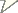(C) 2011 Diego J. Páez-Moscoso. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Combining a molecular phylogeny and morphological data, we discovered a new species of Osornophryne from the Amazonian slope of the Ecuadorian Andes. Morphologically, the new taxon is distinguished from all others species in Osornophryne by having the Toes IV and V longer than Toes I–III, a short and rounded snout with a small rostral papilla, and conical pustules on flanks. The new species previously was confused with Osornophryne guacamayo. A taxonomic key is provided for all known species of Osornophryne.
Andes, Bufonidae, Ecuador, new species, Osornophryne , phylogeny
Osornophryne
is endemic to the Andes of Colombia and Ecuador, where it occurs in
mountain forests and paramo at elevations between 2100 and 4000 m (
Given the complex topography of the Andes and the opportunity for allopatric speciation in areas with similar climatic conditions, it is possible that morphologically similar populations are evolving independently. Herein, we report and describe a new species of Osornophryne, previously confused with Osornophryne guacamayo.
Material and methodsMorphology. We examined alcohol-preserved
specimens from the herpetological collections at Museo de Zoología of
the Pontificia Universidad Católica del Ecuador (QCAZ), Escuela
Politécnica Nacional (EPN), and Museo Ecuatoriano de Ciencias Naturales
(DH-MECN), all based in Quito, Ecuador. Specimens examined are listed
in Appendix I. Fingers are numbered preaxially to postaxially from I–IV
to facilitate comparison with previous literature dealing with anurans;
however, we stress that in an evolutionary perspective anuran fingers
should be numbered from II–V, consistent with the hypothesis that Digit
I was lost in anurans (
Molecular data. Fresh liver samples were preserved
in 90% alcohol, and stored at –80°C. We used salt-precipitation
protocols to extract genomic DNA from ethanol-preserved tissues (M.
Fujita, unpubl. data). To amplify the mitochondrial gene 12S, we used
the primers MVZ59 and tRNA-val, developed by
Phylogenetics. Analyses were conducted using
Maximum Parsimony (MP), Maximum Likelihood (ML), and Bayesian Analyses
(BA). Parsimony analyses were performed in PAUP (
For most of the species and population of Osonophryne and three species of Atelopus, we obtained a total of 800 bp from the mitochondrial marker 12S rRNA (Table 1).
Parameter value estimates for best-fit models for 12S gene generated by
jModeltest 1.1 are TIM2 + I (0.001) + G (0.4700). The only taxon for
which we could not obtain molecular information was Osornophryne talipes, a species that, in Ecuador, is only know from a specimen collected on 02 August 1970 (
urn:lsid:zoobank.org:act:9BFBC919-F698-4FDE-8994-9154FF9E99ED
QCAZ 49774 (Figs 2, 3), an adult male near San Rafael-Chontayacu (1°16'34.61"S, 78°4'21.14"W, 2266 m.a.s.l.), Reserve Ankaku-Zona, Río Challuwayacu, Provincia de Pastaza, Ecuador, by Elicio Tapia on 21 October 2009.
QCAZ 48781, 49777, 45899 obtained with holotype.
DH-MECN 5660 adult female, DH-MECN 5261, 5263, 5258–59, adult male obtained near Reserva Biológica Río Zuñac (1°20'57.87"S, 78°09'31.37"W, 2250 m.a.s.l.), Parroquia Río Negro, Cantón Baños, Provincia de Tungurahua, Ecuador, by MYM, M. Urgiles y A. Laguna on 17 May 2008; QCAZ 39769 also obtained near Reserva Biológica Río Zuñac, by DJP, A. Narváez, and J. P. Reyes-Puig on 21 January 2009.
Summary of specimens sequenced of Osornophryne and Atelopus for the gen 12S and GenBank accession numbers.
| Species and museum no. | Locality | Latitude and Longitude | GenBank No. |
|---|---|---|---|
| Atelopus sp. | |||
| QCAZ 34540 | Limón | JF907488 | |
| QCAZ 41326 | Zuruni | JF907486 | |
| QCAZ 38427 | Las Tres Cruces | JF907487 | |
| Osornophryne angel | |||
| QCAZ 40039 | Páramo del Ángel | 0°41'15"N, 77°52'46"W | JF907459 |
| QCAZ 40036 | Páramo del Ángel | 0°41'15"N, 77°52'46"W | JF907458 |
| QCAZ 40040 | Páramo del Ángel | 0°41'15"N, 77°52'46"W | JF907493 |
| Osornophryne antisana | |||
| QCAZ 40172 | Páramo de Oyacachi | 0°10'34"S, 78°0.6'50"W | JF907453 |
| QCAZ 40173 | Páramo de Oyacachi | 0°10'34"S, 78°0.6'50"W | JF907485 |
| QCAZ 40174 | Páramo de Oyacachi | 0°10'34"S, 78°0.6'50"W | JF907484 |
| DH-MECN 838 | Salvefaccha | 0°13'54"S, 78°0.1'1"W | JF907490 |
| DH-MECN 811 | Salvefaccha | 0°13'54"S, 78°0.1'1"W | JF907489 |
| QCAZ 46204 | Llanganates | 1°15'57"S, 78°26'45"W | JF907450 |
| QCAZ 46212 | Llanganates | 1°15'57"S, 78°26'45"W | JF907449 |
| QCAZ 48035 | Llanganates | 1°15'57"S, 78°26'45"W | JF907448 |
| QCAZ 48223 | Llanganates | 1°15'57"S, 78°26'45"W | JF907445 |
| QCAZ 48220 | Llanganates | 1°15'57"S, 78°26'45"W | JF907446 |
| QCAZ 48034 | Llanganates | 1°15'57"S, 78°26'45"W | JF907447 |
| Osornophryne bufoniformis | |||
| QCAZ 40123 | Santa Bárbara | 0°38'29"N, 77°31'18.5"W | JF907431 |
| QCAZ 40121 | Santa Bárbara | 0°38'29"N, 77°31'18.5"W | JF907432 |
| QCAZ 40003 | Santa Bárbara | 0°38'29.5"N, 77°31'18.5"W | JF907430 |
| QCAZ 45082 | Huaca | 0°40'15"N, 77°46'11"W | JF907460 |
| QCAZ 45083 | Huaca | 0°40'15"N, 77°46'11"W | JF907461 |
| QCAZ 45084 | Huaca | 0°40'15"N, 77°46'11"W | JF907462 |
| DH-MECN 1815 | Playón de San Francisco | 1°37'43"N, 77°54'35"W | JF907455 |
| DH-MECN 1806 | Playón de San Francisco | 1°37'43"N, 77°54'35"W | JF907457 |
| DH-MECN 1807 | Playón de San Francisco | 1°37'43"N, 77°54'35"W | JF907456 |
| DH-MECN 1808 | Playón de San Francisco | 1°37'42.6"N, 77°54'35"W | JF907454 |
| Osornophryne cf. bufoniformis | |||
| QCAZ 9316 | Vía Tulcán-Maldonado | 0°47'31"N, 77°54'25"W | AF375498 |
| Osornophryne cofanorum | |||
| DH-MECN 1591 | La Bonita | 00°29'19"N, 77°35'11"W | JF907440 |
| DH-MECN 1579 | La Bonita | 00°29'19"N, 77°35'11"W | JF907439 |
| DH-MECN 1629 | La Bonita | 00°29'19"N, 77°35'11"W | JF907441 |
| Osornophryne guacamayo | |||
| QCAZ 40138 | Poblado de Oyacachi | 0°10'34"S, , 78°0'50"W | JF907463 |
| QCAZ 40143 | Poblado de Oyacachi | 0°10'34"S, , 78°0'50"W | JF907464 |
