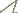(C) 2011 Wei-Guang Lian. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The genus Plistobunus Pocock, 1903 and its type species Plistobunus rapax Pocock, 1903 are redescribed based on the type material deposited in the British Museum of Natural History (BMNH), London. In addition, a new Plistobunus species from Hainan Island is described and illustrated of Plistobunus columnarius sp. n. The new species is diagnosed by having a row of 12 setiferous tubercles on anterior margin of carapace, and the femur of pedipalpus ventrally with 13 setiferous tubercles in male.
taxonomy, Arachnida, harvestmen, Plistobunus, China
The Epedanidae Sørensen, 1886 includes 73 genera and 188 species, and they are endemic to Asia (
This family was removed from the Phalangodidae Simon, 1879 by
The genus Plistobunus of Epedaninae was erected by
In 2009, we explored Hainan Island, China, and collected some laniatorid harvestmen specimens by sieving the leaf in the forest. Among the collected specimens, we recognized a species new to science, and describe it under the name Plistobunus columnarius sp. n. here. Additionally, we loaned the type specimen of Plistobunus rapax from the British Museum of Natural History (BMNH), London, examined, redescribed and illustrated it, and revised the generic characters based on the two species above.
Materials and methodsTaxonomic methods follow outline proposed by
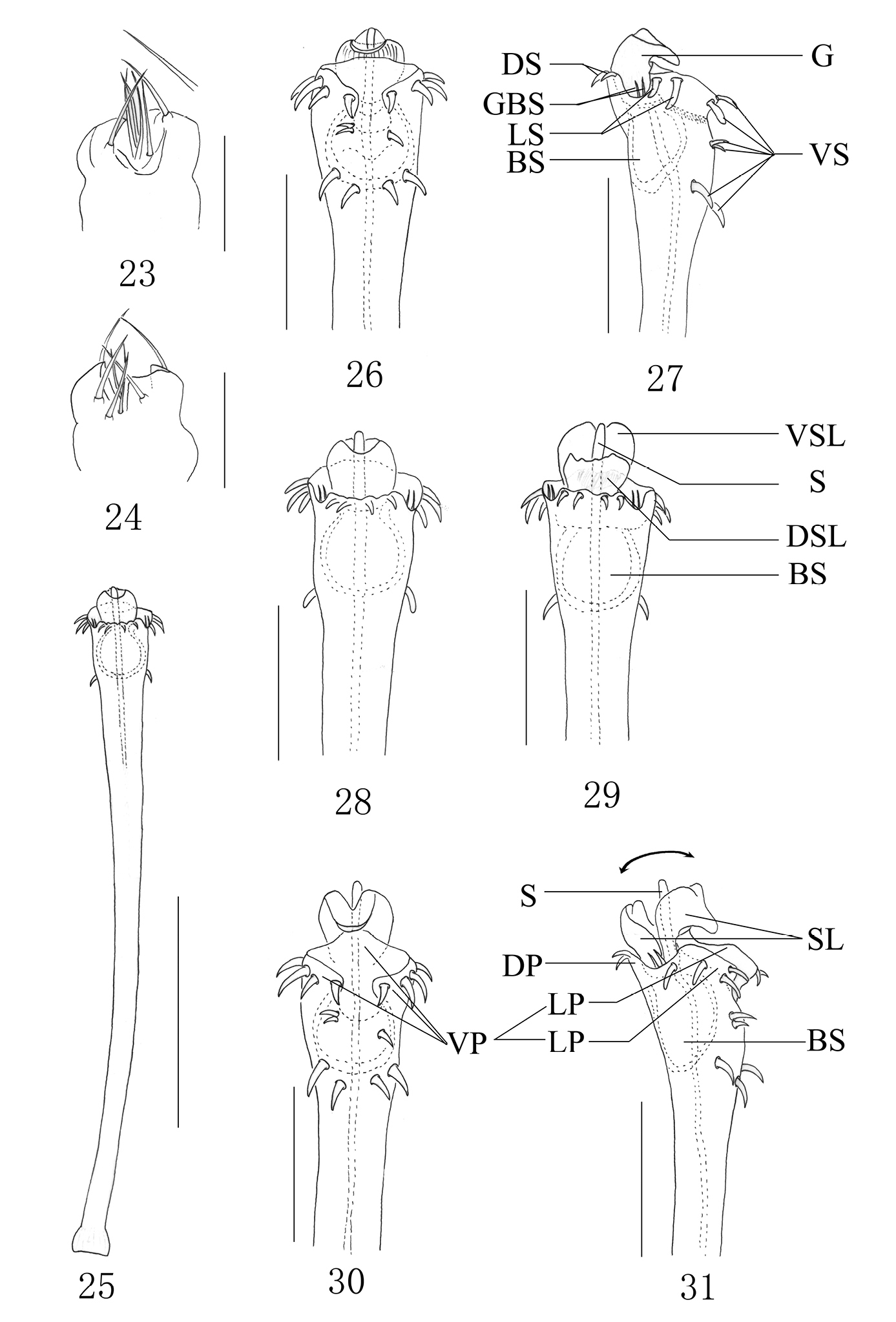
The following abbreviations are used in the text: BS, basal sac; DP, dorsal plate; DS, dorsal setae; DSL, dorsal stylar lobe; G, glans; GBS, basal setae of glans; LP, lateral plate; LS, lateral setae; S, stylus; SL, stylar lobe; VP, ventral plate; VS, ventral setae; VSL, ventral stylar lobe.
Taxonomyhttp://species-id.net/wiki/Plistobunus
Plistobunus rapax Pocock, 1903, by original designation.
