestinal coils are perceptible with a regularly spiral shape. The tail musculature is pale and covered by dark brown spots which condense to form reticulations. Fins are transparent, with few brown spots on the dorsal fin, and the ventral fin is free from pigment.
Drawings of the preserved DNA voucher tadpole of Boophis englaenderi (FGZC 2244-ZSM 623/2008): A Dorsal view B Lateral view C Oral disc.
Boophis englaenderi [Ca23
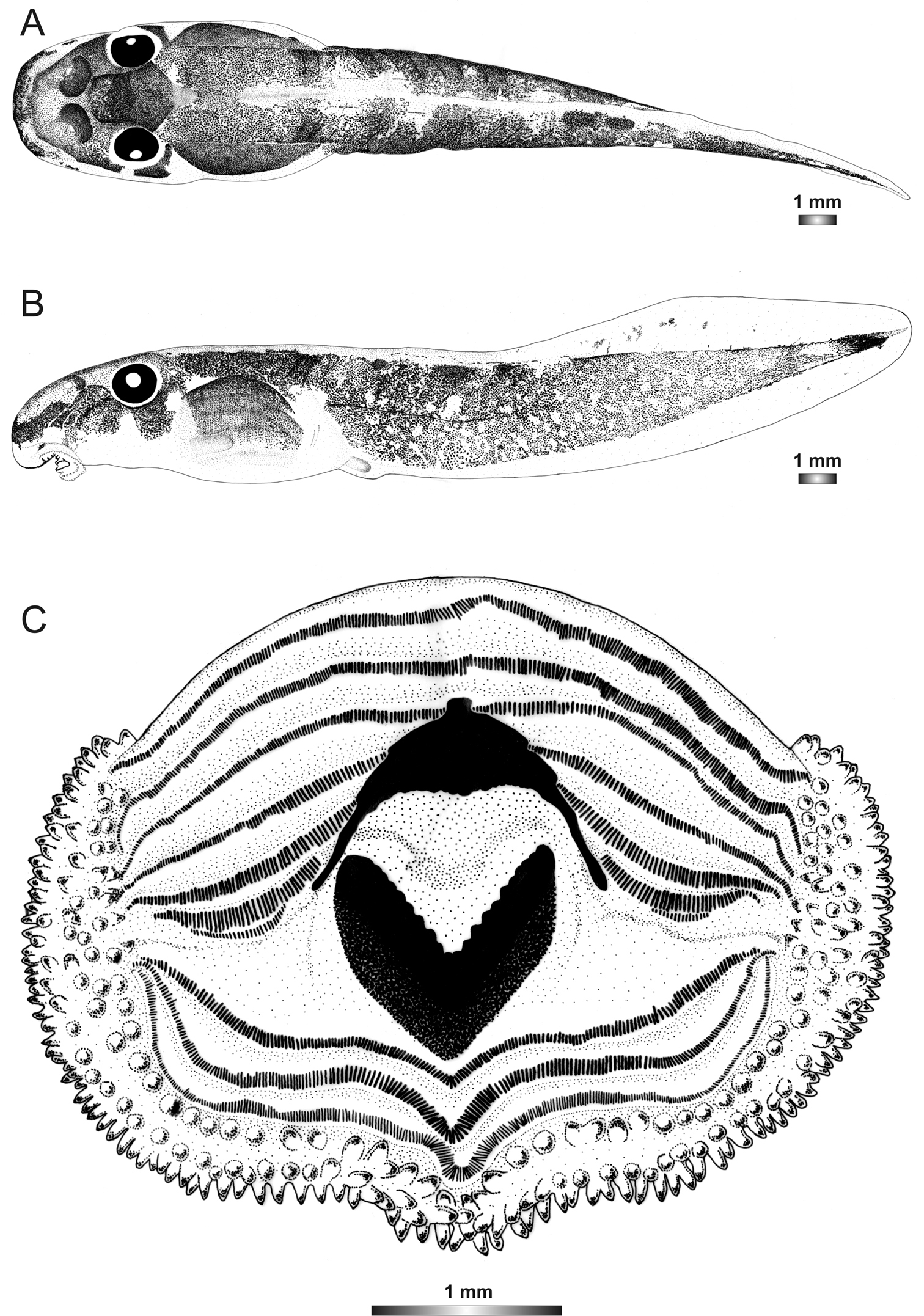
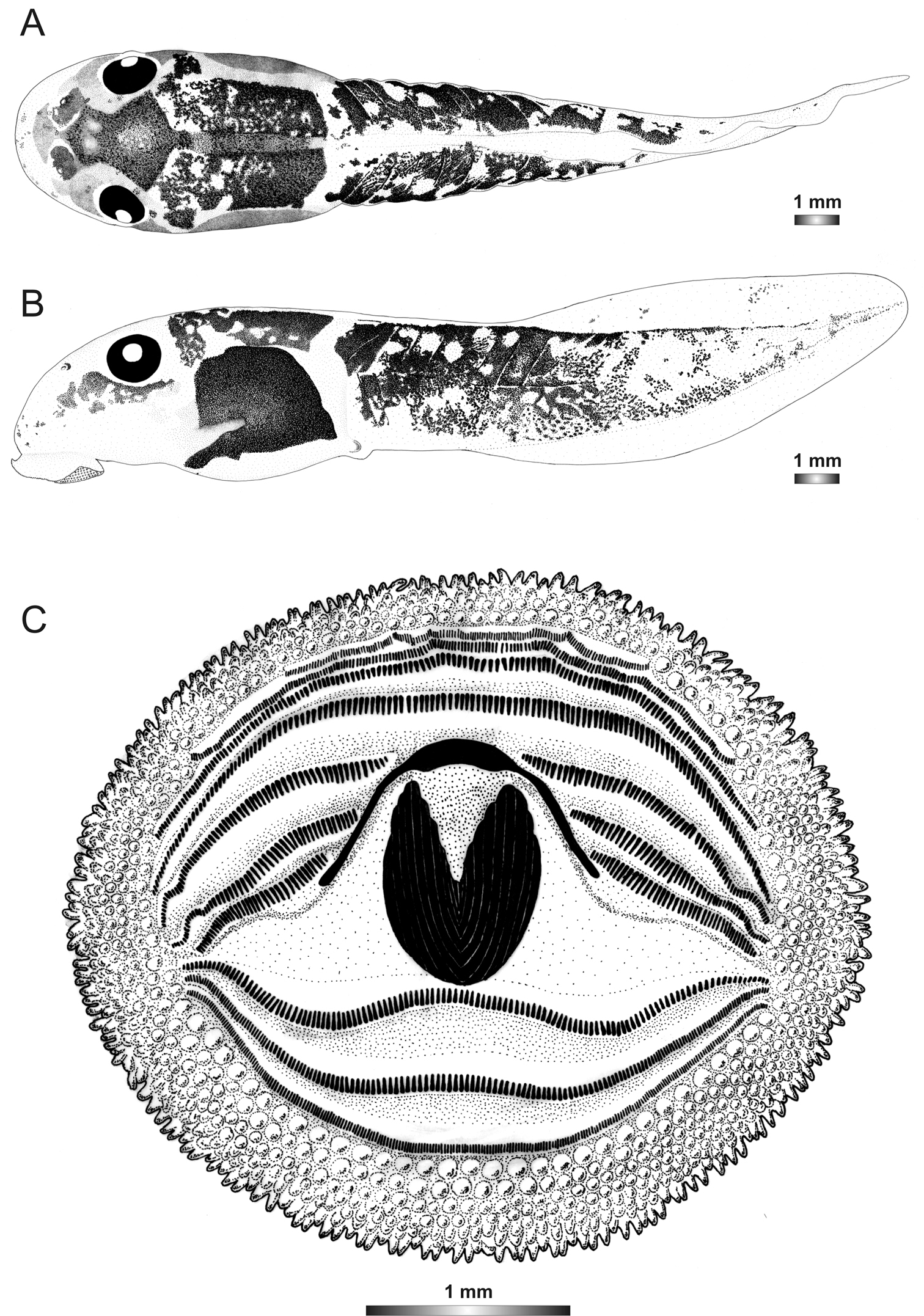
Morphological data were assessed in one tadpole (Figures 2 and 4) in developmental stage 30 (field number FGZC 2957, ZSM 1632/2007, BL 10.5 mm, TL 29.5 mm, Genbank accession number JQ518193) from Marojejy National Park. The 16S rDNA sequence of this specimen is 94% identical to a reference sequence of an adult Boophis englaenderi (accession AY848474) from Ilampy. Nine other voucher specimens agree in morphology with the voucher specimen described herein.
The external morphology of this tadpole has a very close similarity with that of Boophis englaenderi, except that it has a distinctly longer tail (TAL/BL 183% vs. 153%) and a lighter pigmentation. Additional differences between the two tadpoles are found in the oral disc structure. It is bulged laterally and has one more interrupted upper keratodont row and a first uninterrupted lower row giving the keratodont row formula LTRF 7(3–7)/3 vs. 6(3–6)/3(1). The number of papillae is higher than inBoophis englaenderi with 175 marginal papillae (vs. 128), and 94 submaginal papillae (vs. 33), although the examined tadpole is still in a developmental stage inferior to that of the examined tadpole of Boophis englaenderi. The submarginal papillae are complete on the lower labium. This tadpole is also characterized by a light brown coloration in preservative. The jugal area is covered by scarce light brown patches, and the tail musculature is covered by light brown spots which group in some areas to form patches or sparse reticulations. The intestinal coils are visible. The examination of nine other voucher specimens (see Table 2) confirms the differences to Boophis englaenderi.
