(C) 2011 Nesrine Akkari. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Two remarkable genera and species of the millipede family Julidae, Titanophyllum spiliarum gen. n., sp. n. and Mammamia profuga gen. n. sp., n., are described from caves in Greece and Italy, respectively. The presence of a flagellum and the absence of a ‘pro-mesomerital forceps’ on the gonopods place them in the tribe Brachyiulini Verhoeff, 1909, an unnatural grouping based on plesiomorphic characters. Both are outstanding in being the only hitherto known blind julidans having such gonopodal features. A dichotomous key to the nine valid brachyiulinine genera based on peripheral and gonopodal characters is presented. Moreover, notes on subanal hooks and anchors in Julida are provided with hypotheses on their possible function.
Greece, Italy, cave, millipedes, new genera, new species, Titanophyllum, Mammamia
We describe here two new genera and species of Julidae, collected from caves in Italy and Greece, which we tentatively assign to the poorly characterised tribe Brachyiulini. In addition to the taxonomic description we discuss the status of the tribe and provide a dichotomous key to its nine currently valid genera. Notes on the presence of subanal hooks and anchors in Julida are also given with hypotheses on their possible function.
Material and methodsSpecimens were collected in two caves in Italy and Greece and preserved in 70% ethanol. All measurements were made using a Leica Wild M10 microscope equipped with an ocular micrometer. Vertical body diameter was measured at midbody. Antennae, legs and gonopods were mounted in glycerin for temporary microscope preparations.
Microphotographs were obtained using a Leica digital camera M205A mounted on a stereomicroscope Leica DFC 420. Images were processed with a Leica Application Suite program and final stacking made with Helicon Focus 4.60.2 Pro software. SEM micrographs were obtained using a JEOL JSM-6335F scanning electron microscope. Drawings were made using a camera lucida mounted on a Leica DMRXE microscope. All pictures were later assembled for a final layout with Adobe Photoshop CS.
Results TaxonomyOrder Julida Brandt, 1833
Family Julidae Leach, 1814
Tribe Brachyiulini Verhoeff, 1909
urn:lsid:zoobank.org:act:16F56F2A-E20D-43FA-815D-2C1E242401E1
Differs from all other genera of Brachyiulini by lacking ocelli and by having a distally expanded promerite and a slightly shorter posterior gonopod, the latter with a basally broad and distally slender mesomerital process mostly lodged in an opisthomerital furrow.
The name derives from the Italian exclamation “Mamma mia” which came to our mind when we first saw this astonishing species. Gender feminine.
urn:lsid:zoobank.org:act:C319EC4E-FB69-4756-B1CE-609ACDE9F347
http://species-id.net/wiki/Mammamia_profuga
Figs 1–8Holotype: adult ♂ (broken into head and 6 body parts), Italy, Taranto, Grotta della Cava, iii.1964, P. Parenzan leg. (Natural History Museum of Denmark, Zoological Museum, University of Copenhagen – ZMUC).
The new species was collected in a cave in Taranto Province (south-eastern Italy). P. Parenzan (1984, in a letter to HE) wrote that the cave where the species have been collected was subsequently destroyed.
‘profuga’ in Latin means homeless; the name emphasizes the destroyed type locality of the species.
(all measurements in mm). Body uniformly pale yellowish, approximately 26 mm in length, vertical body diameter (height, H) 1.5, length/height ratio 17. Head: ocelli absent, frontal setae and setal sockets missing; gnathochilarium with 2 setae in apical parts of the stipites and with a seta on each lamella lingualis; 4 supralabral setae and a row of ca 12 labral setae; mandibular stipital lobes not expanded in males; antennal length ca 1.5×H. Body with 51 podous + 2 apodous rings and telson; striation moderately dense; setae apparently missing, probably broken off; legs yellowish, their length ca 1.5×H. Male first leg-pair reduced and hook-shaped. Telson blunt, preanal ring without projection, with at least 5 long setae; subanal scale with 2 long setae; anal valves pilose.