Medium-sized epedanines (3.03–3.57) with a long median spine on the ocularium. Carapace with a row of 4–6 setiferous tubercles on each side of the frontal margin. Area II with a pair of spines. Area IV with a median spine. Area IV and all free tergites with a transverse row of hair-tipped tubercles. The proximal segment of chelicera elongated and armed above with numerous tubercles, of which distal one enlarged the largest on the dorsal surface. Pedipalpus elongated; femur of male with 9–13 setiferous tubercles ventrally, a longitudinal row of 7–9 setiferous tubercles dorsally, and with two tubercles on medial side distally; patella of male with two setiferous tubercle disto-medially and three setiferous tubercles ectally. Distitarsus of leg I with two segments. Shaft of penis widened distally. DP conspicuous, VP complex. G protrude sideways beyond the distal penis and near the DP. S is surrounded and protected by SL. BS globular, immovable and entire hidden into truncus.
China (Hongkong, Hainan).
The male genitalia of Plistobunus rapax remains unknown, because the penis was lost (see remarks below). According to study of Plistobunus columnarius sp. n., we tentatively supplemented the male genital structure to the generic characters.
http://species-id.net/wiki/Plistobunus_rapax
Figs 1–6Holotype ♂, in 75% Industrial Methylated Spirit (IMS), labelled as follows: “56. 113, Plistobunus rapax Pocock, Hong Kong” (BMNH 56. 113).
Male holotype (habitus see Fig. 1): Coloration. Body yellowish brown and appendages yellow. Lateral margins and free tergites banded with dark brown. Chelicerae dorsally reticulated with dark brown.
Dorsum. Dorsal scutum nearly trapezoid in shape; widest portion at fourth scutal area; anterior margin of carapace armed with a transverse row of four to five setiferous tubercles. Ocularium long oval, armed with a short median spine. Opisthosomal region of scutum with four areas, first area completely smooth, without a median furrow or line; second area has four hair-tipped tubercles, of which two median ones are longer than others; third area covered with two relatively tubercles; fourth area with a transverse row of seven tubercles, of which the median one is longest. Free tergites with hair-tipped granules arraged in a transverse; each lateral margin of the scutum with a longitudinal row of granules.
