(C) 2011 Alan Cassidy. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The taxonomy and distribution of the five species of Hypolycaena in Maluku are discussed and new locality records given. Corrections are made to the published taxonomy and distribution of Hypolycaena phorbas (Fabricius, 1793). This clarification enables a better understanding of the biogeography of the genus. Hypolycaena asahi Okubo, 2007, was originally described from a single female from Ambon and is here recorded from Seram. The male is described for the first time.
Hypolycaena, asahi, danis, dictaea, erylus, phorbas, pigres, silo, sipylus, Indonesia, Maluku, Lepidoptera, Lycaenidae
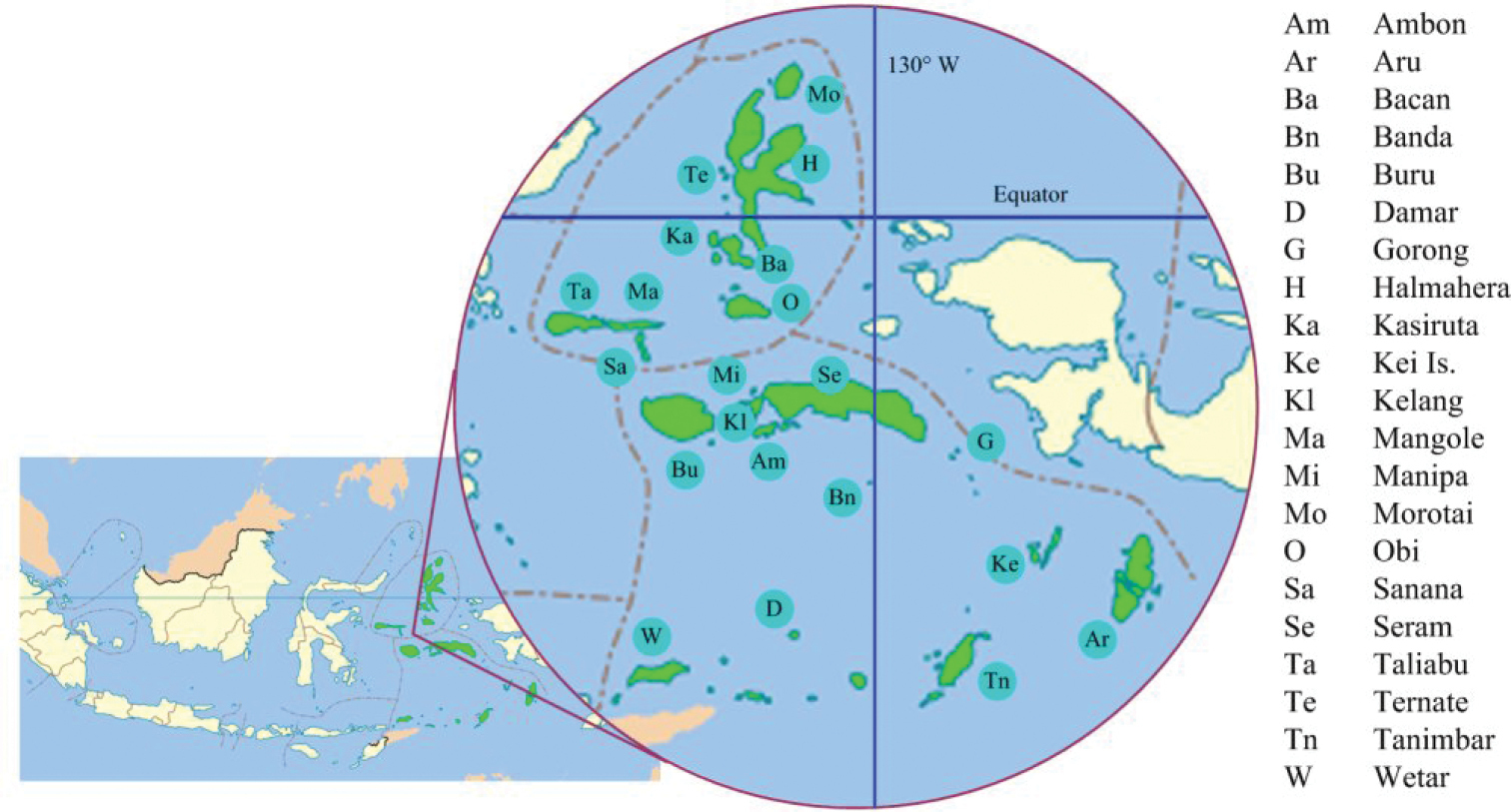
The Indonesian provinces of North Maluku and Maluku
consist of numerous islands, yet their butterfly fauna remains less
well described than those of the principal surrounding areas of the
Philippines, Sulawesi and New Guinea.
