(C) 2012 Polona Mrak. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
An important adaptation to land habitats in terrestrial isopod crustaceans is development of embryos in a fluid-filled female brood pouch, marsupium. The study brings insight into the structure and protective role of egg envelopes and cuticle renewal during ontogenetic development of Porcellio embryos and marsupial mancas. Egg envelopes cover embryos, the outer chorion until late-stage embryo and the inner vitelline membrane throughout the whole embryonic development. Egg envelopes of Porcellio have relatively simple ultrastuctural architecture compared to Drosophila egg envelopes. Exoskeletal cuticle is produced in late embryonic development by hypodermal cells of the embryo and is renewed in further development in relation to growth of developing embryos and mancas. Cuticle structure and renewal in prehatching late-stage embryos and marsupial mancas exhibit main features of cuticle in adults. Epicuticle is thin and homogenous. The characteristic arrangement of chitin-protein fibers and the dense distal layer in exocuticle are hardly discernible in prehatching embryo and distinct in marsupial mancas. Endocuticle consists of alternating electron dense and electron lucent sublayers and is perforated by pore canals in both stages. Differences from adult cuticle are evident in cuticle thickness, ultrastructure and mineralization. Signs of cuticle renewal in prehatching embryo and marsupial mancas such as detachment of cuticle from hypodermis, partial disintegration of endocuticle and assembly of new cuticle are described.
Chorion, vitelline membrane, cuticle, molting, ontogenetic development, terrestrial isopods
The unique feature of embryonic development in isopod and amphipod crustaceans (Peracarida) is its location in the brood pouch on the ventral side of female body (marsupium). The marsupium has likely been of a great adaptive significance in the colonization of land by crustacean species as it allows embryonic development to occur in aqueous environment within the protected chamber (
Two egg envelopes, the outer chorion and the inner vitelline membrane, are produced during oogenesis by the somatic follicle cells of the female reproductive system and cover the embryo until the transition to the late-stage embryo and hatching, respectively. Arthropods evolved egg envelopes of different morphologies and complexities as a consequence of embryonic development in different environments. The data on egg envelopes (chorion and vitelline membrane) structure derive mainly from the studies on the fruit fly Drosophila melanogaster, as this model species offers wide opportunities for genetic studies (
Cuticle is the innermost protective layer, formed later during intramarsupial development and is produced by hypodermal cells of the embryo. In adult arthropods the cuticle is a complex hierarchically structured extracellular matrix, consisting of chitin, proteins and lipids, hardened mostly by mineralization in crustaceans in contrast to insect cuticle which is only sclerotized. It comprises the distal epicuticle, the exocuticle in the middle and the proximal endocuticle. Several reports were published on cuticle structure in adult isopods, mostly in Porcellio scaber (
Cuticle renewal is related to growth in arthropods. In crustaceans molting frequently recurs during adult life. Isopod crustaceans molt in two phases, separately molting posterior and anterior parts of the body. Molt cycle begins with premolt stage, when remarkable morphological changes of the integument occur. The old cuticle separates from the underlying epithelium (apolysis). Epithelial cells secrete a new cuticle, starting with the epicuticle and followed by pre-ecdysal exocuticle. The old and the new cuticles are separated by an extracellular compartment, the ecdysal space, containing different material involved in cuticle renewal. At molting the old cuticle is shed and the new cuticle is further produced, forming post-ecdysal endocuticle. Postmolt stage is marked by soft body surface with progressive hardening of the exoskeleton until the intermolt stage.
In this study we present new data on the ultrastructural architecture of egg envelopes, including chorion and vitelline membrane, and on the ultrastructural characteristics of cuticle renewal in embryos and marsupial mancas of isopod crustaceans Porcellio scaber and Porcellio dilatatus. Comparison of envelopes structure in terrestrial crustaceans and insects will bring new insights into the protective role of egg envelopes and cuticle renewal in developing embryos of these two terrestrial arthropod groups with different developmental strategies.
MethodsAnimals were maintained and bred in a laboratory culture. Staging system of Porcellio scaber ontogenetic development, based on morphological characteristics of embryos and marsupial mancas, was used in this study (
The embryos and marsupial mancas of different developmental stages are shown in Figures 1A, 2A, 3A, 4A and 5A-C in the Results. The term early-stage embryo is used for embryos with large amount of yolk mass in the central part and no visible limb buds. A mid-stage embryo has visible developing limb buds and two midgut glands primordia, which enclose yolk. After bending ventrally and shedding of chorion, embryos are termed late-stage embryos. Prior to hatching swelled embryo inside the vitelline membrane is described as a prehatching late-stage embryo. When late embryos hatch from the vitelline membrane they become marsupial mancas. The progress of development of Porcellio scaber marsupial mancas is characterized by the following morphologically discernible modifications: reduction of the midgut glands size due to yolk consumption, increase in exoskeleton pigmentation, enlargement of body size and pronounced locomotion (
Embryos and mancas at different stages of development were isolated from the marsupium and fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2). Prior to fixation, the egg envelopes of embryos were either carefully perforated with a thin needle or removed. After washing in cacodylate buffer, the samples were postfixed in 1% osmium tetroxide for 2 hours, washed again and dehydrated in a graded series of ethanol.