Venter. Coxae I–III armed with a row of hair-tipped tubercles, additionally coxa I covered with a row of relatively small hair-tipped tubercles. Coxa III with a row of low humps along front and hind margins. Coxa IV with a row of small hair-tipped granules. Some small hair-tipped granules scttered over surfaces of coxae I–IV. Tracheal stigma clearly visible.
Chelicera (Figs 2–4). Proximal segment fairly strong, distinctly armed with two prominent spines dorsally, numerous hair-tipped tubercles scattered over ventral and lateral surface. Second segment distinctly expanded, armed with a row of four strong hair-tipped bifid tubercles on the prodorsal surface. A few hair-tipped granules scattered over the prodorsal surface. Fingers relatively strong, cutting edges dentate (Fig. 4).
Pedipalpus (Figs 5–6). Relatively long and slender. Trochanter with a single setiferous tubercle dorsally, three ventrally. Femur dorsally with a longitudinal row of seven setiferous tubercles; ventrally with a longitudinal row of nine setiferous tubercles; distally with two setiferous tubercles medially. Patella ectally with three setiferous tubercles, disto-medially with two ones. Tibia with three medial and five ectal setiferous tubercles. Tarsus with four setiferous tubercles on both sides of ventral surface. Tibia with a longitudinal row of three granules ventrally. Tarsal claw long, strongly curved.
Legs. All of legs were destroyed and missing but their trochanters ventrally with two hair-tipped tubercles, their femora armed with a row of setiferous tubercles.
Penis. Lost.
Measurements. Body 3.03 long, scutum 2.64 long, 2.25 with at the widest portion; ocularium 0.65 long, 0.35 wide.
China: Hong Kong.
Plistobunus rapax
is only known from the type specimen. To make matters worse, the type
specimen is in an incomplete state. All legs were missing and the penis
is lost. Roewer at first (
Plistobunus rapax Pocock, 1903, male (holotype) 1 Body, dorsal view 2 Left chelicera, medial view 3 Same, ectal view 4 Distal segment of the left chelicera, frontal view 5 Left pedipalpus, ectal view 6 Same, medial view. Scale bars: 0.5 mm (1–6).