Drawings of the preserved DNA voucher tadpole of Boophis englaenderi [Ca23](FGZC 2957-ZSM 1632/2007): A Dorsal view B Lateral view C Oral disc.
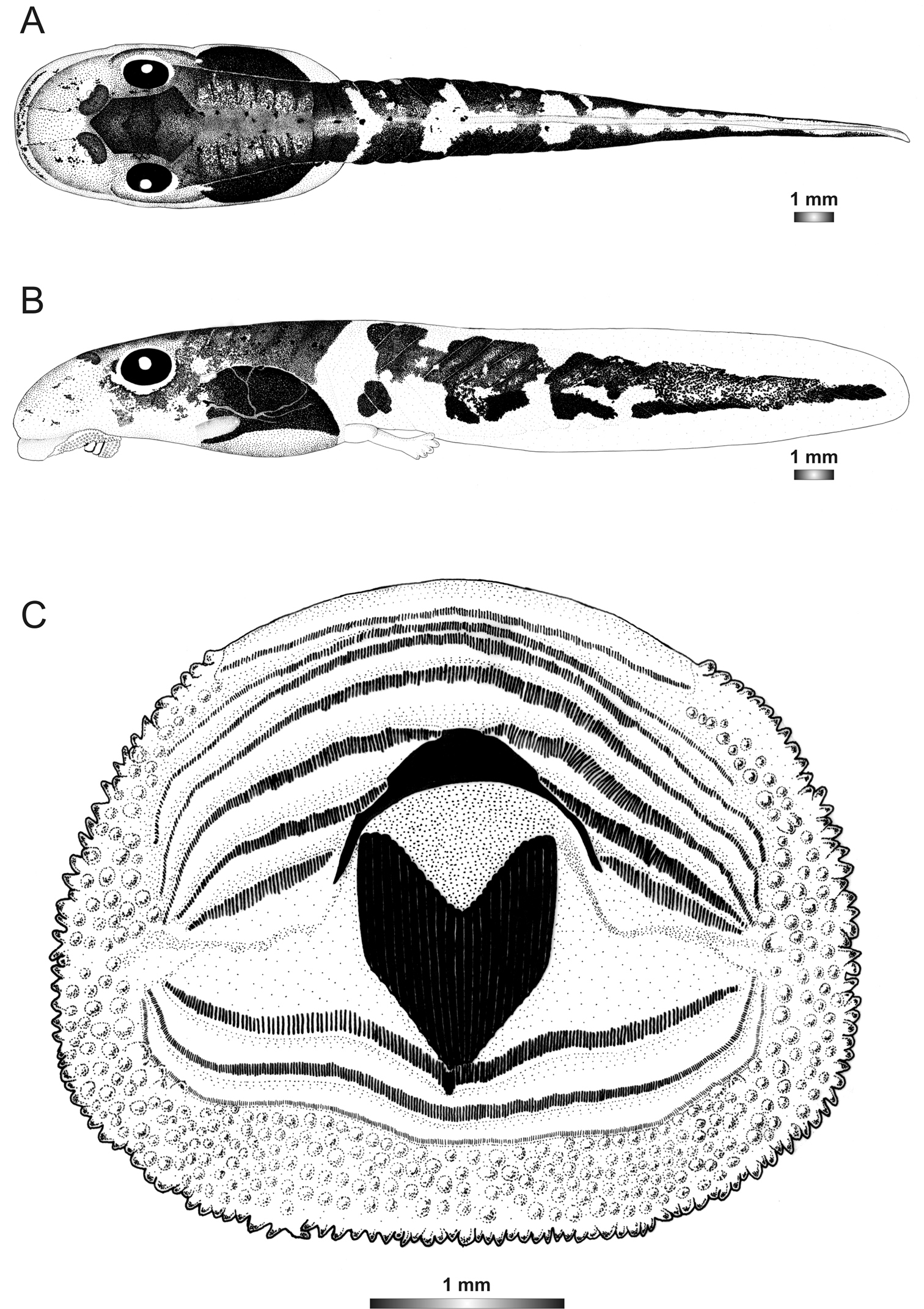
Boophis andohahela Andreone, Nincheri & Piazza, 1995
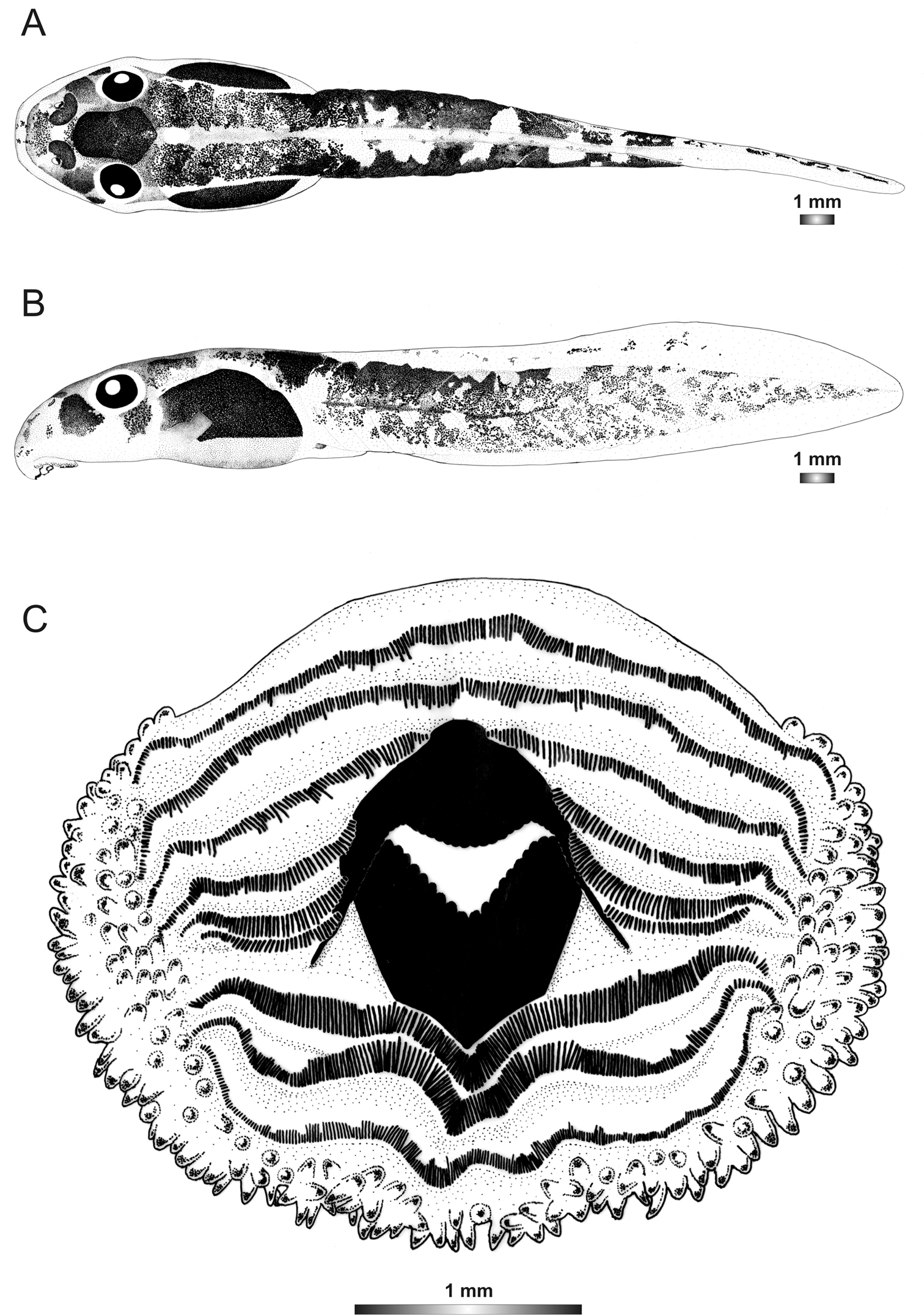
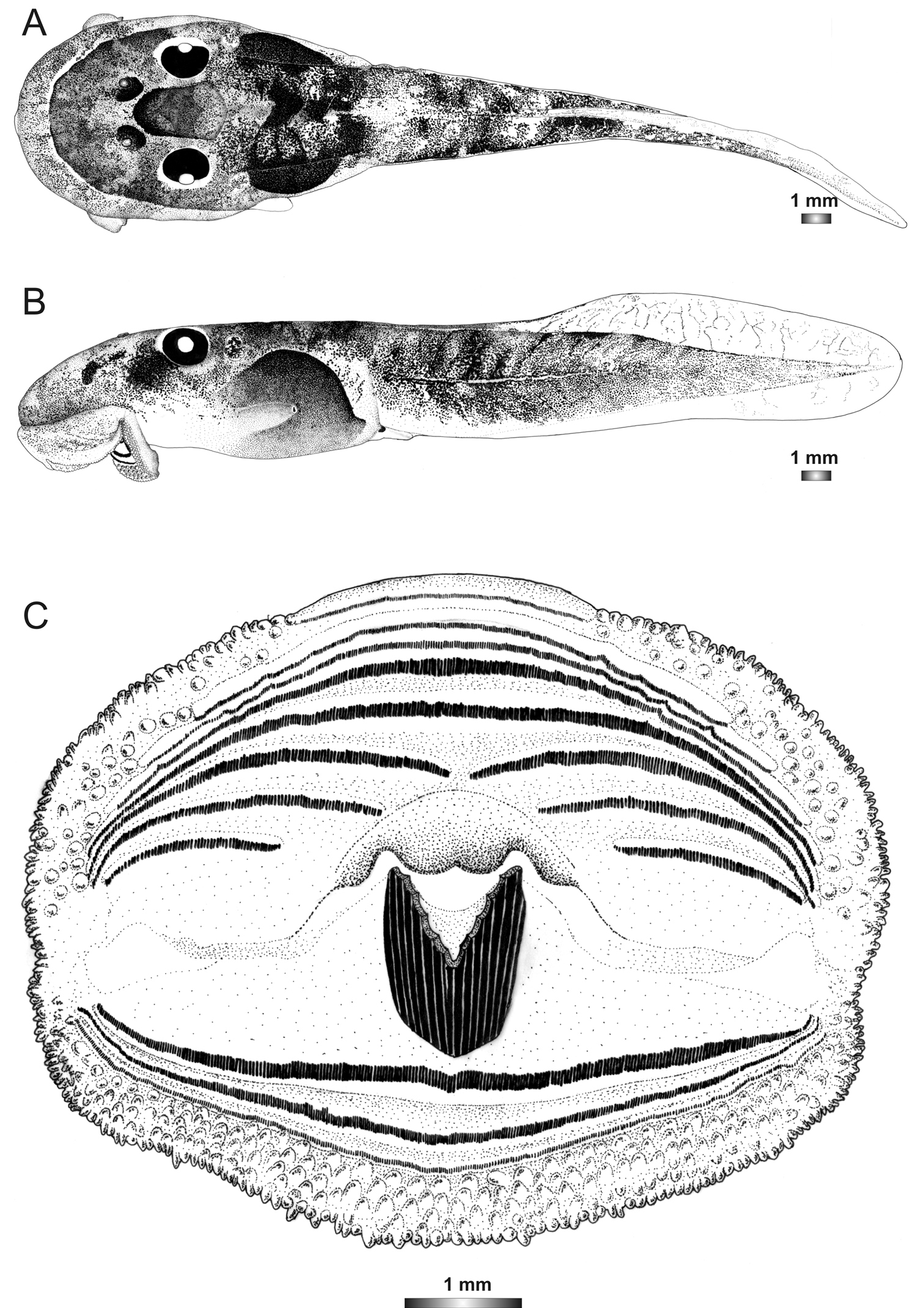
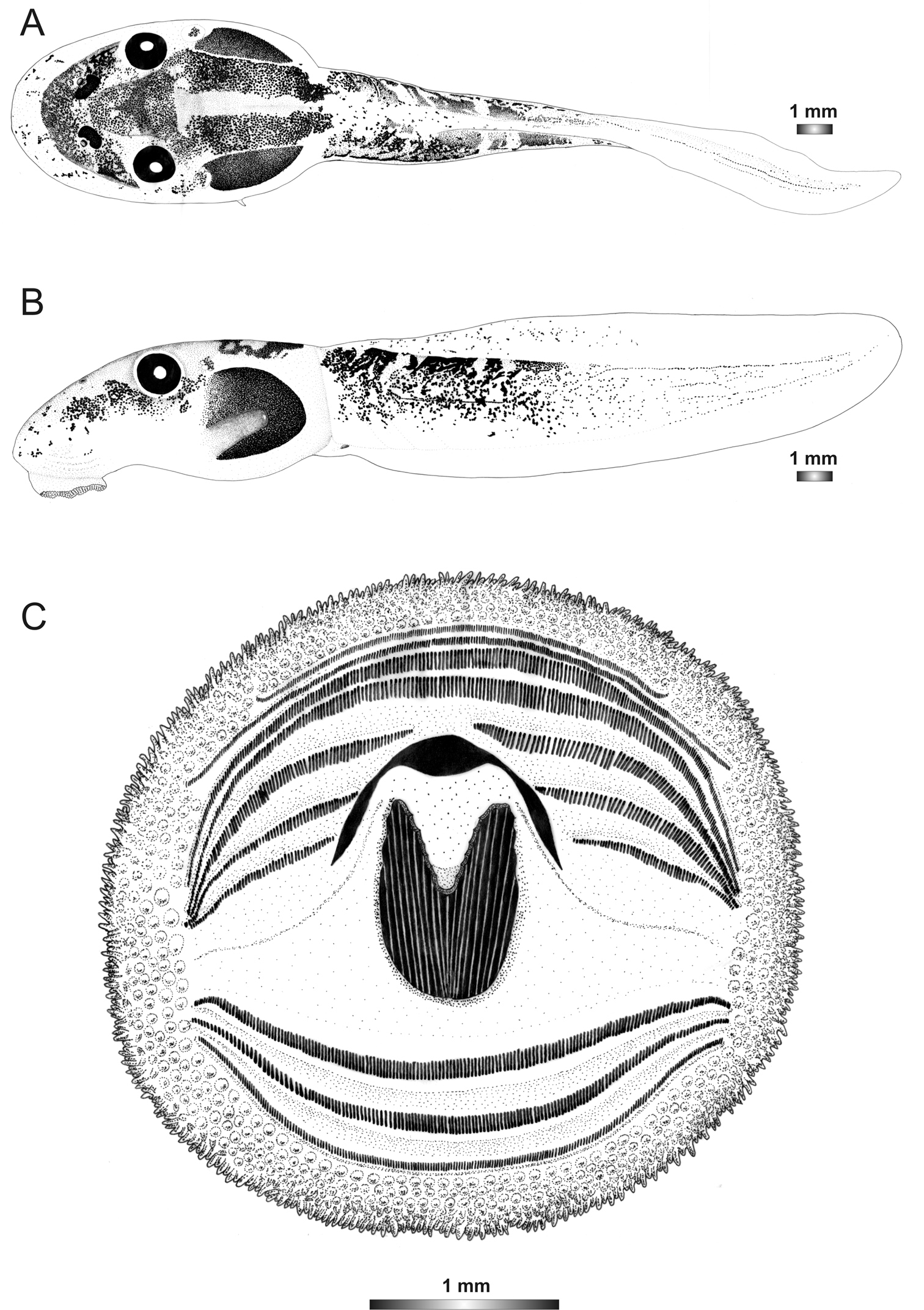
Morphological data were assessed in one tadpole (Figures 2 and 5) in developmental stage 26 (field number T 428; ZSM 998/2007, BL 11.8 mm, TL 25.4 mm, Genbank accession number GU974449) from Ranomafana National Park. The 16S rDNA sequence of this specimen is 100% identical to a reference sequence of an adult Boophis andohahela (accession AY848456) from the same locality. Five out of six other voucher specimens have the morphological characteristics of this species, whereas one tadpole has a difference in the oral disc configuration.
The general morphology of this tadpole is similar to that of Boophis englaenderi and Boophis englaenderi [Ca23], but it is characterized by the presence of a white patch posterior to the hexagonal mark above the neurocranium in life and even in preservative (Figure 1). The non-visibility of its intestinal coils is shared with Boophis englaenderi. The LTRF 6(3–6)/3 is identical to that of some specimens of Boophis englenderi but differs from that of Boophis englaenderi [Ca 23]. On the other hand, the absence of papillae on the ventral area of the lower labium is similar to that of Boophis englaenderi. The oral disc of this tadpole has a slightly developed lateral bulge.
Drawings of the preserved DNA voucher tadpole of Boophis andohahela (T 428-ZSM 998/2007): A Dorsal view B Lateral view C Oral disc.
This group is characterized by tadpoles having an enlarged oral disc without lateral emargination (but bulged laterally in some species) and ventral gap of marginal papillae. The dorsal gap is moderately wide. The anterior margin of the oral disc is separated by a deep crevice to the snout; i.e., the entire margin is free from the snout. LTRF 8(5–8)/3 or 7(5–7)/3. The jaw sheaths are moderately strong and completely keratinized. The upper sheath has a medial convexity in some species. The lower sheath is U or V-shaped and ribbed. Dorsolateral glands are present.