| QCAZ 40147 | Poblado de Oyacachi | 0°10'34"S, , 78°0'50"W | JF907465 |
| QCAZ 43370 | Volcán Sumaco | 0°34'11"S, 77°35'39"W | JF907474 |
| QCAZ 4576 | Volcán Sumaco | 0°34'11"S, 77°35'39"W | JF907491 |
| QCAZ 40106 | Cordillera de los Guacamayos | 0°37'26.5"S, 77°50'27"W | JF907468 |
| QCAZ 40102 | Cordillera de los Guacamayos | 0°37'26.5"S, 77°50'27"W | JF907492 |
| QCAZ 43554 | Cordillera de los Guacamayos | 0°37'26.5"S, 77°50'27"W | JF907467 |
| QCAZ 17295 | Volcán Reventador | 0°6'43"S, 77°40'44"W | JF907471 |
| QCAZ 17294 | Volcán Reventador | 0°6'43"S, 77°40'44"W | JF907473 |
| QCAZ 17293 | Volcán Reventador | 0°6'43"S, 77°40'44"W | JF907472 |
| QCAZ 12240 | Río Angel | 0°37'26.5"S, 77°50'27"W | JF907469 |
| QCAZ 12241 | Río Angel | 0°37'26.5"S, 77°50'27"W | JF907470 |
| QCAZ 2735 | Jondachi (Río Angel) | 0°37'26.5"S, 77°50'27"W | JF907466 |
| QCAZ 46662 | Santa Bárbara | 0°33'51"N, 77°31'38"W | JF907475 |
| Osornophryne occidentalis | |||
| QCAZ 40028 | Chilma | 0°51'50"N, 78°4'1"W | JF907436 |
| QCAZ 43652 | Cuellaje | 0°27'30"N, 78°32'43"W | JF907444 |
| QCAZ 43498 | Cuellaje | 0°27'30"N, 78°32'43"W | JF907443 |
| QCAZ 43653 | Cuellaje | 0°27'30"N, 78°32'43"W | JF907442 |
| Osornophryne puruanta | |||
| QCAZ 13271 | Laguna de San Marcos | 0°7'36"N, 78°15'22"W | JF907451 |
| QCAZ 13320 | Laguna de San Marcos | 0°7'36"N, 78°15'22"W | JF907452 |
| QCAZ 11471 | Laguna de Puruanta | 00°12’ N, 77°57’ W | AF375499.1 |
| Osornophryne simpsoni | |||
| QCAZ 49779 | Llanganates | 1°16'35"S, 78°4'21"W | JF907482 |
| QCAZ 49777 | Llanganates | 1°16'35"S, 78°4'21"W | JF907477 |
| QCAZ 49781 | Llanganates | 1°16'35"S, 78°4'21"W | JF907483 |
| QCAZ 49776 | Llanganates | 1°16'35"S, 78°4'21"W | JF907476 |
| QCAZ 39774 | Río Zuñac | 1°20'58"S, 78°09'31"W | JF907478 |
| DH-MECN 5262 | Río Zuñac | 1°20'58"S, 78°09'31"W | JF907480 |
| QCAZ 39778 | Rio Zuñac | 1°20'58"S, 78°09'31"W | JF907479 |
| QCAZ 39773 | Rio Zuñac | 1°20'58"S, 78°09'31"W | JF907481 |
| Osornophryne sumacoensis | |||
| QCAZ 41243 | Volcán Sumaco | 0°34'11"S, 77°35'39"W | JF907434 |
| QCAZ 41250 | Volcán Sumaco | 0°34'11"S, 77°35'39"W | JF907433 |
| QCAZ 41246 | Volcán Sumaco | 0°34'11"S, 77°35'39"W | JF907437 |
| QCAZ 41249 | Volcán Sumaco | 0°34'11"S, 77°35'39"W | JF907438 |
| QCAZ 43379 | Volcán Sumaco | 0°34'11"S, 77°35'39"W | JF907435 |
Osornophryne simpsoni differs from all other species in Osornophryne (except for Osornophryne guacamayo and Osornophryne cofanorum) by having Toes IV and V longer than Toes I–III (Fig. 4). Morphologically, Osornophryne simpsoni is most similar to Osornophryne guacamayo; both species have Toes IV and V longer than Toes I–III, pustular dorsal skin, and dark brown dorsal coloration. However, Osornophryne simpsoni lacks the conspicuos proboscis present in Osornophryne guacamayo; males of Osornophryne simpsoni can be distinguished from males of Osornophryne guacamayo by having ventral skin with conical pustules (non-conic pustules in Osornophryne guacamayo), and light brown to orange conical pustules on the flanks (dark brown to black non-conical pustules in Osornophryne guacamayo); the venter of female Osornophryne guacamayo is mostly whitish to yellowish with brown marks, whereas that of female Osornophryne simpsoni is orange-brown. Osornophryne cofanorum differs from Osornophryne simpsoni by having its vertebrae and urostlyle coosified with the overlying skin (not co-osified in Osornophryne simpsoni) and vertebral neural spines that are visible dorsally (not visible in Osornophryne simpsoni); also, males of Osornophryne cofanorum have yellow pustules on the tip of the snout, upper eyelid, limbs, and dorsolateral pustular clusters (absent in Osornophryne simpsoni). Finally, Osornophryne simpsoni is distinguished from its sister species, Osornophryne occidentalis, by having a rounded snout in lateral view (protruding in Osornophryne occidentalis), brown dorsum with some lighter patches (dark brown dorsum with dark ochre-brown warts in Osornophryne occidentalis), orange-brown venter (white in Osornophryne occidetalis), and by inhabiting in the Amazonian slopes of the Andes (Osornophryne occidentalis is found on the Pacific slopes of the Andes).
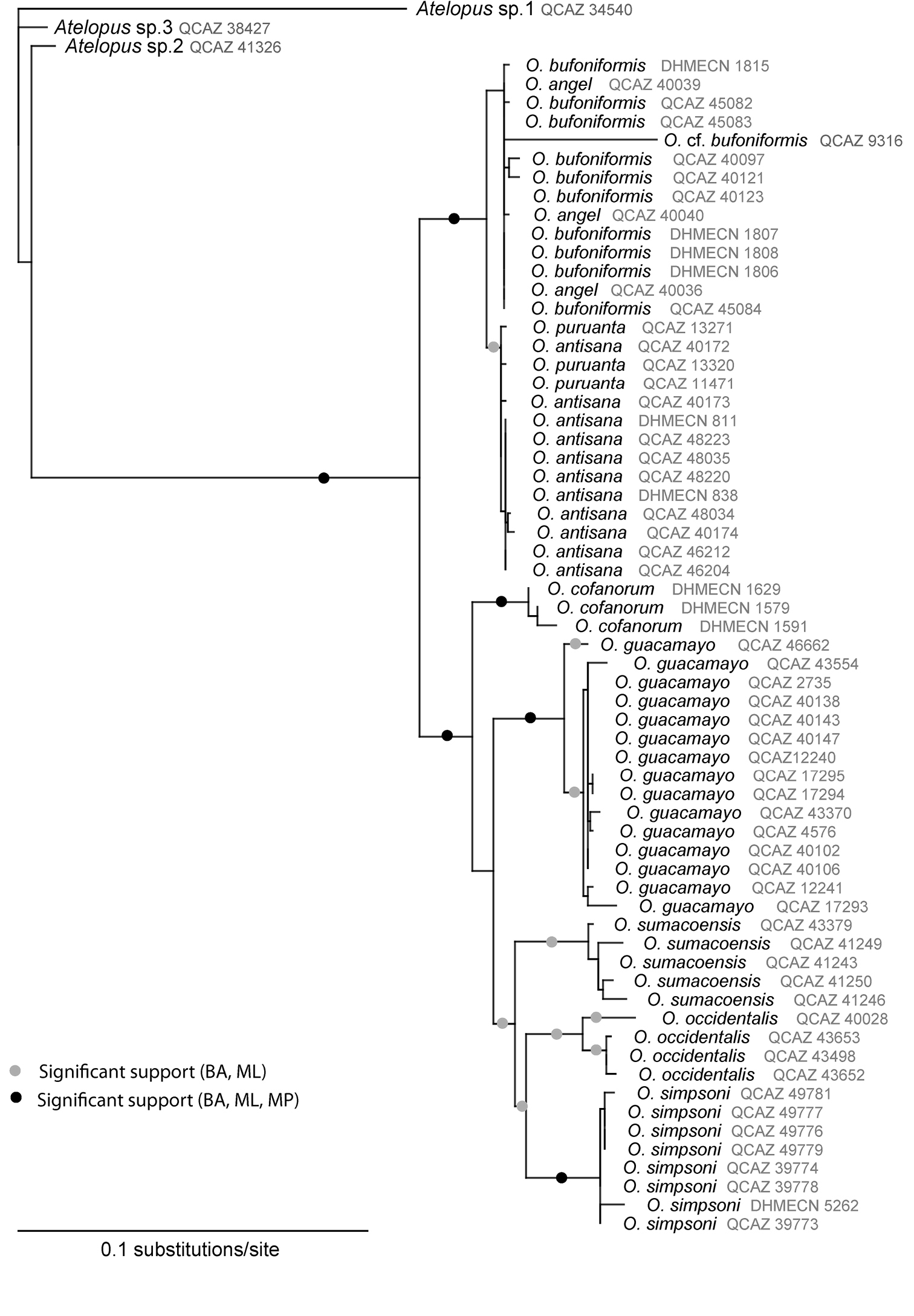
Ten adult males and one adult female. Females of medium size (SVL = 33.0 mm, n = 1); males small (SVL = 17.6–26.1 mm; mean = 21.1 ±2.40, n = 10; Table 2). Head length 77.2–95.1% head width; male head width 34.9–40.8% SVL; female head width 37.3% SVL; width of head greater at level of posterior margin of mouth; snout short, rounded, with rostral papilla in dorsal and lateral views; nostrils slightly swollen; each nostril oblique, oval, directed laterally; internarial area concave in males and slightly concave in female; interorbital region with skin co-osified with underlying bone, which has few low tubercles; occipital region mostly flat, but with few bony tubercles and cranial crests in males and females; upper eyelids finely tuberculate in females, with conical tubercles in males; interorbital region wider than the upper eyelid (upper eyelid 73.0–87.5% of interorbital distance in males, n = 9; 64.4% in female); outer edge of the eyelid delineated by a continuous row of warts, which are more conical in males than in female; canthus rostralis straight; loreal region slightly concave, with small warts in males and female; pale brown lips; eyes with oval horizontally pupil; infraorbital and postorbital regions with some prominent tubercles of variable size in males and females. Skin of dorsum highly tuberculate, with discontinuous row of conical tubercles starting at level of posterolateral edge of cranium and ending at level of sacrum in males and females; in males, ventral skin with several small pustules and few conical tubercles on gular region and toward the flanks, pustules much denser on chest and abdomen and less conical; in females, ventral skin smooth, with small, non-conical isolated pustules, pustules more numerous on abdomen.