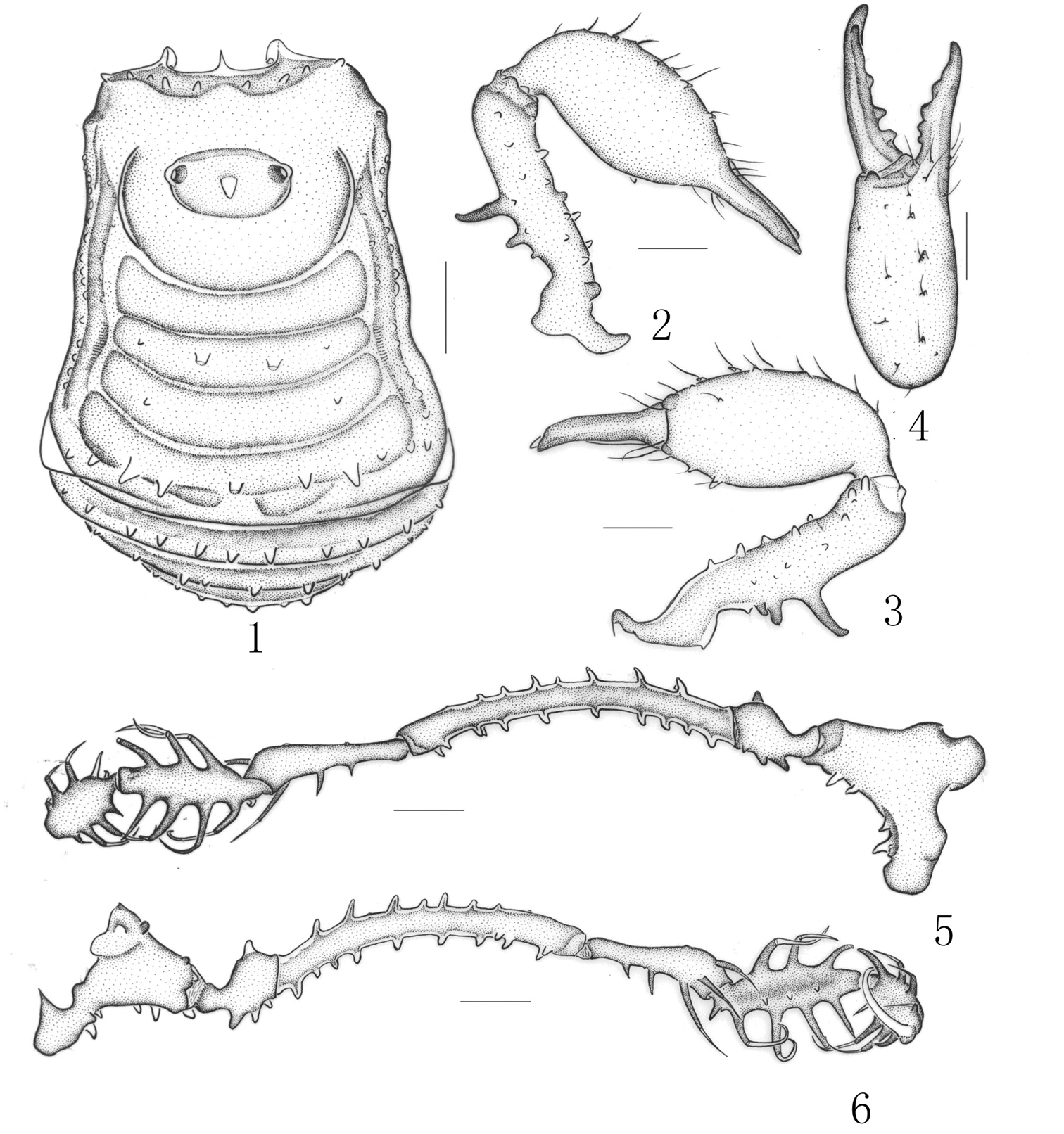
Plistobunus columnarius sp. n. male 7 Body, dorsal view 8 Same, lateral view 9 Left chelicera, medial view 10 Same, ectal view 11 Distal segment of the left chelicera, frontal view 12 Proximal cheliceral segment, dorsal view 13 Left pedipalpus, ectal view 14 Same, medial view. Scale bars: 1 mm (7–14).
urn:lsid:zoobank.org:act:1E2AB18D-7941-495F-A8C5-1406864D6F79
http://species-id.net/wiki/Plistobunus_columnarius
Figs 7–23Holotype male (Opi.11061601), CHINA: Hainan Province, Tunchang County, Xichang Town [N19°26´, E 110°02´], June 16, 2009, C. Zhang leg. (MHBU), paratype 1♀(Opi.11061602), same data as holotype.
The new species is similar to the type species Plistobunus rapax Pocock, 1903, but can be easily distinguished from the latter by: 1) anterior margin of carapace with a row of six setiferous tubercles on either side; 2) the femur of pedipalpus ventrally with 13 setiferous tubercles in male and seven setiferous tubercles in female; 3) the medial surface of cheliceral proximal segment with a huge protuberance at base.
The specific name is derived from the Latin adjectives “columnaris” meaning columnar, refers to shape of the stylus in male penis.
Male (holotype) habitus as in Figs 7–8. Coloration: entire body rusty yellow, with somewhat dark brown to blackish brown patches on the dorsum; median area of carapace with blackish brown reticulations; each side of carapace dark brown; lateral ridges of the scute margined with blackish brown; venter concolorous with dorsum; coxae with dark brown reticulations; free sternites with transverse band of blackish brown; chelicerae rusty yellow, with blackish brown reticulate markings above; pedipalpus dark brown, tibia and tarsus paler, tarsus with dark brown reticulations dorsally; legs brown, trochanters yellow, femur, patella and tibia with blackish brown reticulations, metatarsus and tarsus lighter.