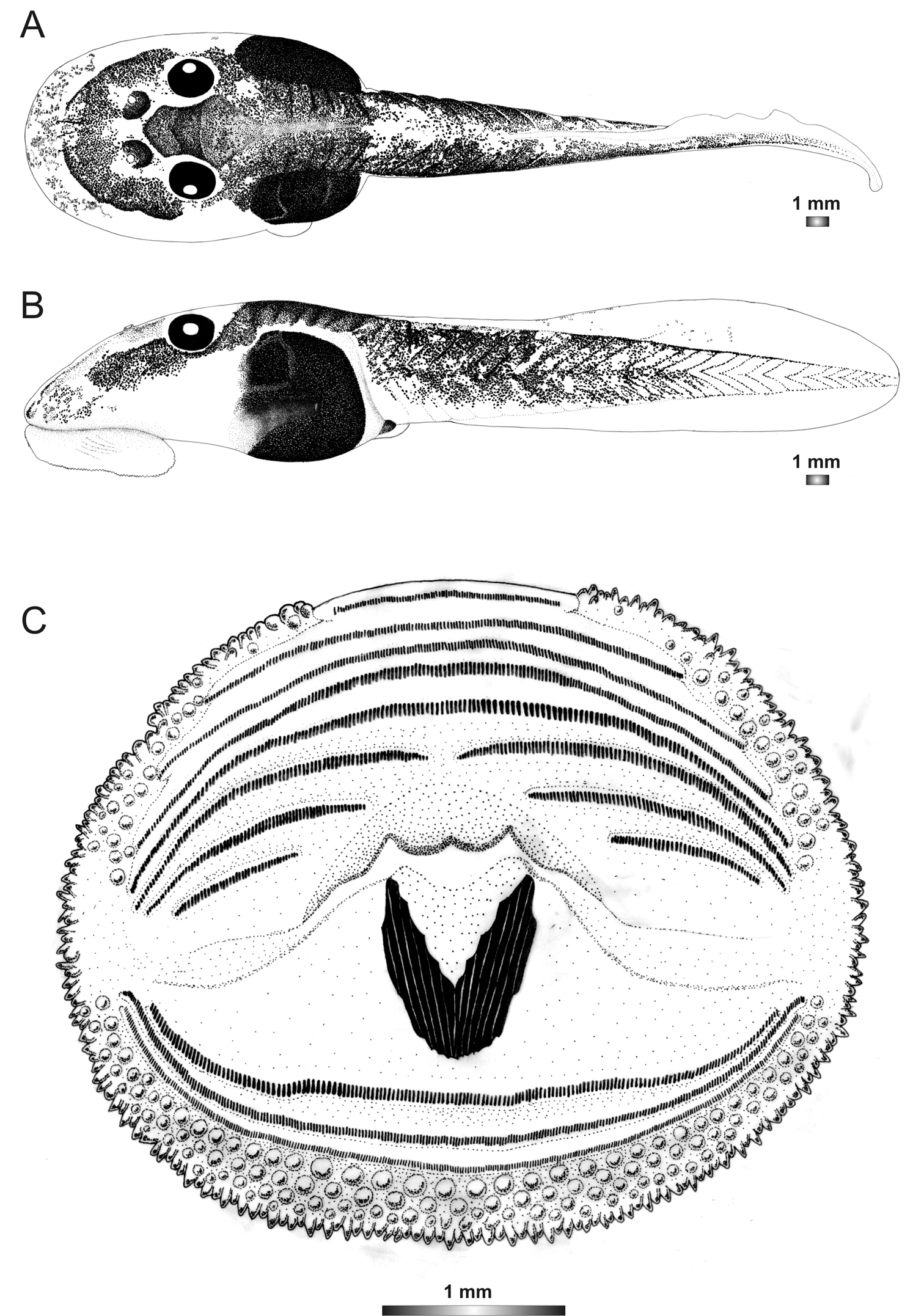
Boophis ankaratra Andreone, 1993
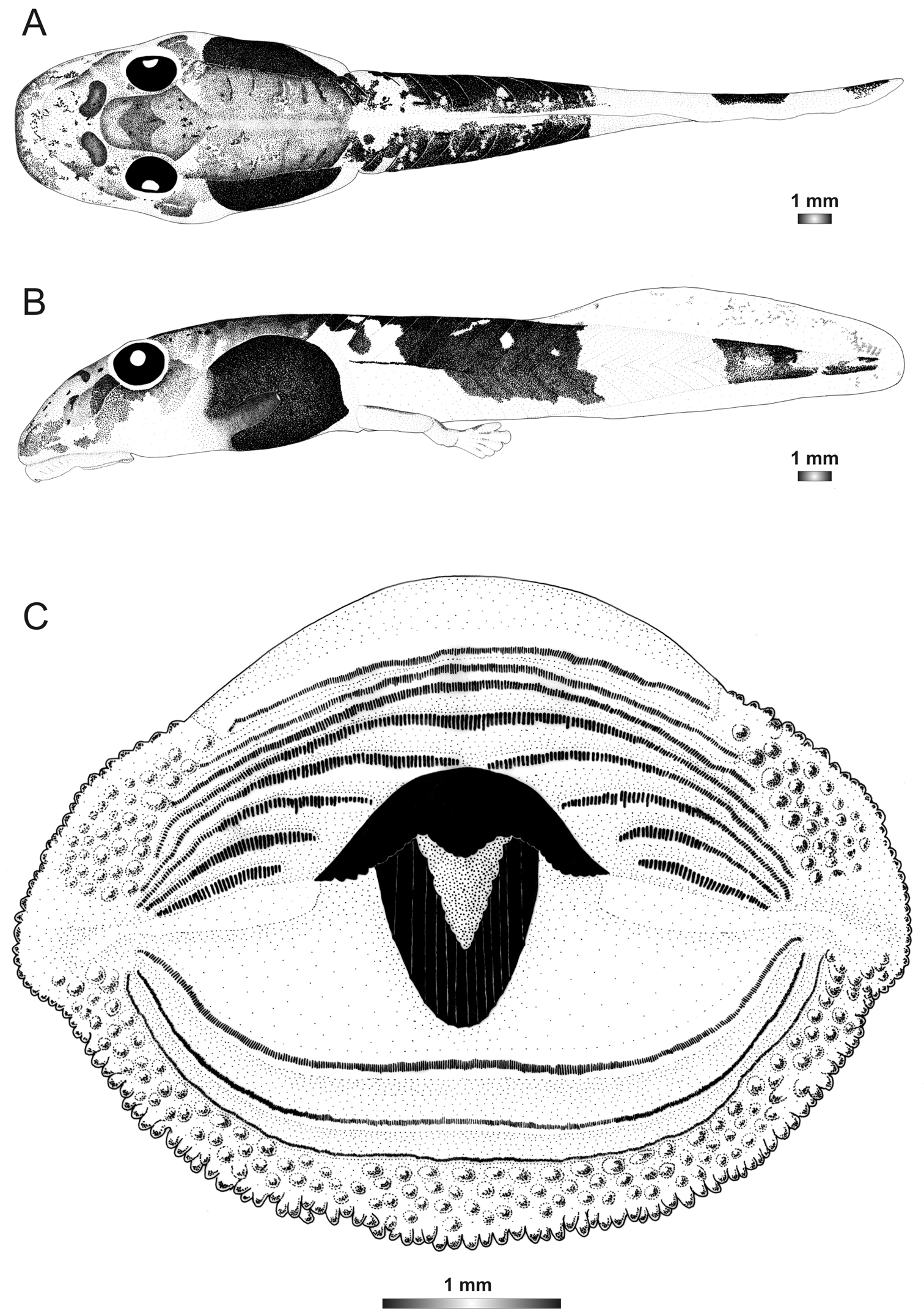
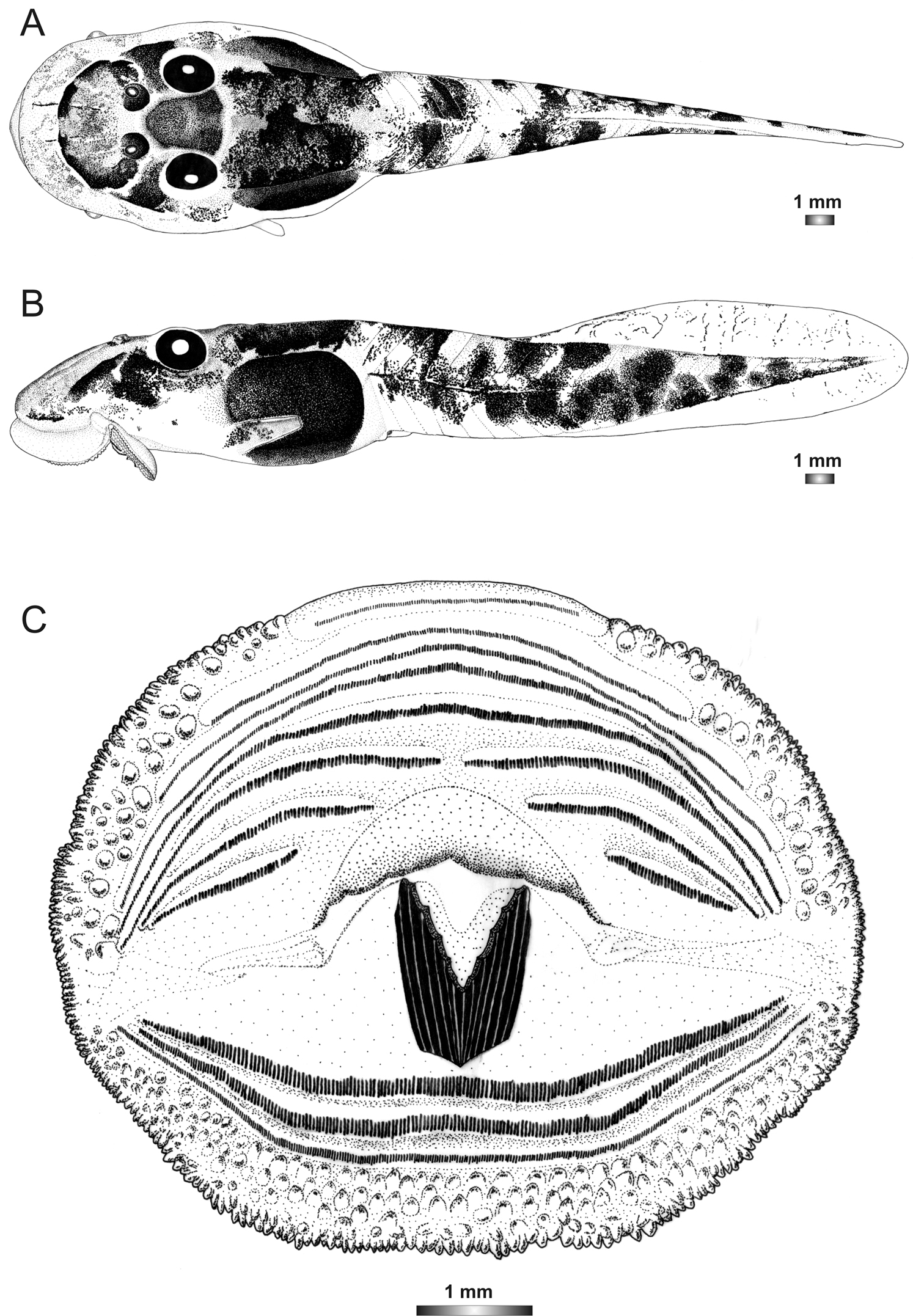
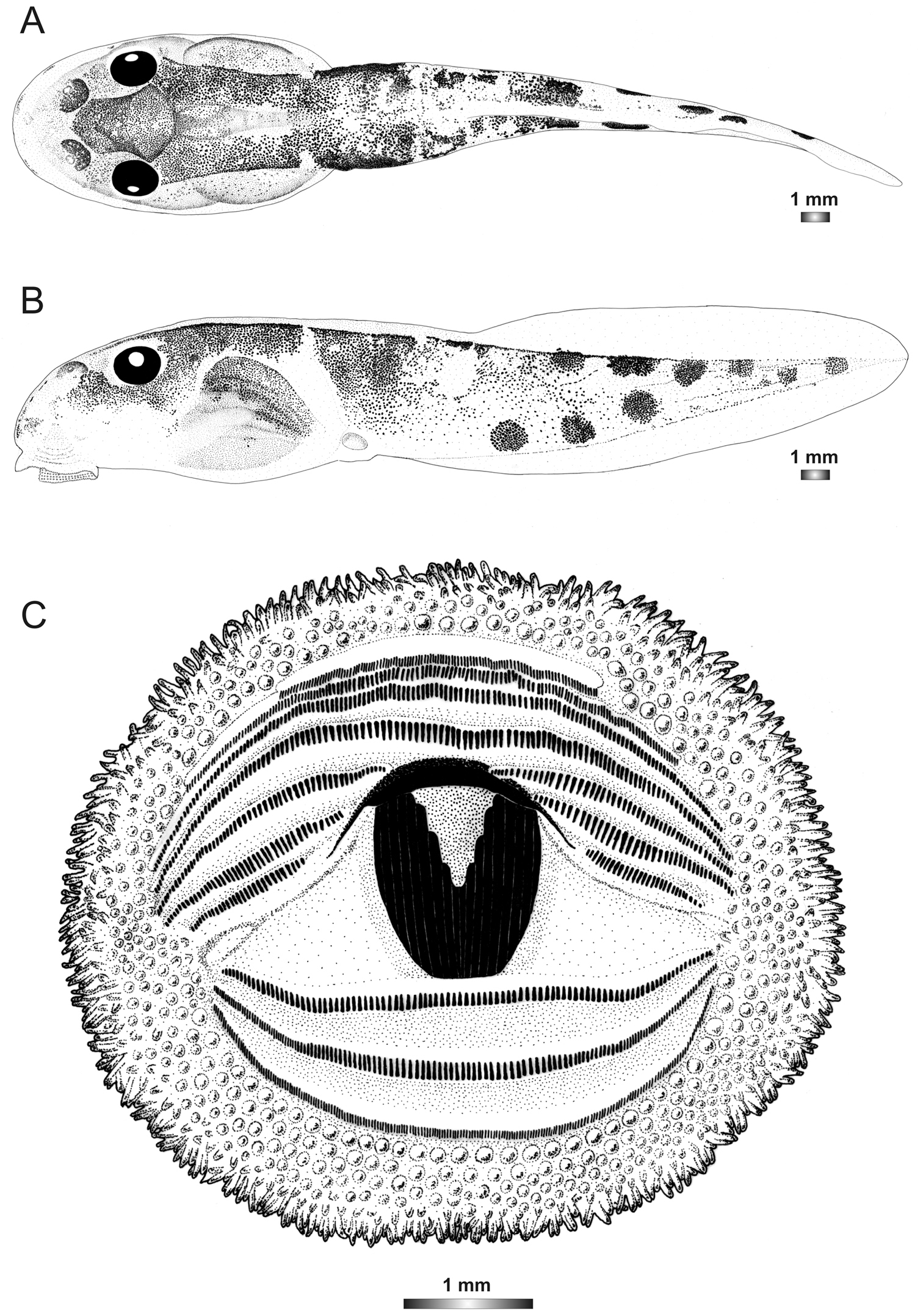
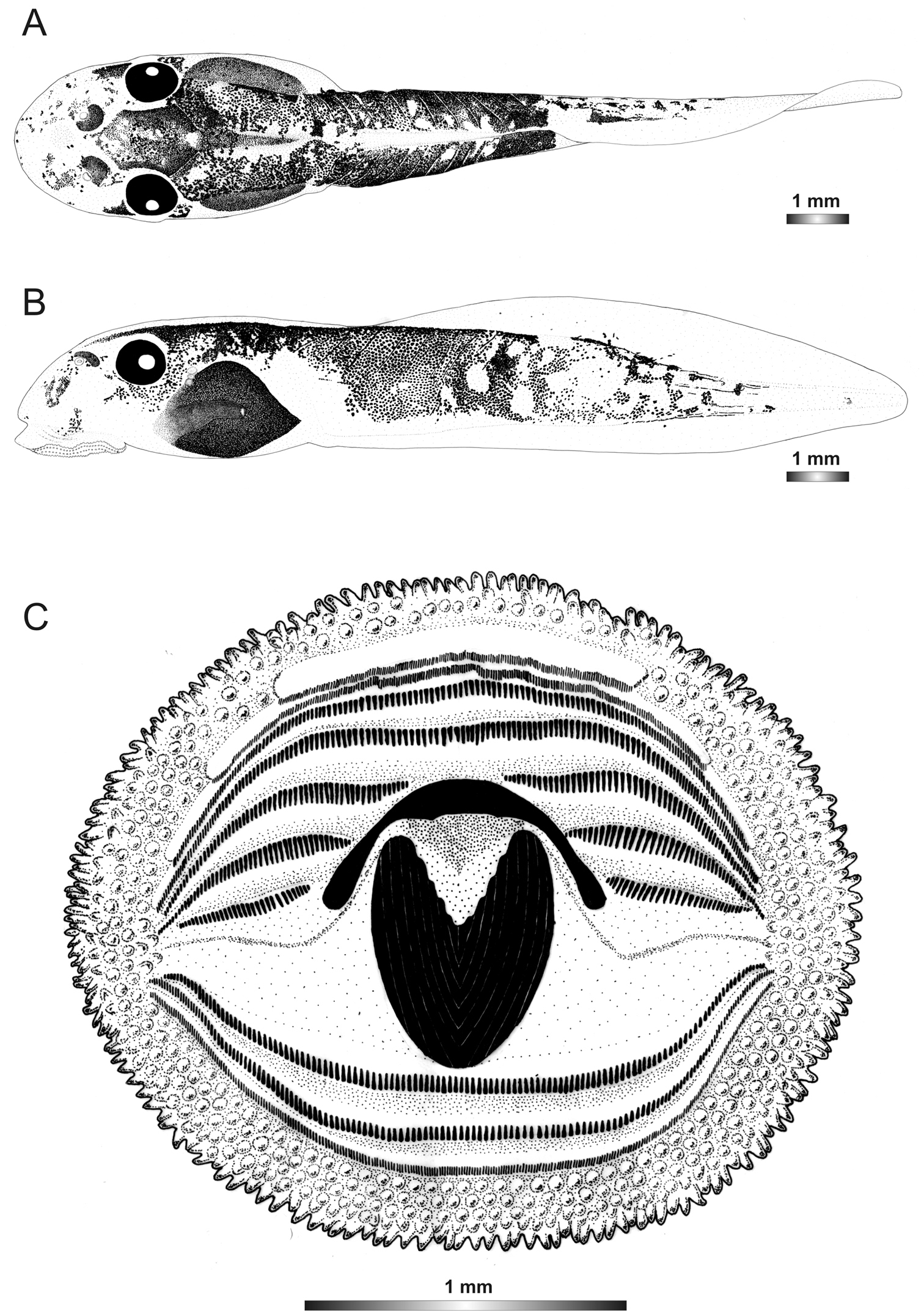
Morphological data were assessed in one tadpole (Figures 2 and 6) in developmental stage 28 (field number ZCMV 4917, ZSM 876/2007, BL 11.3 mm, TL 25.5 mm, Genbank accession number GU974476) from Ranomafana National Park. The 16S rDNA sequence of this specimen is 100% identical to a reference sequence of an adult Boophis ankaratra (accession AJ315909) from Mandraka. Two other voucher specimens possess the typical morphological characters of the species.