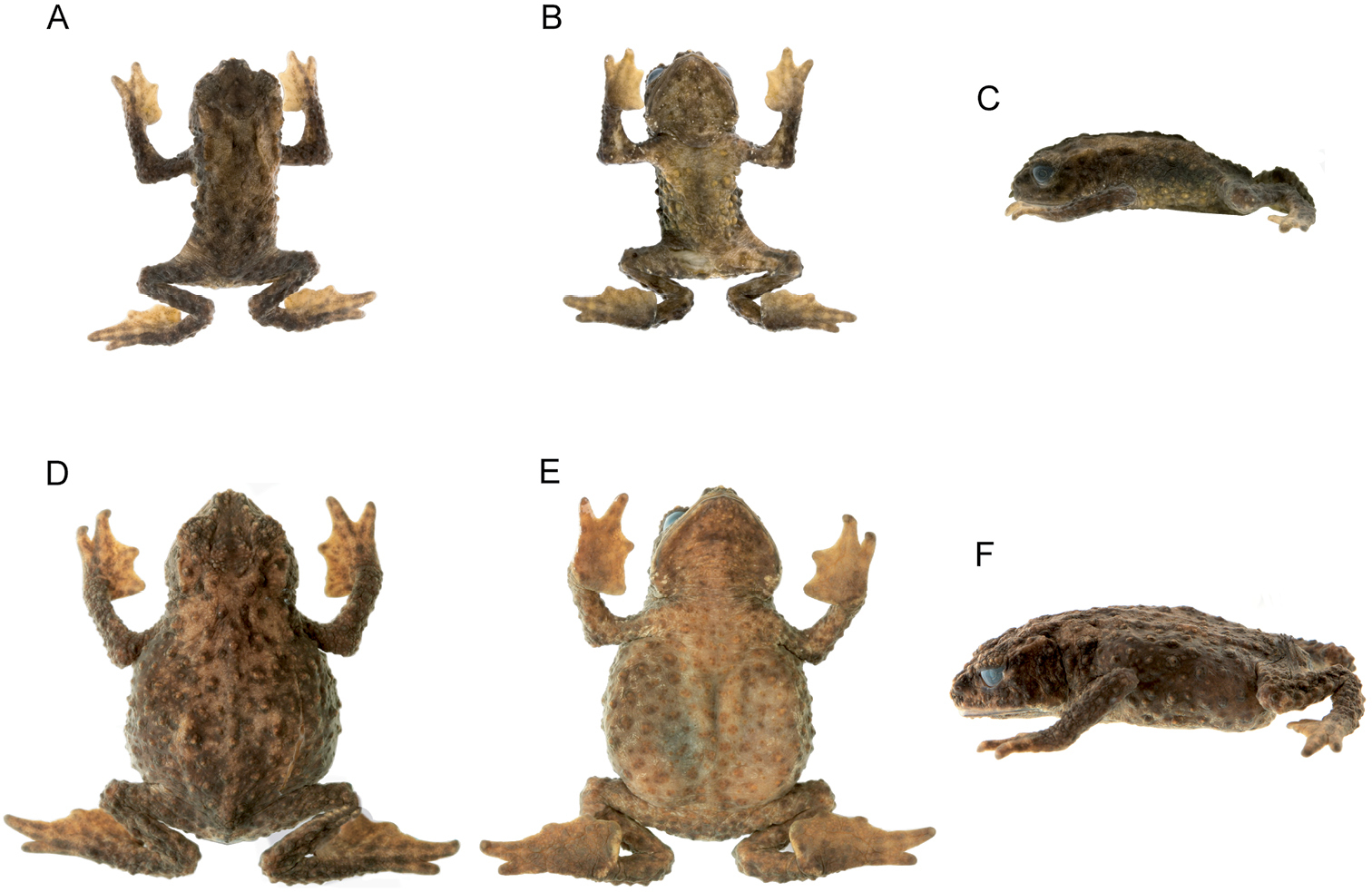
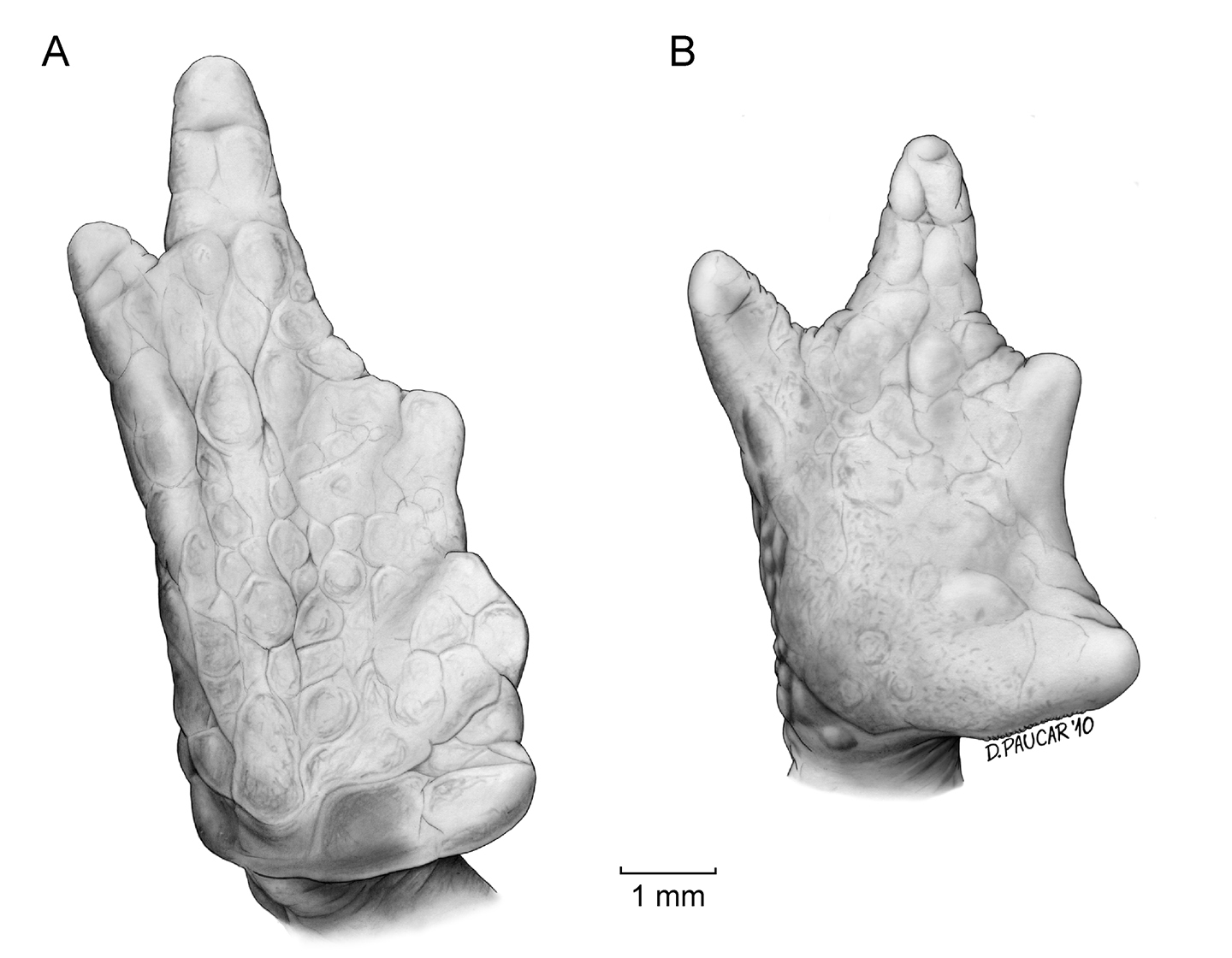
Forelimb long, slender, finely granular, with several larger tubercles extending along inner and outer edges of fingers in males; in females, tubercles smaller than in males. Hand of moderate length, representing 25.0–30.4% (n = 10) of SVL in males and 28.5% in female; extensive webbing between fingers (Fig. 4); lengths of fingers in order of increasing length : I < II < IV < III; palms with numerous tubercles; subarticular tubercles not distinguishable; palmar tubercle rounded, thenar tubercle almost undistinguishable.
Hind limbs long and slender; well-defined pustules present on inner and outer edges of fingers in males, females with less pronounced pustules than those in males; tibia and foot, respectively, 32.8–36.9% and 34.9–42.5% of male SVL, and 33.9% and 41.9% of female SVL; webbing between Toes I–III more extensive that webbing between Toes IV–V (Fig. 4); lengths of Toes: I < II < III < V < IV; Toe V much longer than Toe III, soles with numerous tubercles; subarticular tubercles indistinguishable; inner metatarsal tubercle oval. Choanae slightly rounded; adult males lacking vocal sacs; vocal slits absent; nuptial pads on proximal surfaces of Toes I and II, not pigmented; cloacal opening medial to thighs.
Maximum likelihood phylogeny of the species in Osornophryne inferred from the mitochondrial gene 12S (lnL = –1384, 3649).
Osornophryne simpsoni sp. n. in life (male holotype, QCAZ 49774).
Osornophryne simpsoni sp. n. in alcohol. A–C Dorsal, ventral and lateral views of holotype, adult male, QCAZ 49774, SVL 20.1 mm D–F Dorsal, ventral and lateral views of adult female, DH-MECN 5260, SVL 33.0 mm.
Foot (A) and hand (B) of Osornophryne simpsoni sp. n. (holotype, adult male, QCAZ 49774).
Dorsum, head, forearms, and hind limbs brown to dark brown, with some orange patches; tubercles on upper eyelid, proboscis, and flanks pale yellow. Throat pale yellow; venter cream with brown tubercles.
