(C) 2012 Bastian H. M. Seidl. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
The crustacean cuticle consists of a complex organic matrix and a mineral phase. The physical and chemical properties of the cuticle are corellated to the specific functions of cuticular elements, leading to a large variety in its structure and composition. Investigation of the structure-function relationship in crustacean cuticle requires sophisticated methodological tools for the analysis of different aspects of the cuticular architecture. In the present paper we report improved preparation methods that, in combination with various electron microscopic techniques, have led to new insights of cuticle structure and composition in the tergite cuticle of Porcellio scaber. We used thin sections of non-decalcified tergites and decalcified resin embedded material for transmission electron microscopy and scanning transmission electron microscopy. Etched sagittal planes of bulk tergite samples were analysed with field emission scanning electron microscopy. We have found a distinct distal region within the exocuticle that differs from the subjacent proximal exocuticle in the arrangement of fibres. Within this distal exocuticle chitin-protein fibrils assemble to fibres with diameters between 15 and 50 nm that are embedded in a mineral matrix. In the proximal exocuticle and the endocuticle fibrils do not assemble to fibres and are surrounded by mineral individually. Furthermore, we show that the pore canals are filled with mineral, and demonstrate that mild etching of polished sagittal cuticle surfaces reveals regions containing mineral of diverse solubility.
Isopoda, cuticle, ultrastructure, Porcellio scaber
The structural organisation of the crustacean cuticle has recently led to increasing interest because of its high structural and chemical variability. Furthermore, the outstanding mechanical properties of the crustacean cuticle have attracted attention to its high potential for biomimetic technical applications. The organic matrix of the arthropod cuticle is hierarchically organised (
The crustacean cuticle is an exoskeleton that provides support, protection against predation and environmental strains, sites for muscle attachment, and structures involved in receiving sensory information. The cuticle surrounds the whole animal and is subdivided into skeletal elements such as segments of the main body and legs, claws, mouthparts, eye lenses, etc. Flexible cuticular membranes often connect skeletal elements to allow for relative movements. The structure and composition of the cuticle are adapted to the function of a specific skeletal element and to the habitat of the animal (
In the present paper we describe improved preparation methods for the cuticle of terrestrial isopods using the tergite cuticle of Porcellio scaber as an experimental model. The cuticle of Porcellio scaber and other related species has been studied previously (
Porcellio scaberLatreille, 1804 were collected from biotopes near Ulm, Germany, kept in plastic containers filled with soil and bark, and fed with fresh potatoes and dry oak leaves. Animals in the intermoult stage were identified by the lack of external signs of moulting such as sternal CaCO3 deposits. The intermoult stage was further verified during tergite sample preparation and structural analysis. Specimens with any signs of apolysis and samples with soft or incompletely developed cuticle were not used. We used the tergites of pereonites 2–7.
For transmission and low voltage scanning transmission electron microscopy (TEM, STEM) of the organic phase, in decalcified EPON resin embedded samples, dissection of the cuticle was performed in a decalcification/fixation solution containing 0.05 mol L-1 EDTA for decalcification, 2.5% glutaraldehyde and 2% paraformaldehyde for fixation of organic material, and 0.25 mol L-1 HEPES (pH 7.8) for buffering pH during decalcification. The samples were incubated in fresh solution for 34 days at 4 °C. During simultaneous decalcification and fixation, parts of the organic matrix, which emerge slowly from surrounding mineral and are thus exposed to the solution, are immediately fixed by the aldehyde. The decalcified tergites were then washed 3 times in bi-distilled H2O for 10 minutes each and postfixed for 1 h in a solution containing 1% OsO4 and 0.8% K4[Fe(CN)6] (
STEM is a method where the electron beam is focused in a spot which is raster scanned across a sample. We used a high-resolution FE-SEM equipped with a dark field STEM-detector to perform STEM analysis at low voltages (30 kV), which considerably enhances contrast and still yields spatial resolution of about 1 nm. The contrast arises from local density of the sample that is in particular useful for the analysis of unstained sections of non-demineralised material.