Dorsum. Dorsal scutum trapezoid in shape; widest portion of body at forth scutal area. Carapace with a row of six small setiferous tubercles on each side of front margin. Surface of dorsum almost smooth. Ocularium wide oval, remote from anterior border of scutum, armed with a long erect median spine. Opisthosomal region of scutum with four areas, first area well defined, entire. Second area with two long median spines. Third with a pair of hair-tipped tubercles removed from each other. Fourth with a transverse row of nine tubercles, median tubercle enlarged into a spine. Each lateral margin of the scutum with a longitudinal row of minute hair-tipped granules. Free tergites each with a transverse row of hair-tipped granules spread over its entire width.
Venter. All coxae and genital operculum granulate. Coxae I–III disto-dorsally with two coarse tubercles on anterior and posterior sides respectively. Coxa IV with a reduced one on medio-prolaterally. Coxa I medio-ventrally and prolaterally with transverse rows of hair-tipped tubercles. Coxae II–III medio-ventrally with a transverse row of same tubercles. Coxa III with prolateral and retrolateral rows of small humps. Coxa IV widened, with hair-tipped granules. Free sternites each with a transverse of hair-tipped granules. Tracheal stigma clearly visible.
Chelicera (Figs 9–12). Fairly strong. Proximal segment elongated and with numerous hair-tipped tubercles above; the dorsal surface centrally with six hair-tipped tubercles, of which distal one the largest, three medium-sized tubercles posterior to it, and two smaller ones toward the medial side; the ventral surface and the medial surface with rows of eight hair-tipped tubercles respectively, the medial surface with a huge protuberance at base; the ectal surface with a row of seven tubercles. Second segment considerably widened, medially with five enlarged hair-tipped bifid tubercles and ectally with five reduced ones on prodorsal surface, medially with many small hair-tipped granules on ventral surface, the largest one towards the base of fingers. Fingers relatively strong, inner edges toothed as illustrated (Fig. 11): moveable finger with four teeth, the proximal one square, the middle with two crest teeth, the distal one rectangular; fixed finger with five teeth, the proximal two formed one bifid tooth, the middle with one conical tooth, the distal with two lower than the middle one.
Pedipalpus (Figs 13–14). Coxa dorsally with one proximal and one strong distal bifurcate setiferous tubercles, ventrally with a row of five setiferous tubercles, two enlarged ones additionally at base. Trochanter ventrally with three setiferous tubercles, dorsally with one. Femur elongate, ventrally with 13 setiferous tubercles, dorsally with nine ones of which the distal two inconspicuous, distally on medial side with two setiferous tubercles of which the distal one tipped and the other one conical. Patella very long, widening abruptly distally, with two setiferous tubercle disto-medially and three setiferous tubercles ectally, dorsally with five inconspicuous granules. Tibia with three medial and five ectal setiferous tubercles, with a row of six hair-tipped granules in the ventral surface. Tarsus with four setiferous tubercles on both sides of ventral surface, with three granules in the ventral surface. Tarsal claw nearly as long as tarsus, strongly curved.
Legs. Legs I–II slender and legs III–IV strong. Trochanters I–II each with one hair-tipped tubercles arising distally on the dorsal surface, the ventral surface with three hair-tipped tubercles. Trochanters III-IV smooth dorsally, with inconspicuous granules ventrally. Femur I–II ventrally with a row of 10 or 17 setiferous tubercles respectively (Fig. 15). Femur III ventrally with two rows of 12 and 14 setiferous tubercles respectively. Femur IV ventrally with two rows of many granules. The remaining leg-segments unarmed, smooth, with hairs. Tarsi III–IV with bare double claws, without scopulae. Tarsal formula: 8/17/7/8. Distitarsus of the first and second tarsi with two segments.
