(C) 2011 Gil Wizen. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Species identification using the characteristics of developmental stages is challenging. However, for insect taxonomy the coloration of larval stages can be an informative feature. The use of live specimens is recommended for this because the color fades in preserved specimens. In this study we examine the possibility of using variation in coloration and color pattern of larvae in order to distinguish between twoground beetlesspecies Epomis dejeani (Dejean, 1831) and Epomis circumscriptus (Duftschmid, 1812). We present an atlas and describe the coloration and body size of the three larval stages of the above species based on live specimens. An identification key is given for the three larval instars of the two Epomis species.
The first instar larvae of the two Epomis species can be easily distinguished based on their color. From the second instar on, the variability in coloration and color patterns increases, creating an overlap in these attributes between larvae of the two species. Except for minor differences in color of the antennae and the base of the mandibles, larvae of the two species are indistinguishable at the second and third larval stages. To the best of our knowledge this is the first attempt to use variation in coloration and color pattern in live larvae in order to identify coleopterans. The color atlas of the larvae enables simple separation of the two Epomis species without requiring sophisticated magnifying devices, although it is less straightforward at the second and third larval stages.
We found similar body lengths between the two species for all developmental stages, except for third instar larvae prior to pupation. In the two species the difference in larval body length before pupation positively correlated with that of the adult beetles. More than 70% of the adults’ length can be explained by the length of the late third-instar larva; i.e. the large larvae develop into large adults. The larger specimens are the females.
Epomis larvae , Carabidae, color atlas, body size
Coloration can be an informative feature for insect taxonomy (
In a recent study of two Chlaeniini (Carabidae) species of Epomis, Epomis dejeani (Dejean, 1831) and Epomis circumscriptus (Duftschmid, 1812), in Israel, we noticed that the larvae display color variation. The genus Epomis
Bonelli, 1810 consists in ca. 20 species, mostly known from tropical
Africa and south and south eastern Asia. Five of the species are from
the Palaearctic region (Kryzhanovskij 1983). In mediterranean lands in
Europe, this genus is rare and considered endangered (
The taxonomic status of Epomis is under debate.
We describe the variation in coloration and color pattern and body size of the three larval stages of Epomis dejeani and Epomis circumscriptus.
MethodsThe three larval stages of Epomis are referred to as L1, L2 and L3. Larvae were obtained ex-ovo in the laboratory from a dozen females of each species collected in the wild. The two Epomis species do not coexist at the same sites. Adults of each species were collected in a different locality from the largest population known for each species. The larvae were reared in a room with constant temperature (25oC ± 1oC) and artificial light. They were kept in one liter plastic containers (10.5 cm high; 14.5 cm diameter) with moist peat as substrate and were fed with live amphibian metamorphs. Freshly hatched or molted larvae appear uniformly white; the final color appears after about two hours. For this reason we used larvae 10 hours after hatching or 10 hours post-molt for documenting color variability and patterns. We photographed them using a Canon EOS 50D camera with Canon MP-E 65 mm and EF-S 60 mm macro lenses and Canon MT-24EX flash. We also measured larval body length from the tip of the mandible to the end of the abdomen (mandible-abdomen length, MAL) using a caliper (±0.05 mm). L1 was measured after hatching and before molting into L2; L2 was measured after molting and before molting into L3; and L3 was measured after molting and before pupation. We measured the body length of the adults that emerged (from the tip of the mandible to the end of the elytra), and examined the correlation between their body length with that of the late L3.
