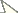ZooKeys 115: 27–38, doi: 10.3897/zookeys.115.1135
A new species of Buthus Leach, 1815 from Cyprus (Scorpiones, Buthidae)
Ersen Aydın Yağmur1,†, Halil Koç2,‡, Wilson R. Lourenço3,§
1 Ege University, Science Faculty, Biology Department, Zoology Section, İzmir, Turkey
2 Sinop University, Science and Art Faculty, Biology Department, Sinop, Turkey
3 Muséum national d’Histoire naturelle, Département Systématique et Evolution, UMR7205, CP 053, 57 rue Cuvier 75005 Paris, France
Abstract
During the last decade, several contributions to the genus Buthus Leach, 1815 (family Buthidae) and especially to the ‘Buthus occitanus’
species complex were proposed. These contributions led to the
definition of several species, previously considered only as subspecies
or varieties, and also to the description of new species. In the
present study, the questionable presence of the genus Buthus in the Cyprus is rediscussed and a new species Buthus kunti sp. n. is described.
KeywordsScorpion, Buthus, new species, Cyprus
Introduction
The genus Buthus was described by Leach, 1815 with the type species (by original designation), Scorpio occitanus
Amoreux, 1789. The type species was described from Sauvignargues in
the South of France. In his study about the scorpions of North Africa,
Vachon (see Vachon 1952), revised the composition of the genus Buthus and proposed a revised diagnosis, closer to the generic type Buthus occitanus. Consequently, quite many species placed in the genus Buthus
have been transferred to other genera. Some were already available as
subgenera, while others have been described by Vachon at this occasion.
Can be cited, Androctonus Ehrenberg, 1828, Buthacus Birula, 1908, Leiurus Ehrenberg, 1828, Compsobuthus Vachon, 1949 and Buthotus Vachon, 1949 (= Hottentotta Birula, 1908). (see Lourenço 2002, 2003 for details). However, the classification proposed by Vachon (1952) for the species of Buthus, and in particular for those belonging to the “Buthus occitanus” species complex, remained unsatisfactory. A more precise definition of the Buthus species has been attempted recently by (Lourenço (2002, 2003) which was followed by the elevation of several subspecies to species rank and the description of a new species.
Buthus occitanus (Amoreux, 1789) was first recorded from Cyprus by Kraepelin (1891). Levy and Amitai (1980) confirmed it to Cyprus, and also stated that this population was distinct from that of Buthus israelis (Shulov and Amitai, 1959), as follows: “Some specimens of Buthus from our region resemble specimens of the Moroccan Buthus occitanus mardochei”. - “On the other hand, specimens from Cyprus, Tunisia, Libya and Somalia are different”. Subsequently, the presence of a Buthus population in the island was again questioned (Gantenbein et al. 2000).
During this study, the third author (WRL) was able to
find one adult female previously studied by E. Simon by the end of the
19th century (Simon’s Collection N° 3228) in the collections of the
Muséum national d’Histoire naturelle, Paris. In his notes, Simon
indicates that the specimen was collected in Cyprus and represented a
new species, ‘Buthus orientalis’. This species name, however, was never published.
Here we confirm the presence of a Buthus population in Cyprus, and a new species belonging to the “Buthus occitanus” complex is described. This new Buthus population is certainly endemic to Cyprus.
Materials and methods
Illustrations and measurements were made with the aid of a
Wild M5 stereo-microscope with a drawing tube (camera lucida) and an
ocular micrometer. Measurements follow Stahnke (1970) and are given in mm. Trichobothrial notations follow Vachon (1974), and morphological terminology mostly follows Vachon (1952) and Hjelle (1990).
Specimens were photographed using a Nikon d100 (lens AF micro-NIKKOR 60
mm f/2.8D). Digital images were edited with the assistance of Photoshop
CS3 software.
Abbreviations
MNHN - Museum National d’Histoire Naturelle, Paris, France.
MTAS - Museum of Turkish Arachnology Society, Ankara, Turkey.