Penis (Figs 25–31) slender, its shaft widened distally. The apical structure is divided by lateral incisions into both DP and VP. The VP with complex structures, is separated again by a median cleft, consists of two LPs and the membrane between both sides. The DP simple, distal margin corrugate. G protruding beyond the anterior margin of the dorsal surface. SL somewhat as the shape of tulip ventrally and dorsally, consists of VSL and DSL. The VSL slightly curved toward ventral surface and with a labiate protrusion distally. The DSL petaloid. S smooth, columnar and arising straight from the glans, SL almost entire surrounding the S. BS globular, well developed, immovable and entire sunken into truncus. Setae arranged as follow: 11 VS, four DS, four LS, four GBS.
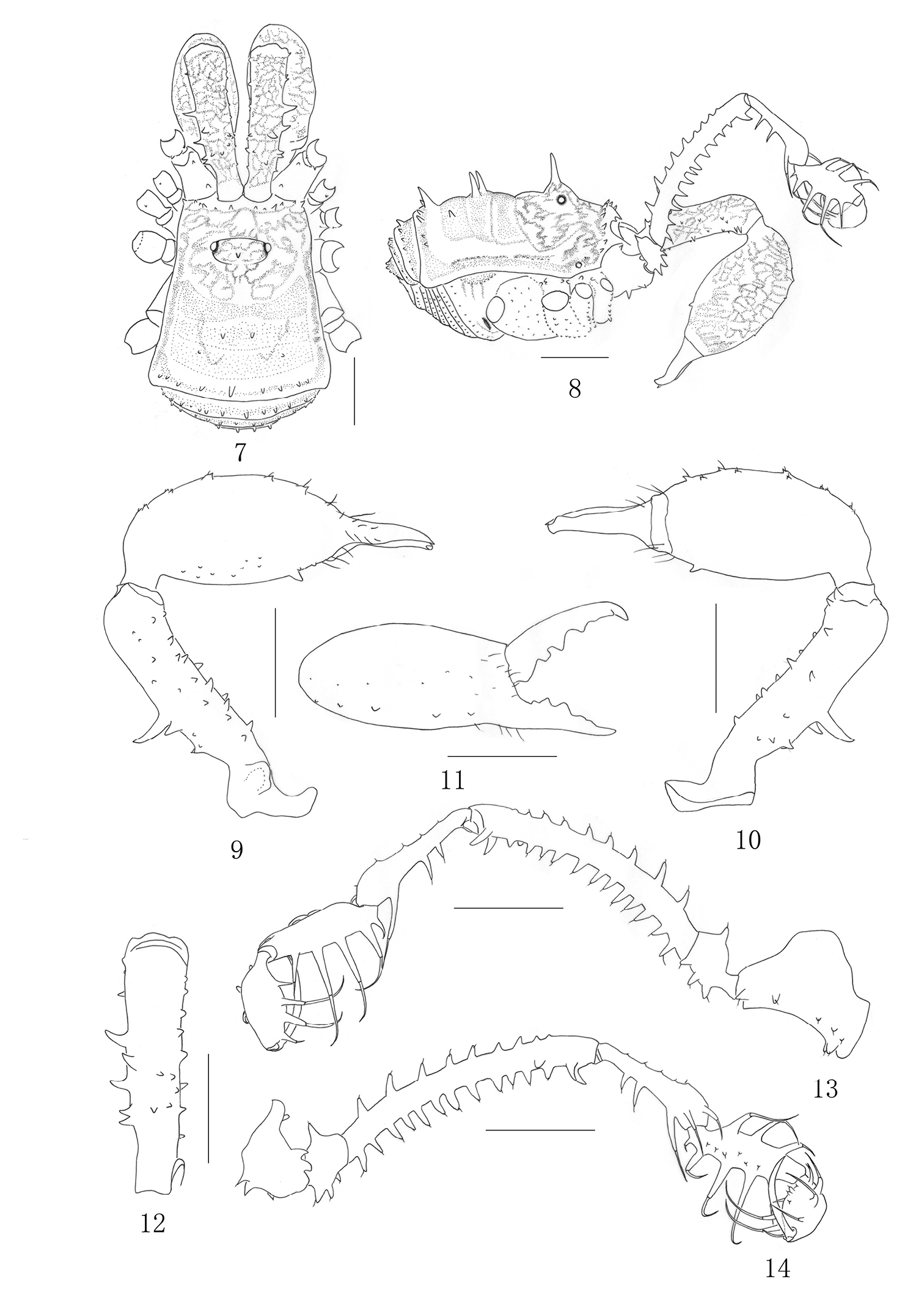
Female (Figs 16–24). In general appearance similar to the male but smaller and with abdomen more rounded posteriorly (Fig. 17). Chelicera (Figs 18–20) smaller and with reduced tubercles, the proximal segment shorter than those of the male, the second segment is not so greatly enlarged as in the male, inner edges of finger toothed as illustrated (Fig. 20). The pedipalpus (Figs 21–22) femur with seven reduced setiferous tubercles ventrally and two conspicuous setiferous tubercles dorsally, distally on medial side without any setiferous tubercle; Patella with two setiferous tubercle disto-medially and one setiferous tubercles disto-ectally. Setiferous tubercles of leg I (Fig. 16), as well as leg II–IV inconspicuous. Tarsal formula: 7/17/7/8. Distitarsus of the first and second tarsi with two segments.
Ovipositor (Figs 23–24). Ventral surface with five setae and dorsal surface with six setae. Tip of each seta pinpoint (Fig. 23).