Embryos and mancas of Porcellio scaber for light microscopy (LM) and transmission electron microscopy (TEM) were embedded in Agar 100 resin. Prior to embedding, mancas were perforated with a thin needle for better infiltration of resin. Semithin sections were made with a glass knife, stained with Azure II - Methylene Blue and imaged by Zeiss AxioImager Z.1 light microscope, equipped with a HRC Axiocam camera. Ultrathin sections were made with a Reichert Ultracut S ultramicrotome (Leica), contrasted with 4% uranyl acetate for 10 minutes and 10% lead citrate for 5 minutes and inspected with a Philips CM100 transmission electron microscope, equipped by BioScan 792 camera (Gatan).
After dehydration in a graded series of ethanol and in acetone, Porcellio dilatatus specimens for scanning electron microscopy (SEM) were transferred into hexamethyldisilazane (HMDS) to perform chemical drying. Mounted specimens were coated with gold and observed with scanning electron microscopes (Philips XL20 and Philips XL30).
For histochemical detection of calcified tissue, mancas of Porcellio scaber were isolated from the marsupium and fixed in 3.7% formaldehyde in 0.1 M cacodylate buffer (pH 7.2). Specimens were washed in cacodylate buffer and embedded in tissue freezing medium (Jung). Transversal sections (10 µm) were cut with a Leica CM1850 cryostat at –18°C and stained with Alizarin red S solution in 0.2 M Trihydroxylmethyl aminomethane (Tris) - HCl buffer (pH 9). Cuticle ofadult Porcellio scaber was used as a positive control. Sections were imaged by Zeiss AxioImager Z.1 light microscope, equipped with a HRC Axiocam camera.
ResultsOntogenetic development of Porcellio scaber in the marsupium, from released fertilized eggs to marsupial mancas, was recently described morphologically (
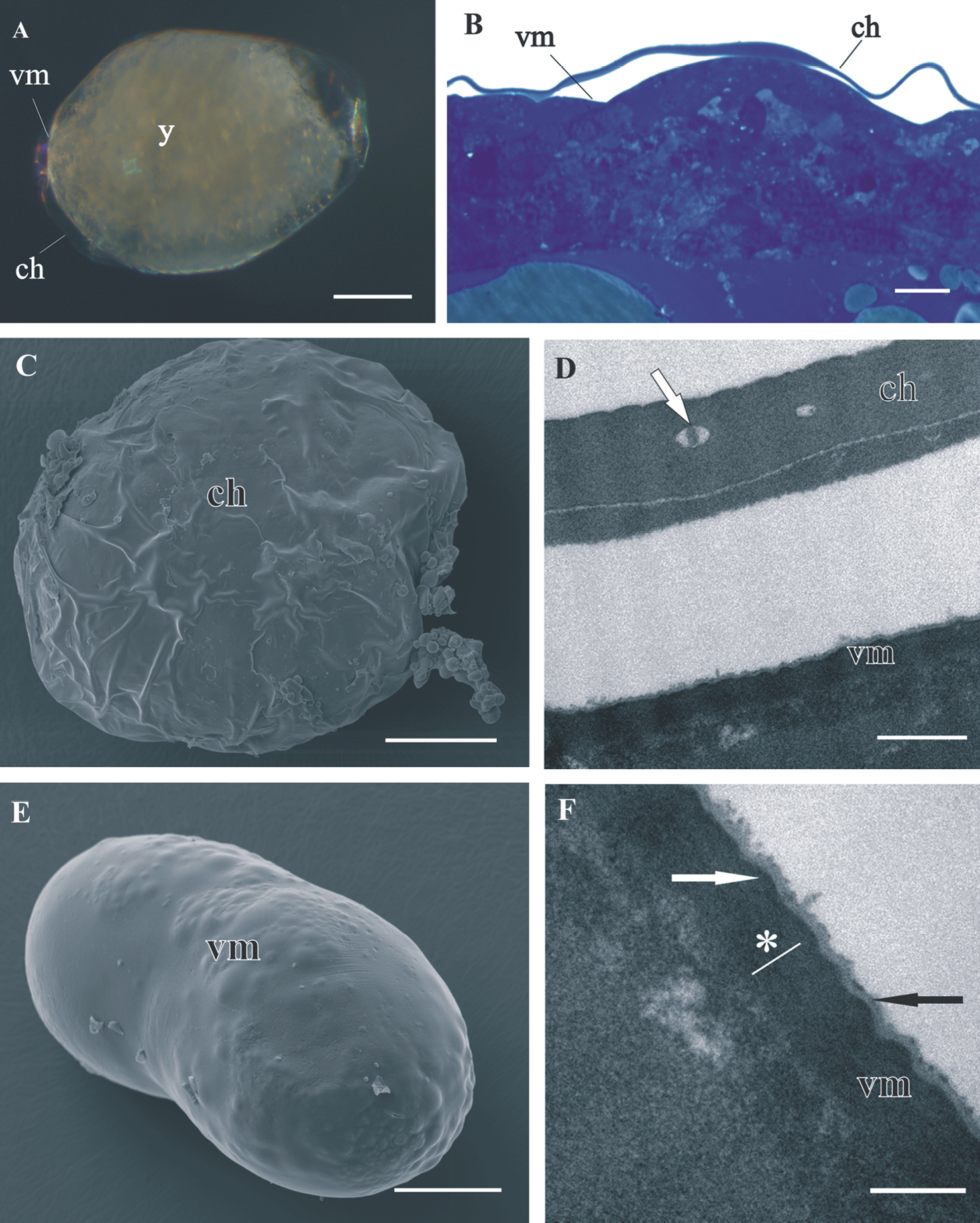
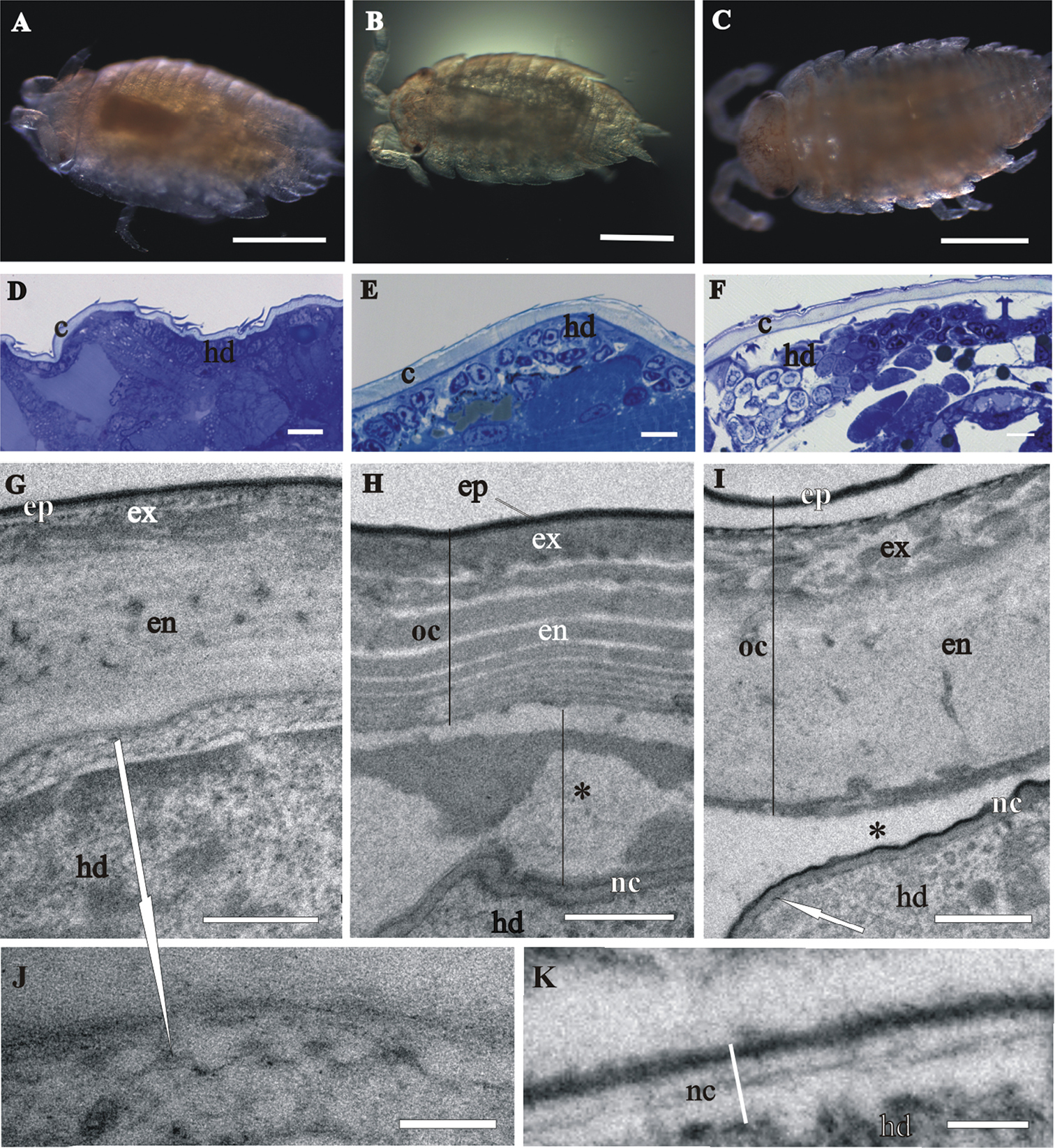
Both egg envelopes, distal chorion and proximal vitelline membrane, surround early-stage (Figs 1A, B, C, E) and mid-stage embryos (Figs 2A, B). Chorion has a similar appearance in both stages. It is a one-layered envelope, separated from the embryo surface and is approximately 500 nm thick. Ultrastructurally chorion consists of an electron dense matrix with sparse electron lucent “lacunae” (Figs 1D, 2C, 2D).