Results
Description of the new species
Type material:
Cyprus, 1 female holotype, Karpaz Region, Dipkarpaz Town (İskele), 2 km south east, 35°35'05"N, 34°25'23"E, leg. H. Koç (MTAS). Paratypes: 1 subadult male, Karpaz Region, Zafer headland, 2 km west, 35°41'29"N, 34°33'43"E,
leg. M. Z. Yıldız and B. Göçmen (MTAS). 1 subadult male, Güzelyurt
District (Morphou), about 5 km south east of Güzelyurt town, leg. H.
Koç (MNHN) (Fig. 13).
Note:
Although Simon’s female specimen may belong
to the new species, we decided not to include it among the type
material because (i) it is poorly preserved (ii) the precise collecting
site is unknown.
Derivatio nominis:
The species is dedicated to Kadir Boğaç Kunt who is the founder of the Turkish Arachnological Society.
Diagnosis:
Scorpion of medium to large size, reaching a
total length of 73 mm. General coloration yellow to pale yellow, with
brownish spots on the carinae of carapace; legs with diffused brownish
spots. Carinae moderately to strongly marked; granulations moderately to
weakly marked. Fixed and movable fingers with 12 rows of granules.
Pectines with 27 to 29 teeth in males, 24–25 in female.
Relationships:
Buthus kunti sp. n., belongs to the “Buthus occitanus” species complex. It can be distinguished from the other species of Buthus and in particular from Buthus israelis Shulov & Amitai, 1959, a species distributed in the nearby region of the Middle East, by the following characters: (i) Buthus israelis is smaller, measuring up to 62 mm in total length for females; (ii) according to Levy and Amitai (1980)
pectinal teeth 28–33 in males, 22–28 in females, the new species has a
slightly reduced number of pectinal teeth; (iii) metasomal segment II
is longer than wide in the female of the new species, whereas it is
wider than long in the female of Buthus israelis; (iv) pedipalp segments are oligotrichous (sense Vachon 1952) in the new species, whereas they are polytrichous in Buthus israelis.
Taxonomic note:
As already exposed in a recent paper (Lourenço et al. 2010), the Israeli and Sinai populations were originally described only as a variety: Buthus occitanus mardochei var. israelis Shulov & Amitai, 1959. Subsequently, this form was raised to subspecies level as Buthus occitanus israelis (Levy and Amitai 1980). This decision followed the previous taxonomic position adopted by Vachon (1952), who considered almost all Buthus populations from North Africa and Middle East as subspecies of Buthus occitanus. However, a revision of the genus Buthus (Lourenço 2003) revealed that the species Buthus occitanus is limited to France and Spain. Most of the populations of Buthus,
previously defined as subspecies and even varieties, were raised to
the species level, or described as new species. In the case of Buthus occitanus israelis, it seemed that this population could no longer be considered as a subspecies of Buthus occitanus, both for morphological and especially geographical reasons. Consequently, it was raised to species level, as Buthus israelis (Lourenço et al. 2010). Kovařík (2006) examined material from Egypt and Israel and synonimized Buthus occitanus mardochei var. israelis Shulov & Amitai, 1959 and Buthus occitanus israelis with Buthus intumescens. But Lourenço et al. (2010) didn’t follow this synonimization and accept Buthus occitanus israelis as valid and elevated to species range.
Description based on female holotype:
Measurements in Table 1. Coloration basically yellowish to pale yellow (Figs 1–3). Prosoma: carapace yellowish; carinae and eyes marked by dark pigment (Figs 1–2).
Table 1.
Morphometric values (in mm) of the female holotype of Buthus kunti sp. n.
| Total length |
73.3 |
|---|
| Carapace: |
| - length |
8.2 |
| - anterior width |
5.8 |
| - posterior width |
9.4 |
| Mesosoma length: |
21.4 |
| Metasomal segment I: |
| - length |
5.5 |
| - width |
5.7 |
| Metasomal segment II: |
| - length |
6.6 |
| - width |
5.7 |
| Metasomal segment V: |
| - length |
8.7 |
| - width |
4.8 |
| - depth |
3.7 |
| Telson: |
| - length |
8.0 |
| - width |
4.0 |
| - depth |
3.5 |
| Pedipalp: |
| - Femur length |
6.6 |
| - Femur width |
2.2 |
| - Patella length |
7.6 |
| - Patella width |
3.1 |
| - Chela length |
13.2 |
| - Chela width |
3.4 |
| - Chela depth |
3.6 |
| Movable finger: length |
8.9 |
Figures 1–3.