The statistical analysis was performed using Statistica
ver. 8. Due to a small sample size and non-normal distribution of the
data we used non-parametric statistical tests (
We prepared a key to the larvae, incorporating morphological characters described by
L1, L2 and L3 differ in coloration. Moreover, we found variability in coloration within L2 and L3 stages (Fig. 2, 3 and 7). Median mandible-abdomen length (MAL) of L1 after hatching is 4.8 mm and before molting into L2 it is 8.3 mm (Table 1). The larva is mostly black-dark brown, with the last two or three segments of the abdomen being yellow. The tergite preceding the latter segments is medially yellow and its sides are black. The base of the mandible is pale brown or pale yellow. The retinaculum is reddish-brown and the apex is of similar but paler color. The remaining mouthparts, antennae, legs and urogomphi are pale yellow (Fig. 1). Median MAL of L2 after molting is 8.3 mm and before molting into L3 it is 13.2 mm (Table 1). The main body color of L2 ranges from pale brown with black spots to completely black. The legs and urogomphi are pale yellow and sometimes their base is black. The mandibles are pale brown or black, apex and retinaculum are reddish-brown. The base of the mandible is pale brown. The remaining mouthparts and antennae are pale brown or grey (Fig. 2 and 7). Median MAL of L3 after molting is 13.5 mm and before pupation it is 18.2 mm (Table 1). L3 resembles L2 in coloration, with the main body color ranging from pale brown with black spots to completely black. The legs are pale yellow. The urogomphi are mostly pale yellow but in dark colored larvae the base of the urogomphi is black. The mandibles are pale brown or black, apex and retinaculum are reddish-brown. The base of the mandible is pale brown. The remaining mouthparts and antennae are pale brown or grey (Fig. 3 and 7).
Morphs presenting color variability of L1 larvae of Epomis dejeani.Scale bar 2 mm.
Morphs presenting color variability of L2 larvae of Epomis dejeani. Scale bar 5 mm.
Morphs presenting color variability of L3 larvae of Epomis dejeani.Scale bar 10 mm.
Median body length (mm) of Epomis dejeani and Epomis circumscriptus larvae and inter-species MAL comparison (Mann-Whitney test) at different developmental stages. Number in parentheses indicates number of individuals.
| Taxa | L1 | L2 | L3 | |||
|---|---|---|---|---|---|---|
| After hatching | Prior to molting | After molting | Prior to molting | After molting | Prior to pupation | |
| E. dejeani | 4.8 (12) | 8.3 (12) | 8.3 (10) | 13.2 (10) | 13.5 (12) | 18.2 (11) |
| E. circumscriptus | 5 (11) | 8.5 (9) | 8.9 (10) | 13 (9) | 14 (19) | 20.2 (14) |
| p-value | 0.102 | 0.522 | 0.088 | 0.595 | 0.542 | 0.00084 |
L1, L2 and L3 differ in coloration. Here too we found variability in coloration within L2 and L3 stages (Fig. 5, 6 and 7). Median MAL of L1 after hatching is 5 mm and before molting into L2 it is 8.5 mm (Table 1). The larva L1 is mostly pale yellow or brownish and on rare occasions brown. In some larvae the sides of the body are grayish-black. The apex and retinaculum are reddish. The remaining mouthparts, antennae, legs and urogomphi are pale yellow. The dark eyes are prominent against the background of the brighter body (Fig. 4). Median MAL of L2 after molting is 8.9 mm and before molting into L3 it is 13 mm (Table 1). The main body color ranges from yellow-brown or white with black and orange spots to completely black with orange spots. The legs and urogomphi are yellow. The mandibles are black, apex and retinaculum are red. The remaining mouthparts are grey or black. The antennae are mostly black except for the two apical segments which are pale yellow (Fig. 5 and 7). Median MAL of L3 after molting is 14 mm and before pupation it is 20.2 mm (Table 1). The main body color ranges from dark brown or white with black and orange spots to completely black with orange spots. On rare occasions the orange spots are missing. The legs and urogomphi are yellow. The mandibles are black, apex and retinaculum are reddish-brown. The remaining mouthparts are grey or black. The antennae are mostly black except for the two last segments which are pale yellow (Fig. 6 and 7).
Morphs presenting color variability of L1 larvae of Epomis circumscriptus. Scale bar 2 mm.
Morphs presenting color variability of L2 larvae of Epomis circumscriptus.Scale bar 5 mm.
Morphs presenting color variability of L3 larvae of Epomis circumscriptus. Scale bar 10 mm.