This tadpole can be differentiated from Boophis luteus goup tadpoles by the general state of the oral disc. It is characterized by an enlarged and laterally bulged oral disc. There is a double row of marginal papillae interrupted by a moderately wide dorsal gap. Papillae are short, small, conical with protuberance, and their tip is rounded. There are 148 and 190 marginal and submarginal papillae, respectively. The LTRF is 8(5–8)/3 and A1 is moderately long. The jaw sheaths are moderately strong and totally keratinized. The upper sheath is characterized by a short narrowly pointed medial convexity. The lower sheath is U-shaped, ribbed, higher than wide, and partially hidden by the upper one.
In life this tadpole is generally dark brown. Dorsally, body and tail covered by dense brown spots. A hexagonal mark above the neurocranium and a dark semicircular patch posterior to each narial opening are obvious. The domino-like structures between the vertebral area and the abdominal region are recognizable. Few irregular dark blotches and silvery spots scattered on the skin. Laterally, jugal area is covered by dense brown patches and the abdominal region is very dark leaving a transparent noticeable spiracle. The tail musculature is yellowish and covered by sparse brown spots which coalesce to form patches. Their density diminishes toward the tail tip. Fins are transparent with few brown blotches on the dorsal fin and the ventral fin is almost free from pigment. Ventrally, intestinal coils are invisible (Figure 1). In preservative, the tadpole is similar except that it is paler and the silver tissue which covers the heart and the venter has become whitish.
Drawings of the preserved DNA voucher tadpole of Boophis ankaratra (ZCMV 4917-ZSM 876/2007): A Dorsal view B Lateral view C Oral disc.
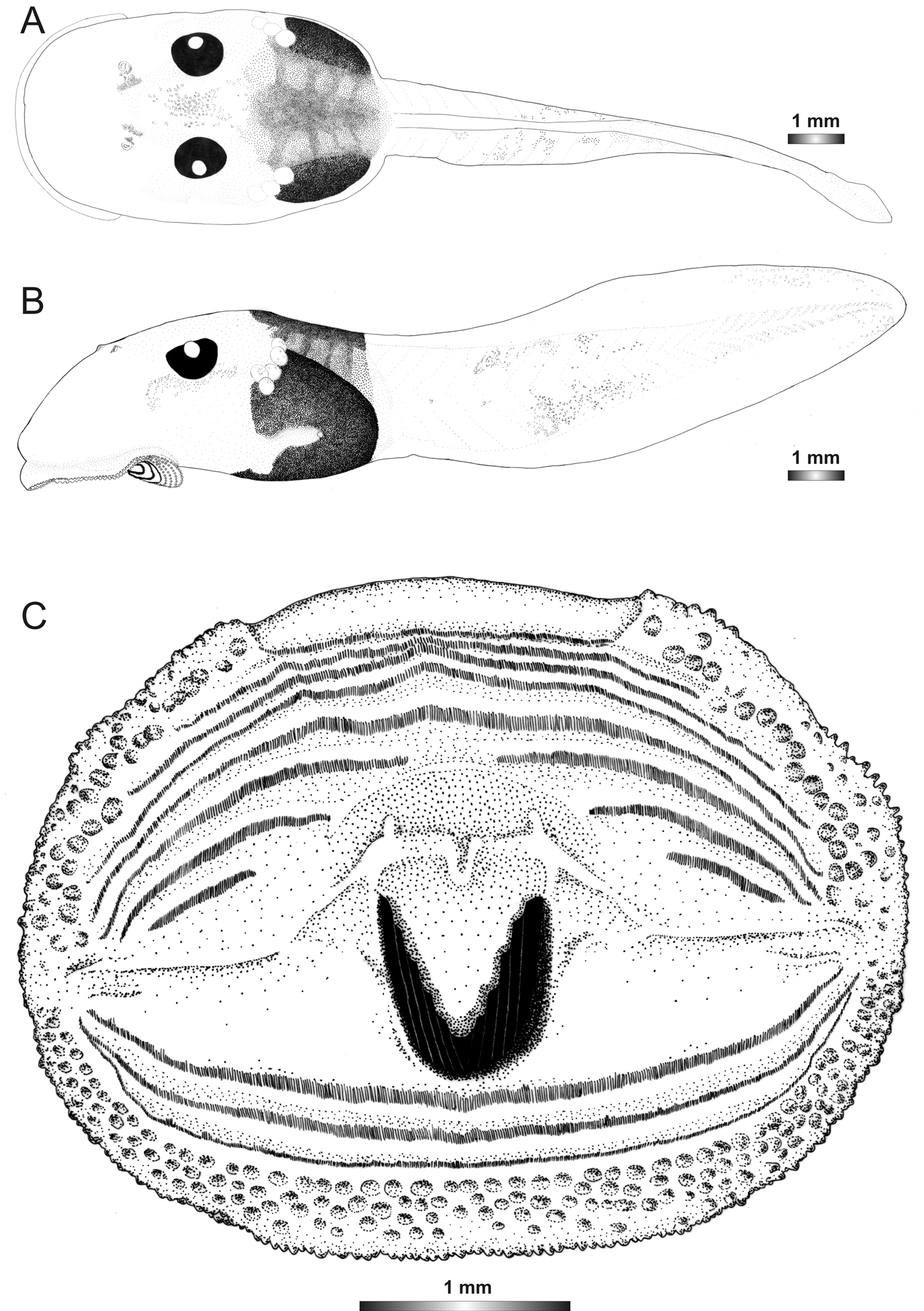
Boophis schuboeae Glaw & Vences, 2002
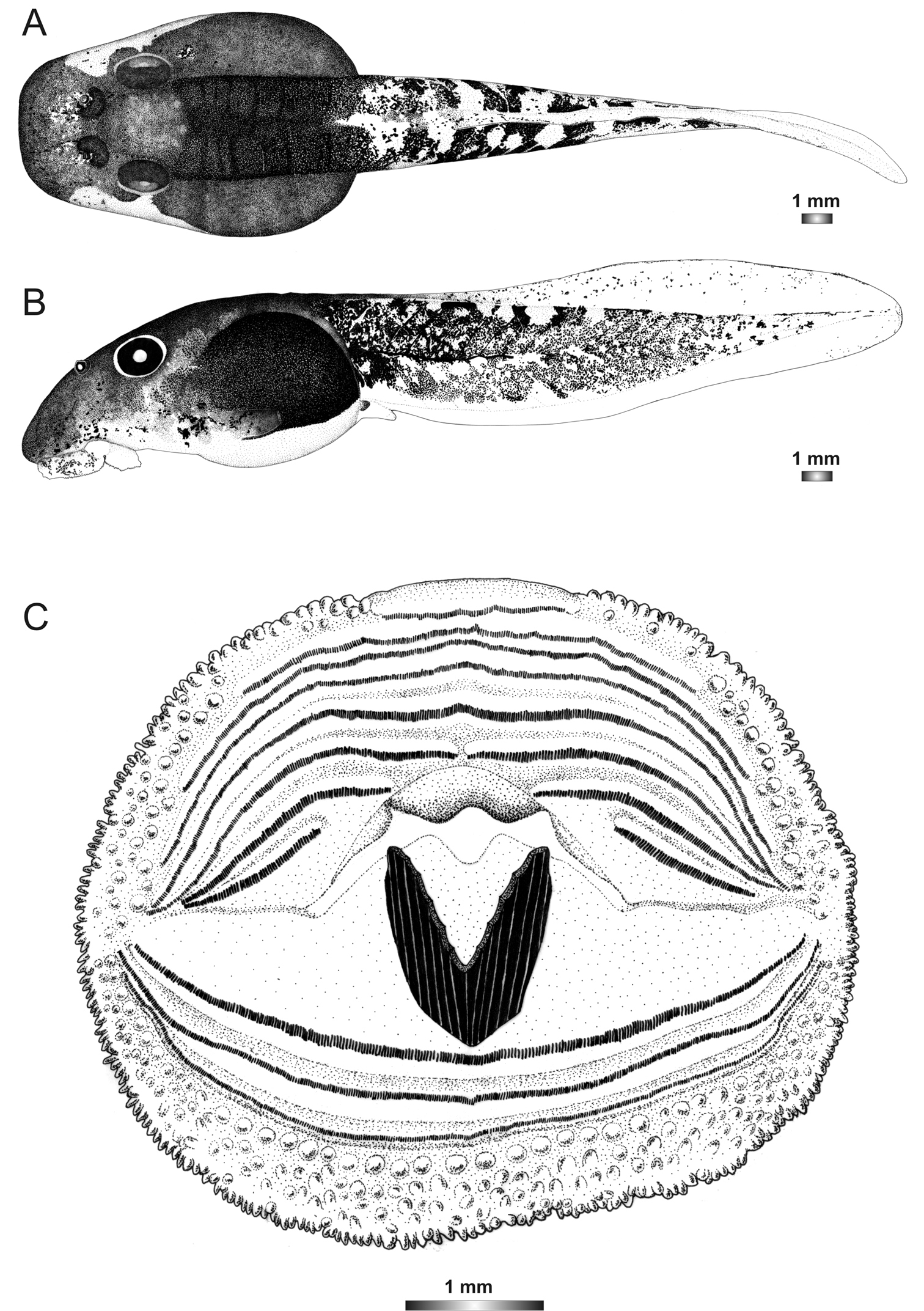
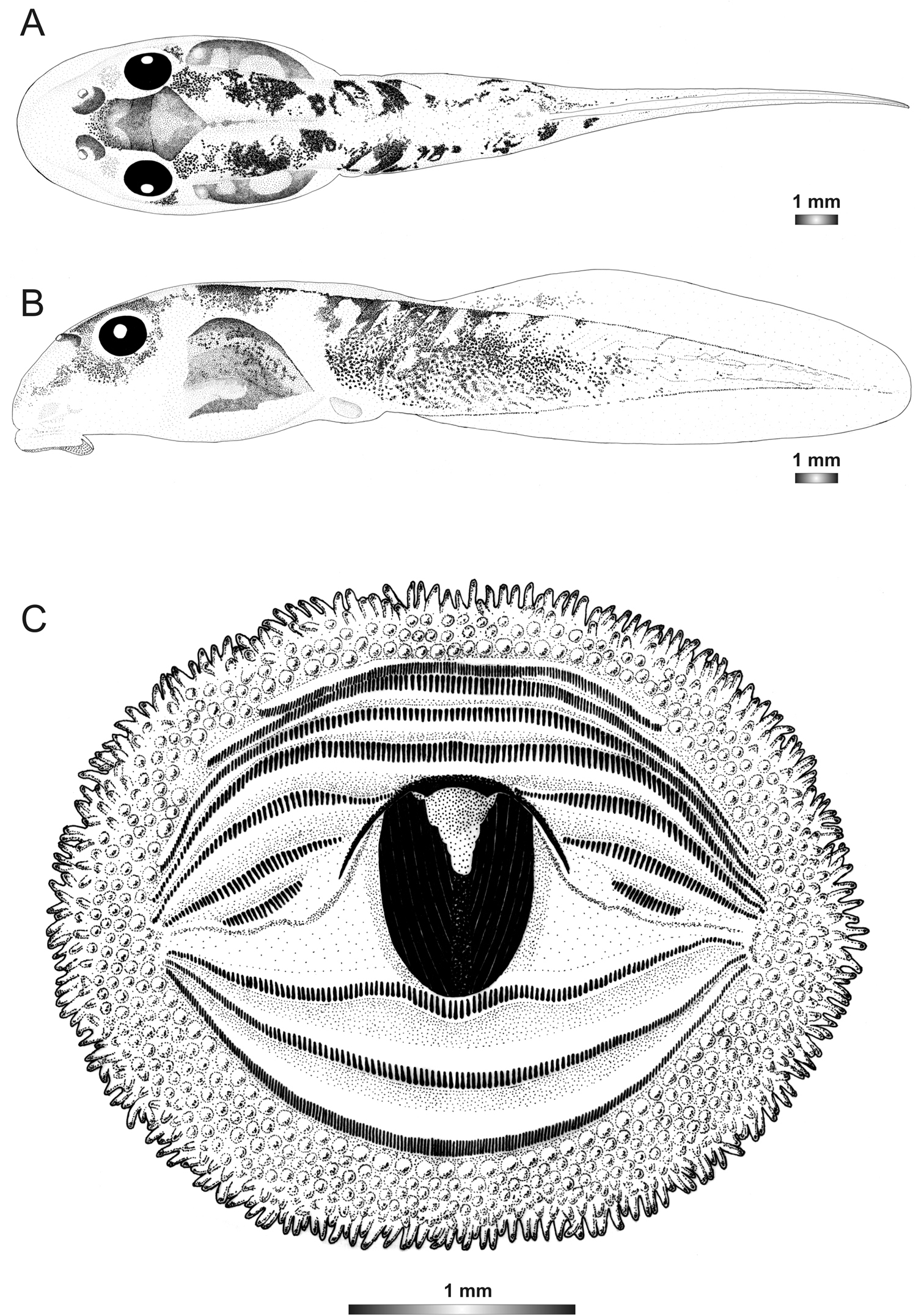
Morphological data were assessed in one tadpole (Figures 2 and 7) in developmental stage 36 (field number FGMV 2002–1800, ZSM 978/2004, BL 12.1 mm, TL 25.5 mm, Genbank accession number DQ068394) from Ranomafana National Park. The 16S rDNA sequence of this specimen is 100% identical to a reference sequence of an adult Boophis schuboeae (accession AJ315912) from the same locality. Six other voucher specimens from the same locality show the typical coloration pattern and oral disc configuration of the species.