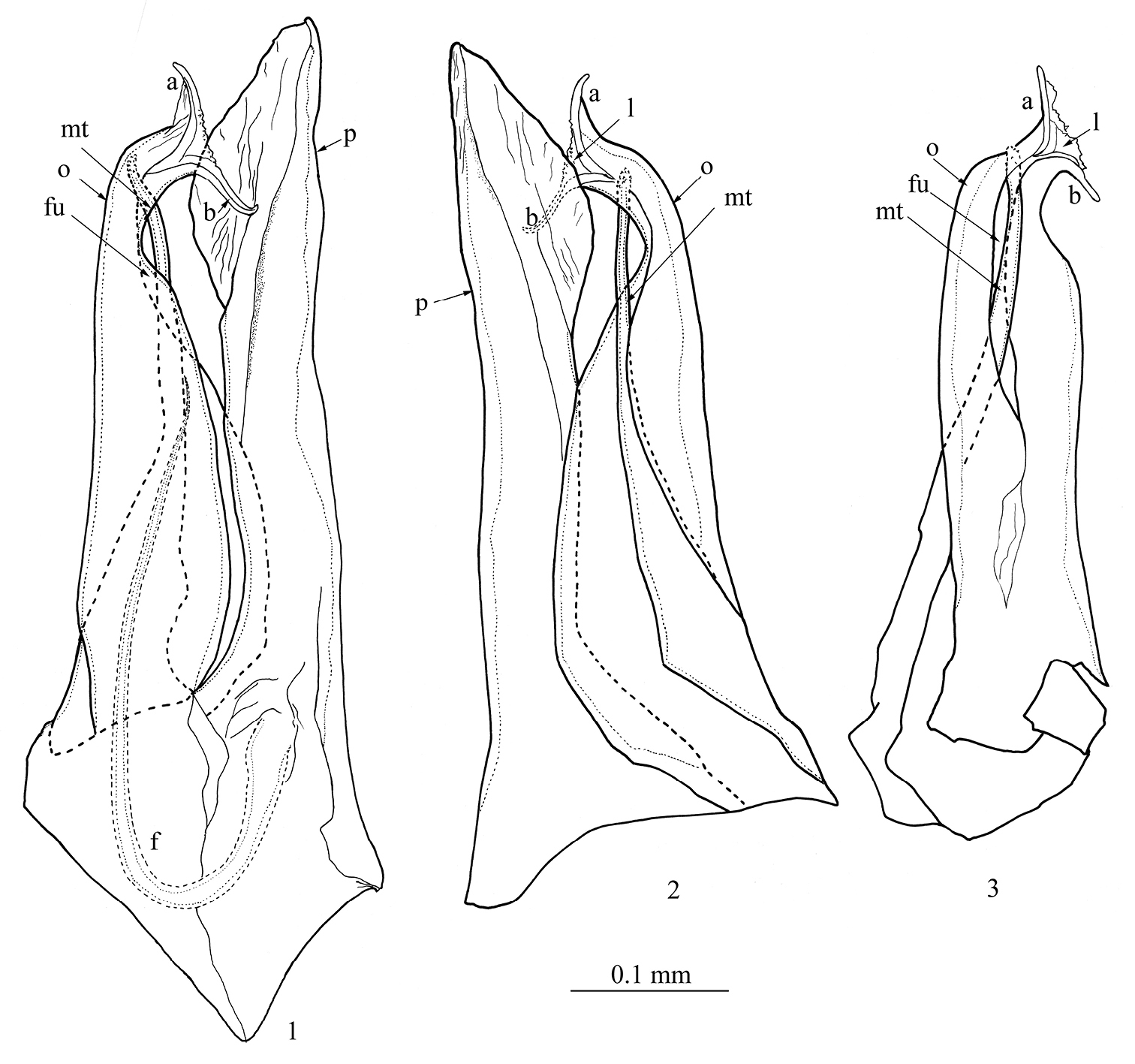
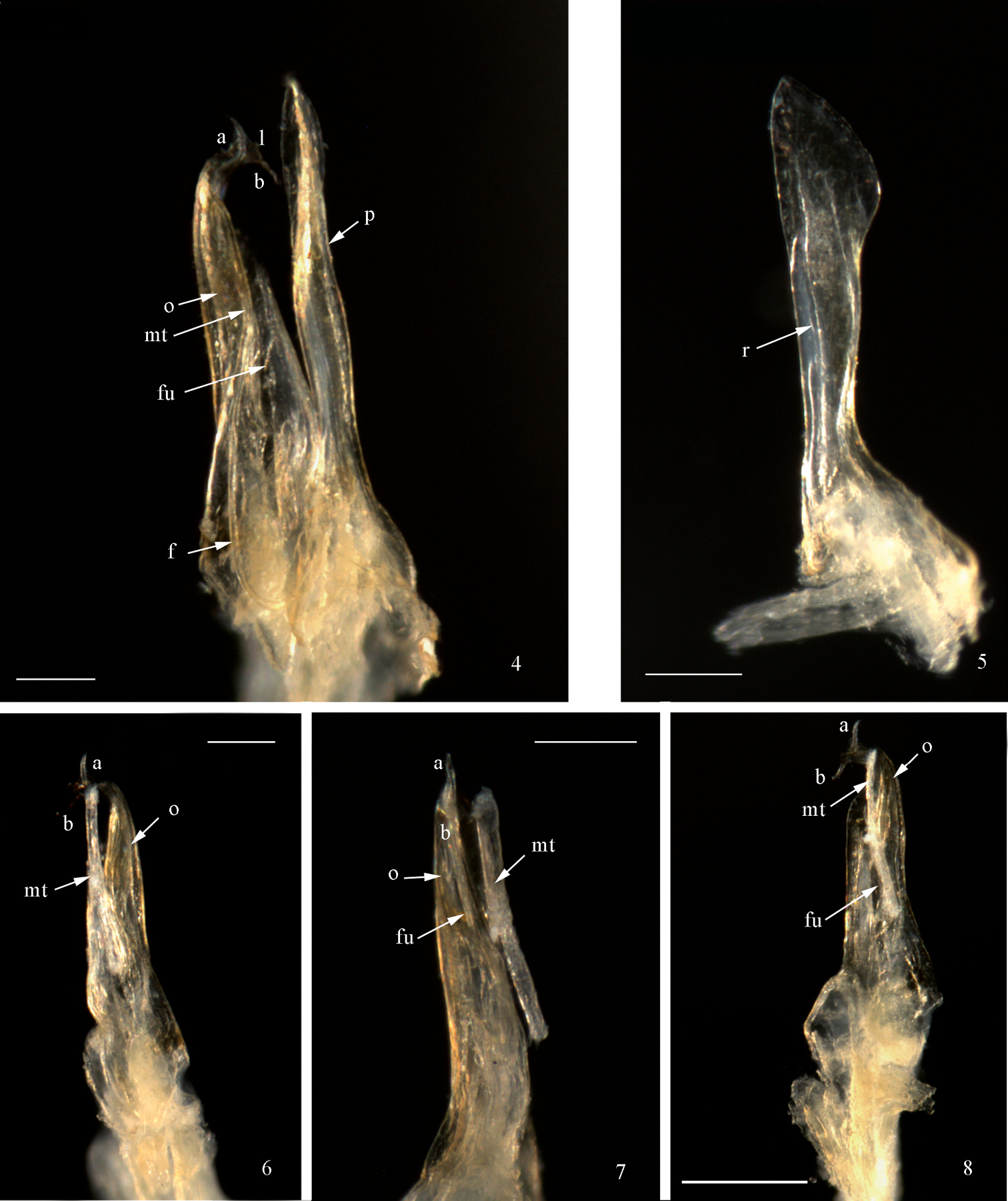
Anterior gonopod (promerite, p) slightly longer than posterior gonopod (Figs 1, 2, 4); broad at base, abruptly narrowing at about 1/4 of its height, then gradually broadening distally to form a spatula-like process (Figs 1, 2, 5); apically blunt and mesally with a quite high ridge (r) (Fig. 5). Flagellum (f) (Figs 1, 4) moderately long, falcate, emerging from the promerite’s base, its tip reaching about 2/3 the height of the posterior gonopod. Posterior gonopod: Opisthomerite (o) (Figs 1−4, 6−8) broadest at base, gently tapering up to about 3/4 of its height, then abruptly narrowing and curving anterolaterad; mesally with a wide furrow (Figs 1, 2, fu) running along its length; apex resembling a fish-tail, with two processes (a, b) pointing in opposite directions (Figs 1−4, 6−8) connected by a thin, marginally serrated lamella (l) bearing several small spines on the surface (Figs 2−4). Mesomerital process (mt) (Figs 1−3, 4, 6−8) emerging from the anterior side of the opisthomerite, mostly lodged in the opisthomerital furrow, broad at base, narrowing at about midlength, thereafter becoming very slender and bent, apical margin gently serrated.