Thus Maluku sensu lato remains an area of immense biogeographical interest, with the largest of its islands forming the northeasterly part of Vane-Wright’s “Wallacea”: the land between the Sunda and Sahul shelves. To facilitate testing of biogeographical hypotheses, it is important that the taxonomy and distributional data of all butterfly families represented in Maluku is accurate and as comprehensive as possible. The extensive lycaenid fauna is perhaps the least understood.
The genus Hypolycaena C. & R. Felder, 1862, (Lycaenidae, Theclinae, Hypolycaenini) consists of about 25 species in the Indo-Australian region as well as about 20 species in Africa.
This paper is primarily concerned with Hypolycaena species in Maluku. However, it is necessary to discuss in some detail the taxonomy and wider distribution of Hypolycaena phorbas (Fabricius, 1793) and its allies. These taxa form a species group in which the males exhibit a large circular dark brand of apparently normal scales on the upperside of the forewing and in which the early stages are polyphagous and strongly myrmecophilic (Fiedler, 1992). This study will confirm the identity of the taxon found on Aru Islands and also clarify D’Abrera’s record of Hypolycaena erasmus Grose-Smith, 1900, in Halmahera. A more accurate understanding of the taxonomy and distribution of the phorbas species group will in turn lead to a better understanding of the biogeography of the Papua mainland and the islands to the East and West of it.
Note that the frequent references to Parsons and D’Abrera refer to
The Indonesian western half of the Island of New Guinea and its associated offshore islands, which has previously been known as Irian Jaya, now consists of two provinces: Papua and West Papua. However “Papua” has also been used to denote this whole area. For simplicity we will use the term “Papua mainland” to describe the whole area excluding offshore islands.
Equipment and methodsThe preserved material forming the basis of this study is primarily that of the collections of the Natural History Museum London (BMNH) and of the second author. Where their reliability is assured, other distributional data have been accepted in correspondence from curators of other private collections.
Male genitalia were prepared by soaking in 0.1N potassium hydroxide solution for 24 hours at room temperature prior to dissection. Micro-photography of the genitalia, while suspended in 80% Iso-Propanol, was with an AIGO GE-5 digital microscope and the images were subsequently processed using Helicon-Focus 5.0 software (Helicon Soft Ltd. 2010) to enhance depth of field.
All photographs of preserved adult specimens, except those kindly provided by Mr. Yusuke Takanami, were taken using a Nikon D80 digital SLR camera fitted with a Micro-Nikkor 60mm macro lens. The photographic images presented were post-processed for exposure compensation, cropping, resizing and sharpening using Adobe Photoshop Elements 6.0. The scale on photographs represents multiples of 5mm.
Hypolycaena asahi Okubo, 2007
♂Figs 1, 2, 10, Fig. 9 genitalia; ♀ Figs 3–8, 10.
The holotype female was captured in March 2000. The type location was given by Okubo as “Mt. Tuna, ca 900m, Ambon Island, North Moluccas [sic], Indonesia”. Four further specimens of Hypolycaena were captured in Central Maluku in 2002 and 2004, comprising three females and one male. These four specimens are illustrated in Figs 1–8. Two of the three female specimens are from the Hypolycaena asahi type location in Ambon, while a male and female are recorded for the first time from Salemon in Seram.
Hypolycaena asahi, top row recto, bottom row verso. 1, 2 male, Seram 3, 4 female, Seram 5–8 females, Ambon.
The external morphology of all of these new females is inseparable from that of the Hypolycaena asahi holotype, and their identification as examples of Hypolycaena asahi is assured. We propose the hypothesis that the male specimen is also Hypolycaena asahi because of its underside markings and its sympatry with the aforementioned female from Seram.
♂ Upperside. Forewing length 13mm. Both fore and hindwings metallic blue with dark borders. The forewing black border about 1mm wide at the tornus but rapidly widening along the termen to meet the costa at its mid-point, then running down to the base above vein 12, but not quite entering the cell. The forewing also with basal swelling of veins 2, 3 and 4 with a faint brand of seemingly normal (not androconial) scales surrounding these swollen veins. The hindwing black between veins 7 and 8, and with a dark grey dorsal border in spaces 1 and 1a. In space 1a a small black tornal lobe with a white marginal streak. Filamentous white-tipped black tails at veins 1b and 2, 2mm and 3mm long respectively.
Underside. No significant differences exist between the undersides of the females from Ambon and those of both sexes from Seram.
♂ Genitalia. Saccus short, bluntly pointed. Brachia long and tapering to a fine point, with a broad elbow and a pronounced lobe at the proximal junction with the tegumen. Valvae short, broad and conjoined basally, tapering distally with the apex rounded and the inner margins finely serrate. Aedeagus medium length, the sub-zonal portion shorter that the supra-zonal portion.
Male genitalia of Hypolycaena asahi Okubo, 2007, from Seram, showing ventral view of armature and lateral view of aedeagus.