The oral disc of the tadpoles belonging to this species is identical to those of Boophis ankaratra, except that it has a lower number of rather smaller papillae, and the lateral area where the oral disc folds is free from submarginal papillae. However, the tadpoles of these two species are easy to distinguish by their particular coloration pattern (Figure 1) (see also
Drawings of the preserved DNA voucher tadpole of Boophis schuboeae (FG/MV 2003.1800-ZSM 978/2004): A Dorsal view B Lateral view C Oral disc.
Boophis albipunctatus Glaw & Thiesmeier, 1993
Morphological data were assessed in one tadpole (Figures 2 and 8) in developmental stage 25 (field number ZCMV 4946, ZSM 82/2008, BL 7.5 mm, TL 15.5 mm, Genbank accession number GU974374) from Ambohitsara-Tsitolaka. The 16S rDNA sequence of this specimen is 99% identical to a reference sequence of a Boophis albipunctatus adult specimen (accession AY848446) from Manantantely. One other voucher tadpole of Boophis albipunctatus from the same locality is morphologically very similar to the described voucher specimen.
Boophis albipunctatus tadpoles can be distinguished from those of Boophis ankaratra and Boophis schuboeae by the absence of the lateral bulge on the oral disc, the absence of the medial convexity on the upper sheath, the high number of papillae, and the LTRF 7(5–7)/3, but they share the ribbed pattern, the U-shape, and the partially hidden state of the lower jaw sheath. These tadpole are also characterized by their less pigmented state in preservative which makes them easy to identify. The absence of silver pigment covering the heart in life is also typical for these tadpoles.
Drawings of the preserved DNA voucher tadpole of Boophis albipunctatus (ZCMV 4946-ZSM 82/2008): A Dorsal view B Lateral view C Oral disc.
Boophis sibilans Glaw & Thiesmeier, 1993
Morphological data were assessed in one tadpole (Figures 2 and 9) in developmental stage 29 (field number FGZC 2956, ZSM 1631/2007, BL 11 mm, TL 26 mm, Genbank accession number JQ518194) from Marojejy National Park. The 16S rDNA sequence of this specimen is 99.4% identical to a reference sequence of a Boophis sibilans adult specimen (accession AY341718) from Andasibe. Two other voucher tadpoles have similar morphological characteristics.
Boophis sibilans tadpoles have the same oral disc feature (absence of lateral bulge, LTRF) as Boophis albipunctatus, except for a lower number of submarginal papillae and a V-shaped lower sheath. These tadpoles are characterized by their rather long tail (up to 200% of BL) and their unique tail pattern which is composed of dark spots separated by a clear unpigmented area. The inner part of the spots is usually free from pigment (Figure 1).
Drawings of the preserved DNA voucher tadpole of Boophis sibilans (FGZC 2956-ZSM 1631/2007): A Dorsal view B Lateral view C Oral disc.
Boophis luciae Glaw, Köhler, de la Riva, Vieites & Vences, 2010
Morphological data were assessed in one tadpole (Figures 2 and 10) in developmental stage 36 (field number ZCMV 5146, ZSM 730/2007, BL 10.4 mm, TL 22.2 mm, Genbank accession number GU975069) from Ranomafana National Park. The 16S rDNA sequence of this specimen is 100% identical to a reference sequence of a Boophis luciae adult specimen (accession AY848444) from the same locality. Ten other voucher tadpoles are morphologically very similar to the described voucher specimen.
The tadpoles of Boophis luciae are similar to those of Boophis sibilans by their oral disc structure and the general external pattern except that they have a rather short tail. They can be characterized by the state of the spots on the tail musculature which are connected to each other (Figure 1).
Drawings of the preserved DNA voucher tadpole of Boophis luciae (ZCMV 5146-ZSM 730/2007): A Dorsal view B Lateral view C Oral disc.
This group is characterized by tadpoles having an enlarged oral disc without lateral emargination and ventral gap of papillae. The dorsal gap of papillae is narrow to very narrow, and the lateral area where the oral disc folds is free of submarginal papillae. The anterior margin of the oral disc is separated by a deep crevice to the snout; i.e., the entire margin is free from the snout. The upper labium has always five uninterrupted and three interrupted keratodont rows, and the three lower rows are always uninterrupted giving a unique LTRF 8(6–8)/3. The upper sheath is always absent. The lower sheaths are moderately strong and completely keratinized, U-shaped, ribed, and higher than wide. Dorsolateral glands are present.
Boophis sambirano Vences & Glaw, 2005
Morphological data were assessed in one tadpole (Figures 2 and 11) in developmental stage 25 (field number FG/MV 2002.1902, ZSM 672/2004, BL 6.5 mm, TL 12.7 mm, Genbank accession number EU717861) from a site locally named “Camp Norbert” in Manongarivo Special Reserve. The 16S rDNA sequence of this specimen is 96% identical to a reference sequence of Boophis sambirano adult specimen (accession AY848544), and because of this 4% difference its identity and belonging to the “true” Boophis sambirano needs further confirmation. Since this specimen was collected next to the type locality of Boophis sambirano in Manongarivo, following a parsimonious approach we here assign it to this species, although the large numbers of distinct lineages in Boophis sambirano make it likely that yet another candidate species of this complex occurs in Manongarivo. Many non-voucher specimens of the same series present morphological similarities to the voucher specimen.
Boophis sambirano tadpoles are easy to distinguish from all other tadpoles described above by the state of their oral disc which has no upper jaw sheath, a short keratodont row A1, and a narrow dorsal gap of papillae. The absence of submarginal papillae on the lateral area where the oral disc folds is shared with Boophis schuboeae. The tadpoles of this species are also characterized by the extension of an obvious lateral transparent area of the body wall only on the anterior 2/3 of the body, but not surrounding the whole body like in other tadpoles.
Drawings of the preserved DNA voucher tadpole of Boophis sambirano (FG/MV 2002.1904-ZSM 678/2004): A Dorsal view B Lateral view C Oral disc.
Boophis mandraka [Ca38
Morphological data were assessed in one tadpole (Figures 2 and 12) in developmental stage 26 (field number ZCMV 4261, ZSM 456/2007, BL 7.6 mm, TL 15.8 mm, Genbank accession number FJ559153) from Ranomafana National Park. The 16S rDNA sequence of this specimen is 93.3 % identical to a reference sequence of a Boophis sambirano adult specimen (accession EU717863) from Manongarivo Special Reserve.
The single tadpole of this candidate species has a similar oral disc structure to Boophis sambirano except that it has a slightly wider dorsal gap of papillae (DG/ODW 39% vs. 34%). The typical coloration, yellowish in life (Figure 1) and whitish in preservative and the good visibility of the 10 (5 right and 5 left) dorsolateral glands allow its distinction from other tadpoles.
Drawings of the preserved DNA voucher tadpole of Boophis mandraka [Ca38](ZCMV 4261-ZSM 456/2007): A Dorsal view B Lateral view C Oral disc.
Boophis mandraka [Ca46 JQ518195]
Morphological data were assessed in one tadpole (Figures 2 and 13) in developmental stage 25 (field number ZCMV 3479, ZSM 1784/2007, BL 6.8 mm, TL 14.3 mm, Genbank accession number JQ518195) from An'Ala. The 16S rDNA sequence of this specimen is 90.4 % identical to a reference sequence of Boophis sp. aff. mandraka adult specimen (accession AY848542) from Ilampy.
The oral disc of the single tadpole of this candidate species is similar to those of Boophis sambirano and Boophis mandraka [Ca38] except that it has the narrowest dorsal gap of papillae with DG 14% of ODW and the shortest A1 with 21% of ODW. Within the Boophis mandraka group tadpoles, it has also the lowest number of papillae. The external morphology of the single tadpole of this candidate species is similar to that of tadpoles of Boophis sambirano, except that the ratio RN/NP is much higher (194 vs. 125) and the pigmentation pattern is slightly different.
Drawings of the preserved DNA voucher tadpole of Boophis mandraka [Ca46](ZCMV 3479-ZSM 1784/2007): A Dorsal view B Lateral view C Oral disc.
Boophis sambirano [Ca47 JQ518203]
Morphological data were assessed in one tadpole (Figures 2 and 14) in developmental stage 27 (field number ZCMV 13105, ZSM 482/2010, BL 13.5 mm, TL 27.1 mm, Genbank accession number JQ518203) from Anjingo river (bridge 57 km from Antsohihy to Bealanana). The 16S rDNA sequence of this specimen is 97% identical to a reference sequence of Boophis sambirano tadpoles (accession EU717861) from Manongarivo Special Reserve. Two other voucher tadpoles are morphologically very similar to the described voucher specimen.
The tadpoles assigned to this candidate species have a similar oral disc structure as Boophis sambirano exept that they have a higher number of marginal papillae (377 vs. 248) and of keratodonts on A3 (1193 vs. 740). These tadpoles have a rather large size in comparison to others of the Boophis mandraka group, and their pigmentation pattern distinguishes them also. Their tail musculature is covered by dissipated distinct patches following mainly the lateral tail vein and the myosepta on the anterior half of the tail musculature, and irregularly dispersed on the posterior half (Figure 1), whereas it is just covered by dense spots on the anterior half in Boophis sambirano tadpoles. The dorsal fin of these tadpoles begins usually on the anterior 1/5 of the tail musculature, vs. beginning more or less at the dorsal body-tail junction in Boophis sambirano.
Drawings of the preserved DNA voucher tadpole of Boophis sambirano [Ca47](ZCMV 13105-ZSM 482/2010): A Dorsal view B Lateral view C Oral disc.
Boophis sambirano [Ca48 JQ518205]
Morphological data were assessed in one tadpole (Figures 2 and 15) in developmental stage 27 (field number ZCMV 13109, ZSM 485/2010, BL 12.7 mm, TL 24.7 mm, Genbank accession number JQ518205) from Anjingo river (bridge at 57 km on the road from Antsohihy to Bealanana). The 16S rDNA sequence of this specimen was 94% identical to a reference sequence of Boophis sambirano tadpoles (accession EU717861) from Manongarivo Special Reserve. Two other voucher tadpoles are very similar to the described voucher specimen.
The tadpoles assigned to this candidate species have a similar oral disc as Boophis sambirano and Boophis sambirano [Ca47]. The higher number of marginal papillae (336) and of keratodonts on A3 (1052) differentiate these tadpoles from those of Boophis sambirano but are similar to Boophis sambirano [Ca47]. The ovoidal body form in dorsal view and the pigmentation pattern – variegated spots on the body and less coalesced spots on the tail musculature (Figure 1) – differentiate these tadpoles from those of Boophis sambirano [Ca47]. The beginning of the dorsal fin on the anterior 1/5 of the tail musculature is similar to that of Boophis sambirano [Ca47] but different from Boophis sambirano.
Drawings of the preserved DNA voucher tadpole of Boophis sambirano [Ca48](ZCMV 13109-ZSM 485/2010): A Dorsal view B Lateral view C Oral disc.
Boophis sambirano [Ca49 JQ518208]
Morphological data were assessed in one tadpole (Figures 2 and 16) in developmental stage 27 (field number ZCMV 13155, ZSM 528/2010, BL 11.7 mm, TL 26.7 mm, Genbank accession number JQ518208) from Ankijagna Lagnana. The 16S rDNA sequence of this specimen is 94.1% identical to a reference sequence of Boophis sambirano tadpoles (accession EU717861) from Manongarivo Special Reserve. Three other voucher specimens and many non-voucher specimens of the same series are morphologically very similar to the described specimen.
The oral disc of the tadpoles assigned to this candidate species is the typical one of the Boophis mandraka group, characterized by a narrow dorsal gap of papillae (DG 23% of ODW) which is here wider than in Boophis mandraka [Ca46] but smaller than in the other tadpoles, and the short keratodont row A1 which is similar to that of Boophis mandraka [Ca46] tadpoles. The number of papillae is similar to that of Boophis sambirano and Boophis mandraka [Ca38]. These tadpoles can be easily distinguished from all Boophis sambirano-like tadpoles by their particular pigmentation pattern which is uniformly dark (Figure 1), by the non visibility of the lateral transparent area of the body wall, the ovoidal form of the body in dorsal view, and the eye position between the anterior 3/10 and 4/10 of the body.
Drawings of the preserved DNA voucher tadpole of Boophis sambirano [Ca49](ZCMV 13155-ZSM 528/2010): A Dorsal view B Lateral view C Oral disc.
Boophis sambirano [Ca50 JQ518211]
Morphological data were assessed in one tadpole (Figures 2 and 17) in developmental stage 27 (field number ZCMV 13172, ZSM 545/2010, BL 11.7 mm, TL 25.7 mm, Genbank accession number JQ518211) from Ambinanitelo. The 16S rDNA sequence of this specimen is 94.9% identical to a reference sequence of Boophis sambirano tadpoles (accession EU717861) from Manongarivo Special Reserve. Three other voucher tadpoles are morphologically very similar to the described voucher specimen.