Mammamia profuga gen. n., sp. n., gonopods: 1 right gonopod, mesal view 2 right gonopod, lateral view 3 left posterior gonopod, anterior view. Abbreviations: a, b: opisthomerital processes a and b, f: flagellum, fu: furrow, l: lamella, mt: mesomerital process, o: opisthomerite, p: promerite.
Mammamia profuga gen. n., sp. n., gonopods: 4 right gonopod, mesal view 5 left promerite, posterior view 6 left posterior gonopod, lateral view 7 left posterior gonopod, postero-lateral view 8 left posterior gonopod, posterior view. Abbreviations: a, b: opisthomerital processes a and b, f: flagellum, fu: furrow, l: lamella, mt: mesomerital process, o: opisthomerite, p: promerite, r: ridge. Scale bar: 0.1 mm.
urn:lsid:zoobank.org:act:C09ADA27-45ED-49E0-A2CB-1457D0CDF629
Differs from all other genera of Brachyiulini by lacking ocelli and by having a rather simple, apically incised promerite devoid of any filamentous processes or apical appendages, and a simple unipartite posterior gonopod with a proximal lobe laterally and a subbasal fold and a groove mesally, the latter ending in a subapical opening.
The name combines the type locality, Titanospilia (the cave of Titans) and the suffix – phyllum – referring to the simple, leaf-shaped posterior gonopods. Gender neuter.
urn:lsid:zoobank.org:act:C4B8738C-470F-4F54-A4F4-B69741C20EF4
http://species-id.net/wiki/Titanophyllum_spiliarum
Figs 9, 10−14Holotype: adult ♂, Greece, Magnesia, Othris Mts., village of Kofi, Titanospilia (Cave of Titans), 13.VII.2003, P. Beron leg. (National Museum of Natural History Sofia − NMNHS); Paratypes: 4 ♂♂, 5 ♀♀, same locality, date and collector (ZMUC); 12 adult ♂♂, 11 adult ♀♀, 2 subadult ♂♂, 2 subadult ♀♀, same locality, date and collector (NMNHS).
Titanospilia is an approximately 100 m long vertical cave composed of a single voluminous hall. All material was collected at the bottom of the shaft (P. Beron, pers. comm.).
The names means “of caves” in Greek and emphasizes the troglomorphic character of the species.
(all measurements in mm). Body uniformly pale to yellowish, legs brownish, metazonites with a slightly darker posterior band; length: 17.3−33.5 mm, vertical body diameter (height, H) 1–1.2 (♂) and 1–1.4 (♀); length/height ratio 16 (♂). Head: ocelli absent (Fig. 9); frons with 2 setae; gnathochilarium with 3 setae in apical part of each stipites, and with a long seta on each lamella lingualis; 4 supralabral setae and a row of ca 8 labral setae; mandibular stipites not expanded; male first leg-pair reduced and hook-shaped; antennal length about 1.6×H. Body rings with more or less dense striation and a whorl of moderately dense long setae; 50–61 (♂) and 47–54 (♀) podous rings, 1–2 apodous rings + telson. Defense glands visible as dark spots opening on the suture. Length of legs ca 0.83×H. Preanal ring dorsally only slightly protruding beyond anal margin; subanal scale with a small hook pointing anteriad (Fig. 14); anal valves pilose, with long setae. Male 7th body ring with well developed ventral lobes.