Hypolycaena asahi Okubo, compared with Hypolycaena shirozui Hayashi and Hypolycaena toshikoae Hayashi from the Philippines. Philippine photos courtesy of Mr. Yusuke Takanami.
Remarks. The early stages are unknown. The females of Hypolycaena asahi (Figs 3–8) from both locations show varying amounts of basal blue scaling not evident in the holotype. Otherwise, they conform closely to Okubo’s description.
Okubo notes the similarity between this species and two allied species from the Philippines: Hypolycaena shirozui (Hayashi, 1981) and Hypolycaena toshikoae Hayashi, 1984 (Fig. 10). In all three species the underside hindwing tornal orange area extends into space 3 and the sub-marginal black spot in space 3 is much larger and darker than in space 4. On the underside in both sexes, asahi shows a much more marked dislocation of the post-discal band than in shirozui, while this dislocation is absent in toshikoae. On the male upperside, the apical black border in asahi is much broader than in either of the other two species, most notably in spaces 2, 3 and 4. On the female upperside, neither Hypolycaena shirozui nor Hypolycaena toshikoae exhibits the white forewing discal patch of Hypolycaena asahi. Both Philippine species have orange inwardly surrounding the hindwing sub-marginal lunules, which is absent in Hypolycaena asahi from both Seram and Ambon.
In the male genitalia, Hypolycaena asahi is distinguished from these other species by the more elongate valvae, which have more rounded apices, and by the shorter suprazonal portion of the aedeagus.
Taxonomy and distribution of other Hypolycaena species in MalukuIn addition to Hypolycaena asahi there are four other Hypolycaena species in Maluku. Fiedler, 1992, pointed out that two of these, Hypolycaena phorbas and Hypolycaena erylus (Godart, [1824]) shared characteristics of larval polyphagy and strong mymecophily and referred to them as the phorbas species group. Adult males in this group also have a large circular black brand on the upperside of the forewing. It group includes the taxa erylus, phorbas, dictaea C. & R. Felder, 1865, (TL: Waigeo) and periphorbas Butler, 1882 (TL: New Britain), all of which Parsons treated as separate species.
D’Abrera treated Hypolycaena erasmus (TL: New Ireland) as a valid species name and moutoni Ribbe, 1899 (TL: Duke of York Island) as a subspecies of Hypolycaena erylus. Parsons synonymised erasmus and moutoni with periphorbas, giving periphorbas species status. D’Abrera had listed periphorbas as a subspecies of phorbas. Parsons noted that Hypolycaena periphorbas is restricted to the Bismarck Archipelago. We accept Parsons’ synonymies.
D’Abrera illustrated the upperside of a female Hypolycaena specimen from Halmahera which he labelled “Hypolycaena erasmus subsp.?” We examined this specimen in BMNH which carries a label stating: “Specimen photographed by B. D’Abrera, 1970”. The specimen is clearly Hypolycaena erylus thyrius and matches other thyrius specimens in the same column. This specimen is shown here in Figs 29 and 30 which should be compared with Figs 17 and 18 from Bacan.
Therefore we conclude there is no record of Hypolycaena periphorbas (= Hypolycaena erasmus) occurring in Maluku. In the Maluku fauna, Hypolycaena phorbas can be readily distinguished from Hypolycaena erylus by its broader dark forewing margin in the male (Fig. 31) and its white forewing patch in the female (Figs 33, 35).
Hypolycaena erylus (Godart, [1824]) (Type Locality (TL): “De Java”)
This species ranges from India to Indonesia, the Philippines and New Guinea. Within Maluku Hypolycaena erylus is known from N. Maluku, the Sula Islands and there are a few specimens in BMNH (The Natural History Museum in London) from S.E. Maluku (see below).
Hypolycaena erylus gamatius Fruhstorfer, [1912] (TL: Toli-Toli, N. Sulawesi). Figs 11–14.
Hypolycaena erylus gamatius Fruhstorfer has been recorded from Mangole (Vane-Wright & de Jong, 2003) in the Sula Islands.
Specimens received by the second author from Taliabu (Jorjoga - 1♂, 1♀, ii/2001, 1♂, 1♀ x/2001, 1♂ v/2002, 1♂ iii/2004) represent a new island record. We also add the islands of Muna (1♂, 1♀ iii/2008) and Timpaus (5♂♂, 7♀♀ vii/2006) as new locality records, although not in Maluku Province.
Hypolycaena erylus thyrius Fruhstorfer, [1912] (TL: Halmahera). Figs 15–18.
= Hypolycaena erylus pigres Fruhstorfer, [1912] syn. nov. (TL: Obi). Figs 19–22.
