(C) 2010 Benoit Vincent. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Two new species of Lophocampa Harris are described from the Dominican Republic, Lophocampa lineata sp. n. based on two males, and Lophocampa albitegula sp. n. based on three females. The habitus and genitalia are illustrated. The following nomenclatural changes are also proposed: Lophocampa albiguttata Boisduval, 1870, stat. rev. and Lophocampa brunnea Vincent, nom. n.
Phaegopterini, Arctiidae, Neotropics, new species, Dominican Republic, Lophocampa
The genus Lophocampa Harris includes 66 species and 10 subspecies in the most recent catalogue (
Specimens were collected in the Dominican Republic by
attraction to a mercury vapour light bulb, powered by a portable
generator. Trapping was done throughout the night from 6:30 pm to 6:30
am. Specimens were injected with ammonia and stored in labelled paper
envelopes. Dried specimens were subsequently relaxed in a humid
container, mounted and spread. Genitalia were prepared using a hot KOH
solution (10%). Illustrations were made using a camera attached to a
Leica MZ16 stereomicroscope. The genitalia were stained with
chlorazol-black to enhance membrane contrast with the cuticle, and were
mounted on slides using Euparal. Terminology for the genitalic
characters follows
The treatment of the Arctiidae at the family level follows that of
The “barcode” fragment of the mitochondrial cytochrome c
oxidase subunit 1 (CO1) gene was used to compare molecular variation
among taxa. Dried specimen legs, from the collection of the author,
were sent to the University of Guelph (Ontario, Canada) and sequenced
in the “All Leps Barcodes of Life Campaign” (BOLD). Extraction,
amplification and sequencing protocols are described in
Repository abbreviations are as follows:
AMNH American Museum of Natural History, New York, New York, USA
ANSP Academy of Natural Sciences, Philadelphia, Pennsylvania, USA
BMNH The Natural History Museum (formerly British Museum [Natural History]), London, UK
CMNH Carnegie Museum of Natural History, Pittsburgh, Pennsylvania, USA
MNHN Muséum National d’Histoire Naturelle, Paris, France
USNM National Museum of Natural History (formerly United States National Museum), Washington, DC, USA
ZMHB Museum für Naturkunde (formerly Zoologisches Museum, Humboldt Universität), Berlin, Germany
BVC Personal collection of Benoit Vincent, Saint-Denis, France
Systematicsurn:lsid:zoobank.org:act:B379F5E6-D5BF-4897-816A-4561CEB03FA9
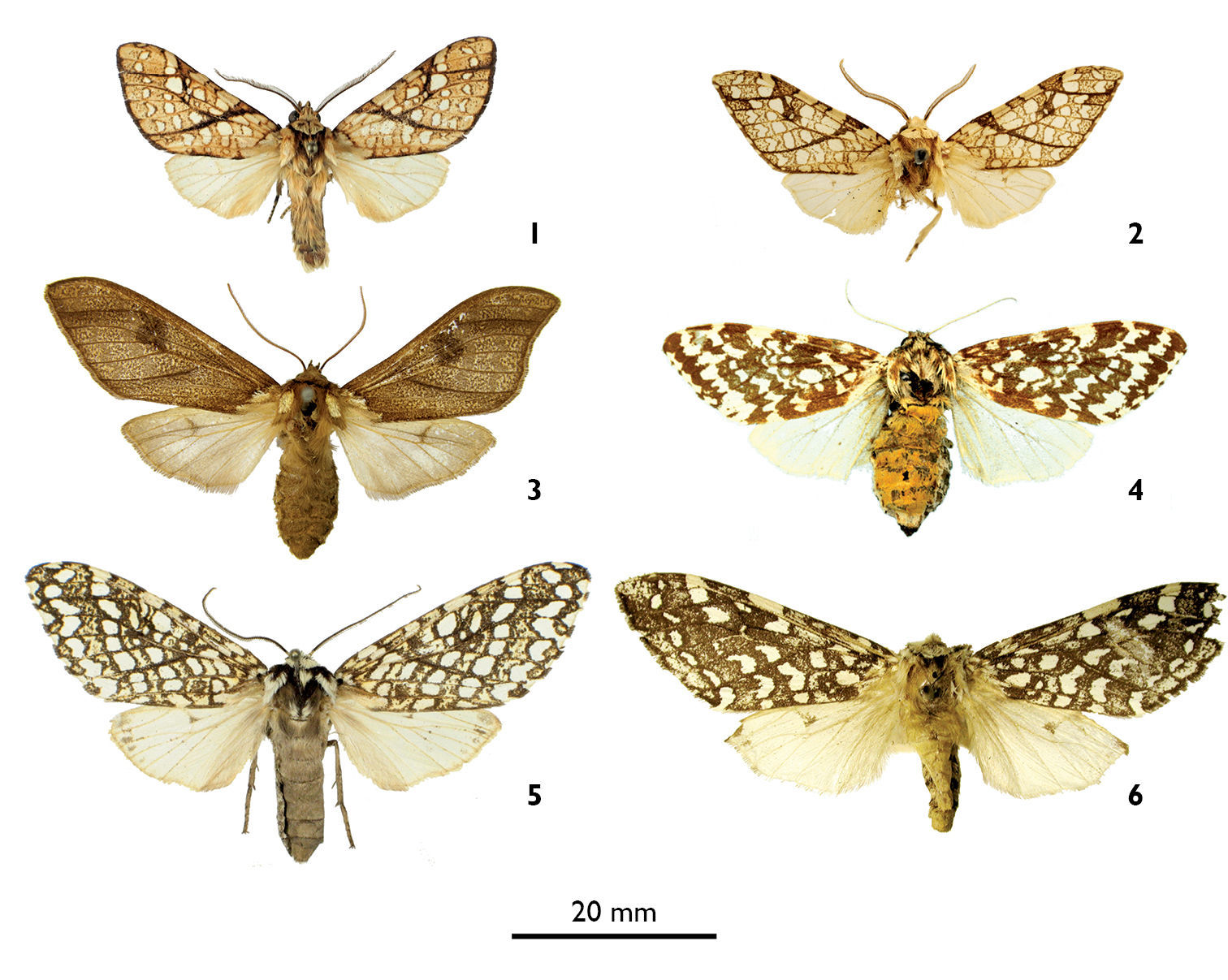
Figs 1, 7, 9Holotype – ♂, Dominican Republic, Monseñor Nouel, Road El Blanco to Constanza pK [kilometer post] 10, Ebano Verde Scientific Reserve, 1360 m, 15-VIII-2007, 19°01.729'N, 70°30.988'W, J. Haxaire and O. Paquit leg. prep gen BV 355, Barcode ID ARCTB 641–08, Sample ID BEVI0551, Genbank # HQ682628. Deposited in MNHN. Paratype. 1 ♂, same data as holotype, Barcode ID ARCTB 873–09, Sample ID BEVI0768, Genbank # HQ682627 ; in [BVC].
The name refers to the two brown transverse lines crossing the forewing.
Lophocampa lineata Vincent, sp. n. is externally similar to Lophocampa propinqua
1 Lophocampa lineata male holotype 2 Lophocampa propinqua male holotype 3 Lophocampa brunnea femaleholotype 4 Lophocampa alternata female holotype 5 Lophocampa albitegula female holotype 6 Lophocampa albiguttata female syntype potential.
Head. Labial palpi curved upward, the third segment shorter than the first two. Yellow ventrally, brown dorsally. Frons yellow with a transverse large brown band. Vertex and scape yellow. Male antenna yellow with dark pectinations, longest rami 3.0 x longer than segment length.