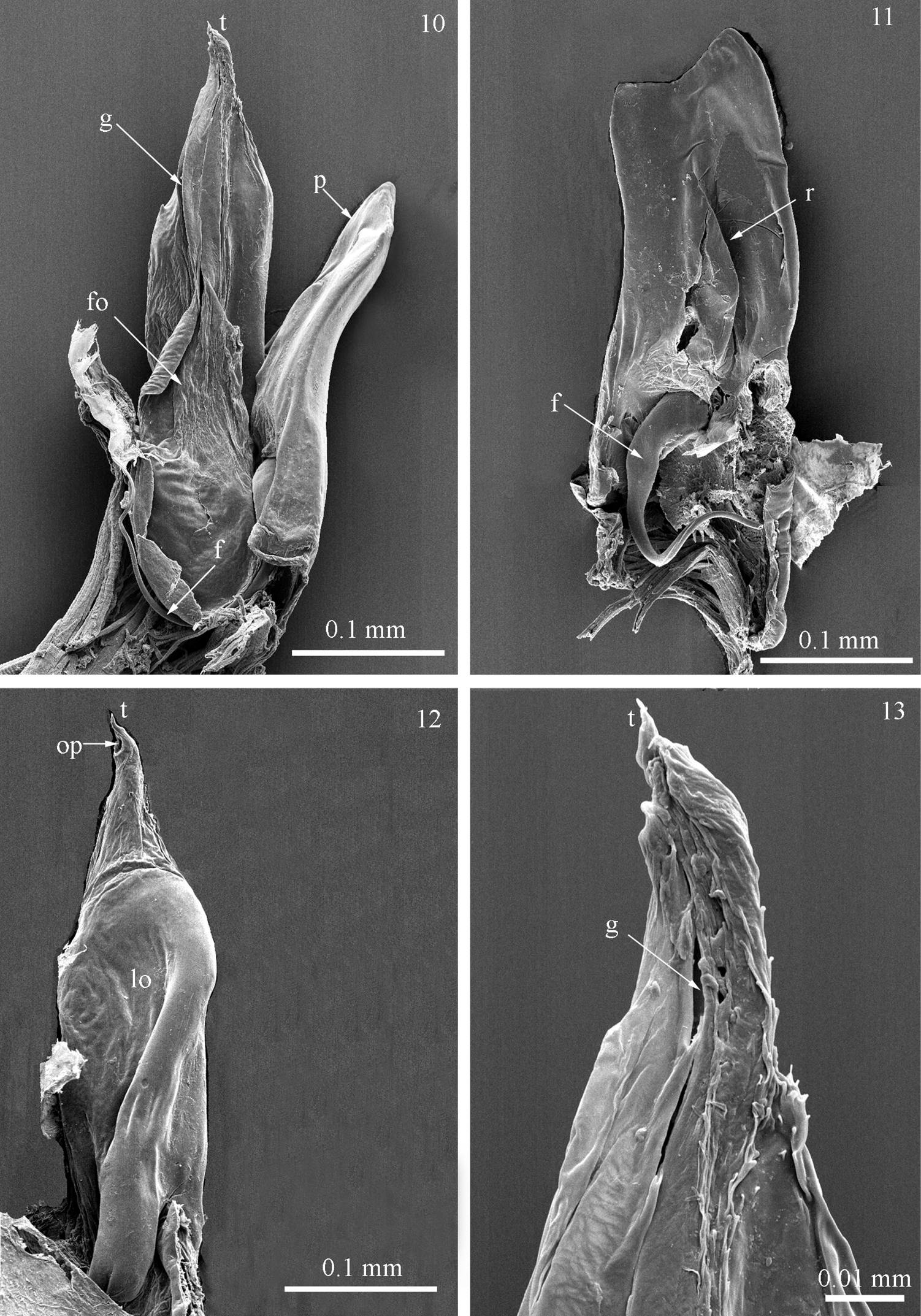
Gonopods protruding from the 7th body ring. Anterior gonopod (promerite, p) much shorter than posterior gonopod (Figs 10, 11), uniformly broad along its length, slightly expanded at midlength; apex incised, with a lower angular process mesally and a higher triangular one laterally (Fig. 11). Posterior side of promerite at middle with a quite high, semitriangular ridge (r) pointing postero-laterad (Fig. 11). Flagellum (f) (Figs 10, 11) moderately long, falcate, pointing ventrad, emerging from the base of ridge.