Dorsum, head, forearms, and hind limbs dark brown to light brown with some lighter patches; tubercles on upper eyelid, proboscis and flanks orange to yellow. Throat cream yellow, with small dark marks; venter orange-brown.
The following osteological description of Osornophryne simpsoni is based on a cleared-and-double stained adult male (QCAZ 45899, SVL = 19.5 mm). The osteological description of females was not possible because only one female is known.
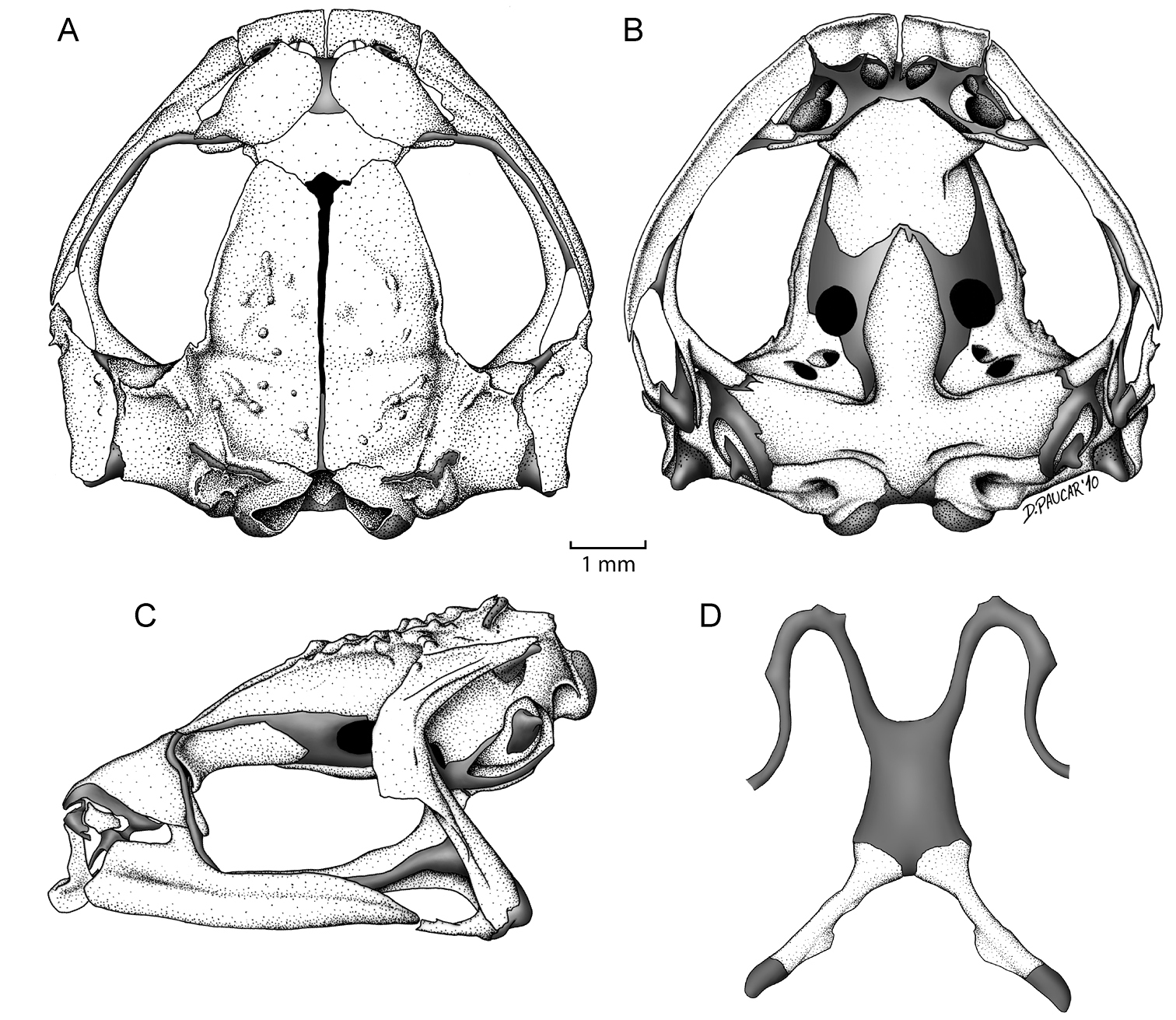
Cranium. Shape and proportions. The skull is widest posterior to the orbit at the level of the articulation of the maxilla with the quadratojugal. The braincase is broad; at the level of the midorbit, the width of the braincase is about 41.2% of the greatest width of the skull and 26.2% of the medial skull length.
Neurocranium. The neurocranium is formed by five bones—the sphenethmoid, and the paired prootics and exoccipitals. Anteriorly, the neurocranium is completely ossified. A minute septomaxilla is embedded in the anterior nasal capsule cartilage. In dorsal aspect, the cartilaginous planum antorbitale has a perpendicular orientation in relation to the longitudinal axis of the skull. In lateral and ventral views, a broad cartilaginous separation between bony sphenethmoid and prootic is evident. The frontoparietal fontanelle is partially exposed medially between the frontoparietals. Distally, the otic capsules are cartilaginous. Medially, the exoccipitals are slightly separated from one another. The dorsal surface of each prootic is smooth. The epiotic eminences are prominent.
Auditory apparatus. The stapes and tympanic annulus are absent. The operculum is oval and cartilaginous.
Dermal investing bones. Dorsal investing bones are well developed. The nasals are separated from one another and cover most of the nasal capsules dorsally. The maxillary process of the nasal overlaps the pars fascialis of the maxilla to form a bony anterior margin of the orbit. The frontoparietals are well developed and have a narrow separation between one another along its longitudinal axis. The posteriormedial margin of each frontoparietal contacts the exoccipital, but is not fused to it. Posterolateraly, each frontoparietal bears a bony extension that reaches the epiotic eminence. Each frontoparietal has a lamina perpendicularis that is narrow anteriorly and greatly expanded posteriorly (Fig. 5C). The dorsal surface of each frontoparietal bears small, bony tubercles that are visible externally; the tubercles seem to be co-ossified with the overlying skin.
Ventral investing and palatal bones. The parasphenoid has the shape of an inverted T. The broad cultriform process extends anteriorly to about the mid-level of the orbit, where it is narrowly separated from the posterior border of the sphenethmoid. The cultriform process reaches its maximum width at a level that is coincident with the posterior margin of the optic fenestra. The parasphenoid alae are robust, investing the cartilaginous floor of the otic capsule anterior to the exoccipitals; the length of each ala is 61.8% the length of the cultriform process. A broadly acuminate posteromedial process of the parasphenoid terminates just anterior to the margin of the foramen magnum. The vomers are small, arcuate, broadly separated bones that support the medial margins of the choanae; the bones are unornamented, edentate, and lack dentigerous processe; the prechoanal ramus of the vomer is especially short. The neopalatine is short and narrow; medially, it reaches the anterolateral margin of the sphenethmoid; medially, the neopalatine does not contact the maxilla (Fig. 5B).
Maxillary arcade. The premaxillae and maxillae lack teeth. The arcade is complete and has a tenuous articulation with the short quadratojugals. The pars palatinae of the premaxillae are broad. The premaxilla bears two palatine processes, a narrow longer medial and a broad lateral process. There is a simple, juxtaposed articulation between the anterior end of the maxilla and the premaxilla. The pars facialis of the maxilla is well-developed anteriorly, covering the the posterior region of the olfactory capsule; also, the pars facialis has a well-developed preorbital process, which covers most of the planum antorbitale (Fig. 5B, C).
Suspensory apparatus. The tridiate pterygoid bears a slightly curved anterior ramus that is orientated anterolaterally toward the maxilla, with which it articulates. The pterygoid is in close proximity to the maxilla and the narrow space between them is filled by the pterygoid cartilage. The medial and posterior rami of the pterygoid are about equal in length; however, the medial ramus is more robust than the posterior. The lateral end of the medial ramus overlaps the lateral edge of the prootic. The squamosal has the shape of an inverted L; the zygomatic ramus is almost absent, whereas the otic ramus is long and almost reaches the posterior end of the skull. The otic ramus overlaps the lateral margin of the crista parotica slightly. The ventral ramus invests the lateral surface of the palatoquadrate, and articulates with the quadratojugal (Fig. 5A, C); along its anterior margin, the ventral ramus has a conspicuos flange, which extends along the upper border of the otic ramus (Fig. 5C).
Hyoid.The width of the cartilaginous hyoid corpus is narrower than its medial length (width 63.1% of length). The anterolateral and posterolateral processes of the hyoid are absent. The bony posteromedial processes are slightly expanded proximally; each process has a bony flange along the posteromedial margin. The hypoglossal sinus is broadly U-shaped. The hyalia are simple and lack any processes (Fig. 5D).
