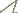(C) 2011 Olavi Kurina. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Both males and females of Baeopterogyna mihalyiiMatile, 1975 are recorded from northern Greece. Females are described for the first time providing photographs of the general facies and terminalia. In contrast to the single congener with stenopterous females – Baeopterogyna nudipes Vockeroth, 1972 – Baeopterogyna mihalyii is shown to have normally developed wings in both sexes. Association of sexes is based on both morphological characters and sequence data from cytochrome oxidase subunit one (COI). DNA sequences are used for the first time for the association of sexes in Mycetophilidae.
Diptera, Mycetophilidae, Baeopterogyna, systematics, Europe, COI
Baeopterogyna Vockeroth, 1972 is a small genus of Mycetophilidae including only two species: Baeopterogyna nudipes Vockeroth, 1972 from the Nearctic and Baeopterogyna mihalyiiMatile, 1975 from the Palaearctic region, respectively. The genus belongs to the subfamily Sciophilinae and is most closely related to the Neuratelia Rondani (
Since the introduction of the ‘DNA barcoding’ approach (
The current study was initiated by finding both sexes of Baeopterogyna mihalyii in Malaise trap samples from northern Greece. The aims of this article are to describe the so far unknown female of Baeopterogyna mihalyii and introduce a possibility of using COI sequence data for association of females and males of fungus gnat species.
Material and methods Collection, illustration and morphological studyAll Baeopterogyna mihalyii material was collected by GR from the Kerkini Lake area in Northern Greece south of the Bulgarian border. Despite an extensive Mycetophilidae material collected from the area during a survey of invertebrates from 2003 to 2009 (for details see
The material has been deposited in IZBE (Institute of
Agricultural and Environmental Science, Estonian University of Life
Sciences, former Institute of Zoology and Botany) and all specimen data
have been inserted into the database of Estonian animal collections (
The genomic DNA was extracted using a High Pure PCR Template Preparation Kit (Roche Diagnostics GmbH, Mannheim, Germany). Anterior segments of the abdomen that had been stored after genitalia dissection were crushed and used for the extraction. This process was carried out following the manufacturer’s instructions for extraction of genetic material from mammalian tissue.
A 762-bp fragment of cytochrome C oxidase subunit 1 (COI), corresponding to positions 2228–2989 of the mitochondrial genome of Drosophila melanogaster
Meigen, 1830 (RefSeq NC_001709) was amplified and sequenced using
primers C1-J-2195 (5’-TTGATTTTTTGGTCACCCTGAAGT-3’) and TL2-N-3014
(5’-TCCAATGCACTAATCTGCCATATTA-3’) (
In total, 9 specimens including three species of fungus gnats from the subfamily Sciophilinae were analysed. In addition to 3 males and 2 females of Baeopterogyna mihalyii, both sexes of Allocotocera pulchella (Curtis 1837) and both sexes of Sciophila nigronitida Landrock, 1925, the latter as an outgroup, were included. For detailed information about specimens see Table 1.
Consensus sequences were created with the program Consed (
Bayesian phylogenetic analysis implementing the GTR+I model was performed using MrBayes 3.1 (
For determination of male material of Baeopterogyna mihalyii, the key to mycetophilid genera by
All specimens identified preliminarily as Baeopterogyna mihalyii according to their morphological characteristics carried identical COI haplotypes, and the same applied for both Allocotocera pulchella individuals, thus proving that morphology-based identification was correct. The Sciophila nigronitida
specimens, however, carried different COI haplotypes at one locus
corresponding to position 2508 of the full mitochondrial genome of Drosophila melanogaster
(RefSeq NC_001709); the male had an adenine nucleotide, whereas the
female had a guanine nucleotide. Since the genetic distance between
these two specimens is only 0, 13%, i. e. significantly below the
average pairwise distance between individuals belonging to different
species (
Among the species used for phylogenetic analysis also Sciophila nigronitida is representing the first record from Greece (for collecting details see Table 1).