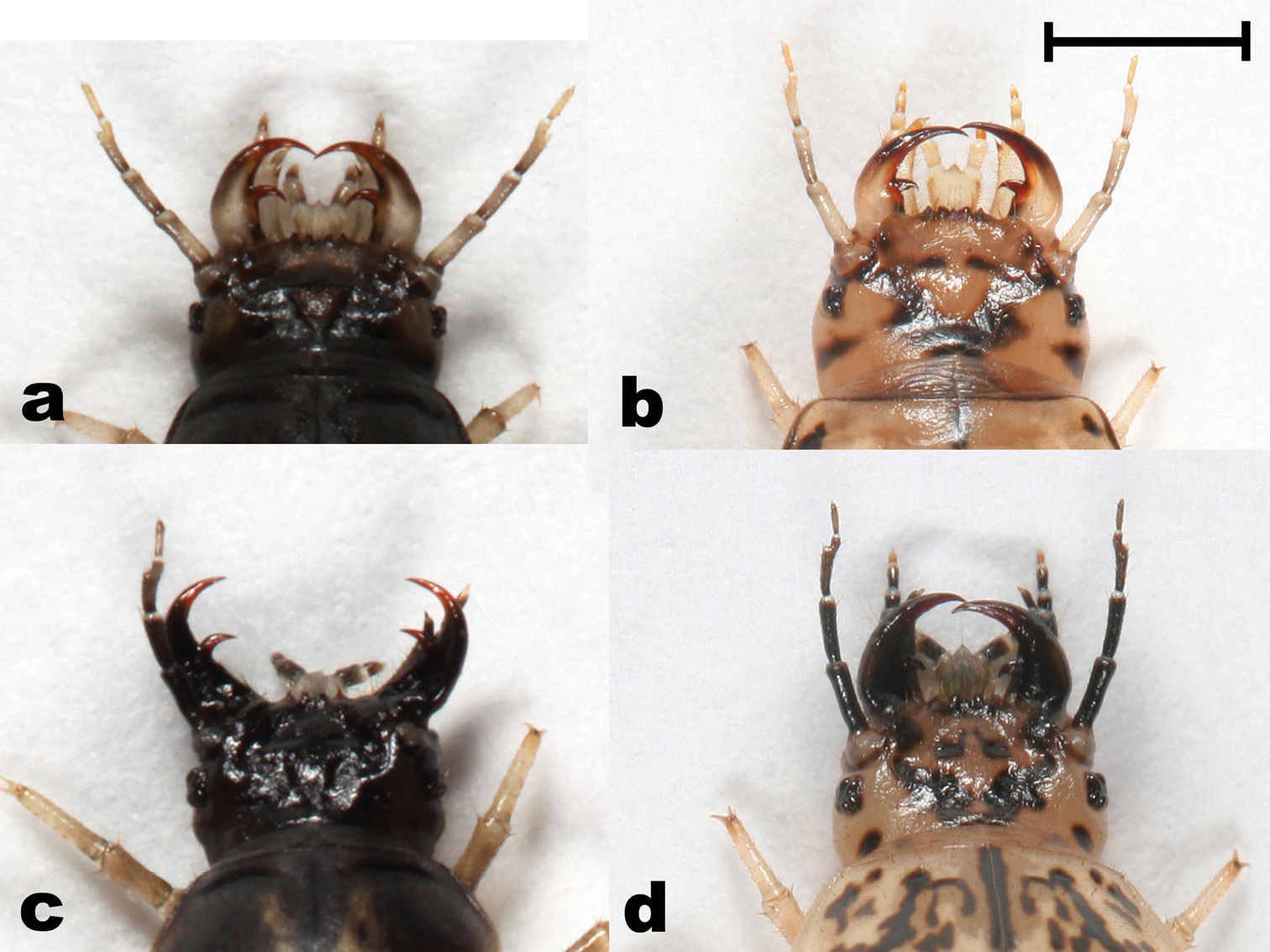
Head (dorsal view) of Epomis larvae showing differences in color of antennae and base of mandibles between the two species a Epomis dejeani, L2 dark morph b Epomis dejeani, L3 pale morph c Epomis circumscriptus, L2 dark morph d Epomis circumscriptus, L3 pale morph. Scale bar 2 mm.
Except for L3 before pupation, the body length of L1, L2 and L3 of the two Epomis species is similar (Mann-Whitney, p>0.09, Table 1). Prior to pupation the body length of Epomis circumscriptus L3 is larger than that of Epomis dejeani at the same larval stage (Mann-Whitney, p<0.001; Table 1).