Thorax.Collar light yellow marked with two brown transverse lines located on each side of the median axis. Tegulaelight yellow with the internal side brown and with a light brownish spot centrally. Dorsal surface brown, with light yellow triangle centrally and the outer margins posteriorly light yellow. Ventral surface light yellow, very hairy. Prothorax brown. Meso- and metathorax light yellow. Legs yellow ringed with brown.
Forewing. Length: 18 mm. Ground yellow with white spot bands as follows: basal band with one white spot highlighted with brown. Postbasal, antemedian, median, postmedian and subterminal bands formed by rounded white spots intercalating between veins. Postbasal band broken The postmedian band contains a large spot between veins M1 and M2 which is out of alignment with the rest. Subterminal band does not reach the anal angle, with the last spot reduced and triangular. The wing is crossed by two narrow brown transverse lines, the longest one is wide at the costa then narrows following vein CuA2. The shorter line follows vein M1. Fringe brown.
Hindwing. White, semi-translucent; anal margin more densely scaled with yellow.
Abdomen. Light yellow both dorsally and ventrally.
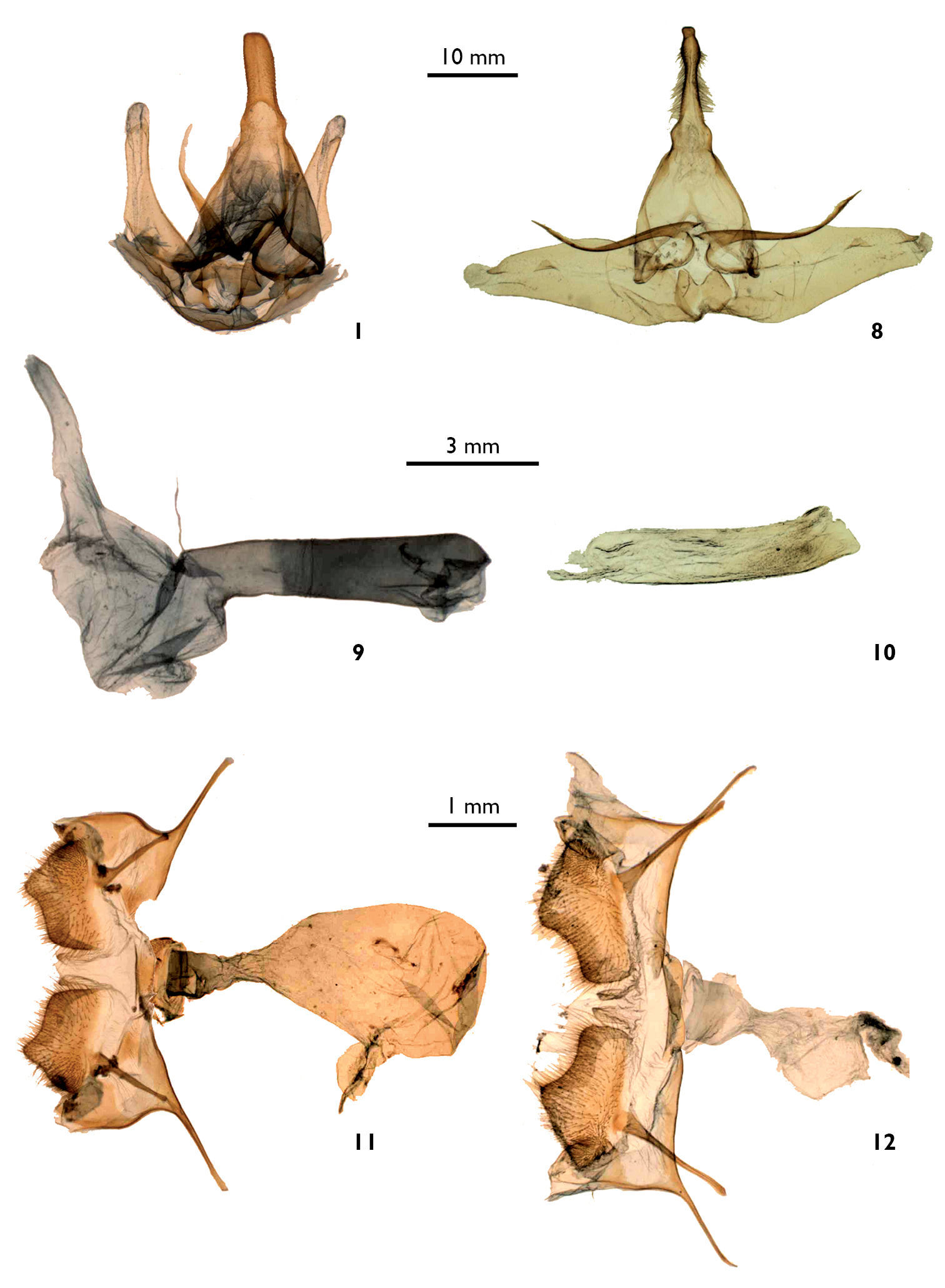
Male genitalia. Tegumen triangular, large basally. Uncus elongated and rectilinear, slightly enlarged basally, bearing lateral setae except on the apical third; apex truncated. Valvae symmetrical, elongated, reaching the middle of the uncus, bearing a small, rounded protuberance near the apex. Basally, on the dorsal face, presence of two elongated, thin and symmetrical protuberances reaching the base of the uncus. Juxta reduced, trapezoidal with a small median notch. Vinculum slender. Penis straight, caecum penis reduced. Vesica large consisting of three smaller elongated lobes, lacking spines.
Female genitalia. Unknown.