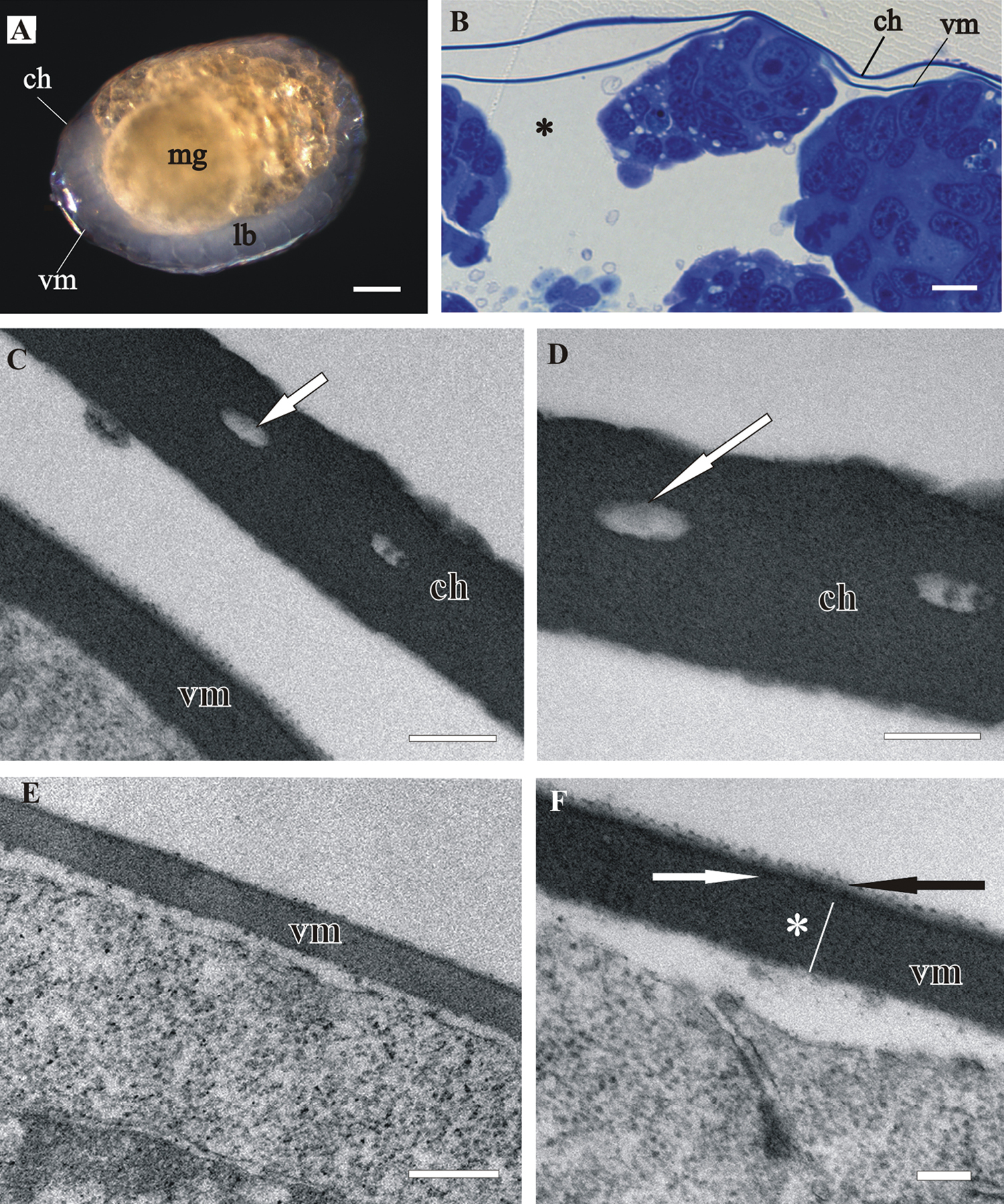
The vitelline membrane surrounds embryos throughout the whole developmental period (Figs 1A, 1B, 1E, 2A, 2B, 3A, 3B, 3E, 4A). It maintains the same thickness of approximately 200 nm from early-stage till late-stage embryo. It is closely apposed to the embryo surface in early-stage embryos (Figs 1B, D, F), while it is slightly detached from the embryo surfaces of mid-stage embryos (Figs 2B, E, F) and late-stage embryos (Figs 3B, C, F, G). Above the embryo limb buds a wider space appears between embryo surface and vitelline membrane due to intense cell rearrangement during limb buds formation (Fig. 2B). The vitelline membrane consists of a thick proximal homogenous electron dense matrix superposed by a thin middle electron dense layer and a superficial corrugated lucent layer (Figs 1F, 2E, 2F, 3C). In non-osmicated specimens of late-stage embryo, the main thick layer is evidently lighter and superficial layer is not discerned (Fig. 3D). Between the outer embryo surface and the vitelline membrane of late-stage embryo a network of fibers was observed by SEM, presumably functioning as connective elements (Figs 3F, G).
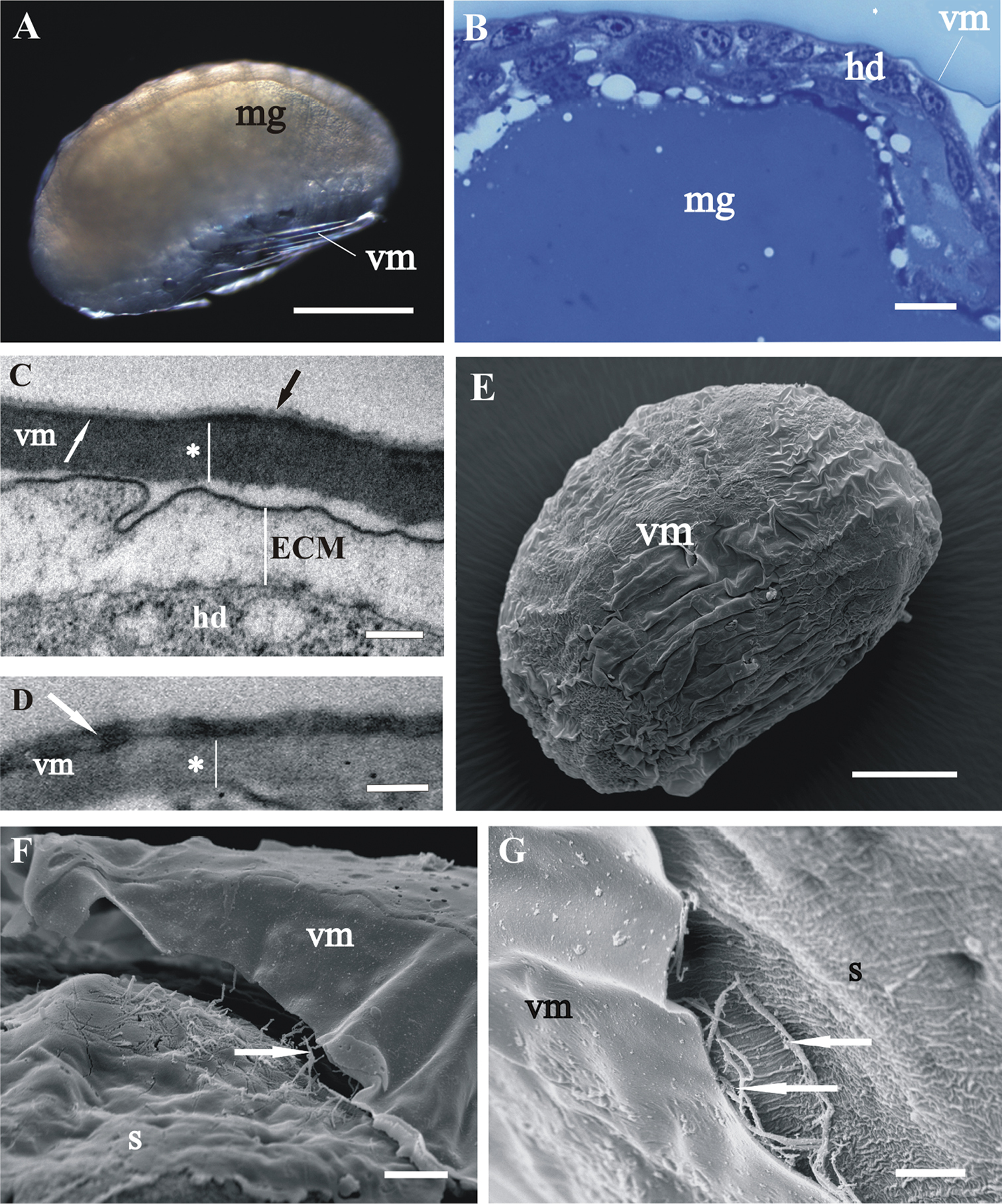
Structure and renewal of cuticleIn late-stage embryo the embryo surface is covered with a homogenous extracellular matrix (Figs 3C, F, G). The prehatching late-stage embryo is the earliest developmental stage, in which the overall ultrastructural architecture of the cuticle is similar to the adult crustacean cuticle and first morphological evidence of cuticle renewal is evident. Cuticle is from 2 to 3 µm thick, which is significantly thinner than in adults. It is composed of three layers, the outermost thin electron dense epicuticle, the middle exocuticle and the innermost endocuticle (Figs 4C, D, E). Cuticular scales are