Buthus kunti sp. n. 1 Carapace of female holotype 2 female holotype from Karpaz 3 Ditto, ventral view.
Mesosoma yellowish with carinae also marked
by dark pigment, but less conspicuous than carapace. Metasomal segments
yellowish; vesicle yellowish; aculeus yellowish at its base and dark
reddish at its extremity. Venter yellowish; pectines pale yellow.
Chelicerae yellowish with vestigial variegated spots; fingers yellowish
with dark reddish to blackish teeth. Pedipalps yellowish; fingers with
dark oblique rows of denticles. Legs pale yellow with diffuse brownish
spots.
Morphology:
Carapace moderately to strongly granular;
anterior margin almost straight and without a median concavity. Carinae
strong; anterior median, central median and posterior median carinae
strongly granular, with ‘lyre’ configuration. All furrows moderate to
strong. Median ocular tubercle at the centre of carapace. Eyes separated
by almost three ocular diameters (one median eye absent on the
holotype). Three pairs of lateral eyes of moderate size (Fig. 1).
Sternum triangular, wider than long. Mesosoma: tergites moderately
granular. Three longitudinal carinae moderately crenulate in all
tergites; lateral carinae reduced in tergites I and II. Tergite VII
pentacarinate. Venter: genital operculum divided longitudinally, which
plate with a semi-triangular shape. Pectines: pectinal tooth count:
25–24 in female holotype (28–27, 29–29 in male paratypes); middle basal
lamella of the pectines not dilated. Sternites without granules,
smooth with elongated spiracles; four carinae on sternite VII; other
sternites acarinated and with two vestigial furrows. Metasomal segments I
to III with ten crenulated carinae, ventral strongly marked on II-III
with lobate granules; segment IV with eight carinae, crenulated; the
first four segments with a smooth dorsal depression; segment V with five
carinae; the latero-ventral carinae crenulate with 2–3 lobate denticles
posteriorly (Fig. 5);
ventral median carina not divided posteriorly; anal arc composed of 5–6
ventral teeth, and two lateral lobes. Intercarinal spaces weakly
granular. Telson almost smooth; aculeus curved and only slightly shorter