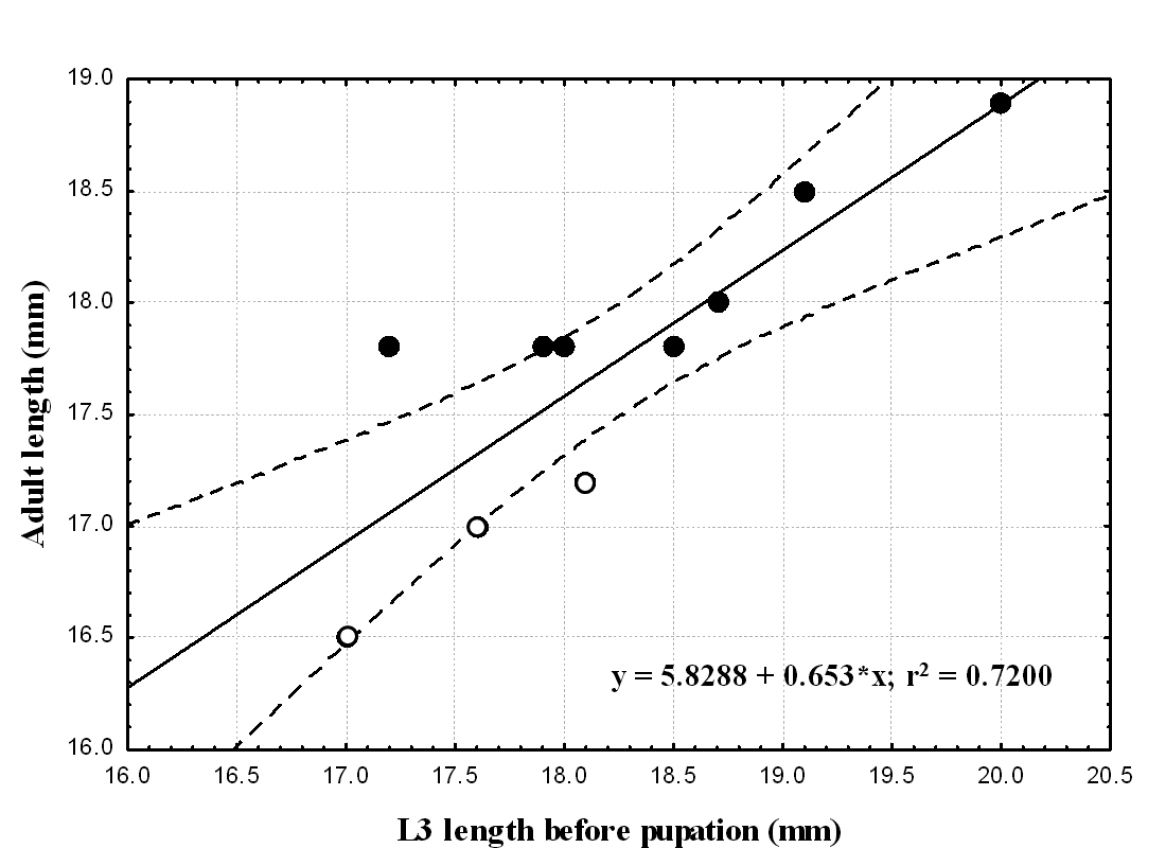
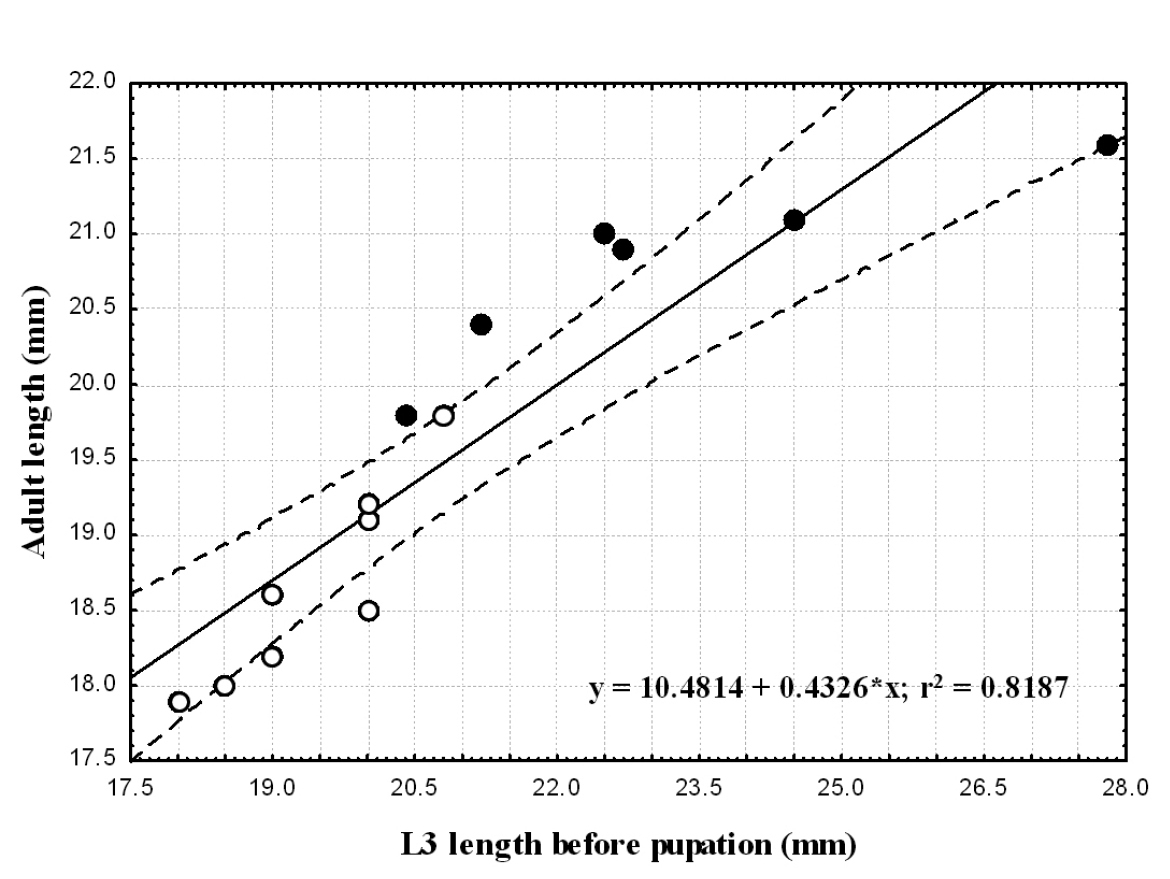
Relationship between pre-pupal instar and adult body lengthFor each species we compared body length of pre-pupal instar to that of the adult that emerged (Table 2). We found a positive significant correlation between larval and adult body length in each of the two species (Spearman correlation; p≤0.005). Larger larvae metamorphosed into larger adults. Larval size explains 72 and 82% of the variability in adult length in Epomis dejeani and Epomis circumscriptus, respectively (Table 2; Fig. 8 and 9). The largest larvae developed into females.
Correlation between adults’ body length (mm) and that of third-instar larvae (mm) of Epomis dejeani males (open circles) and females (black circles). Regression equation and coefficient of variation are shown.
Correlation between adults’ body length (mm) and that of third-instar larvae (mm) of Epomis circumscriptus males (open circles) and females (black circles). Regression equation and coefficient of variation are shown.
Range and median of body length (mm) of third instar larvae before pupation and of adults (male and female) of Epomis dejeani and Epomis circumscriptus.
| Taxa | Body length (mm) | Sex | Individual number | |||
|---|---|---|---|---|---|---|
| L3 before pupation | Adult | |||||
| range | median | range | median | |||
| Epomis dejeani | 17–18.1 | 17.6 | 16.5–17.2 | 17 | M | 3 |
| 17.2–20 | 18.5 | 17.8–18.9 | 17.8 | F | 8 | |
| Epomis circumscriptus | 18–20.8 | 19.5 | 17.9–19.8 | 18.6 | M | 8 |
| 20.4–27.8 | 22.6 | 19.8–21.6 | 21 | F | 6 | |
Key to the larvae
| 1 | Body length of the larva is up to 8.5 mm; egg bursters present on forehead, consisting of two rows of denticles; first instar | 2 |
| – | Body length of the larva is larger than 8.5 mm; no egg bursters present | 3 |
| 2 | Body length is 5 to 8.5 mm; color is mostly pale yellow or brownish, sometimes brown; body sides can sometimes be grayish-black; mandibles’ color is similar to body color, apex and retinaculum are darker and reddish; retinaculum on the same plane as the apical tooth and about the same length as the latter; both teeth strongly chitinized and curved, forming a strong double complanar hook; the remaining mouthparts, antennae, legs and urogomphi are pale yellow; stemmata are dark and clearly visible against the bright body color (Fig. 1) | Epomis circumscriptus |