Details of specimens used for taxonomic study and molecular analysis
| Voucher No | Species | Sex | Collecting site, collecting method and collector | Date | Method of preservation | GenBank acc. code for COI |
|---|---|---|---|---|---|---|
| IZBE0200002 | Allocotocera pulchella (Curtis, 1837) | ♂ | Estonia, Palupõhja 58°25'54.68"N 26°14'28.90"E, Malaise trap, Soon, V. leg. | 25.vii – 4. viii 2009 | Abdomen used for DNA sequencing; terminalia in glycerin; rest of body dry mounted from ethanol | JN007851 |
| IZBE0200003 | ♀ | Abdomen used for DNA sequencing; terminalia in glycerin; rest of body dry mounted from ethanol | JN007851 | |||
| IZBE0200004 | Baeopterogyna mihalyii Matile, 1975 | ♂ | Greece, Central Macedonia, Kerkini lakes area, village Neo Petritsi, Sultanitsa site, 41°19'02.1"N 023°12'05.0"E, 1485 m a.s.l., Malaise trap, Ramel G. leg. | 12 – 18.v 2008 | In ethanol | |
| IZBE0200005 | ♂ | In ethanol | ||||
| IZBE0200006 | ♀ | Abdomen used for DNA sequencing; terminalia in glycerin; rest of body in ethanol | JN007850 | |||
| IZBE0200007 | ♂ | 19 – 25. v 2008 | Abdomen used for DNA sequencing; rest of body and terminalia slide mounted | JN007850 | ||
| IZBE0200008 | ♂ | Abdomen used for DNA sequencing; terminalia in glycerin; rest of body in ethanol | JN007850 | |||
| IZBE0200009 | ♂ | Slide mounted under 5 different coverslips | ||||
| IZBE0200010 | ♂ | In ethanol | ||||
| IZBE0200011 | ♂ | In ethanol | ||||
| IZBE0200012 | ♂ | 25.v – 1. vi 2008 | Abdomen used for DNA sequencing; rest of body and terminalia slide mounted | JN007850 | ||
| IZBE0200013 | ♂ | In ethanol | ||||
| IZBE0200014 | ♀ | Abdomen used for DNA sequencing; terminalia in glycerin; rest of body slide mounted | JN007850 | |||
| IZBE0200015 | Sciophila nigronitida Landrock, 1925 | ♂ | Greece, Central Macedonia, Kerkini lakes area, village Neo Petritsi, Farfara site, 41°19'30.5"N 023°15'00.1"E, 750 m a.s.l., Malaise trap, Ramel G. leg. | 16 – 22. vi 2008 | Abdomen used for DNA sequencing; terminalia in glycerin; rest of body dry mounted from ethanol | JN007853 |
| IZBE0200016 | ♀ | Abdomen used for DNA sequencing; terminalia in glycerin; rest of body dry mounted from ethanol | JN007852 |
9♂♂ 2♀♀, for collecting data see Table 1: voucher numbers from IZBE0200004 to IZBE0200014.
Female (Figs 2a, 2c – 2f, 3a – 3c).
Length of body 4.65 – 4.94 mm (n=2).
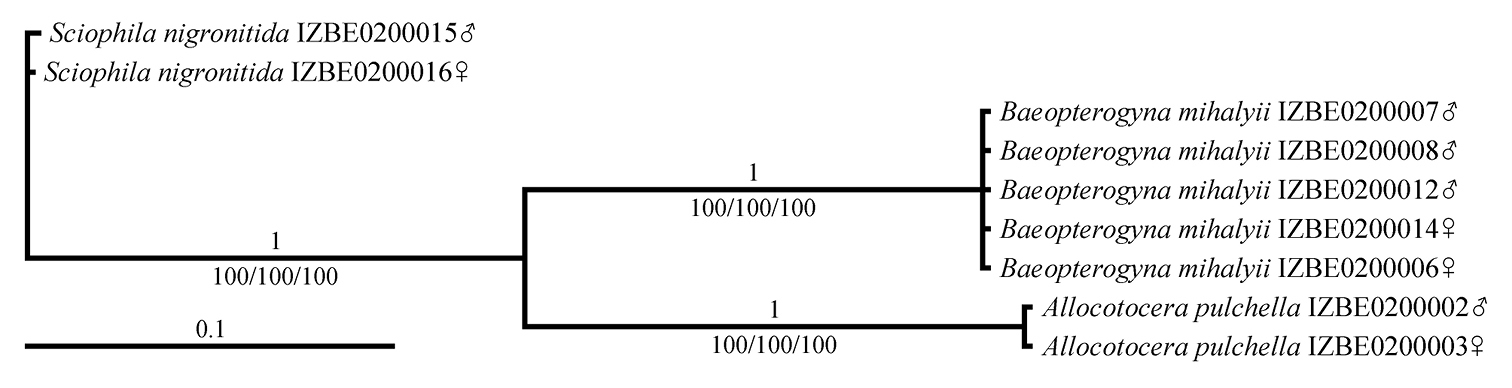
Head brown with dark setae. Three equally sized ocelli in a triangular arrangement. Clypeus subrounded. Palpus with 5 light brown setose segments with ratios of 1: 1.17:1.58: 2.25:4.33. Mouthparts brownish. Antenna with 2+14 segments. Scape, pedicel and base of first flagellar segment light brown, rest of flagellomeres brown. Scape with sparse setae including dorsoapicals extending to middle of pedicel. Pedicel with sparse and all flagellomeres with dense setae. First flagellomere 3 times as long as wide, succeeding segments gradually shorter. Apical flagellomere cylindrical, about three times as long as wide.