fully elaborated (Fig. 4B, D). Several transverse sections of completely structured sensilla are observed in the hypodermis (Fig. 4F). Dendritic outer segments and enveloping cells are clearly differentiated. The characteristic pattern of chitin-protein fibers arrangement in the exocuticle is resolved in some regions and hardly discernible in other regions of the same specimen. The endocuticle is subdivided in several electron dense sublayers alternating with electron lucent sublayers (Figs 4C, D, E). In some regions pore canals are visible running through the endocuticle, consisting of electron lucent central part and electron dense margins (Fig. 4C). Several morphological features in prehatching late-stage embryo show the exoskeletal cuticle renewal already in this stage of development: partly disintegrated proximal portion of endocuticle in some regions (Fig. 4C); cuticle detachment from the hypodermis (Figs 4B, C, D, E); rough apical plasma membranes of hypodermal cells and irregularly arranged electron dense particles on their outer surface (Figs 4C, E).
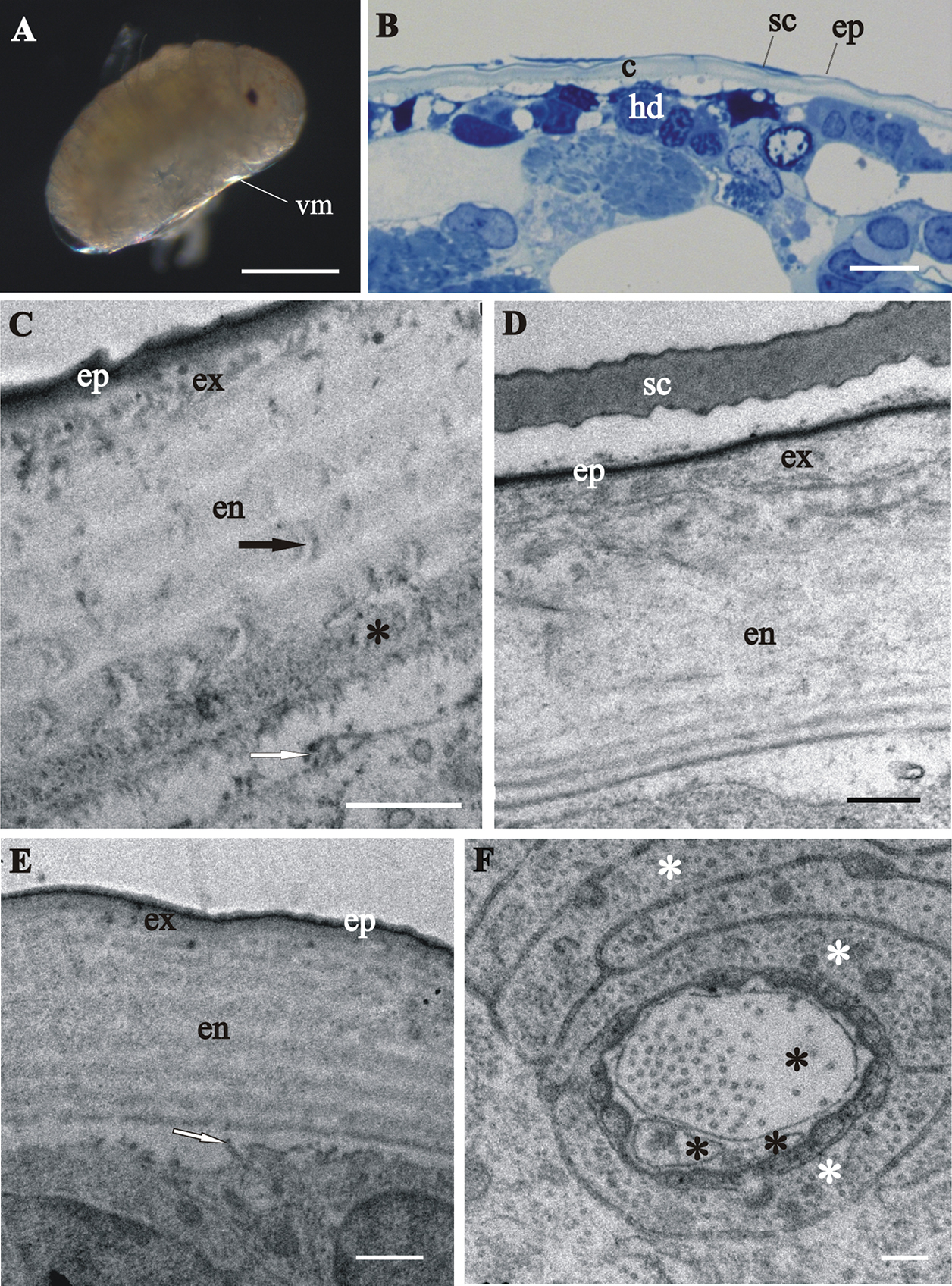
Next, we present evidence of cuticle renewal in different stages of marsupial mancas, namely: (I) early-stage marsupial manca, immediately after hatching (Fig. 5A); (II) mid-stage marsupial manca (Fig. 5B) and (III) late-stage marsupial manca, just prior to release from the marsupium (Fig. 5C). In the marsupial mancas the cuticle has similar architecture as the cuticle of prehatching embryo (Figs 5D–I). The main difference is the more pronounced characteristic chitin-protein pattern of exocuticle with a dense distal layer in marsupial mancas in comparison to embryo (Figs 5G, I). The micrograph of the late-stage marsupial manca cuticle displays well-formed pattern of exocuticle, resembling the adult helicoidal