= Hypolycaena erasmus ssp;
Fruhstorfer described Hypolycaena erylus thyrius from “Halmaheira, Batjan” but listed only a single female type. This taxon is known from Halmahera, Ternate and Bacan. To this we add Morotai (Daeo – 2♂♂ 3/vi/1992, 1♂ ii/1998, 1♂ iii/1998, 2♀♀ iv/2004, 1♀ 25/viii/1995, 1♀ 2/xi/1995), Buho-Buho (1♂ 8/xii/90) and Kasiruta (1♂ iv/2003).
In the same paper Fruhstorfer described Hypolycaena erylus pigres from Obi, based on a series of eight males. Having examined the pigres and thyrius holotypes, as well as a long series of Obi and Halmahera specimens, we can not see any clear differences between the two taxa and therefore consider pigres to be a synonym of thyrius, which appears earlier in Fruhstorfer’s work.
Hypolycaena erylus incertae sedis. Figs 23–28.
Although Hypolycaena erylus is widespread in the South-East Asian islands and into New Guinea, material from South and South-East Maluku is scarce. We have seen a single male from Banda and BMNH has three males of Hypolycaena erylus from Tanimbar (20 miles north of Saumlaki, Yamdena - 1917–1918, Frost). These are all difficult to assign to a particular named subspecies (the males of the different subspecies tend to be fairly similar whilst the females vary more). See Figs 23–26.
In addition BMNH holds one female from Manawoka Island (label reads: Manovolka. 13.xi.(18)99. H. Kühn) in the Gorong Islands, which is unlike any other subspecies, having extensive pale areas on the upperside - especially the forewing - and may represent a new subspecies. See Figs 27, 28.
We await further confirmatory material before naming any further subspecies based on these few specimens.
Hypolycaena phorbas (Fabricius, 1793) (TL: “Ins. Papuanae”). Figs 31–42.
Parsons reviewed Hypolycaena phorbas from Papua New Guinea stating that two subspecies occur there: silo Frustorfer, [1912] and infumata Fruhstorfer, 1910. He synonymised latostrigatus van Eecke, 1915, pseudophorbas Fruhstorfer, [1914], phorbanta Rothschild, 1916 and walteri Fruhstorfer, [1916a] with silo. He gave the range of phorbas as Waigeo, Biak, Roon, mainland New Guinea, various outlying islands and Australia.
D’Abrera and Seitz, 1926, considered Felder’s taxon dictaea to be a subspecies of phorbas found on Waigeo only, whereas Parsons “provisionally treated” dictaea as a separate species and stated the range to include Aru, Waigeo, mainland New Guinea and its varying outlying islands as far south east as Australia. He went on to specify a number of island localities.
Therefore according to Parsons, two “species”, Hypolycaena phorbas and Hypolycaena dictaea, occur on Waigeo as well as mainland New Guinea. However, we consider that phorbas and dictaea are conspecific and that only one subspecies, Hypolycaena phorbas silo, occurs in political Maluku, on Aru, its type locality and on mainland Papua. As Aru is separated from mainland New Guinea only by shallow water, and may well have been directly connected at the surface during the last glaciation, it can be regarded biogeographically as part of Papua.
Hypolycaena phorbas dictaea C. & R. Felder, 1865 (TL: Waigeo) Figs 37, 38, 41, 42.
Parsons did not locate the holotype female of dictaea although it is deposited in BMNH. We have examined this specimen along with a series of female specimens in BMNH from Waigeo and it is clear that they all have undersides that are significantly paler in ground colour and weaker in the post discal striae than the underside of the holotype female of silo (also at BMNH).
We have also examined females, whose undersides are dark and therefore match that of the silo holotype, from Papua mainland, Aru, Roon, Biak and Yapen in BMNH and the collection of the 2nd author.
Additionally, we have studied a series of males from Waigeo. Unlike the females, the Waigeo males’ undersides vary, ranging from the paleness of the holotype female dictaea to the much darker underside of the holotype female of silo.
Hypolycaena phorbas silo Fruhstorfer, [1912] (TL: “Neu-Guinea Fr. Wilh. Hafen.”) Figs 31–36, 39, 40.
Within Maluku this subspecies is only found on Aru Islands. We have examined five males and five females from Aru. All display the typical silo phenotype with the exception of one female from Wokam (Figs 35, 36), which has a slightly lighter underside than the other four.
We present new records of this subspecies from Wokam Island in the Aru group, (1♂ xi/2004, 1♂ xii/2005, 1♀ x/2006).
Other phorbas material examined, from outside MalukuUndersides of series of males from Batanta, Papua mainland, Aru, Roon, Biak and Yapen are all of the darker form matching the holotype female silo underside. The three males and three females from Misool in BMNH all have the paler undersides matching the dictaea type specimen. There is one male from Salawati in BMNH whose underside is of this form.