For microscopy of non-decalcified samples, dissection in aqueous solutions should be avoided because this would lead to dissolution of the mineral phase within the endocuticle. In vitro experiments have demonstrated that the ACC polymorph is ten times more soluble than its crystalline counterparts (
To obtain polished sagittal faces and sections of native (non-demineralised) cuticle, tergites were first glued to cylindrical aluminium holders (diameter 3 mm) using super glue gel. Sagittal planes were cut with a glass knife and then polished with a Diatome diamond knife according to the method described previously (
To expose the organic matrix in non-decalcified tergite cuticle, samples were first polished as described above and then etched for 20 seconds with an aqueous solution of 2.5% glutaraldehyde and 0.01 mol L-1 MOPS buffer adjusted to pH 6.5 or 0.1 mol L-1 HEPES buffer adjusted to 8.0. Then, the samples were washed 3 times for 10 minutes with isopropanol, critical point dried, rotary shadowed with 4 nm of platinum (BAF 300, Balzers) and analysed with a Hitachi S-5200 FE-SEM at 4 kV using the secondary electron detector. Secondary electrons (SE) have a low energy and thus only those elicited from near the sample surface can reach the detector and can thus contribute to imaging of the surface topography. A SEM with a field emission gun was used to obtain high resolution (nominally 1.8 to 0.5 nm at 1 to 30 kV, respectively) at low acceleration voltages as a result of the small diameter of the focused electron beam (spot size).
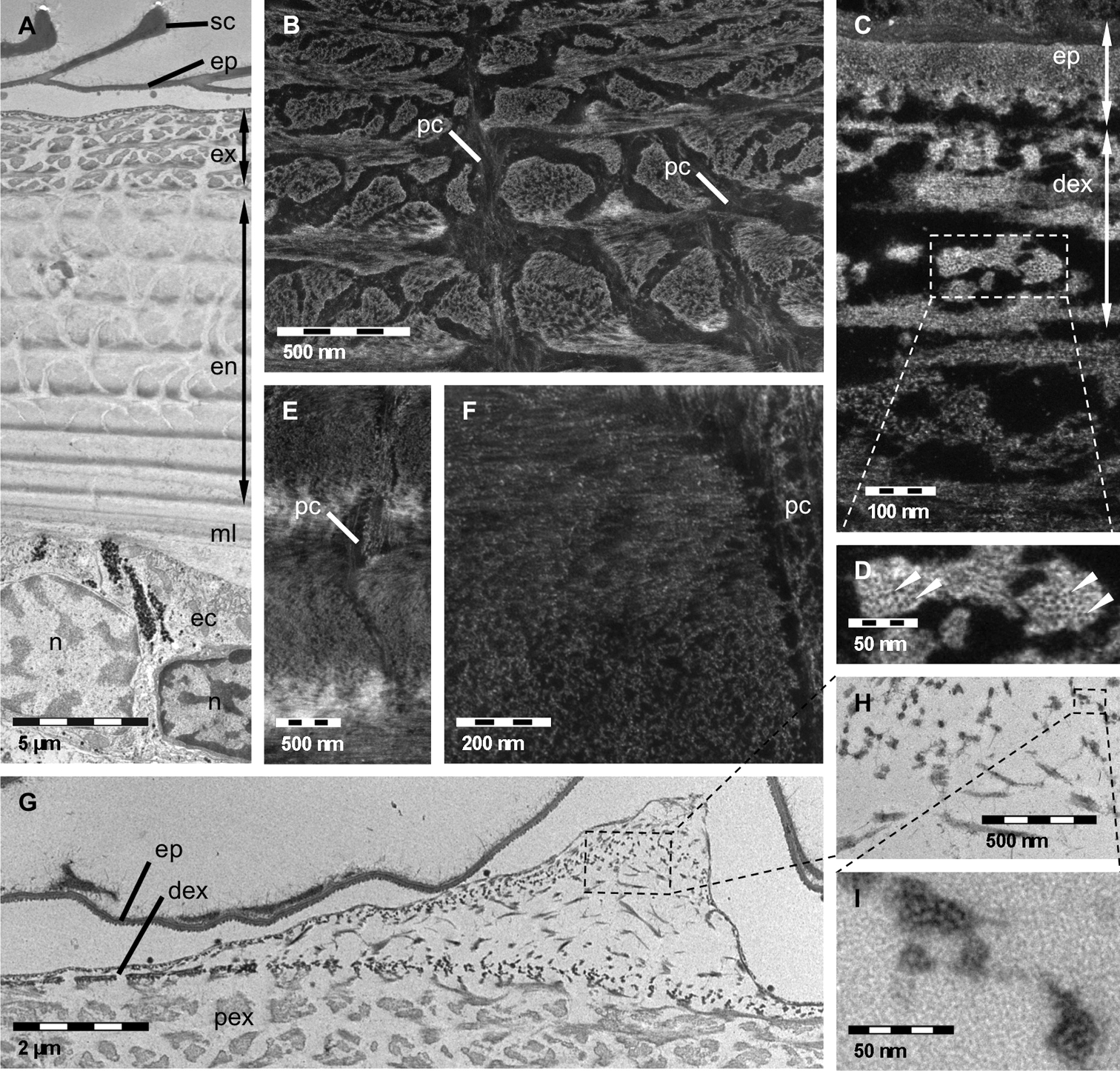
Results Decalcified thin sectionsTEM and STEM analyses of simultaneously decalcified and fixed samples provide detailed information about the specific hierarchical organisation of the organic matrix of the cuticle from its principal layers down to single protein fibrils and chitin crystallites. Exo- and endocuticle can be clearly distinguished by differences in contrast and morphology (Figure 1). The organic matrix of the exocuticle is more intensely stained than the endocuticle one, resulting in a sharp border between them (Figure 1A). In the proximal exocuticle pore canals are more branched than in the endocuticle and a high variation in the width of pore canals can be observed, probably due to twisting of spindle-shaped pore canals. In