The oral disc of the tadpoles of this candidate species is similar to that of other Boophis mandraka group species. The tadpoles belonging to this candidate species have an elliptical body form in dorsal view but differ from those of Boophis sambirano [Ca49] by their pigmentation pattern. The presence of a lateral transparent area of the body wall surrounding the anterior 2/3 of the body is similar to those of Boophis sambirano, but the absence of contrasted integumental patches limiting the transparent body wall area surrounding the snout is a difference to Boophis sambirano, Boophis sambirano [Ca47], and Boophis sambirano [Ca48]. The tadpoles of this candidate species can thus be distinguished from those of other candidate species close to Boophis sambirano mainly by their coloration pattern (Figure 1).
Drawings of the preserved DNA voucher tadpole of Boophis sambirano [Ca50](ZCMV 13172-ZSM 545/2010): A Dorsal view B Lateral view; C Oral disc.
This group is heterogeneous in larval morphology and probably non monophyletic (e.g.,
Boophis marojezensis Glaw & Vences, 1994
Morphological data were assessed in one tadpole (Figures 2 and 18) in developmental stage 27 (field number FGZC 2277, ZSM 1528/2007, BL 7.1 mm, TL 18.3 mm, Genbank accession number JQ518196), from Marojejy National Park. The 16S rDNA sequence of this specimen is 99.8% identical to a reference sequence of a Boophis marojezensis adult specimen (accession FJ559127) from the same locality. Three other voucher tadpoles are morphologically very similar to the described voucher specimen.
The tadpoles of this species are easily to distinguish from those belonging to other species groups (as described above) by the general structure of their oral disc which has no dorsal gap of papillae, and a LTRF of 7(5–7)/3. These tadpoles are also characterized by the highest number of submarginal papillae in Boophis, with 290 marginal and 606 submarginal papillae. The lateral transparent area of the body wall area is visible and the dorsolateral gland is obvious. The tail muscle is spotted and the spots fused to form patches mainly on the upper half of tail musculature, the density of the spots diminishes toward the tail tip. The posterior 1/3 of the tail has few pigments.
Drawings of the preserved DNA voucher tadpole of Boophis marojezensis (FGZC 2277-ZSM 1528/2007): A Dorsal view B Lateral view C Oral disc.
Boophis marojezensis [Ca25
Morphological data were assessed in one tadpole (Figures 2 and 19) in developmental stage 29 (field number FGZC 2929, ZSM 1611/2007, BL 7.8 mm, TL 18.5 mm, Genbank accession number FJ559146), from Marojejy National Park. The 16S rDNA sequence of this specimen is 97% identical to a reference sequence of Boophis marojezensis adult specimen (accession AY848596) from Vohidrazana, and less similar to other tadpoles from Marojejy. Two non-voucher specimens from the same series have the particular caudal pattern present in the voucher specimen.
Tadpoles assigned to this candidate species have the same oral disc structure as those of Boophis marojezensis, but with a lower number of papillae (222 marginal and 315 submarginal). The presence of seven more or less rounded patches formed by condensation of spots on the posterior half of the tail musculature of these tadpoles is a further useful character to differentiate them from those of Boophis marojezensis.
Drawings of the preserved DNA voucher tadpole of Boophis marojezensis [Ca25](FGZC 2929-ZSM 1611/2007): A Dorsal view B Lateral view C Oral disc.
Boophis marojezensis [Ca26
Morphological data were assessed in one tadpole (Figures 2 and 20) in developmental stage 29 (field number FGZC 2930, ZSM 1612/2007, BL 8.8 mm, TL 20.6 mm, Genbank accession number JQ518197), from Marojejy National Park. The 16S rDNA sequence of this specimen is 96.6% identical to a reference sequence of a Boophis marojezensis adult specimen (accession AY848595) from Tsaratanana.
The single tadpole belonging to this candidate species has the typical marojezensis-like oral disc structure with 234 marginal and 430 submarginal papillae. It has almost the same pigmentation pattern as Boophis marojezensis, but the patches are more striking on the upper limit of tail musculature. It is differentiated from Boophis marojezensis [Ca25] by the absence of distinct patches on the tail musculature.
Drawings of the preserved DNA voucher tadpole of Boophis marojezensis [Ca26](FGZC 2930-ZSM 1612/2007): A Dorsal view B Lateral view C Oral disc.
Boophis marojezensis [Ca51 JQ518198]
Morphological data were assessed in one tadpole (Figures 2 and 21) in developmental stage 25 (field number ZCMV 3691, ZSM 267/2008, BL 6 mm, TL 20 mm, Genbank accession number JQ518198) from Ranomafana National Park. The 16S rDNA sequence of this specimen is 99.7% identical to a reference sequence of a Boophis marojezensis adult specimen (accession AY848594) from Vohiparara (but with >5% divergence to all other Boophis marojezensis-like forms). Twenty-one other tadpoles assigned to this candidate species reveal a similar morphological pattern and oral disc configuration as the described voucher specimen.
The tadpoles assigned to this candidate species have the typical marojezensis-like oral disc structure with 297 marginal and 309 submarginal papillae. They can be distinguished from the other marojezensis-like tadpoles by the absence of a lateral transparent area of the body wall area surrounding the body. They have also the widest inter-orbital distance (IOD) in the group, and they are also the only marojezensis-like tadpoles with eyes situated between the anterior 3/10 and 4/10 of the body. The tail muscle is covered by reticulations, mainly on the anterior half.
Drawings of the preserved DNA voucher tadpole of Boophis marojezensis [Ca51](ZCMV 3691-ZSM 267/2008): A Dorsal view B Lateral view C Oral disc.
Boophis marojezensis [Ca52 JQ518215]
Morphological data were assessed in one tadpole (Figures 2 and 22) in developmental stage 28 (field number ZCMV 13168, ZSM 541/2010, BL 10.5 mm, TL 26.1 mm, Genbank accession number JQ518215) from Ambinanitelo. The 16S rDNA sequence of this specimen is 100% identical to a reference sequence of anadult specimen assigned to Boophis marojezensis (accession AY848595) from Tsaratanana (but with >5% divergence to all other Boophis marojezensis-like forms). One other voucher specimen is morphologically very similar to the described one.
Tadpoles of this candidate species have the typical marojezensis-like oral disc structure with 258 marginal and 522 submarginal papillae. These tadpoles are distinguished from other marojezensis-like tadpoles by the only poorly recognizable lateral transparent body wall area surrounding the body, and by their tail pigmentation pattern which lacks melanophoric pigments (Figure 1). The position of the eyes is in the range of most other Boophis marojezensis-like tadpoles.
Drawings of the preserved DNA voucher tadpole of Boophis marojezensis [Ca52](ZCMV 13168-ZSM 541/2010): A Dorsal view B Lateral view C Oral disc.
Boophis marojezensis [Ca53 JQ518216]
Morphological data were assessed in one tadpole (Figures 2 and 23) in developmental stage 27 (field number ZCMV 13200, ZSM 573/2010, BL 9.6 mm, TL 23 mm, Genbank accession number JQ518216) from Tsaratanana Integral Reserve. The 16S rDNA sequence of this specimen is 98.8% identical to a reference sequence of a Boophis marojezensis adult specimen (accession FJ559127) from Marojejy. Five other voucher specimens attributed to the same candidate species are morphologically very similar to the described one.
The tadpoles of this candidate species have also a marojezensis-like oral disc with 243 marginal and 452 submarginal papillae. They are similar to Boophis marojezensis, Boophis marojezensis [Ca25], and Boophis marojezensis [Ca26], but different from Boophis marojezensis [Ca51] and Boophis marojezensis [Ca52] by the presence of a distinct lateral clear area surrounding the body (Figure 1). The general pigmentation pattern is similar to that of Boophis marojezensis [Ca26].
Drawings of the preserved DNA voucher tadpole of Boophis marojezensis [Ca53](ZCMV 13200-ZSM 573/2010): A Dorsal view B Lateral view C Oral disc.
Boophis vittatus Glaw, Vences, Andreone & Vallan, 2001
Morphological data were assessed in one tadpole (Figures 2 and 24) in developmental stage 29 (field number FGZC 2238, ZSM 1906/2007, BL 7.8 mm, TL 18.5 mm, Genbank accession number JQ518200), from Marojejy National Park - Camp Mantella. The 16S rDNA sequence of this specimen is 100% identical to a reference sequence of a Boophis vittatus adult specimen (accession FJ559158) from the same locality. Three other voucher tadpoles of Boophis vittatus are morphologically very similar to the described voucher specimen.
The tadpoles of Boophis vittatus are the smallest tadpoles in this group. They have also a marojezensis-like oral disc structure with 289 marginal and 326 submarginal papillae. The tadpoles of this species are provided with a lateral transparent area of the body wall which is more pronounced surrounding the 2/3 anterior of the body. The tail musculature is reticulated like in Boophis marojezensis [Ca51].
Drawings of the preserved DNA voucher tadpole of Boophis vittatus (FGZC 2238-ZSM 1906/2007): A Dorsal view B Lateral view C Oral disc.
In streams of Ranomafana National Park, during the wet season, tadpoles of 44 frog species were found of which five had the morphological characteristics of the strongly rheophilous Boophis. These species were found in eleven out of 33 streams. Boophis andohahela occurred in eight streams with a mean of 9.9 specimens (min=1 to max=31 specimens), Boophis ankaratra occurred in two streams each with one single specimen, Boophis marojezensis [Ca51] was found in seven streams with a mean of 6.3 specimens (1 to 16 specimens), and only a single specimen of Boophis schuboeae was found. The tadpoles of Boophis luciae (named Boophis sp. 17 in
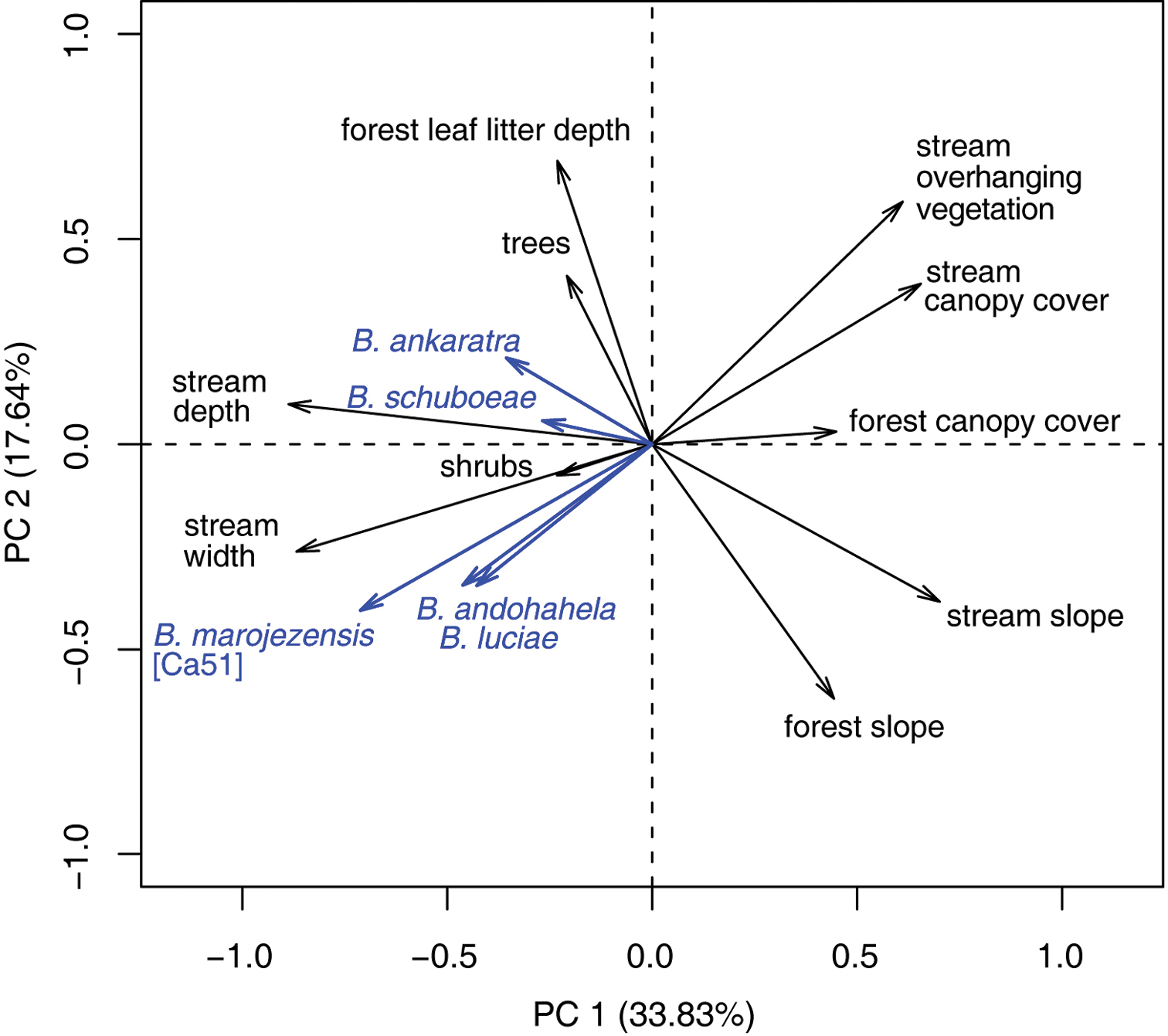
Principal Component Analysis on the habitat variables of the stream and the surrounding forest at Ranomafana resulted in three principal components, explaining together 65.5% of the variation in the data. We identified the following habitat variables being well represented (Figure 25): PC1 (33.8%) positive: slope and canopy cover of forest and stream, overhanging vegetation; negative: width and depth of the stream. Also four of the strongly rheophilous tadpole species, Boophis ankaratra, Boophis andohahela, Boophis luciae, and Boophis marojezensis [Ca51] are negatively correlated with this PC. The strongest contributors to PC2 (17.6%) were positive: forest leaf litter depth, stream overhanging plants, trees, and stream canopy cover; negative: slope of forest and stream. Boophis andohahela and Boophis marojezensis [Ca51] are negatively correlated with this PC. To PC3 (14.1%), the following variables were positive: number of small trees and shrubs in the forest and overhanging vegetation. Due to its rareness, no correlation of Boophis schuboeae incidence and PCs can be statistically assessed.
PCA biplot of variables of stream and surrounding habitat as recorded during a tadpole community study in Ranomafana National Park. The five present species of strongly rheophilous tadpoles are included as supplementary variables. Length and direction of vectors can be interpreted as correlations.