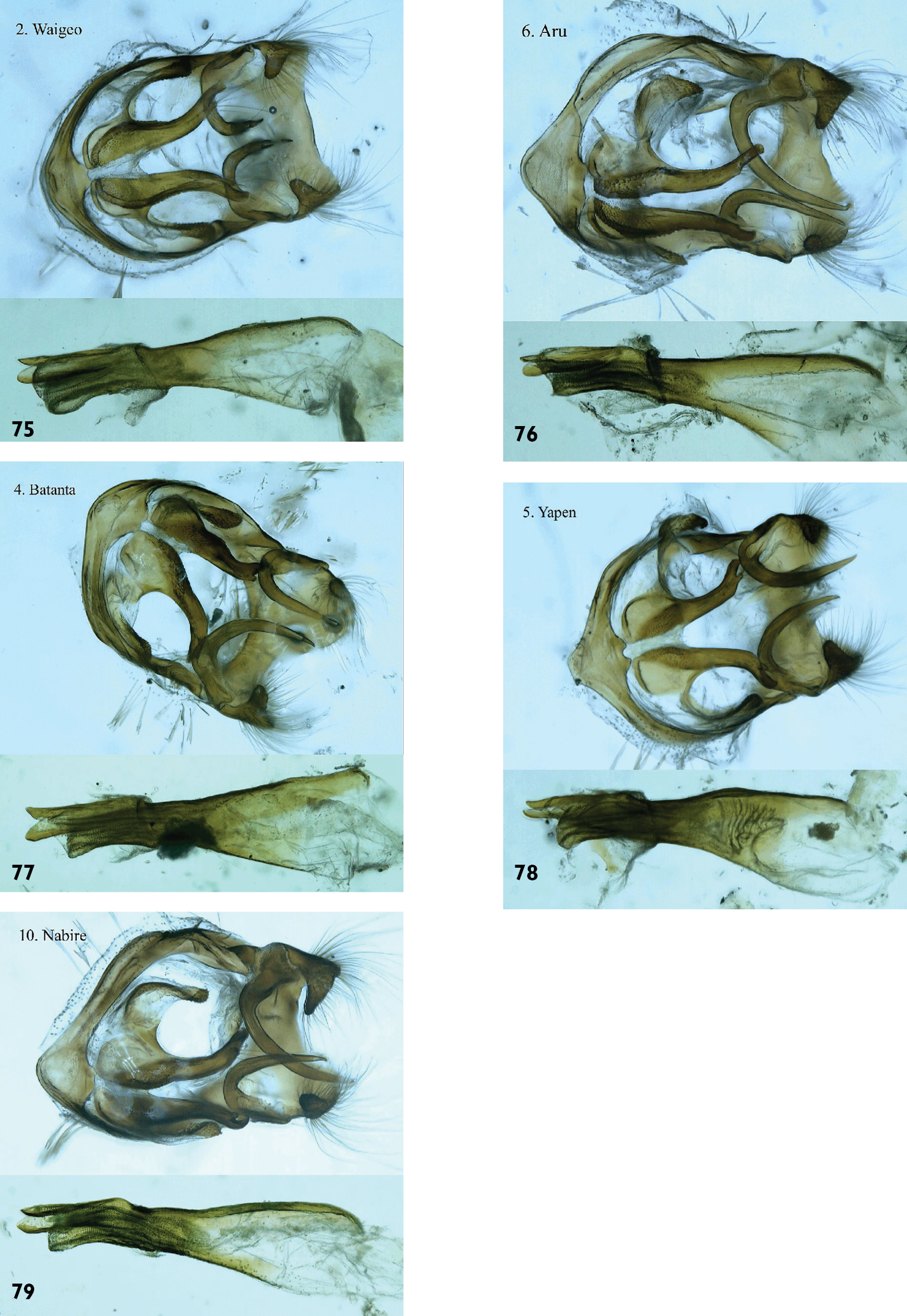
We have also examined male genitalia from specimens from Waigeo (Fig. 75), Aru (Fig. 76), Batanta (Fig. 77), Yapen (Fig. 78) and Papua mainland (Fig. 79). We can find no consistent differences between them. We therefore conclude that these should all be considered conspecific.
We therefore consider that Hypolycaena dictaea is not a separate species but is a subspecies (or possibly just a form) of Hypolycaena phorbas occurring on the islands of Waigeo, Misool and possibly Salawati, which all lie to the west of mainland New Guinea. We consider that the taxon present in Papua mainland, Aru, Roon, Biak and Yapen is Hypolycaena phorbas silo.
Batanta is a new distribution record for Hypolycaena phorbas. We have examined four males collected in October 2009 on the South Coast of the island. These all have the darker underside pattern. In the absence of females we prefer not to assign subspecific status.