Measurements. Male holotype (female paratype): body 3.57 (3.37) long, 2.70 (2.65) wide at the widest portion, scutum 3.21 (2.75) long. Ocularium 0.38 (0.33) long, 0.90 (0.68) wide. Pedipalpus claw 0.90 (0.83) long. Penis 1.55 long. Measurements of left pedipalpus and legs as in Table 1.
Collected by leaf litter sieving in the rubber forest.
China: Hainan (Tunchang County).
A certain similarity in ornamentation of the new species may be noted with that of Plistobunus rapax as figured and described by (
In this paper, we tentatively explain the movement of the penis in the new species briefly. The S is mainly exposed by the movement of VSL and DSL in the opposite direction, DSL tends to move dorsally wider than that of VSL. DSL and VSL like the petal, S is similar to the stamen. The expansion of G resembles the blooming flower (Fig. 31).
Plistobunus columnarius sp. n. 15 Right first leg, male, retrolateral view 16 Left first leg, female, retrolateral view 17 Female body, dorsal view 18 Left chelicera, female, medial view 19 Same, ectal view 20 left cheliceral fingers, female, frontal view 21 Left pedipalpus, ectal view 22 Same, medial view. Scale bars: 1 mm (15–19, 21–22); 0.5 mm (20).
Plistobunus columnarius sp. n. 23 Ovipositor, dorsal view 24 Ditto, ventral view 25 Entire penis, dorsal view 26 Penis tip, ventral view 27 Ditto, lateral view 28 Ditto, dorsal view 29 Expanded penis, dorsal view 30 Ditto, ventral view 31 Ditto, lateral view. Abbreviations: BS basal sac DP dorsal plate DS dorsal setae DSL dorsal stylar lobe G glans GBS basal setae of glans LP lateral plate LS lateral setae S stylus SL stylar lobe VP ventral plate VS ventral setae VSL ventral stylar lobe. Scale bars: 0.5 mm (25); 0.20 mm (26–31); 0.25 (23–24).