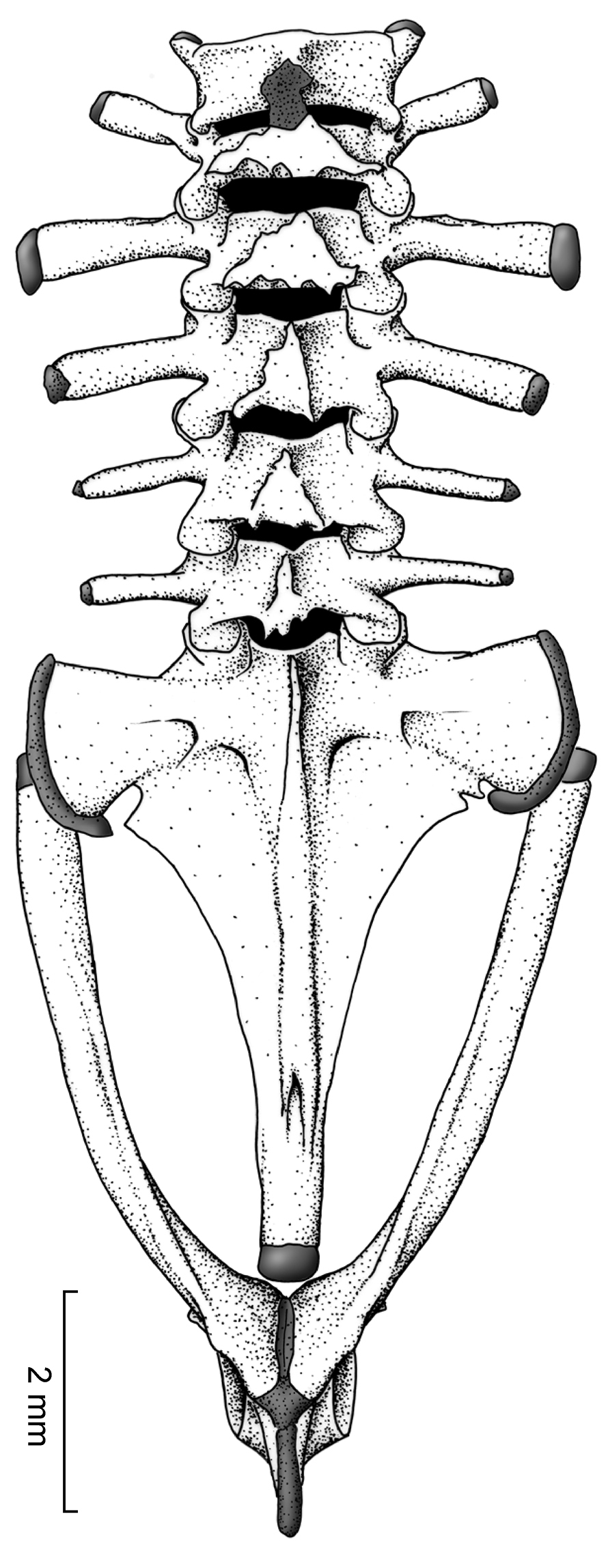
Postcranium. Vertebral column. There are six prepresacral vertebrae. Presacrals I and II are not fused and are notably shorter than Presacrals III–VI. The vertebral profile in decreasing order of overall width of bony parts is: Sacrum > III > IV > V > VI > II > I. Presacral I, or the atlas, lacks transverse processes. All presacrals are non-imbricate. The transverse processes of Presacral II have a anterolateral orientation, Presacrals III–V have a slightly posterolateral orientation, and Presacral VI is approximately perpendicular to the longitudinal axis of the body. The bony sacral diapophyses are broadly expanded; posteriorly, the sacrum is broadly fused with the urostyle, which is greatly expanded laterally. The urostyle bears a well-developed dorsal crest throughout most of its length (Fig. 6).
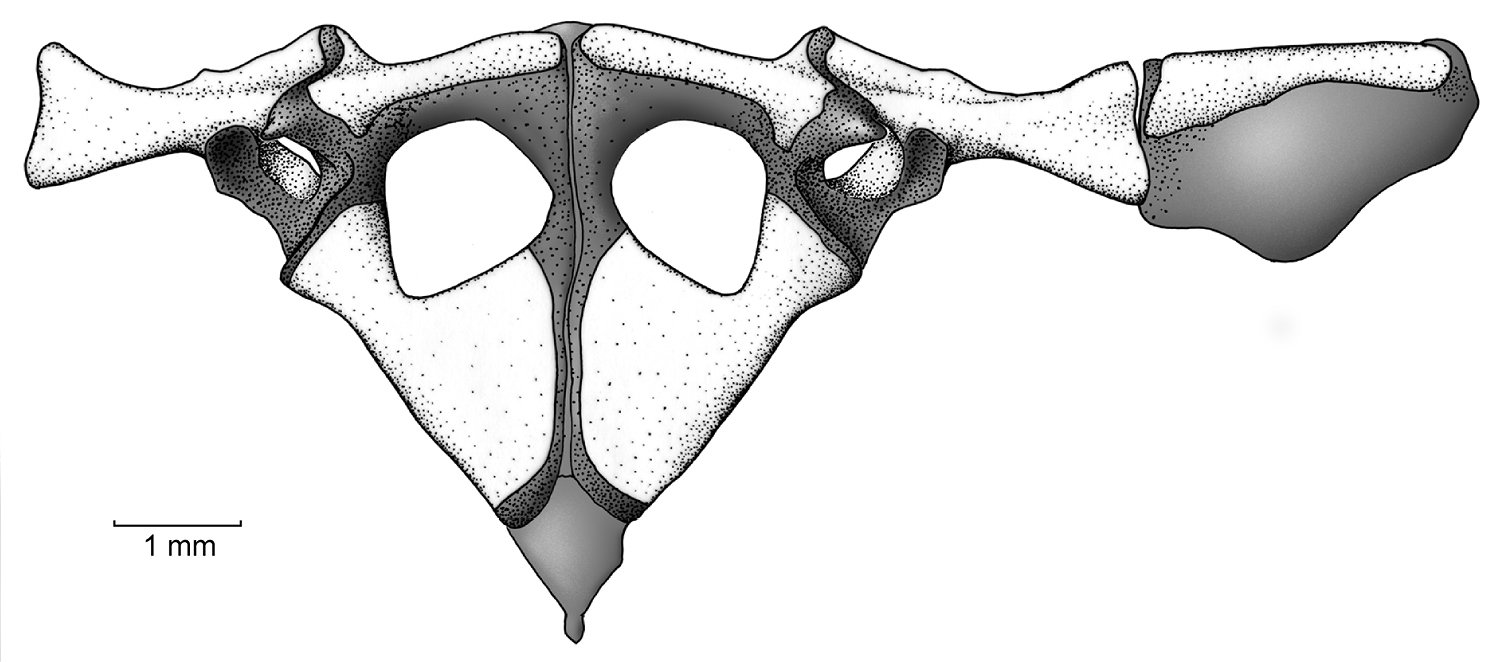
Pectoral girdle. The clavicles have a slight orientation, with the medial tips distinctly separated from one another and located at about the same level of the anterolateral end of the clavicle, which articulates with the pars acromialis of the scapula (Fig. 7). The coracoid is notably stout, with the sternal end having a moderate expansion and the sternal end being heavily expanded (sternal end 45% of glenoid end); the inner edge of coracoid has an angle of about 45˚, wheras the external edge is straight (no angle). The pectoral fenestra is has a tringular shape, in which the base is anteriorly convex. The scapula is moderately long with a prominent pars acromialis that is separated from the pars glenoidalis; the leading and posterior edges of the scapula are slightly concave. The suprascapula is mostly cartilaginous, but it is mineralized at both ends, with the ossified cleithrum apparent as a slender bone along the leading edge of the suprascapular blade and with a proximal end that is wider than its distal end. The sternum is small and completely cartilaginous; it contacts the epicoracoid cartilage, which is extensive, and the posterior margin of the coracoid. The omosternum is absent.
Pelvic girdle.The long, slightly concave, and slender ilial shafts bear small dorsal crests, which extend from the anterior third to the posterior end of the shafts (Fig. 6). The ilial prominence is broad and low; the pubes is highly mineralized.