We also make the following comments on Parsons’ suggested wider eastern distribution of dictaea, in the sense that he uses that name. The males from Waigeo, Misool, Batanta, Salawati, Papua mainland, Aru, Roon, Biak and Yapen, all localities within the western part of the species’ range, share the same shade of dark blue upperside. These contrast with the more purple colour of nominate phorbas and a number of un-named specimens in BMNH from the eastern islands of Papua New Guinea including Yule, Woodlark and Kiriwina (= Trobriand Islands).
In addition these more purple males have much darker undersides than the dictaea type. The origins of these un-named specimens match many of the localities given by Parsons included in his distribution of dictaea.
We believe he mistakenly included these together with Waigeo specimens in his provisional assessment of dictaea. As this latter, more purple, group is beyond the geographical scope of this article, we do not describe any of these specimens further, but await a more comprehensive revision of the genus. Nevertheless, the clarification herein of the status of the Maluku fauna should aid in such a revision.
Hypolycaena sipylus(Felder, 1860) (TL: Ambon)
Hypolycaena sipylus is widespread in Indonesia as well as occurring in the Philippines and New Guinea region (
Hypolycaena sipylus giscon Fruhstorfer, 1912 (TL: Sulawesi). Figs 43–46.
Within Maluku Hypolycaena sipylus giscon is known from Mangole and Sanana in the Sula Islands (Vane-Wright & de Jong, 2003). To this we add Taliabu (1♂ i/2005).
Hypolycaena sipylus sipylus (Felder, 1860) (TL: Ambon). Figs 47–50.
The range of Hypolycaena sipylus sipylus is recorded by D’Abrera as “The Moluccas generally”. We have specific records from Morotai, Halmahera, Bacan and Obi in N. Maluku as well as Buru, Manipa, Kelang, Ambon, Seram and Kasa Island (off Seram) in C. Maluku.
Hypolycaena sipylus numa Fruhstorfer, [1912] (TL: Sumbawa, Flores). Figs 51–54.
Hypolycaena sipylus numa occurs on Wetar Island (
Hypolycaena danis (C. & R. Felder, 1865) (TL: Halmahera)
This species occurs in Maluku Province in Indonesia as well as the New Guinea region and N. E. Australia.
Hypolycaena danis danis (C. & R. Felder, 1865) (TL: Halmahera). Figs 55–58.
= Hypolycaena danis batjana Fruhstorfer, [1916b] (TL: Bacan)
D’Abrera records Hypolycaena danis danis from Bacan and Halmahera in N. Maluku. To this we add Morotai (Daeo – 1♂ ii/1998, 1♀ v/2005, 1♀ vi/2005).
Hypolycaena danis danisoides de Nicéville, 1897 (TL: “Key” Islands). Figs 59–70.
Hypolycaena danis danisoides occurs on the Kei Islands. BMNH has specimens from Little Kei (Kei Kecil) and to this we add Kei Besar (Yamtimur - 3♀♀ v/2002).
Neither D’Abrera nor Parsons record Hypolycaena danis as occurring in C. Maluku but there are four males from Seram in BMNH. The second author has received several further specimens from Seram (1♂ and 1♀ vii/2001, 1♀ viii/2001, 1♀ vii/2002, 1♂ viii/2002, 2♂♂ and 1♀ x/2002, 1♀ x/2003, 1♀ vii/2004, 1♂ x/2007) and Ambon is added as a new locality record (Hila – 5♂♂ iv/2003, 1♀ ii/2008). The Seram specimens show a slight variation in phenotype of both males and females (Figs 63-66) but we include them within subspecies danisoides.
BMNH also has one male and three females from Obi which match this taxon. However there is a second male labelled Obi which is typical of the nominate subspecies from Halmahera. The specimen bears two labels:
1. “Obi, ex J. Waterstradt, 1904”.
2. “Ex Oberth Coll, Brit. Mus. 1927–3”.
Without further males to examine it is hard to draw a conclusion from this, but based on the other four Obi specimens we include these within danisoides. Therefore we extend the range of Hypolycaena danis danisoides to include Obi, Seram and Ambon as well as Kei.
Hypolycaena danis derpiha (Hewitson, [1878]) (TL: Aru). Figs 71–74.
= Hypolycaena danis deripha [sic] D’Abrera, 1978.
D’Abrera records distribution as: “Aru (?) Papua and islands of Louisade Archipelago”. We assume he intends the “(?)” to refer to Aru, although Hewitson states the holotype to have been collected in Aru by Wallace. Although we could find no specimens from Aru in BMNH, M. Nagai (pers. comm.) says his son, K. Nagai, has collected three males and five females in Aru, confirming the type locality. This subspecies also occurs widely on the island of New Guinea including both Papua New Guinea (Parsons) and Papua mainland (Timika – 1♂ vi/2002, Nabire – 1♀ ii/2003, 1♀ iii/2003, Fak Fak – iv/2003).