Posterior gonopod (Figs 12, 13) unipartite, long, broad at base and midlength, gradually tapering distally; with a proximal lobe (lo) laterally (Fig. 12) and a subbasal fold (fo) mesally (Fig. 10), the latter giving rise to a mesal groove (g) running proximal and distal (Figs 10, 13) and ending in a subapical opening (op) (Fig. 12). Gonopodal apex (t) with a pointed tip (Figs 12, 13).
Titanophyllum spiliarum gen. n., sp. n., habitus. Scale bar: 2mm.
Titanophyllum spiliarum gen. n., sp. n., gonopods: 10 right gonopod, mesal view 11 left promerite, posterior view 12 right posterior gonopod, lateral view 13 close up of the tip of posterior gonopods, mesal view. Abbreviations: f: flagellum, fo: fold, g: groove, lo: lobe, op: opening, p: promerite, r: ridge, t: tip.
The tribe Brachyiulini
Verhoeff, 1909, is a grouping of julid genera based exclusively on
plesiomorphic gonopod characters: presence of a flagellum and absence of
a ‘pro-mesomerital forceps’ (
The assignment of Titanophyllum gen. n. and Mammamia gen. n. to Brachyiulini
is based on purely typological considerations. They have gonopodal
flagella and no mesomerites and therefore fall into the brachyiulinine
‘pigeonhole’. In the classical key to subfamilies/tribes of Julidae by
Non-monophyly of Brachyiulini as currently defined is quite probable and is indeed suggested by recent molecular analyses of the phylogeny of the family Julidae (
Even though all genera of Brachyiulini agree in the absence of a ‘pro-mesomerital’ forceps and the presence of a flagellum, the brachyiulinine gonopods exhibit great variation in the degree of complexity, size and position of the processes (see Table 1). The nine valid genera also vary in peripheral characters such as the presence/absence of ocelli, frontal setae and preanal hook, as well as the length of the preanal projection. The peripheral character that best characterises the new genera described here vis-a-vis the rest of the tribe is the lack of eyes.