sagittal sections, this leads to an islelike appearance of the part of the organic matrix that is organised in a twisted plywood structure (Figures 1A, B). In the sagittal sections of the endocuticle however, pore canals have a lunulate appearance. The width of the pore canals decreases towards proximal stacks (Figure 1A). There is a clear difference in the organisation at the level of fibrils and fibres between a distinct distal and the proximal region of the exocuticle. Within the distal region of the exocuticle chitin-protein fibrils form fibres consisting of electronlucent approximately 3 nm thick chitin crystallites surrounded by densely stained protein (Figures 1C, D). The fibres vary in size and shape. Most fibres have a diameter of approximately 25 nm but fibres with diameters up to 50 nm also appear regularly. Within the proximal part of the exocuticle as well as in the endocuticle, fibrils appear not to form fibres (Figures 1E, F). Local thickenings of the cuticle, regularly observed in thin sections and probably representing edges of tubercles, derive from an increase in the height of stacks in the distal exocuticle, whereas the thickness of stacks of the endocuticle remains unchanged (Figures 1G–I). The 150 nm thick epicuticle consisting of an electron-lucent outer and an electron-dense inner epicuticle (Figure 1C) remains unaltered as well (Figure 1G).
TEM A, G, H, I and STEM B, C, D, E, F micrographs of decalcified and EPON embedded tergites of Porcellio scaber. A Sagittal overview showing epicuticle (ep), exocuticle (ex), endocuticle (en) and membranous layer (ml), ec, epithelial cell; n, nucleus; sc, epicuticular scale. B Proximal exocuticle. Dense network of pore canals (pc) containing fibrils or fibres following the direction of the pore canal. C, D Fibres of the distal exocuticle (dex) consisting of approximately 3 nm thick unstained chitin crystallites (arrowheads) surrounded by densely stained proteins. E, F In the endocuticle single fibrils form the twisted plywood structure. The pore canals contain vertical fibrils or fibres. G, H, I Section through a cuticular thickening. The increase in cuticle thickness is brought about by an increase of the stacking height in the distal exocuticle only. H, I Details of G confirm the typical structure of fibres within the distal exocuticle.