than the vesicle, without a subaculear tubercle (Fig. 5). Cheliceral dentition as defined by Vachon (1963) for the family Buthidae; external distal and internal distal teeth approximately the same length; basal teeth on movable finger small but not fused (Fig. 7);
ventral aspect of both fingers and manus covered with long dense setae.
Pedipalps: Femur pentacarinate; patella with eight carinae; all faces
weakly granular; chela smooth, without carinae. Fixed and movable
fingers with 12 oblique rows of granules. Internal and external
accessory granules present, strong; three accessory granules on the
distal end of the movable finger next to the terminal denticles (Fig. 6).
Legs: Tarsus with two longitudinal rows of thin and long setae
ventrally; tibial spur strong on legs III and IV; pedal spurs moderate
on legs I to IV. Trichobothriotaxy: trichobothrial pattern of Type A,
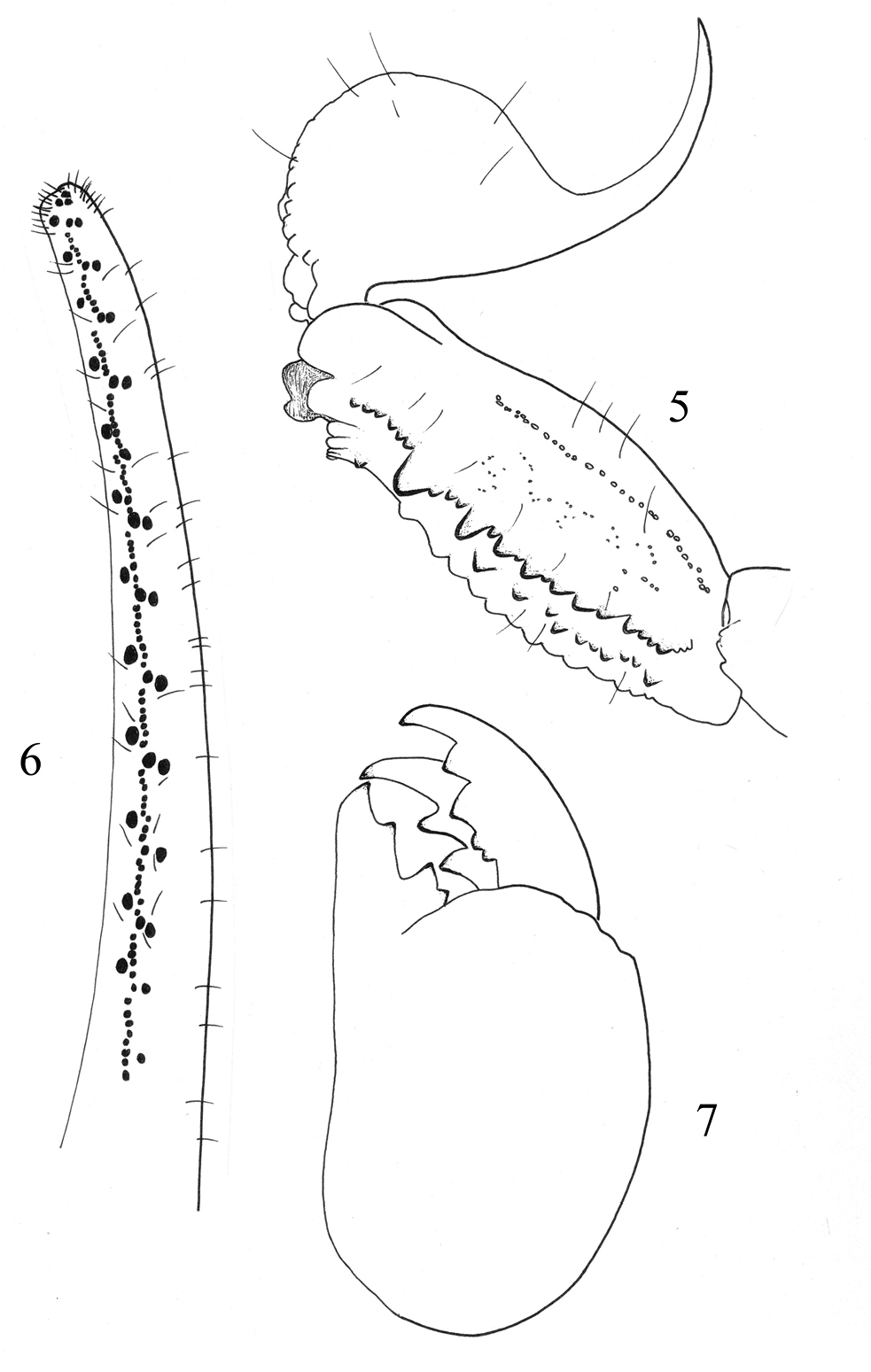
orthobothriotaxic as defined by Vachon (1974). Dorsal trichobothria of femur arranged in b-configuration (Vachon 1975) (Figs 8–12).
Figure 4.
Buthus kunti sp. n., subadult male paratype from Zafer headland.
Figures 5–7.
Buthus kunti sp. n. Female holotype 5 Metasomal segments V and telson, lateral aspect 6 Movable finger of pedipalp chela with rows of granules 7 Chelicera, dorsal aspect.
Figures 8–12.
Trichobothrial pattern of Buthus kunti sp. n., female holotype. 8–9 Chela, dorso-external and ventral aspects 10 Femur, dorsal aspect 11–12 Patella, dorsal and external aspects.
Ecological notes and biogeography:
Cyprus Island exhibits the Mediterranean
climate which is warm and rainy in winter and hot and dry in summer.
Rainy season is rare and only occurs in winter in plain areas (İlseven et al. 2006). Sandy soil exists at Zafer headland locality, where the vegetation is composed of Pancratium maritimum, Cakile maritima, Limonium albidum and Pistacia lentiscus (Fig. 14). Redzina soil is present at Güzelyurt, where the habitat was steppe vegetation with small bushes. Buthus kunti sp. n. has allopatric distribution with another species endemic to Cyprus, Mesobuthus cyprius Gantenbein & Kropf, 2000. Interestingly, Cyprus Island is the only territory where representatives of Buthus and Mesobuthus genera have been found together.