| – | Body length is 4.8 to 8.3 mm; color is mostly black-dark brown, the last two or three segments of the abdomen are yellow, the tergite preceding these segments is medially yellow with black sides; mandible base is pale brown or pale yellow, retinaculum reddish-brown and apex of similar but paler color; retinaculum as robust as the apex, bent dorsally and backwards, not at the same plain as the apex, especially on the left side; both teeth strongly chitinized and curved, forming a strong double hook; the remaining mouthparts, antennae, legs and urogomphi are pale yellow; stemmata are poorly pigmented (Fig. 4) | Epomis dejeani |
| 3 | Body length of the larva is up to 13.5 mm; second instar | 4 |
| – | Body length of the larva is larger than 13.5 mm; third instar | 5 |
| 4 | Body length is 8.9 to 13.5 mm; color ranges from yellow-brown or white with black and orange spots to uniformly black with orange spots; the mandibles are black, apex and retinaculum are red; the remaining mouthparts are grey or black; the antennae are mostly black except for the two apical segments which are pale yellow; legs and urogomphi are yellow (Fig. 2 and 7) | Epomis circumscriptus |
| – | Body length is 8.3 to 13.2 mm; color ranges from pale brown with black spots to uniformly black; the mandibles are pale brown or black, mandible base is pale brown, apex and retinaculum are reddish-brown; the remaining mouthparts and antennae are pale brown or grey; legs and urogomphi are pale yellow, urogomphi sometimes black at base (Fig. 5 and 7) | Epomis dejeani |
| 5 | Body length is 14 to 20.2 mm; color ranges from dark brown or white with black and orange spots to uniformly black with or without orange spots; the mandibles are black, apex and retinaculum are reddish-brown; the remaining mouthparts are grey or black; the antennae are mostly black with the two last segments which are yellow; legs and urogomphi are yellow (Fig. 3 and 7) | Epomis circumscriptus |
| – | Body length is 13.5 to 18.2 mm; color ranges from pale brown with black spots to uniformly black; the mandibles are pale brown or black, mandible base is pale brown, apex and retinaculum are reddish-brown; the remaining mouthparts and antennae are pale brown or grey; legs pale yellow; urogomphi mostly pale yellow, and with a black base in uniformly black colored larvae (Fig. 6 and 7) | Epomis dejeani |
Epomis dejeani and Epomis circumscriptus appear in the key to European Carabidae (