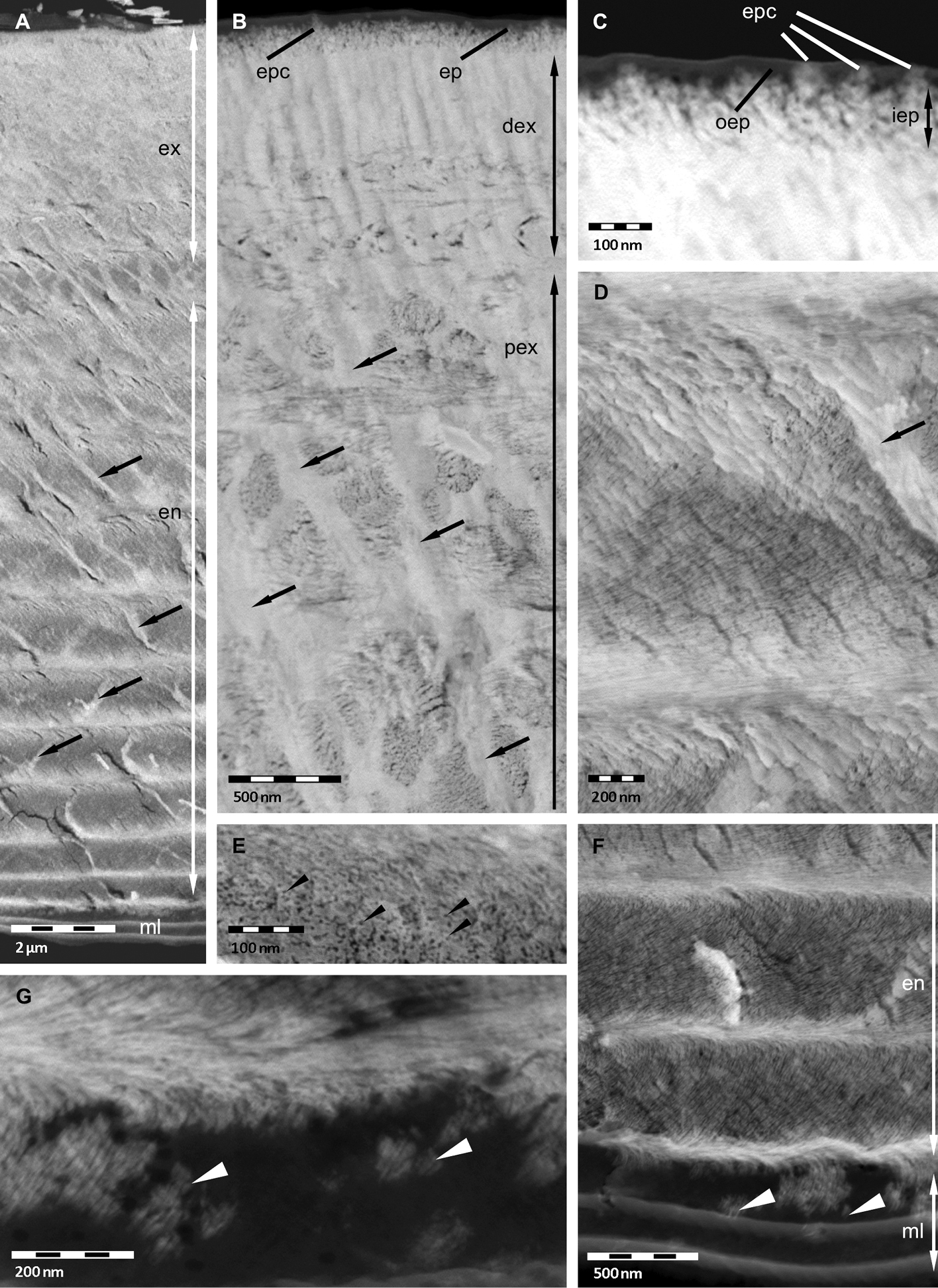
Thin sections of non-decalcified cuticle are of value for studying the distribution of mineral and its spatial relation to the organic phase. Figure 2A shows a sagittal section through the whole cuticle. We confirm that the membranous layer and most regions of the outer epicuticle are devoid of mineral (Figures 2A, C). It appears that parts of the inner epicuticle and perhaps epicuticular pore canals may well contain mineral (Figures 2B, C). It is of interest that within the whole exo- and endocuticle pore canals are filled with mineral (Figures 2A, B, D). Thin pore canals within proximal stacks of the endocuticle can well be seen (Figure 2A). In the 11.5 µm thick distal exocuticle mineral surrounds electron-lucent organic fibres approximately 25 nm in diameter indicating that the proteinaceous material around the chitin crystallites shown in Figures 1C and 1D remains unmineralised (Figure 2B). Within the proximal exocuticle and the whole endocuticle, we observe electron-lucent structures with diameters of approximately 6 nm, corresponding to single chitin-protein fibrils (Figures 2D, E). They are surrounded by mineral that forms interconnected tube-like structures, confirming that in these layers the fibrils do not assemble to fibres. At the border between the endocuticle and the membranous layer rod-like mineral structures occur in a partly mineralised region (Figures 2F, G).
STEM micrographs of non-decalcified tergites of Porcellio scaber. A Overview showing the mineralised exocuticle (ex) and endocuticle en and the unmineralised membranous layer (ml). The pore canals (arrows) are mineralised. B, C Exocuticle and epicuticle (ep). Approximately 25 nm thick fibres in the distal exocuticle (dex) and approximately 6 nm thick fibrils in the proximal exocuticle (pex). Pore canals arrows contain mineral. The inner epicuticle (iep) appears partly mineralised, and the outer epicuticle (oep) unmineralised, except epicuticular pore canals (epc). D, E Endocuticle with mineralised pore canal (arrow). Approximately 6 nm thick chitin-protein fibrils (black arrowheads) individually surrounded by mineral forming a twisted plywood structure. F, G Single mineral rods (white arrowheads) at the border between membranous layer and endocuticle.