exocuticle structure and with a dense distal layer (Fig. 5I). The endocuticle of marsupial mancas is perforated by pore canals. The thickness of the marsupial manca cuticles is up to 3 µm. Morphological characteristics of cuticle renewal are observed in all three stages of marsupial mancas (Figs 5G-K). Apolysis, detachment of the old cuticle from the hypodermis is clearly visible in several stages (Figs 5F, H, I). The detached cuticle is partly degraded and much thinner in certain regions of the same specimen. The ecdysal space between the detached cuticle and the newly forming cuticle is also well evident and it contains homogenous electron dense material in some specimens (Fig. 5H), while in others it appears devoid of electron dense material (Fig. 5I). The newly assembling cuticle, covering hypodermal cells, consists of two layers, a thin electron dense external layer - epicuticle and an electron lucent procuticle (Figs 5H, I, K). In some regions of late-stage marsupial manca the procuticle appears homogenous (Fig. 5I), while in other regions helicoidal chitin-protein fibers arrangement is clearly discernible (Fig. 5K). The surface of the new cuticle is slightly (Fig. 5H) or highly wrinkled (Fig. 5I). Protrusions with electron dense tips are formed on apical surfaces of hypodermal cells. These characteristic features of cuticle synthesis are clearly visible underneath the assembling cuticle in all stages of marsupial mancas (Figs 5G, I, J).
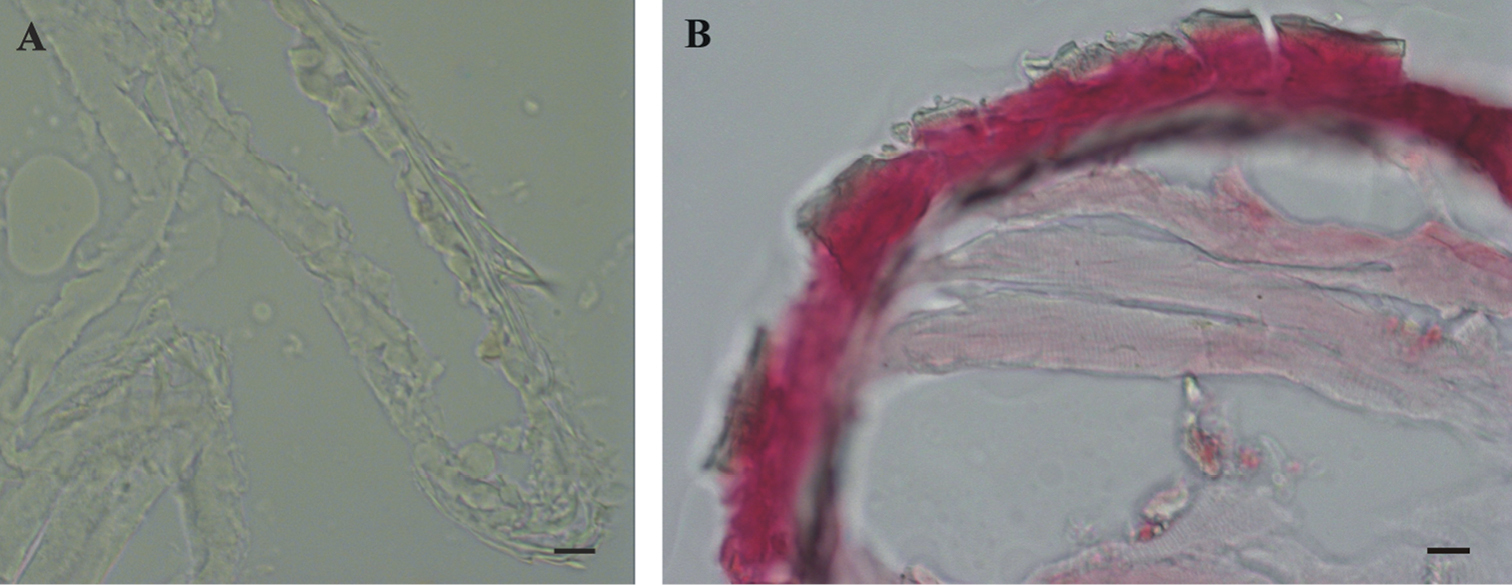
The results of histochemical reaction with Alizarin red S for calcified tissues localization indicate that the cuticle of marsupial mancas is not strongly mineralized (Fig. 6A) in comparison to adult cuticle (Fig. 6B). The ventral calcium deposits, characteristic for adult premolt animals, were not observed in molting marsupial mancas.