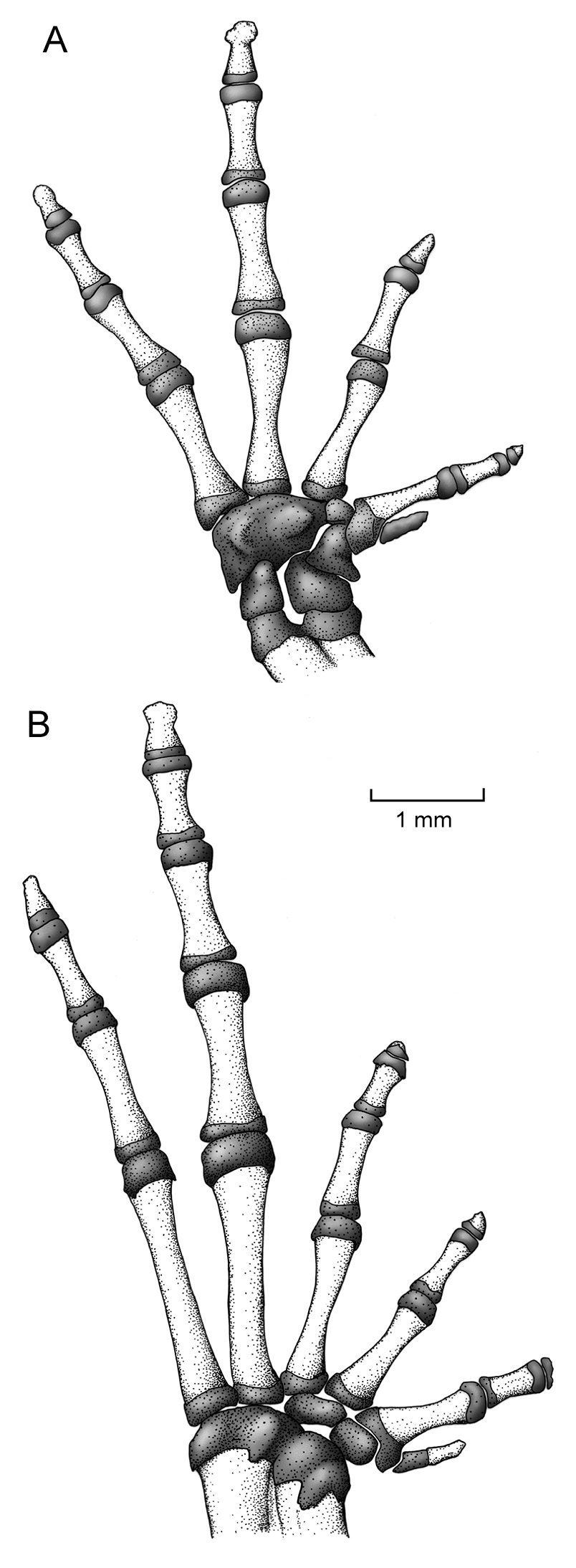
Manus and pes. The phalangeal formulae for the hand and foot are standard—i.e., 2-2-3-3 and 2-2-3-4-3, respectively; however, the distal phalange of Finger I, Toe I, and Toe III are greatly reduced and formed mostly by cartilage (Fig. 8). Relative length of fingers, in increasing order, is: I-II-IV-III, and of the foot is: I-II-III-V-IV. The carpus is composed of a radiale, ulnare, Element Y, Carpal 2, and a large postaxial assumed to represent a fusion of Carpals 3–5. Element Y is about 3 times the size of Carpal 2, and the prepollex is an elongated cartilage. The terminal phalanges are acuminate, except Finger III that is slightly T-shaped. The tarsus is composed of two tarsal elements, presumably Tarsal 1 and Tarsal 2 + 3. The prehallux is presented by a proximal mineralized cartilage element associated with a small bony element.
The specific name simpsoni is a patronym for Dr. Nigel Simpson in recognition for his continual efforts in protecting the Andean cloud forests of Ecuador. Dr. Simpson is a collaborator of two of the most important conservation NGOs in Ecuador, EcoMinga Foundation (www.ecominga.net) and Jocotoco Foundation (www.fjocotoco.org). As the common name of the species, we suggest “Simpson’s Plumb Toad.” In Spanish, we suggest the name “Osornosapo de Simpson.”
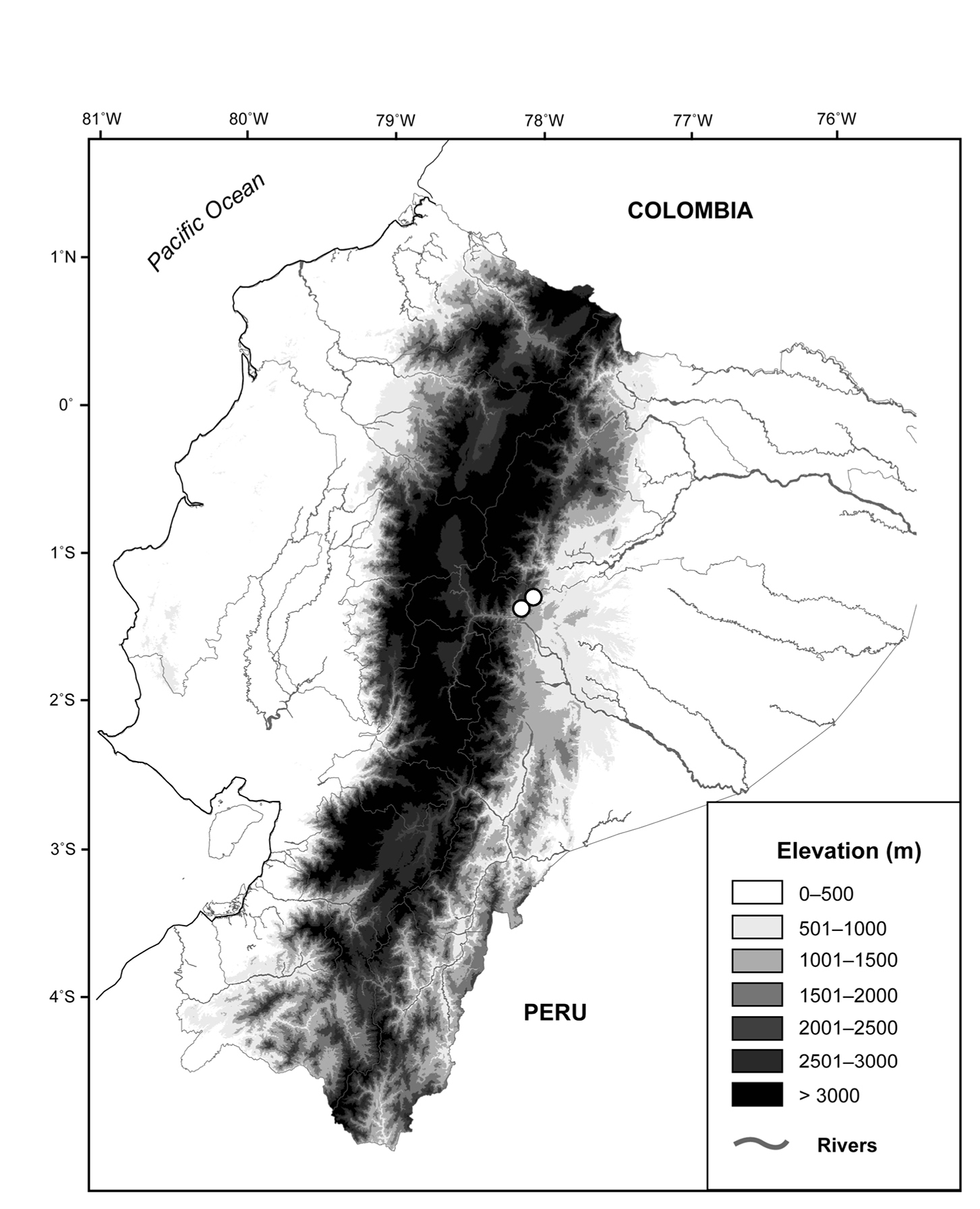
Osornoprhyne simpsoni is only known from the type locality and surrounding areas, Reserva Zuñac (1°20'58"S, 78°09'31"W) and Reserve Ankaku-Zona (1°16'35"S, 78°4'21"W; Fig. 9). These localities are included in the Bosque de Niebla Montano (Montane Cloud Forest) according to the classification proposed by Valencia et al.(1999). Vegetation is dominated by Clusia spp. trees. All individuals of Osornophryne simpsoni have been found on leaves of bromeliads and ferns during the night. Simpatric anurans include Pristimantis altamis, Pristimantis bicantus, Pristimantis imcomptus and Pristimantis galdi. Following the IUCN (2001) criteria, we consider Osornophryne simpsoni as Data Deficient; however, it is likely that Osornophryne simpsoni has a restricted distribution, as observed in other Osornophryne species.
It has become increasingly evident that lineage
independence is not always accompanied by morphological change when
ecological conditions remain similar (
Skull and hyoid of Osornophryne simpsoni sp. n., adult male, QCAZ 45899. A Dorsal view of skull B Ventral view of skull C Lateral view of skull D Ventral view of hyoid.
Vertebral column of Osornophryne simpsoni sp. n. in dorsal view; adult male, QCAZ 45899.
Pectoral girdle of Osornophryne simpsoni sp. n. in ventral view; adult male, QCAZ 45899.
Osteology of hand and foot of Osornophryne simpsoni sp. n. in ventral view; adult male, QCAZ 45899.
Morphometrics of adult males and female of Osonophryne simpsoni sp. n.