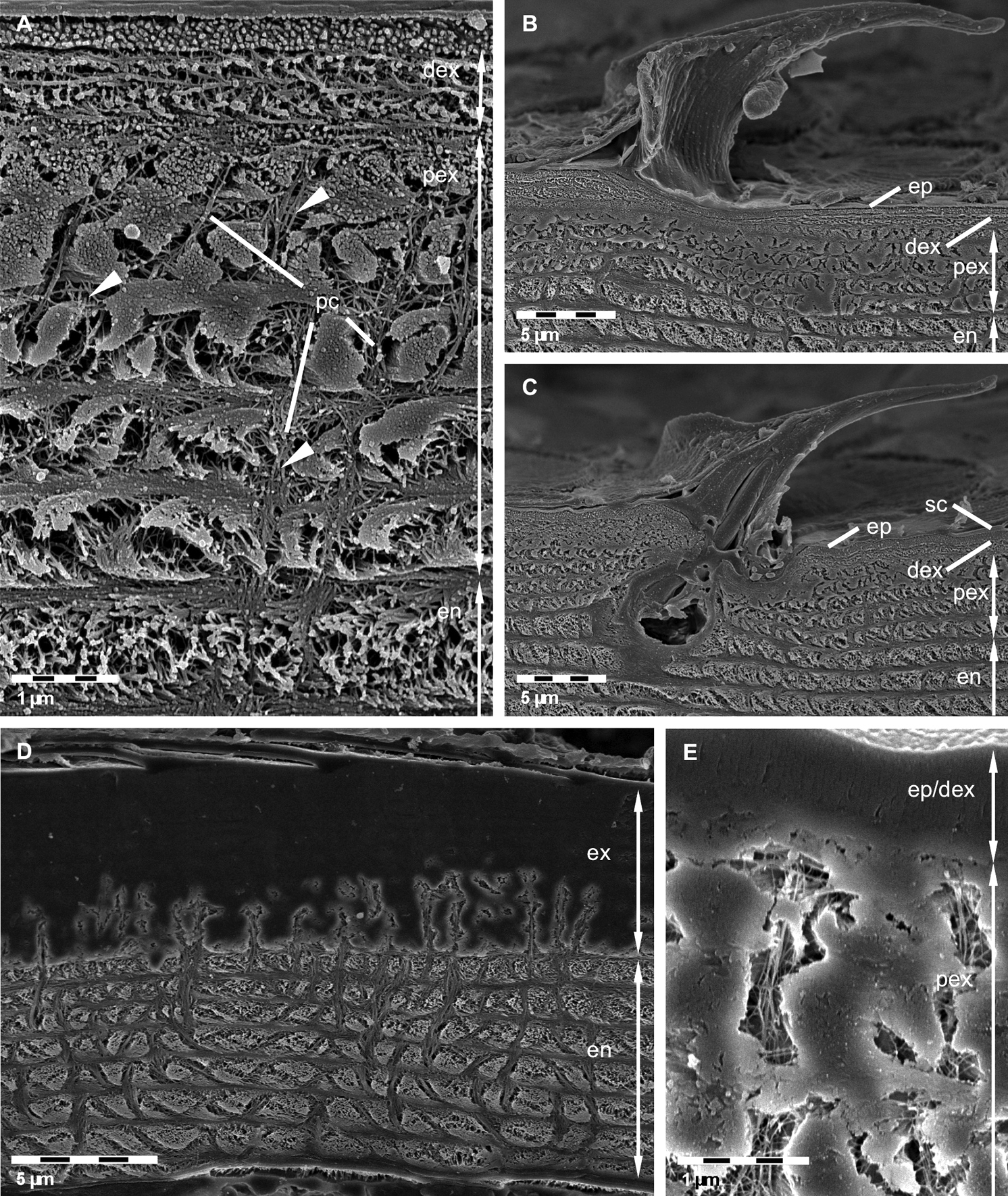
Etched surfaces are in particular suitable to visualize the distribution of organic fibrils or fibres within the pore canal system. In addition, because shrinkage due to demineralisation followed by drying of the cuticle can be minimised, some quantitative aspects of the cuticle, such as the stacking height and thickness of exo- and endocuticle can be measured more accurately. Just as in non-decalcified thin sections the distal exocuticle is approximately 1 µm thick. Fibres within the pore canals that are only faintly visible in TEM sections can be well observed (Figure 3A). Etching of polished samples can also be used to distinguish mineralised from non-mineralised structures. A side view of a tricorn sensillum is shown in Figure 3B. Non-mineralised epicuticular structures in the cutting plane can well be distinguished from surrounding mineralised cuticular layers (Figures 3B, C). Depending on the pH used for etching polished and etched cuticle surfaces can be used to discern regions differing in the solubility of the mineral phases e.g. between ACC and calcite. Etched at pH 6.5, fibres or fibrils in all principal layers are visible (Figures 3A–C). At pH 8.0, mineral was etched away in the endocuticle and partly in the exocuticle (Figure 3D). In the latter etching occurs mostly in proximal regions of the pore canals, sometimes also more distally, but not in the distal exocuticle (Figures 3D, E).