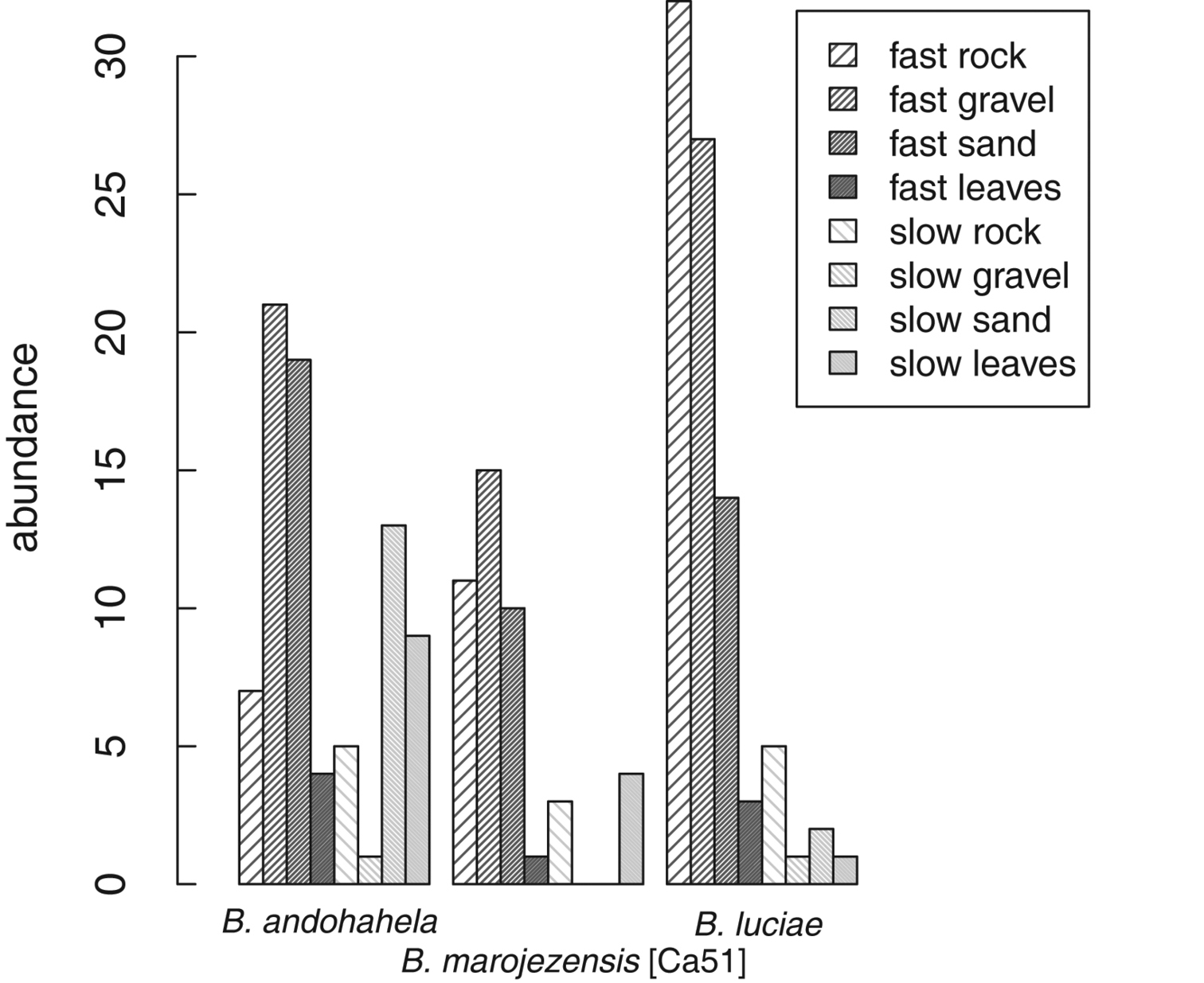
Strongly rheophilous Boophis tadpoles were found in all microhabitats available in streams of Ranomafana National Park (Figure 26). A considerable amount of specimens was found in microhabitats characterised by fast flowing water and substrates of rock, gravel, and sand which generally do not harbour many tadpoles (own unpublished data). Tadpoles of Boophis andohahela were also relatively often found in slow moving parts of the streams with leaves and sand as substrates. Of the two locally rare species, Boophis ankaratra and Boophis schuboeae, one specimen of each was found in fast rock and fast sand microhabitat, and one specimen in slow rock microhabitat, respectively.
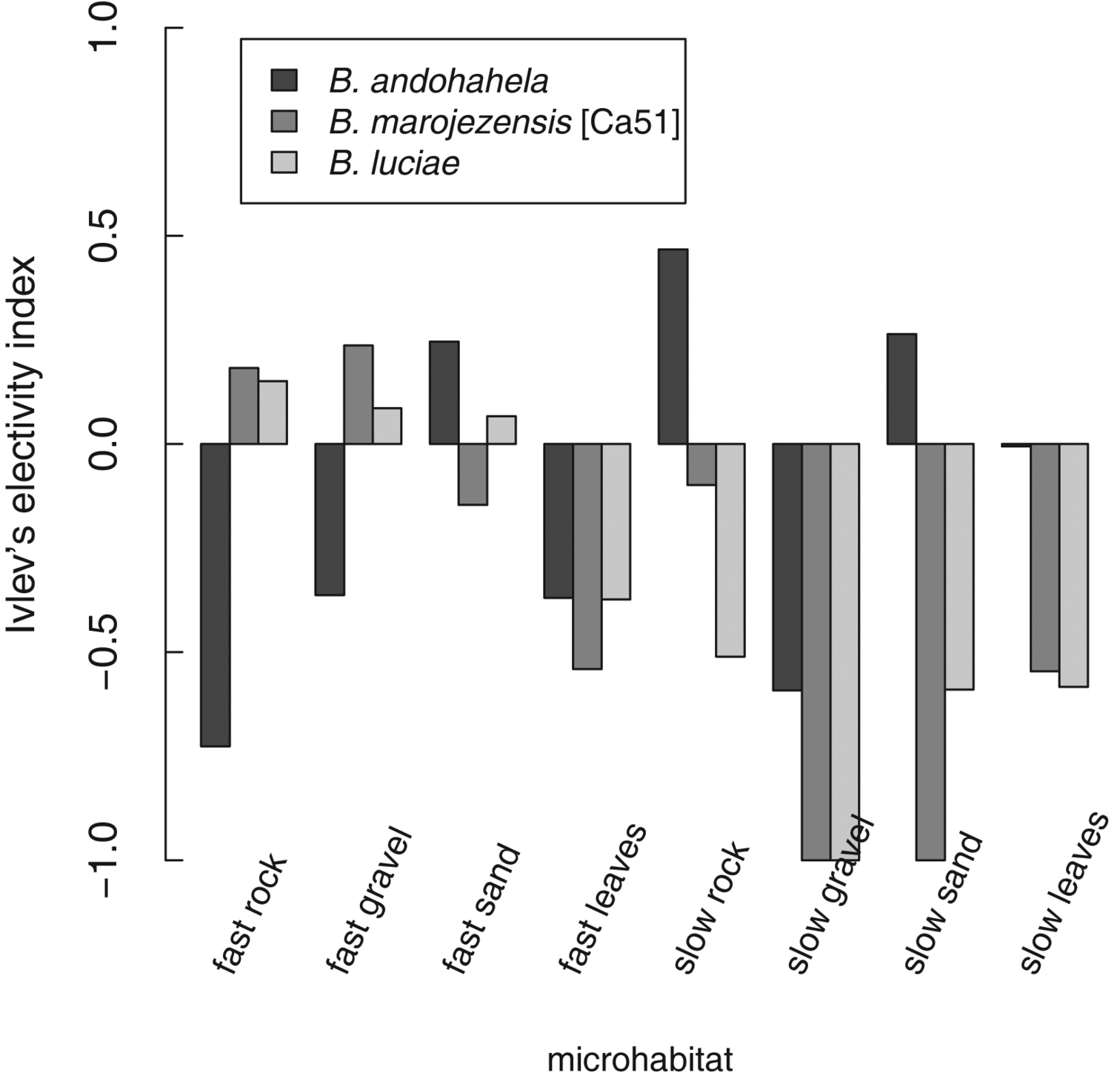
Considering the availability of microhabitats in the streams, Ivlev's electivity index (E,
Tadpole distribution across the eight microhabitats (defined using water current and stream substrat) of the three most abundant strongly rheophilous Boophis that were sampled in Ranomafana National Park in wet season 2008. Boophis andohahela: N=8 , Boophis marojezensis [Ca51]: N=7, Boophis luciae N=10 with N= the number of streams.
Barplot displaying tadpole microhabitat use of three most abundant Boophis species with strongly rheophilous tadpole morphology. Microhabitat use is calculated using Ivlev's electivity index (E,
Twenty-two strongly rheophilous tadpoles are characterized morphologically in this study, including fourteen tadpoles that are described for the first time and eight other species that had been previously described by other authors. Strongly rheophilous Boophis tadpoles have long been known by the work of
The tadpoles of Boophis majori described by
The tadpoles of Boophis sp. (Blommers-Schlösser, 1979) are similar to the Boophis luteus group tadpoles described herein according to their general oral disc structure. The LTRF 6(3–6)/3(1) corresponds to those of Boophis englaenderi tadpoles and 6(3–6)/3 to those of Boophis englaenderi [Ca23] tadpoles: This indicates that these tadpoles might belong to two different Boophis species. Since Boophis englaenderi and Boophis englaenderi [Ca23] do not occur in the site where
Tadpoles having narial openings closer to the eyes than to the snout, a sinistral spiracle situated on the 3/4 of the body, a well developed caudal musculature, a rounded oral disc with a LTRF of 7(5–7)/3, a dorsal gap of papillae and a complete jaw sheath were also described and assigned to Boophis erythrodactylus, a species of the Boophis rappiodes group, by
As described by
Tadpoles of Boophis andohahela from Ranomafana were described by
The Boophis sibilans tadpoles from Andasibe that
Boophis englaenderi, Boophis vittatus and Boophis luciae were described by
As decribed by
This type of tadpoles can be found in different Boophis species groups: Boophis luteus group, Boophis albipunctatus group, Boophis mandraka group, and Boophis majori group (its occurrence in the Boophis rappiodes group is in need of confirmation). As described by
Within the main groups of morphologically similar tadpoles, some can be very similar, but usually there are morphological details to differentiate them, whether in the external morphology or in the oral disc configuration; i.e., tadpoles that are very similar in external morphology can be differentiated in oral disc structure and vice versa:
(1) Three tadpoles belonging to the Boophis luteus group (Boophis englaenderi, Boophis englaenderi [Ca23], and Boophis andohahela) look alike in external morphology but can be differentiated easily by their keratodont row formula. Of these, Boophis englaenderi and Boophis englaenderi [Ca23] occur syntopically. The tadpoles of Boophis englaenderi [Ca23] can be distinguished from those of Boophis englaenderi by their relative tail length, by their pigmentation pattern and mainly by their oral disc structure (LTRF and number of papillae).
(2) In the Boophis albipunctatus group, Boophis ankaratra, Boophis schuboeae, Boophis sibilans and Boophis luciae are similar. Boophis ankaratra and Boophis schuboeae occur sympatrically, and they can be differentiated by the presence of dark pigmented bands on the tail muscle in Boophis schuboeae, and also by the absence of papillae on the lateral area where the oral disc folds in Boophis schuboeae. Boophis sibilans and Boophis luciae differ by the presence of a dark bridge which connects the dark sections on the tail muscle in Boophis luciae.
(3) All tadpoles known from the species of the Boophis mandraka group have a similar oral disc configuration, characterized by the absence of the upper jaw sheath and a LTRF of 8(6–8)/3. The tadpoles of Boophis sambirano and Boophis mandraka [Ca46] are very similar, except that Boophis mandraka [Ca46] has the narrowest dorsal gap of marginal papillae. The fact that these two tadpoles live allopatrically can help also to identify them. Five species of this group are distributed in close proximity in the North of Madagascar. Of these, Boophis sambirano [Ca47] and Boophis sambirano [Ca48] are sympatric, and can be differentiated by the patched vs. spotted pattern on the tail. Boophis sambirano [Ca49] and Boophis sambirano [Ca50] live also sympatrically. Boophis sambirano [Ca49] can be distinguished to the three other species by its generally dark coloration pattern, the ovoidal form of the body in dorsal view and the wide inter-orbital distance. Boophis sambirano [Ca50] can be differentiated by the intensity of the golden pigments which may cover the whole body and overlay the dark pigment in some specimens. Boophis mandraka [Ca38] is very typical by its weakly expressed pigmentation.
(4) Two tadpoles belonging to two different groups, Boophis albipuncatus (Boophis albipunctatus group) and Boophis mandraka [Ca38] (Boophis mandraka group) are similar in their weak expression of pigmentation, but they can easily differentiated by their oral disc morphology.
(5) Two cases of similarity are also found in Boophis majori group tadpoles. Boophis marojezensis, Boophis marojezensis [Ca26], Boophis marojezensis [Ca53] and Boophis vittatus are very similar in the presence of a clear, not pigmented lateral area surrounding the body, and in the tail pigmentation pattern. The fact that several of these species can occur sympatrically increases also the chance to confound them. On the other hand, the tadpoles of Boophis marojezensis [Ca51] and Boophis marojezensis [Ca52] are similar in the absence of a lateral clear area surrounding the body, and in their general pigmentation pattern. Only the tadpole of Boophis marojezensis [Ca25] is easily distinguishable by the presence of clear and more or less rounded patches on the tail muscle. As the three Boophis marojezensis-like tadpoles, Boophis marojezensis, Boophis marojezensis [Ca25] and Boophis marojezensis [Ca26], live syntopically in Marojejy National Park, Boophis marojezensis [Ca25] tadpoles will not be confounded with those of the two other species.
Morphological clusters of strongly rheophilous Boophis tadpolesAnalyzing the structure of the oral disc of all these tadpoles allows classifying them into three clusters:
(1) The first cluster including three Boophis luteus group tadpoles is characterized by a moderately wide to very wide (ODW 56 to 84% of BW), non emarginated, ventrally positioned and oriented oral disc, which has an anterior margin connected directly to the snout, two uninterrupted upper rows of keratondonts (LTRF is 6(3–6)/3(1) for Boophis englaenderi but 6(3–6)/3 for the tadpoles of Boophis englaenderi [Ca23] and Boophis andohahela); a very long A1 (82 to 90% of ODW); a high number of keratodonts in A1 (220 to 301), totally keratinized; typically narrow to moderate sized jaw sheaths (JW 31 to 46% of ODW) with a very short medial convexity (MCL 0.04 to 0.11% of JW); a wide to very wide dorsal gap of papillae (DG 67 to 85% of BW); a low number of submarginal papillae (33 to 94) and a medium number of marginal papillae (101 to 175); a high positioned eye (EH 69 to 85% of BH) that is situated not far from midbody (SE 32 to 39% of BL); very high positioned nares (NH 57 to 82% of BH) that are situated below or at eye level (NH 82 to 97% EH) and closer to the snout than to the eye (RN 60 to 92% of NP); a short tail (TAL 155 to 183% of BL), and a developed caudal musculature.