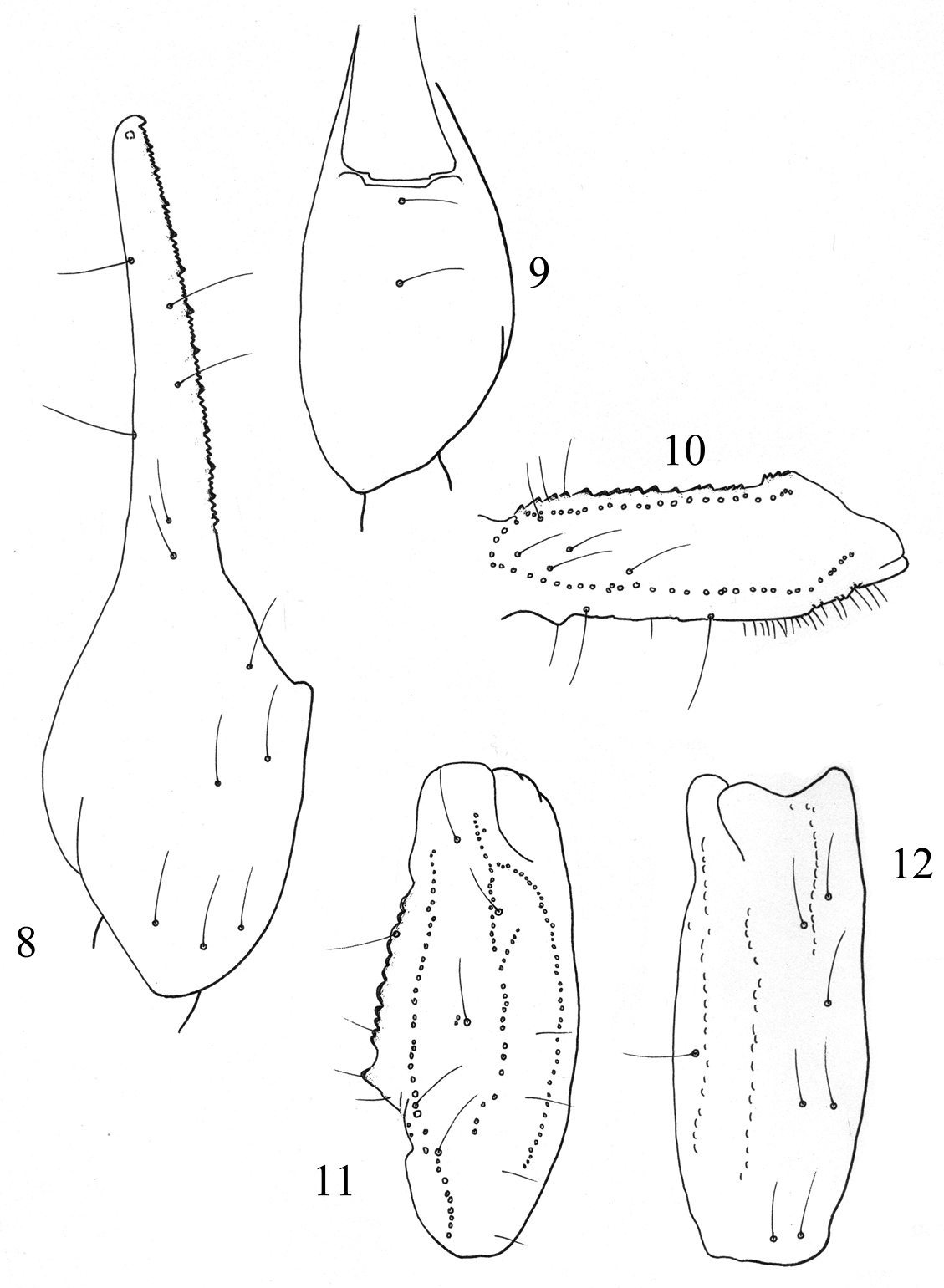
Figure 13.