It is reasonable to think that Lophocampa lineata sp. n. is a species restricted to middle elevations of the central cordillera in the Dominican Republic. Previous exploration in this area in April and May of 2004 failed to find this taxon. The habitat is montane cloud forest. Early stages and foodplants are unknown.
Based on a single male from Mexico, Edwards described Lophocampa propinqua as a variation of Lophocampa caryae Harris, 1841. While examining the holotype in the AMNH collection, the specimen labelled propinqua
from Jalapa, Mexico was examined. It has a white label with an
unjustified lectotype designation by Allan Watson dated from 1967. The
taxon propinqua was placed by
Lophocampa caryae form montana Gaede, 1928 (Fig. 3) was described from Guatemala. In the catalogue of
The nomenclatural changes proposed here are summarized as follows:
urn:lsid:zoobank.org:act:7651F821-F591-46F8-88E0-D57107FFF38A
Figs 5, 11Holotype – ♀, Dominican Republic, Pedernales, track from los Arroyos to El Aguacate pK 5, 2, 1990m, 11-VIII-2007, 18°16.269'N, 71°43.496'W, J. Haxaire and O. Paquit leg. prep gen BV 386, Barcode ID ARCTB 638–08, Sample ID BEVI0548, HQ682631. Deposited in [MNHN]. Paratypes. 2 ♀, same data as holotype, Barcode ID ARCTB 648–08, Sample ID BEVI0558, HQ682630, prep gen BV 369, Barcode ID ARCTB 639–08, Sample ID BEVI0549, HQ682629 ; in [BVC].
The name reflects the white tegulae of the species.
This species is superficially most similar to Lophocampa albiguttata (Boisduval, 1870) (Fig. 6).It has a dark ground colour on the forewings, a subterminal band formed by irregular and flattened spots, a postmedian band formed by spots rounded at the costa then similar to those of the subterminal band. The legs are ringed brown and white. Female genitalia have posterior apophysis shorter than the anterior (longer than the anterior apophysis in Lophocampa albiguttata) and a smaller bursa(Figs 11 and 12).