DiscussionOur extensive study of the phorbas species group taxa from Maluku, Papua Mainland and the islands of West Papua has clarified the status of the taxa dictaea and erasmus. This new information, when combined with further study of the related specimens from the islands to the East of Papua Mainland, should provide valuable evidence about the biogeography of the island arc from North Maluku to the East of Papua New Guinea and confirm the apparent monophyletic status of the species group.
ConclusionsExamination of the male confirms the specific status of Hypolycaena asahi which is now recorded from Seram as well as the type locality Ambon. The species of Hypolycaena most closely resembling asahi occur in Sulawesi, Mindanao and Mindoro.
There is no confirmed record of Hypolycaena periphorbas (= Hypolycaena erasmus) occurring in Maluku. The distribution of this species remains extralimital, to the East of the region studied.
We synonymise Hypolycaena erylus pigres Fruhstorfer, [1912], with Hypolycaena erylus thyrius Fruhstorfer, [1912], the latter having page priority. The low number of specimens of Hypolycaena erylus available at this time from South and South East Maluku, especially of females, makes determination at subspecific rank for those islands speculative.
The phorbas species group sensu Fielder comprises Hypolycaena phorbas, Hypolycaena erylus and Hypolycaena periphorbas. Hypolycaena dictaea sensu Parsons, 1998, deserves at most subspecific rank and is restricted to certain islands to the west of Papua mainland.
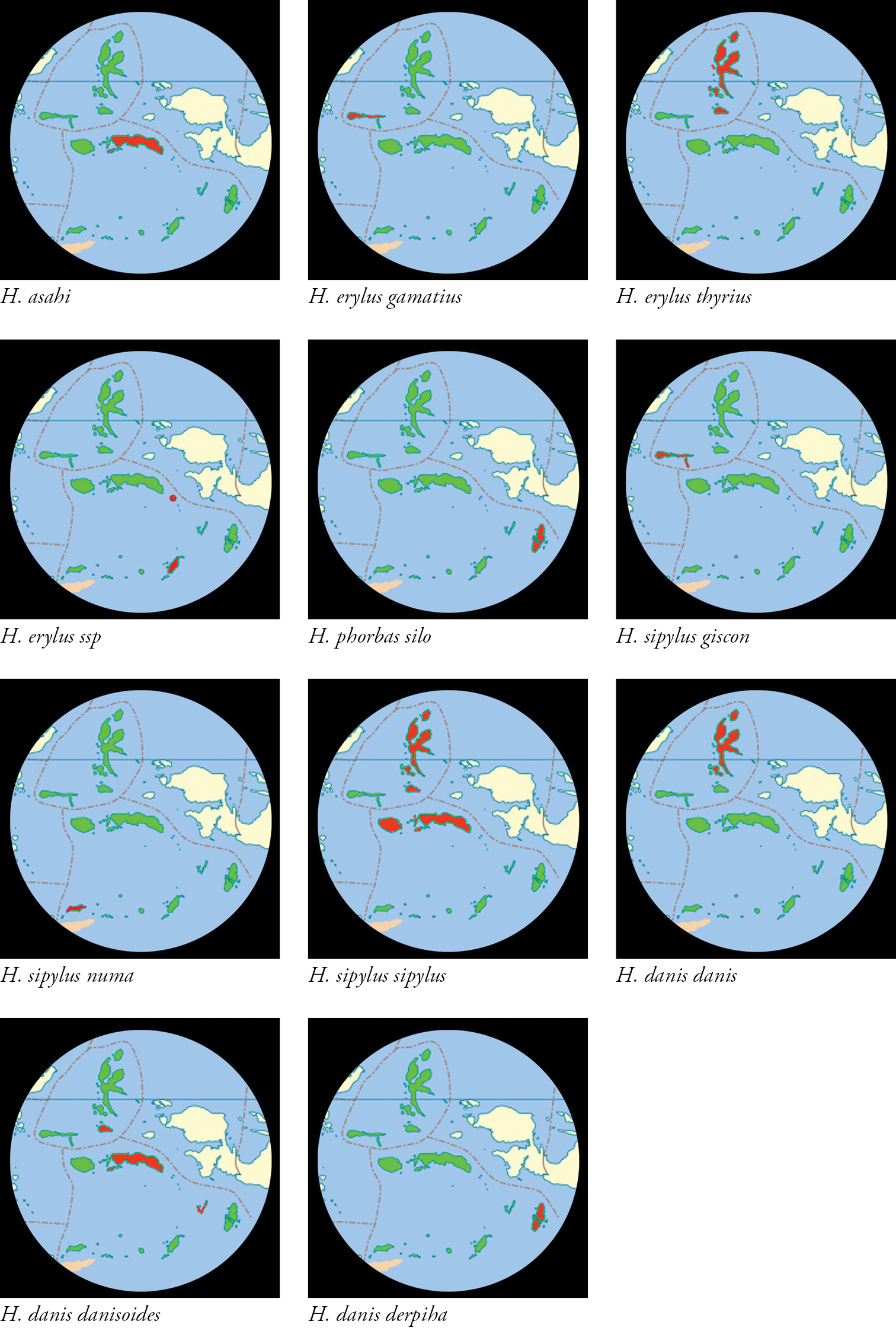
Summary of distribution of Hypolycaena species and subspecies in Maluku| Hypolycaena asahi | C. Maluku: Ambon, Seram. |
| Hypolycaena erylus gamatius | Sula Islands: Taliabu, Mangole. |
| Hypolycaena erylus thyrius | N. Maluku: Morotai, Halmahera, Ternate, Bacan, Kasiruta, Obi. |
| Hypolycaena erylus ssp? | SE. Maluku: Tanimbar - Yamdena Island, Gorong Islands - Manawoka Island. |
| Hypolycaena phorbas silo | SE. Maluku: Aru. |
| Hypolycaena sipylus giscon | Sula Islands: Taliabu, Mangole, Sanana. |
| Hypolycaena sipylus sipylus | N. Maluku: Morotai, Halmahera, Bacan, Obi. |
| C. Maluku: Buru, Manipa, Kelang, Ambon, Seram, Kasa Is. | |
| Hypolycaena sipylus numa | SW. Maluku: Wetar. |
| Hypolycaena danis danis | N. Maluku: Morotai, Halmahera, Bacan. |