Map of Cyprus, showing the site where the new species was collected. 1 Collecting locality of holotype, Karpaz Region, Dipkarpaz Town (İskele) 2 Collecting locality of paratype, Karpaz Region, Zafer headland 3 Ditto, Güzelyurt District (Morphou).
Figure 14.
Buthus kunti sp. n. Habitat from Zafer headland (Sandy soil habitat).
The geological evolution of the eastern
Mediterranean region, has run a series of prominent geological
movements, together with the world wide sea levels rising and falling
accompanying the continental glaciations leading to join and split of
Cyprus and Anatolia (Robertson 1998). It is thus clear that no consensus yet as to the geological history of Cyprus; Schmidt (1960)
express Cyprus was part of a united landmass of the mainland and then
was broken piece of the mainland, but according to the modern
geological history of the eastern Mediterranean region, Cyprus became
due to tectonic movements occurring in the area, Gass (1987)
supports during Mesozoic time Mt. Troodos is originated a submarine
volcano that arise an oceanic island which occured at
Cretaceous-Palaeocene. Whereas Kyrenia Mts (which include Pentadactylos
Mt.) maybe as a second island or as a part of the southern Taurus Mts
range originated in Eocene then later separated from each other to the
south (Cavazza and Wezel 2003).
According to widely accepted theory is Mediterranean salinity crisis
that the Mediterranean sea dried out and these two island or the Trodos
island and southern Tauruian-Kyrenian peninsula connected via
landbridges about 5.6 Myrs (Hsü et al. 1977; Cavazza and Wezel 2003). When the refilling of the Mediterranean basin, Cyprus terrestrial animals was isolated for around 5.2 – 5.3 Myrs (Robertson 1998; Gantenbein and Keightley 2004
). This isolation played a major role in forming actual scorpion fauna
of Cyprus and molecular and morphological phylogenetic analysis has
revealed that populations of the island of Cyprus represent a divergent
lineage; so these have been assigned to the species rank (i.e., Mesobuthus cyprius
Gantenbein and Kropf, 2000). On the other hand, the other discussions
about endemism of some snake species occurring in the two island origin
of Cyprus (Troodos and Kyrenia island); Hierophis cypriensis, in only southern Cyprus (i.e., Throodos island) while Platyceps najadum (non-endemic)and Natrix tessellata (non-endemic) is distributed only in northern Cyprus (i.e., Kyrenia island) and also on the mainland (Göçmen et al. 2009). Gantenbein and Keightley (2004) stated his analyses shows that Mesobuthus cyprius occurring in Cyprus is autochthonous. Mesobuthus cyprius recorded in both southern and northern Cyprus. While Mesobuthus cyprius recorded at high elevation in Cyprus, Buthus kunti
sp. n. collected at low altitude in dry condition. It is not yet clear
if the distribution of new species restricted to Kyrenia island
(northern Cyprus). However, Mt. Troodos run vertically and Kyrenia Mts.
lay horizontally with less high in Cyprus, are not usually a
zoogeographic barrier there. When we take in consideration for this
situation we expect the distribution of new species is all over Cyprus.
Another point of view explains that as a result of the geological
process, it is a localized endemic species in Kyrenia island
(Pentadactylos Mt.).
Since the second record of scorpion species,
a museum material, Simon’s material the precise collecting site is
unknown and poorly preserved, no other species have been seen in
several recent field works, so the species might be very rare on the
island, and should be investigated again for male specimens under
suitable seasonal conditions.
Unplanned urban settlement destroys the
habitats of these endemic species. Government agencies are required to
take precautions to not destroy habitats.
Acknowledgements
We are most grateful to Dr. Victor Fet, Marshal
University, Huntington, West Virginia, USA for useful comments to the
manuscript. The first two authors wish to thank Dr. Bayram Göçmen and
Mehmet Zülfü Yıldız (Ege University, Turkey) for providing specimens,
photographs and literature.
ReferencesCavazza W, Wezel FC (2003) The Mediterranean region – a geological primer. Episodes 26:160-168.
Gantenbein B, Kropf C, Largiadèr CR, Scholl A
(2000) Molecular and morphological evidence for the presence of a new
buthid taxon (Scorpiones: Buthidae) on the Island of Cyprus. Rev. Suisse
Zool. 107: 213–232.