We found variability in color patterns in all larval stages of the two Epomis species. A couple of hours after hatching the first instar larvae of the two species can be easily distinguished based on their color. From the second instar on, the variability in coloration and color patterns increases, creating an overlap in these characteristics between larvae of the two species. Except for minor differences in color of the antennae and the base of the mandibles (Fig. 7), larvae of the two species are indistinguishable at the second and third larval stages. We found no significant difference in body length of larvae of the two Epomis species, except for the third instar larvae prior to pupation. At this stage the median body length of Epomis circumscriptus larvae is 2 mm longer than that of Epomis dejeani at the same stage. In the two species the difference in larval body length before pupation positively correlated with that of the adult beetles.
Epomis adults are the largest among the Chlaeniini (
According to the Catalogue of Palaearctic Coleoptera (
It should be pointed out that Epomis is an endangered taxon in the European Mediterranean region. Epomis circumscriptus was classified as a critically endangered species in Italy (
In conclusion, the color atlas of Epomis
larvae that we provide present the color pattern of yet little known
larval stages of two ground beetle species, moreover it enables a
simple separation of the two species without requiring sophisticated
magnifying devices (for example ESM). The two species are easily
distinguishable at the first larval stage. While the separation is less
straightforward at the later stages, it is nonetheless possible by
focusing on the color of the antennae and base of the mandibles.
Accurate identification requires use of additional taxonomic tools. In
another study (
We thank Pietro Brandmayr and Tullia Zetto-Brandmayr (University of Calabria, Italy) for describing the larvae and for constructive remarks. We thank Thorsten Assman (Leuphana University, Germany) for providing information on past taxonomic status of Epomis. Alex Shlagman (Department of Zoology, Tel-Aviv University, Israel) provided essential information on larvae rearing. We are indebted to Naomi Paz (Tel-Aviv University) for editorial assistance. Permit for collecting and rearing the beetles was issued by the Nature and Parks Authority, Israel.