Structure of distal chorion (ch) and proximal vitelline membrane (vm), covering Porcellio scaber A, B, D, F and Porcellio dilatatus C, E early-stage embryo. A The early-stage embryo with large amount of yolk (y) and no visible limb buds. B Semithin section of the embryo peripheral region. Chorion is separated from the embryo surface. The vitelline membrane is closely apposed to the embryo surface. C SEM micrograph of the early-stage embryo. The outer egg envelope, chorion, is visible. D TEM micrograph of one-layered chorion, including electron lucent “lacunae” (white arrow). There is a layer of artificially spilt yolk underneath the chorion. E SEM micrograph of the early-stage embryo. Chorion is artificially removed and the inner egg envelope, vitelline membrane, is exposed. F TEM micrograph of vitelline membrane, composed of three layers: main proximal homogenous layer (*), thin middle electron dense layer (white arrow) and superficial corrugated lucent layer (black arrow). Bars: A, C, E 200 µm; B 10 µm; D 0.5 µm; F 200 nm.
Structure of distal chorion (ch) and proximal vitelline membrane (vm), covering Porcellio scaber mid-stage embryo. A The mid-stage embryo with visible limb buds (lb) and midgut glands primordia (mg). B Semithin section of the embryo peripheral region. Chorion is separated from the embryo surface. The vitelline membrane is slightly detached from the embryo cells; * - a wider space between embryo surface and vitelline membrane. C, D TEM micrographs of one-layered chorion, including electron lucent “lacunae” (white arrow). E, F TEM micrographs of vitelline membrane, composed of three layers: main proximal homogenous layer (*), thin middle electron dense layer (white arrow) and superficial corrugated lucent layer (black arrow). Bars: A 200 µm; B 10 µm; C, E 0.5 µm; D, F 200 nm.
Structure of vitelline membrane (vm), covering Porcellio scaber A–D and Porcellio dilatatus E–G late-stage embryo. A Ventrally bent late-stage embryo, yolk is completely enclosed into the midgut glands (mg). B Semithin section of the embryo peripheral region. Vitelline membrane is slightly detached from the hypodermis (hd). C, D TEM micrographs of the vitelline membrane in osmicated specimen C and in non-osmicated specimen D Main proximal homogenous layer (*), thin middle electron dense layer (white arrow) and superficial corrugated lucent layer (black arrow). Hypodermis is covered with an extracellular matrix (ECM). E SEM micrograph of the late-stage embryo surrounded by vitelline membrane. F, G SEM micrographs of the late-stage embryo surface area. The vitelline membrane is artificially slit and fibers (arrows) between the outer embryo surface, covered with an extracellular matrix (s), and the vitelline membrane are exposed. Bars: A 500 µm; B, F 10 µm; C, D 200 nm; E 200 µm; G 5 µm.
Cuticle structure and renewal in Porcellio scaber prehatching late-stage embryo. A Swelled embryo inside the vitelline membrane (vm), prior to hatching. B Semithin section of the embryo peripheral region. The vitelline membrane is artificially removed. Clearly discernible exoskeletal cuticle (c), detached from the underlying hypodermis (hd). C, D, E TEM micrographs of exoskeletal cuticle in different regions of the same specimen, composed of three principal layers: the outermost thin electron dense epicuticle (ep), the middle exocuticle (ex) and the innermost endocuticle with several sublayers (en). The micrographs show features of cuticle renewal: cuticle detachment from the hypodermis, partial disintegration of proximal portion of endocuticle (*) and irregularly arranged electron dense particles on outer apical plasma membrane surface (white arrows). Pore canals (black arrow) in the endocuticle consist of electron lucent central part and electron dense margins C. Cuticular scales (sc) are fully elaborated and the exocuticle has the characteristic pattern of chitin-protein fibers arrangement D. Exocuticle is hardly discernible E. F TEM micrograph of completely structured sensillum transverse section in the hypodermis. Dendritic outer segments (*) and enveloping cells (white *). Bars: A 500 µm; B 10 µm; C, E 1 µm; D 0.5 µm; F 200 nm.