7–8. Dorsal view of male genitalia. 7 Lophocampa lineata male holotype (dissection # BV 355) 8 Lophocampa propinqua male holotype (A. Watson dissection # 1246). 9–10. Lateral view of penis. 9 Lophocampa lineata male holotype 10 Lophocampa propinqua male holotype. 11–12. Female genitalia, ventral view. 11 Lophocampa albitegula female holotype (dissection # BV 386) 12 Lophocampa albigutata female (dissection # BV 388), (Mexico).
Head. Labial palpi dark, the third segment largely shorter than the two first. Frons dark, vertex white. Scape whitish. Antenna bipectinate, dark except the two first articles which are ochre.
Thorax. Collar white except a wide median dark spot on the anterior side. Tegulae white, hairy, with the internal side dark and the center marked with a wide dark spot. Dorsal side dark with a central white spot and the median axis whitish on the posterior side. Legs dark with some whitish spots.
Forewing. Length: 25 mmGround colour light beige, largely speckled with brown and ornamented with bands of white rounded spots underlined with brown.Basal band formed by an unique spot on the costa. Postbasal, antemedian, median, postmedian and subterminal bands formed by an alignment of white spots underlined with brown and intercalating between veins. Postbasal band broken. Antemedian formed by two white spots and two brown spots. Median band formed by two spots on the reniform spot. Postmedain almost straight and complete. Terminal band formed by irregular white spotssometimes fused with the sinuous subterminal spots.
Hindwing. White, semi-translucent; anal margin more densely scaled with brown scales. The fringe is checkered white and brownish from apex to vein CuA2.
Abdomen. Abdomen greyish.
Female genitalia. Pseudopapillae analeswholly fused. Papillae anales trapezoidandstrongly setose. Anterior apophysis straight, 1 mm in length.Posterior apophysis with a long basis, 1, 2 mm. Ductus bursaenarrow, non-sclerotized, reduced and wrinkled at the insertion with the corpus bursae. Corpus bursae large, ovoid, smooth without sigma.In its median area, insertion of a large ductus seminalis.
Male genitalia. Unknown.
It is reasonable to think that Lophocampa albitegula sp. n. is a species restricted to high elevations of the Sierra de Bahoruco in the Dominican Republic. It is probable that the new taxon could be present in the Sierra de Neiba. Previous exploration in this two area in April and May 2004 failed to find this taxon. The habitat is montane cloud forest. Early stages and foodplants are unknown.
Lophocampa albiguttata (Boisduval, 1870) is treated as a synonym of Lophocampa alternata (Grote, 1867) by
Lophocampa alternata was described and illustrated based on a single female specimen (“Number 743, Gundlach’s MS. Catalogue” Grote 1867: 319) from Cuba, and the holotype was in the ANSP. Jason Weintraub, collection manager of this Institution, provided pictures of a female specimen labelled “TYPE n° 7695 by A.R. Grote” and “HOLOTYPE ♀ by A[llan] W[atson] 1967”. This specimen not currently in the ANSP. It may have been transferred to CMNH by mistake, during a major exchange of specimens between ANSP and CMNH in the mid-1960s (J. Weintraub, pers. comm.). The presence of this specimen, and other type specimens described by Grote from Cuba, is not yet confirmed by the CMNH’s curator.
I thank Jean Haxaire and Odile Paquit for providing specimens of the two new species. I also thank Susan Rab-Green (AMNH), Don Harvey (USNM) and Jason Weintraub (ANSP) for providing images of the type specimens, Wolfang Mey (ZMHB), Alessandro Guisti (BMNH), Martin Honey (BMNH) for their hospitality during my visit in their Institutions and my friends Michel Laguerre and Antoine Lévêque for the reviews of this manuscript. The campaign ‘DNA barcoding’ for Arctiidae receives funding from Genome Canada and the Natural Sciences and Engineering Research Council of Canada (CRSNG), the molecular biology studies have been made to the CCDB (Canadian Centre for DNA Barcoding) by Rodolphe Rougerie, under the supervision of Professor Paul D. N. Hebert.