Pedipalpus and leg measurements of the male holotype (female paratype).
| Trochanter | Femur | Patella | Tibia | Metatarsus | Tarsus | Total | |
|---|---|---|---|---|---|---|---|
| Pedipalpus | 0.63(0.50) | 2.63(2.00) | 1.35(1.13) | 1.18(1.00) | 0.95(0.88) | 6.74(5.51) | |
| Leg I | 0.50(0.45) | 2.10(1.68) | 0.80(0.58) | 1.50(1.25) | 2.48(1.88) | 1.38(1.20) | 8.76(7.04) |
| Leg II | 0.50(0.45) | 2.98(2.45) | 0.83(0.75) | 2.35(1.88) | 3.13(2.43) | 2.60(2.38) | 12.39(10.34) |
| Leg III | 0.50(0.45) | 2.38(2.00) | 0.85(0.63) | 1.63(1.38) | 2.95(2.45) | 1.43(1.25) | 9.74(8.16) |
| Leg IV | 0.50(0.45) | 3.00(2.70) | 0.95(0.75) | 2.00(1.75) | 3.95(3.25) | 1.75(1.45) | 12.15(10.35) |
It is obvious to assign Plistobunus to the Epedanidae
because its penis possesses immovable sac, and protruding glans which
only consists of stylus and stylar lobe. Furthermore, according to
The external morphology of Plistobunus is similar to the genera Euepedanus Roewer, 1915 and Pseudoepedanus Suzuki, 1969. Plistobunus is distinct from Euepedanus and Pseuduoepedanus by the number of tubercles on the femur of pedipalpus ventrally and dorsally. The most significant difference concerns the male genitalia. Euepedanus has conspicuous ventral plate which is absent in Plistobunus; and glans in Euepedanus protrude from the center at the top of penis, while glans in Plistobunus protrude sideways beyond the distal penis and near the dorsal plate; dorsal plate of penis has incision in Pseudonepedanus, while absent incision in Plistobunus; and the shape of glans are different in both Pseudonepedanus and Plistobunus.
The male genitalia of Plistobunus columnarius sp. n. is very similar to some other species of Epedaninae, Acrobuninae, Sarasinicinae and even incertae sedis of Epedanidae, respectively Alloepedanus robustus Suzuki, 1985 (Epedaninae), Zepedanulus ishikawai Suzuki, 1971 (Epedaninae), Zepedanulus watanabei Suzuki, 1981 (Epedaninae), Toccolus chibai Suzuki, 1976 (Epedaninae), Toccolus globitarsis Suzuki, 1969 (Epedaninae), Paracrobunus bimaculatus Suzuki, 1977 (Acrobuninae), Opelytus spinichelis Roewer, 1938 (Sarasinicinae), Pasohnus bispinosus Suzuki, 1976 (Sarasinicinae), Tokunosia tenuipes tenuipes Suzuki, 1964 (incertae sedis), Tokunosia tenuipes taiwana Suzuki, 1977 (incertae sedis).
Moreover, Alloepedanus robustus (
Similar concerns apply to Toccolus globitarsis (
The external morphology of other four species (i. e., Zepedanulus watanabei, Zepedanulus ishikawai, Tokunosia tenuipes tenuipes, Tokunosia tenuipes taiwana and Paracrobunus bimaculatus ) is distinct from Plistobunus columnarius sp. n. except Alloepedanus robustus, Toccolus globitarsis, Toccolus chibai and Pasohnus bispinosus.
They are all absent a long median spine on ocularium and without
conspicuous enlarged tubercles on proximal segment of chelicera. They
are distributed mainly in the Thailand (e. g., Zepedanulus watanabei (
Based on the references cited above, these species are distributed mainly in Southeast Asia. Furthermore, the male genital morphology of most species also shows great similarity to Plistobunus columnarius sp. n., although they belong to different subfamilies presently. For these reasons, we presume they may have a relatively close phylogenetic relationship, not depend only on the external morphological characters which currently support the subfamilies.
We are grateful to Mrs. Janet Beccaloni for the loan of the valuable type specimen. This work was supported by the Ministry of Science and Technology of the People’s Republic of China (MOST Grant No. 2006FY110500), and in part by the Open Project Program of Key Laboratory of Hebei University (09265631D–10) and the Program of Science & Technology Bureau of Baoding City (11ZF071) to Chao Zhang.