(2) The second cluster is characterized by a wide to hyper-wide (ODW 74 to 108% of BW) non emarginated, ventrally positioned and oriented oral disc with an anterior margin separated from the snout by a shallow crevice or free; four or five uninterrupted upper rows of keratondonts giving a LTRF of 7(5–7)/3 or 8(6–8)/3); a short to moderately sized A1 (21 to 59% of ODW); a low to medium number of keratodonts in A1 (95 to 241), totally keratinized; U-shaped, ribbed narrow upper jaw sheaths (JW 30 to 34% of ODW which can present a small medial convexity or not); a U-shaped and "ribbed" lower sheath, a moderately wide to very narrow dorsal gap of papillae (DG 14 to 59% of BW); a medium to high number of marginal papillae (148 to 377), many submarginal papillae (190 to 368); high to very high positioned eyes (EH 71 to 84% of BH) that are situated closer to the snout than to midbody (SE 35 to 49% of BL), high to very high positioned nares (NH 64 to 92% of BH) that are situated below or above the eye level (NH 86 to 112% of EH) and closer to the eye than to the snout (RN 107 to 194% of NP); a short to very short tail (TAL 146 to 184% of BL); and a developed caudal musculature. Tadpoles of the Boophis albipuncatus group (Boophis schuboeae, Boophis ankaratra, Boophis albipunctatus, Boophis sibilans, and Boophis luciae) and Boophis mandraka group (Boophis sambirano, Boophis mandraka [Ca38], Boophis mandraka [Ca46], Boophis sambirano [Ca47], Boophis sambirano [Ca48], Boophis sambirano [Ca49], and Boophis sambirano [Ca50]) belong to this cluster. All Boophis mandraka group tadpoles lack a keratinized upper jaw sheath.
(3) The third cluster is characterized by a wide (ODW 68 to 79% of BW) non emarginated, ventrally positioned and oriented oral disc without a dorsal gap of papillae and with the anterior margin being free from the snout; four uninterrupted upper keratodont rows (LTRF 7(5–7)/3); a moderately sized A1 (45 to 52% of ODW); a medium number of keratodonts in A1 (126 to 235); a totally keratinized upper jaw sheath (JW 30 to 38% of ODW) without medial convexity; a U-shaped and ribbed lower sheath, many submarginal (222 to 318) and marginal (206 to 522) papillae; high positioned eyes (EH 68 to 80% of BH) that are situated closer to midbody (SE 35 to 49% of BL); very high positioned nares (NH 68 to 80% of BH) that are situated below eye level except for Boophis vittatus and Boophis marojezensis [Ca25] (NH 89 to 101% of EH) and closer to the snout for the most (RN 78 to 109 % of NP); a very short to short tail (TAL 140 to 188% of BL), and a developed caudal musculature. Tadpoles of the Boophis majori group (Boophis marojezensis, Boophis marojezensis [Ca25], Boophis marojezensis [Ca26], Boophis marojezensis [Ca51], Boophis marojezensis [Ca52], Boophis marojezensis [Ca53], and Boophis vittatus) belong to this cluster.
Although we lack at this time an explicit and well supported phylogenetic hypothesis for the relationships among all these species of Boophis, the morphological characters of these three morphological clusters can serve to develop a possible evolutionary scenario of the origin of the specializations in strongly rheophilous Boophis tadpoles, departing from the structures in more generalized Boophis tadpoles. All of the latter are characterized by having one (the first) uninterrupted upper keratodont row and one (the first) interrupted lower keratodont row, typically smooth (non-ribbed) jaw sheaths and a medial convexity in the upper jaw sheath (see
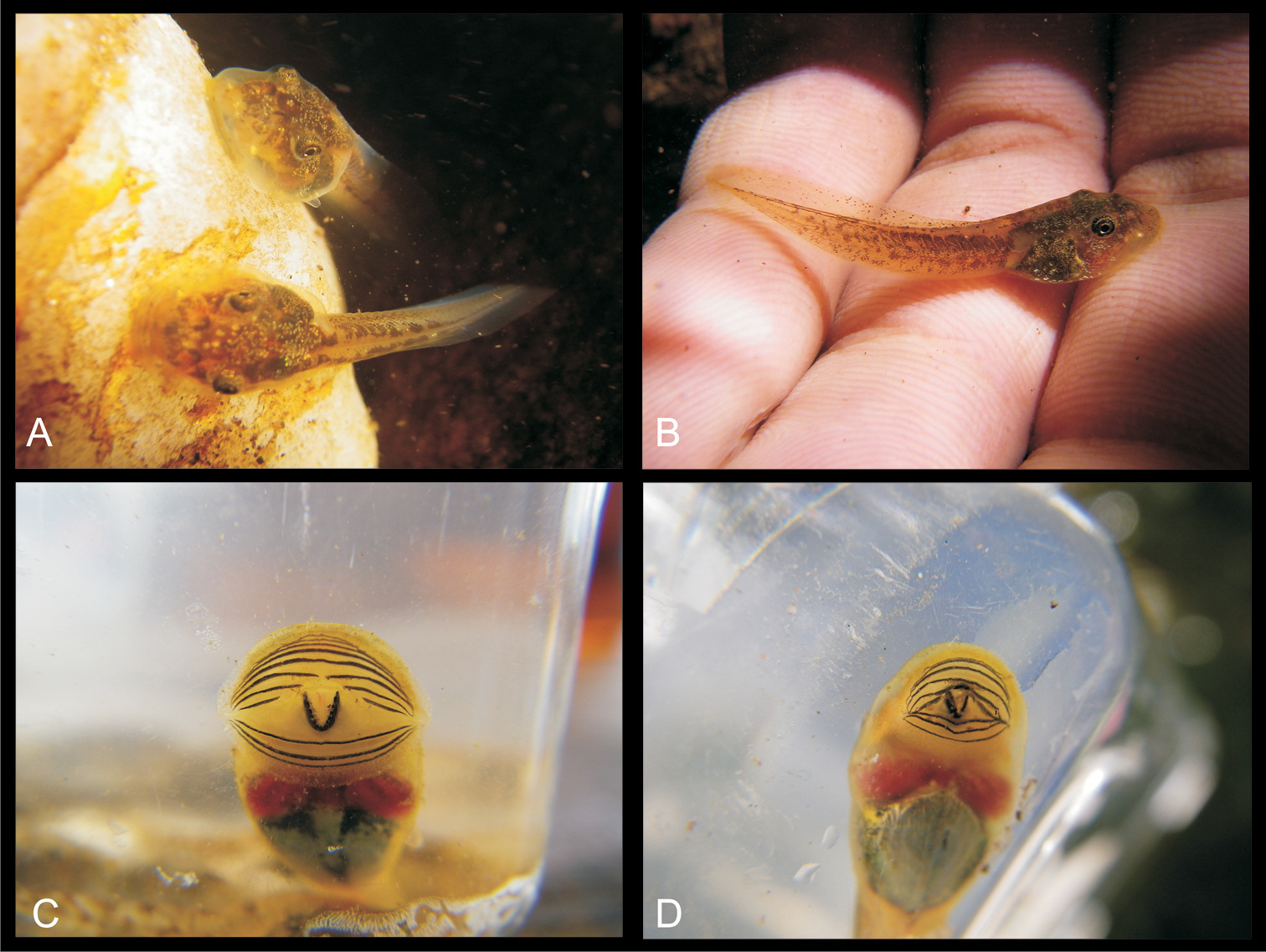
The decrease of the size of the jaw sheaths may provoke the fading of its medial convexity on one hand and leaves a place for many dorsal and lateral, even ventral submarginal papillae, and new uninterrupted upper keratodont rows on the other hand. Also, the reduction of the size of the dorsal gap leads to a higher number of marginal papillae. The development of many dorsal marginal papillae reduces the area available for the first upper keratodont row and thus may cause the reduction of its length, which in turn leads to the decrease of the number of the teeth. However, the loss of the upper jaw sheath in all species and candidate species of the Boophis sambirano complex is still unclear. This characteristic is neither caused by a fixation artifact nor by the transportation of the specimens because we observed it already in the living tadpoles in the field (Figure 29), and because it is consistent within series. The absence of the upper jaw sheath was found even in young tadpoles (Gosner 25) indicating that it occurs very early in larval development. It remains to be tested (e.g., by a study on embryonic development), however, if this structure never develops, or is initially formed but then disappears at some early developmental stage.
Ecomorphological guilds in Boophis tadpolesA magnitude of descriptions of the larval stages of Madagascan frogs have been recently published (
According to
In this study, no new guild names are defined, but we suggest to adapt in a preliminary way the guilds already defined by
(1) We do not consider the Boophis luteus group tadpoles truely strongly rheophilous, due to their more generalized and intermediate characteristics. These tadpoles (Boophis englaenderi, Boophis englaenderi [Ca23], Boophis andohahela) can possible be considered to be part of the “clasping” guild.
(2) The first guild of strongly rheophilous tadpoles, here considered as “adherent”, is the second category of tadpoles classified in the previous section which is composed by the tadpoles of the Boophis albipuncatus group (Boophis schuboeae, Boophis ankaratra, Boophis albipunctatus, Boophis sibilans, and Boophis luciae) and the Boophis mandraka group (Boophis sambirano, Boophis mandraka [Ca38], Boophis mandraka [Ca46], Boophis sambirano [Ca47], Boophis sambirano [Ca48], Boophis sambirano [Ca49], and Boophis sambirano [Ca50]), because they inhabit faster running water and the maintenance of the position in the water via their oral disc is common to continuous. This guild is characterized mainly by the presence of a dorsal gap of papillae and two typical LTRF-s which are 8(5–8)/3 and 8(6–8)/3. All Boophis mandraka group tadpoles lack an upper jaw sheath, while this structure is present in the Boophis albipuncatus group tadpoles.
(3) The second guild that we define as “suctorial” is the third category of tadpoles classified in the previous section which is composed of all Boophis marojezensis-like tadpoles (Boophis marojezensis, Boophis marojezensis [Ca25], Boophis marojezensis [Ca26], Boophis marojezensis [Ca51], Boophis marojezensis [Ca52], Boophis marojezensis [Ca53], and Boophis vittatus). They probably inhabit faster running water and maintain continuously their position in the water with the help of their oral disc because of the complete state of the papillae that they have. This guild is characterized by the absence of a dorsal gap of papillae and a LTRF of 7(5–7)/3.
Habitat selection and ecology of strongly rheophilous Boophis tadpolesIn the tropical rainforest of Ranomafana National Park, strongly rheophilous Boophis tadpoles occur throughout the whole year (own unpublished data) with clearly higher abundances in the wet season. Whereas some species are relatively common (e.g., Boophis marojezensis and Boophis luciae), others are locally extremely rare (e.g., Boophis ankaratra, Boophis schuboeae). In this area, strongly rheophilous Boophis do neither include the most common tadpoles species nor is the group itself as common as other groups (
Within the streams, however, strongly rheophilous Boophis tadpoles are quite outstanding regarding their microhabitat choice compared to other abundant and well observed tadpole groups. This is especially true for two of the most common of these species, Boophis marojezensis [Ca51] and Boophis luciae, and less pronounced for Boophis andohahela, consistent with the more generalized oral disc structure of this latter species. Whereas we could not show true preferences for fast running sections, we could at least show that a considerable number of specimens are indeed using these faster parts of the streams. This clearly separates these tadpoles from other abundant groups (
Their large ventral oral disc allows attaching on substrate (Figure 29) such as rocks and gravel, and the presence of numerous short papillae presumably aids in forming a tight seal between the oral disc and the irregularities of substrate (
As already demonstrated by
(1) Boophis englaenderi [Ca23] lives syntopically with Boophis englaenderi, and these two forms show clear and constant differences genetically and in larval morphology, as described above, including characters of the oral disc, relative tail length, and coloration.
(2) In the Boophis mandraka group, Boophis sambirano [Ca49] tadpoles are very deviant and can easily be differentiated by coloration and the position of the eyes from the lineage Boophis sambirano [Ca50] occurring at a nearby locality in the same stream.
(3) Boophis marojezensis [Ca25] is very distinct by the presence of more or less rounded patches on the posterior half of the tail musculature which distinguishes it from the two syntopic forms, Boophis marojezensis and Boophis marojezensis [Ca26].
As a conclusion, this extraordinary and surprising diversity of Boophis marojezensis-like and Boophis sambirano-like candidate species especially in northern Madagascar probably indeed reflects a high number of yet undescribed species, and claims for a biogeographic and evolutionary explanation. It further confirms that stream-breeding frogs apparently show a higher geographical structuring of their diversity (e.g.,
Pictures showing tadpole capture sites inside a primary forest in Ranomafana National Park (A in Fompohonina river, B in Piste E 100 stream), and outside the forest (C in Anjingo river and D in Ankijagna Lagnana).
Photographs in life of strongly rheophilous Boophis tadpoles: A and B Underwater pictures of Boophis sambirano-like tadpoles (from Antevialambazaha - Tsaratanana Integral Reserve) C and D Oral disc of Boophis sambirano [Ca48] (ZCMV 13109 -ZSM 485/2010) and Boophis marojezensis [Ca52](ZCMV 13169-ZSM 542/2010) (from Ambinanitelo) fixing on the sides of an aquarium.
We are grateful to L. Raharivololoniaina, P. Bora, A.F. Ranjanaharisoa, T.J. Razafindrabe, J. Patton, C. Patton, E. Reeve, T. Rasolonjatovo-H, S. Ndriantsoa, A.S. Rasamison, A. Rakotoarison, F.M Ratsoavina, F. Glaw, and D.R. Vieites who assisted during fieldwork. We also acknowledge the help of the Madagascar Institut pour la Conservation des Ecosystems Tropicaux (MICET), of Valbio biological station, and of Madagascar National Parks for their invaluable help with logistics. This study was carried out in the framework of a cooperation accord between the Département de Biologie Animale of the University of Antananarivo, Madagascar and the Technische Universität Braunschweig. Financial support was granted by the Volkswagen Foundation to MV, FG and RDR, the Deutsche Forschungsgemeinschaft (grant VE247/2-1) to AS and JG, and by the Deutscher Akademischer Austauschdienst to RDR.
Explanation note: Appendix I contains Table 3–5.
Explanation note: Appendix II contains complete morphological description of the rheophilous Boophis tadpoles.