FE-SEM micrographs of polished sagittal plane through bulk tergite samples etched at pH 6.5 A, B, C and 8.0 D, E. A Fibres in the distal exocuticle (dex) and the isle-like structure of the proximal exocuticle (pex) caused by large pore canals (pc). Fibrils or fibres (arrowheads) in the pore canals are well visible. en, endocuticle. B, C Side views of tricorn sensilla. The epicuticular unmineralised material forming the sensilla is well distinguishable from the mineralised exo- and endocuticle. ep, epicuticle; sc, epicuticular scale. D, E Mild etching reveals regions containing mineral of different solubility. Mineral within the endocuticle appears etched whereas most regions within the exocuticle (ex) remain unaltered. Note etching within pore canals of the exocuticle.
Using improved preparation methods for conventional TEM, low voltage STEM and FESEM, we can confirm all general features of the tergite cuticle described in several previous publications on the integument cuticle of terrestrial isopods (
Simultaneous decalcification and fixing of samples allows for visualising the structure on fibre level within the distal exocuticle such as chitin crystallites embedded in a proteinaceous matrix. In Porcellio scaber these fibres have the same structure as those reported previously for the distal exocuticle in the tergites of Tylos europaeus (
Studies on the distribution of calcite and ACC within the cuticle of three terrestrial isopods using Raman spectroscopy and Raman imaging techniques (
Previous structural analysis of broken non-demineralised cuticle revealed two regions in the exocuticle of Porcellio scaber, Armadillidium vulgare, Titanethes albus und Tylos europaeus: a distal region in which broken faces appear smooth, hardly exposing any organic fibres (smooth layer), and a subjacent exocuticular layer in which stacks of mineralised twisted fibrils are well visible (
According to the model for insect cuticle, proteins form a helicoidal sheath around 19 anti-parallel chitin chains that form the approximately 3 nm thick crystallites (
Other important structural features of the crustacean cuticle are the distribution, shape and size of pore canals. The pore canal system serves not only as a transport system during cuticle mineralisation (
The present paper shows that investigation of crustacean cuticle by using an improved protocol for demineralisation and fixation for TEM/STEM, the analysis of thin sections of non-demineralised cuticle, and etching of polished cuticle samples can reveal new aspects of cuticular structure and mineral distribution. Combining the results from decalcified resin embedded specimens and those from thin sections of non-decalcified cuticle has led to the demonstration of a distinct distal exocuticle that differs in the organisation of the organic matrix at the level of chitin-protein fibrils and fibres, from those in the proximal exocuticle and the whole endocuticle. Thin sectioning of non-decalcified cuticle samples in combination with low voltage STEM is also a valuable method to gain new and detailed information about the distribution of mineral and organic fibrils/fibreswith high spatial resolution. In combination with TEM/STEM of resin embedded material, this method revealed details such as mineral filled pore canals and chitin-protein fibrils forming a twisted plywood structure without assembling in fibres. Etching of polished samples has the advantage that shrinkage of mineralised regions is minimised. This is of particular interest when length scales have to be quantified with high accuracy. Furthermore, etching reveals well the orientation of fibrous organic structures that, in combination with differential etching of amorphous and crystalline mineral phases, allows for the allocation of mineral phases to specific cuticular structures.
We like to thank Sukhum Ruangchai for his help on tergite preparation. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) within the priority program SPP 1420 (Zi 368/ 7-1).