Characters matrix for the brachyiulinine genera. Ac – Acropoditius; An – Anaulaciulus; Ba – Balkanophoenix; Br – Brachyiulus; Gr – Grusiniulus; Me – Megaphyllum; Rh – Rhamphidoiulus; Tit – Titanophyllum; Mam – Mammamia
| Character | Ac | An | Ba | Br | Gr | Me | Rh | Tit | Mam |
| Eyes | + | + | + | + | + | + | + | - | - |
| Frontal setae | + | + | + | + | +/- | + | -? | ||
| Subanal hook | - | - | - | - | - | - | - | + | - |
| Male mandibles with protruding lobe | + | - | - | +(all?) | + | +(all?) | + | - | - |
| Length of anterior gonopods compared to posterior gonopods | ~as long | ~half as long | ~as long | ~half as long | ~half as long | ~as long | ~as long | ¾ as long | longer |
| Anterior gonopods: flagelliferous lobe with very long, almost filamentous appendage | - | - | - | - | - | - | + | - | - |
| Anterior gonopods with a distal very long, almost filamentous appendage | + | - | - | - | - | - | - | - | - |
| Posterior gonopods simple, unipartite (S) vs. complex, bearing one or more processes distally (C) | S | C | C | C | C | C | S | S | C |
| 1(4) | Ocelli absent | 2 |
| 2(3) | Subanal scale with a hook; promerite broad, incised apically, much shorter than posterior gonopods | Titanophyllum gen. n. |
| 3(2) | Subanal scale without a hook; promerite slenderer, distally expanded, slightly longer than posterior gonopods | Mammamia gen. n. |
| 4(1) | Ocelli present | 5 |
| 5(6) | Promerite with a long, falcate appendage apically | Acropoditius |
| 6(5) | Promerite without such an appendage | 7 |
| 7(8) | Promerite with a long, straight filamentous process at midlength; posterior gonopods simple | Rhamphidoiulus |
| 8(7) | Promerite a filamentous process; posterior gonopods complex | 9 |
| 9(15) | Promerite half as long as posterior gonopod | 10 |
| 10(11) | Male mandibular stipes with expanded lobes (Southeast Asia) | Anaulaciulus |
| 11(12) | Male mandibular stipes without a lobe (Europe, Caucasus) | 13 |
| 13(14) | Promerite simple; posterior gonopods with an anterior ‘mesomerital’ process | Brachyiulus |
| 14(13) | Promerite complex, with a well developed ridge and a basal cavity mesally, apex subconcave; posterior gonopods without anterior ‘mesomerital’ process | Grusiniulus |
| 15(9) | Promerite nearly as long as posterior gonopod | 16 |
| 16(17) | Male mandibular stipes without a lobe; metazonital striation absent; mesomerital process lying well apart from the main stem of the posterior gonopods, separated by a deep, broad concavity | Balkanophoenix |
| 17(16) | Male mandibular stipes with a lobe; metazonital striation present; mesomerital process usually lying in close proximity to the main stem of posterior gonopods | Megaphyllum |
Apart from the male gonopods, julid millipedes are
relatively uniform in structure. There are some non-gonopodal characters
in males that exhibit a certain degree of variability, as do the