Gantenbein B, Keightley PD (2004) Rates of
molecular evolution in nuclear genes of east mediterranean scorpions.
Evolution 58:2486-2497.
Gass IG (1987) Ophiolite: Ozeankruste an Land.
In: Giesse PP (Ed) Ozeane und Kontinente, Spektrum der Wissenschaft,
Heidelberg, Germany, 172–181.
Göçmen B, Kasot N, Yıldız MZ, Sas I, Akman B,
Yalçınkaya D, Gücel S (2008) Results of the herpetological trips to
Northern Cyprus. North-West J. Zool. 4: 139–149.
Göçmen B, Atatür MK, Budak A, Bahar H, Yıldız
MZ, Alpagut-Keskin N (2009) Taxonomic notes on the snakes of Northern
Cyprus, with observations on their morphologies and ecologies. Animal
Biology 59:1-30.
doi: 10.1163/157075609X417062
Hjelle JT (1990) Anatomy and morphology. In:
Polis GA (Ed) The Biology of Scorpions, Stanford University Press,
Stanford, 9–63.
Hsü KJ, Montadert L, Bernoulli D, Cita MB,
Erickson A, Garrison RE, Kidd RB, Mèlierés F, Müller C, Wright R (1977)
History of the Mediterranean salinity crisis. Nature 267:399-403.
doi: doi:10.1038/267399a0
İlseven S, Hıdırer G, Tümer A (2006) Kıbrıs Coğrafyası (Geography of Cyprus), K.T. Eğitim Vakfı, Lefkoşa.
Kovařík F (2006) Review of Tunisian species of
the genus Buthus with descriptions of two new species and a discussion
of Ehrenberg’s types (Scorpiones: Buthidae). Euscorpius 34:1-16.
Kraepelin K (1891) Revision der Skorpione. I.
Die Familie der Androctonidae. Jahrbuch der Hamburgischen
wissenschaftlichen Anstalten 8:1-144.
Levy G, Amitai P (1980) Fauna Palaestina,
Arachnida I: Scorpiones, Israel Academy of Sciences and Humanities,
Jerusalem, 130pp.
Lourenço WR (2002) Considérations sur les modèles de distribution et différentiation du genre
Buthus
Leach, 1815, avec la description d’une nouvelle espèce des montagnes
du Tassili des Ajjer, Algérie (Scorpiones, Buthidae). Biogeographica
78:109-127.
Lourenço WR (2003) Compléments à la faune de scorpions (Arachnida) de l’Afrique du Nord, avec des considérations sur le genre
Buthus Leach, 1815. Rev. Suisse Zool. 110: 875–912.
Lourenço WR, Yağmur EA, Duhem B (2010) A new species of
Buthus Leach, 1815 from Jordan (Scorpiones, Buthidae). Zoology in the Middle East 49:95-99.
Robertson AHF (1998) Mesozoic–Tertiary
tectonic evolution of the easternmost Mediterranean area: integration of
marine and land evidence. In: Robertson AHF, Emeis KC, Richter C,
Camerlenghi A (Eds) Proceedings of the Ocean Drilling Program,
Scientific Results, Vol. 160. Collage Station, TX (Ocean Drilling
Program), 723–782.
Stahnke HL (1970) Scorpion nomenclature and mensuration. Entomol News 81:297-316.
Vachon M (1952) Etudes sur les scorpions. Publications de l’Institut Pasteur d’Algérie, Algeria, 782pp.
Vachon M (1963) De l’utilité, en
systématique, d’une nomenclature des dents des chélicères chez les
Scorpions. Bulletin du Muséum national d’Histoire naturelle 35:161-166.
Vachon M (1974) Etude des caractères utilisés
pour classer les familles et les genres de Scorpions (Arachnides). 1. La
trichobothriotaxie en arachnologie. Sigles trichobothriaux et types de
trichobothriotaxie chez les Scorpions. Bulletin du Muséum national
d’Histoire naturelle 104:857-958.
Vachon M (1975) Sur l’utilisation de la
trichobothriotaxie du bras des pédipalpes des Scorpions (Arachnides)
dans le classement des genres de la famille des Buthidae Simon. Comptes
Rendus des Séances de l’Académie de Sciences 281:1597-1599.