| Museum number | Sex | SVL | HL | HW | FL | TIB | IOD | EW | IND | IND | FIIIL | FIVL | LM | LF | TL | EN | ED |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DH-MECN 5260 | Female | 33.0 | 9.5 | 12.3 | 13.8 | 11.4 | 5.9 | 3.8 | 3.1 | 3.1 | 8.4 | 9.4 | 8.3 | 13.0 | 11.2 | 2.8 | 3.2 |
| QCAZ 49774 | Male | 20.1 | 7.2 | 8.2 | 8.4 | 6.8 | 3.4 | 2.8 | 2.3 | 2.3 | 5.3 | 5.7 | 5.4 | 8.0 | 7.2 | 1.7 | 2.9 |
| QCAZ 45899 | Male | 19.5 | 6.4 | 7.6 | 7.0 | 5.7 | 3.7 | 2.7 | 1.9 | 1.9 | 4.5 | 5.2 | 4.9 | 6.6 | 6.5 | 1.7 | 2.8 |
| QCAZ 49777 | Male | 17.6 | 5.9 | 6.7 | 7.0 | 5.6 | 3.2 | 2.8 | 2.0 | 2.0 | 4.2 | 4.6 | 4.4 | 6.3 | 5.9 | 1.5 | 2.8 |
| QCAZ 49781 | Male | 18.6 | 6.0 | 7.0 | 7.1 | 5.6 | 3.1 | 2.6 | 2.1 | 2.1 | 4.6 | 5.0 | 4.5 | 6.5 | 6.1 | 1.6 | 2.1 |
| QCAZ 39774 | Male | 23.2 | 7.7 | 8.1 | 8.5 | 7.4 | 4.0 | 1.8 | 2.1 | 2.1 | 5.3 | 5.8 | 5.3 | 7.6 | 7.9 | 1.9 | 2.6 |
| QCAZ 39769 | Male | 26.1 | 8.2 | 9.4 | 9.1 | 7.7 | 4.0 | 3.0 | 3.0 | 3.0 | 6.3 | 6.6 | 5.8 | 8.9 | 8.8 | 2.0 | 2.7 |
| DH-MECN 5261 | Male | 21.5 | 7.2 | 8.0 | 8.7 | 7.4 | 4.1 | 3.2 | 2.2 | 2.2 | 5.7 | 6.2 | 5.4 | 7.6 | 7.1 | 1.9 | 4.1 |
| DH-MECN 5259 | Male | 21.4 | 6.8 | 8.1 | 9.1 | 7.7 | 4.0 | 3.1 | 2.6 | 2.6 | 5.8 | 6.5 | 5.8 | 9.3 | 7.9 | 2.0 | 4.0 |
| DH-MECN 5258 | Male | 21.2 | 7.2 | 8.4 | 8.1 | 6.8 | 3.8 | 2.8 | 2.6 | 2.6 | 5.3 | 5.8 | 5.4 | 8.8 | 7.6 | 2.1 | 3.8 |
| DH-MECN 5263 | Male | 21.3 | 6.8 | 8.0 | 8.8 | 7.5 | 3.6 | 3.0 | 2.7 | 2.7 | 5.5 | 5.8 | 5.3 | 7.9 | 7.5 | 1.8 | 3.6 |
| 1 | Toe V longer than Toes I–III (Fig. 4A) | 2 |
| – | Toe V shorter than Toes I–III | 4 |
| 2 | Vertebrae and urostyle co-ossified with overlying skin; in life, males with yellow pustules on upper eyelid and tip of snout | Osornophryne cofanorum |
| – | Vertebrae and urostyle not coossified with overlying skin; males lacking yellow pustules on tip of snout | 3 |
| 3 | Head acuminate, with a long proboscis (Figs 10, 11); dorsal skin lacking conical tubercles in most populations (except population from Volcan Sumaco); dorsum dark brown to black, sometimes with grayish-yellow dorsolateral stripes | Osornophryne guacamayo |
| – | Head with short and round snout, with small papillae at tip (Figs 10, 11); dorsal skin with conical tubercles; dorsum lacking dorsolateral stripes (Fig. 2) | Osornophryne simpsoni |
| 4 | Dorsum covered with numerous round pustules of different sizes (lacking space among pustules) | 5 |
| – | Dorsum with sparsely distributed pustules (space among pustules clearly evident) | 6 |
| 5 | Female dorsal skin highly tuberculate, with prominent dorsolateral ridges; flanks with large rounded pustules; males and females with prominent occipital ridges; in males, head acuminated to subacuminated in lateral view (Figs 10, 11) | Osornophryne angel |
| – | Female dorsal skin highly tuberculate, with faintly defined dorsolateral ridges; flanks with scattered and small pustules; males and females with low (or lacking) occipital ridges; in males, head rounded or truncated in lateral view (Figs 10, 11) | Osornophryne bufoniformis |
| 6 | Dorsolateral, occipital, and pelvic ridges separated by smooth skin | 7 |
| – | Dorsolateral, occipital, and pelvic ridges separated by flat pustules | 9 |
| 7 | Males and females large (in males, SVL > 23.5 mm; in females, SVL > 40 mm); males and females with a continuous dorsolateral ridges, and acuminate snout in dorsal view (Figs 10, 11) | 8 |
| – | Males and females small (in males, SVL < 19.0 mm; in females, SVL < 30 mm). Males and females with discontinuous dorsalateral ridges | Osornophryne antisana |
| 8 | Males and females with prominent dorsolateral, occipital, and pelvic ridges | Osornophryne talipes |
| – | Females with comparatively lower dorsolateral, occipital, and pelvic ridges (males unknown) | Osornophryne puruanta |
| 9 | Females with yellow, orange, or white venter in life | 10 |
| – | Females with blue to silver venter in life; males with a yellow to orange papillae at tip of snout (Figs 10, 11) | Osornophryne sumacoensis |
| 10 | Females with yellow to orange venter in life; males and females lacking dorsolateral ridges | Osornophryne percrassa |
| – | Females with yellow to white venter; males and females with clearly defined dorsolateral ridges | Osornophryne occidentalis |
Distribution of Osornophryne simpsoni sp. n. (white circles) in Ecuador.
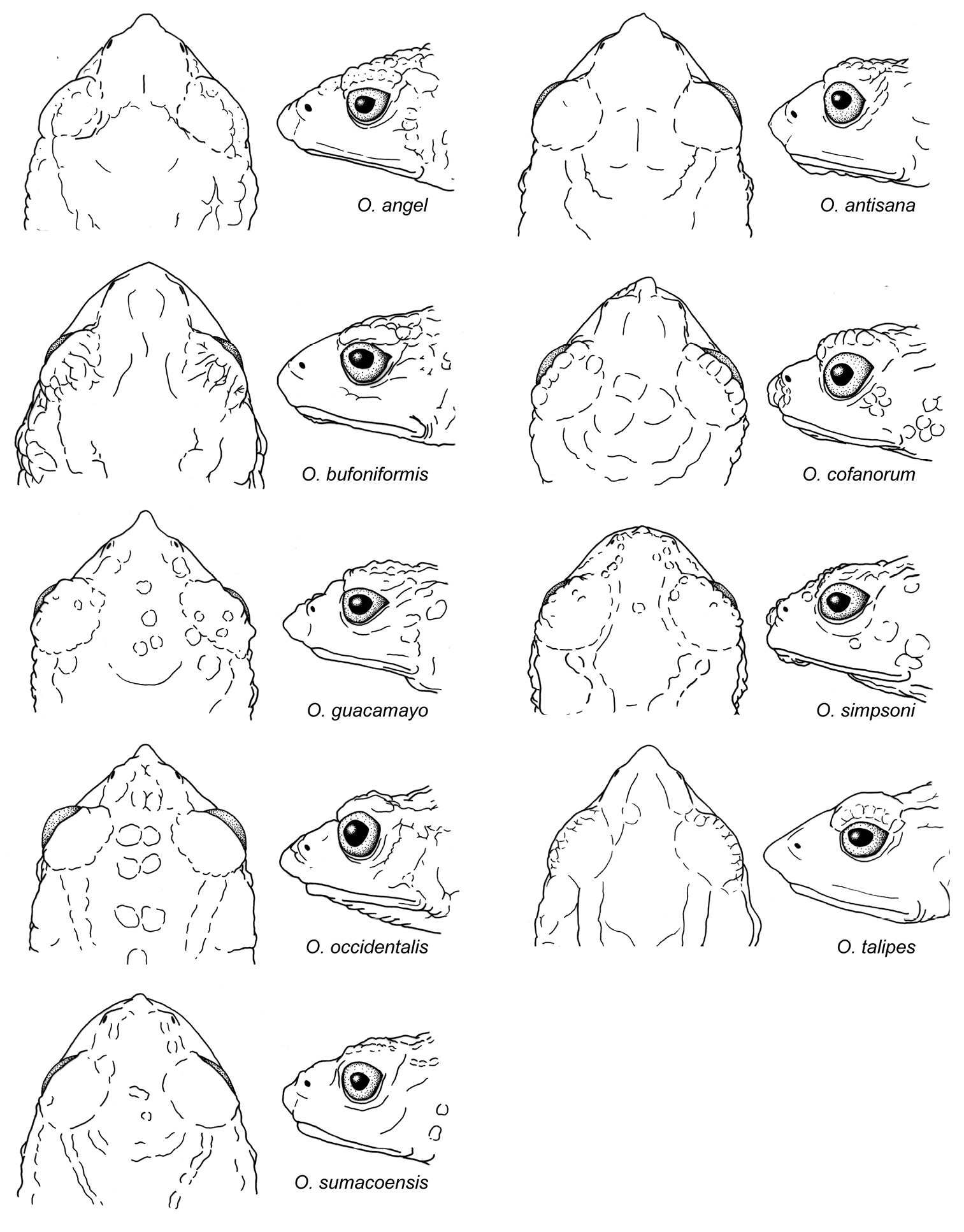
Head shape in dorsal and lateral views of Osornophryne males. Illustrated species are: Osornophryne angel, QCAZ 40048; Osornophryne antisana, QCAZ 48209; Osornophryne bufoniformis, QCAZ 45084; Osornophryne cofanorum, DH-MECN 6248; Osornophryne guacamayo, QCAZ 40106; Osornophryne simpsoni, QCAZ 49774; Osornophryne occidentalis, QCAZ 43529; Osornophryne sumacoensis, QCAZ 41246; Osornophryne talipes, ICN 12256. Not drawn at scale.