Thorax brown. Scutum covered with pale setae including long lateral hairs. Lateral parts of thorax slightly paler than scutum. Antepronotum with numerous long pale hairs. Proepisternum with numerous shorter setae. Laterotergite and mediotergite with upward directed hairs. Anepimeron and metepisternum with short setae, anepisternum bare. Scutellum with setae not in distinct pairs.
Legs. Fore coxa light brown with hind margin and apical fourth yellow. Mid and hind coxae brown, apically slightly paler. All trochanters brown. All femora and tibiae yellow with apical brown markings. All tarsi dark brown. Tibiae with irregularly arranged setae but without distinct bristles. Fore tibia with a spur 0.18 of basitarsus length. Mid and hind tibiae both with two equal spurs, 0.19 and 0.21 of basitarsus length, respectively. Ratio of femur to tibia for fore, mid and hind legs: 1.00; 0.82; 0.81. Ratio of tibia to basitarsus for fore, mid and hind legs: 1.15; 1.57; 1.86.
Wing hyaline. Length of wing 4.00 – 4.23 mm (n=2). Ratio of length to width 2.83. Veins light brown, setose on both surfaces. Wing membrane with dense irregularly arranged microtrichia and with few macrotrichia in anal area and close to wing tip below of R1 and R5. C not produced beyond apex of R5, which is strongly sinuate. Sc ends in C at the level of beginning of medial fork. Sc2 situated at the level of middle of bM-Cu. M1basally obsolete. Cubital fork begins slightly before the base of r-m. Haltere pale with brownish knob. Both, stem and knob with short setae.
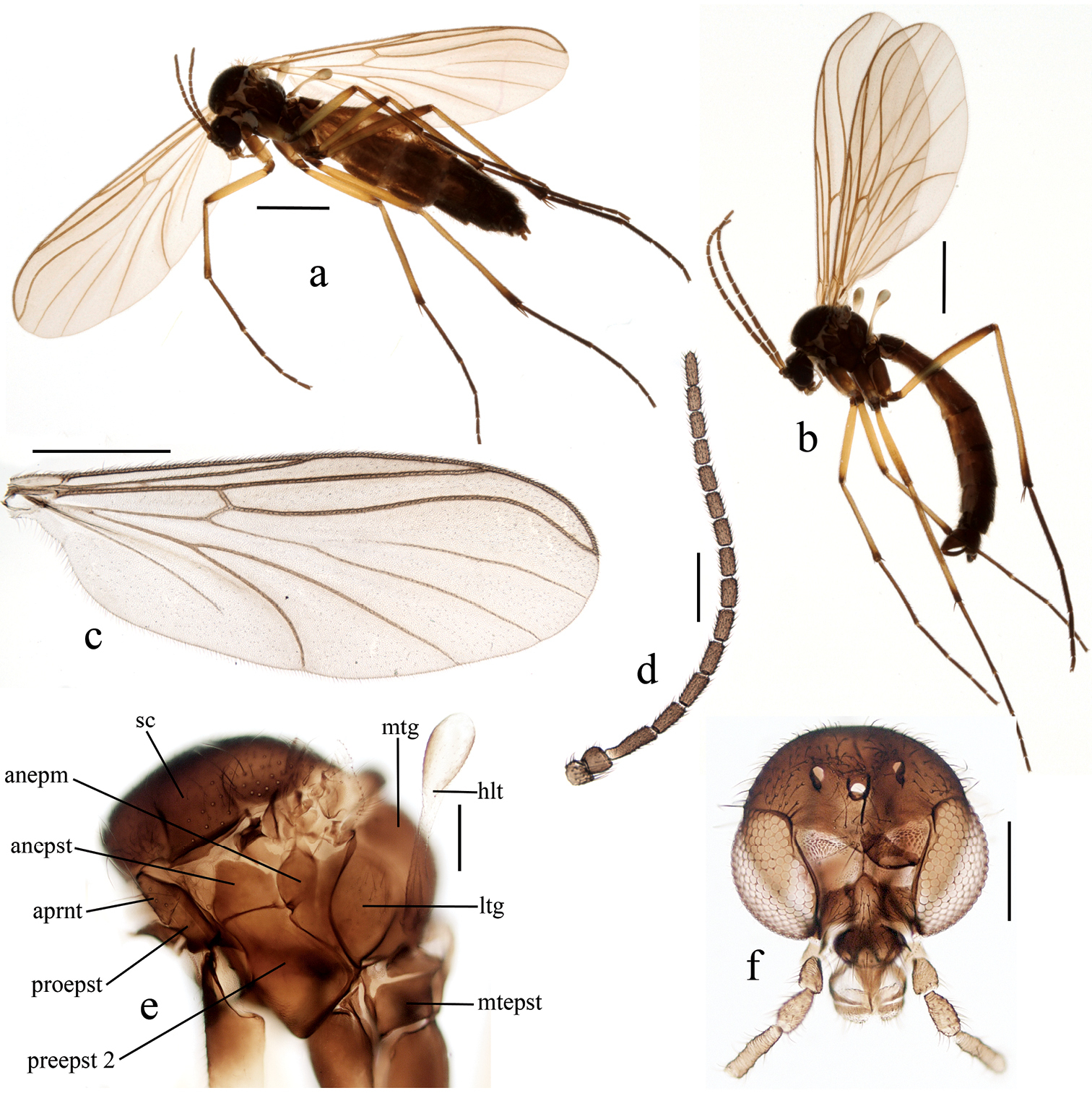
Abdomen brown with segments I–IV lighter. Terminalia (Figs 3a, 3b, 3c) light brown. Cercus distinctly two-segmented, segments with subequal length, proximal segment more than twice as wide as distal. Tergite VIII subquadrate, as large as tergite IX and tergite X together. Sternite VIII with deep ventral cleft. Gonapophysis IX well sclerotized and visible in lateral view. Hypoproct with apical incision, exposed in ventral view.
Male (Figs 2b, 3d).