Cuticle structure and renewal in Porcellio scaber marsupial mancas. A The early-stage marsupial manca, immediately after hatching B The mid-stage marsupial manca C The late-stage marsupial manca, just prior to release from the marsupium D–F Semithin sections of the manca peripheral region in the early-stage marsupial manca D in the mid-stage marsupial manca E and in the late-stage marsupial manca F. Cuticle (c), overlying the hypodermis (hd), becomes progressively more similar to adult cuticle. G–K TEM micrographs of exoskeletal cuticle in the early-stage marsupial manca G, J in the mid-stage marsupial manca H and in the late-stage marsupial manca I, K Three main layers are distinguished: epicuticle (ep), exocuticle (ex) and endocuticle (en). The micrographs show morphological characteristics of cuticle renewal: detachment of the old cuticle (oc) from the hypodermis, ecdysal space (*) between the detached cuticle and the newly forming cuticle (nc) and partial degradation of the old cuticle H, I protrusions with electron dense tips (white arrows) on apical surfaces of hypodermal cells G, I, J. The new cuticle consists of two layers, external electron dense epicuticle and internal electron lucent procuticle H, I, K. Helicoidal chitin-protein fibers arrangement is discernible in some regions of late-stage marsupial manca K. Bars: A–C 500 µm; D–F 10 µm; G–I 1 µm; J, K 200 nm.
Histochemical reaction for calcium – Alizarin red S. A No reaction in Porcellio scaber late marsupial manca B Positive control (red-pink) in adult Porcellio scaber cuticle. Bars: 10 µm.
Schematic representation of different protective envelopes, coating Porcellio scaber embryos and marsupial mancas during development (lasting 35 days), namely egg envelopes (chorion and vitelline membrane) and exoskeletal cuticle. During growth of embryos and mancas egg envelopes are shed and cuticle is renewed. ch – chorion; vm – vitelline membrane; ECM – extracellular matrix; epi – epicuticle; exo – exocuticle; endo – endocuticle; nc – newly assembling cuticle.
The structure of egg envelopes and the ultrastructural architecture of the exoskeletal cuticle in Porcellio embryos and marsupial mancas share some common features with those described in other arthropods, but there are also some significant differences.
In comparison to egg envelopes of Drosophila eggs from ovaries, described by
We show here that the late embryo is already covered with a homogenous extracellular matrix, possibly first cuticle. In the prehatching late-stage embryo of Porcellio scaber the exoskeletal cuticle has already main features of the adult crustacean cuticle, but minor differences are evident. It is composed of three principal layers - epicuticle, exocuticle and endocuticle. Endocuticle shows the typical arrangement of chitin lamellae in sublayers which are not so distinct as in adult cuticle. The characteristic pattern of chitin-protein fibers arrangement in the exocuticle of adults is not discernible in exocuticle of the prehatching late-stage embryo. A distal layer, similar to the dense distal exocuticular layer, described in adult isopods (
Next, the issue of cuticle renewal during intramarsupial development was addressed in this study. Partly disintegrated proximal endocuticle and cuticle detachment from the underlying hypodermis indicate that cuticle renewal takes place already in the prehatching embryonic stage. Prehatching embryo is thus the earliest stage of Porcellio scaber development, where renewal of exoskeleton, i.e. initiation of molting, was observed so far. These results are in agreement with the previous observation of apolysis on appendage tips in late-stage embryo of Porcellio scaber (
Ultrastructural research of isopod egg envelopes and cuticle structure during ontogenetic development in a fluid-filled marsupium is a valuable approach to get insight into their differentiation and function and contribute to comparative analysis of ontogenetic development in different arthropods. Evaluation of the data on the newly forming cuticle structure and composition obtained in sequential phases of the ontogenetic development is important to get insight into the mechanisms of mineralized biological matrix assembly.
ConclusionsDuring marsupial development of Porcellio scaber embryos and marsupial mancas in different stages are coated by different protective envelopes (Fig. 7).
Egg envelopes of isopod crustacean Porcellio scaber embryos are thinner and structurally less complex in comparison to egg envelopes of insect Drosophila melanogaster. These are expected differences due to different embryonic developmental strategies of these arthropods. Similarities in egg envelopes of these two species appear particularly in their inner egg envelope, the vitelline membrane.
Exoskeletal cuticles of Porcellio scaber prehatching late-stage embryo and marsupial mancas have already some features of the adult crustacean cuticle, but are significantly thinner. Three principal layers are distinguished, the outermost epicuticle, the middle exocuticle and the innermost endocuticle. Characteristic chitin-protein patterns of adult cuticle, particularly regarding the exocuticle, are not very distinct in prehatching late-stage embryo and become progressively more explicit in marsupial mancas. Cuticular scales and sensilla are fully elaborated already in prehatching late-stage embryo. In the distal portion of the exocuticle a dense layer is observed in marsupial mancas, which could correspond to the dense distal exocuticular layer of adult cuticle. Marsupial manca cuticle is not strongly calcified.
Cuticle renewal takes place already in prehatching late-stage embryo, where detachment of cuticle from hypodermis and partial disintegration of proximal endocuticle occur. Old cuticle detachment and new cuticle assembly appear in marsupial mancas of several stages. Morphological changes, related to calcium storage during molt cycle in adult isopods, were not observed in premolt marsupial stages.
We would like to thank Jožica Murko Bulić, Miloš Vittori and dr. Rok Kostanjšek for their laboratory assistance and all help and suggestions. This work was financed by the Slovenian Research Agency (ARRS), grand No. P1-0184 and 1000-11-310087.