female cyphopods, but when it comes to non-sexual morphology, the
diversity is modest and mostly concerns such details as size, colour,
presence/absence of eyes, presence/absence of frontal and metazonital
setae, length of preanal projection, etc. There are a few julid
species, however, which stand out by having some remarkable
apomorphies. The first such species to be described was Unciger foetidus (C.L. Koch, 1838). The generic name Unciger Brandt, 1841, refers to its peculiar character: a stout forward-pointing hook on the subanal scale (Fig. 15). The hook occurs in both sexes.
Occurring in adults (both sexes) as well as
juveniles, this hook has probably no function in courtship or
copulation. One possibility could be that the hook is a protective
device: when a julid rolls up into a spiral, as many julids do when
disturbed, the hook might be inserted under one metazonital hind
margin, ‘locking’ the spiral and making it more difficult for a
would-be predator to uncoil. The only structure remotely similar to the
julid hook we have been able to identify in other millipedes is the
paxillus in certain Gomphodesmidae, order Polydesmida (
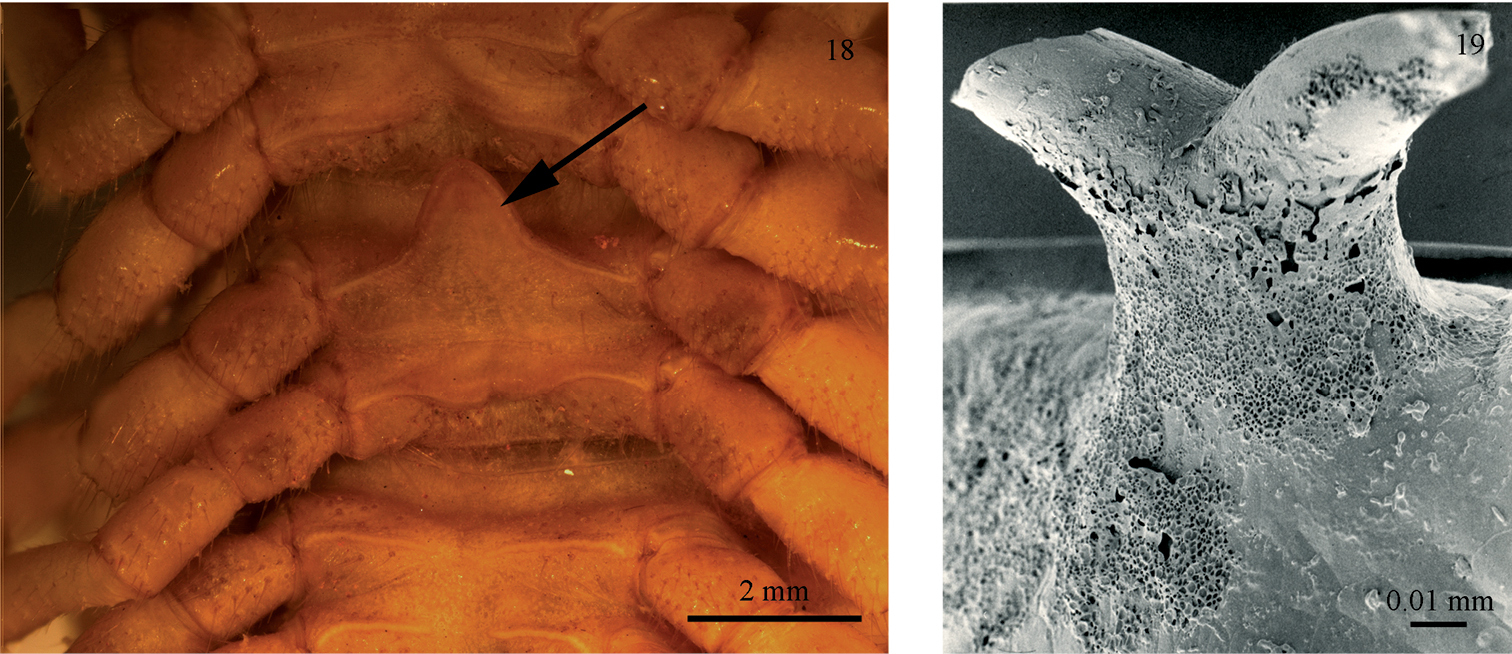
The julid hook may be a spiral-locking device, but there would be a different way obtaining the same effect if a structure on the dorsal side of the body rings could anchor itself to the leg coxae on the spiralled millipede. In fact there is a single species with such a structure, namely Chersoiulus sphinx Strasser, 1940, from north-eastern Italy, Slovenia and Croatia. In this species, each body ring carries a tiny mid-dorsal ‘anchor’ on the posterior margin of the metazonites (Fig. 19). The function of the anchors, which are missing in the only known congener, Chersoiulus ciliatus (Strasser, 1938), could very well be as suggested above for the subanal hooks. However, until somebody makes observations on behaviour of the hook- or anchor-bearing julids, the functional explanation given above remains entirely hypothetical.
Hooks in julids: 14 Titanophyllum spiliarum 15 Unciger transsilvanicus (ZMUC) 16 Syrioiulus sp., Crete (ZMUC) 17 Syrioiulus andreevi (paratype, National Museum of Natural History, Sofia).
Anchors and sternal processes in millipedes: 18 Sternal process (indicated by an arrow) in Astrodesmus laxus (Gerstäcker, 1873) (Gomphodesmidae) (ZMUC) 19 Anchor in Chersoiulus sphinx (ZMUC).
We want to express our gratitude to the late Professor Pietrio Parenzan, Taranto, and Dr. Petar Beron, Sofia, who made the specimens here described available to us. We are grateful to Peter Decker (Senckenberg Museum, Görlitz) for his kind assistance in taking some of the photographs. Many thanks are also due to two anonymous reviewers for their helpful comments. This project has partly been made possible with funding to PS from the EC FP6 Integrated Infrastructure Initiative Synthesys (DK-TAF).