| Hypolycaena danis danisoides | N. Maluku: Obi; C. Maluku: Ambon, Seram; SE. Maluku: Kei. |
| Hypolycaena danis derpiha | SE. Maluku: Aru. |
Islands of North Maluku and Maluku Provinces, shaded green, with key to named islands.
North Maluku and Maluku Provinces shown in green. Provincial boundaries chain dotted in red .Ranges of taxa shown in red.
The authors would like to acknowledge the help of the following individuals in the preparation of this paper:
Blanca Huertas, British Museum (Natural History) for access to the collections. Stefan Schröder for comments on the manuscript. Makoto Nagai for collection records and Yusuke Takanami for supplying photographs of some species.
Hypolycaena erylus gamatius, Sula Islands, Mangole, l. to r.: ♂ Up, Un, ♀ Up, Un.
Hypolycaena erylus thyrius, l. to r.: ♂ Halmahera Up, Un, ♀ Bacan Up, Un.
Hypolycaena erylus thyrius (= pigres), Obi, l. to r.: ♂ Up, Un, ♀ Up, Un.
l. to r.: Hypolycaena erylus ssp ♂ Banda Up, Un, ♂ Tanimbar Up, Un
“Hypolycaena erasmus ssp” D’Abrera, 1977: 304 recte Hypolycaena erylus thyrius ♀, Halmahera.
Hypolycaena phorbas silo, l. to r.: Aru, Wokam, ♂ Up, Un, Aru, ♀ Up, Un.
Hypolycaena phorbas silo Aru, Wokam, ♀; Hypolycaena phorbas dictaea Up, Un, Waigeo, ♂ Up, Un.
Hypolycaena phorbas silo ♀ Holoype Up, Un, New Guinea; Hypolycaena phorbas dictaea ♀ holotype, Up, Un, Waigeo.
Hypolycaena sipylus giscon, l. to r.: Sula Islands, Mangole, ♂ Up, Un, Sula Islands, Sanana, ♀ Up, Un.
l. to r.: Hypolycaena sipylus sipylus, Bacan, l. to r.: ♂ Up, Un, ♀ Up, Un.
l. to r.: Hypolycaena sipylus numa, l. to r.: Wetar, ♂ Up, Un, Timor, Dili, ♀ Up, Un.
Hypolycaena danis danis, l. to r.: ♂ Halmahera, Up, Un, ♀ Bacan, Up, Un.
Hypolycaena danis danisoides, Obi, l. to r.: ♂ Up, Un, ♀ Up, Un.
Hypolycaena danis danisoides, Seram, l. to r.: ♂ Up, Un, ♀ Up, Un.
Hypolycaena danis danisoides, Kei, l. to r. ♂ Up, Un, ♀ Up, Un.
Hypolycaena danis derpiha, Papua mainland, Arfak, l. to r.: ♂ Up, Un, ♀ Up, Un. Similar to specimens to be found in Aru.
Hypolycaena phorbas, ♂ genitalia. Above: Armature in ventral or latero-ventral aspect. Below: Aedeagus in lateral aspect. 75. Hypolycaena phorbas dictaea Waigeo Is. 76. Hypolycaena phorbas silo Aru Is. 77. Hypolycaena phorbas silo Batanta Is. 78. Hypolycaena phorbas silo Yapen Is. 79. Hypolycaena phorbas silo Nabire, Papua mainland.