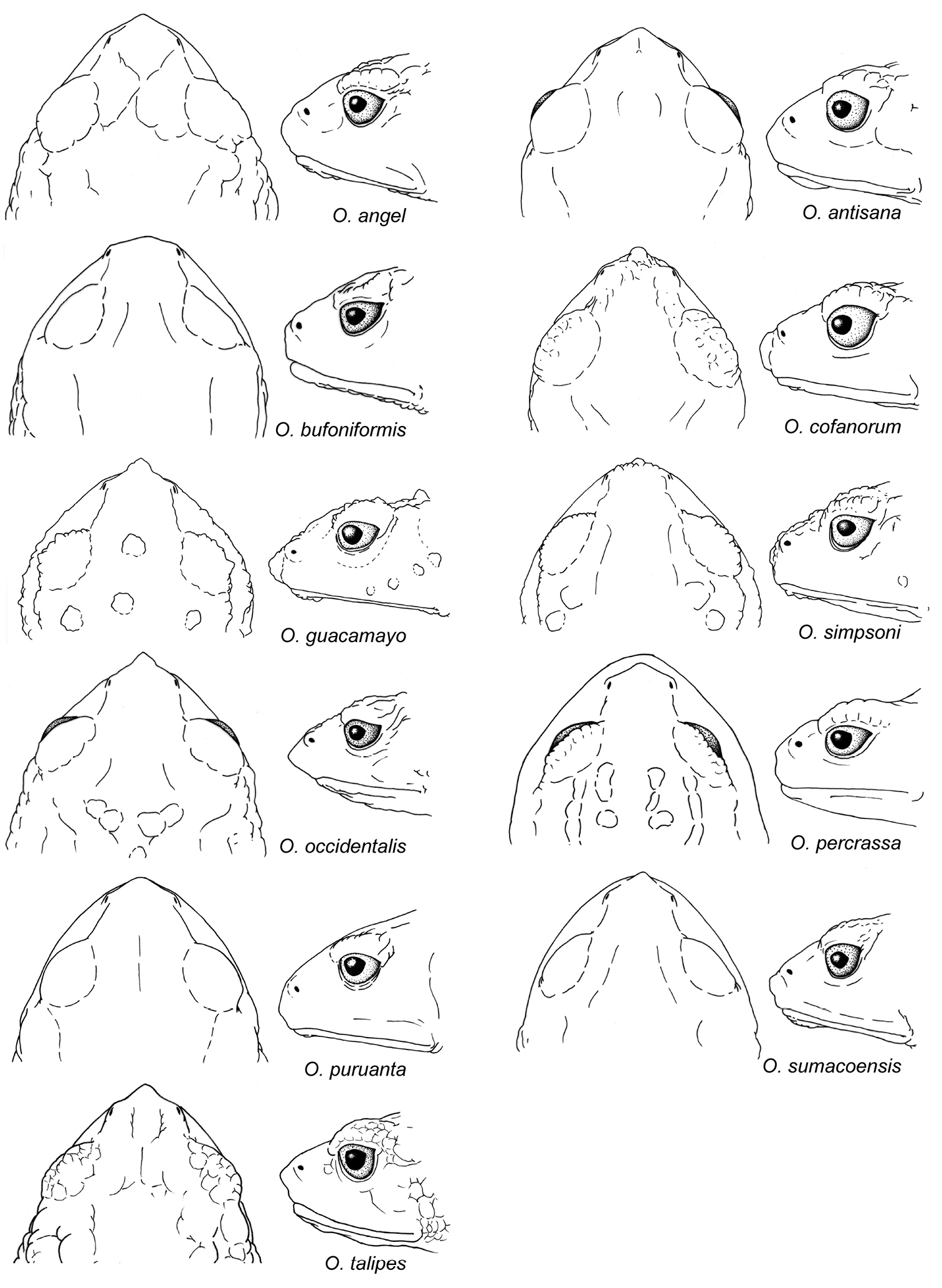
Head shape in dorsal and lateral views of Osornophryne females. Illustrated species are: Osornophryne angel, QCAZ 43560; Osornophryne antisana, QCAZ 48221; Osornophryne bufoniformis, QCAZ 40122; Osornophryne cofanorum, DH-MECN 6194; Osornophryne guacamayo, QCAZ 26047; Osornophryne simpsoni, DH-MECN 5260; Osornophryne occidentalis, QCAZ 43498; Osornophryne percrassa, ICN 319; Osornophryne puruanta, QCAZ 11471; Osornophryne sumacoensis, QCAZ 41244; Osornophryne talipes, EPN 2823. Not drawn at scale.
This article was greatly improved by comments from Linda Trueb, Franco Andreone and Andrew Gluesenkamp. Initial field work has possible thanks to the project “Evaluación de la Herpetofauna de las Reservas Biológicas de la Fundación Ecominga. Cuenca Alta del Río Pastaza. Ecuador”, executed by the Museo Ecuatoriano de Ciencias Naturales and funded by Fundación Jocotoco and Fundación Ecominga. Research was supported by the projects “Inventario y Caracterización Genética y Morfológica de la Diversidad de Anfibios, Aves y Reptiles de los Andes del Ecuador” and “Diversidad Críptica de los Géneros Pristimantis, Osornophryne e Hypsiboas, ” granted by the Secretaría Nacional de Ciencia y Tecnología del Ecuador (PIC-08-0000470) and the Pontificia Universidad Católica del Ecuador, respectively. Universidad Tecnológica Indoamérica supported the final phase of this investigation. For access to collection specimens, we thank the Museo Ecuatoriano de Ciencias Naturales, Museo de Zoología of the Pontificia Universidad Católica del Ecuador, and the Museo de Zoología de la Escuela Politécnica Nacional. Research was conducted under collection permit No. 021–08 IC-FAU-DNBAPVS/MA, issued by Ministerio del Ambiente del Ecuador. Fieldwork at Reserva Zuñac was possible thanks to the collaboration of Lou Jost, Francisco Sornoza M., Rocío and Javier Robayo. MYM thanks Miguél Urgiles, Juan P. Reyes Puig and Andrés Laguna for assistance during field work, and Mauro Yánez C., Joaquín Yánez C., and Alejandra Bejarano for their continuous support. Special thanks go to Elicio Tapia, who found many of the specimens herein reported, Luis A. Coloma and Eduardo Toral for the photographs shown in Figures 2 and 3, and Diego Paucar, who rendered most of the figures shown in this article (Figs 4–8, Figs 10–11).
Osornophryne angel: Carchi: El Voladero (0°41'15"N, 77°52'46"W), DH-MECN 4617, 4606, 4629–30, 4626, 6079, QCAZ 40030–31, 40040, 40043–49.
Osornophryne antisana: Napo: Oyacachi (0°10'34"S, 78°0.6'50"W, 3913 m) QCAZ 40172, 40174; Salvefaccha (0°13'54"S, 78°0.1'01"W, 3900 m) EPN 8937. Tungurahua: Llanganates (1°15'57"S, 78°26'45"W, 3600 m), QCAZ 515, 46282, 48231, 46207, 46204–5, 48230, 48487, 48233–36, 48222, 48215, 48217, 48213, 46212.
Osornophryne bufoniformis: Carchi: Huaca (0°40'15"N, 77°46'11"W, 3780 m) QCAZ 45082–4, 45080–81, 43351; El Chamizo QCAZ 14597–98; Tulcán-Maldonado (0°47'31"N, 77°54'25"W, 3817 m) QCAZ 735, 9316–17. Sucumbios: El Playón de San Francisco (00°36'44"N, 77°40'13"W, 3400–4100 m), DH-MECN 6067–70, 6073, 6079, 6082; La Bonita (00°29'19"N, 77°35'11.4"W, 2614 m) QCAZ 46640–43; Santa Bárbara (0°38'29.47"N, 77°31'18.55"W, 2700 m), QCAZ 40003, 40122, 14080–81, 40118–19.
Osornophryne cofanorum: Sucumbios: La Bonita (0°29'19"N, 77°35'11"W, 2614 m), DH-MECN 6192, 6194, 6205, 6214, 6219, 6232, 6237, 6243, 6250, 6296–97, 6300, 6303–04, 6315, 6325, 6328, 6334, 6337, 6339.
Osornophryne guacamayo: Napo: Cordillera de los Guacamayos (0°37'26.5"S, 77°50'27.09"W, 2238 m), QCAZ 3266, 4889, 9894, 12245, 12249, 13260, 12240, 13259, 26047, 26049, 39081, 33197, 10457, 40106, 40111, 10465, 12241, EPN 6822, 6821, 7438, 7806; Oyacachi (0°15'25"S, 77°57'53"W, 2253 m), QCAZ 40158, 40169, 40138, 40143, 40160, 40148, 40137; Volcán Sumaco (0°34'11"S, 77°35'39"W, 2479 m), QCAZ 41211, 41206, 41210, 41194, 41207, 41229, 41196, 41223, 41203, 41190, 43378, 43370, 43377, 43551–54, 43557, 43550, 43557–59. Sucumbios: Santa Bárbara (0°33'51.4"N, 77°32'50"W, 2388 m), QCAZ 46661.
Osornophryne occidentalis: Carchi: Chilma Bajo (0°51'50"N, 78°4'01"W, 2237 m), QCAZ 40028. Imbabura: Rosario (0°27'29"N, 78°32'50"W, 2296 m) QCAZ 36894, 43498, 43649, 43647, 10141, 43652, 43529, 43650, 43646, 43651, 43653. Pichincha: Guarumos (00°02'S, 78°39'W, 2550–2600 m) EPN 1239.
Osornophryne puruanta: Imbabura: Laguna de Puruanta (00°12'N, 77°57'W, 3000–3500 m) QCAZ 11471, 7685. Pichincha: Laguna de San Marcos (0°7'36"N, 78°15'22"W, 3834 m), EPN7081–83, QCAZ 13271.
Osornophryne sumacoensis: Napo: Volcán Sumaco (0°34'11"S, 77°35'39"W, 2479 m), QCAZ 41247–50, 41243–54, 41233–34, 43379, 4574.