Length of body 5.36 – 6.32, 5.65 mm (n=5). Length of wing 4.37 – 5.30, 4.76 mm (n=5), ratio of length to width 2.55 – 2.82, 2.64 (n=5). Coloration and other non-terminal characters similar to female except anepisternum, which has short setae on upper part. Terminalia brown. Gonostylus simple, without any additional lobes or spines, slender, tapering, curved medially and covered with short setae.
Bayesian phylogenetic tree (GTR+I model) of selected Mycetophilidae taxa, based on a 762 bp fragment of a COI gene. Bayesian posterior probabilities are given above the branches; bootstrap support for the ML/NJ/MP trees, which exhibited identical topology, are presented below the branches.
Baeopterogyna mihalyii. a female b male c female wing d female antenna e female thorax f female head (last palpal segments absent). Scale = 1 mm (a, b, c), 0.2 mm (d, e, f).<br/> anepm = anepimeron; anepst = anepisternum; aprnt = antepronotum; htl = halter; ltg = laterotergite; mtepst = metepisternum; mtg = mediotergite; proepst = proepisternum; preepst = preepisternum; sc = scutum.
Baeopterogyna mihalyii. a female terminalia, lateral view b female terminalia, dorsal view c female terminalia, ventral view d male terminalia, ventral view. Scale = 0.2 mm.<br/> cerc = cercus; gc = gonocoxite; gp = gonapophysis; gst = gonostylus; hyp = hypoproct; st = sternite; tg = tergite
The study was funded by Estonian Science Foundation grants 7558 and 8583, targeted financing projects SF0170160s08 and SF0180122s08, and by the European Union through the European Regional Development Fund (Center of Excellence FIBIR). OK is grateful to the European Commission’s Research Infrastructure for funding the study visit to MNHN via the SYNTHESYS programme (FR-TAF-5005) and is much obliged to the curator Dr. Christophe Daugeron (MNHN) for the opportunity to work with the collections in Paris. We thank Tuuli Reisberg (Estonian Biocentre, Tartu, Estonia) for technical assistance with sequencing. Peter Chandler (Melkshamn, United Kingdom) and Peter Kerr (Sacramento, USA) are acknowledged for their comments and suggestions to the manuscript.